JP5405486B2 - 燃料電池システム - Google Patents
燃料電池システム Download PDFInfo
- Publication number
- JP5405486B2 JP5405486B2 JP2010540644A JP2010540644A JP5405486B2 JP 5405486 B2 JP5405486 B2 JP 5405486B2 JP 2010540644 A JP2010540644 A JP 2010540644A JP 2010540644 A JP2010540644 A JP 2010540644A JP 5405486 B2 JP5405486 B2 JP 5405486B2
- Authority
- JP
- Japan
- Prior art keywords
- fuel cell
- carbon dioxide
- conduit
- anode
- fuel
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000000446 fuel Substances 0.000 title claims description 394
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 claims description 294
- 239000001569 carbon dioxide Substances 0.000 claims description 147
- 229910002092 carbon dioxide Inorganic materials 0.000 claims description 147
- 239000012530 fluid Substances 0.000 claims description 81
- 239000000463 material Substances 0.000 claims description 68
- 238000001816 cooling Methods 0.000 claims description 41
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 34
- 229910052760 oxygen Inorganic materials 0.000 claims description 30
- 238000000034 method Methods 0.000 claims description 29
- 239000001301 oxygen Substances 0.000 claims description 29
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 27
- 238000004891 communication Methods 0.000 claims description 24
- 239000007789 gas Substances 0.000 claims description 23
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 21
- 239000003054 catalyst Substances 0.000 claims description 20
- 229910002091 carbon monoxide Inorganic materials 0.000 claims description 17
- 238000010438 heat treatment Methods 0.000 claims description 16
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 claims description 14
- 238000011069 regeneration method Methods 0.000 claims description 11
- 229910052739 hydrogen Inorganic materials 0.000 claims description 10
- 239000001257 hydrogen Substances 0.000 claims description 10
- 230000008929 regeneration Effects 0.000 claims description 10
- 238000006243 chemical reaction Methods 0.000 claims description 9
- 239000002737 fuel gas Substances 0.000 claims description 9
- 230000003134 recirculating effect Effects 0.000 claims description 7
- 230000002950 deficient Effects 0.000 claims description 5
- 150000003464 sulfur compounds Chemical class 0.000 claims description 3
- 210000004027 cell Anatomy 0.000 description 228
- 239000003570 air Substances 0.000 description 33
- 239000012528 membrane Substances 0.000 description 21
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 18
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical group NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 16
- 239000000919 ceramic Substances 0.000 description 12
- 229930195733 hydrocarbon Natural products 0.000 description 11
- 150000002430 hydrocarbons Chemical class 0.000 description 11
- 239000002250 absorbent Substances 0.000 description 10
- 230000002745 absorbent Effects 0.000 description 10
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 10
- 239000011261 inert gas Substances 0.000 description 9
- 239000003345 natural gas Substances 0.000 description 8
- -1 oxygen ions Chemical class 0.000 description 8
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 7
- 229910001882 dioxygen Inorganic materials 0.000 description 7
- 239000003792 electrolyte Substances 0.000 description 7
- 239000007787 solid Substances 0.000 description 7
- 239000004215 Carbon black (E152) Substances 0.000 description 6
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 6
- 230000005611 electricity Effects 0.000 description 6
- 238000010521 absorption reaction Methods 0.000 description 5
- 229910000288 alkali metal carbonate Inorganic materials 0.000 description 5
- 150000008041 alkali metal carbonates Chemical class 0.000 description 5
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 5
- 239000011575 calcium Substances 0.000 description 5
- 229940058020 2-amino-2-methyl-1-propanol Drugs 0.000 description 4
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 4
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 4
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 4
- CBTVGIZVANVGBH-UHFFFAOYSA-N aminomethyl propanol Chemical compound CC(C)(N)CO CBTVGIZVANVGBH-UHFFFAOYSA-N 0.000 description 4
- LVTYICIALWPMFW-UHFFFAOYSA-N diisopropanolamine Chemical compound CC(O)CNCC(C)O LVTYICIALWPMFW-UHFFFAOYSA-N 0.000 description 4
- 229910052759 nickel Inorganic materials 0.000 description 4
- 229920005597 polymer membrane Polymers 0.000 description 4
- 238000002407 reforming Methods 0.000 description 4
- 229910052717 sulfur Inorganic materials 0.000 description 4
- 239000011593 sulfur Substances 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 3
- 229910052788 barium Inorganic materials 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- VUZPPFZMUPKLLV-UHFFFAOYSA-N methane;hydrate Chemical compound C.O VUZPPFZMUPKLLV-UHFFFAOYSA-N 0.000 description 3
- CRVGTESFCCXCTH-UHFFFAOYSA-N methyl diethanolamine Chemical compound OCCN(C)CCO CRVGTESFCCXCTH-UHFFFAOYSA-N 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 3
- 229910052712 strontium Inorganic materials 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 239000006096 absorbing agent Substances 0.000 description 2
- 239000010405 anode material Substances 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 239000010406 cathode material Substances 0.000 description 2
- 239000011195 cermet Substances 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 239000004205 dimethyl polysiloxane Substances 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 229910044991 metal oxide Inorganic materials 0.000 description 2
- 150000004706 metal oxides Chemical class 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 239000007800 oxidant agent Substances 0.000 description 2
- 230000000737 periodic effect Effects 0.000 description 2
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000001172 regenerating effect Effects 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 229910001233 yttria-stabilized zirconia Inorganic materials 0.000 description 2
- 229910000975 Carbon steel Inorganic materials 0.000 description 1
- 229910002215 La0.9Sr0.1Ga0.8Mg0.2O3 Inorganic materials 0.000 description 1
- 229910052779 Neodymium Inorganic materials 0.000 description 1
- 229910052777 Praseodymium Inorganic materials 0.000 description 1
- 229910052772 Samarium Inorganic materials 0.000 description 1
- 229910021536 Zeolite Inorganic materials 0.000 description 1
- MCMNRKCIXSYSNV-UHFFFAOYSA-N ZrO2 Inorganic materials O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- BRPQOXSCLDDYGP-UHFFFAOYSA-N calcium oxide Chemical compound [O-2].[Ca+2] BRPQOXSCLDDYGP-UHFFFAOYSA-N 0.000 description 1
- ODINCKMPIJJUCX-UHFFFAOYSA-N calcium oxide Inorganic materials [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 description 1
- 239000000292 calcium oxide Substances 0.000 description 1
- 239000010962 carbon steel Substances 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 210000003850 cellular structure Anatomy 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- TVZPLCNGKSPOJA-UHFFFAOYSA-N copper zinc Chemical compound [Cu].[Zn] TVZPLCNGKSPOJA-UHFFFAOYSA-N 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 125000004386 diacrylate group Chemical group 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 1
- 239000002019 doping agent Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000002001 electrolyte material Substances 0.000 description 1
- 238000011066 ex-situ storage Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- JJSINHRPPHLULR-UHFFFAOYSA-N gadolinium(3+);oxygen(2-);titanium(4+) Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[O-2].[O-2].[Ti+4].[Ti+4].[Gd+3].[Gd+3] JJSINHRPPHLULR-UHFFFAOYSA-N 0.000 description 1
- 230000020169 heat generation Effects 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- SZVJSHCCFOBDDC-UHFFFAOYSA-N iron(II,III) oxide Inorganic materials O=[Fe]O[Fe]O[Fe]=O SZVJSHCCFOBDDC-UHFFFAOYSA-N 0.000 description 1
- 229910052747 lanthanoid Inorganic materials 0.000 description 1
- 150000002602 lanthanoids Chemical class 0.000 description 1
- 229910052746 lanthanum Inorganic materials 0.000 description 1
- 239000002905 metal composite material Substances 0.000 description 1
- 239000002808 molecular sieve Substances 0.000 description 1
- 239000002071 nanotube Substances 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920006254 polymer film Polymers 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 238000010248 power generation Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 239000007784 solid electrolyte Substances 0.000 description 1
- 239000002594 sorbent Substances 0.000 description 1
- 238000000629 steam reforming Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000008646 thermal stress Effects 0.000 description 1
- 239000010457 zeolite Substances 0.000 description 1
- 229910001928 zirconium oxide Inorganic materials 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04082—Arrangements for control of reactant parameters, e.g. pressure or concentration
- H01M8/04089—Arrangements for control of reactant parameters, e.g. pressure or concentration of gaseous reactants
- H01M8/04097—Arrangements for control of reactant parameters, e.g. pressure or concentration of gaseous reactants with recycling of the reactants
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04082—Arrangements for control of reactant parameters, e.g. pressure or concentration
- H01M8/04089—Arrangements for control of reactant parameters, e.g. pressure or concentration of gaseous reactants
- H01M8/04119—Arrangements for control of reactant parameters, e.g. pressure or concentration of gaseous reactants with simultaneous supply or evacuation of electrolyte; Humidifying or dehumidifying
- H01M8/04156—Arrangements for control of reactant parameters, e.g. pressure or concentration of gaseous reactants with simultaneous supply or evacuation of electrolyte; Humidifying or dehumidifying with product water removal
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/06—Combination of fuel cells with means for production of reactants or for treatment of residues
- H01M8/0662—Treatment of gaseous reactants or gaseous residues, e.g. cleaning
- H01M8/0668—Removal of carbon monoxide or carbon dioxide
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/06—Combination of fuel cells with means for production of reactants or for treatment of residues
- H01M8/0662—Treatment of gaseous reactants or gaseous residues, e.g. cleaning
- H01M8/0675—Removal of sulfur
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2257/00—Components to be removed
- B01D2257/50—Carbon oxides
- B01D2257/504—Carbon dioxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2258/00—Sources of waste gases
- B01D2258/02—Other waste gases
- B01D2258/0208—Other waste gases from fuel cells
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M8/12—Fuel cells with solid electrolytes operating at high temperature, e.g. with stabilised ZrO2 electrolyte
- H01M2008/1293—Fuel cells with solid oxide electrolytes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/04—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids
- H01M8/04007—Auxiliary arrangements, e.g. for control of pressure or for circulation of fluids related to heat exchange
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/06—Combination of fuel cells with means for production of reactants or for treatment of residues
- H01M8/0606—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants
- H01M8/0612—Combination of fuel cells with means for production of reactants or for treatment of residues with means for production of gaseous reactants from carbon-containing material
- H01M8/0618—Reforming processes, e.g. autothermal, partial oxidation or steam reforming
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02C—CAPTURE, STORAGE, SEQUESTRATION OR DISPOSAL OF GREENHOUSE GASES [GHG]
- Y02C20/00—Capture or disposal of greenhouse gases
- Y02C20/40—Capture or disposal of greenhouse gases of CO2
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Sustainable Development (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Sustainable Energy (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Fuel Cell (AREA)
Description
本願は、2007年12月28日出願の米国仮特許出願第61/009,418号明細書の利益を主張するものである。この特許出願の教示全体は参照により本明細書に援用される。
量%−70重量%のNiのようなNiを含むセラミック金属複合体を意味する。Niサーメットの例は、約15重量%のY2O3を含有するZrO2などのイットリア−安定化ジルコニア(YSZ)とNiを含む材料とNiとYSr−ジルコニアを含む材料である。
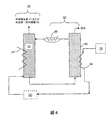
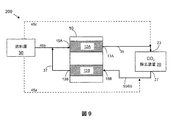
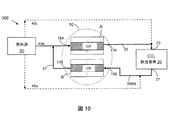
この実施例において、約50%電気効率を有するkW(キロワット)固体酸化物燃料電池(SOFC)システムは、MEAを含む吸収機22を含むCO2−除去装置および構造化充填材料またはポールリングのような充填材料ならびに冷却用部品24のための熱交換器で設計される。この実施例のSOFCシステムは、再生機62を含む再生装置60、加熱用部品64としての熱交換器、二酸化炭素除去装置と再生装置との間で循環する液体のために用いられるバブルポンプ、冷却用部品66のための熱交換器も含む。この実施例のCO2−除去装置20および再生装置60は以下の仕様を有する。
・燃料:天然ガス
・陽極排気におけるCO2成分流速:約0.01kgモル/hr=約0.44kg/hr=約4slpm
・吸収溶液のMEA濃度:約15重量%−50重量%MEA
・CO2捕捉能力:約0.3モルCO2/モルMEA
・MEA溶液循環速度:約4.1kg/hr
・MEA吸収溶液の吸収温度:約20℃−60℃の間
・使用済みMEAの再生温度:約80℃−約150℃の間
本発明の実例的な実施形態に関して本発明を詳細に示すとともに記載してきた一方で、添付した特許請求の範囲によって包含された本発明の範囲から逸脱せずに、形態および詳細事項における種々の変更を実施形態においてなし得ることは当業者によって理解されるであろう。
Claims (13)
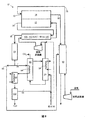
- a)陽極および陰極を各々が含む少なくとも1個の燃料電池を含む燃料電池組立品と、
b)二酸化炭素除去材料を含み、気相にある二酸化炭素を除去するように適合された二酸化炭素除去装置と、
c)前記燃料電池組立品と前記二酸化炭素除去装置を接続する陽極排気導管と、
d)それぞれが独立して前記燃料電池組立品に流体連通している燃料源および酸素源と、
e)前記燃料源を少なくとも部分的に前記燃料電池組立品に接続する燃料導管と、
f)前記燃料電池組立品、前記燃料導管および前記燃料源の少なくとも1つに前記二酸化炭素除去装置を接続する再循環導管であって、前記再循環導管が、前記燃料導管と一体化され、前記燃料電池組立品に伸びる単一の再循環−燃料導管になり、前記再循環導管および/または再循環導管−燃料導管がすべての気体状流体を前記二酸化炭素除去装置から前記燃料電池組立品に送る再循環導管と、
g)水および一酸化炭素を水素および二酸化炭素に転化する1種以上の触媒を含む水−ガス−転化装置であって、前記燃料電池組立品と前記二酸化炭素除去装置との間で伸びる前記陽極排気導管の一部にある水−ガス−転化装置と、
h)前記二酸化炭素除去装置と流体連通する再生装置であって、使用済み二酸化炭素除去材料を再生するように適合され、使用済み二酸化炭素除去材料を加熱するための加熱用部品を更に含み、且つ、前記燃料電池組立品と前記二酸化炭素除去装置との間の陽極排気導管の少なくとも一部が、該再生装置の少なくとも一部を覆って前記加熱用部品として機能する、再生装置と、
を含む、燃料電池システム。 - 前記燃料電池組立品と前記燃料源との間の点で前記陽極排気導管から分岐する流出導管であって、前記陽極排気導管から流出排気に流体の少なくとも一部を送る流出導管を更に含む請求項1に記載の燃料電池システム。
- 前記流出導管の下流にある点での空気−熱交換器装置であって、熱交換が前記流出導管から受領した流体と前記流出導管から受領した流体より低い温度を有する第2の流体との間で起きる空気−熱交換器装置を更に含む請求項2に記載の燃料電池システム。
- 前記燃料電池組立品と前記二酸化炭素除去装置との間で伸びる前記陽極排気導管の一部で冷却装置を更に含む請求項2に記載の燃料電池システム。
- 前記二酸化炭素除去装置はハウジングを含み、該ハウジングが二酸化炭素除去材料入口および二酸化炭素除去材料出口を更に画定し、前記再生装置が前記二酸化炭素除去材料出口および前記二酸化炭素除去材料入口に流体連通する請求項4に記載の燃料電池システム。
- 加熱用部品として機能する前記陽極排気導管の一部が陽極熱交換器であって、熱交換が前記陽極排気導管の流体と前記陽極排気導管の流体より低い温度を有する第2の流体との間で起きる、請求項5に記載の燃料電池システム。
- 燃料ガスを水素ガスに転化する触媒を含む改質装置またはプレ改質装置であって、前記再循環−燃料導管または前記再循環導管および前記燃料導管のいずれかにある改質装置またはプレ改質装置を更に含む請求項6に記載の燃料電池システム。
- 前記燃料電池組立品が第1の燃料電池および第2の燃料電池を含み、前記第1の燃料電池が第1の陽極入口および第1の陽極出口を含み、前記第1の陽極入口および第1の陽極出口の各々が、独立して、前記第1の燃料電池の陽極に流体連通しており、
前記第2の燃料電池が第2の陽極入口及び第2の陽極出口を含み、前記第2の陽極入口および第2の陽極出口の各々が、独立して、前記第2の燃料電池の陽極に流体連通している、請求項1に記載の燃料電池システム。 - 燃料電池システムにおいて陽極排気を再循環する方法であって、
a)水と一酸化炭素を水素と二酸化炭素に転化する1種以上の触媒を含む水−ガス−転化装置で、燃料電池組立品の少なくとも1個の燃料電池由来の陽極排気からの一酸化炭素と水の少なくとも一部を二酸化炭素と水素に転化する工程と、
b)前記水−ガス−転化装置から二酸化炭素除去材料を含む二酸化炭素除去装置に、前記陽極排気と二酸化炭素とを含むガス流を送る工程と、
c)前記二酸化炭素除去装置から再生装置に前記ガス流を送る工程と、
d)前記陽極排気からの熱を用いて前記使用済み二酸化炭素除去材料を加熱することにより、前記ガス流から二酸化炭素の少なくとも一部を除去して、それによって二酸化炭素欠乏陽極排気を形成する工程と、
e)前記燃料電池組立品の少なくとも1個の燃料電池に前記二酸化炭素欠乏陽極排気のすべてを送り、それによって前記陽極排気を再循環する工程と
を含む方法。 - 前記二酸化炭素除去装置が燃料ガスから硫黄化合物の少なくとも一部を更に除去する請求項9に記載の燃料電池システム。
- 前記水−ガス−転化装置を通り抜けた陽極排気の少なくとも一部を流出排気に送り、前記陽極排気の残りを前記二酸化炭素除去装置に送る工程を更に含む請求項9に記載の方法。
- 前記流出排気に送られる前記陽極排気の一部を、空気熱交換器を介して前記流出排気に送り、前記方法が、酸素源からの酸素ストリームを、前記空気熱交換器を介して少なくとも1個の前記燃料電池に送り、よって、前記陽極排気と前記酸素ストリームとの間で熱交換が起きる請求項11に記載の方法。
- 前記二酸化炭素除去装置での二酸化炭素の除去の前に、前記水−ガス−転化装置から前記二酸化炭素除去装置に送られた前記陽極排気を冷却装置で冷却して、それによって前記陽極排気から水の少なくとも一部を除去する工程を更に含む請求項11に記載の方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US941807P | 2007-12-28 | 2007-12-28 | |
| US61/009,418 | 2007-12-28 | ||
| PCT/US2008/013764 WO2009085155A1 (en) | 2007-12-28 | 2008-12-16 | Fuel cell system |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2011508949A JP2011508949A (ja) | 2011-03-17 |
| JP2011508949A5 JP2011508949A5 (ja) | 2013-11-07 |
| JP5405486B2 true JP5405486B2 (ja) | 2014-02-05 |
Family
ID=40445625
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010540644A Expired - Fee Related JP5405486B2 (ja) | 2007-12-28 | 2008-12-16 | 燃料電池システム |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8343671B2 (ja) |
| EP (1) | EP2235777A1 (ja) |
| JP (1) | JP5405486B2 (ja) |
| KR (1) | KR101299930B1 (ja) |
| WO (1) | WO2009085155A1 (ja) |
Families Citing this family (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20100063994A (ko) * | 2008-12-04 | 2010-06-14 | 삼성전자주식회사 | 연료 전지 시스템 내부의 연결 장치 및 이를 구비한 연료 전지 시스템 |
| TWI414101B (zh) * | 2010-11-09 | 2013-11-01 | Iner Aec Executive Yuan | 一種二氧化碳使用於固態氧化物燃料電池-二氧化碳能源轉化循環方法及其裝置 |
| CA2869384C (en) * | 2011-12-22 | 2017-11-21 | Renaissance Energy Research Corporation | Co shift conversion device and shift conversion method |
| US8945368B2 (en) | 2012-01-23 | 2015-02-03 | Battelle Memorial Institute | Separation and/or sequestration apparatus and methods |
| WO2013178430A1 (en) * | 2012-05-29 | 2013-12-05 | Topsøe Fuel Cell A/S | Pre-reforming of sulfur-containing fuels to produce syngas for use in fuel cell systems |
| US20140302410A1 (en) * | 2013-04-09 | 2014-10-09 | Arun K.S. Iyengar | High efficiency fuel cell system with anode gas chemical recuperation and carbon capture |
| US9755258B2 (en) * | 2013-09-30 | 2017-09-05 | Exxonmobil Research And Engineering Company | Integrated power generation and chemical production using solid oxide fuel cells |
| US10156373B1 (en) | 2015-05-15 | 2018-12-18 | The United States Of America, As Represented By The Secretary Of The Navy | Thermal integration of a catalytic burner and a carbon dioxide removal unit |
| EP3156363A1 (en) * | 2015-10-15 | 2017-04-19 | Casale SA | Process for making a synthesis gas by reforming of a hydrocarbon and including recovery of carbon dioxide at high pressure |
| JP6084314B1 (ja) * | 2016-01-26 | 2017-02-22 | 東京瓦斯株式会社 | 燃料電池システム |
| JP6322219B2 (ja) * | 2016-03-31 | 2018-05-09 | 本田技研工業株式会社 | 燃料電池システム |
| CA3021637C (en) | 2016-04-21 | 2021-07-06 | Fuelcell Energy, Inc. | Molten carbonate fuel cell anode exhaust post-processing for carbon dioxide capture |
| KR102153398B1 (ko) * | 2016-04-21 | 2020-09-08 | 퓨얼 셀 에너지, 인크 | 냉각/응축에 의해 연료전지의 애노드 배기물로부터 이산화탄소 제거 |
| US10840530B2 (en) * | 2016-04-21 | 2020-11-17 | Fuelcell Energy, Inc. | High efficiency fuel cell system with intermediate CO2 recovery system |
| CN116435559A (zh) | 2016-04-29 | 2023-07-14 | 燃料电池能有限公司 | 甲烷化阳极废气以提高二氧化碳捕获 |
| AT519416B1 (de) | 2016-11-29 | 2019-01-15 | Avl List Gmbh | Brennstoffzellensystem |
| DE102017001056A1 (de) * | 2017-02-04 | 2018-08-09 | Diehl Aerospace Gmbh | Verfahren und Vorrichtung zur Erzeugung von elektrischer Energie |
| JP6945315B2 (ja) * | 2017-03-24 | 2021-10-06 | 三菱重工業株式会社 | 発電プラント及び発電プラントの運転方法 |
| US10693158B2 (en) | 2017-10-26 | 2020-06-23 | Lg Electronics, Inc. | Methods of operating fuel cell systems with in-block reforming |
| US12355085B2 (en) | 2018-11-30 | 2025-07-08 | ExxonMobil Technology and Engineering Company | Cathode collector structures for molten carbonate fuel cell |
| WO2020112895A1 (en) | 2018-11-30 | 2020-06-04 | Exxonmobil Research And Engineering Company | Reforming catalyst pattern for fuel cell operated with enhanced co2 utilization |
| CA3121538C (en) | 2018-11-30 | 2023-09-12 | Exxonmobile Research And Engineering Company | Method for producing electricity in a molten carbonate fuel cell |
| KR102541753B1 (ko) * | 2018-12-18 | 2023-06-14 | 레르 리키드 쏘시에떼 아노님 뿌르 레드 에렉스뿔라따시옹 데 프로세데 조르즈 클로드 | 연료 전지와 증기 메탄 개질기를 통합하는 방법 |
| KR102153212B1 (ko) * | 2019-01-25 | 2020-09-07 | 제이플래닛 주식회사 | 겔형 구리 전구체의 제조방법 |
| JP7370792B2 (ja) * | 2019-09-30 | 2023-10-30 | 東京瓦斯株式会社 | 燃料電池システム、及び燃料電池システムの運転方法 |
| AU2019476338B2 (en) | 2019-11-26 | 2024-04-04 | ExxonMobil Technology and Engineering Company | Fuel cell module assembly and systems using same |
| CN120497393A (zh) | 2019-11-26 | 2025-08-15 | 埃克森美孚技术与工程公司 | 具有用于平行流动的外部歧管的燃料电池组件 |
| JP2021093320A (ja) * | 2019-12-12 | 2021-06-17 | 東京瓦斯株式会社 | 燃料電池システム、及び燃料電池システム運転方法 |
| CN118026095A (zh) | 2020-03-11 | 2024-05-14 | 燃料电池能有限公司 | 用于碳捕获的蒸汽甲烷重整单元 |
| KR102480268B1 (ko) * | 2020-12-22 | 2022-12-22 | 전북대학교산학협력단 | 고체산화물 연료전지 및 고체산화물 연료전지 활용 시스템 |
| US11555446B2 (en) * | 2021-06-11 | 2023-01-17 | Mitsubishi Power Americas, Inc. | Hybrid power plant with C02 capture |
| EP4511898A1 (en) * | 2022-04-21 | 2025-02-26 | Nuovo Pignone Tecnologie - S.R.L. | Low-emission power generation system and method |
| DK181747B1 (en) * | 2022-12-19 | 2024-11-22 | Blue World Technologies Holding ApS | Fuel cell system with separation of hydrogen gas from anode exhaust gas and method of its operation as well as use thereof |
| WO2024197095A2 (en) * | 2023-03-20 | 2024-09-26 | Solugen, Inc. | Compositions and methods for carbon dioxide capture and fluid sweetening |
Family Cites Families (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2886405A (en) | 1956-02-24 | 1959-05-12 | Benson Homer Edwin | Method for separating co2 and h2s from gas mixtures |
| US3144301A (en) | 1961-04-21 | 1964-08-11 | Girdler Corp | Removal of carbon dioxde from gaseous mixtures |
| US3932582A (en) | 1966-02-01 | 1976-01-13 | Eickmeyer Allen Garland | Method and compositions for removing acid gases from gaseous mixtures and reducing corrosion of ferrous surface areas in gas purification systems |
| US3615839A (en) | 1968-07-12 | 1971-10-26 | United Aircraft Corp | Fuel cell system with recycle stream |
| AU506199B2 (en) | 1975-06-26 | 1979-12-20 | Exxon Research And Engineering Company | Absorbtion of co2 from gaseous feeds |
| US4160810A (en) | 1978-03-07 | 1979-07-10 | Benfield Corporation | Removal of acid gases from hot gas mixtures |
| DE3117084A1 (de) | 1981-04-29 | 1982-11-18 | Linde Ag, 6200 Wiesbaden | Verfahren zum auswaschen von sauren gasen |
| JPS60165063A (ja) * | 1984-02-07 | 1985-08-28 | Ishikawajima Harima Heavy Ind Co Ltd | 燃料電池発電方法 |
| US4675035A (en) | 1986-02-24 | 1987-06-23 | Apffel Fred P | Carbon dioxide absorption methanol process |
| US4751151A (en) * | 1986-12-08 | 1988-06-14 | International Fuel Cells Corporation | Recovery of carbon dioxide from fuel cell exhaust |
| JP2802072B2 (ja) | 1988-06-08 | 1998-09-21 | 松下電器産業株式会社 | 溶融炭酸塩型燃料電池 |
| JPH02172159A (ja) * | 1988-12-24 | 1990-07-03 | Ishikawajima Harima Heavy Ind Co Ltd | 溶融炭酸塩型燃料電池発電方法及び装置 |
| DE3913581A1 (de) | 1989-04-25 | 1990-10-31 | Linde Ag | Verfahren zum betrieb von brennstoffzellen |
| DK162245C (da) | 1989-06-19 | 1992-02-17 | Haldor Topsoe As | Braendselscellesystem |
| EP0733396B1 (en) | 1991-10-09 | 2009-07-22 | The Kansai Electric Power Co., Inc. | Recovery of carbon dioxide from combustion exhaust gas |
| EP0553643B1 (en) | 1992-01-17 | 1998-05-13 | The Kansai Electric Power Co., Inc. | Method for treating combustion exhaust gas |
| JP3000118B2 (ja) * | 1992-08-04 | 2000-01-17 | 運輸省船舶技術研究所長 | 固体酸化物燃料電池を用い電力発生と同時に二酸化炭素を分離回収する方法 |
| JPH07302609A (ja) | 1994-05-06 | 1995-11-14 | Toshiba Corp | 燃料電池発電プラント |
| JPH09262432A (ja) | 1996-03-29 | 1997-10-07 | Kansai Electric Power Co Inc:The | 脱炭酸塔排ガス中の塩基性アミン化合物の回収方法 |
| AR010696A1 (es) | 1996-12-12 | 2000-06-28 | Sasol Tech Pty Ltd | Un metodo para la eliminacion del dioxido de carbono de un gas de proceso |
| US6277508B1 (en) | 1998-07-17 | 2001-08-21 | International Fuel Cells Corporation | Fuel cell power supply with exhaust recycling for improved water management |
| DE19908905C2 (de) | 1999-03-02 | 2003-03-20 | Daimler Chrysler Ag | Brennstoffzellensystem mit zugeordneter Wasserstofferzeugungsanlage |
| NO320939B1 (no) | 2002-12-10 | 2006-02-13 | Aker Kvaerner Engineering & Te | Fremgangsmate for eksosgassbehandling i brenselcellesystem basert pa oksider i fast form |
| JP4211434B2 (ja) | 2003-03-04 | 2009-01-21 | トヨタ自動車株式会社 | 水素分離装置 |
| JP2004288417A (ja) * | 2003-03-20 | 2004-10-14 | Mitsubishi Heavy Ind Ltd | 燃料電池発電システム |
| US6924053B2 (en) | 2003-03-24 | 2005-08-02 | Ion America Corporation | Solid oxide regenerative fuel cell with selective anode tail gas circulation |
| JP2004342413A (ja) | 2003-05-14 | 2004-12-02 | Toshiba Corp | 燃料電池システム |
| DE10339079A1 (de) | 2003-08-26 | 2005-03-24 | Forschungszentrum Jülich GmbH | Verfahren zur Erzeugung elektrischer Energie mit Hilfe einer Festelekrolyt-Brennstoffzelle |
| US20050123810A1 (en) * | 2003-12-09 | 2005-06-09 | Chellappa Balan | System and method for co-production of hydrogen and electrical energy |
| US7396603B2 (en) * | 2004-06-03 | 2008-07-08 | Fuelcell Energy, Inc. | Integrated high efficiency fossil fuel power plant/fuel cell system with CO2 emissions abatement |
| JP2006031989A (ja) * | 2004-07-13 | 2006-02-02 | Tokyo Gas Co Ltd | 固体酸化物形燃料電池による発電方法及びシステム |
| JP2006114373A (ja) * | 2004-10-15 | 2006-04-27 | Dainippon Printing Co Ltd | 単室型固体酸化物形燃料電池発電システム |
| JP4681277B2 (ja) * | 2004-11-11 | 2011-05-11 | 三菱重工業株式会社 | 燃料電池システム |
| US9911989B2 (en) * | 2005-07-25 | 2018-03-06 | Bloom Energy Corporation | Fuel cell system with partial recycling of anode exhaust |
| JP4687886B2 (ja) * | 2005-09-30 | 2011-05-25 | 三洋電機株式会社 | 燃料電池用水素発生装置 |
-
2008
- 2008-12-16 JP JP2010540644A patent/JP5405486B2/ja not_active Expired - Fee Related
- 2008-12-16 EP EP08868253A patent/EP2235777A1/en not_active Withdrawn
- 2008-12-16 US US12/316,802 patent/US8343671B2/en not_active Expired - Fee Related
- 2008-12-16 KR KR1020107015771A patent/KR101299930B1/ko not_active Expired - Fee Related
- 2008-12-16 WO PCT/US2008/013764 patent/WO2009085155A1/en not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| WO2009085155A1 (en) | 2009-07-09 |
| US20090169931A1 (en) | 2009-07-02 |
| EP2235777A1 (en) | 2010-10-06 |
| KR101299930B1 (ko) | 2013-08-27 |
| JP2011508949A (ja) | 2011-03-17 |
| US8343671B2 (en) | 2013-01-01 |
| KR20100093122A (ko) | 2010-08-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5405486B2 (ja) | 燃料電池システム | |
| JP2011508949A5 (ja) | ||
| JP5011673B2 (ja) | 燃料電池発電システム | |
| US8101307B2 (en) | Fuel cell system with electrochemical anode exhaust recycling | |
| US7833668B2 (en) | Fuel cell system with greater than 95% fuel utilization | |
| US9373856B2 (en) | Method of recycling and tapping off hydrogen for power generation apparatus | |
| US6623880B1 (en) | Fuel cell-fuel cell hybrid system | |
| US7883803B2 (en) | SOFC system producing reduced atmospheric carbon dioxide using a molten carbonated carbon dioxide pump | |
| US8530101B2 (en) | Anode exhaust recycle system | |
| US7846599B2 (en) | Method for high temperature fuel cell system start up and shutdown | |
| JP4450623B2 (ja) | 燃料電池システム | |
| JP4464692B2 (ja) | 燃料電池システム | |
| JP2005537621A (ja) | シフト膜バーナー/燃料電池の組み合わせ | |
| JP2004171802A (ja) | 燃料電池システム | |
| JP4166561B2 (ja) | 燃料電池発電システム | |
| JP2003317778A (ja) | 燃料電池の排ガス燃焼器、及び燃料電池発電システム | |
| JP5496504B2 (ja) | 水素生成装置を多段に備える燃料電池システム | |
| JP4741568B2 (ja) | 水素製造発電システムの水素製造方法 | |
| JP2003317772A (ja) | 燃料電池発電システム | |
| JP3969976B2 (ja) | 燃料改質システムおよび燃料電池システム | |
| JP2005203194A (ja) | 燃料電池システム | |
| JP2003100327A (ja) | 燃料電池システム |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20120809 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20121214 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20121214 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20121217 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130115 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20121228 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130415 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130422 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130514 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130521 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20130531 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130702 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20130917 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20131015 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20131030 |
|
| LAPS | Cancellation because of no payment of annual fees |