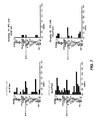
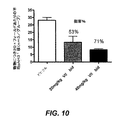
JP5204761B2 - ピリミジンジアミン化合物を使用することによる細胞増殖性障害を処置する方法 - Google Patents
ピリミジンジアミン化合物を使用することによる細胞増殖性障害を処置する方法 Download PDFInfo
- Publication number
- JP5204761B2 JP5204761B2 JP2009506662A JP2009506662A JP5204761B2 JP 5204761 B2 JP5204761 B2 JP 5204761B2 JP 2009506662 A JP2009506662 A JP 2009506662A JP 2009506662 A JP2009506662 A JP 2009506662A JP 5204761 B2 JP5204761 B2 JP 5204761B2
- Authority
- JP
- Japan
- Prior art keywords
- cell
- syk
- composition
- cells
- tumor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 230000002062 proliferating effect Effects 0.000 title claims abstract description 54
- 238000000034 method Methods 0.000 title abstract description 33
- YAAWASYJIRZXSZ-UHFFFAOYSA-N pyrimidine-2,4-diamine Chemical class NC1=CC=NC(N)=N1 YAAWASYJIRZXSZ-UHFFFAOYSA-N 0.000 title description 8
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 161
- 150000001875 compounds Chemical class 0.000 claims abstract description 155
- 101150110875 Syk gene Proteins 0.000 claims abstract description 114
- 102000000551 Syk Kinase Human genes 0.000 claims abstract description 89
- 108010016672 Syk Kinase Proteins 0.000 claims abstract description 89
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 81
- 229940043355 kinase inhibitor Drugs 0.000 claims abstract description 59
- 239000003757 phosphotransferase inhibitor Substances 0.000 claims abstract description 59
- 238000011282 treatment Methods 0.000 claims abstract description 46
- 206010027476 Metastases Diseases 0.000 claims abstract description 28
- 230000009401 metastasis Effects 0.000 claims abstract description 26
- 208000002250 Hematologic Neoplasms Diseases 0.000 claims abstract description 23
- 210000004027 cell Anatomy 0.000 claims description 244
- 230000000694 effects Effects 0.000 claims description 99
- -1 [1,4] oxazin-6-yl Chemical group 0.000 claims description 67
- 239000003112 inhibitor Substances 0.000 claims description 60
- 239000000203 mixture Substances 0.000 claims description 54
- 230000002401 inhibitory effect Effects 0.000 claims description 44
- 241000700605 Viruses Species 0.000 claims description 43
- 108090000623 proteins and genes Proteins 0.000 claims description 39
- 208000031261 Acute myeloid leukaemia Diseases 0.000 claims description 34
- 102000006495 integrins Human genes 0.000 claims description 31
- 108010044426 integrins Proteins 0.000 claims description 31
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 claims description 29
- 230000001404 mediated effect Effects 0.000 claims description 27
- 102000004169 proteins and genes Human genes 0.000 claims description 26
- 201000010099 disease Diseases 0.000 claims description 17
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 claims description 15
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 claims description 13
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 claims description 12
- 241000701044 Human gammaherpesvirus 4 Species 0.000 claims description 12
- 201000003793 Myelodysplastic syndrome Diseases 0.000 claims description 11
- 150000003839 salts Chemical class 0.000 claims description 11
- 230000011664 signaling Effects 0.000 claims description 11
- 206010006187 Breast cancer Diseases 0.000 claims description 10
- 208000019420 lymphoid neoplasm Diseases 0.000 claims description 10
- 208000012526 B-cell neoplasm Diseases 0.000 claims description 9
- 208000025317 T-cell and NK-cell neoplasm Diseases 0.000 claims description 8
- 208000026310 Breast neoplasm Diseases 0.000 claims description 7
- 208000011691 Burkitt lymphomas Diseases 0.000 claims description 7
- 206010061187 Haematopoietic neoplasm Diseases 0.000 claims description 7
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 claims description 7
- 201000005787 hematologic cancer Diseases 0.000 claims description 7
- 208000008720 Bone Marrow Neoplasms Diseases 0.000 claims description 6
- 102000008607 Integrin beta3 Human genes 0.000 claims description 6
- 108010020950 Integrin beta3 Proteins 0.000 claims description 6
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 6
- 206010038389 Renal cancer Diseases 0.000 claims description 6
- 201000010982 kidney cancer Diseases 0.000 claims description 6
- 208000014767 Myeloproliferative disease Diseases 0.000 claims description 5
- 150000001204 N-oxides Chemical class 0.000 claims description 5
- 208000029052 T-cell acute lymphoblastic leukemia Diseases 0.000 claims description 5
- 201000006491 bone marrow cancer Diseases 0.000 claims description 5
- 108020001507 fusion proteins Proteins 0.000 claims description 5
- 102000037865 fusion proteins Human genes 0.000 claims description 5
- 210000004072 lung Anatomy 0.000 claims description 5
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 5
- 239000012453 solvate Substances 0.000 claims description 5
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 claims description 4
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 claims description 4
- 108010059108 CD18 Antigens Proteins 0.000 claims description 4
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 4
- 238000003745 diagnosis Methods 0.000 claims description 4
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 4
- 201000002528 pancreatic cancer Diseases 0.000 claims description 4
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 4
- 206010005003 Bladder cancer Diseases 0.000 claims description 3
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 claims description 3
- 241001502974 Human gammaherpesvirus 8 Species 0.000 claims description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 3
- 102000012355 Integrin beta1 Human genes 0.000 claims description 3
- 108010022222 Integrin beta1 Proteins 0.000 claims description 3
- 206010033128 Ovarian cancer Diseases 0.000 claims description 3
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 3
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 claims description 3
- 208000009956 adenocarcinoma Diseases 0.000 claims description 3
- XGXNTJHZPBRBHJ-UHFFFAOYSA-N n-phenylpyrimidin-2-amine Chemical group N=1C=CC=NC=1NC1=CC=CC=C1 XGXNTJHZPBRBHJ-UHFFFAOYSA-N 0.000 claims description 3
- 206010041823 squamous cell carcinoma Diseases 0.000 claims description 3
- 238000012546 transfer Methods 0.000 claims description 3
- 201000005112 urinary bladder cancer Diseases 0.000 claims description 3
- 208000033833 Myelomonocytic Chronic Leukemia Diseases 0.000 claims description 2
- 201000010902 chronic myelomonocytic leukemia Diseases 0.000 claims description 2
- 208000003747 lymphoid leukemia Diseases 0.000 claims 2
- 241000714260 Human T-lymphotropic virus 1 Species 0.000 claims 1
- 201000007224 Myeloproliferative neoplasm Diseases 0.000 claims 1
- 101100335081 Mus musculus Flt3 gene Proteins 0.000 abstract description 110
- 230000005764 inhibitory process Effects 0.000 abstract description 16
- 230000001225 therapeutic effect Effects 0.000 abstract description 13
- 230000000069 prophylactic effect Effects 0.000 abstract description 7
- 241000699670 Mus sp. Species 0.000 description 84
- 208000035475 disorder Diseases 0.000 description 57
- 210000004881 tumor cell Anatomy 0.000 description 57
- 241001465754 Metazoa Species 0.000 description 55
- 239000003981 vehicle Substances 0.000 description 52
- 210000001185 bone marrow Anatomy 0.000 description 40
- 125000001424 substituent group Chemical group 0.000 description 38
- 241000282414 Homo sapiens Species 0.000 description 37
- 230000004083 survival effect Effects 0.000 description 34
- 208000032839 leukemia Diseases 0.000 description 32
- 230000002159 abnormal effect Effects 0.000 description 29
- 125000003118 aryl group Chemical group 0.000 description 28
- 108091000080 Phosphotransferase Proteins 0.000 description 25
- 102000020233 phosphotransferase Human genes 0.000 description 25
- 235000018102 proteins Nutrition 0.000 description 24
- 210000005259 peripheral blood Anatomy 0.000 description 23
- 239000011886 peripheral blood Substances 0.000 description 23
- 108700020796 Oncogene Proteins 0.000 description 22
- 230000004913 activation Effects 0.000 description 22
- 210000004369 blood Anatomy 0.000 description 22
- 239000008280 blood Substances 0.000 description 22
- 210000003719 b-lymphocyte Anatomy 0.000 description 21
- 230000014509 gene expression Effects 0.000 description 21
- 210000000130 stem cell Anatomy 0.000 description 21
- 230000006870 function Effects 0.000 description 20
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 20
- 210000001519 tissue Anatomy 0.000 description 20
- 230000012010 growth Effects 0.000 description 19
- 125000001072 heteroaryl group Chemical group 0.000 description 19
- 241000699666 Mus <mouse, genus> Species 0.000 description 17
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 description 15
- 125000000217 alkyl group Chemical group 0.000 description 15
- 238000002513 implantation Methods 0.000 description 15
- 238000011789 NOD SCID mouse Methods 0.000 description 14
- 238000011887 Necropsy Methods 0.000 description 14
- 230000004663 cell proliferation Effects 0.000 description 14
- 239000007924 injection Substances 0.000 description 14
- 229940090044 injection Drugs 0.000 description 14
- 238000002347 injection Methods 0.000 description 14
- 239000000758 substrate Substances 0.000 description 14
- 210000001744 T-lymphocyte Anatomy 0.000 description 13
- 201000011510 cancer Diseases 0.000 description 13
- 230000037361 pathway Effects 0.000 description 13
- 210000001948 pro-b lymphocyte Anatomy 0.000 description 13
- 230000035755 proliferation Effects 0.000 description 13
- 208000017604 Hodgkin disease Diseases 0.000 description 12
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 12
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 description 12
- 102000043276 Oncogene Human genes 0.000 description 12
- 238000003556 assay Methods 0.000 description 12
- 230000010261 cell growth Effects 0.000 description 12
- 238000009472 formulation Methods 0.000 description 12
- 239000000047 product Substances 0.000 description 12
- 238000010186 staining Methods 0.000 description 12
- 239000002246 antineoplastic agent Substances 0.000 description 11
- 125000003710 aryl alkyl group Chemical group 0.000 description 11
- 230000034994 death Effects 0.000 description 11
- 238000011161 development Methods 0.000 description 11
- 230000018109 developmental process Effects 0.000 description 11
- 238000001727 in vivo Methods 0.000 description 11
- 230000002829 reductive effect Effects 0.000 description 11
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 10
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 10
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 10
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 10
- 150000001413 amino acids Chemical group 0.000 description 10
- 229940127089 cytotoxic agent Drugs 0.000 description 10
- 239000003814 drug Substances 0.000 description 10
- 208000015181 infectious disease Diseases 0.000 description 10
- 230000001394 metastastic effect Effects 0.000 description 10
- 230000026731 phosphorylation Effects 0.000 description 10
- 238000006366 phosphorylation reaction Methods 0.000 description 10
- 238000002360 preparation method Methods 0.000 description 10
- 230000019491 signal transduction Effects 0.000 description 10
- 230000009466 transformation Effects 0.000 description 10
- 208000016261 weight loss Diseases 0.000 description 10
- 230000004580 weight loss Effects 0.000 description 10
- 125000003342 alkenyl group Chemical group 0.000 description 9
- 230000027455 binding Effects 0.000 description 9
- 230000032823 cell division Effects 0.000 description 9
- 230000001413 cellular effect Effects 0.000 description 9
- 238000002474 experimental method Methods 0.000 description 9
- 230000003394 haemopoietic effect Effects 0.000 description 9
- 206010061289 metastatic neoplasm Diseases 0.000 description 9
- 125000002950 monocyclic group Chemical group 0.000 description 9
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 8
- 108020004414 DNA Proteins 0.000 description 8
- 241001529936 Murinae Species 0.000 description 8
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 8
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 8
- 239000006180 TBST buffer Substances 0.000 description 8
- 108010040002 Tumor Suppressor Proteins Proteins 0.000 description 8
- 102000001742 Tumor Suppressor Proteins Human genes 0.000 description 8
- 230000001594 aberrant effect Effects 0.000 description 8
- 125000000304 alkynyl group Chemical group 0.000 description 8
- 238000004458 analytical method Methods 0.000 description 8
- 238000011888 autopsy Methods 0.000 description 8
- 229910052799 carbon Inorganic materials 0.000 description 8
- 229960004397 cyclophosphamide Drugs 0.000 description 8
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 8
- 230000003993 interaction Effects 0.000 description 8
- 210000004698 lymphocyte Anatomy 0.000 description 8
- 210000004379 membrane Anatomy 0.000 description 8
- 239000012528 membrane Substances 0.000 description 8
- 230000035772 mutation Effects 0.000 description 8
- 239000000243 solution Substances 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- 102100035634 B-cell linker protein Human genes 0.000 description 7
- 102000004190 Enzymes Human genes 0.000 description 7
- 108090000790 Enzymes Proteins 0.000 description 7
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 7
- 241000598436 Human T-cell lymphotropic virus Species 0.000 description 7
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 7
- FIWILGQIZHDAQG-UHFFFAOYSA-N NC1=C(C(=O)NCC2=CC=C(C=C2)OCC(F)(F)F)C=C(C(=N1)N)N1N=C(N=C1)C1(CC1)C(F)(F)F Chemical compound NC1=C(C(=O)NCC2=CC=C(C=C2)OCC(F)(F)F)C=C(C(=N1)N)N1N=C(N=C1)C1(CC1)C(F)(F)F FIWILGQIZHDAQG-UHFFFAOYSA-N 0.000 description 7
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 7
- 159000000007 calcium salts Chemical class 0.000 description 7
- 150000001721 carbon Chemical group 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- 229940079593 drug Drugs 0.000 description 7
- 229940088598 enzyme Drugs 0.000 description 7
- 125000004446 heteroarylalkyl group Chemical group 0.000 description 7
- 238000000338 in vitro Methods 0.000 description 7
- 238000001990 intravenous administration Methods 0.000 description 7
- 229940002612 prodrug Drugs 0.000 description 7
- 239000000651 prodrug Substances 0.000 description 7
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 7
- 230000009385 viral infection Effects 0.000 description 7
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical compound C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 description 6
- 101710083670 B-cell linker protein Proteins 0.000 description 6
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- 208000031404 Chromosome Aberrations Diseases 0.000 description 6
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 6
- LYAVXWPXKIFHBU-UHFFFAOYSA-N N-{2-[(1,2-diphenylhydrazinyl)carbonyl]-2-hydroxyhexanoyl}-6-aminohexanoic acid Chemical compound C=1C=CC=CC=1N(C(=O)C(O)(C(=O)NCCCCCC(O)=O)CCCC)NC1=CC=CC=C1 LYAVXWPXKIFHBU-UHFFFAOYSA-N 0.000 description 6
- 108010029485 Protein Isoforms Proteins 0.000 description 6
- 102000001708 Protein Isoforms Human genes 0.000 description 6
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 6
- RWRDLPDLKQPQOW-UHFFFAOYSA-N Pyrrolidine Chemical compound C1CCNC1 RWRDLPDLKQPQOW-UHFFFAOYSA-N 0.000 description 6
- 208000036142 Viral infection Diseases 0.000 description 6
- XSCHRSMBECNVNS-UHFFFAOYSA-N benzopyrazine Natural products N1=CC=NC2=CC=CC=C21 XSCHRSMBECNVNS-UHFFFAOYSA-N 0.000 description 6
- 238000004364 calculation method Methods 0.000 description 6
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 6
- 150000002148 esters Chemical class 0.000 description 6
- GVEPBJHOBDJJJI-UHFFFAOYSA-N fluoranthene Chemical compound C1=CC(C2=CC=CC=C22)=C3C2=CC=CC3=C1 GVEPBJHOBDJJJI-UHFFFAOYSA-N 0.000 description 6
- 210000003714 granulocyte Anatomy 0.000 description 6
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 231100000590 oncogenic Toxicity 0.000 description 6
- 230000002246 oncogenic effect Effects 0.000 description 6
- 239000008194 pharmaceutical composition Substances 0.000 description 6
- 102000005962 receptors Human genes 0.000 description 6
- 108020003175 receptors Proteins 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 229920006395 saturated elastomer Polymers 0.000 description 6
- 230000005945 translocation Effects 0.000 description 6
- 238000002054 transplantation Methods 0.000 description 6
- 230000003612 virological effect Effects 0.000 description 6
- 208000016683 Adult T-cell leukemia/lymphoma Diseases 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 5
- 206010011906 Death Diseases 0.000 description 5
- 102100033636 Histone H3.2 Human genes 0.000 description 5
- 108010033040 Histones Proteins 0.000 description 5
- 101000935040 Homo sapiens Integrin beta-2 Proteins 0.000 description 5
- 102100025390 Integrin beta-2 Human genes 0.000 description 5
- 108010002586 Interleukin-7 Proteins 0.000 description 5
- 208000007766 Kaposi sarcoma Diseases 0.000 description 5
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 5
- 201000006966 adult T-cell leukemia Diseases 0.000 description 5
- 230000010307 cell transformation Effects 0.000 description 5
- 125000004122 cyclic group Chemical group 0.000 description 5
- 230000001086 cytosolic effect Effects 0.000 description 5
- 230000004069 differentiation Effects 0.000 description 5
- 210000003743 erythrocyte Anatomy 0.000 description 5
- 239000003102 growth factor Substances 0.000 description 5
- 125000000623 heterocyclic group Chemical group 0.000 description 5
- 238000001114 immunoprecipitation Methods 0.000 description 5
- 238000001802 infusion Methods 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 125000005647 linker group Chemical group 0.000 description 5
- 210000001165 lymph node Anatomy 0.000 description 5
- 239000006166 lysate Substances 0.000 description 5
- 210000001616 monocyte Anatomy 0.000 description 5
- 201000000050 myeloid neoplasm Diseases 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 125000004433 nitrogen atom Chemical group N* 0.000 description 5
- 239000002243 precursor Substances 0.000 description 5
- 108090000765 processed proteins & peptides Proteins 0.000 description 5
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 230000009885 systemic effect Effects 0.000 description 5
- 231100000588 tumorigenic Toxicity 0.000 description 5
- 230000000381 tumorigenic effect Effects 0.000 description 5
- YBYIRNPNPLQARY-UHFFFAOYSA-N 1H-indene Chemical compound C1=CC=C2CC=CC2=C1 YBYIRNPNPLQARY-UHFFFAOYSA-N 0.000 description 4
- AXAVXPMQTGXXJZ-UHFFFAOYSA-N 2-aminoacetic acid;2-amino-2-(hydroxymethyl)propane-1,3-diol Chemical compound NCC(O)=O.OCC(N)(CO)CO AXAVXPMQTGXXJZ-UHFFFAOYSA-N 0.000 description 4
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 4
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 4
- UJOBWOGCFQCDNV-UHFFFAOYSA-N 9H-carbazole Chemical compound C1=CC=C2C3=CC=CC=C3NC2=C1 UJOBWOGCFQCDNV-UHFFFAOYSA-N 0.000 description 4
- 108700028369 Alleles Proteins 0.000 description 4
- 206010061818 Disease progression Diseases 0.000 description 4
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 4
- PCNDJXKNXGMECE-UHFFFAOYSA-N Phenazine Natural products C1=CC=CC2=NC3=CC=CC=C3N=C21 PCNDJXKNXGMECE-UHFFFAOYSA-N 0.000 description 4
- KYQCOXFCLRTKLS-UHFFFAOYSA-N Pyrazine Chemical compound C1=CN=CC=N1 KYQCOXFCLRTKLS-UHFFFAOYSA-N 0.000 description 4
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 description 4
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 4
- 206010042971 T-cell lymphoma Diseases 0.000 description 4
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 description 4
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical compound C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 description 4
- 150000001408 amides Chemical class 0.000 description 4
- 150000001412 amines Chemical class 0.000 description 4
- 235000001014 amino acid Nutrition 0.000 description 4
- 238000010171 animal model Methods 0.000 description 4
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 4
- 230000000259 anti-tumor effect Effects 0.000 description 4
- 230000006907 apoptotic process Effects 0.000 description 4
- CUFNKYGDVFVPHO-UHFFFAOYSA-N azulene Chemical compound C1=CC=CC2=CC=CC2=C1 CUFNKYGDVFVPHO-UHFFFAOYSA-N 0.000 description 4
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 230000036952 cancer formation Effects 0.000 description 4
- 125000004432 carbon atom Chemical group C* 0.000 description 4
- 238000012754 cardiac puncture Methods 0.000 description 4
- 238000000423 cell based assay Methods 0.000 description 4
- 230000033077 cellular process Effects 0.000 description 4
- 230000005754 cellular signaling Effects 0.000 description 4
- WDECIBYCCFPHNR-UHFFFAOYSA-N chrysene Chemical compound C1=CC=CC2=CC=C3C4=CC=CC=C4C=CC3=C21 WDECIBYCCFPHNR-UHFFFAOYSA-N 0.000 description 4
- VPUGDVKSAQVFFS-UHFFFAOYSA-N coronene Chemical compound C1=C(C2=C34)C=CC3=CC=C(C=C3)C4=C4C3=CC=C(C=C3)C4=C2C3=C1 VPUGDVKSAQVFFS-UHFFFAOYSA-N 0.000 description 4
- 230000000875 corresponding effect Effects 0.000 description 4
- 125000006165 cyclic alkyl group Chemical group 0.000 description 4
- 125000000753 cycloalkyl group Chemical group 0.000 description 4
- 230000003247 decreasing effect Effects 0.000 description 4
- 238000001514 detection method Methods 0.000 description 4
- 230000005750 disease progression Effects 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 125000000524 functional group Chemical group 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 4
- YLMAHDNUQAMNNX-UHFFFAOYSA-N imatinib methanesulfonate Chemical compound CS(O)(=O)=O.C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 YLMAHDNUQAMNNX-UHFFFAOYSA-N 0.000 description 4
- 238000011532 immunohistochemical staining Methods 0.000 description 4
- 238000000099 in vitro assay Methods 0.000 description 4
- PQNFLJBBNBOBRQ-UHFFFAOYSA-N indane Chemical compound C1=CC=C2CCCC2=C1 PQNFLJBBNBOBRQ-UHFFFAOYSA-N 0.000 description 4
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 4
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 4
- 238000011081 inoculation Methods 0.000 description 4
- 230000009545 invasion Effects 0.000 description 4
- AWJUIBRHMBBTKR-UHFFFAOYSA-N isoquinoline Chemical compound C1=NC=CC2=CC=CC=C21 AWJUIBRHMBBTKR-UHFFFAOYSA-N 0.000 description 4
- 239000003550 marker Substances 0.000 description 4
- 235000013336 milk Nutrition 0.000 description 4
- 239000008267 milk Substances 0.000 description 4
- 210000004080 milk Anatomy 0.000 description 4
- 210000000440 neutrophil Anatomy 0.000 description 4
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 4
- 230000002093 peripheral effect Effects 0.000 description 4
- YNPNZTXNASCQKK-UHFFFAOYSA-N phenanthrene Chemical compound C1=CC=C2C3=CC=CC=C3C=CC2=C1 YNPNZTXNASCQKK-UHFFFAOYSA-N 0.000 description 4
- RDOWQLZANAYVLL-UHFFFAOYSA-N phenanthridine Chemical compound C1=CC=C2C3=CC=CC=C3C=NC2=C1 RDOWQLZANAYVLL-UHFFFAOYSA-N 0.000 description 4
- GBROPGWFBFCKAG-UHFFFAOYSA-N picene Chemical compound C1=CC2=C3C=CC=CC3=CC=C2C2=C1C1=CC=CC=C1C=C2 GBROPGWFBFCKAG-UHFFFAOYSA-N 0.000 description 4
- 230000036470 plasma concentration Effects 0.000 description 4
- BBEAQIROQSPTKN-UHFFFAOYSA-N pyrene Chemical compound C1=CC=C2C=CC3=CC=CC4=CC=C1C2=C43 BBEAQIROQSPTKN-UHFFFAOYSA-N 0.000 description 4
- 108091008598 receptor tyrosine kinases Proteins 0.000 description 4
- 102000027426 receptor tyrosine kinases Human genes 0.000 description 4
- 238000007920 subcutaneous administration Methods 0.000 description 4
- 208000008732 thymoma Diseases 0.000 description 4
- 230000005740 tumor formation Effects 0.000 description 4
- AIFRHYZBTHREPW-UHFFFAOYSA-N β-carboline Chemical compound N1=CC=C2C3=CC=CC=C3NC2=C1 AIFRHYZBTHREPW-UHFFFAOYSA-N 0.000 description 4
- ZIIUUSVHCHPIQD-UHFFFAOYSA-N 2,4,6-trimethyl-N-[3-(trifluoromethyl)phenyl]benzenesulfonamide Chemical compound CC1=CC(C)=CC(C)=C1S(=O)(=O)NC1=CC=CC(C(F)(F)F)=C1 ZIIUUSVHCHPIQD-UHFFFAOYSA-N 0.000 description 3
- 125000004974 2-butenyl group Chemical group C(C=CC)* 0.000 description 3
- DQSBZDLZCZUJCJ-UHFFFAOYSA-N 2h-triazole-4,5-diamine Chemical compound NC=1N=NNC=1N DQSBZDLZCZUJCJ-UHFFFAOYSA-N 0.000 description 3
- GJCOSYZMQJWQCA-UHFFFAOYSA-N 9H-xanthene Chemical compound C1=CC=C2CC3=CC=CC=C3OC2=C1 GJCOSYZMQJWQCA-UHFFFAOYSA-N 0.000 description 3
- 208000009746 Adult T-Cell Leukemia-Lymphoma Diseases 0.000 description 3
- 108090000672 Annexin A5 Proteins 0.000 description 3
- 102000004121 Annexin A5 Human genes 0.000 description 3
- 102100026008 Breakpoint cluster region protein Human genes 0.000 description 3
- 208000005623 Carcinogenesis Diseases 0.000 description 3
- 206010008805 Chromosomal abnormalities Diseases 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 3
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 3
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical compound C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 3
- 108010064785 Phospholipases Proteins 0.000 description 3
- 102000015439 Phospholipases Human genes 0.000 description 3
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 3
- 102100020718 Receptor-type tyrosine-protein kinase FLT3 Human genes 0.000 description 3
- 241000283984 Rodentia Species 0.000 description 3
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 3
- 241000863480 Vinca Species 0.000 description 3
- 108700005077 Viral Genes Proteins 0.000 description 3
- 102000007624 ZAP-70 Protein-Tyrosine Kinase Human genes 0.000 description 3
- 108010046882 ZAP-70 Protein-Tyrosine Kinase Proteins 0.000 description 3
- 239000013543 active substance Substances 0.000 description 3
- 230000001154 acute effect Effects 0.000 description 3
- 229940100198 alkylating agent Drugs 0.000 description 3
- 239000002168 alkylating agent Substances 0.000 description 3
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 3
- 239000003242 anti bacterial agent Substances 0.000 description 3
- 230000000340 anti-metabolite Effects 0.000 description 3
- 229940088710 antibiotic agent Drugs 0.000 description 3
- 229940100197 antimetabolite Drugs 0.000 description 3
- 239000002256 antimetabolite Substances 0.000 description 3
- BVUSIQTYUVWOSX-UHFFFAOYSA-N arsindole Chemical compound C1=CC=C2[As]C=CC2=C1 BVUSIQTYUVWOSX-UHFFFAOYSA-N 0.000 description 3
- 125000004429 atom Chemical group 0.000 description 3
- 230000035578 autophosphorylation Effects 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 208000035269 cancer or benign tumor Diseases 0.000 description 3
- 231100000504 carcinogenesis Toxicity 0.000 description 3
- 230000021164 cell adhesion Effects 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- VZWXIQHBIQLMPN-UHFFFAOYSA-N chromane Chemical compound C1=CC=C2CCCOC2=C1 VZWXIQHBIQLMPN-UHFFFAOYSA-N 0.000 description 3
- QZHPTGXQGDFGEN-UHFFFAOYSA-N chromene Chemical compound C1=CC=C2C=C[CH]OC2=C1 QZHPTGXQGDFGEN-UHFFFAOYSA-N 0.000 description 3
- 125000004093 cyano group Chemical group *C#N 0.000 description 3
- 230000007547 defect Effects 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 3
- 231100000673 dose–response relationship Toxicity 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 210000002919 epithelial cell Anatomy 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 3
- 210000002744 extracellular matrix Anatomy 0.000 description 3
- RMBPEFMHABBEKP-UHFFFAOYSA-N fluorene Chemical compound C1=CC=C2C3=C[CH]C=CC3=CC2=C1 RMBPEFMHABBEKP-UHFFFAOYSA-N 0.000 description 3
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 3
- 108010003374 fms-Like Tyrosine Kinase 3 Proteins 0.000 description 3
- 239000012737 fresh medium Substances 0.000 description 3
- 210000002216 heart Anatomy 0.000 description 3
- 230000011132 hemopoiesis Effects 0.000 description 3
- 125000005842 heteroatom Chemical group 0.000 description 3
- 125000000592 heterocycloalkyl group Chemical group 0.000 description 3
- 102000056982 human CD33 Human genes 0.000 description 3
- 150000004677 hydrates Chemical class 0.000 description 3
- 229960002411 imatinib Drugs 0.000 description 3
- 230000036039 immunity Effects 0.000 description 3
- 125000001041 indolyl group Chemical group 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 3
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 3
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical compound C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 3
- QDLAGTHXVHQKRE-UHFFFAOYSA-N lichenxanthone Natural products COC1=CC(O)=C2C(=O)C3=C(C)C=C(OC)C=C3OC2=C1 QDLAGTHXVHQKRE-UHFFFAOYSA-N 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 210000002751 lymph Anatomy 0.000 description 3
- 239000012139 lysis buffer Substances 0.000 description 3
- 230000003211 malignant effect Effects 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 208000017869 myelodysplastic/myeloproliferative disease Diseases 0.000 description 3
- NIHNNTQXNPWCJQ-UHFFFAOYSA-N o-biphenylenemethane Natural products C1=CC=C2CC3=CC=CC=C3C2=C1 NIHNNTQXNPWCJQ-UHFFFAOYSA-N 0.000 description 3
- WCPAKWJPBJAGKN-UHFFFAOYSA-N oxadiazole Chemical compound C1=CON=N1 WCPAKWJPBJAGKN-UHFFFAOYSA-N 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- XDJOIMJURHQYDW-UHFFFAOYSA-N phenalene Chemical compound C1=CC(CC=C2)=C3C2=CC=CC3=C1 XDJOIMJURHQYDW-UHFFFAOYSA-N 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 210000001778 pluripotent stem cell Anatomy 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 102000004196 processed proteins & peptides Human genes 0.000 description 3
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 3
- 210000003079 salivary gland Anatomy 0.000 description 3
- 208000011581 secondary neoplasm Diseases 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 3
- 210000000952 spleen Anatomy 0.000 description 3
- 102000009076 src-Family Kinases Human genes 0.000 description 3
- 108010087686 src-Family Kinases Proteins 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- 238000004448 titration Methods 0.000 description 3
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 150000003852 triazoles Chemical class 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- 238000001262 western blot Methods 0.000 description 3
- 125000006708 (C5-C14) heteroaryl group Chemical group 0.000 description 2
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical group C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 2
- FLBAYUMRQUHISI-UHFFFAOYSA-N 1,8-naphthyridine Chemical compound N1=CC=CC2=CC=CN=C21 FLBAYUMRQUHISI-UHFFFAOYSA-N 0.000 description 2
- 125000004973 1-butenyl group Chemical group C(=CCC)* 0.000 description 2
- MFJCPDOGFAYSTF-UHFFFAOYSA-N 1H-isochromene Chemical compound C1=CC=C2COC=CC2=C1 MFJCPDOGFAYSTF-UHFFFAOYSA-N 0.000 description 2
- AAQTWLBJPNLKHT-UHFFFAOYSA-N 1H-perimidine Chemical compound N1C=NC2=CC=CC3=CC=CC1=C32 AAQTWLBJPNLKHT-UHFFFAOYSA-N 0.000 description 2
- VEPOHXYIFQMVHW-XOZOLZJESA-N 2,3-dihydroxybutanedioic acid (2S,3S)-3,4-dimethyl-2-phenylmorpholine Chemical compound OC(C(O)C(O)=O)C(O)=O.C[C@H]1[C@@H](OCCN1C)c1ccccc1 VEPOHXYIFQMVHW-XOZOLZJESA-N 0.000 description 2
- UXGVMFHEKMGWMA-UHFFFAOYSA-N 2-benzofuran Chemical compound C1=CC=CC2=COC=C21 UXGVMFHEKMGWMA-UHFFFAOYSA-N 0.000 description 2
- VHMICKWLTGFITH-UHFFFAOYSA-N 2H-isoindole Chemical compound C1=CC=CC2=CNC=C21 VHMICKWLTGFITH-UHFFFAOYSA-N 0.000 description 2
- MGADZUXDNSDTHW-UHFFFAOYSA-N 2H-pyran Chemical compound C1OC=CC=C1 MGADZUXDNSDTHW-UHFFFAOYSA-N 0.000 description 2
- JSIAIROWMJGMQZ-UHFFFAOYSA-N 2h-triazol-4-amine Chemical class NC1=CNN=N1 JSIAIROWMJGMQZ-UHFFFAOYSA-N 0.000 description 2
- KDOPAZIWBAHVJB-UHFFFAOYSA-N 5h-pyrrolo[3,2-d]pyrimidine Chemical compound C1=NC=C2NC=CC2=N1 KDOPAZIWBAHVJB-UHFFFAOYSA-N 0.000 description 2
- ZKHQWZAMYRWXGA-KQYNXXCUSA-J ATP(4-) Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)[C@H]1O ZKHQWZAMYRWXGA-KQYNXXCUSA-J 0.000 description 2
- 208000033496 Acute myeloid leukaemia with myelodysplasia-related features Diseases 0.000 description 2
- 208000036762 Acute promyelocytic leukaemia Diseases 0.000 description 2
- ZKHQWZAMYRWXGA-UHFFFAOYSA-N Adenosine triphosphate Natural products C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C(O)C1O ZKHQWZAMYRWXGA-UHFFFAOYSA-N 0.000 description 2
- NPDLYUOYAGBHFB-WDSKDSINSA-N Asn-Arg Chemical compound NC(=O)C[C@H](N)C(=O)N[C@H](C(O)=O)CCCN=C(N)N NPDLYUOYAGBHFB-WDSKDSINSA-N 0.000 description 2
- 108091008875 B cell receptors Proteins 0.000 description 2
- 238000000846 Bartlett's test Methods 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 125000001313 C5-C10 heteroaryl group Chemical group 0.000 description 2
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 108010067225 Cell Adhesion Molecules Proteins 0.000 description 2
- 102000016289 Cell Adhesion Molecules Human genes 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- FKLJPTJMIBLJAV-UHFFFAOYSA-N Compound IV Chemical compound O1N=C(C)C=C1CCCCCCCOC1=CC=C(C=2OCCN=2)C=C1 FKLJPTJMIBLJAV-UHFFFAOYSA-N 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 2
- 206010064571 Gene mutation Diseases 0.000 description 2
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 108060003951 Immunoglobulin Proteins 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 206010025323 Lymphomas Diseases 0.000 description 2
- 102000043136 MAP kinase family Human genes 0.000 description 2
- 108091054455 MAP kinase family Proteins 0.000 description 2
- 102100024193 Mitogen-activated protein kinase 1 Human genes 0.000 description 2
- 206010027906 Monocytosis Diseases 0.000 description 2
- 108091008606 PDGF receptors Proteins 0.000 description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 description 2
- 102000011653 Platelet-Derived Growth Factor Receptors Human genes 0.000 description 2
- 102000001253 Protein Kinase Human genes 0.000 description 2
- 102000003923 Protein Kinase C Human genes 0.000 description 2
- 108090000315 Protein Kinase C Proteins 0.000 description 2
- 108091005682 Receptor kinases Proteins 0.000 description 2
- 208000009527 Refractory anemia Diseases 0.000 description 2
- 206010072684 Refractory cytopenia with unilineage dysplasia Diseases 0.000 description 2
- 102000014400 SH2 domains Human genes 0.000 description 2
- 108050003452 SH2 domains Proteins 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 206010041660 Splenomegaly Diseases 0.000 description 2
- 241000282887 Suidae Species 0.000 description 2
- 108091008874 T cell receptors Proteins 0.000 description 2
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- SLGBZMMZGDRARJ-UHFFFAOYSA-N Triphenylene Natural products C1=CC=C2C3=CC=CC=C3C3=CC=CC=C3C2=C1 SLGBZMMZGDRARJ-UHFFFAOYSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- QVXFGVVYTKZLJN-KHPPLWFESA-N [(z)-hexadec-7-enyl] acetate Chemical compound CCCCCCCC\C=C/CCCCCCOC(C)=O QVXFGVVYTKZLJN-KHPPLWFESA-N 0.000 description 2
- DGEZNRSVGBDHLK-UHFFFAOYSA-N [1,10]phenanthroline Chemical compound C1=CN=C2C3=NC=CC=C3C=CC2=C1 DGEZNRSVGBDHLK-UHFFFAOYSA-N 0.000 description 2
- 230000005856 abnormality Effects 0.000 description 2
- 125000004054 acenaphthylenyl group Chemical group C1(=CC2=CC=CC3=CC=CC1=C23)* 0.000 description 2
- SQFPKRNUGBRTAR-UHFFFAOYSA-N acephenanthrylene Chemical group C1=CC(C=C2)=C3C2=CC2=CC=CC=C2C3=C1 SQFPKRNUGBRTAR-UHFFFAOYSA-N 0.000 description 2
- HXGDTGSAIMULJN-UHFFFAOYSA-N acetnaphthylene Natural products C1=CC(C=C2)=C3C2=CC=CC3=C1 HXGDTGSAIMULJN-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 208000016544 acute myeloid leukemia with multilineage dysplasia Diseases 0.000 description 2
- 230000001464 adherent effect Effects 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 150000001336 alkenes Chemical class 0.000 description 2
- 150000001345 alkine derivatives Chemical class 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 2
- 230000001093 anti-cancer Effects 0.000 description 2
- 230000030741 antigen processing and presentation Effects 0.000 description 2
- KNNXFYIMEYKHBZ-UHFFFAOYSA-N as-indacene Chemical compound C1=CC2=CC=CC2=C2C=CC=C21 KNNXFYIMEYKHBZ-UHFFFAOYSA-N 0.000 description 2
- 210000002469 basement membrane Anatomy 0.000 description 2
- 210000003651 basophil Anatomy 0.000 description 2
- ZYGHJZDHTFUPRJ-UHFFFAOYSA-N benzo-alpha-pyrone Natural products C1=CC=C2OC(=O)C=CC2=C1 ZYGHJZDHTFUPRJ-UHFFFAOYSA-N 0.000 description 2
- 125000002619 bicyclic group Chemical group 0.000 description 2
- 238000010256 biochemical assay Methods 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 210000000601 blood cell Anatomy 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 125000005510 but-1-en-2-yl group Chemical group 0.000 description 2
- CREMABGTGYGIQB-UHFFFAOYSA-N carbon carbon Chemical compound C.C CREMABGTGYGIQB-UHFFFAOYSA-N 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 230000003197 catalytic effect Effects 0.000 description 2
- 230000003915 cell function Effects 0.000 description 2
- 239000002771 cell marker Substances 0.000 description 2
- OTAFHZMPRISVEM-UHFFFAOYSA-N chromone Chemical compound C1=CC=C2C(=O)C=COC2=C1 OTAFHZMPRISVEM-UHFFFAOYSA-N 0.000 description 2
- WCZVZNOTHYJIEI-UHFFFAOYSA-N cinnoline Chemical compound N1=NC=CC2=CC=CC=C21 WCZVZNOTHYJIEI-UHFFFAOYSA-N 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 238000011109 contamination Methods 0.000 description 2
- 238000012937 correction Methods 0.000 description 2
- 230000002559 cytogenic effect Effects 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 210000004443 dendritic cell Anatomy 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 231100000676 disease causative agent Toxicity 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 108700004025 env Genes Proteins 0.000 description 2
- 101150030339 env gene Proteins 0.000 description 2
- 210000003979 eosinophil Anatomy 0.000 description 2
- 230000008029 eradication Effects 0.000 description 2
- 235000019441 ethanol Nutrition 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 239000013604 expression vector Substances 0.000 description 2
- 210000000416 exudates and transudate Anatomy 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 229910052736 halogen Inorganic materials 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 150000002367 halogens Chemical class 0.000 description 2
- QSQIGGCOCHABAP-UHFFFAOYSA-N hexacene Chemical compound C1=CC=CC2=CC3=CC4=CC5=CC6=CC=CC=C6C=C5C=C4C=C3C=C21 QSQIGGCOCHABAP-UHFFFAOYSA-N 0.000 description 2
- PKIFBGYEEVFWTJ-UHFFFAOYSA-N hexaphene Chemical compound C1=CC=C2C=C3C4=CC5=CC6=CC=CC=C6C=C5C=C4C=CC3=CC2=C1 PKIFBGYEEVFWTJ-UHFFFAOYSA-N 0.000 description 2
- 208000010726 hind limb paralysis Diseases 0.000 description 2
- 210000003630 histaminocyte Anatomy 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 125000002883 imidazolyl group Chemical group 0.000 description 2
- 150000002466 imines Chemical class 0.000 description 2
- 102000018358 immunoglobulin Human genes 0.000 description 2
- 238000003364 immunohistochemistry Methods 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000005462 in vivo assay Methods 0.000 description 2
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 2
- 150000002475 indoles Chemical class 0.000 description 2
- HOBCFUWDNJPFHB-UHFFFAOYSA-N indolizine Chemical compound C1=CC=CN2C=CC=C21 HOBCFUWDNJPFHB-UHFFFAOYSA-N 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 239000007928 intraperitoneal injection Substances 0.000 description 2
- 238000011835 investigation Methods 0.000 description 2
- GWVMLCQWXVFZCN-UHFFFAOYSA-N isoindoline Chemical compound C1=CC=C2CNCC2=C1 GWVMLCQWXVFZCN-UHFFFAOYSA-N 0.000 description 2
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 2
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical compound C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 description 2
- 238000000021 kinase assay Methods 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 231100000225 lethality Toxicity 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 230000000873 masking effect Effects 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 210000003593 megakaryocyte Anatomy 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 210000000822 natural killer cell Anatomy 0.000 description 2
- 230000009826 neoplastic cell growth Effects 0.000 description 2
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 2
- 102000037979 non-receptor tyrosine kinases Human genes 0.000 description 2
- 108091008046 non-receptor tyrosine kinases Proteins 0.000 description 2
- 230000036963 noncompetitive effect Effects 0.000 description 2
- 150000007523 nucleic acids Chemical group 0.000 description 2
- 210000004287 null lymphocyte Anatomy 0.000 description 2
- PFTXKXWAXWAZBP-UHFFFAOYSA-N octacene Chemical compound C1=CC=CC2=CC3=CC4=CC5=CC6=CC7=CC8=CC=CC=C8C=C7C=C6C=C5C=C4C=C3C=C21 PFTXKXWAXWAZBP-UHFFFAOYSA-N 0.000 description 2
- OVPVGJFDFSJUIG-UHFFFAOYSA-N octalene Chemical compound C1=CC=CC=C2C=CC=CC=CC2=C1 OVPVGJFDFSJUIG-UHFFFAOYSA-N 0.000 description 2
- WTFQBTLMPISHTA-UHFFFAOYSA-N octaphene Chemical compound C1=CC=C2C=C(C=C3C4=CC5=CC6=CC7=CC=CC=C7C=C6C=C5C=C4C=CC3=C3)C3=CC2=C1 WTFQBTLMPISHTA-UHFFFAOYSA-N 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 238000011275 oncology therapy Methods 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- PMJHHCWVYXUKFD-UHFFFAOYSA-N penta-1,3-diene Chemical compound CC=CC=C PMJHHCWVYXUKFD-UHFFFAOYSA-N 0.000 description 2
- SLIUAWYAILUBJU-UHFFFAOYSA-N pentacene Chemical compound C1=CC=CC2=CC3=CC4=CC5=CC=CC=C5C=C4C=C3C=C21 SLIUAWYAILUBJU-UHFFFAOYSA-N 0.000 description 2
- GUVXZFRDPCKWEM-UHFFFAOYSA-N pentalene Chemical compound C1=CC2=CC=CC2=C1 GUVXZFRDPCKWEM-UHFFFAOYSA-N 0.000 description 2
- 125000002080 perylenyl group Chemical group C1(=CC=C2C=CC=C3C4=CC=CC5=CC=CC(C1=C23)=C45)* 0.000 description 2
- CSHWQDPOILHKBI-UHFFFAOYSA-N peryrene Natural products C1=CC(C2=CC=CC=3C2=C2C=CC=3)=C3C2=CC=CC3=C1 CSHWQDPOILHKBI-UHFFFAOYSA-N 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 210000004214 philadelphia chromosome Anatomy 0.000 description 2
- 150000003905 phosphatidylinositols Chemical class 0.000 description 2
- LFSXCDWNBUNEEM-UHFFFAOYSA-N phthalazine Chemical compound C1=NN=CC2=CC=CC=C21 LFSXCDWNBUNEEM-UHFFFAOYSA-N 0.000 description 2
- LYKMMUBOEFYJQG-UHFFFAOYSA-N piperoxan Chemical compound C1OC2=CC=CC=C2OC1CN1CCCCC1 LYKMMUBOEFYJQG-UHFFFAOYSA-N 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 125000006238 prop-1-en-1-yl group Chemical group [H]\C(*)=C(/[H])C([H])([H])[H] 0.000 description 2
- 108060006633 protein kinase Proteins 0.000 description 2
- CPNGPNLZQNNVQM-UHFFFAOYSA-N pteridine Chemical compound N1=CN=CC2=NC=CN=C21 CPNGPNLZQNNVQM-UHFFFAOYSA-N 0.000 description 2
- PBMFSQRYOILNGV-UHFFFAOYSA-N pyridazine Chemical compound C1=CC=NN=C1 PBMFSQRYOILNGV-UHFFFAOYSA-N 0.000 description 2
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 2
- 230000002285 radioactive effect Effects 0.000 description 2
- 230000000306 recurrent effect Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 125000006413 ring segment Chemical group 0.000 description 2
- FMKFBRKHHLWKDB-UHFFFAOYSA-N rubicene Chemical compound C12=CC=CC=C2C2=CC=CC3=C2C1=C1C=CC=C2C4=CC=CC=C4C3=C21 FMKFBRKHHLWKDB-UHFFFAOYSA-N 0.000 description 2
- WEMQMWWWCBYPOV-UHFFFAOYSA-N s-indacene Chemical compound C=1C2=CC=CC2=CC2=CC=CC2=1 WEMQMWWWCBYPOV-UHFFFAOYSA-N 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- 108091006024 signal transducing proteins Proteins 0.000 description 2
- 102000034285 signal transducing proteins Human genes 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 125000003107 substituted aryl group Chemical group 0.000 description 2
- 229940124530 sulfonamide Drugs 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 208000011580 syndromic disease Diseases 0.000 description 2
- 150000003536 tetrazoles Chemical class 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- VLLMWSRANPNYQX-UHFFFAOYSA-N thiadiazole Chemical compound C1=CSN=N1.C1=CSN=N1 VLLMWSRANPNYQX-UHFFFAOYSA-N 0.000 description 2
- 229930192474 thiophene Natural products 0.000 description 2
- 210000001541 thymus gland Anatomy 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 102000035160 transmembrane proteins Human genes 0.000 description 2
- 108091005703 transmembrane proteins Proteins 0.000 description 2
- 125000005580 triphenylene group Chemical group 0.000 description 2
- 230000004614 tumor growth Effects 0.000 description 2
- 238000013414 tumor xenograft model Methods 0.000 description 2
- 241001529453 unidentified herpesvirus Species 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 1
- 125000006570 (C5-C6) heteroaryl group Chemical group 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 1
- YJZSUCFGHXQWDM-UHFFFAOYSA-N 1-adamantyl 4-[(2,5-dihydroxyphenyl)methylamino]benzoate Chemical compound OC1=CC=C(O)C(CNC=2C=CC(=CC=2)C(=O)OC23CC4CC(CC(C4)C2)C3)=C1 YJZSUCFGHXQWDM-UHFFFAOYSA-N 0.000 description 1
- 102100025573 1-alkyl-2-acetylglycerophosphocholine esterase Human genes 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 description 1
- XWIYUCRMWCHYJR-UHFFFAOYSA-N 1h-pyrrolo[3,2-b]pyridine Chemical compound C1=CC=C2NC=CC2=N1 XWIYUCRMWCHYJR-UHFFFAOYSA-N 0.000 description 1
- VOXBZHOHGGBLCQ-UHFFFAOYSA-N 2-amino-3,7-dihydropurine-6-thione;hydrate Chemical compound O.N1C(N)=NC(=S)C2=C1N=CN2.N1C(N)=NC(=S)C2=C1N=CN2 VOXBZHOHGGBLCQ-UHFFFAOYSA-N 0.000 description 1
- CTRPRMNBTVRDFH-UHFFFAOYSA-N 2-n-methyl-1,3,5-triazine-2,4,6-triamine Chemical compound CNC1=NC(N)=NC(N)=N1 CTRPRMNBTVRDFH-UHFFFAOYSA-N 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- RZFZKQGEQZUQKS-UHFFFAOYSA-N 2-pyrimidin-4-yl-1,3-thiazole Chemical group C1=CSC(C=2N=CN=CC=2)=N1 RZFZKQGEQZUQKS-UHFFFAOYSA-N 0.000 description 1
- 102100037263 3-phosphoinositide-dependent protein kinase 1 Human genes 0.000 description 1
- BWCDLEQTELFBAW-UHFFFAOYSA-N 3h-dioxazole Chemical compound N1OOC=C1 BWCDLEQTELFBAW-UHFFFAOYSA-N 0.000 description 1
- 206010068532 5q minus syndrome Diseases 0.000 description 1
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 1
- 208000016557 Acute basophilic leukemia Diseases 0.000 description 1
- 206010000871 Acute monocytic leukaemia Diseases 0.000 description 1
- 206010000890 Acute myelomonocytic leukaemia Diseases 0.000 description 1
- 208000016585 Acute panmyelosis with myelofibrosis Diseases 0.000 description 1
- 206010001413 Adult T-cell lymphoma/leukaemia Diseases 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 235000019489 Almond oil Nutrition 0.000 description 1
- 206010073478 Anaplastic large-cell lymphoma Diseases 0.000 description 1
- 101000719121 Arabidopsis thaliana Protein MEI2-like 1 Proteins 0.000 description 1
- IYMAXBFPHPZYIK-BQBZGAKWSA-N Arg-Gly-Asp Chemical class NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O IYMAXBFPHPZYIK-BQBZGAKWSA-N 0.000 description 1
- 108010024976 Asparaginase Proteins 0.000 description 1
- 102000004000 Aurora Kinase A Human genes 0.000 description 1
- 108090000461 Aurora Kinase A Proteins 0.000 description 1
- 102000003989 Aurora kinases Human genes 0.000 description 1
- 108090000433 Aurora kinases Proteins 0.000 description 1
- NOWKCMXCCJGMRR-UHFFFAOYSA-N Aziridine Chemical compound C1CN1 NOWKCMXCCJGMRR-UHFFFAOYSA-N 0.000 description 1
- 208000025324 B-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000003950 B-cell lymphoma Diseases 0.000 description 1
- 208000032568 B-cell prolymphocytic leukaemia Diseases 0.000 description 1
- 206010003908 B-cell small lymphocytic lymphoma Diseases 0.000 description 1
- 208000033775 Basophilic Acute Leukemia Diseases 0.000 description 1
- 208000035462 Biphenotypic Acute Leukemia Diseases 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 208000031648 Body Weight Changes Diseases 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 206010055113 Breast cancer metastatic Diseases 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- NKDLAMYVMOWZOK-UHFFFAOYSA-N C1=CC=C2CCCC2=C1.C1=CC=C2CC=CC2=C1 Chemical compound C1=CC=C2CCCC2=C1.C1=CC=C2CC=CC2=C1 NKDLAMYVMOWZOK-UHFFFAOYSA-N 0.000 description 1
- 229940045513 CTLA4 antagonist Drugs 0.000 description 1
- 206010006895 Cachexia Diseases 0.000 description 1
- 102000000905 Cadherin Human genes 0.000 description 1
- 108050007957 Cadherin Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 102100040428 Chitobiosyldiphosphodolichol beta-mannosyltransferase Human genes 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 102000010958 Cortactin Human genes 0.000 description 1
- 108010037663 Cortactin Proteins 0.000 description 1
- 108010068192 Cyclin A Proteins 0.000 description 1
- 102000002427 Cyclin B Human genes 0.000 description 1
- 108010068150 Cyclin B Proteins 0.000 description 1
- 108010058545 Cyclin D3 Proteins 0.000 description 1
- 102000003909 Cyclin E Human genes 0.000 description 1
- 108090000257 Cyclin E Proteins 0.000 description 1
- 102000002495 Cyclin H Human genes 0.000 description 1
- 108010068237 Cyclin H Proteins 0.000 description 1
- 102100025191 Cyclin-A2 Human genes 0.000 description 1
- 108010024986 Cyclin-Dependent Kinase 2 Proteins 0.000 description 1
- 108010025454 Cyclin-Dependent Kinase 5 Proteins 0.000 description 1
- 108010025468 Cyclin-Dependent Kinase 6 Proteins 0.000 description 1
- 102100032857 Cyclin-dependent kinase 1 Human genes 0.000 description 1
- 101710106279 Cyclin-dependent kinase 1 Proteins 0.000 description 1
- 102100036239 Cyclin-dependent kinase 2 Human genes 0.000 description 1
- 102100036329 Cyclin-dependent kinase 3 Human genes 0.000 description 1
- 102100026804 Cyclin-dependent kinase 6 Human genes 0.000 description 1
- 102100026810 Cyclin-dependent kinase 7 Human genes 0.000 description 1
- 102100026805 Cyclin-dependent-like kinase 5 Human genes 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- 241000450599 DNA viruses Species 0.000 description 1
- 102000052510 DNA-Binding Proteins Human genes 0.000 description 1
- 108700020911 DNA-Binding Proteins Proteins 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- 101100015729 Drosophila melanogaster drk gene Proteins 0.000 description 1
- 102100031480 Dual specificity mitogen-activated protein kinase kinase 1 Human genes 0.000 description 1
- 101710146526 Dual specificity mitogen-activated protein kinase kinase 1 Proteins 0.000 description 1
- 206010058314 Dysplasia Diseases 0.000 description 1
- 208000006586 Ectromelia Diseases 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 206010015108 Epstein-Barr virus infection Diseases 0.000 description 1
- 208000031637 Erythroblastic Acute Leukemia Diseases 0.000 description 1
- 102000016955 Erythrocyte Anion Exchange Protein 1 Human genes 0.000 description 1
- 108010014384 Erythrocyte Anion Exchange Protein 1 Proteins 0.000 description 1
- 208000036566 Erythroleukaemia Diseases 0.000 description 1
- 208000032027 Essential Thrombocythemia Diseases 0.000 description 1
- 108090000371 Esterases Proteins 0.000 description 1
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical group CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
- 108010007457 Extracellular Signal-Regulated MAP Kinases Proteins 0.000 description 1
- 208000016937 Extranodal nasal NK/T cell lymphoma Diseases 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 102100027842 Fibroblast growth factor receptor 3 Human genes 0.000 description 1
- 101710182396 Fibroblast growth factor receptor 3 Proteins 0.000 description 1
- 238000000729 Fisher's exact test Methods 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- 102100037859 G1/S-specific cyclin-D3 Human genes 0.000 description 1
- 208000034951 Genetic Translocation Diseases 0.000 description 1
- 208000031448 Genomic Instability Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 102000019058 Glycogen Synthase Kinase 3 beta Human genes 0.000 description 1
- 108010051975 Glycogen Synthase Kinase 3 beta Proteins 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- 102100033067 Growth factor receptor-bound protein 2 Human genes 0.000 description 1
- 101150113453 Gsk3a gene Proteins 0.000 description 1
- 241000700586 Herpesviridae Species 0.000 description 1
- 102000002268 Hexosaminidases Human genes 0.000 description 1
- 108010000540 Hexosaminidases Proteins 0.000 description 1
- 101000600756 Homo sapiens 3-phosphoinositide-dependent protein kinase 1 Proteins 0.000 description 1
- 101000803266 Homo sapiens B-cell linker protein Proteins 0.000 description 1
- 101000891557 Homo sapiens Chitobiosyldiphosphodolichol beta-mannosyltransferase Proteins 0.000 description 1
- 101000715946 Homo sapiens Cyclin-dependent kinase 3 Proteins 0.000 description 1
- 101000911952 Homo sapiens Cyclin-dependent kinase 7 Proteins 0.000 description 1
- 101000871017 Homo sapiens Growth factor receptor-bound protein 2 Proteins 0.000 description 1
- 101001035237 Homo sapiens Integrin alpha-D Proteins 0.000 description 1
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 1
- 101000578774 Homo sapiens MAP kinase-activated protein kinase 5 Proteins 0.000 description 1
- 101001052493 Homo sapiens Mitogen-activated protein kinase 1 Proteins 0.000 description 1
- 101001074439 Homo sapiens Polycystin-2 Proteins 0.000 description 1
- 101000573199 Homo sapiens Protein PML Proteins 0.000 description 1
- 101000857677 Homo sapiens Runt-related transcription factor 1 Proteins 0.000 description 1
- 101000777293 Homo sapiens Serine/threonine-protein kinase Chk1 Proteins 0.000 description 1
- 101000777277 Homo sapiens Serine/threonine-protein kinase Chk2 Proteins 0.000 description 1
- 101001026882 Homo sapiens Serine/threonine-protein kinase D2 Proteins 0.000 description 1
- 101000987310 Homo sapiens Serine/threonine-protein kinase PAK 2 Proteins 0.000 description 1
- 101000604583 Homo sapiens Tyrosine-protein kinase SYK Proteins 0.000 description 1
- 101001117146 Homo sapiens [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial Proteins 0.000 description 1
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 1
- 102000009438 IgE Receptors Human genes 0.000 description 1
- 108010073816 IgE Receptors Proteins 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 102100039904 Integrin alpha-D Human genes 0.000 description 1
- 102100022338 Integrin alpha-M Human genes 0.000 description 1
- 102100022297 Integrin alpha-X Human genes 0.000 description 1
- 108010047852 Integrin alphaVbeta3 Proteins 0.000 description 1
- 102000005755 Intercellular Signaling Peptides and Proteins Human genes 0.000 description 1
- 108010070716 Intercellular Signaling Peptides and Proteins Proteins 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 102000000588 Interleukin-2 Human genes 0.000 description 1
- 108010055717 JNK Mitogen-Activated Protein Kinases Proteins 0.000 description 1
- 102000019145 JUN kinase activity proteins Human genes 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 description 1
- 208000006404 Large Granular Lymphocytic Leukemia Diseases 0.000 description 1
- 208000032004 Large-Cell Anaplastic Lymphoma Diseases 0.000 description 1
- 244000178870 Lavandula angustifolia Species 0.000 description 1
- 235000010663 Lavandula angustifolia Nutrition 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 206010024503 Limb reduction defect Diseases 0.000 description 1
- 208000007433 Lymphatic Metastasis Diseases 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- 241000712899 Lymphocytic choriomeningitis mammarenavirus Species 0.000 description 1
- 208000030289 Lymphoproliferative disease Diseases 0.000 description 1
- 201000003791 MALT lymphoma Diseases 0.000 description 1
- 102000019149 MAP kinase activity proteins Human genes 0.000 description 1
- 108040008097 MAP kinase activity proteins Proteins 0.000 description 1
- 102100034069 MAP kinase-activated protein kinase 2 Human genes 0.000 description 1
- 101710141394 MAP kinase-activated protein kinase 2 Proteins 0.000 description 1
- 102100028396 MAP kinase-activated protein kinase 5 Human genes 0.000 description 1
- 229940124647 MEK inhibitor Drugs 0.000 description 1
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 description 1
- 208000035490 Megakaryoblastic Acute Leukemia Diseases 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 208000035489 Monocytic Acute Leukemia Diseases 0.000 description 1
- 208000034578 Multiple myelomas Diseases 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 101100351501 Mus musculus Cbfb gene Proteins 0.000 description 1
- 101100268066 Mus musculus Zap70 gene Proteins 0.000 description 1
- 241000202944 Mycoplasma sp. Species 0.000 description 1
- 208000033835 Myelomonocytic Acute Leukemia Diseases 0.000 description 1
- HSHXDCVZWHOWCS-UHFFFAOYSA-N N'-hexadecylthiophene-2-carbohydrazide Chemical compound CCCCCCCCCCCCCCCCNNC(=O)c1cccs1 HSHXDCVZWHOWCS-UHFFFAOYSA-N 0.000 description 1
- GXCLVBGFBYZDAG-UHFFFAOYSA-N N-[2-(1H-indol-3-yl)ethyl]-N-methylprop-2-en-1-amine Chemical compound CN(CCC1=CNC2=C1C=CC=C2)CC=C GXCLVBGFBYZDAG-UHFFFAOYSA-N 0.000 description 1
- 208000033755 Neutrophilic Chronic Leukemia Diseases 0.000 description 1
- 206010029461 Nodal marginal zone B-cell lymphomas Diseases 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 102000007999 Nuclear Proteins Human genes 0.000 description 1
- 108010089610 Nuclear Proteins Proteins 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 206010033661 Pancytopenia Diseases 0.000 description 1
- 206010033799 Paralysis Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 208000027190 Peripheral T-cell lymphomas Diseases 0.000 description 1
- 102000003993 Phosphatidylinositol 3-kinases Human genes 0.000 description 1
- 108090000430 Phosphatidylinositol 3-kinases Proteins 0.000 description 1
- 108700019535 Phosphoprotein Phosphatases Proteins 0.000 description 1
- 102000045595 Phosphoprotein Phosphatases Human genes 0.000 description 1
- 208000007452 Plasmacytoma Diseases 0.000 description 1
- 102000001393 Platelet-Derived Growth Factor alpha Receptor Human genes 0.000 description 1
- 108010068588 Platelet-Derived Growth Factor alpha Receptor Proteins 0.000 description 1
- 102100036142 Polycystin-2 Human genes 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 102000000434 Pre-B Cell Receptors Human genes 0.000 description 1
- 108010016231 Pre-B Cell Receptors Proteins 0.000 description 1
- 208000017414 Precursor T-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000032758 Precursor T-lymphoblastic lymphoma/leukaemia Diseases 0.000 description 1
- 206010036711 Primary mediastinal large B-cell lymphomas Diseases 0.000 description 1
- 208000035416 Prolymphocytic B-Cell Leukemia Diseases 0.000 description 1
- 208000033759 Prolymphocytic T-Cell Leukemia Diseases 0.000 description 1
- 208000033826 Promyelocytic Acute Leukemia Diseases 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 102100026375 Protein PML Human genes 0.000 description 1
- 239000012083 RIPA buffer Substances 0.000 description 1
- 102000004278 Receptor Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000873 Receptor Protein-Tyrosine Kinases Proteins 0.000 description 1
- 102100023606 Retinoic acid receptor alpha Human genes 0.000 description 1
- 102100025373 Runt-related transcription factor 1 Human genes 0.000 description 1
- 108010029477 STAT5 Transcription Factor Proteins 0.000 description 1
- 101100545004 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) YSP2 gene Proteins 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 102100031081 Serine/threonine-protein kinase Chk1 Human genes 0.000 description 1
- 102100031075 Serine/threonine-protein kinase Chk2 Human genes 0.000 description 1
- 102100027939 Serine/threonine-protein kinase PAK 2 Human genes 0.000 description 1
- 208000009359 Sezary Syndrome Diseases 0.000 description 1
- 208000021388 Sezary disease Diseases 0.000 description 1
- 102100024481 Signal transducer and activator of transcription 5A Human genes 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 206010042434 Sudden death Diseases 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 208000031672 T-Cell Peripheral Lymphoma Diseases 0.000 description 1
- 208000026651 T-cell prolymphocytic leukemia Diseases 0.000 description 1
- 241000710209 Theiler's encephalomyelitis virus Species 0.000 description 1
- 241000906446 Theraps Species 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-M Thiocyanate anion Chemical compound [S-]C#N ZMZDMBWJUHKJPS-UHFFFAOYSA-M 0.000 description 1
- 102000001400 Tryptase Human genes 0.000 description 1
- 108060005989 Tryptase Proteins 0.000 description 1
- 102000004243 Tubulin Human genes 0.000 description 1
- 108090000704 Tubulin Proteins 0.000 description 1
- 102000044209 Tumor Suppressor Genes Human genes 0.000 description 1
- 108700025716 Tumor Suppressor Genes Proteins 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 108010067390 Viral Proteins Proteins 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 1
- 150000001241 acetals Chemical class 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 208000017733 acquired polycythemia vera Diseases 0.000 description 1
- 125000000641 acridinyl group Chemical group C1(=CC=CC2=NC3=CC=CC=C3C=C12)* 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 208000036677 acute biphenotypic leukemia Diseases 0.000 description 1
- 208000021841 acute erythroid leukemia Diseases 0.000 description 1
- 208000013593 acute megakaryoblastic leukemia Diseases 0.000 description 1
- 208000020700 acute megakaryocytic leukemia Diseases 0.000 description 1
- 208000026784 acute myeloblastic leukemia with maturation Diseases 0.000 description 1
- 208000010816 acute myeloblastic leukemia without maturation Diseases 0.000 description 1
- 208000011912 acute myelomonocytic leukemia M4 Diseases 0.000 description 1
- 125000002015 acyclic group Chemical group 0.000 description 1
- 125000004423 acyloxy group Chemical group 0.000 description 1
- 230000004721 adaptive immunity Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 102000019997 adhesion receptor Human genes 0.000 description 1
- 108010013985 adhesion receptor Proteins 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 239000003470 adrenal cortex hormone Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 208000015230 aggressive NK-cell leukemia Diseases 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 150000001335 aliphatic alkanes Chemical class 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 125000001118 alkylidene group Chemical group 0.000 description 1
- 239000008168 almond oil Substances 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 150000001409 amidines Chemical class 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 229960001220 amsacrine Drugs 0.000 description 1
- 230000003698 anagen phase Effects 0.000 description 1
- 229960002932 anastrozole Drugs 0.000 description 1
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 1
- 230000033115 angiogenesis Effects 0.000 description 1
- 206010002449 angioimmunoblastic T-cell lymphoma Diseases 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 239000003080 antimitotic agent Substances 0.000 description 1
- 229940034982 antineoplastic agent Drugs 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 108010072041 arginyl-glycyl-aspartic acid Proteins 0.000 description 1
- 210000000784 arm bone Anatomy 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 229940046844 aromatase inhibitors Drugs 0.000 description 1
- 125000005018 aryl alkenyl group Chemical group 0.000 description 1
- 125000005015 aryl alkynyl group Chemical group 0.000 description 1
- 238000003149 assay kit Methods 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- 229960002170 azathioprine Drugs 0.000 description 1
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 1
- 150000001540 azides Chemical class 0.000 description 1
- 125000000751 azo group Chemical group [*]N=N[*] 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- RFRXIWQYSOIBDI-UHFFFAOYSA-N benzarone Chemical compound CCC=1OC2=CC=CC=C2C=1C(=O)C1=CC=C(O)C=C1 RFRXIWQYSOIBDI-UHFFFAOYSA-N 0.000 description 1
- DZBUGLKDJFMEHC-UHFFFAOYSA-N benzoquinolinylidene Natural products C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 description 1
- DPECLNSLLIQJJO-UHFFFAOYSA-M benzyl-diethyl-[2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethyl]azanium;chloride Chemical compound [Cl-].C=1C=CC=CC=1C[N+](CC)(CC)CCOC1=CC=C(C(C)(C)CC(C)(C)C)C=C1 DPECLNSLLIQJJO-UHFFFAOYSA-M 0.000 description 1
- 102000000072 beta-Arrestins Human genes 0.000 description 1
- 108010080367 beta-Arrestins Proteins 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 210000003969 blast cell Anatomy 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 239000002981 blocking agent Substances 0.000 description 1
- 230000004579 body weight change Effects 0.000 description 1
- 210000002798 bone marrow cell Anatomy 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 201000008274 breast adenocarcinoma Diseases 0.000 description 1
- 210000000069 breast epithelial cell Anatomy 0.000 description 1
- FMWLUWPQPKEARP-UHFFFAOYSA-N bromodichloromethane Chemical compound ClC(Cl)Br FMWLUWPQPKEARP-UHFFFAOYSA-N 0.000 description 1
- 239000000337 buffer salt Substances 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000000480 butynyl group Chemical group [*]C#CC([H])([H])C([H])([H])[H] 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 230000001275 ca(2+)-mobilization Effects 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 1
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 1
- 229940127093 camptothecin Drugs 0.000 description 1
- 230000000711 cancerogenic effect Effects 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 229960004424 carbon dioxide Drugs 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 231100000315 carcinogenic Toxicity 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000006369 cell cycle progression Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 239000002458 cell surface marker Substances 0.000 description 1
- 230000008614 cellular interaction Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 208000021668 chronic eosinophilic leukemia Diseases 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 201000010903 chronic neutrophilic leukemia Diseases 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 108091036078 conserved sequence Proteins 0.000 description 1
- 230000006552 constitutive activation Effects 0.000 description 1
- 230000037011 constitutive activity Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 108010010201 core binding factor alpha Proteins 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 238000013211 curve analysis Methods 0.000 description 1
- XLJMAIOERFSOGZ-UHFFFAOYSA-M cyanate Chemical compound [O-]C#N XLJMAIOERFSOGZ-UHFFFAOYSA-M 0.000 description 1
- 125000000058 cyclopentadienyl group Chemical group C1(=CC=CC1)* 0.000 description 1
- 229940108605 cyclophosphamide injection Drugs 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 208000024389 cytopenia Diseases 0.000 description 1
- 210000005220 cytoplasmic tail Anatomy 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 239000003405 delayed action preparation Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 229940042935 dichlorodifluoromethane Drugs 0.000 description 1
- 229940087091 dichlorotetrafluoroethane Drugs 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 230000007783 downstream signaling Effects 0.000 description 1
- 239000003651 drinking water Substances 0.000 description 1
- 235000020188 drinking water Nutrition 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 230000005584 early death Effects 0.000 description 1
- 230000002900 effect on cell Effects 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- ZSWFCLXCOIISFI-UHFFFAOYSA-N endo-cyclopentadiene Natural products C1C=CC=C1 ZSWFCLXCOIISFI-UHFFFAOYSA-N 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940095399 enema Drugs 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 230000029578 entry into host Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 210000003414 extremity Anatomy 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 235000013861 fat-free Nutrition 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 108700014844 flt3 ligand Proteins 0.000 description 1
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 201000003444 follicular lymphoma Diseases 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 229960004421 formestane Drugs 0.000 description 1
- OSVMTWJCGUFAOD-KZQROQTASA-N formestane Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CCC2=C1O OSVMTWJCGUFAOD-KZQROQTASA-N 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 208000024386 fungal infectious disease Diseases 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 210000004475 gamma-delta t lymphocyte Anatomy 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 229930182478 glucoside Natural products 0.000 description 1
- 150000008131 glucosides Chemical class 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- 210000002149 gonad Anatomy 0.000 description 1
- 101150098203 grb2 gene Proteins 0.000 description 1
- 201000009277 hairy cell leukemia Diseases 0.000 description 1
- 125000001188 haloalkyl group Chemical group 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000005802 health problem Effects 0.000 description 1
- 230000009033 hematopoietic malignancy Effects 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 125000004447 heteroarylalkenyl group Chemical group 0.000 description 1
- 229960001340 histamine Drugs 0.000 description 1
- 102000045613 human SYK Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 150000002430 hydrocarbons Chemical group 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N hydrogen thiocyanate Natural products SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 1
- 229950000801 hydroxyprogesterone caproate Drugs 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 1
- 229960001101 ifosfamide Drugs 0.000 description 1
- 229960003685 imatinib mesylate Drugs 0.000 description 1
- 230000005931 immune cell recruitment Effects 0.000 description 1
- 102000027596 immune receptors Human genes 0.000 description 1
- 108091008915 immune receptors Proteins 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 125000003392 indanyl group Chemical group C1(CCC2=CC=CC=C12)* 0.000 description 1
- 230000006882 induction of apoptosis Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 201000006747 infectious mononucleosis Diseases 0.000 description 1
- 238000011850 initial investigation Methods 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 102000030582 inositol polyphosphate 5-phosphatase Human genes 0.000 description 1
- 108060004006 inositol polyphosphate 5-phosphatase Proteins 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 208000028774 intestinal disease Diseases 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 208000030776 invasive breast carcinoma Diseases 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 150000003951 lactams Chemical class 0.000 description 1
- 150000002596 lactones Chemical class 0.000 description 1
- 239000001102 lavandula vera Substances 0.000 description 1
- 235000018219 lavender Nutrition 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 210000001930 leg bone Anatomy 0.000 description 1
- GWNVDXQDILPJIG-NXOLIXFESA-N leukotriene C4 Chemical compound CCCCC\C=C/C\C=C/C=C/C=C/[C@H]([C@@H](O)CCCC(O)=O)SC[C@@H](C(=O)NCC(O)=O)NC(=O)CC[C@H](N)C(O)=O GWNVDXQDILPJIG-NXOLIXFESA-N 0.000 description 1
- 150000002617 leukotrienes Chemical class 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000012160 loading buffer Substances 0.000 description 1
- 210000005014 lower spinal cord Anatomy 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000005265 lung cell Anatomy 0.000 description 1
- 230000001926 lymphatic effect Effects 0.000 description 1
- 201000011649 lymphoblastic lymphoma Diseases 0.000 description 1
- 230000000527 lymphocytic effect Effects 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 208000020968 mature T-cell and NK-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229960004616 medroxyprogesterone Drugs 0.000 description 1
- FRQMUZJSZHZSGN-HBNHAYAOSA-N medroxyprogesterone Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](O)(C(C)=O)CC[C@H]21 FRQMUZJSZHZSGN-HBNHAYAOSA-N 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 230000006510 metastatic growth Effects 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 210000005087 mononuclear cell Anatomy 0.000 description 1
- 230000004899 motility Effects 0.000 description 1
- 201000005962 mycosis fungoides Diseases 0.000 description 1
- 208000025113 myeloid leukemia Diseases 0.000 description 1
- 210000003643 myeloid progenitor cell Anatomy 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- 125000004923 naphthylmethyl group Chemical group C1(=CC=CC2=CC=CC=C12)C* 0.000 description 1
- 210000003928 nasal cavity Anatomy 0.000 description 1
- 239000006225 natural substrate Substances 0.000 description 1
- 230000017066 negative regulation of growth Effects 0.000 description 1
- 210000005155 neural progenitor cell Anatomy 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- 125000000018 nitroso group Chemical group N(=O)* 0.000 description 1
- 208000025275 nodular sclerosis classical Hodgkin lymphoma Diseases 0.000 description 1
- 210000004967 non-hematopoietic stem cell Anatomy 0.000 description 1
- 230000000683 nonmetastatic effect Effects 0.000 description 1
- 238000010606 normalization Methods 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 230000008266 oncogenic mechanism Effects 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 125000001181 organosilyl group Chemical group [SiH3]* 0.000 description 1
- LSQODMMMSXHVCN-UHFFFAOYSA-N ovalene Chemical compound C1=C(C2=C34)C=CC3=CC=C(C=C3C5=C6C(C=C3)=CC=C3C6=C6C(C=C3)=C3)C4=C5C6=C2C3=C1 LSQODMMMSXHVCN-UHFFFAOYSA-N 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- SOWBFZRMHSNYGE-UHFFFAOYSA-N oxamic acid Chemical class NC(=O)C(O)=O SOWBFZRMHSNYGE-UHFFFAOYSA-N 0.000 description 1
- 150000002923 oximes Chemical class 0.000 description 1
- 125000004043 oxo group Chemical group O=* 0.000 description 1
- NRZWYNLTFLDQQX-UHFFFAOYSA-N p-tert-Amylphenol Chemical compound CCC(C)(C)C1=CC=C(O)C=C1 NRZWYNLTFLDQQX-UHFFFAOYSA-N 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- JQQSUOJIMKJQHS-UHFFFAOYSA-N pentaphene Chemical compound C1=CC=C2C=C3C4=CC5=CC=CC=C5C=C4C=CC3=CC2=C1 JQQSUOJIMKJQHS-UHFFFAOYSA-N 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- LCPDWSOZIOUXRV-UHFFFAOYSA-N phenoxy-acetic acid Natural products OC(=O)COC1=CC=CC=C1 LCPDWSOZIOUXRV-UHFFFAOYSA-N 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- LFGREXWGYUGZLY-UHFFFAOYSA-N phosphoryl Chemical group [P]=O LFGREXWGYUGZLY-UHFFFAOYSA-N 0.000 description 1
- DCWXELXMIBXGTH-UHFFFAOYSA-N phosphotyrosine Chemical compound OC(=O)C(N)CC1=CC=C(OP(O)(O)=O)C=C1 DCWXELXMIBXGTH-UHFFFAOYSA-N 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 210000002826 placenta Anatomy 0.000 description 1
- 208000031223 plasma cell leukemia Diseases 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 230000010118 platelet activation Effects 0.000 description 1
- 208000037244 polycythemia vera Diseases 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 238000010837 poor prognosis Methods 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 208000017426 precursor B-cell acute lymphoblastic leukemia Diseases 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 208000003476 primary myelofibrosis Diseases 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 1
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 1
- 125000002568 propynyl group Chemical group [*]C#CC([H])([H])[H] 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 230000006337 proteolytic cleavage Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- LNKHTYQPVMAJSF-UHFFFAOYSA-N pyranthrene Chemical compound C1=C2C3=CC=CC=C3C=C(C=C3)C2=C2C3=CC3=C(C=CC=C4)C4=CC4=CC=C1C2=C34 LNKHTYQPVMAJSF-UHFFFAOYSA-N 0.000 description 1
- 125000003226 pyrazolyl group Chemical group 0.000 description 1
- UXBRLLSHFJWECW-UHFFFAOYSA-N pyridine 2H-triazol-4-amine Chemical class C1=CC=NC=C1.NC1=CNN=N1 UXBRLLSHFJWECW-UHFFFAOYSA-N 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- UXWCKTJNCAIYBQ-UHFFFAOYSA-N pyrimidine;2h-triazol-4-amine Chemical class C1=CN=CN=C1.NC1=CNN=N1 UXWCKTJNCAIYBQ-UHFFFAOYSA-N 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 125000002112 pyrrolidino group Chemical group [*]N1C([H])([H])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 150000004944 pyrrolopyrimidines Chemical group 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000003134 recirculating effect Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000008844 regulatory mechanism Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 201000006845 reticulosarcoma Diseases 0.000 description 1
- 208000029922 reticulum cell sarcoma Diseases 0.000 description 1
- 108091008726 retinoic acid receptors α Proteins 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 229960004641 rituximab Drugs 0.000 description 1
- 102220005659 rs121913486 Human genes 0.000 description 1
- 102220198098 rs121913490 Human genes 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 210000002832 shoulder Anatomy 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 230000036555 skin type Effects 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000003393 splenic effect Effects 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 229940032147 starch Drugs 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 210000001562 sternum Anatomy 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 125000000547 substituted alkyl group Chemical group 0.000 description 1
- 238000009495 sugar coating Methods 0.000 description 1
- NVBFHJWHLNUMCV-UHFFFAOYSA-N sulfamide Chemical compound NS(N)(=O)=O NVBFHJWHLNUMCV-UHFFFAOYSA-N 0.000 description 1
- 150000003456 sulfonamides Chemical class 0.000 description 1
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 description 1
- 150000003457 sulfones Chemical class 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- 125000004963 sulfonylalkyl group Chemical group 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000002511 suppository base Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 125000004001 thioalkyl group Chemical group 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 125000000464 thioxo group Chemical group S=* 0.000 description 1
- 210000000779 thoracic wall Anatomy 0.000 description 1
- 201000002341 thymus lymphoma Diseases 0.000 description 1
- 210000002303 tibia Anatomy 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 230000002463 transducing effect Effects 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 230000010474 transient expression Effects 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 125000003258 trimethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])[*:1] 0.000 description 1
- 238000003211 trypan blue cell staining Methods 0.000 description 1
- 230000004565 tumor cell growth Effects 0.000 description 1
- 230000005748 tumor development Effects 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 210000001631 vena cava inferior Anatomy 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 210000000605 viral structure Anatomy 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000012224 working solution Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5383—1,4-Oxazines, e.g. morpholine ortho- or peri-condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/54—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame
- A61K31/542—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one sulfur as the ring hetero atoms, e.g. sulthiame ortho- or peri-condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
- A61P31/22—Antivirals for DNA viruses for herpes viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Virology (AREA)
- Oncology (AREA)
- Engineering & Computer Science (AREA)
- Hematology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Communicable Diseases (AREA)
- Diabetes (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/407,233 | 2006-04-18 | ||
| US11/407,233 US8227455B2 (en) | 2005-04-18 | 2006-04-18 | Methods of treating cell proliferative disorders |
| PCT/US2007/064511 WO2007124221A1 (en) | 2006-04-18 | 2007-03-21 | Methods of treating cell proliferative disorders by using pyrimidinediamine compounds |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009534397A JP2009534397A (ja) | 2009-09-24 |
| JP2009534397A5 JP2009534397A5 (cg-RX-API-DMAC7.html) | 2011-05-12 |
| JP5204761B2 true JP5204761B2 (ja) | 2013-06-05 |
Family
ID=38477280
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009506662A Active JP5204761B2 (ja) | 2006-04-18 | 2007-03-21 | ピリミジンジアミン化合物を使用することによる細胞増殖性障害を処置する方法 |
Country Status (10)
| Country | Link |
|---|---|
| US (2) | US8227455B2 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2010181B1 (cg-RX-API-DMAC7.html) |
| JP (1) | JP5204761B2 (cg-RX-API-DMAC7.html) |
| AT (1) | ATE499102T1 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2649549C (cg-RX-API-DMAC7.html) |
| DE (1) | DE602007012677D1 (cg-RX-API-DMAC7.html) |
| DK (1) | DK2010181T3 (cg-RX-API-DMAC7.html) |
| ES (1) | ES2364657T3 (cg-RX-API-DMAC7.html) |
| SI (1) | SI2010181T1 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2007124221A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK1663242T3 (da) * | 2003-08-07 | 2011-08-01 | Rigel Pharmaceuticals Inc | 2,4-Pyrimidindiamin-forbindelser og anvendelse som antiproliferative midler |
| US8227455B2 (en) * | 2005-04-18 | 2012-07-24 | Rigel Pharmaceuticals, Inc. | Methods of treating cell proliferative disorders |
| US20090048214A1 (en) * | 2006-11-15 | 2009-02-19 | Rigel Pharmaceuticals, Inc | Methods for Treating Renal Tumors Using 2, 4-Pyrimidinediamine Drug and Prodrug Compounds |
| WO2009003136A1 (en) * | 2007-06-26 | 2008-12-31 | Rigel Pharmaceuticals, Inc. | Substituted pyrimidine-2, 4 -diamines for treating cell proliferative disorders |
| WO2009029682A1 (en) * | 2007-08-28 | 2009-03-05 | Rigel Pharmaceuticals, Inc. | Combination therapy with syk kinase inhibitor |
| TR201807879T4 (tr) * | 2007-11-07 | 2018-06-21 | Rigel Pharmaceuticals Inc | Bir su ayırma maddesi kullanılarak ıslak granülasyon. |
| AR071891A1 (es) | 2008-05-30 | 2010-07-21 | Imclone Llc | Anticuerpos humanos anti-flt3 (receptor tirosina cinasa 3 tipo fms humano) |
| DK2305300T3 (en) | 2008-07-10 | 2016-06-06 | Toray Industries | Pharmaceutical composition for treatment and prevention of cancer |
| JP5603881B2 (ja) * | 2009-01-15 | 2014-10-08 | ライジェル ファーマシューティカルズ, インコーポレイテッド | プロテインキナーゼc阻害剤とその使用 |
| US8389515B2 (en) * | 2009-11-20 | 2013-03-05 | Rigel Pharmaceuticals, Inc. | 2,4-pyrimidinediamine compounds and prodrugs and their uses |
| US8618095B2 (en) | 2010-04-13 | 2013-12-31 | Rigel Pharmaceuticals, Inc. | 2, 4-pyrimidinediamine compounds and prodrugs thereof and their uses |
| MX2012013274A (es) | 2010-05-21 | 2013-05-28 | Chemilia Ab | Novedosos derivados de la pirimidina. |
| JP5744021B2 (ja) | 2010-06-30 | 2015-07-01 | 富士フイルム株式会社 | 新規なニコチンアミド誘導体またはその塩 |
| JP5886411B2 (ja) | 2011-03-24 | 2016-03-16 | ノビガ・リサーチ・エービーNoviga Research AB | 新規のピリミジン誘導体 |
| WO2012167423A1 (en) | 2011-06-08 | 2012-12-13 | Hutchison Medipharma Limited | Substituted pyridopyrazines as novel syk inhibitors |
| MX339685B (es) | 2011-07-28 | 2016-06-06 | Rigel Pharmaceuticals Inc | Formulaciones de (trimetoxifenilamino) pirimidinilo nuevas. |
| ES2661444T3 (es) | 2011-12-28 | 2018-04-02 | Fujifilm Corporation | Nuevo derivado de nicotinamida o sal del mismo |
| US20130310387A1 (en) | 2012-05-16 | 2013-11-21 | Novartis Ag | Monocyclic Heteroaryl Cycloalkyldiamine Derivatives |
| WO2013189241A1 (zh) * | 2012-06-20 | 2013-12-27 | 上海恒瑞医药有限公司 | 嘧啶二胺类衍生物、其制备方法及其在医药上的应用 |
| CN103804382A (zh) * | 2012-11-05 | 2014-05-21 | 韩文毅 | 一类治疗湿疹的化合物及其用途 |
| CN103102412B (zh) * | 2013-01-29 | 2014-03-26 | 陈仁杰 | 人源抗鼻咽癌LMP2A胞外区抗体Fab及其应用 |
| AU2016209737A1 (en) * | 2015-01-23 | 2017-08-17 | Erasmus University Medical Center Rotterdam | Anti-senescence compounds and uses thereof |
| CN108025013A (zh) * | 2015-08-12 | 2018-05-11 | 博尔托拉制药公司 | 用于治疗骨髓瘤的赛度替尼 |
| IL288519B2 (en) | 2015-11-02 | 2023-09-01 | Blueprint Medicines Corp | inhibitors of ret |
| RU2621187C1 (ru) * | 2016-05-13 | 2017-06-01 | Общество с ограниченной ответственностью "Молекулярные Технологии" | Новая кристаллическая солевая форма 2,2-диметил-6-((4-((3,4,5-триметоксифенил)амино)-1,3,5-триазин-2-ил)амино)-2н-пиридо[3,2-в][1,4]оксазин-3(4н)-она для медицинского применения |
| AU2017325844A1 (en) | 2016-09-14 | 2019-03-07 | Gilead Sciences, Inc. | SYK inhibitors |
| TW201822764A (zh) | 2016-09-14 | 2018-07-01 | 美商基利科學股份有限公司 | Syk抑制劑 |
| WO2018195471A1 (en) | 2017-04-21 | 2018-10-25 | Gilead Sciences, Inc. | Syk inhibitors in combination with hypomethylating agents |
| HRP20240124T1 (hr) | 2018-04-03 | 2024-04-12 | Blueprint Medicines Corporation | Inhibitori za ret za uporabu u liječenju raka koji ima ret alteraciju |
| US12448366B2 (en) | 2020-05-29 | 2025-10-21 | Rigel Pharmaceuticals, Inc. | Solid forms of pralsetinib |
| JPWO2023182460A1 (cg-RX-API-DMAC7.html) * | 2022-03-24 | 2023-09-28 | ||
| WO2024037910A1 (en) * | 2022-08-17 | 2024-02-22 | Institut National de la Santé et de la Recherche Médicale | Syk inhibitors for use in the treatment of cancer |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9523675D0 (en) | 1995-11-20 | 1996-01-24 | Celltech Therapeutics Ltd | Chemical compounds |
| ATE261730T1 (de) * | 1997-12-31 | 2004-04-15 | Univ Kansas | Wasserlösliche medikamentenvorstufen tertiärer amine enthaltender medikamente und verfahren zu ihrer herstellung |
| GB9828511D0 (en) | 1998-12-24 | 1999-02-17 | Zeneca Ltd | Chemical compounds |
| CN1307173C (zh) | 2000-12-21 | 2007-03-28 | 葛兰素集团有限公司 | 作为血管生成调节剂的嘧啶胺 |
| WO2003030909A1 (en) | 2001-09-25 | 2003-04-17 | Bayer Pharmaceuticals Corporation | 2- and 4-aminopyrimidines n-substtituded by a bicyclic ring for use as kinase inhibitors in the treatment of cancer |
| WO2003026664A1 (en) | 2001-09-26 | 2003-04-03 | Bayer Corporation | 2-phenylamino-4- (5-pyrazolylamino) -pyramidine derivatives as kinase inhibitors, in particular, src kinase inhibitors |
| WO2003040141A1 (en) | 2001-09-28 | 2003-05-15 | Bayer Pharmaceuticals Corporation | Oxazolyl-phenyl-2,4-diamino-pyrimidine compounds and methods for treating hyperproliferative disorders |
| WO2003055489A1 (en) | 2001-12-21 | 2003-07-10 | Bayer Pharmaceuticals Corporation | 2,4-diamino-pyrimidine derivative compounds as inhibitors of prolylpeptidase, inducers of apoptosis and cancer treatment agents |
| MXPA04008999A (es) * | 2002-03-20 | 2004-12-07 | Squibb Bristol Myers Co | Profarmacos de fosfato de fluorooxindoles. |
| ES2445208T3 (es) | 2002-07-29 | 2014-02-28 | Rigel Pharmaceuticals, Inc. | Compuestos de 2,4-pirimidindiamina para uso en métodos para tratar o prevenir enfermedades autoinmunitarias |
| WO2004014832A2 (en) | 2002-08-02 | 2004-02-19 | Vanetta S.P.A. | Redox process particularly for the production of menadione and use of polyoxometalates |
| WO2004087698A2 (en) | 2003-03-25 | 2004-10-14 | Vertex Pharmaceuticals Incorporated | Thiazoles useful as inhibitors of protein kinases |
| PT1656372E (pt) * | 2003-07-30 | 2013-06-27 | Rigel Pharmaceuticals Inc | Métodos para o tratamento ou prevenção de doenças autoimunes com compostos de 2,4-pirimidinadiamina |
| DK1663242T3 (da) | 2003-08-07 | 2011-08-01 | Rigel Pharmaceuticals Inc | 2,4-Pyrimidindiamin-forbindelser og anvendelse som antiproliferative midler |
| WO2005027848A2 (en) | 2003-09-19 | 2005-03-31 | Barnes-Jewish Hospital | Methods for screening osteogenic compounds targeting syk kinase and/or vav3 and uses of syk modulators and/or vav modulators |
| US20050267182A1 (en) * | 2003-11-13 | 2005-12-01 | Ambit Biosciences Corporation | Urea derivatives as FLT-3 modulators |
| JP5095409B2 (ja) | 2004-11-24 | 2012-12-12 | ライジェル ファーマシューティカルズ, インコーポレイテッド | スピロ2,4−ピリミジンジアミン化合物およびその使用 |
| AU2006206458B2 (en) | 2005-01-19 | 2012-10-25 | Rigel Pharmaceuticals, Inc. | Prodrugs of 2,4-pyrimidinediamine compounds and their uses |
| US8227455B2 (en) | 2005-04-18 | 2012-07-24 | Rigel Pharmaceuticals, Inc. | Methods of treating cell proliferative disorders |
| US20090048214A1 (en) | 2006-11-15 | 2009-02-19 | Rigel Pharmaceuticals, Inc | Methods for Treating Renal Tumors Using 2, 4-Pyrimidinediamine Drug and Prodrug Compounds |
-
2006
- 2006-04-18 US US11/407,233 patent/US8227455B2/en active Active
-
2007
- 2007-03-21 CA CA2649549A patent/CA2649549C/en active Active
- 2007-03-21 JP JP2009506662A patent/JP5204761B2/ja active Active
- 2007-03-21 AT AT07759007T patent/ATE499102T1/de not_active IP Right Cessation
- 2007-03-21 EP EP07759007A patent/EP2010181B1/en active Active
- 2007-03-21 WO PCT/US2007/064511 patent/WO2007124221A1/en not_active Ceased
- 2007-03-21 DE DE602007012677T patent/DE602007012677D1/de active Active
- 2007-03-21 SI SI200730591T patent/SI2010181T1/sl unknown
- 2007-03-21 DK DK07759007.3T patent/DK2010181T3/da active
- 2007-03-21 ES ES07759007T patent/ES2364657T3/es active Active
-
2012
- 2012-06-04 US US13/487,979 patent/US8481521B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CA2649549C (en) | 2011-10-18 |
| JP2009534397A (ja) | 2009-09-24 |
| US8227455B2 (en) | 2012-07-24 |
| EP2010181B1 (en) | 2011-02-23 |
| CA2649549A1 (en) | 2007-11-01 |
| DK2010181T3 (da) | 2011-06-14 |
| SI2010181T1 (sl) | 2011-06-30 |
| US8481521B2 (en) | 2013-07-09 |
| ATE499102T1 (de) | 2011-03-15 |
| ES2364657T3 (es) | 2011-09-08 |
| EP2010181A1 (en) | 2009-01-07 |
| WO2007124221A1 (en) | 2007-11-01 |
| US20120245127A1 (en) | 2012-09-27 |
| DE602007012677D1 (de) | 2011-04-07 |
| US20060276459A1 (en) | 2006-12-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5204761B2 (ja) | ピリミジンジアミン化合物を使用することによる細胞増殖性障害を処置する方法 | |
| Siveen et al. | Role of non receptor tyrosine kinases in hematological malignances and its targeting by natural products | |
| Lospinoso Severini et al. | The SHH/GLI signaling pathway: a therapeutic target for medulloblastoma | |
| Massimino et al. | Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia | |
| US9259399B2 (en) | Targeting CDK4 and CDK6 in cancer therapy | |
| Spurgeon et al. | The selective SYK inhibitor P505-15 (PRT062607) inhibits B cell signaling and function in vitro and in vivo and augments the activity of fludarabine in chronic lymphocytic leukemia | |
| Bartok et al. | Phosphoinositide 3-kinase δ regulates migration and invasion of synoviocytes in rheumatoid arthritis | |
| Han et al. | Ruxolitinib synergistically enhances the anti-tumor activity of paclitaxel in human ovarian cancer | |
| Howe et al. | Focal adhesion kinase inhibitors in combination with erlotinib demonstrate enhanced anti-tumor activity in non-small cell lung cancer | |
| US20160058741A1 (en) | Thiadiazolidinone Derivatives | |
| Hofmann et al. | PI 3K‐dependent multiple myeloma cell survival is mediated by the PIK 3 CA isoform | |
| Fei et al. | Treatment of human pre-B acute lymphoblastic leukemia with the Aurora kinase inhibitor PHA-739358 (Danusertib) | |
| WO2016196991A1 (en) | Therapeutic targeting of myeloproliferative neoplasms by dusp1 inhibition | |
| KR20080004495A (ko) | 암을 치료하기 위한 조합물, 방법 및 조성물 | |
| HUP0302913A2 (hu) | Eljárások ráksejt-halál és tumor visszafejlődés indukálására és ezek kezelésére alkalmas gyógyszerkészítmények előállítására | |
| CN101222850A (zh) | 治疗对药物有抗性的癌症的方法 | |
| CA2890086C (en) | Combination of an anti-cancer agent such as a tyrosinekinase inhibitor and a stat5 antagonist preferably a thiazolid nedione, for eliminating hematologic cancer stem cells in vivo and for preventing hematologic cancer relapse | |
| Santos et al. | New drugs for chronic myelogenous leukemia | |
| CA3138876A1 (en) | Compositions and methods for treating cancer | |
| US20240335449A1 (en) | Combination therapy for vav3 cancer | |
| WO2021086912A1 (en) | Combined pikfyve and p38 map kinase inhibition for treating cancer | |
| JP2021127291A (ja) | Mef2d融合型急性リンパ性白血病の治療方法及び治療剤 | |
| Pandit | Study of structure activity relationship of analogs derived from SU-5416 and thalidomide and mechanism of antiproliferative activity | |
| Dai et al. | Vorinostat synergistically potentiates MK-0457 lethality in chronic | |
| EP2482820A2 (en) | New use of pdgfrbeta inhibitors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100323 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100323 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110309 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120926 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121226 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130123 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130215 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5204761 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20160222 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20160222 Year of fee payment: 3 |
|
| S201 | Request for registration of exclusive licence |
Free format text: JAPANESE INTERMEDIATE CODE: R314201 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R370 | Written measure of declining of transfer procedure |
Free format text: JAPANESE INTERMEDIATE CODE: R370 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |