JP4792388B2 - 改良型カニューレ - Google Patents
改良型カニューレ Download PDFInfo
- Publication number
- JP4792388B2 JP4792388B2 JP2006503969A JP2006503969A JP4792388B2 JP 4792388 B2 JP4792388 B2 JP 4792388B2 JP 2006503969 A JP2006503969 A JP 2006503969A JP 2006503969 A JP2006503969 A JP 2006503969A JP 4792388 B2 JP4792388 B2 JP 4792388B2
- Authority
- JP
- Japan
- Prior art keywords
- cannula
- blood
- adapter
- hardness
- shaft portion
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000008280 blood Substances 0.000 claims description 52
- 210000004369 blood Anatomy 0.000 claims description 52
- 238000003780 insertion Methods 0.000 claims description 3
- 230000037431 insertion Effects 0.000 claims description 3
- 238000000465 moulding Methods 0.000 description 8
- 208000007536 Thrombosis Diseases 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- 210000005240 left ventricle Anatomy 0.000 description 7
- 239000000463 material Substances 0.000 description 6
- 230000017531 blood circulation Effects 0.000 description 5
- 238000000034 method Methods 0.000 description 5
- 230000002787 reinforcement Effects 0.000 description 5
- 241001631457 Cannula Species 0.000 description 4
- 230000036772 blood pressure Effects 0.000 description 4
- 229920002379 silicone rubber Polymers 0.000 description 4
- 239000004945 silicone rubber Substances 0.000 description 4
- 230000000747 cardiac effect Effects 0.000 description 3
- 229920000728 polyester Polymers 0.000 description 3
- 229920001296 polysiloxane Polymers 0.000 description 3
- 239000012779 reinforcing material Substances 0.000 description 3
- 230000035602 clotting Effects 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000002107 myocardial effect Effects 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 230000003014 reinforcing effect Effects 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 238000009958 sewing Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 206010007559 Cardiac failure congestive Diseases 0.000 description 1
- 206010019280 Heart failures Diseases 0.000 description 1
- 229920000271 Kevlar® Polymers 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000000227 bioadhesive Substances 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 210000003361 heart septum Anatomy 0.000 description 1
- 210000003709 heart valve Anatomy 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 239000004761 kevlar Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 229920002529 medical grade silicone Polymers 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 210000004115 mitral valve Anatomy 0.000 description 1
- 210000004165 myocardium Anatomy 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000000541 pulsatile effect Effects 0.000 description 1
- 210000005241 right ventricle Anatomy 0.000 description 1
- 239000013464 silicone adhesive Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 230000002861 ventricular Effects 0.000 description 1
- 238000004073 vulcanization Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M60/00—Blood pumps; Devices for mechanical circulatory actuation; Balloon pumps for circulatory assistance
- A61M60/10—Location thereof with respect to the patient's body
- A61M60/122—Implantable pumps or pumping devices, i.e. the blood being pumped inside the patient's body
- A61M60/126—Implantable pumps or pumping devices, i.e. the blood being pumped inside the patient's body implantable via, into, inside, in line, branching on, or around a blood vessel
- A61M60/148—Implantable pumps or pumping devices, i.e. the blood being pumped inside the patient's body implantable via, into, inside, in line, branching on, or around a blood vessel in line with a blood vessel using resection or like techniques, e.g. permanent endovascular heart assist devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/04—Surgical instruments, devices or methods, e.g. tourniquets for suturing wounds; Holders or packages for needles or suture materials
- A61B17/0469—Suturing instruments for use in minimally invasive surgery, e.g. endoscopic surgery
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/11—Surgical instruments, devices or methods, e.g. tourniquets for performing anastomosis; Buttons for anastomosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/34—Trocars; Puncturing needles
- A61B2017/348—Means for supporting the trocar against the body or retaining the trocar inside the body
- A61B2017/3482—Means for supporting the trocar against the body or retaining the trocar inside the body inside
- A61B2017/3484—Anchoring means, e.g. spreading-out umbrella-like structure
- A61B2017/3488—Fixation to inner organ or inner body tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/33—Controlling, regulating or measuring
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/33—Controlling, regulating or measuring
- A61M2205/3303—Using a biosensor
Landscapes
- Health & Medical Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Engineering & Computer Science (AREA)
- Cardiology (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Anesthesiology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Mechanical Engineering (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- External Artificial Organs (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Description
本発明はカニューレ・アセンブリに係り、特に血液輸送、好ましくは、患者の心臓あるいは循環器系と、埋込型の血液ポンプ機器との間の血液輸送に適したカニューレ・アセンブリに係るものである。
血液ポンプは、通常、うっ血性心不全および患者の循環器系に関わるその他の疾患を治療するために使用される。血液ポンプは、一般的に、カニューレを介して、心臓および循環器系に接続される必要がある。
血栓形成部位となるからである。
本発明の1つの広義の形態のカニューレは、以下のような血液輸送用カニューレである。すなわち、該カニューレは、第一端部と第二端部との間の血液経路を定める、細長く柔軟性を有するチューブ状の軸部を備え、上記軸部は、上記軸部が実質的に損傷することなく、上記血液経路を閉塞するように固定されるようになっているとともに、ねじれ応力に対して抵抗性を有するものである。
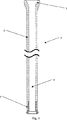
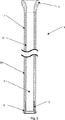
本発明に係る第一の好適な実施形態は、左心室と血液ポンプとをつなぐ、脱血カニューレ(inflow cannula)である。下記にこの実施形態について、複数の図を参照して説明する。
中空穴21内に固定されている。
Claims (4)
- 血液経路を定める、細長く柔軟性を有するチューブ状の軸部を備え、
上記軸部は、弾力性を有する漏斗形のアダプタを含んでおり、
上記アダプタは、心臓へ少なくとも部分的に挿入可能に取り付けられるものであり、アダプタの内側に凸面を有しており、上記軸部の挿入側の先端に設けられ、心臓へ挿入される、血液輸送用カニューレの先端に向けて末広に形成されるものであり、
上記軸部が実質的に損傷することなく、上記血液経路を閉塞するようにクランプで締められるようになっているとともに、ねじれ応力に対して抵抗性を有するものである血液輸送用カニューレ。 - 上記柔軟性を有するチューブ状の軸部の外側部は、50〜65ショアの硬度を有するものである請求項1に記載のカニューレ。
- 上記チューブ状の軸部の外側部が外層であり、
上記柔軟性を有するチューブ状の軸部は、さらに、上記外層よりも実質的に硬度が低い内層を有する請求項1に記載のカニューレ。 - 上記内層は、約35ショアの硬度を有するものである請求項3に記載のカニューレ。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2003901345A AU2003901345A0 (en) | 2003-03-21 | 2003-03-21 | Improved cannula |
| AU2003901345 | 2003-03-21 | ||
| PCT/AU2004/000340 WO2004082742A1 (en) | 2003-03-21 | 2004-03-19 | Improved cannula |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2006520621A JP2006520621A (ja) | 2006-09-14 |
| JP2006520621A5 JP2006520621A5 (ja) | 2011-08-04 |
| JP4792388B2 true JP4792388B2 (ja) | 2011-10-12 |
Family
ID=31500388
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006503969A Expired - Fee Related JP4792388B2 (ja) | 2003-03-21 | 2004-03-19 | 改良型カニューレ |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20060235357A1 (ja) |
| EP (1) | EP1605988B1 (ja) |
| JP (1) | JP4792388B2 (ja) |
| AU (1) | AU2003901345A0 (ja) |
| CA (1) | CA2517876C (ja) |
| WO (1) | WO2004082742A1 (ja) |
Families Citing this family (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7850676B2 (en) * | 2004-04-19 | 2010-12-14 | The Invention Science Fund I, Llc | System with a reservoir for perfusion management |
| US9283314B2 (en) | 2005-09-21 | 2016-03-15 | Abiomed, Inc. | Cannula systems |
| US8157720B2 (en) * | 2006-01-27 | 2012-04-17 | Circulite, Inc. | Heart assist system |
| US8333686B2 (en) | 2006-08-30 | 2012-12-18 | Circulite, Inc. | Cannula insertion devices, systems, and methods including a compressible member |
| CA2666881C (en) | 2006-08-30 | 2015-03-24 | Circulite, Inc. | Devices, methods and systems for establishing supplemental blood flow in the circulatory system |
| US7905823B2 (en) * | 2006-08-30 | 2011-03-15 | Circulite, Inc. | Devices, methods and systems for establishing supplemental blood flow in the circulatory system |
| WO2008034068A2 (en) * | 2006-09-14 | 2008-03-20 | Circulite, Inc. | Intravascular blood pump and catheter |
| AU2008229639A1 (en) * | 2007-03-20 | 2008-09-25 | Thoratec Corporation | Heart assist device, cannula and filter therefor |
| WO2009011993A1 (en) * | 2007-07-19 | 2009-01-22 | Circulite, Inc. | Cannula for heart chamber implantation and related systems and methods |
| US8343029B2 (en) | 2007-10-24 | 2013-01-01 | Circulite, Inc. | Transseptal cannula, tip, delivery system, and method |
| JP4996578B2 (ja) * | 2008-10-28 | 2012-08-08 | 株式会社サンメディカル技術研究所 | 多孔性構造体を具備する医療用装置又は器具 |
| WO2010071810A1 (en) * | 2008-12-19 | 2010-06-24 | Andy Christopher Kiser | Methods and devices for endoscopic access to the heart |
| US20100249491A1 (en) * | 2009-03-27 | 2010-09-30 | Circulite, Inc. | Two-piece transseptal cannula, delivery system, and method of delivery |
| US8460168B2 (en) | 2009-03-27 | 2013-06-11 | Circulite, Inc. | Transseptal cannula device, coaxial balloon delivery device, and methods of using the same |
| US20110040241A1 (en) * | 2009-08-11 | 2011-02-17 | Dongfang Wang | Blood access assembly for artificial lung and right ventricular assist device |
| US20110112353A1 (en) * | 2009-11-09 | 2011-05-12 | Circulite, Inc. | Bifurcated outflow cannulae |
| US8308715B2 (en) * | 2009-11-13 | 2012-11-13 | Circulite, Inc. | Cannula stabilizer |
| JP5380312B2 (ja) * | 2010-01-08 | 2014-01-08 | 株式会社サンメディカル技術研究所 | 多孔性構造体を具備する医療用装置又は器具 |
| US9750866B2 (en) | 2010-02-11 | 2017-09-05 | Circulite, Inc. | Cannula lined with tissue in-growth material |
| WO2011100552A1 (en) * | 2010-02-11 | 2011-08-18 | Circulte, Inc. | Devices, methods and systems for establishing supplemental blood flow in the circulatory system |
| US20120143141A1 (en) * | 2010-08-03 | 2012-06-07 | Verkaik Josiah E | Conformal cannula device and related methods |
| WO2012051454A2 (en) * | 2010-10-13 | 2012-04-19 | Thoratec Corporation | Pumping blood |
| WO2012119073A1 (en) | 2011-03-02 | 2012-09-07 | Thoratec Corporation | Ventricular cuff |
| US8905030B2 (en) | 2011-03-31 | 2014-12-09 | Covidien Lp | Tracheal tube with connector insert |
| JP5809359B2 (ja) * | 2011-08-05 | 2015-11-10 | サーキュライト・インコーポレーテッド | 組織内部成長材料でライニング加工されたカニューレおよびその使用方法 |
| US8821527B2 (en) * | 2011-09-07 | 2014-09-02 | Circulite, Inc. | Cannula tips, tissue attachment rings, and methods of delivering and using the same |
| CA2856400C (en) * | 2011-11-23 | 2018-03-06 | Abiomed, Inc. | Use of a counterpulsation device and counterpulsation |
| CN105188797B (zh) * | 2013-03-07 | 2017-09-08 | 塞考利特公司 | 经中隔套管、尖端、递送系统和方法 |
| US10449274B2 (en) * | 2013-06-26 | 2019-10-22 | Circulite, Inc. | System and method of facilitating connection between cannulae and a blood pump |
| US10667854B2 (en) | 2013-09-24 | 2020-06-02 | Adagio Medical, Inc. | Endovascular near critical fluid based cryoablation catheter and related methods |
| WO2015160574A1 (en) | 2014-04-17 | 2015-10-22 | Adagio Medical, Inc. | Endovascular near critical fluid based cryoablation catheter having plurality of preformed treatment shapes |
| JP6607938B2 (ja) | 2014-11-13 | 2019-11-20 | アダージョ メディカル インコーポレイテッド | 圧力調整型冷凍アブレーションシステム及び関連方法 |
| WO2016086137A1 (en) | 2014-11-26 | 2016-06-02 | Thoratec Corporation | Pump and method for mixed flow blood pumping |
| WO2017048965A1 (en) | 2015-09-18 | 2017-03-23 | Adagio Medical Inc. | Tissue contact verification system |
| US9717830B2 (en) | 2015-10-28 | 2017-08-01 | Circulite, Inc. | Inflow cannula and blood flow assist system |
| WO2017095756A1 (en) | 2015-11-30 | 2017-06-08 | Adagio Medical, Inc. | Ablation method for creating elongate continuous lesions enclosing multiple vessel entries |
| EP3205360B1 (en) * | 2016-02-11 | 2018-08-29 | Abiomed Europe GmbH | Blood pump |
| EP3436105B1 (en) * | 2016-03-30 | 2021-04-28 | Heartware, Inc. | Flanged heart tissue blocker |
| CN106388799B (zh) * | 2016-09-26 | 2023-06-06 | 柳州市人民医院 | 有创压力监测传感器注液管及与其配合的针管 |
| EP3311860A1 (de) * | 2016-10-20 | 2018-04-25 | Berlin Heart GmbH | Kanüle, kanülensystem, herzpumpensystem und verfahren zur volumenentlastung eines herzens |
| US10856745B2 (en) * | 2017-05-16 | 2020-12-08 | Heartware, Inc. | Intra ventricular ambulatory implantable PV loop system |
| WO2018226991A1 (en) | 2017-06-07 | 2018-12-13 | Shifamed Holdings, Llc | Intravascular fluid movement devices, systems, and methods of use |
| US11564725B2 (en) | 2017-09-05 | 2023-01-31 | Adagio Medical, Inc. | Ablation catheter having a shape memory stylet |
| DE202017106098U1 (de) * | 2017-10-09 | 2019-01-10 | Deutsches Herzzentrum Berlin | Künstliche Herzunterstützungspumpe |
| CN111556763B (zh) | 2017-11-13 | 2023-09-01 | 施菲姆德控股有限责任公司 | 血管内流体运动装置、系统 |
| IL275963B1 (en) | 2018-01-10 | 2024-09-01 | Adagio Medical Inc | Freeze extinction component with internal conductor |
| EP4085965A1 (en) | 2018-02-01 | 2022-11-09 | Shifamed Holdings, LLC | Intravascular blood pumps and methods of use and manufacture |
| FR3081333B1 (fr) * | 2018-05-22 | 2020-06-05 | Fineheart | Dispositif d'ancrage d'une pompe cardiaque |
| JP2022540616A (ja) | 2019-07-12 | 2022-09-16 | シファメド・ホールディングス・エルエルシー | 血管内血液ポンプならびに製造および使用の方法 |
| US11654275B2 (en) | 2019-07-22 | 2023-05-23 | Shifamed Holdings, Llc | Intravascular blood pumps with struts and methods of use and manufacture |
| WO2021062270A1 (en) | 2019-09-25 | 2021-04-01 | Shifamed Holdings, Llc | Catheter blood pumps and collapsible pump housings |
| EP4034192A4 (en) | 2019-09-25 | 2023-11-29 | Shifamed Holdings, LLC | INTRAVASCULAR BLOOD PUMP SYSTEMS AND METHODS OF USE AND CONTROL THEREOF |
| CN115666708A (zh) | 2021-03-17 | 2023-01-31 | 怡忠生命科学有限公司 | 具无缝合导流管组件的植入式心室外延共搏循环支持系统 |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS56104801A (en) * | 1980-01-18 | 1981-08-20 | Sealy | Vein vasodilating device |
| JPH0295377A (ja) * | 1988-09-30 | 1990-04-06 | Nippon Zeon Co Ltd | 医療用樹脂組成物、及び医療用成形品の製造方法 |
| US5084064A (en) * | 1989-06-15 | 1992-01-28 | Abiomed Cardiovascular, Inc. | Surgical cuff |
| US5234455A (en) * | 1992-02-20 | 1993-08-10 | Arkansas Knee Clinic, P.A. | Lipped cannula and methods of introducing surgical instruments in arthroscopic surgical procedures |
| JPH11500642A (ja) * | 1995-02-24 | 1999-01-19 | ハートポート インコーポレイテッド | 血管吻合を行なう装置および方法 |
| WO2000015146A1 (en) * | 1998-09-10 | 2000-03-23 | Percardia, Inc. | Transmyocardial shunt for left ventricular revascularization |
| JP2000084086A (ja) * | 1998-09-10 | 2000-03-28 | Nippon Cable Syst Inc | 医療用カニューレ |
| US6186999B1 (en) * | 1998-08-27 | 2001-02-13 | The Cleveland Clinic Foundation | Rigid clampable cannula |
| JP2002113009A (ja) * | 2000-08-05 | 2002-04-16 | Heartport Inc | 心臓の固定器具およびその使用方法 |
| WO2003020173A1 (en) * | 2001-09-04 | 2003-03-13 | Graeme Cocks | A stent |
| JP2003510143A (ja) * | 1999-08-04 | 2003-03-18 | パーカーディア インコーポレイテッド | 直接的冠状血管再生方法及び装置 |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4086665A (en) * | 1976-12-16 | 1978-05-02 | Thermo Electron Corporation | Artificial blood conduit |
| US4323072A (en) * | 1980-01-18 | 1982-04-06 | Shiley, Incorporated | Cannula for a vein distention system |
| US4639252A (en) * | 1985-04-05 | 1987-01-27 | Research Medical, Inc. | Venous return catheter |
| US4795446A (en) * | 1986-01-30 | 1989-01-03 | Sherwood Medical Company | Medical tube device |
| US5013296A (en) * | 1989-09-21 | 1991-05-07 | Research Medical, Inc. | Antegrade cardioplegia cannula |
| JP2830440B2 (ja) * | 1990-09-21 | 1998-12-02 | 東洋紡績株式会社 | カニューレ |
| US5984908A (en) * | 1997-04-10 | 1999-11-16 | Chase Medical Inc | Venous return catheter having integral support member |
| US6123725A (en) * | 1997-07-11 | 2000-09-26 | A-Med Systems, Inc. | Single port cardiac support apparatus |
| WO2000012148A2 (en) * | 1998-08-27 | 2000-03-09 | A-Med Systems Inc. | Intravascular cannulation apparatus and methods of use |
| US6533770B1 (en) * | 1998-01-21 | 2003-03-18 | Heartport, Inc. | Cannula and method of manufacture and use |
| US6152911A (en) * | 1998-08-27 | 2000-11-28 | Chase Medical, Inc. | Venous return catheter having multiple helical support members |
| US6001056A (en) | 1998-11-13 | 1999-12-14 | Baxter International Inc. | Smooth ventricular assist device conduit |
| US7258679B2 (en) * | 2002-08-09 | 2007-08-21 | Vascor, Inc. | Inflow conduit for ventricular assist device |
-
2003
- 2003-03-21 AU AU2003901345A patent/AU2003901345A0/en not_active Abandoned
-
2004
- 2004-03-19 CA CA2517876A patent/CA2517876C/en not_active Expired - Fee Related
- 2004-03-19 US US10/548,868 patent/US20060235357A1/en not_active Abandoned
- 2004-03-19 JP JP2006503969A patent/JP4792388B2/ja not_active Expired - Fee Related
- 2004-03-19 EP EP04721776.5A patent/EP1605988B1/en not_active Expired - Lifetime
- 2004-03-19 WO PCT/AU2004/000340 patent/WO2004082742A1/en active Application Filing
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS56104801A (en) * | 1980-01-18 | 1981-08-20 | Sealy | Vein vasodilating device |
| JPH0295377A (ja) * | 1988-09-30 | 1990-04-06 | Nippon Zeon Co Ltd | 医療用樹脂組成物、及び医療用成形品の製造方法 |
| US5084064A (en) * | 1989-06-15 | 1992-01-28 | Abiomed Cardiovascular, Inc. | Surgical cuff |
| US5234455A (en) * | 1992-02-20 | 1993-08-10 | Arkansas Knee Clinic, P.A. | Lipped cannula and methods of introducing surgical instruments in arthroscopic surgical procedures |
| JPH11500642A (ja) * | 1995-02-24 | 1999-01-19 | ハートポート インコーポレイテッド | 血管吻合を行なう装置および方法 |
| US6186999B1 (en) * | 1998-08-27 | 2001-02-13 | The Cleveland Clinic Foundation | Rigid clampable cannula |
| WO2000015146A1 (en) * | 1998-09-10 | 2000-03-23 | Percardia, Inc. | Transmyocardial shunt for left ventricular revascularization |
| JP2000084086A (ja) * | 1998-09-10 | 2000-03-28 | Nippon Cable Syst Inc | 医療用カニューレ |
| JP2003510143A (ja) * | 1999-08-04 | 2003-03-18 | パーカーディア インコーポレイテッド | 直接的冠状血管再生方法及び装置 |
| JP2002113009A (ja) * | 2000-08-05 | 2002-04-16 | Heartport Inc | 心臓の固定器具およびその使用方法 |
| WO2003020173A1 (en) * | 2001-09-04 | 2003-03-13 | Graeme Cocks | A stent |
Also Published As
| Publication number | Publication date |
|---|---|
| US20060235357A1 (en) | 2006-10-19 |
| CA2517876C (en) | 2012-01-03 |
| JP2006520621A (ja) | 2006-09-14 |
| EP1605988A4 (en) | 2011-03-02 |
| EP1605988A1 (en) | 2005-12-21 |
| AU2003901345A0 (en) | 2003-04-03 |
| EP1605988B1 (en) | 2014-08-13 |
| WO2004082742A1 (en) | 2004-09-30 |
| CA2517876A1 (en) | 2004-09-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4792388B2 (ja) | 改良型カニューレ | |
| JP5629576B2 (ja) | 心房内に埋め込むためのカニューレ、およびそれに関連したシステム、ならびにその方法 | |
| JPH025799Y2 (ja) | ||
| JP3189974B2 (ja) | カテーテル | |
| EP3735280B1 (en) | Fluid treatment system for a driveline | |
| CN109069792B (zh) | 经皮导管及经皮导管用管的制造方法 | |
| CA2587809C (en) | Catheter having reinforcing rings and method of use | |
| US20120143141A1 (en) | Conformal cannula device and related methods | |
| US20040193004A1 (en) | Method and apparatus for adjusting a length of the inflow conduit on a ventricular assist device | |
| WO2017122377A1 (ja) | 経皮カテーテル、経皮カテーテルの使用方法 | |
| US11318281B2 (en) | Catheter for extracorporeal blood circulator | |
| US11260159B2 (en) | Percutaneous catheter and percutaneous catheter assembly | |
| JP4900242B2 (ja) | 大動脈内バルーンポンピングセット | |
| AU2004222674B2 (en) | Improved cannula | |
| JP2009225895A (ja) | 瘻孔用カテーテル | |
| US10799263B2 (en) | Catheter design for use in treating pleural diseases | |
| JPS6096260A (ja) | 変形自在のカニユ−レ | |
| JPH04193181A (ja) | 医療用チューブ及び医療用チューブ保持具 | |
| CN216456525U (zh) | 一种用于血泵的支撑装置及辅助血液循环系统 | |
| JPH1015061A (ja) | 腹腔内留置カテーテル | |
| EP4272796A1 (en) | Stylet and catheter assembly | |
| WO2020137899A1 (ja) | カテーテル組立体 | |
| JP2019176919A (ja) | カテーテル用補強体 | |
| JPH0228984B2 (ja) | ||
| JP2016154606A (ja) | カテーテル |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070220 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20070220 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20091027 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20100127 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100203 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20100225 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100304 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100426 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100727 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20101007 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20101007 Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20101026 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110315 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20110614 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20110712 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20110725 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140729 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4792388 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |