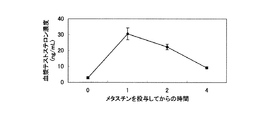
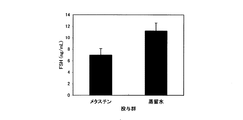
JP4639052B2 - 性腺機能改善剤 - Google Patents
性腺機能改善剤 Download PDFInfo
- Publication number
- JP4639052B2 JP4639052B2 JP2004068255A JP2004068255A JP4639052B2 JP 4639052 B2 JP4639052 B2 JP 4639052B2 JP 2004068255 A JP2004068255 A JP 2004068255A JP 2004068255 A JP2004068255 A JP 2004068255A JP 4639052 B2 JP4639052 B2 JP 4639052B2
- Authority
- JP
- Japan
- Prior art keywords
- amino acid
- acid sequence
- polypeptide
- receptor
- seq
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Landscapes
- Peptides Or Proteins (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2004068255A JP4639052B2 (ja) | 2003-03-12 | 2004-03-11 | 性腺機能改善剤 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2003067283 | 2003-03-12 | ||
| JP2003311892 | 2003-09-03 | ||
| JP2004068255A JP4639052B2 (ja) | 2003-03-12 | 2004-03-11 | 性腺機能改善剤 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2005097237A JP2005097237A (ja) | 2005-04-14 |
| JP2005097237A5 JP2005097237A5 (enExample) | 2007-04-19 |
| JP4639052B2 true JP4639052B2 (ja) | 2011-02-23 |
Family
ID=34468253
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004068255A Expired - Lifetime JP4639052B2 (ja) | 2003-03-12 | 2004-03-11 | 性腺機能改善剤 |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4639052B2 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2009131191A1 (ja) * | 2008-04-24 | 2011-08-25 | 武田薬品工業株式会社 | メタスチン誘導体およびその用途 |
| IL265696B2 (en) * | 2016-09-30 | 2024-08-01 | Sumitomo Pharma Switzerland Gmbh | 2-(n-acetyl-d-tyrosyl-trans-4-hydroxy-l-prolyl-l-asparaginyl-l-threonyl-l-phenylalanyl) hydrazinocarbonyl-l-leucyl-nω-methyl-l-arginyl-l-tryptophanamide, for use in assisted reproductive technology. |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4486187B2 (ja) * | 1998-10-27 | 2010-06-23 | 武田薬品工業株式会社 | 新規g蛋白質共役型レセプター蛋白質、そのdnaおよびそのリガンド |
| JP2001340094A (ja) * | 2000-03-30 | 2001-12-11 | Takeda Chem Ind Ltd | 新規蛋白質、そのdnaおよびその製造法 |
| JP2003002841A (ja) * | 2001-04-20 | 2003-01-08 | Takeda Chem Ind Ltd | ペプチド含有製剤 |
-
2004
- 2004-03-11 JP JP2004068255A patent/JP4639052B2/ja not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| JP2005097237A (ja) | 2005-04-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20110052563A1 (en) | Gonadal function improving agents | |
| US7556926B2 (en) | Methods for screening insulin-sensitizing agents | |
| EP1239039A1 (en) | Novel polypeptide and dna thereof | |
| JP5317318B2 (ja) | 新規ポリペプチドおよびその用途 | |
| JP4639052B2 (ja) | 性腺機能改善剤 | |
| EP1191096A1 (en) | Novel protein and dna thereof | |
| JP4184875B2 (ja) | 新規スクリーニング方法 | |
| JP2004073182A (ja) | インスリン抵抗性改善剤 | |
| JP4184697B2 (ja) | スクリーニング方法 | |
| EP1600165A1 (en) | Medicinal use of mip-3alpha inhibitor and method of screening brain/nerve cell protective agent | |
| JP4761812B2 (ja) | マスクリン受容体およびその用途 | |
| JP4353697B2 (ja) | がんの予防・治療剤 | |
| JP4445291B2 (ja) | 新規タンパク質およびそのdna | |
| JP4676608B2 (ja) | 新規タヒキニン様ポリペプチドおよびその用途 | |
| JP2004198202A (ja) | 新規スクリーニング方法 | |
| JP4300008B2 (ja) | 新規タンパク質およびそのdna | |
| JP4167868B2 (ja) | 新規分泌蛋白質およびそのdna | |
| JP4320355B2 (ja) | 新規Fasリガンド様タンパク質およびそのDNA | |
| EP1602380A1 (en) | Preventive/remedy for diseases in upper digestive tract | |
| JP2004173677A (ja) | 新規タンパク質およびそのdna | |
| JP2004089182A (ja) | がんの予防・治療剤 | |
| JP2005210924A (ja) | アルカデイン結合タンパク質およびその用途 | |
| JPWO2006101273A1 (ja) | 癌の予防・治療剤 | |
| EP1300467A1 (en) | Novel polypeptide and dna thereof | |
| JP2004323506A (ja) | MIP−3α抑制薬の医薬用途および脳・神経細胞保護剤のスクリーニング方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20061011 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070302 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20070302 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100427 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100625 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100727 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100831 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20101021 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20101116 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20101129 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131203 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4639052 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20131203 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |