JP2009517015A - 組換えポリペプチドならびにtshレセプターに対する自己抗体を検出及び/又は定量化する方法 - Google Patents
組換えポリペプチドならびにtshレセプターに対する自己抗体を検出及び/又は定量化する方法 Download PDFInfo
- Publication number
- JP2009517015A JP2009517015A JP2008541846A JP2008541846A JP2009517015A JP 2009517015 A JP2009517015 A JP 2009517015A JP 2008541846 A JP2008541846 A JP 2008541846A JP 2008541846 A JP2008541846 A JP 2008541846A JP 2009517015 A JP2009517015 A JP 2009517015A
- Authority
- JP
- Japan
- Prior art keywords
- tsh receptor
- amino acid
- acid sequence
- isolated
- purified recombinant
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 102000003911 Thyrotropin Receptors Human genes 0.000 title claims abstract description 329
- 108090000253 Thyrotropin Receptors Proteins 0.000 title claims abstract description 329
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 181
- 102000004196 processed proteins & peptides Human genes 0.000 title claims abstract description 163
- 229920001184 polypeptide Polymers 0.000 title claims abstract description 153
- 238000000034 method Methods 0.000 title claims abstract description 91
- 230000027455 binding Effects 0.000 claims abstract description 82
- 102000037865 fusion proteins Human genes 0.000 claims abstract description 67
- 108020001507 fusion proteins Proteins 0.000 claims abstract description 67
- 238000009739 binding Methods 0.000 claims abstract description 66
- 208000023275 Autoimmune disease Diseases 0.000 claims abstract description 59
- 230000028993 immune response Effects 0.000 claims abstract description 53
- 230000004044 response Effects 0.000 claims abstract description 52
- 150000001413 amino acids Chemical group 0.000 claims description 187
- 230000036961 partial effect Effects 0.000 claims description 90
- 210000004027 cell Anatomy 0.000 claims description 61
- 239000012634 fragment Substances 0.000 claims description 52
- 239000013598 vector Substances 0.000 claims description 47
- 108010070675 Glutathione transferase Proteins 0.000 claims description 44
- 150000007523 nucleic acids Chemical group 0.000 claims description 39
- 238000003556 assay Methods 0.000 claims description 27
- 239000007787 solid Substances 0.000 claims description 25
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 24
- 108090000190 Thrombin Proteins 0.000 claims description 22
- 229960004072 thrombin Drugs 0.000 claims description 22
- 238000002965 ELISA Methods 0.000 claims description 21
- 239000013060 biological fluid Substances 0.000 claims description 20
- 239000003153 chemical reaction reagent Substances 0.000 claims description 18
- 239000006166 lysate Substances 0.000 claims description 18
- 230000015572 biosynthetic process Effects 0.000 claims description 16
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 claims description 15
- 210000000805 cytoplasm Anatomy 0.000 claims description 14
- 239000008194 pharmaceutical composition Substances 0.000 claims description 14
- 230000035772 mutation Effects 0.000 claims description 13
- 101000772267 Homo sapiens Thyrotropin receptor Proteins 0.000 claims description 12
- 239000004094 surface-active agent Substances 0.000 claims description 12
- 238000003776 cleavage reaction Methods 0.000 claims description 11
- 230000007017 scission Effects 0.000 claims description 11
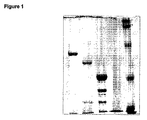
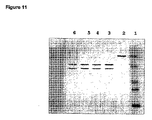
- 238000001262 western blot Methods 0.000 claims description 11
- 102000005962 receptors Human genes 0.000 claims description 9
- 108020003175 receptors Proteins 0.000 claims description 9
- 108010024636 Glutathione Proteins 0.000 claims description 8
- 238000004519 manufacturing process Methods 0.000 claims description 8
- 238000003127 radioimmunoassay Methods 0.000 claims description 8
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 7
- 238000001042 affinity chromatography Methods 0.000 claims description 7
- 229960003180 glutathione Drugs 0.000 claims description 7
- 238000000338 in vitro Methods 0.000 claims description 7
- 102000053187 Glucuronidase Human genes 0.000 claims description 6
- 108010060309 Glucuronidase Proteins 0.000 claims description 6
- 108010043121 Green Fluorescent Proteins Proteins 0.000 claims description 6
- 102000004144 Green Fluorescent Proteins Human genes 0.000 claims description 6
- 101710175625 Maltose/maltodextrin-binding periplasmic protein Proteins 0.000 claims description 6
- 239000003814 drug Substances 0.000 claims description 6
- 238000000684 flow cytometry Methods 0.000 claims description 6
- 239000005090 green fluorescent protein Substances 0.000 claims description 6
- 238000001114 immunoprecipitation Methods 0.000 claims description 6
- 238000012258 culturing Methods 0.000 claims description 5
- 238000001699 lithographically induced self-assembly Methods 0.000 claims description 5
- 239000002736 nonionic surfactant Substances 0.000 claims description 5
- 239000002671 adjuvant Substances 0.000 claims description 4
- 239000003085 diluting agent Substances 0.000 claims description 4
- 229930182470 glycoside Natural products 0.000 claims description 4
- 150000002338 glycosides Chemical class 0.000 claims description 4
- 238000002955 isolation Methods 0.000 claims description 4
- 230000002934 lysing effect Effects 0.000 claims description 4
- 108091008324 binding proteins Proteins 0.000 claims description 3
- 239000001913 cellulose Substances 0.000 claims description 3
- 229920002678 cellulose Polymers 0.000 claims description 3
- 238000003745 diagnosis Methods 0.000 claims description 3
- 239000003937 drug carrier Substances 0.000 claims description 3
- 238000005858 glycosidation reaction Methods 0.000 claims description 3
- 238000012123 point-of-care testing Methods 0.000 claims description 3
- 239000013543 active substance Substances 0.000 claims description 2
- 230000002265 prevention Effects 0.000 claims description 2
- 229940124597 therapeutic agent Drugs 0.000 claims description 2
- 230000001225 therapeutic effect Effects 0.000 claims description 2
- 102000005720 Glutathione transferase Human genes 0.000 claims 6
- 230000001131 transforming effect Effects 0.000 claims 2
- 102100029337 Thyrotropin receptor Human genes 0.000 claims 1
- 102000023732 binding proteins Human genes 0.000 claims 1
- 229940079593 drug Drugs 0.000 claims 1
- 230000008105 immune reaction Effects 0.000 claims 1
- 235000001014 amino acid Nutrition 0.000 description 46
- 210000002966 serum Anatomy 0.000 description 46
- 229940024606 amino acid Drugs 0.000 description 43
- 102100029100 Hematopoietic prostaglandin D synthase Human genes 0.000 description 38
- 108090000623 proteins and genes Proteins 0.000 description 36
- 102000004169 proteins and genes Human genes 0.000 description 32
- 235000018102 proteins Nutrition 0.000 description 31
- 241000282414 Homo sapiens Species 0.000 description 30
- 239000000872 buffer Substances 0.000 description 24
- 102000011923 Thyrotropin Human genes 0.000 description 21
- 108010061174 Thyrotropin Proteins 0.000 description 21
- 150000001875 compounds Chemical class 0.000 description 15
- 238000001514 detection method Methods 0.000 description 15
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 14
- 239000002953 phosphate buffered saline Substances 0.000 description 14
- 239000003656 tris buffered saline Substances 0.000 description 13
- 238000004458 analytical method Methods 0.000 description 12
- 239000011248 coating agent Substances 0.000 description 12
- 238000000576 coating method Methods 0.000 description 12
- 239000000203 mixture Substances 0.000 description 11
- 239000000427 antigen Substances 0.000 description 10
- 102000036639 antigens Human genes 0.000 description 10
- 108091007433 antigens Proteins 0.000 description 10
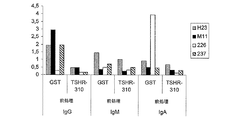
- 102000051345 human TSHR Human genes 0.000 description 10
- 239000000523 sample Substances 0.000 description 10
- 241000588724 Escherichia coli Species 0.000 description 9
- 241000894006 Bacteria Species 0.000 description 8
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 8
- 229940098773 bovine serum albumin Drugs 0.000 description 8
- 230000004927 fusion Effects 0.000 description 8
- 229910052751 metal Inorganic materials 0.000 description 8
- 239000002184 metal Substances 0.000 description 8
- 230000036963 noncompetitive effect Effects 0.000 description 8
- 239000013612 plasmid Substances 0.000 description 8
- 230000009257 reactivity Effects 0.000 description 8
- 238000006467 substitution reaction Methods 0.000 description 8
- 239000000758 substrate Substances 0.000 description 8
- 241000283973 Oryctolagus cuniculus Species 0.000 description 7
- 230000001580 bacterial effect Effects 0.000 description 7
- 238000010586 diagram Methods 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 239000004033 plastic Substances 0.000 description 7
- 229920003023 plastic Polymers 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 7
- 210000001685 thyroid gland Anatomy 0.000 description 7
- 208000023328 Basedow disease Diseases 0.000 description 6
- 102000004190 Enzymes Human genes 0.000 description 6
- 108090000790 Enzymes Proteins 0.000 description 6
- 208000015023 Graves' disease Diseases 0.000 description 6
- 241000699666 Mus <mouse, genus> Species 0.000 description 6
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 6
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 6
- 102000009843 Thyroglobulin Human genes 0.000 description 6
- 108010034949 Thyroglobulin Proteins 0.000 description 6
- 238000004587 chromatography analysis Methods 0.000 description 6
- 229940088598 enzyme Drugs 0.000 description 6
- 238000002372 labelling Methods 0.000 description 6
- 108020004707 nucleic acids Proteins 0.000 description 6
- 102000039446 nucleic acids Human genes 0.000 description 6
- 229960002175 thyroglobulin Drugs 0.000 description 6
- -1 152 Eu Chemical class 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 5
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 5
- 108060003951 Immunoglobulin Proteins 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 102000001708 Protein Isoforms Human genes 0.000 description 5
- 108010029485 Protein Isoforms Proteins 0.000 description 5
- 230000000903 blocking effect Effects 0.000 description 5
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 5
- 238000010367 cloning Methods 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 5
- 102000018358 immunoglobulin Human genes 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 239000013642 negative control Substances 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 241000238631 Hexapoda Species 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 4
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 4
- QPCDCPDFJACHGM-UHFFFAOYSA-N N,N-bis{2-[bis(carboxymethyl)amino]ethyl}glycine Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(=O)O)CCN(CC(O)=O)CC(O)=O QPCDCPDFJACHGM-UHFFFAOYSA-N 0.000 description 4
- 230000004071 biological effect Effects 0.000 description 4
- 230000008827 biological function Effects 0.000 description 4
- 239000007853 buffer solution Substances 0.000 description 4
- 150000001720 carbohydrates Chemical class 0.000 description 4
- 235000014633 carbohydrates Nutrition 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 230000002759 chromosomal effect Effects 0.000 description 4
- 238000012875 competitive assay Methods 0.000 description 4
- 239000002299 complementary DNA Substances 0.000 description 4
- 238000010790 dilution Methods 0.000 description 4
- 239000012895 dilution Substances 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 238000003018 immunoassay Methods 0.000 description 4
- 238000011534 incubation Methods 0.000 description 4
- 238000002761 liquid phase assay Methods 0.000 description 4
- 229960003330 pentetic acid Drugs 0.000 description 4
- 230000009870 specific binding Effects 0.000 description 4
- 230000004936 stimulating effect Effects 0.000 description 4
- 239000005495 thyroid hormone Substances 0.000 description 4
- 229940036555 thyroid hormone Drugs 0.000 description 4
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 241000283690 Bos taurus Species 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 3
- 102000003839 Human Proteins Human genes 0.000 description 3
- 108090000144 Human Proteins Proteins 0.000 description 3
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 3
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 3
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 3
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical compound CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 3
- 150000001408 amides Chemical class 0.000 description 3
- 235000003704 aspartic acid Nutrition 0.000 description 3
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- VHJLVAABSRFDPM-QWWZWVQMSA-N dithiothreitol Chemical compound SC[C@@H](O)[C@H](O)CS VHJLVAABSRFDPM-QWWZWVQMSA-N 0.000 description 3
- 231100000673 dose–response relationship Toxicity 0.000 description 3
- 238000001502 gel electrophoresis Methods 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 239000003547 immunosorbent Substances 0.000 description 3
- 238000011065 in-situ storage Methods 0.000 description 3
- 239000003446 ligand Substances 0.000 description 3
- 230000009871 nonspecific binding Effects 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 238000000527 sonication Methods 0.000 description 3
- 230000000638 stimulation Effects 0.000 description 3
- 230000009466 transformation Effects 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- 229920000936 Agarose Polymers 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 102000014914 Carrier Proteins Human genes 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 2
- 229920002307 Dextran Polymers 0.000 description 2
- 206010060742 Endocrine ophthalmopathy Diseases 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 239000004472 Lysine Substances 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- 108010038988 Peptide Hormones Proteins 0.000 description 2
- 102000003992 Peroxidases Human genes 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- 229920002684 Sepharose Polymers 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- 102000013090 Thioredoxin-Disulfide Reductase Human genes 0.000 description 2
- 108010079911 Thioredoxin-disulfide reductase Proteins 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- AUYYCJSJGJYCDS-LBPRGKRZSA-N Thyrolar Chemical class IC1=CC(C[C@H](N)C(O)=O)=CC(I)=C1OC1=CC=C(O)C(I)=C1 AUYYCJSJGJYCDS-LBPRGKRZSA-N 0.000 description 2
- 239000013504 Triton X-100 Substances 0.000 description 2
- 229920004890 Triton X-100 Polymers 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 125000001931 aliphatic group Chemical group 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 238000003491 array Methods 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 210000000170 cell membrane Anatomy 0.000 description 2
- 230000002860 competitive effect Effects 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 238000004925 denaturation Methods 0.000 description 2
- 230000036425 denaturation Effects 0.000 description 2
- 239000003599 detergent Substances 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- 210000005256 gram-negative cell Anatomy 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 230000003100 immobilizing effect Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 229940072221 immunoglobulins Drugs 0.000 description 2
- 238000013198 immunometric assay Methods 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 238000004255 ion exchange chromatography Methods 0.000 description 2
- 238000001155 isoelectric focusing Methods 0.000 description 2
- 229960000310 isoleucine Drugs 0.000 description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 2
- 238000004020 luminiscence type Methods 0.000 description 2
- 210000002751 lymph Anatomy 0.000 description 2
- RLSSMJSEOOYNOY-UHFFFAOYSA-N m-cresol Chemical compound CC1=CC=CC(O)=C1 RLSSMJSEOOYNOY-UHFFFAOYSA-N 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 2
- 239000002808 molecular sieve Substances 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- AQIXEPGDORPWBJ-UHFFFAOYSA-N pentan-3-ol Chemical compound CCC(O)CC AQIXEPGDORPWBJ-UHFFFAOYSA-N 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- 230000001766 physiological effect Effects 0.000 description 2
- 210000002381 plasma Anatomy 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920002704 polyhistidine Polymers 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 238000010188 recombinant method Methods 0.000 description 2
- 230000006798 recombination Effects 0.000 description 2
- 238000005215 recombination Methods 0.000 description 2
- GHMLBKRAJCXXBS-UHFFFAOYSA-N resorcinol Chemical compound OC1=CC=CC(O)=C1 GHMLBKRAJCXXBS-UHFFFAOYSA-N 0.000 description 2
- 210000003296 saliva Anatomy 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 2
- 235000002639 sodium chloride Nutrition 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 108090000721 thyroid hormone receptors Proteins 0.000 description 2
- 102000004217 thyroid hormone receptors Human genes 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 241000701447 unidentified baculovirus Species 0.000 description 2
- 210000002700 urine Anatomy 0.000 description 2
- 239000004474 valine Substances 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- BHNQPLPANNDEGL-UHFFFAOYSA-N 2-(4-octylphenoxy)ethanol Chemical compound CCCCCCCCC1=CC=C(OCCO)C=C1 BHNQPLPANNDEGL-UHFFFAOYSA-N 0.000 description 1
- HUDPLKWXRLNSPC-UHFFFAOYSA-N 4-aminophthalhydrazide Chemical compound O=C1NNC(=O)C=2C1=CC(N)=CC=2 HUDPLKWXRLNSPC-UHFFFAOYSA-N 0.000 description 1
- 102100033639 Acetylcholinesterase Human genes 0.000 description 1
- 108010022752 Acetylcholinesterase Proteins 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- 108010000239 Aequorin Proteins 0.000 description 1
- 102000007698 Alcohol dehydrogenase Human genes 0.000 description 1
- 108010021809 Alcohol dehydrogenase Proteins 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 108010024976 Asparaginase Proteins 0.000 description 1
- 102000015790 Asparaginase Human genes 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 102100035882 Catalase Human genes 0.000 description 1
- 108010053835 Catalase Proteins 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- IVOMOUWHDPKRLL-KQYNXXCUSA-N Cyclic adenosine monophosphate Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=CN=C2N)=C2N=C1 IVOMOUWHDPKRLL-KQYNXXCUSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 1
- 101710088194 Dehydrogenase Proteins 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- BVTJGGGYKAMDBN-UHFFFAOYSA-N Dioxetane Chemical compound C1COO1 BVTJGGGYKAMDBN-UHFFFAOYSA-N 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 108010073178 Glucan 1,4-alpha-Glucosidase Proteins 0.000 description 1
- 102100022624 Glucoamylase Human genes 0.000 description 1
- 108010015776 Glucose oxidase Proteins 0.000 description 1
- 239000004366 Glucose oxidase Substances 0.000 description 1
- 102000000587 Glycerolphosphate Dehydrogenase Human genes 0.000 description 1
- 108010041921 Glycerolphosphate Dehydrogenase Proteins 0.000 description 1
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 1
- 206010020850 Hyperthyroidism Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical class C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 1
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 1
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 1
- 102000004195 Isomerases Human genes 0.000 description 1
- 108090000769 Isomerases Proteins 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- 125000000998 L-alanino group Chemical group [H]N([*])[C@](C([H])([H])[H])([H])C(=O)O[H] 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- 102000013460 Malate Dehydrogenase Human genes 0.000 description 1
- 108010026217 Malate Dehydrogenase Proteins 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 108010059724 Micrococcal Nuclease Proteins 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 206010028665 Myxoedema Diseases 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 108700026244 Open Reading Frames Proteins 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108700020962 Peroxidase Proteins 0.000 description 1
- 108010053210 Phycocyanin Proteins 0.000 description 1
- 108010004729 Phycoerythrin Proteins 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 102000006382 Ribonucleases Human genes 0.000 description 1
- 108010083644 Ribonucleases Proteins 0.000 description 1
- 239000012722 SDS sample buffer Substances 0.000 description 1
- 241000242677 Schistosoma japonicum Species 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 208000024799 Thyroid disease Diseases 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- 108700015934 Triose-phosphate isomerases Proteins 0.000 description 1
- 102100033598 Triosephosphate isomerase Human genes 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- IVOMOUWHDPKRLL-UHFFFAOYSA-N UNPD107823 Natural products O1C2COP(O)(=O)OC2C(O)C1N1C(N=CN=C2N)=C2N=C1 IVOMOUWHDPKRLL-UHFFFAOYSA-N 0.000 description 1
- 108010046334 Urease Proteins 0.000 description 1
- 229940022698 acetylcholinesterase Drugs 0.000 description 1
- DZBUGLKDJFMEHC-UHFFFAOYSA-N acridine Chemical class C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 108010004469 allophycocyanin Proteins 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 150000001371 alpha-amino acids Chemical class 0.000 description 1
- 235000008206 alpha-amino acids Nutrition 0.000 description 1
- VREFGVBLTWBCJP-UHFFFAOYSA-N alprazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NN=C2CN=C1C1=CC=CC=C1 VREFGVBLTWBCJP-UHFFFAOYSA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- 230000003302 anti-idiotype Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 101150010487 are gene Proteins 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960003272 asparaginase Drugs 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-M asparaginate Chemical compound [O-]C(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-M 0.000 description 1
- 230000001357 autoimmunogenic effect Effects 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 229960003872 benzethonium Drugs 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 238000005415 bioluminescence Methods 0.000 description 1
- 230000029918 bioluminescence Effects 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- HUTDDBSSHVOYJR-UHFFFAOYSA-H bis[(2-oxo-1,3,2$l^{5},4$l^{2}-dioxaphosphaplumbetan-2-yl)oxy]lead Chemical compound [Pb+2].[Pb+2].[Pb+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O HUTDDBSSHVOYJR-UHFFFAOYSA-H 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 229940041514 candida albicans extract Drugs 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 230000010307 cell transformation Effects 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 238000004182 chemical digestion Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000011097 chromatography purification Methods 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- NKLPQNGYXWVELD-UHFFFAOYSA-M coomassie brilliant blue Chemical compound [Na+].C1=CC(OCC)=CC=C1NC1=CC=C(C(=C2C=CC(C=C2)=[N+](CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=2C=CC(=CC=2)N(CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=C1 NKLPQNGYXWVELD-UHFFFAOYSA-M 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 230000009260 cross reactivity Effects 0.000 description 1
- 229940095074 cyclic amp Drugs 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000012470 diluted sample Substances 0.000 description 1
- SIYLLGKDQZGJHK-UHFFFAOYSA-N dimethyl-(phenylmethyl)-[2-[2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy]ethyl]ammonium Chemical compound C1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 SIYLLGKDQZGJHK-UHFFFAOYSA-N 0.000 description 1
- 150000002016 disaccharides Chemical class 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- DNJIEGIFACGWOD-UHFFFAOYSA-N ethyl mercaptane Natural products CCS DNJIEGIFACGWOD-UHFFFAOYSA-N 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- ZFKJVJIDPQDDFY-UHFFFAOYSA-N fluorescamine Chemical compound C12=CC=CC=C2C(=O)OC1(C1=O)OC=C1C1=CC=CC=C1 ZFKJVJIDPQDDFY-UHFFFAOYSA-N 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 238000001215 fluorescent labelling Methods 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000010363 gene targeting Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229940116332 glucose oxidase Drugs 0.000 description 1
- 235000019420 glucose oxidase Nutrition 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 125000003147 glycosyl group Chemical group 0.000 description 1
- 210000005255 gram-positive cell Anatomy 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 238000000703 high-speed centrifugation Methods 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 238000001597 immobilized metal affinity chromatography Methods 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 230000000984 immunochemical effect Effects 0.000 description 1
- 229940127121 immunoconjugate Drugs 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 239000002563 ionic surfactant Substances 0.000 description 1
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 1
- 150000002540 isothiocyanates Chemical class 0.000 description 1
- 229910052747 lanthanoid Inorganic materials 0.000 description 1
- 150000002602 lanthanoids Chemical class 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000012417 linear regression Methods 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 208000003786 myxedema Diseases 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 230000000050 nutritive effect Effects 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 229940054441 o-phthalaldehyde Drugs 0.000 description 1
- HEGSGKPQLMEBJL-RKQHYHRCSA-N octyl beta-D-glucopyranoside Chemical compound CCCCCCCCO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O HEGSGKPQLMEBJL-RKQHYHRCSA-N 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 229940055729 papain Drugs 0.000 description 1
- 235000019834 papain Nutrition 0.000 description 1
- 239000013610 patient sample Substances 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 230000002572 peristaltic effect Effects 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- RXNXLAHQOVLMIE-UHFFFAOYSA-N phenyl 10-methylacridin-10-ium-9-carboxylate Chemical compound C12=CC=CC=C2[N+](C)=C2C=CC=CC2=C1C(=O)OC1=CC=CC=C1 RXNXLAHQOVLMIE-UHFFFAOYSA-N 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- ZWLUXSQADUDCSB-UHFFFAOYSA-N phthalaldehyde Chemical compound O=CC1=CC=CC=C1C=O ZWLUXSQADUDCSB-UHFFFAOYSA-N 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 235000019419 proteases Nutrition 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 238000003908 quality control method Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000012723 sample buffer Substances 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 238000005063 solubilization Methods 0.000 description 1
- 230000007928 solubilization Effects 0.000 description 1
- 239000011537 solubilization buffer Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 238000002798 spectrophotometry method Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- SFVFIFLLYFPGHH-UHFFFAOYSA-M stearalkonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1 SFVFIFLLYFPGHH-UHFFFAOYSA-M 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 208000021510 thyroid gland disease Diseases 0.000 description 1
- 229940098465 tincture Drugs 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- 239000012138 yeast extract Substances 0.000 description 1
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/72—Receptors; Cell surface antigens; Cell surface determinants for hormones
- C07K14/723—G protein coupled receptor, e.g. TSHR-thyrotropin-receptor, LH/hCG receptor, FSH receptor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/564—Immunoassay; Biospecific binding assay; Materials therefor for pre-existing immune complex or autoimmune disease, i.e. systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, rheumatoid factors or complement components C1-C9
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/20—Fusion polypeptide containing a tag with affinity for a non-protein ligand
- C07K2319/23—Fusion polypeptide containing a tag with affinity for a non-protein ligand containing a GST-tag
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/20—Fusion polypeptide containing a tag with affinity for a non-protein ligand
- C07K2319/24—Fusion polypeptide containing a tag with affinity for a non-protein ligand containing a MBP (maltose binding protein)-tag
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/60—Fusion polypeptide containing spectroscopic/fluorescent detection, e.g. green fluorescent protein [GFP]
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/72—Assays involving receptors, cell surface antigens or cell surface determinants for hormones
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Cell Biology (AREA)
- Biochemistry (AREA)
- Urology & Nephrology (AREA)
- General Physics & Mathematics (AREA)
- Pharmacology & Pharmacy (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Rheumatology (AREA)
- Pathology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Rehabilitation Therapy (AREA)
- Food Science & Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Endocrinology (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US73802105P | 2005-11-21 | 2005-11-21 | |
| PCT/IB2006/003394 WO2007057778A2 (en) | 2005-11-21 | 2006-11-21 | Recombinant polypeptides and methods for detecting and/or quantifying autoantibodies against tsh receptor |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009517015A true JP2009517015A (ja) | 2009-04-30 |
| JP2009517015A5 JP2009517015A5 (enExample) | 2010-01-14 |
Family
ID=37998447
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008541846A Withdrawn JP2009517015A (ja) | 2005-11-21 | 2006-11-21 | 組換えポリペプチドならびにtshレセプターに対する自己抗体を検出及び/又は定量化する方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20080305098A1 (enExample) |
| EP (2) | EP2080772A3 (enExample) |
| JP (1) | JP2009517015A (enExample) |
| WO (1) | WO2007057778A2 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2011136911A (ja) * | 2009-12-25 | 2011-07-14 | Tosoh Corp | 甲状腺刺激ホルモンレセプターの安定化方法 |
| JP2016527304A (ja) * | 2013-08-06 | 2016-09-08 | アピトープ インターナショナル エヌブイ | ペプチド |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8470554B2 (en) * | 2008-12-22 | 2013-06-25 | St. Jude Children's Research Hospital | Prokaryotic expression of soluble, active Dkk |
| EP2617735A3 (en) * | 2008-12-24 | 2013-10-16 | Rsr Limited | Human anti tshr antibodies |
| US20130189707A1 (en) * | 2010-01-26 | 2013-07-25 | Svetlana Alexandrovna Sergeeva | Method of determination of autoantibody level by means of enzyme immunoassay |
| JP5908590B2 (ja) | 2011-09-09 | 2016-04-26 | クイデル コーポレーション | 自己抗体を検出するための組成物および方法 |
| JP6472789B2 (ja) | 2013-05-14 | 2019-02-20 | シーメンス・ヘルスケア・ダイアグノスティックス・インコーポレーテッドSiemens Healthcare Diagnostics Inc. | 受容体含有安定化液剤 |
| RU2728963C2 (ru) * | 2014-12-24 | 2020-08-03 | Эпитоп Интернэшнл Нв | Композиция |
| CN114601913B (zh) * | 2022-03-09 | 2025-03-04 | 陕西慧普德泰生物科技有限责任公司 | 人TSHR A亚基在预防Graves病中的应用 |
| CN119192331A (zh) * | 2024-11-11 | 2024-12-27 | 郑州伊美诺生物技术有限公司 | 促甲状腺激素受体的突变体及其制备方法和应用 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030022826A1 (en) * | 1987-09-08 | 2003-01-30 | Duke University | Use of synthetic peptides to induce tolerance to pathogenic T and B cell epitopes of autoantigens or infectious agents |
| JPH0789991A (ja) * | 1993-09-28 | 1995-04-04 | Mitsubishi Chem Corp | 新規なポリペプチドおよびそれを用いるtsh受容体抗体の測定法 |
| EP1078986B1 (en) | 1999-08-24 | 2007-02-28 | Tosoh Corporation | Secretory thyroid stimulating hormone receptor (TSHR), and method for assaying anti-TSHR antibody using the same |
| ES2732276T3 (es) * | 2001-08-23 | 2019-11-21 | Rsr Ltd | Regiones del epítopo de un receptor de tirotropina (TSH), usos del mismo y anticuerpos para el mismo |
| US8110664B2 (en) * | 2002-11-29 | 2012-02-07 | Rsr Limited | Binding partners for the thyrotropin receptor and uses thereof |
-
2006
- 2006-11-21 US US12/094,533 patent/US20080305098A1/en not_active Abandoned
- 2006-11-21 WO PCT/IB2006/003394 patent/WO2007057778A2/en not_active Ceased
- 2006-11-21 EP EP09005494A patent/EP2080772A3/en not_active Withdrawn
- 2006-11-21 JP JP2008541846A patent/JP2009517015A/ja not_active Withdrawn
- 2006-11-21 EP EP06820995A patent/EP1951753A2/en not_active Withdrawn
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2011136911A (ja) * | 2009-12-25 | 2011-07-14 | Tosoh Corp | 甲状腺刺激ホルモンレセプターの安定化方法 |
| JP2016527304A (ja) * | 2013-08-06 | 2016-09-08 | アピトープ インターナショナル エヌブイ | ペプチド |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2007057778A2 (en) | 2007-05-24 |
| US20080305098A1 (en) | 2008-12-11 |
| EP2080772A3 (en) | 2009-10-07 |
| EP1951753A2 (en) | 2008-08-06 |
| WO2007057778A3 (en) | 2007-11-08 |
| EP2080772A2 (en) | 2009-07-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2021223701B2 (en) | Methods and reagents for diagnosis of SARS-CoV-2 infection | |
| EP3855186B1 (en) | A method for determining the efficacy of a sars-cov-2 vaccine | |
| EP2853898B1 (en) | Analysis of myostatin in serum | |
| US9840551B2 (en) | Blood markers for diagnosing epithelium derived cancers and monoclonal antibodies thereof | |
| JP5379698B2 (ja) | アビバクテリウム・パラガリナラム抗体の検出方法およびキット | |
| JP5442641B2 (ja) | 全インフルエンザ菌の測定方法 | |
| EP2238167A2 (en) | Anti-t. cruzi antibodies and methods of use | |
| CN110684740A (zh) | 一种抗人泛素羧基末端水解酶-1(uch-l1)的单克隆抗体及其应用 | |
| JP2009517015A (ja) | 組換えポリペプチドならびにtshレセプターに対する自己抗体を検出及び/又は定量化する方法 | |
| JP2001507447A (ja) | H. pylori診断薬 | |
| WO2021237534A1 (zh) | 抗新型冠状病毒的抗体、抗体组合、制备方法、包含其的检测试剂盒 | |
| EP4148429A2 (en) | Method and kit for the detection of bovine herpes virus type 1 (bohv-1) antibodies | |
| WO2008068906A1 (ja) | 中皮腫診断剤、中皮腫診断キットおよび中皮腫診断方法 | |
| WO2013071127A1 (en) | Monoclonal antibodies against serotransferrin antigens, and uses therefor | |
| KR102244121B1 (ko) | 브루셀라균의 omp28에 특이적으로 결합하는 단일클론항체, 상기 항체를 생산하는 하이브리도마 세포주 및 이의 용도 | |
| WO2006073133A1 (ja) | 新規タンナーゼ遺伝子およびそのタンパク質 | |
| WO2022057254A1 (zh) | Hcv重组抗原及其突变体 | |
| JP6407990B2 (ja) | アウグリン免疫学的検定 | |
| CA2571673A1 (en) | Novel methods of diagnosis of treatment of p. aeruginosa infection and reagents therefor | |
| CN101133085B (zh) | 人乳清酸磷酸核糖基转移酶蛋白质的测定方法 | |
| US20040122213A1 (en) | Chlamydia heat shock protein | |
| TW202323286A (zh) | 抗EphA4抗體 | |
| CN119684430A (zh) | 一种抗原多肽、由其制备的抗体及其在检测cd8中的应用 | |
| CN112180096A (zh) | 用于诊断线虫感染的新测定法 | |
| JP2012131713A (ja) | ヒトdapitを免疫原として得られたポリクローナル抗体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20091119 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20091119 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20101221 |