JP2006525099A - 子宮頚部組織のバイオインピーダンス測定のためのデバイス、システムおよび方法、並びにヒト子宮頚部の診断および処置のための方法 - Google Patents
子宮頚部組織のバイオインピーダンス測定のためのデバイス、システムおよび方法、並びにヒト子宮頚部の診断および処置のための方法 Download PDFInfo
- Publication number
- JP2006525099A JP2006525099A JP2006514248A JP2006514248A JP2006525099A JP 2006525099 A JP2006525099 A JP 2006525099A JP 2006514248 A JP2006514248 A JP 2006514248A JP 2006514248 A JP2006514248 A JP 2006514248A JP 2006525099 A JP2006525099 A JP 2006525099A
- Authority
- JP
- Japan
- Prior art keywords
- electrodes
- tissue
- electrode
- voltage
- array
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 85
- 238000005259 measurement Methods 0.000 title claims description 141
- 238000003745 diagnosis Methods 0.000 title claims description 8
- 210000003679 cervix uteri Anatomy 0.000 title description 16
- 238000011282 treatment Methods 0.000 title description 14
- 239000000523 sample Substances 0.000 claims description 120
- 238000001514 detection method Methods 0.000 claims description 59
- 238000003491 array Methods 0.000 claims description 33
- 238000012545 processing Methods 0.000 claims description 26
- 230000035935 pregnancy Effects 0.000 claims description 20
- 238000000691 measurement method Methods 0.000 claims description 11
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 claims description 8
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 claims description 6
- 230000004044 response Effects 0.000 claims description 6
- 239000003814 drug Substances 0.000 claims description 4
- 229940079593 drug Drugs 0.000 claims description 4
- 229940127291 Calcium channel antagonist Drugs 0.000 claims description 3
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 claims description 3
- 239000000808 adrenergic beta-agonist Substances 0.000 claims description 3
- 239000000480 calcium channel blocker Substances 0.000 claims description 3
- 239000003246 corticosteroid Substances 0.000 claims description 3
- 229960001334 corticosteroids Drugs 0.000 claims description 3
- 230000000977 initiatory effect Effects 0.000 claims description 3
- 229960004194 lidocaine Drugs 0.000 claims description 3
- 229910052943 magnesium sulfate Inorganic materials 0.000 claims description 3
- 229960003390 magnesium sulfate Drugs 0.000 claims description 3
- 235000019341 magnesium sulphate Nutrition 0.000 claims description 3
- 239000002599 prostaglandin synthase inhibitor Substances 0.000 claims description 3
- GQPLMRYTRLFLPF-UHFFFAOYSA-N Nitrous Oxide Chemical compound [O-][N+]#N GQPLMRYTRLFLPF-UHFFFAOYSA-N 0.000 claims 2
- 229940122204 Cyclooxygenase inhibitor Drugs 0.000 claims 1
- 208000007914 Labor Pain Diseases 0.000 claims 1
- 208000035945 Labour pain Diseases 0.000 claims 1
- 239000001272 nitrous oxide Substances 0.000 claims 1
- 230000008520 organization Effects 0.000 claims 1
- 208000037805 labour Diseases 0.000 abstract description 38
- 230000005856 abnormality Effects 0.000 abstract description 2
- 230000003902 lesion Effects 0.000 abstract description 2
- 238000010998 test method Methods 0.000 abstract 1
- 210000001519 tissue Anatomy 0.000 description 101
- 238000004891 communication Methods 0.000 description 30
- 230000007274 generation of a signal involved in cell-cell signaling Effects 0.000 description 21
- 208000006399 Premature Obstetric Labor Diseases 0.000 description 10
- 230000006870 function Effects 0.000 description 10
- 239000000463 material Substances 0.000 description 10
- 238000012384 transportation and delivery Methods 0.000 description 10
- 230000008859 change Effects 0.000 description 8
- 210000003754 fetus Anatomy 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 7
- 238000010586 diagram Methods 0.000 description 7
- 230000001605 fetal effect Effects 0.000 description 7
- 238000003032 molecular docking Methods 0.000 description 7
- 238000007634 remodeling Methods 0.000 description 7
- 208000005107 Premature Birth Diseases 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 230000002028 premature Effects 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 102000008186 Collagen Human genes 0.000 description 5
- 108010035532 Collagen Proteins 0.000 description 5
- 206010036600 Premature labour Diseases 0.000 description 5
- 229920001436 collagen Polymers 0.000 description 5
- 208000015181 infectious disease Diseases 0.000 description 5
- 208000026440 premature labor Diseases 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 230000001954 sterilising effect Effects 0.000 description 5
- 238000004659 sterilization and disinfection Methods 0.000 description 5
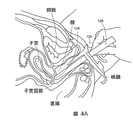
- 210000001215 vagina Anatomy 0.000 description 5
- 208000036029 Uterine contractions during pregnancy Diseases 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 230000000241 respiratory effect Effects 0.000 description 4
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 229920002683 Glycosaminoglycan Polymers 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 238000007796 conventional method Methods 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000002567 electromyography Methods 0.000 description 3
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 3
- 229910052737 gold Inorganic materials 0.000 description 3
- 239000010931 gold Substances 0.000 description 3
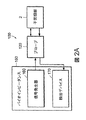
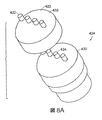
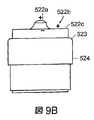
- 238000002847 impedance measurement Methods 0.000 description 3
- 238000001453 impedance spectrum Methods 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 230000008774 maternal effect Effects 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- 230000003287 optical effect Effects 0.000 description 3
- 238000002604 ultrasonography Methods 0.000 description 3
- 210000004291 uterus Anatomy 0.000 description 3
- 206010000234 Abortion spontaneous Diseases 0.000 description 2
- 206010001052 Acute respiratory distress syndrome Diseases 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 208000018522 Gastrointestinal disease Diseases 0.000 description 2
- 208000032843 Hemorrhage Diseases 0.000 description 2
- 206010022840 Intraventricular haemorrhage Diseases 0.000 description 2
- 208000013616 Respiratory Distress Syndrome Diseases 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 210000001691 amnion Anatomy 0.000 description 2
- 210000003484 anatomy Anatomy 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 238000001574 biopsy Methods 0.000 description 2
- 208000034158 bleeding Diseases 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000002405 diagnostic procedure Methods 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 238000005538 encapsulation Methods 0.000 description 2
- 230000003054 hormonal effect Effects 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000007040 lung development Effects 0.000 description 2
- 230000004199 lung function Effects 0.000 description 2
- 230000035800 maturation Effects 0.000 description 2
- 208000015994 miscarriage Diseases 0.000 description 2
- 239000002840 nitric oxide donor Substances 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 230000032633 organ maturation Effects 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 230000001850 reproductive effect Effects 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 208000000995 spontaneous abortion Diseases 0.000 description 2
- 230000035882 stress Effects 0.000 description 2
- 230000007704 transition Effects 0.000 description 2
- 230000036266 weeks of gestation Effects 0.000 description 2
- PROQIPRRNZUXQM-UHFFFAOYSA-N (16alpha,17betaOH)-Estra-1,3,5(10)-triene-3,16,17-triol Natural products OC1=CC=C2C3CCC(C)(C(C(O)C4)O)C4C3CCC2=C1 PROQIPRRNZUXQM-UHFFFAOYSA-N 0.000 description 1
- 206010008267 Cervical incompetence Diseases 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229910000881 Cu alloy Inorganic materials 0.000 description 1
- 208000005189 Embolism Diseases 0.000 description 1
- 102000016359 Fibronectins Human genes 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 1
- 206010021718 Induced labour Diseases 0.000 description 1
- 125000002066 L-histidyl group Chemical group [H]N1C([H])=NC(C([H])([H])[C@](C(=O)[*])([H])N([H])[H])=C1[H] 0.000 description 1
- 208000036626 Mental retardation Diseases 0.000 description 1
- 208000034702 Multiple pregnancies Diseases 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 101710158668 Placental protein Proteins 0.000 description 1
- 201000009916 Postpartum depression Diseases 0.000 description 1
- 208000006994 Precancerous Conditions Diseases 0.000 description 1
- 102000008217 Pregnancy Proteins Human genes 0.000 description 1
- 206010036590 Premature baby Diseases 0.000 description 1
- 102000003946 Prolactin Human genes 0.000 description 1
- 108010057464 Prolactin Proteins 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 208000001435 Thromboembolism Diseases 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- NEIHULKJZQTQKJ-UHFFFAOYSA-N [Cu].[Ag] Chemical compound [Cu].[Ag] NEIHULKJZQTQKJ-UHFFFAOYSA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 210000004381 amniotic fluid Anatomy 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 238000010876 biochemical test Methods 0.000 description 1
- 239000000560 biocompatible material Substances 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000003990 capacitor Substances 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 238000013500 data storage Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 231100000517 death Toxicity 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000012631 diagnostic technique Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 230000010339 dilation Effects 0.000 description 1
- PROQIPRRNZUXQM-ZXXIGWHRSA-N estriol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H]([C@H](O)C4)O)[C@@H]4[C@@H]3CCC2=C1 PROQIPRRNZUXQM-ZXXIGWHRSA-N 0.000 description 1
- 229960001348 estriol Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000005802 health problem Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 238000010562 histological examination Methods 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 230000008376 long-term health Effects 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 230000004118 muscle contraction Effects 0.000 description 1
- 230000009826 neoplastic cell growth Effects 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 238000002559 palpation Methods 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000009984 peri-natal effect Effects 0.000 description 1
- 239000012466 permeate Substances 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 230000010363 phase shift Effects 0.000 description 1
- 238000013439 planning Methods 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 229940097325 prolactin Drugs 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/42—Gynaecological or obstetrical instruments or methods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/05—Detecting, measuring or recording for diagnosis by means of electric currents or magnetic fields; Measuring using microwaves or radio waves
- A61B5/053—Measuring electrical impedance or conductance of a portion of the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/05—Detecting, measuring or recording for diagnosis by means of electric currents or magnetic fields; Measuring using microwaves or radio waves
- A61B5/053—Measuring electrical impedance or conductance of a portion of the body
- A61B5/0538—Measuring electrical impedance or conductance of a portion of the body invasively, e.g. using a catheter
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/43—Detecting, measuring or recording for evaluating the reproductive systems
- A61B5/4306—Detecting, measuring or recording for evaluating the reproductive systems for evaluating the female reproductive systems, e.g. gynaecological evaluations
- A61B5/4318—Evaluation of the lower reproductive system
- A61B5/4331—Evaluation of the lower reproductive system of the cervix
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6846—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be brought in contact with an internal body part, i.e. invasive
- A61B5/6885—Monitoring or controlling sensor contact pressure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B2017/00017—Electrical control of surgical instruments
- A61B2017/00022—Sensing or detecting at the treatment site
- A61B2017/00026—Conductivity or impedance, e.g. of tissue
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Surgery (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pathology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Gynecology & Obstetrics (AREA)
- Reproductive Health (AREA)
- Radiology & Medical Imaging (AREA)
- Pregnancy & Childbirth (AREA)
- Measurement And Recording Of Electrical Phenomena And Electrical Characteristics Of The Living Body (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US46745603P | 2003-05-02 | 2003-05-02 | |
| US49730003P | 2003-08-22 | 2003-08-22 | |
| PCT/US2004/013720 WO2004098389A2 (en) | 2003-05-02 | 2004-05-03 | Devices, systems and methods for bioimpendence measurement of cervical tissue and methods for diagnosis and treatment of human cervix |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2006525099A true JP2006525099A (ja) | 2006-11-09 |
| JP2006525099A5 JP2006525099A5 (enExample) | 2011-09-08 |
Family
ID=34316211
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006514248A Pending JP2006525099A (ja) | 2003-05-02 | 2004-05-03 | 子宮頚部組織のバイオインピーダンス測定のためのデバイス、システムおよび方法、並びにヒト子宮頚部の診断および処置のための方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US8060195B2 (enExample) |
| EP (1) | EP1620006A4 (enExample) |
| JP (1) | JP2006525099A (enExample) |
| WO (1) | WO2004098389A2 (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012509724A (ja) * | 2008-11-28 | 2012-04-26 | インぺディメッド リミテッド | インピーダンス測定処理 |
| WO2012070569A1 (ja) * | 2010-11-22 | 2012-05-31 | 国立大学法人大阪大学 | 子宮着床能の測定方法、測定装置及び測定装置の作動方法 |
| KR20190091701A (ko) * | 2018-01-29 | 2019-08-07 | 고려대학교 산학협력단 | 비침습형 신경 전극 조립체 및 이를 이용한 신경 전극 제어 시스템 |
| WO2023090533A1 (en) * | 2021-11-22 | 2023-05-25 | Dc Medical Inc | System and methods for providing information for diagnosing preterm birth risk |
| JP2023550878A (ja) * | 2020-10-13 | 2023-12-06 | インペリアル・カレッジ・イノベーションズ・リミテッド | 術中リアルタイム腫瘍組織識別装置および方法 |
| WO2024242172A1 (ja) * | 2023-05-23 | 2024-11-28 | 国立大学法人大阪大学 | 子宮の着床能の予測及び分娩時期の推定のための装置及び方法 |
Families Citing this family (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1765161B1 (en) | 2004-06-18 | 2019-09-25 | Impedimed Limited | Oedema detection |
| GB0511323D0 (en) | 2005-06-03 | 2005-07-13 | Sheffield Teaching Hospitals | Apparatus for measuring tissue sample electrical impedance |
| GB2426824A (en) * | 2005-06-03 | 2006-12-06 | Sheffield Teaching Hospitals | Body tissue impedance measuring probe with wireless transmitter |
| JP5034028B2 (ja) | 2005-07-01 | 2012-09-26 | インペダイムド・リミテッド | 肺モニタリングシステム |
| CA2609111C (en) | 2005-07-01 | 2016-10-18 | Scott Chetham | A method and apparatus for performing impedance measurements in accordance with determining an electrode arrangement using a displayed representation |
| AU2006265761B2 (en) | 2005-07-01 | 2011-08-11 | Impedimed Limited | Monitoring system |
| US20100292603A1 (en) * | 2005-09-21 | 2010-11-18 | Beth Israel Deaconess Medical Center, Inc. | Electrical Impedance Myography |
| WO2007041783A1 (en) | 2005-10-11 | 2007-04-19 | Impedance Cardiology Systems, Inc. | Hydration status monitoring |
| EP2091425A4 (en) | 2006-11-30 | 2012-07-25 | Impedimed Ltd | Measurement apparatus |
| ES2543967T3 (es) | 2007-01-15 | 2015-08-26 | Impedimed Limited | Método para efectuar mediciones de impedancia en un sujeto |
| EP2098828A4 (en) * | 2007-04-19 | 2011-10-19 | Hosiden Corp | ROTATION INPUT DEVICE AND ROTATION DETECTION DEVICE USING THE SAME |
| EP2148613B9 (en) | 2007-04-20 | 2014-12-10 | Impedimed Limited | Monitoring system and probe |
| US7930022B2 (en) * | 2007-05-07 | 2011-04-19 | Cardiac Pacemakers, Inc. | System and method to determine hemodynamic tolerability |
| JP4893515B2 (ja) * | 2007-07-19 | 2012-03-07 | オムロンヘルスケア株式会社 | 生体インピーダンス測定用胴部装着ユニットおよび体脂肪測定装置 |
| US20110046505A1 (en) * | 2007-08-09 | 2011-02-24 | Impedimed Limited | Impedance measurement process |
| CA2704061C (en) | 2007-11-05 | 2017-06-20 | Impedimed Limited | Impedance determination |
| AU2008207672B2 (en) | 2008-02-15 | 2013-10-31 | Impedimed Limited | Impedance Analysis |
| WO2009154758A2 (en) | 2008-06-19 | 2009-12-23 | Cardiac Pacemakers, Inc. | Cardiac rhythm management system with hemodynamic tolerability analyzer |
| EP2419159A4 (en) | 2009-04-16 | 2017-07-19 | Bivacor Pty Ltd | Heart pump controller |
| JP2012523856A (ja) | 2009-04-16 | 2012-10-11 | ビバコール プロプライエタリー リミテッド | 心臓ポンプコントローラ |
| EP2493378A4 (en) | 2009-10-26 | 2014-07-09 | Impedimed Ltd | DETERMINATION OF A LIQUID LEVEL INDICATOR |
| US9585593B2 (en) | 2009-11-18 | 2017-03-07 | Chung Shing Fan | Signal distribution for patient-electrode measurements |
| WO2011103473A2 (en) * | 2010-02-18 | 2011-08-25 | The Johns Hopkins University | Preterm labor monitor |
| US20170035347A1 (en) * | 2012-09-12 | 2017-02-09 | Mb Device Llc | Method for monitoring pregnancy in mammals |
| EP2912458B1 (en) | 2012-10-24 | 2018-07-18 | NYU Winthrop Hospital | Non-invasive biomarker to identify subjects at risk of preterm delivery |
| US20150038872A1 (en) * | 2013-08-02 | 2015-02-05 | The Trustees Of Dartmouth College | Multiple-electrode electrical impedance sensing biopsy sampling device and method |
| JP6414713B2 (ja) | 2014-03-28 | 2018-11-07 | 国立大学法人大阪大学 | 膣評価装置及び子宮評価装置 |
| CN103876738B (zh) * | 2014-04-03 | 2016-03-09 | 思澜科技(成都)有限公司 | 基于频谱特性的生物阻抗测量探针、测量系统及方法 |
| US10966601B2 (en) * | 2015-05-27 | 2021-04-06 | Ob Tools Ltd. | Vaginal speculum with electromyographic sensors |
| GB2545703B (en) * | 2015-12-22 | 2019-01-09 | Univ Sheffield | Apparatus and methods for determining force applied to the tip of a probe |
| EP3400033B1 (en) | 2016-01-06 | 2024-06-05 | Bivacor Inc. | Heart pump with impeller axial position control |
| CN110709114B (zh) | 2017-04-05 | 2023-10-31 | 毕瓦克公司 | 心脏泵驱动器和轴承 |
| US11207509B2 (en) | 2017-06-15 | 2021-12-28 | Wiesman Holdings, LLC | Method and device for delivery of a solution into a body orifice |
| JP2020533595A (ja) | 2017-09-13 | 2020-11-19 | プロジェニティ, インコーポレイテッド | 子癇前症バイオマーカならびに関連するシステムおよび方法 |
| USD918388S1 (en) | 2018-06-15 | 2021-05-04 | Wiesman Holdings, LLC | Solution diffusing head |
| CN109700459B (zh) * | 2018-11-19 | 2022-03-15 | 重庆大学附属肿瘤医院 | 用于宫颈筛查的电阻抗检测系统 |
| US12102303B2 (en) * | 2019-04-05 | 2024-10-01 | Gynetronics Ltd. | Method and apparatus for direct in-vivo, electrical and chemical monitoring and stimulation of the endometrial cavity |
| EP4070113A4 (en) | 2019-12-04 | 2023-12-20 | Biora Therapeutics, Inc. | ASSESSMENT OF PREECAMPSIA USING FREE AND DISSOCIATE PLACENTAL GROWTH FACTOR ASSAYS |
| TWI723696B (zh) * | 2019-12-20 | 2021-04-01 | 金上達科技股份有限公司 | 人體生物訊息檢測裝置及其方法 |
| FR3143593A1 (fr) * | 2022-12-20 | 2024-06-21 | Aequilliving Inc Limited | Procédé de fabrication d’un produit de traitement. |
| CN116869505A (zh) * | 2023-08-25 | 2023-10-13 | 杭州堃博生物科技有限公司 | 阻抗检测导管、阻抗检测系统以及阻抗检测方法 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01113645A (ja) * | 1987-10-27 | 1989-05-02 | Nippon Syst Kenkyusho:Kk | 導電性測定装置 |
| DE4100568A1 (de) * | 1991-01-11 | 1992-07-16 | Fehling Guido | Vorrichtung zur ueberwachung eines patienten auf abstossungsreaktionen eines implantierten organs |
| WO2000019894A1 (en) * | 1998-10-08 | 2000-04-13 | Polartechnics Limited | Apparatus for recognizing tissue types |
| US6169914B1 (en) * | 1998-01-13 | 2001-01-02 | Urometrics, Inc. | Devices and methods for monitoring female arousal |
| US20030060696A1 (en) * | 2001-06-15 | 2003-03-27 | Skladnev Victor Nickolaevich | Apparatus for tissue type recognition using multiple measurement techniques |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4577640A (en) * | 1984-03-20 | 1986-03-25 | Hofmeister John F | Method and apparatus for direct in vivo monitoring of uterine electrical activity |
| US5199442A (en) * | 1991-05-20 | 1993-04-06 | Seager Stephen W J | Apparatus for reduction of spasticity in male and female patients having spinal cord injury as well as obtaining semen from males by stimulation of ejaculatory nerves |
| US6026323A (en) * | 1997-03-20 | 2000-02-15 | Polartechnics Limited | Tissue diagnostic system |
| US6270458B1 (en) * | 1999-03-05 | 2001-08-07 | Barnev Inc. | Cervix dilation and labor progression monitor |
| GB0005247D0 (en) * | 2000-03-03 | 2000-04-26 | Btg Int Ltd | Electrical impedance method for differentiating tissue types |
| JP2005522244A (ja) | 2002-04-09 | 2005-07-28 | メダルティス・アクチェンゲゼルシャフト | 延長器 |
| KR100459903B1 (ko) * | 2002-07-25 | 2004-12-03 | 삼성전자주식회사 | 피부의 국부적인 영역의 임피던스를 측정하는 측정 시스템및 이에 이용되는 임피던스 측정 전극 |
| JP2007532170A (ja) | 2004-04-07 | 2007-11-15 | バーネフ リミテッド | 状態に基づく出産モニタリングシステム |
-
2004
- 2004-05-03 US US10/555,503 patent/US8060195B2/en active Active
- 2004-05-03 EP EP04751210A patent/EP1620006A4/en not_active Withdrawn
- 2004-05-03 WO PCT/US2004/013720 patent/WO2004098389A2/en not_active Ceased
- 2004-05-03 JP JP2006514248A patent/JP2006525099A/ja active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01113645A (ja) * | 1987-10-27 | 1989-05-02 | Nippon Syst Kenkyusho:Kk | 導電性測定装置 |
| DE4100568A1 (de) * | 1991-01-11 | 1992-07-16 | Fehling Guido | Vorrichtung zur ueberwachung eines patienten auf abstossungsreaktionen eines implantierten organs |
| US6169914B1 (en) * | 1998-01-13 | 2001-01-02 | Urometrics, Inc. | Devices and methods for monitoring female arousal |
| WO2000019894A1 (en) * | 1998-10-08 | 2000-04-13 | Polartechnics Limited | Apparatus for recognizing tissue types |
| US20030060696A1 (en) * | 2001-06-15 | 2003-03-27 | Skladnev Victor Nickolaevich | Apparatus for tissue type recognition using multiple measurement techniques |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012509724A (ja) * | 2008-11-28 | 2012-04-26 | インぺディメッド リミテッド | インピーダンス測定処理 |
| WO2012070569A1 (ja) * | 2010-11-22 | 2012-05-31 | 国立大学法人大阪大学 | 子宮着床能の測定方法、測定装置及び測定装置の作動方法 |
| JP5923760B2 (ja) * | 2010-11-22 | 2016-05-25 | 東レ・メディカル株式会社 | 子宮着床能の測定装置 |
| KR20190091701A (ko) * | 2018-01-29 | 2019-08-07 | 고려대학교 산학협력단 | 비침습형 신경 전극 조립체 및 이를 이용한 신경 전극 제어 시스템 |
| KR102040161B1 (ko) | 2018-01-29 | 2019-11-04 | 고려대학교 산학협력단 | 비침습형 신경 전극 조립체 및 이를 이용한 신경 전극 제어 시스템 |
| JP2023550878A (ja) * | 2020-10-13 | 2023-12-06 | インペリアル・カレッジ・イノベーションズ・リミテッド | 術中リアルタイム腫瘍組織識別装置および方法 |
| WO2023090533A1 (en) * | 2021-11-22 | 2023-05-25 | Dc Medical Inc | System and methods for providing information for diagnosing preterm birth risk |
| WO2024242172A1 (ja) * | 2023-05-23 | 2024-11-28 | 国立大学法人大阪大学 | 子宮の着床能の予測及び分娩時期の推定のための装置及び方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2004098389A2 (en) | 2004-11-18 |
| US20090171234A1 (en) | 2009-07-02 |
| EP1620006A2 (en) | 2006-02-01 |
| EP1620006A4 (en) | 2009-12-02 |
| WO2004098389A3 (en) | 2005-03-24 |
| US8060195B2 (en) | 2011-11-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8060195B2 (en) | Devices, systems and methods for bioimpedance measurement of cervical tissue and methods for diagnosis and treatment of human cervix | |
| Kim et al. | Relationship between endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound and pregnancy after intrauterine insemination | |
| US10039472B2 (en) | Preterm labor monitor | |
| JP4053880B2 (ja) | 子宮頸部測定のための装置及び方法 | |
| US20080077053A1 (en) | Method for Vaginal Skin Biomechanical Evaluation | |
| CN102573687A (zh) | 孔探头和一种使用孔探头的方法 | |
| Achiron et al. | Fetal aortic arch measurements between 14 and 38 weeks' gestation: in‐utero ultrasonographic study | |
| US6364844B1 (en) | Diagnostic system for determining and/or monitoring physiological conditions in mammals | |
| Avis et al. | In vitro multifrequency electrical impedance measurements and modelling of the cervix in late pregnancy | |
| US20090137925A1 (en) | Impedance Spectroscopy Cervix Scanning Apparatus and Method | |
| RU2364336C1 (ru) | Способ функциональной диагностики сократительной способности мышц малого таза | |
| JP5923760B2 (ja) | 子宮着床能の測定装置 | |
| JP2002000604A (ja) | 超音波診断装置 | |
| RU2295291C1 (ru) | Способ диагностики варикозного расширения вен малого таза у женщин | |
| Etemadi et al. | Novel device to trend impedance and fluorescence of the cervix for preterm birth detection | |
| Yates | Using ultrasound to detect post-void residual urine | |
| Eldesouky et al. | Clinical audit of normal labor at Al Azhar University Hospitals | |
| Stephens | Imaging Limitations Hinder Early Endometriosis Diagnosis | |
| RU2545889C1 (ru) | Устройство для определения силы сокращения запирательной мышцы влагалища | |
| Başer Seçer et al. | The hidden power in the miracle of pregnancy: the effect of pelvic floor muscle training on fetal and fetal-maternal blood circulation and pelvic floor muscles during pregnancy, a randomized controlled trial | |
| CN114864091A (zh) | 用于足月待引产孕妇宫颈成熟度评价的方法、设备及介质 | |
| Anwar et al. | Saline-infused hysterography versus 3D transvaginal ultrasound in detecting cesarean scar defect: Diagnostic agreement study | |
| Santoso et al. | Use of 2D and multislice transperineal ultrasonography to describe the degree of perineal laceration following vaginal delivery | |
| Stewart et al. | OC27. 07: Translabial ultrasound for localisation of midurethral sling mesh. | |
| Benchimol et al. | OC28. 01: Reference ranges for placental perfusion using dynamic contrast enhancement magnetic resonance imaging. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070501 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20070501 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070914 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100601 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20100901 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100922 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20101001 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20101001 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100913 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20101008 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20101201 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110118 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110418 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110425 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110518 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110525 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110719 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20110719 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120131 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20120703 |