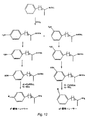
JP2004525359A - 試料中のアンフェタミンおよびメタンフェタミンの新規トレーサーの組成物および合成方法 - Google Patents
試料中のアンフェタミンおよびメタンフェタミンの新規トレーサーの組成物および合成方法 Download PDFInfo
- Publication number
- JP2004525359A JP2004525359A JP2002557773A JP2002557773A JP2004525359A JP 2004525359 A JP2004525359 A JP 2004525359A JP 2002557773 A JP2002557773 A JP 2002557773A JP 2002557773 A JP2002557773 A JP 2002557773A JP 2004525359 A JP2004525359 A JP 2004525359A
- Authority
- JP
- Japan
- Prior art keywords
- group
- amphetamine
- tracer
- sample
- antibody
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000000700 radioactive tracer Substances 0.000 title claims abstract description 96
- 229940025084 amphetamine Drugs 0.000 title claims abstract description 63
- KWTSXDURSIMDCE-QMMMGPOBSA-N (S)-amphetamine Chemical compound C[C@H](N)CC1=CC=CC=C1 KWTSXDURSIMDCE-QMMMGPOBSA-N 0.000 title claims abstract description 61
- 238000000034 method Methods 0.000 title claims abstract description 29
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 title abstract description 30
- 229960001252 methamphetamine Drugs 0.000 title abstract description 30
- 230000015572 biosynthetic process Effects 0.000 title abstract description 17
- 238000003786 synthesis reaction Methods 0.000 title abstract description 17
- 239000000203 mixture Substances 0.000 title description 31
- 238000003018 immunoassay Methods 0.000 claims abstract description 53
- 239000000523 sample Substances 0.000 claims description 21
- 150000001875 compounds Chemical class 0.000 claims description 17
- 210000003296 saliva Anatomy 0.000 claims description 12
- 239000012472 biological sample Substances 0.000 claims description 8
- 239000000427 antigen Substances 0.000 claims description 7
- 102000036639 antigens Human genes 0.000 claims description 7
- 108091007433 antigens Proteins 0.000 claims description 7
- 210000002700 urine Anatomy 0.000 claims description 6
- 150000001412 amines Chemical class 0.000 claims description 5
- 239000007850 fluorescent dye Substances 0.000 claims description 4
- 229920006395 saturated elastomer Polymers 0.000 claims description 4
- 102000004190 Enzymes Human genes 0.000 claims description 2
- 108090000790 Enzymes Proteins 0.000 claims description 2
- 239000008280 blood Substances 0.000 claims description 2
- 210000004369 blood Anatomy 0.000 claims description 2
- 239000000084 colloidal system Substances 0.000 claims description 2
- 210000002381 plasma Anatomy 0.000 claims description 2
- 210000002966 serum Anatomy 0.000 claims description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims 4
- 230000002285 radioactive effect Effects 0.000 claims 1
- 239000007787 solid Substances 0.000 claims 1
- 229940079593 drug Drugs 0.000 abstract description 31
- 239000003814 drug Substances 0.000 abstract description 31
- 239000012491 analyte Substances 0.000 abstract description 30
- 238000006073 displacement reaction Methods 0.000 abstract description 18
- 238000001514 detection method Methods 0.000 abstract description 14
- 239000007858 starting material Substances 0.000 abstract description 12
- 230000001105 regulatory effect Effects 0.000 abstract description 10
- 230000002194 synthesizing effect Effects 0.000 abstract description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 22
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 22
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 18
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 15
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 15
- 239000000047 product Substances 0.000 description 14
- 238000004519 manufacturing process Methods 0.000 description 13
- -1 5 th Ed. Substances 0.000 description 12
- 150000002148 esters Chemical class 0.000 description 12
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 9
- 239000000872 buffer Substances 0.000 description 9
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- 239000011347 resin Substances 0.000 description 8
- 229920005989 resin Polymers 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- 238000004809 thin layer chromatography Methods 0.000 description 8
- 239000012043 crude product Substances 0.000 description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 6
- 239000000377 silicon dioxide Substances 0.000 description 6
- QAEDZJGFFMLHHQ-UHFFFAOYSA-N trifluoroacetic anhydride Chemical compound FC(F)(F)C(=O)OC(=O)C(F)(F)F QAEDZJGFFMLHHQ-UHFFFAOYSA-N 0.000 description 6
- 230000008878 coupling Effects 0.000 description 5
- 238000010168 coupling process Methods 0.000 description 5
- 238000005859 coupling reaction Methods 0.000 description 5
- 239000012044 organic layer Substances 0.000 description 5
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 4
- 239000011541 reaction mixture Substances 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- 229960000074 biopharmaceutical Drugs 0.000 description 3
- 239000003054 catalyst Substances 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 230000009260 cross reactivity Effects 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 150000007925 phenylethylamine derivatives Chemical class 0.000 description 3
- 230000035945 sensitivity Effects 0.000 description 3
- 239000000741 silica gel Substances 0.000 description 3
- 229910002027 silica gel Inorganic materials 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 238000001308 synthesis method Methods 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 2
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical compound ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 2
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 2
- PFYXSUNOLOJMDX-UHFFFAOYSA-N bis(2,5-dioxopyrrolidin-1-yl) carbonate Chemical compound O=C1CCC(=O)N1OC(=O)ON1C(=O)CCC1=O PFYXSUNOLOJMDX-UHFFFAOYSA-N 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 239000000599 controlled substance Substances 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 239000005457 ice water Substances 0.000 description 2
- 238000000691 measurement method Methods 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 239000003068 molecular probe Substances 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 229940014800 succinic anhydride Drugs 0.000 description 2
- 210000004243 sweat Anatomy 0.000 description 2
- WECUIGDEWBNQJJ-UHFFFAOYSA-N 4-phenylbutan-2-amine Chemical compound CC(N)CCC1=CC=CC=C1 WECUIGDEWBNQJJ-UHFFFAOYSA-N 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 208000006550 Mydriasis Diseases 0.000 description 1
- BHHGXPLMPWCGHP-UHFFFAOYSA-N Phenethylamine Chemical compound NCCC1=CC=CC=C1 BHHGXPLMPWCGHP-UHFFFAOYSA-N 0.000 description 1
- 239000002269 analeptic agent Substances 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 239000000805 composite resin Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 206010061428 decreased appetite Diseases 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 238000002290 gas chromatography-mass spectrometry Methods 0.000 description 1
- 210000004209 hair Anatomy 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- 230000003100 immobilizing effect Effects 0.000 description 1
- 230000000622 irritating effect Effects 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- PUAUJIPHHQUZGF-UHFFFAOYSA-N n-methyl-4-phenylbutan-2-amine Chemical compound CNC(C)CCC1=CC=CC=C1 PUAUJIPHHQUZGF-UHFFFAOYSA-N 0.000 description 1
- 210000000282 nail Anatomy 0.000 description 1
- 238000011017 operating method Methods 0.000 description 1
- 210000001428 peripheral nervous system Anatomy 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 239000000021 stimulant Substances 0.000 description 1
- 239000012258 stirred mixture Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 230000002889 sympathetic effect Effects 0.000 description 1
- 239000013076 target substance Substances 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- ZWZVWGITAAIFPS-UHFFFAOYSA-N thiophosgene Chemical compound ClC(Cl)=S ZWZVWGITAAIFPS-UHFFFAOYSA-N 0.000 description 1
- 239000003039 volatile agent Substances 0.000 description 1
- 230000036642 wellbeing Effects 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/94—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving narcotics or drugs or pharmaceuticals, neurotransmitters or associated receptors
- G01N33/946—CNS-stimulants, e.g. cocaine, amphetamines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C331/00—Derivatives of thiocyanic acid or of isothiocyanic acid
- C07C331/16—Isothiocyanates
- C07C331/28—Isothiocyanates having isothiocyanate groups bound to carbon atoms of six-membered aromatic rings
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S436/00—Chemistry: analytical and immunological testing
- Y10S436/815—Test for named compound or class of compounds
- Y10S436/816—Alkaloids, amphetamines, and barbiturates
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Medicinal Chemistry (AREA)
- Analytical Chemistry (AREA)
- Cell Biology (AREA)
- Pharmacology & Pharmacy (AREA)
- Food Science & Technology (AREA)
- Biotechnology (AREA)
- Physics & Mathematics (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/730,095 US6472228B2 (en) | 2000-12-04 | 2000-12-04 | Composition and methods for synthesis of novel tracers for detecting amphetamine and methamphetamine in samples |
| PCT/US2001/045024 WO2002057739A2 (en) | 2000-12-04 | 2001-11-29 | Composition and methods for synthesis of novel tracers for detecting amphetamine and methamphetamine in samples |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004525359A true JP2004525359A (ja) | 2004-08-19 |
| JP2004525359A5 JP2004525359A5 (enExample) | 2005-06-30 |
Family
ID=24933880
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002557773A Withdrawn JP2004525359A (ja) | 2000-12-04 | 2001-11-29 | 試料中のアンフェタミンおよびメタンフェタミンの新規トレーサーの組成物および合成方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US6472228B2 (enExample) |
| EP (1) | EP1340081A4 (enExample) |
| JP (1) | JP2004525359A (enExample) |
| CA (1) | CA2433195A1 (enExample) |
| WO (1) | WO2002057739A2 (enExample) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7314569B2 (en) * | 2003-04-04 | 2008-01-01 | Arrowhead Center, Inc. | Treatment of arsenic-contaminated water using akaganeite adsorption |
| NZ544162A (en) * | 2003-05-29 | 2008-09-26 | Shire Llc | Abuse resistant amphetamine compounds |
| WO2005027714A2 (en) * | 2003-07-12 | 2005-03-31 | Accelr8 Technology Corporation | Sensitive and rapid biodetection |
| US20120077206A1 (en) | 2003-07-12 | 2012-03-29 | Accelr8 Technology Corporation | Rapid Microbial Detection and Antimicrobial Susceptibility Testing |
| US7341841B2 (en) * | 2003-07-12 | 2008-03-11 | Accelr8 Technology Corporation | Rapid microbial detection and antimicrobial susceptibility testing |
| US20050136405A1 (en) * | 2003-12-17 | 2005-06-23 | James Linder | Novel method for the detection of cancer biomarkers in cervical specimens |
| US7037669B2 (en) * | 2004-03-22 | 2006-05-02 | Dade Behring Inc. | Assays for amphetamine and methamphetamine using stereospecific reagents |
| GB2404022B (en) | 2004-06-14 | 2005-08-10 | Cozart Bioscience Ltd | Competitive assays for the detection of methamphetamine group drugs |
| GB2404023B (en) | 2004-07-02 | 2005-07-06 | Cozart Bioscience Ltd | Delta-9-tetrahydrocannabinol detection method |
| JP2008533167A (ja) * | 2005-03-15 | 2008-08-21 | アプレラ コーポレイション | 分析物の検出のための、抗体の代替抗原系の使用 |
| US10254204B2 (en) | 2011-03-07 | 2019-04-09 | Accelerate Diagnostics, Inc. | Membrane-assisted purification |
| US9434937B2 (en) | 2011-03-07 | 2016-09-06 | Accelerate Diagnostics, Inc. | Rapid cell purification systems |
| US9677109B2 (en) | 2013-03-15 | 2017-06-13 | Accelerate Diagnostics, Inc. | Rapid determination of microbial growth and antimicrobial susceptibility |
| US10253355B2 (en) | 2015-03-30 | 2019-04-09 | Accelerate Diagnostics, Inc. | Instrument and system for rapid microorganism identification and antimicrobial agent susceptibility testing |
| KR20170132856A (ko) | 2015-03-30 | 2017-12-04 | 액셀러레이트 다이어그노스틱스, 아이엔씨. | 신속한 미생물 동정 및 항균제 감수성 시험을 위한 기기 및 시스템 |
| CN114890958B (zh) * | 2022-02-23 | 2023-10-20 | 四川警察学院 | 双光子染料化合物、其制备方法及其应用 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4868132A (en) | 1987-02-03 | 1989-09-19 | Abbott Laboratories | Fluorescence polarization immunoassay for amphetamine/methamphetamine |
| DE68924243T2 (de) | 1988-10-28 | 1996-03-28 | Abbott Lab | Verfahren und Reagenzien zum Nachweis von Amphetamin und/oder d-Methamphetamin in biologischen Proben. |
| US5135863A (en) | 1988-12-23 | 1992-08-04 | Syntex (U.S.A.) Inc. | Compositions and methods for determining the presence of amphetamines in a sample suspected of containing amphetamine and/or methamphetamine |
| EP0386644B1 (en) | 1989-03-10 | 1997-06-04 | F. HOFFMANN-LA ROCHE & CO. Aktiengesellschaft | Reagents for the determination of drugs |
| US5101015A (en) * | 1989-04-10 | 1992-03-31 | Abbott Laboratories | Reagents for an amphetamine-class fluorescence polarization immunoassay |
| US5183740A (en) | 1990-02-23 | 1993-02-02 | The United States Of America As Represented By The Secretary Of The Navy | Flow immunosensor method and apparatus |
| CA2096495C (en) * | 1992-06-16 | 2002-07-09 | Kathy Palmer Ordonez | Dual analyte immunoassay |
| US6020209A (en) | 1997-04-28 | 2000-02-01 | The United States Of America As Represented By The Secretary Of The Navy | Microcapillary-based flow-through immunosensor and displacement immunoassay using the same |
-
2000
- 2000-12-04 US US09/730,095 patent/US6472228B2/en not_active Expired - Fee Related
-
2001
- 2001-11-29 JP JP2002557773A patent/JP2004525359A/ja not_active Withdrawn
- 2001-11-29 WO PCT/US2001/045024 patent/WO2002057739A2/en not_active Ceased
- 2001-11-29 CA CA002433195A patent/CA2433195A1/en not_active Abandoned
- 2001-11-29 EP EP01993187A patent/EP1340081A4/en not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| EP1340081A2 (en) | 2003-09-03 |
| CA2433195A1 (en) | 2002-07-25 |
| US6472228B2 (en) | 2002-10-29 |
| WO2002057739A2 (en) | 2002-07-25 |
| WO2002057739A3 (en) | 2003-01-23 |
| EP1340081A4 (en) | 2005-08-03 |
| US20020090661A1 (en) | 2002-07-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6472228B2 (en) | Composition and methods for synthesis of novel tracers for detecting amphetamine and methamphetamine in samples | |
| US4476229A (en) | Substituted carboxyfluoresceins | |
| US4510251A (en) | Fluorescent polarization assay for ligands using aminomethylfluorescein derivatives as tracers | |
| JPH0123061B2 (enExample) | ||
| EP2950104B1 (en) | Immunoassay for compounds of the nbome family | |
| JPS6214064A (ja) | 置換カルボキシフルオレセイン | |
| JPS6176464A (ja) | 化学ルミネッセンス化合物およびこれを用いる発光計量免疫検定法 | |
| EP0371253B1 (en) | Method and reagents for detecting amphetamine and/or d-methamphetamine in biological samples | |
| US9435817B2 (en) | Detection of synthetic cannabinoids | |
| US6686209B2 (en) | Reagents for detecting cannabinoids | |
| JP3899260B2 (ja) | 三環系抗うつ薬誘導体およびイムノアッセイ | |
| EP3067700B1 (en) | Immunoassay for pregabalin | |
| JP2758965B2 (ja) | テトリルータンパク質コンジュゲート | |
| AU2002245045A1 (en) | Composition and methods for synthesis of novel tracers for detecting amphetamine and methamphetamine in samples | |
| US10775394B2 (en) | Immunoassay for phenethylamines of the 2C and DO sub-families | |
| EP2698383A1 (en) | Detection of synthetic Cannabinoids | |
| JP3431825B2 (ja) | 抗pahモノクローナル抗体およびそれを産生する細胞株 | |
| AU2002254093A1 (en) | Novel Reagents for Detecting Cannabinoids | |
| Hirose et al. | Syntheses of antigens conjugated with 3-methoxy-4-hydroxyphenylglycol by Mannich reaction for enzyme immunoassay | |
| JP2003177131A (ja) | 生物学的な結合親和性を検出する方法 | |
| Class et al. | Patent application title: IMMUNOASSAY FOR COMPOUNDS OF THE NBOMe FAMILY Inventors: Ivan Robert Mcconnell (Ardmore Crumlin, GB) Elouard Benchikh (Crumlin, GB) Elouard Benchikh (Crumlin, GB) Peter Fitzgerald (Crumlin, GB) Peter Fitzgerald (Crumlin, GB) Andrew Philip Lowry (Ardmore Crumlin, GB) | |
| JPH07234222A (ja) | 1,5−アンヒドログルシトールの定量方法及び抗体 | |
| Mukkala | Labeling of Steroids on Solid Phase | |
| JPH04232856A (ja) | リガンドのイムノアッセイ | |
| CA2162375A1 (en) | Hapten, tracers, immunogens and antibodies for 3-phenyl-1-adamantaneacetic acids |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20041118 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20060523 |