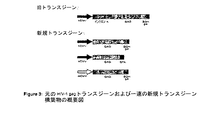
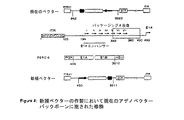
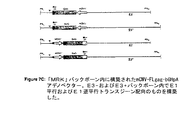
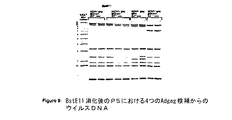
JP2004508064A - コドン最適化hiv1−gag、pol、nefおよび修飾体を発現する増強された第1世代アデノウイルスワクチン - Google Patents
コドン最適化hiv1−gag、pol、nefおよび修飾体を発現する増強された第1世代アデノウイルスワクチン Download PDFInfo
- Publication number
- JP2004508064A JP2004508064A JP2002526335A JP2002526335A JP2004508064A JP 2004508064 A JP2004508064 A JP 2004508064A JP 2002526335 A JP2002526335 A JP 2002526335A JP 2002526335 A JP2002526335 A JP 2002526335A JP 2004508064 A JP2004508064 A JP 2004508064A
- Authority
- JP
- Japan
- Prior art keywords
- adenovirus
- vector
- hiv
- nef
- seq
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/525—Virus
- A61K2039/5256—Virus expressing foreign proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/53—DNA (RNA) vaccination
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/57—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/10011—Adenoviridae
- C12N2710/10311—Mastadenovirus, e.g. human or simian adenoviruses
- C12N2710/10341—Use of virus, viral particle or viral elements as a vector
- C12N2710/10343—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/10011—Adenoviridae
- C12N2710/10311—Mastadenovirus, e.g. human or simian adenoviruses
- C12N2710/10351—Methods of production or purification of viral material
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16111—Human Immunodeficiency Virus, HIV concerning HIV env
- C12N2740/16134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16311—Human Immunodeficiency Virus, HIV concerning HIV regulatory proteins
- C12N2740/16334—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2830/00—Vector systems having a special element relevant for transcription
- C12N2830/42—Vector systems having a special element relevant for transcription being an intron or intervening sequence for splicing and/or stability of RNA
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Virology (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- General Chemical & Material Sciences (AREA)
- Microbiology (AREA)
- Animal Behavior & Ethology (AREA)
- Biophysics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Veterinary Medicine (AREA)
- Communicable Diseases (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Oncology (AREA)
- Tropical Medicine & Parasitology (AREA)
- AIDS & HIV (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US23318000P | 2000-09-15 | 2000-09-15 | |
| PCT/US2001/028861 WO2002022080A2 (en) | 2000-09-15 | 2001-09-14 | Enhanced first generation adenovirus vaccines expressing codon optimized hiv1-gag, pol, nef and modifications |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004508064A true JP2004508064A (ja) | 2004-03-18 |
| JP2004508064A5 JP2004508064A5 (enExample) | 2005-08-04 |
Family
ID=22876220
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002526335A Withdrawn JP2004508064A (ja) | 2000-09-15 | 2001-09-14 | コドン最適化hiv1−gag、pol、nefおよび修飾体を発現する増強された第1世代アデノウイルスワクチン |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP1320621A4 (enExample) |
| JP (1) | JP2004508064A (enExample) |
| AU (2) | AU9456201A (enExample) |
| CA (1) | CA2422882A1 (enExample) |
| WO (1) | WO2002022080A2 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007532656A (ja) * | 2004-04-12 | 2007-11-15 | アメリカ合衆国 | アデノウイルスベクターを用いて免疫応答を誘導するための方法 |
| JP2008539746A (ja) * | 2005-05-12 | 2008-11-20 | グラクソ グループ リミテッド | ワクチン組成物 |
| JP2018035177A (ja) * | 2008-11-18 | 2018-03-08 | ベス イスラエル デアコネス メディカル センター インコーポレイテッド | 細胞性免疫原性が向上した抗ウイルスワクチン |
Families Citing this family (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003516741A (ja) * | 1999-12-17 | 2003-05-20 | メルク エンド カムパニー インコーポレーテッド | コドン最適化HIV−1Nef及び修飾HIV−1Nefを発現するポリヌクレオチドワクチン |
| AU2002337840B2 (en) | 2001-10-11 | 2007-08-09 | Msd Italia S.R.L. | Hepatitis C virus vaccine |
| EP1444350B1 (en) | 2001-10-31 | 2013-06-19 | The South African Medical Research Council | Hiv-1 subtype isolate regulatory/accessory genes, and modifications and derivatives thereof |
| AU2003220237A1 (en) * | 2002-03-13 | 2003-09-29 | Merck & Co., Inc. | Method of inducing an enhanced immune response against hiv |
| EP1492891B1 (en) | 2002-03-29 | 2008-02-20 | Merck & Co., Inc. | Methods of virus production |
| ATE494362T1 (de) | 2002-05-14 | 2011-01-15 | Merck Sharp & Dohme | Verfahren zur reinigung von adenovirus |
| CN1490056A (zh) * | 2002-10-18 | 2004-04-21 | ��¡���ɵ°��̲��о����� | 针对hiv-1的免疫方法和组合物 |
| US20080153083A1 (en) | 2003-10-23 | 2008-06-26 | Crucell Holland B.V. | Settings for recombinant adenoviral-based vaccines |
| AU2003288273A1 (en) | 2002-10-23 | 2004-05-13 | Crucell Holland B.V. | New settings for recombinant adenoviral-based vaccines |
| CA2507915C (en) | 2002-12-17 | 2013-07-02 | Crucell Holland B.V. | Recombinant viral-based malaria vaccines |
| US7282365B2 (en) | 2003-01-03 | 2007-10-16 | Istituto Di Ricerche Di Biologia Molecolare P. Angeletti S.P.A. | Rhesus HER2/neu, nucleotides encoding same, and uses thereof |
| CN101173282B (zh) | 2003-07-21 | 2011-04-06 | P.安杰莱蒂分子生物学研究所 | 编码人表皮生长因子2/neu抗原的合成基因及其用途 |
| GB2406336A (en) * | 2003-09-24 | 2005-03-30 | Oxxon Pharmaccines Ltd | HIV Pharmaccines |
| EP1716173B8 (en) | 2004-02-11 | 2012-06-27 | Istituto di Ricerche di Biologia Molecolare P. Angeletti S.R.L. | Carcinoembryonic antigen fusion protein and uses thereof |
| CA2555412C (en) | 2004-02-23 | 2013-06-25 | Crucell Holland B.V. | Virus purification methods |
| GB0417494D0 (en) | 2004-08-05 | 2004-09-08 | Glaxosmithkline Biolog Sa | Vaccine |
| KR101253363B1 (ko) | 2004-10-13 | 2013-04-15 | 베쓰 이스라엘 디코니스 메디칼 센터 인크 | 개선된 아데노바이러스 벡터 및 그것의 용도 |
| EP1869171B2 (en) | 2005-04-11 | 2015-10-14 | Crucell Holland B.V. | Virus purification using ultrafiltration |
| AU2012201827B2 (en) * | 2005-05-12 | 2014-09-04 | Glaxo Group Limited | Vaccine composition |
| AU2006301582B2 (en) | 2005-10-07 | 2011-10-13 | Msd Italia S.R.L. | Matrix metalloproteinase 11 vaccine |
| US9717788B2 (en) | 2007-03-02 | 2017-08-01 | Glaxosmithkline Biologicals Sa | Method of inducing an immune response against HIV employing HIV immunogens, adenoviral vectors encoding said immunogens, and adjuvant |
| ES2609418T3 (es) * | 2007-03-02 | 2017-04-20 | Glaxosmithkline Biologicals Sa | Procedimiento novedoso y composiciones |
| EP2047861B1 (en) * | 2007-10-12 | 2019-07-31 | Institut Pasteur | Lentiviral gene transfer vectors suitable for iterative administration and their medicinal applications |
| ES2708856T3 (es) | 2007-08-03 | 2019-04-11 | Pasteur Institut | Vectores de transferencia de gen lentivírico y sus aplicaciones medicinales |
| US8071561B2 (en) | 2007-08-16 | 2011-12-06 | Chrontech Pharma Ab | Immunogen platform |
| AP2013007166A0 (en) | 2011-04-06 | 2013-10-31 | Univ Paris Descartes Inst De Rech Pour Le Dev Ird | Pharmaceutical compositions for preventing and/or treating an HIV disease in humans |
| US8932607B2 (en) | 2012-03-12 | 2015-01-13 | Crucell Holland B.V. | Batches of recombinant adenovirus with altered terminal ends |
| DK2825640T3 (en) | 2012-03-12 | 2016-08-01 | Crucell Holland Bv | BATCHES OF RECOMBINANT ADENOVIRUS WITH CHANGED TERMINAL END |
| US10106781B2 (en) | 2012-11-16 | 2018-10-23 | Beth Israel Deaconess Medical Center, Inc. | Recombinant adenoviruses and use thereof |
| SI3271729T1 (sl) | 2015-03-18 | 2021-04-30 | Janssen Vaccines & Prevention B.V. | Testi za rekombinantne ekspresijske sisteme |
| WO2016166088A1 (en) | 2015-04-14 | 2016-10-20 | Janssen Vaccines & Prevention B.V. | Recombinant adenovirus expressing two transgenes with a bidirectional promoter |
| MX393584B (es) | 2016-06-20 | 2025-03-21 | Janssen Vaccines & Prevention Bv | Promotor bidireccional potente y equilibrado. |
| WO2019086466A1 (en) | 2017-10-31 | 2019-05-09 | Janssen Vaccines & Prevention B.V. | Adenovirus and uses thereof |
| SG11202003398SA (en) | 2017-10-31 | 2020-05-28 | Janssen Vaccines & Prevention Bv | Adenovirus vectors and uses thereof |
| AU2018359492B2 (en) | 2017-10-31 | 2023-12-14 | Janssen Vaccines & Prevention B.V. | Adenovirus and uses thereof |
| SG11202003290RA (en) | 2017-10-31 | 2020-05-28 | Janssen Vaccines & Prevention Bv | Adenovirus and uses thereof |
| WO2019118480A1 (en) | 2017-12-11 | 2019-06-20 | Beth Israel Deaconess Medical Center, Inc. | Recombinant adenoviruses and uses thereof |
| CN111057716B (zh) * | 2019-07-27 | 2022-04-15 | 中国人民解放军军事科学院军事医学研究院 | 用于包装重组人4型腺病毒的单质粒载体系统及其应用 |
| MX2022004028A (es) | 2019-10-03 | 2022-05-02 | Janssen Vaccines & Prevention Bv | Vectores de adenovirus y usos de los mismos. |
| CN112522276B (zh) * | 2020-12-15 | 2022-07-15 | 武汉纽福斯生物科技有限公司 | 一种emc1核苷酸序列及其应用 |
| WO2025242657A1 (en) | 2024-05-21 | 2025-11-27 | Shape Biopharmaceuticals Ag | Lipopeptide building blocks and aggregates |
| CN120464596A (zh) * | 2024-10-14 | 2025-08-12 | 南京诺唯赞生物科技股份有限公司 | Rna聚合酶变体及其应用 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5643579A (en) * | 1984-11-01 | 1997-07-01 | American Home Products Corporation | Oral vaccines |
| FR2705686B1 (fr) * | 1993-05-28 | 1995-08-18 | Transgene Sa | Nouveaux adénovirus défectifs et lignées de complémentation correspondantes. |
| AU3551195A (en) * | 1994-09-23 | 1996-04-09 | Somatix Therapy Corporation | Chimeric adenovirus for gene delivery |
| US5872005A (en) * | 1994-11-03 | 1999-02-16 | Cell Genesys Inc. | Packaging cell lines for adeno-associated viral vectors |
| WO1996039178A1 (en) * | 1995-06-05 | 1996-12-12 | The Wistar Institute Of Anatomy And Biology | A replication-defective adenovirus human type 5 recombinant as a vaccine carrier |
| AU6261696A (en) * | 1995-06-05 | 1996-12-24 | Trustees Of The University Of Pennsylvania, The | A replication-defective adenovirus human type 5 recombinant as a vaccine carrier |
| JP2001509375A (ja) * | 1997-07-10 | 2001-07-24 | ヘパヴェック アーゲー フュール ゲンテルアピエ | 最小アデノウイルスベクターを作製するためのクローニングベクター |
| GB9803351D0 (en) * | 1998-02-17 | 1998-04-15 | Oxford Biomedica Ltd | Anti-viral vectors |
| US6670188B1 (en) * | 1998-04-24 | 2003-12-30 | Crucell Holland B.V. | Packaging systems for human recombinant adenovirus to be used in gene therapy |
| WO2000015819A1 (en) * | 1998-09-11 | 2000-03-23 | The Children's Medical Center Corporation | Packaging cell lines for hiv-derived retroviral vector particles |
| CA2360347C (en) * | 1998-12-31 | 2013-05-07 | Chiron Corporation | Improved expression of hiv polypeptides and production of virus-like particles |
| GB9906177D0 (en) * | 1999-03-17 | 1999-05-12 | Oxford Biomedica Ltd | Anti-viral vectors |
| GB9912965D0 (en) * | 1999-06-03 | 1999-08-04 | Oxford Biomedica Ltd | In vivo selection method |
| WO2001045748A1 (en) * | 1999-12-22 | 2001-06-28 | Merck & Co., Inc. | Polynucleotide vaccines expressing codon optimized hiv-1 pol and modified hiv-1 pol |
| ATE318908T1 (de) * | 2000-05-18 | 2006-03-15 | Geneart Gmbh | Synthetische gene für gagpol und deren verwendungen |
| CA2419822C (en) * | 2000-08-14 | 2011-02-08 | Gary J. Nabel | Modifications of hiv env, gag, and pol enhance immunogenicity for genetic immunization |
-
2001
- 2001-09-14 AU AU9456201A patent/AU9456201A/xx active Pending
- 2001-09-14 AU AU2001294562A patent/AU2001294562B2/en not_active Ceased
- 2001-09-14 CA CA002422882A patent/CA2422882A1/en not_active Abandoned
- 2001-09-14 JP JP2002526335A patent/JP2004508064A/ja not_active Withdrawn
- 2001-09-14 WO PCT/US2001/028861 patent/WO2002022080A2/en not_active Ceased
- 2001-09-14 EP EP01975214A patent/EP1320621A4/en not_active Withdrawn
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007532656A (ja) * | 2004-04-12 | 2007-11-15 | アメリカ合衆国 | アデノウイルスベクターを用いて免疫応答を誘導するための方法 |
| JP2008539746A (ja) * | 2005-05-12 | 2008-11-20 | グラクソ グループ リミテッド | ワクチン組成物 |
| JP2013046613A (ja) * | 2005-05-12 | 2013-03-07 | Glaxo Group Ltd | ワクチン組成物 |
| JP2018035177A (ja) * | 2008-11-18 | 2018-03-08 | ベス イスラエル デアコネス メディカル センター インコーポレイテッド | 細胞性免疫原性が向上した抗ウイルスワクチン |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2002022080A9 (en) | 2003-03-06 |
| CA2422882A1 (en) | 2002-03-21 |
| WO2002022080A3 (en) | 2002-05-02 |
| AU2001294562B2 (en) | 2007-05-24 |
| WO2002022080A2 (en) | 2002-03-21 |
| AU9456201A (en) | 2002-03-26 |
| EP1320621A4 (en) | 2005-11-23 |
| EP1320621A2 (en) | 2003-06-25 |
| WO2002022080A8 (en) | 2003-01-16 |
| AU2001294562B8 (en) | 2002-03-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2004508064A (ja) | コドン最適化hiv1−gag、pol、nefおよび修飾体を発現する増強された第1世代アデノウイルスワクチン | |
| US20050070017A1 (en) | Enhanced first generation adenovirus vaccines expressing codon optimized HIV1-Gag, Pol, Nef and modifications | |
| JP2003530307A (ja) | gag遺伝子保有アデノウイルスHIVワクチン | |
| AU2001294562A1 (en) | Enhanced First Generation Adenovirus Vaccines Expressing Codon Optimized HIV1-Gag, Pol, Nef and Modifications | |
| CA2597404A1 (en) | Adenovirus serotype 26 vectors, nucleic acid and viruses produced thereby | |
| AU2003262790A1 (en) | Adenovirus serotype 24 vectors, nucleic acids and virus produced thereby | |
| AU2003298554A1 (en) | Adenovirus serotype 34 vectors, nucleic acids and virus produced thereby | |
| US20080063656A1 (en) | Adenoviral Vector Compositions | |
| US20070054395A1 (en) | Enhanced first generation adenovirus vaccines expressing codon optimized HIV1-Gag, Pol, Nef and modifications | |
| CN1972958B (zh) | 应用腺病毒载体诱导免疫应答的方法 | |
| US20060165664A1 (en) | Method of inducing an enhanced immune response against hiv | |
| US20050106123A1 (en) | Method of inducing an enhanced immune response against hiv | |
| US20070077257A1 (en) | Enhanced first generation adenovirus vaccines expressing condon optimized HIV1-Gag, Pol, Nef and modifications |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A300 | Application deemed to be withdrawn because no request for examination was validly filed |
Free format text: JAPANESE INTERMEDIATE CODE: A300 Effective date: 20081202 |