EP3676410B1 - High-strength, highly formable aluminum alloys and methods of making the same - Google Patents
High-strength, highly formable aluminum alloys and methods of making the same Download PDFInfo
- Publication number
- EP3676410B1 EP3676410B1 EP18797512.3A EP18797512A EP3676410B1 EP 3676410 B1 EP3676410 B1 EP 3676410B1 EP 18797512 A EP18797512 A EP 18797512A EP 3676410 B1 EP3676410 B1 EP 3676410B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- aluminum alloy
- alloy
- alloy product
- alloys
- stage homogenization
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 229910000838 Al alloy Inorganic materials 0.000 title claims description 122
- 238000000034 method Methods 0.000 title claims description 32
- 238000000265 homogenisation Methods 0.000 claims description 41
- 229910052804 chromium Inorganic materials 0.000 claims description 32
- 239000000470 constituent Substances 0.000 claims description 30
- 229910052748 manganese Inorganic materials 0.000 claims description 30
- 229910052726 zirconium Inorganic materials 0.000 claims description 24
- 229910052710 silicon Inorganic materials 0.000 claims description 23
- 229910052802 copper Inorganic materials 0.000 claims description 19
- 229910052749 magnesium Inorganic materials 0.000 claims description 19
- 229910052720 vanadium Inorganic materials 0.000 claims description 19
- 238000005266 casting Methods 0.000 claims description 15
- 229910052742 iron Inorganic materials 0.000 claims description 15
- 238000010438 heat treatment Methods 0.000 claims description 14
- 239000002245 particle Substances 0.000 claims description 14
- 239000012535 impurity Substances 0.000 claims description 13
- 229910052719 titanium Inorganic materials 0.000 claims description 13
- 230000032683 aging Effects 0.000 claims description 12
- 230000008569 process Effects 0.000 claims description 10
- 238000005097 cold rolling Methods 0.000 claims description 4
- 238000005098 hot rolling Methods 0.000 claims description 4
- 229910045601 alloy Inorganic materials 0.000 description 148
- 239000000956 alloy Substances 0.000 description 148
- 239000011651 chromium Substances 0.000 description 44
- 239000011572 manganese Substances 0.000 description 42
- 239000000047 product Substances 0.000 description 28
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 26
- 239000010949 copper Substances 0.000 description 23
- 239000011777 magnesium Substances 0.000 description 23
- 239000000203 mixture Substances 0.000 description 22
- 239000010936 titanium Substances 0.000 description 15
- 238000005275 alloying Methods 0.000 description 12
- 229910052782 aluminium Inorganic materials 0.000 description 12
- 238000009826 distribution Methods 0.000 description 12
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 11
- 230000007704 transition Effects 0.000 description 10
- 239000002244 precipitate Substances 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 7
- 238000005452 bending Methods 0.000 description 6
- 238000001953 recrystallisation Methods 0.000 description 6
- 229910052723 transition metal Inorganic materials 0.000 description 6
- 150000003624 transition metals Chemical class 0.000 description 6
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 230000006911 nucleation Effects 0.000 description 5
- 238000010899 nucleation Methods 0.000 description 5
- 239000010703 silicon Substances 0.000 description 5
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 4
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 4
- 229910019752 Mg2Si Inorganic materials 0.000 description 4
- 238000009749 continuous casting Methods 0.000 description 4
- 238000001000 micrograph Methods 0.000 description 4
- 238000003672 processing method Methods 0.000 description 4
- 238000005096 rolling process Methods 0.000 description 4
- 238000001878 scanning electron micrograph Methods 0.000 description 4
- 230000000694 effects Effects 0.000 description 3
- 238000001556 precipitation Methods 0.000 description 3
- 239000011856 silicon-based particle Substances 0.000 description 3
- 229910001369 Brass Inorganic materials 0.000 description 2
- 229910015136 FeMn Inorganic materials 0.000 description 2
- 241001085205 Prenanthella exigua Species 0.000 description 2
- 238000003917 TEM image Methods 0.000 description 2
- 239000010951 brass Substances 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 238000002149 energy-dispersive X-ray emission spectroscopy Methods 0.000 description 2
- 230000005496 eutectics Effects 0.000 description 2
- 229910052735 hafnium Inorganic materials 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 230000002787 reinforcement Effects 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 238000009864 tensile test Methods 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 229910052718 tin Inorganic materials 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 229910019580 Cr Zr Inorganic materials 0.000 description 1
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 238000003483 aging Methods 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 238000007743 anodising Methods 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000000399 optical microscopy Methods 0.000 description 1
- VSZWPYCFIRKVQL-UHFFFAOYSA-N selanylidenegallium;selenium Chemical compound [Se].[Se]=[Ga].[Se]=[Ga] VSZWPYCFIRKVQL-UHFFFAOYSA-N 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 239000003351 stiffener Substances 0.000 description 1
- 229910052712 strontium Inorganic materials 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/04—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon
- C22F1/043—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon of alloys with silicon as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C21/00—Alloys based on aluminium
- C22C21/02—Alloys based on aluminium with silicon as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C21/00—Alloys based on aluminium
- C22C21/02—Alloys based on aluminium with silicon as the next major constituent
- C22C21/04—Modified aluminium-silicon alloys
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C21/00—Alloys based on aluminium
- C22C21/06—Alloys based on aluminium with magnesium as the next major constituent
- C22C21/08—Alloys based on aluminium with magnesium as the next major constituent with silicon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/04—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon
- C22F1/05—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon of alloys of the Al-Si-Mg type, i.e. containing silicon and magnesium in approximately equal proportions
Definitions
- the present disclosure relates to the fields of material science, materials chemistry, metal manufacturing, aluminum alloys, and aluminum manufacturing.
- the present disclosure relates to high-strength and highly formable aluminum alloys and methods of making and processing the same.
- Aluminum alloys can exhibit high strength due, in part, to the elemental content of the alloys.
- high strength 6xxx series aluminum alloys can be prepared by including high concentrations of certain elements, such as magnesium (Mg), silicon (Si), and/or copper (Cu).
- Mg magnesium
- Si silicon
- Cu copper
- such aluminum alloys containing high concentrations of these elements display poor formability properties.
- precipitates can form along grain boundaries in an aluminum matrix. Precipitate formation along grain boundaries can increase strength in the alloy but negatively affect alloy deformation (e.g., reduce bendability, formability, or any suitable desired deformation).
- the alloys can exhibit reduced yield strength after artificial aging.
- JP H09 202933 A discloses alloys of this type.
- aluminum alloys comprising 0.8 - 1.5 wt. % Si, 0.1 - 0.5 wt. % Fe, 0.5 - 1.0 wt. % Cu, 0.5 - 0.9 wt. % Mg, up to 0.1 wt. % Ti, up to 0.5 wt. % Mn, up to 0.5 wt. % Cr, up to 0.5 wt. % Zr, 0.06 - 0.5 wt. % V, up to 0.15 wt. % impurities, and Al.
- the aluminum alloys can comprise 0.9 - 1.4 wt. % Si, 0.1 - 0.35 wt.
- the aluminum alloys can comprise 1.0 - 1.3 wt. % Si, 0.1 - 0.25 wt. % Fe, 0.7 - 0.9 wt. % Cu, 0.6 - 0.8 wt.
- the aluminum alloy comprises at least one of Mn, Cr, Zr, and V.
- a combined content of Mn, Cr, Zr, and/or V is at least 0.14 wt. % (e.g., from 0.14 wt. % to 0.4 wt. % or from 0.15 wt. % to 0.25 wt. %).
- the aluminum alloy comprises 0.06 - 0.3 wt. % V.
- the aluminum alloy comprises excess Si and the excess Si content is from 0.01 to 1.0.
- the aluminum alloy products comprising the aluminum alloy as described herein.
- the aluminum alloy products comprise a rotated cube crystallographic texture at a volume percent of at least 5 %.
- the aluminum alloy products can comprise dispersoids.
- the dispersoids are present in the aluminum alloy in an amount of at least 1,500,000 dispersoids per mm 2 .
- the dispersoids occupy an area ranging from 0.5 % to 5 % of the aluminum alloy products.
- the aluminum alloy products comprises Fe-constituents.
- the Fe-constituents can comprise Al(Fe,X)Si phase particles.
- the average particle size of the Fe-constituents is up to 4 ⁇ m.
- the aluminum alloy products can exhibit a yield strength of at least 300 MPa when in a T6 temper and/or a uniform elongation of at least 20 % and a minimum bend angle of at least 120° when in a T4 temper.
- the methods comprise casting an aluminum alloy as described herein to provide a cast article, homogenizing the cast article in a two-stage homogenization process, hot rolling and cold rolling the cast article to provide a final gauge aluminum alloy product, solution heat treating the final gauge aluminum alloy product, and pre-aging the final gauge aluminum alloy product.
- the two-stage homogenization process can comprise heating the cast article to a first stage homogenization temperature and holding the cast article at the first stage homogenization temperature for a period of time and further heating the cast article to a second stage homogenization temperature and holding the cast article at the second stage homogenization temperature for a period of time.
- the first stage homogenization temperature is from 470 °C to 530 °C and the second stage homogenization temperature is from 525 °C to 575 °C.
- the second stage homogenization temperature is higher than the first stage homogenization temperature.
- Described herein are novel aluminum alloys and products and methods of preparing the same.
- the alloys exhibit high strength and high formability.
- solute elements including Cu, Mg, and Si
- transition elements e.g., Mn, Cr, Zn, and V
- the transition elements aid in preventing precipitate formation along grain boundaries in the aluminum alloys, as further described below.
- the processing methods used to prepare the alloys and products contribute to the high strength and formability exhibited by the alloys and products.
- room temperature can include a temperature of from about 15 °C to about 30 °C, for example about 15 °C, about 16 °C, about 17 °C, about 18 °C, about 19 °C, about 20 °C, about 21 °C, about 22 °C, about 23 °C, about 24 °C, about 25 °C, about 26 °C, about 27 °C, about 28 °C, about 29 °C, or about 30 °C.
- a plate generally has a thickness of greater than about 15 mm.
- a plate may refer to an aluminum product having a thickness of greater than 15 mm, greater than 20 mm, greater than 25 mm, greater than 30 mm, greater than 35 mm, greater than 40 mm, greater than 45 mm, greater than 50 mm, or greater than 100 mm.
- a shate (also referred to as a sheet plate) generally has a thickness of from about 4 mm to about 15 mm.
- a shate may have a thickness of 4 mm, 5 mm, 6 mm, 7 mm, 8 mm, 9 mm, 10 mm, 11 mm, 12 mm, 13 mm, 14 mm, or 15 mm.
- a sheet generally refers to an aluminum alloy product having a thickness of less than about 4 mm.
- a sheet may have a thickness of less than 4 mm, less than 3 mm, less than 2 mm, less than 1 mm, less than 0.5 mm, less than 0.3 mm, or less than 0.1 mm.
- cast metal article As used herein, terms such as "cast metal article,” “cast article,” “cast aluminum alloy,” and the like are interchangeable and refer to a product produced by direct chill casting (including direct chill co-casting) or semi-continuous casting, continuous casting (including, for example, by use of a twin belt caster, a twin roll caster, a block caster, or any other continuous caster), electromagnetic casting, hot top casting, or any other casting method.
- An F condition or temper refers to an aluminum alloy as fabricated.
- An O condition or temper refers to an aluminum alloy after annealing.
- a T1 condition or temper refers to an aluminum alloy cooled from hot working and naturally aged (e.g., at room temperature).
- a T2 condition or temper refers to an aluminum alloy cooled from hot working, cold worked and naturally aged.
- a T3 condition or temper refers to an aluminum alloy solution heat treated, cold worked, and naturally aged.
- a T4 condition or temper refers to an aluminum alloy solution heat treated and naturally aged.
- a T5 condition or temper refers to an aluminum alloy cooled from hot working and artificially aged (at elevated temperatures).
- a T6 condition or temper refers to an aluminum alloy solution heat treated and artificially aged.
- a T7 condition or temper refers to an aluminum alloy solution heat treated and artificially overaged.
- a T8x condition or temper refers to an aluminum alloy solution heat treated, cold worked, and artificially aged.
- a T9 condition or temper refers to an aluminum alloy solution heat treated, artificially aged, and cold worked.
- the following aluminum alloys are described in terms of their elemental composition in weight percentage (wt. %) based on the total weight of the alloy. In certain examples of each alloy, the remainder is aluminum, with a maximum wt. % of 0.15 % for the sum of the impurities.
- novel aluminum alloys exhibit high strength and high formability. In some cases, the properties of the alloys can be achieved due to the elemental composition of the alloys.
- the aluminum alloys can be precipitation hardened or precipitation hardenable alloys.
- the aluminum alloys can be aluminum alloys classified as 2xxx series aluminum alloys (e.g., wherein copper is a predominant alloying element), 6xxx series aluminum alloys (e.g., wherein magnesium and silicon are predominant alloying elements), or 7xxx series aluminum alloys (e.g., wherein zinc is a predominant alloying element). In some cases, the aluminum alloys can be modified 2xxx series, 6xxx series, or 7xxx series aluminum alloys.
- modified as related to a series of aluminum alloys refers to an alloy composition that would typically be classified within a particular series, but the modification of one or more elements (types or amounts) results in a different predominant alloying element.
- a modified 6xxx series aluminum alloy can refer to an aluminum alloy in which copper and silicon are the predominant alloying elements rather than magnesium and silicon.
- an aluminum alloy can have the following elemental composition as provided in Table 1: Table 1 Element Weight Percentage (wt. %) Si 0.8-1.5 Fe 0.1-0.5 Cu 0.5 - 1.0 Mg 0.5-0.9 Ti 0-0.1 Mn 0 - 0.5 Cr 0 - 0.5 Zr 0 - 0.5 V 0.06 - 0.5 Others 0 - 0.05 (each) 0 - 0.15 (total) Al
- the alloy can have the following elemental composition as provided in Table 2.
- Table 2 Element Weight Percentage (wt. %) Si 0.9 - 1.4 Fe 0.1 - 0.3 Cu 0.6 - 0.9 Mg 0.6 - 0.9 Ti 0.01 - 0.09 Mn 0.01 - 0.3 Cr 0.01 - 0.3 Zr 0.01 - 0.3 V 0.06 - 0.3 Others 0 - 0.05 (each) 0 - 0.15 (total) Al
- the alloy can have the following elemental composition as provided in Table 3.
- Table 3 Element Weight Percentage (wt. %) Si 1.0 - 1.3 Fe 0.1 - 0.25 Cu 0.7 - 0.9 Mg 0.6 - 0.8 Ti 0.01 - 0.05 Mn 0.05 - 0.2 Cr 0.05 - 0.2 Zr 0.05 - 0.2 V 0.06 - 0.2 Others 0 - 0.05 (each) 0 - 0.15 (total) Al
- the alloy described herein includes silicon (Si) in an amount from 0.8 % to 1.5 % (e.g., from 0.9 % to 1.45 %, from 0.9 % to 1.4 %, from 0.9 % to 1.35 %, from 0.9 % to 1.3 %, from 0.9 % to 1.25 %, from 0.9 % to 1.2 %, from 0.95 % to 1.5 %, from 0.95 % to 1.45 %, from 0.95 % to 1.4 %, from 0.95 % to 1.35 %, from 0.95 % to 1.3 %, from 0.95 % to 1.25 %, from 0.95 % to 1.2 %, from 1.0% to 1.5 %, from 1.0% to 1.45 %, from 1.0 % to 1.4 %, from 1.0% to 1.35 %, from 1.0% to 1.3 %, from 1.0% to 1.25 %, or from 1.0 % to 1.2 %) based on the total weight of the alloy.
- the alloy can include 0.8 %, 0.81 %, 0.82 %, 0.83 %, 0.84 %, 0.85 %, 0.86 %, 0.87 %, 0.88 %, 0.89 %, 0.9 %, 0.91 %, 0.92 %, 0.93 %, 0.94 %, 0.95 %, 0.96 %, 0.97 %, 0.98 %, 0.99 %, 1.0 %, 1.01 %, 1.02 %, 1.03 %, 1.04 %, 1.05 %, 1.06 %, 1.07 %, 1.08 %, 1.09 %, 1.1 %, 1.11 %, 1.12 %, 1.13 %, 1.14 %, 1.15 %, 1.16 %, 1.17 %, 1.18 %, 1.19 %, 1.2 %, 1.21 %, 1.22 %, 1.23 %, 1.24 %, 1.25 %, 1.26 %, 1.27 %, 1.28 %, 1.29
- the alloy described herein includes iron (Fe) in an amount from 0.1 % to 0.5 % (e.g., from 0.1 % to 0.45 %, from 0.1 % to 0.4 %, from 0.1 % to 0.35 %, from 0.1 % to 0.3 %, from 0.1 % to 0.25 %, from 0.1 % to 0.2 %, from 0.15 % to 0.45 %, from 0.15 % to 0.4 %, from 0.15 % to 0.35 %, from 0.15 % to 0.3 %, from 0.15 % to 0.25 %, from 0.15 % to 0.2 %, from 0.2 % to 0.45 %, from 0.2 % to 0.4 %, from 0.2 % to 0.35 %, from 0.2 % to 0.3 %, from 0.2 % to 0.25 %, from 0.25 % to 0.45 %, from 0.25 % to 0.4 %, from 0.25 % to 0.35 %, from 0.25
- the alloy can include 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2%, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5 % Fe. All expressed in wt. %.
- the alloy described herein includes copper (Cu) in an amount from 0.5 % to 1.0 % (e.g., from 0.55 % to 1.0 %, from 0.6 % to 1.0 %, from 0.65 % to 1.0 %, from 0.7 % to 1.0 %, from 0.75 % to 1.0 %, from 0.8 % to 1.0 %, from 0.5 % to 0.95 %, from 0.55 % to 0.95 %, from 0.6 % to 0.95 %, from 0.65 % to 0.95 %, from 0.7 % to 0.95 %, from 0.75 % to 0.95 %, from 0.8 % to 0.95 %, from 0.5 % to 0.9 %, from 0.55 % to 0.9 %, from 0.6 % to 0.9 %, from 0.65 % to 0.9 %, from 0.7 % to 0.9 %, from 0.75 % to 0.9 %, from 0.8 % to 0.9 %, from 0.5% to 0.
- the alloy can include 0.5 %, 0.51 %, 0.52 %, 0.53 %, 0.54 %, 0.55 %, 0.56 %, 0.57 %, 0.58 %, 0.59 %, 0.6 %, 0.61 %, 0.62 %, 0.63 %, 0.64 %, 0.65 %, 0.66 %, 0.67 %, 0.68 %, 0.69 %, 0.7 %, 0.71 %, 0.72 %, 0.73 %, 0.74 %, 0.75 %, 0.76 %, 0.77 %, 0.78 %, 0.79 %, 0.8 %, 0.81 %, 0.82 %, 0.83 %, 0.84 %, 0.85 %, 0.86 %, 0.87 %, 0.88 %, 0.89 %, 0.9 %, 0.91 %, 0.92 %, 0.93 %, 0.94 %, 0.95 %, 0.96 %, 0.97 %, 0.98 %, 0.99 %,
- the alloy described herein includes magnesium (Mg) in an amount from 0.5 % to 0.9 % (e.g., from 0.55 % to 0.9 %, from 0.6 % to 0.9 %, from 0.65 % to 0.9 %, from 0.7 % to 0.9 %, from 0.75 % to 0.9 %, from 0.8 % to 0.9 %, from 0.5% to 0.85 %, from 0.55 % to 0.85 %, from 0.6 % to 0.85 %, from 0.65 % to 0.85 %, from 0.7 % to 0.85 %, from 0.75 % to 0.85 %, from 0.8 % to 0.85 %, from 0.5 % to 0.8 %, from 0.55 % to 0.8 %, from 0.6 % to 0.8 %, from 0.65 % to 0.8 %, from 0.7 % to 0.8 %, or from 0.75 % to 0.8 %) based on the total weight of the alloy.
- Mg magnesium
- the alloy can include 0.5 %, 0.51 %, 0.52 %, 0.53 %, 0.54 %, 0.55 %, 0.56 %, 0.57 %, 0.58 %, 0.59 %, 0.6 %, 0.61 %, 0.62 %, 0.63 %, 0.64 %, 0.65 %, 0.66 %, 0.67 %, 0.68 %, 0.69 %, 0.7 %, 0.71 %, 0.72 %, 0.73 %, 0.74 %, 0.75 %, 0.76 %, 0.77 %, 0.78 %, 0.79 %, 0.8 %, 0.81 %, 0.82 %, 0.83 %, 0.84 %, 0.85 %, 0.86 %, 0.87 %, 0.88 %, 0.89 %, or 0.9 % Mg. All expressed in wt. %.
- the alloy described herein includes titanium (Ti) in an amount up to 0.1 % (e.g., from 0.01 % to 0.09 %, from 0.02 % to 0.09 %, from 0.03 % to 0.09 %, from 0.04 % to 0.09 %, from 0.05 % to 0.09 %, from 0.01 % to 0.08 %, from 0.02 % to 0.08 %, from 0.03 % to 0.08 %, from 0.04 % to 0.08 %, from 0.05 % to 0.08 %, from 0.01 % to 0.07 %, from 0.02 % to 0.07 %, from 0.03 % to 0.07 %, from 0.04 % to 0.07 %, from 0.05 % to 0.07 %, from 0.01 % to 0.06 %, from 0.02 % to 0.06 %, from 0.03 % to 0.06 %, from 0.04% to 0.06 %, from 0.05 % to 0.06 %, from 0.01 % to 0.06
- the alloy can include 0.01 %, 0.02 %, 0.03 %, 0.04 %, 0.05 %, 0.06 %, 0.07 %, 0.08 %, 0.09 %, or 0.1 % Ti.
- Ti is not present in the alloy (i.e., 0 % Ti). All expressed in wt. %.
- the alloy described herein includes manganese (Mn) in an amount up to 0.5 % (e.g., from 0.01 % to 0.5 %, from 0.01 % to 0.4 %, from 0.01 % to 0.3 %, from 0.01 % to 0.2 %, from 0.01 % to 0.1 %, from 0.06 % to 0.5 %, from 0.06 % to 0.4 %, from 0.06% to 0.3 %, from 0.06% to 0.2 %, from 0.06 % to 0.1 %, from 0.1% to 0.5 %, from 0.1 % to 0.4 %, from 0.1 % to 0.3 %, or from 0.1 % to 0.2 %) based on the total weight of the alloy.
- Mn manganese
- the alloy can include 0.01 %, 0.02 %, 0.03 %, 0.04 %, 0.05 %, 0.06 %, 0.07 %, 0.08 %, 0.09 %, 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2 %, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5
- the alloy described herein includes chromium (Cr) in an amount up to 0.5 % (e.g., from 0.01 % to 0.5 %, from 0.01 % to 0.4 %, from 0.01 % to 0.3 %, from 0.01 % to 0.2 %, from 0.01 % to 0.1 %, from 0.06 % to 0.5 %, from 0.06 % to 0.4 %, from 0.06% to 0.3 %, from 0.06% to 0.2 %, from 0.06 % to 0.1 %, from 0.1% to 0.5 %, from 0.1 % to 0.4 %, from 0.1 % to 0.3 %, or from 0.1 % to 0.2 %) based on the total weight of the alloy.
- Cr chromium
- the alloy can include 0.01 %, 0.02 %, 0.03 %, 0.04 %, 0.05 %, 0.06 %, 0.07 %, 0.08 %, 0.09 %, 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2 %, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5
- the alloy described herein includes zirconium (Zr) in an amount up to 0.5 % (e.g., from 0.01 % to 0.5 %, from 0.01 % to 0.4 %, from 0.01 % to 0.3 %, from 0.01 % to 0.2 %, from 0.01 % to 0.1 %, from 0.06 % to 0.5 %, from 0.06 % to 0.4 %, from 0.06% to 0.3 %, from 0.06% to 0.2 %, from 0.06 % to 0.1 %, from 0.1% to 0.5 %, from 0.1 % to 0.4 %, from 0.1 % to 0.3 %, or from 0.1 % to 0.2 %) based on the total weight of the alloy.
- Zr zirconium
- the alloy can include 0.01 %, 0.02 %, 0.03 %, 0.04 %, 0.05 %, 0.06 %, 0.07 %, 0.08 %, 0.09 %, 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2 %, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5
- the alloy described herein includes vanadium (V) in an amount 0.06 - 0.5 % (e.g., from 0.06 % to 0.5 %, from 0.06 % to 0.4 %, from 0.06 % to 0.3 %, from 0.06 % to 0.2 %, from 0.06 % to 0.1 %, from 0.1% to 0.5 %, from 0.1% to 0.4 %, from 0.1% to 0.3 %, or from 0.1 % to 0.2 %) based on the total weight of the alloy.
- V vanadium
- the alloy can include 0.06 %, 0.07 %, 0.08 %, 0.09 %, 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2%, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5 % V. All expressed in wt. %.
- the alloy compositions can further include other minor elements, sometimes referred to as impurities, in amounts of about 0.05 % or below, 0.04 % or below, 0.03 % or below, 0.02 % or below, or 0.01 % or below each.
- impurities may include, but are not limited to, Ni, Sc, Sn, Ga, Ca, Hf, Sr, or combinations thereof. Accordingly, Ni, Sc, Sn, Ga, Ca, Hf, or Sr may be present in an alloy in amounts of 0.05 % or below, 0.04 % or below, 0.03 % or below, 0.02 % or below, or 0.01 % or below. In certain aspects, the sum of all impurities does not exceed 0.15 % (e.g., 0.1 %). All expressed in wt. %.
- the alloy composition also includes aluminum. In certain aspects, the remaining percentage of the alloy is aluminum.
- a suitable alloy includes 1.20 % Si, 0.18 % Fe, 0.80 % Cu, 0.70 % Mg, 0.02 % Ti, 0.08 % Cr, 0.11 % V, and up to 0.15 % total impurities, with the remainder Al.
- another non-limiting example of a suitable alloy includes 1.20 % Si, 0.18 % Fe, 0.80 % Cu, 0.70 % Mg, 0.02 % Ti, 0.09 % Zr, 0.10 % V, and up to 0.15 % total impurities, with the remainder Al.
- a suitable alloy includes 1.20 % Si, 0.18 % Fe, 0.80 % Cu, 0.70 % Mg, 0.02 % Ti, 0.09 % Mn, 0.10 % V, and up to 0.15 % total impurities, with the remainder Al.
- the Si, Mg, and Cu content and ratios are controlled to enhance strength and formability.
- the transition element e.g., Mn, Cr, Zr, and/or V
- the transition element e.g., Mn, Cr, Zr, and/or V
- the alloy described herein includes excess Si.
- the Si and Mg content are controlled such that excess Si is present in the alloy as described herein.
- Excess Si content can be calculated according to the method described in U.S. Patent No. 4,614,552 , col. 4, lines 49-52. Briefly, Mg and Si combine as Mg 2 Si, imparting a considerable strength improvement after age-hardening.
- Si-containing constituents such as Al(FeMn)Si, can form. Excess Si is present when the Si content is above the stoichiometric ratio of Mg 2 Si and above the amount included in Al(FeMn)Si constituents.
- the excess Si content can be calculated by subtracting from the total Si content the Si needed for Mg 2 Si (Mg/1.73) and the Fe-containing phase (Fe/3).
- the excess Si content can be 1.0 or less (e.g., from 0.01 to 1.0, from 0.1 to 0.9, or from 0.5 to 0.8).
- the excess Si content can be 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, 0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70, 0.75, 0.80, 0.85, 0.90, 0.95, 1.0, or anywhere in between.
- the alloys described herein include at least one transition element (e.g., at least one of Mn, Cr, Zr, and/or V).
- the combined content of the transition elements in the alloys described herein is at least 0.14 wt. %.
- the combined content of Mn, Cr, Zr, and/or V can be from 0.14 wt. % to 0.40 wt. % (e.g., from 0.15 wt. % to 0.35 wt. % or from 0.25 wt. % to 0.30 wt. %).
- the combined content of Mn, Cr, Zr, and/or V is 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2%, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, or 0.4 %.
- one or more of the transition elements may not be present, as long as the total weight percentage of the present transition elements is at least 0.14 wt. %.
- the presence of one or more of the transition elements, such as Mn, Cr, Zr, and/or V, can advantageously form dispersoids during the processing methods described herein, such as during the homogenization step.
- the dispersoids can function as heterogeneous nucleation sites for precipitates during processing steps, such as during the solution heat treatment step.
- grain boundary (GB) precipitation occurs due to GB misorientation that is favorable for precipitate nucleation.
- the dispersoids reduce or eliminate GB precipitates and also reduce strain localization, thus diffusing strain distribution during deformation.
- the reduced or eliminated GB precipitates and/or the diffused strain distribution during deformation result in an improved bendability of the resulting alloys and alloy products.
- the dispersoids described herein can contain Al and one or more of the alloying elements found in the alloy composition as described above.
- the dispersoids can have a composition according to one or more of the following formulae: AlX, AlXX, AlXSi, Al(Fe,X), Al(Fe,X)Si, or the like, wherein each X is selected from the group consisting of Fe, Si, Mn, Cr, V, or Zr.
- the dispersoid average size and distribution are important factors that result in the desirable strength and formability properties displayed by the alloys and alloy products described herein.
- the size and distribution are influenced by the presence of transition elements, as described above, and also by the methods of processing the alloys, as further described below.
- the dispersoids can be present in the aluminum alloy in an average amount of at least 1,500,000 dispersoids per square millimeter (mm 2 ).
- the dispersoids can be present in an amount of at least 1,600,000 dispersoids per mm 2 , at least 1,700,000 dispersoids per mm 2 , at least 1,800,000 dispersoids per mm 2 , at least 1,900,000 dispersoids per mm 2 , at least 2,000,000 dispersoids per mm 2 , at least 2,100,000 dispersoids per mm 2 , at least 2,200,000 dispersoids per mm 2 , at least 2,300,000 dispersoids per mm 2 , at least 2,400,000 dispersoids per mm 2 , at least 2,500,000 dispersoids per mm 2 , at least 2,600,000 dispersoids per mm 2 , at least 2,700,000 dispersoids per mm 2 , at least 2,800,000 dispersoids per mm 2 , at least 2,900,000 dispersoids per mm 2 , or at least 3,000,000 dispersoids per mm 2 .
- the average number of dispersoids present in the aluminum alloy can be from 1,500,000 dispersoids per mm 2 to 5,000,000 dispersoids per mm 2 (e.g., from 1,750,000 dispersoids per mm 2 to 4,750,000 dispersoids per mm 2 or from 2,000,000 dispersoids per mm 2 to 4,500,000 dispersoids per mm 2 ).
- the dispersoids in the aluminum alloy can occupy an area ranging from 0.5 % to 5 % of the alloy (e.g., from 1 % to 4 % or from 1.5 % to 2.5 % of the alloy).
- the dispersoids can have an average diameter of from 10 nm to 600 nm (e.g., from 50 nm to 500 nm, from 100 nm to 450 nm, from 200 nm to 400 nm, from 10 nm to 200 nm, or from 500 nm to 600 nm).
- the dispersoids can have a diameter of 10 nm, 20 nm, 30 nm, 40 nm, 50 nm, 60 nm, 70 nm, 80 nm, 90 nm, 100 nm, 110 nm, 120 nm, 130 nm, 140 nm, 150 nm, 160 nm, 170 nm, 180 nm, 190 nm, 200 nm, 210 nm, 220 nm, 230 nm, 240 nm, 250 nm, 260 nm, 270 nm, 280 nm, 290 nm, 300 nm, 310 nm, 320 nm, 330 nm, 340 nm, 350 nm, 360 nm, 370 nm, 380 nm, 390 nm, 400 nm, 410 nm, 420 nm, 430 nm, 440 nm, 100 n
- the alloys described herein also include Fe-constituents, which are also referred to herein as Fe-containing particles.
- the Fe-constituents can include one or more of Al, Mn, Si, Cu, Ti, Zr, Cr, and/or Mg.
- the Fe-constituents can be Al(Fe,X)Si phase particles, wherein X can be Mn, Cr, Zr, and/or V, and/or AlFeSi phase particles.
- the Fe-constituents can include one or more of Al 3 Fe, Al x (Fe,Mn), Al 3 Fe, Al 12 (Fe,Mn) 3 Si, Al 7 Cu 2 Fe, Al(Fe,Mn) 2 Si 3 , Al x (Mn,Fe), and Al 12 (Mn,Fe) 3 Si.
- the presence of the transition elements described herein results in the transformation of AlFeSi particles into Al(Fe,X)Si particles.
- the number of Al(Fe,X)Si phase particles, which are spheroid particles is greater than the number of AlFeSi phase particles, which are flake or needle type particles.
- At least 50 % of the Fe-constituents are present as Al(Fe,X)Si particles (e.g., at least 55 %, at least 60 %, at least 65 %, at least 70 %, at least 75 %, at least 80 %, at least 85 %, at least 90 %, or at least 95 % of the Fe-constituents are present as Al(Fe,X)Si particles).
- the Fe-constituents can have an average particle size of up to 4 ⁇ m.
- the Fe-constituents, on average can range in size from 0.1 ⁇ m to 4 ⁇ m (e.g., from 0.5 ⁇ m to 3.5 ⁇ m or from 1 ⁇ m to 3 ⁇ m).
- the Cr, Mn, Zr, and/or V content and ratios are controlled to form the desired size, type, and distribution of dispersoids, which leads to improved formability and strength.
- a ratio of Cr to Mn (also referred to herein as the Cr/Mn ratio) can be from 0.15:1 to 0.7:1 (e.g., from 0.3:1 to 0.6:1 or from 0.4:1 to 0.55:1).
- the Cr/Mn ratio can be 0.15:1, 0.16:1, 0.17:1, 0.18:1, 0.19:1, 0.20:1, 0.21:1, 0.22:1, 0.23:1, 0.24:1, 0.25:1, 0.26:1, 0.27:1, 0.28:1, 0.29:1, 0.30:1, 0.31:1, 0.32:1, 0.33:1, 0.34:1, 0.35:1, 0.36:1, 0.37:1, 0.38:1, 0.39:1, 0.40:1, 0.41:1, 0.42:1, 0.43:1, 0.44:1, 0.45:1, 0.46:1, 0.47:1, 0.48:1, 0.49:1, 0.50:1, 0.51:1, 0.52:1, 0.53:1, 0.54:1, 0.55:1, 0.56:1, 0.57:1, 0.58:1, 0.59:1, 0.60:1, 0.61:1, 0.62:1, 0.63:1, 0.64:1, 0.65:1, 0.66:1, 0.67:1, 0.68:1, 0.69:1, or 0.70:1.
- a ratio of Cr to V (also referred to herein as the Cr/V ratio) can be from 0.5:1 to 1.5:1 (e.g., from 0.6:1 to 1.4:1 or from 0.7:1 to 1.3:1).
- the Cr/V ratio can be 0.50:1, 0.51:1, 0.52:1, 0.53:1, 0.54:1, 0.55:1, 0.56:1, 0.57:1, 0.58:1, 0.59:1, 0.60:1, 0.61:1, 0.62:1, 0.63:1, 0.64:1, 0.65:1, 0.66:1, 0.67:1, 0.68:1, 0.69:1, 0.70:1, 0.71:1, 0.72:1, 0.73:1, 0.74:1, 0.75:1, 0.76:1, 0.77:1, 0.78:1, 0.79:1, 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, 1.4:1, or 1.5:1.
- a ratio of Cr to Zr (also referred to herein as the Cr/Zr ratio) can be from 0.5:1 to 1.5:1 (e.g., from 0.6:1 to 1.4:1 or from 0.7:1 to 1.3:1).
- the Cr/Zr ratio can be 0.50:1, 0.51:1, 0.52:1, 0.53:1, 0.54:1, 0.55:1, 0.56:1, 0.57:1, 0.58:1, 0.59:1, 0.60:1, 0.61:1, 0.62:1, 0.63:1, 0.64:1, 0.65:1, 0.66:1, 0.67:1, 0.68:1, 0.69:1, 0.70:1, 0.71:1, 0.72:1, 0.73:1, 0.74:1, 0.75:1, 0.76:1, 0.77:1, 0.78:1, 0.79:1, 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, 1.4:1, or 1.5:1.
- a ratio of V to Mn can be from 0.8:1 to 1.4:1 (e.g., from 0.9:1 to 1.3:1 or from 0.9:1 to 1.2:1).
- the V/Mn ratio can be 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, or 1.4:1.
- a ratio of V to Zr can be from 0.8:1 to 1.4:1 (e.g., from 0.9:1 to 1.3:1 or from 0.9:1 to 1.2:1).
- the V/Zr ratio can be 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, or 1.4:1.
- a ratio of V to Cr can be from 0.8:1 to 1.4:1 (e.g., from 0.9:1 to 1.3:1 or from 0.9:1 to 1.2:1).
- the V/Cr ratio can be 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, or 1.4:1.
- the mechanical properties of the aluminum alloy can be controlled by various aging conditions depending on the desired use.
- the alloy can be produced (or provided) in a T4 temper or a T6 temper.
- the proposed alloy has very high formability and bendability in the T4 temper and very high strength in the T6 temper.
- the aluminum alloy may have a T4 yield strength ranging from 150 MPa to 250 MPa (e.g., 150 MPa, 160 MPa, 170 MPa, 180 MPa, 190 MPa, 200 MPa, 210 MPa, 220 MPa, 230 MPa, 240 MPa, or 250 MPa). In some cases, the yield strength is from 185 MPa to 195 MPa.
- the alloy in the T4 temper provides a uniform elongation of at least 20 % (e.g., from 20% to 30% or from 22 % to 26%).
- the uniform elongation can be 20 %, 21 %, 22 %, 23 %, 24 %, 25 %, 26 %, 27 %, 28 %, 29 %, or 30 %.
- the uniform elongation is measured in the longitudinal (L) direction.
- the alloy in the T4 temper provides a bend angle, as tested according to VDA 238-100, of at least 120°.
- the bend angle can be from 120° to 140° (e.g., 120°, 121°, 122°, 123°, 124°, 125°, 126°, 127°, 128°, 129°, 130°, 131°, 132°, 133°, 134°, 135°, 136°, 137°,138°, 139°, or 140°).
- including V can improve the formability of the alloys.
- alloys that include V exhibit an increase in bend angle of up to 10° (e.g., showing an improvement of at least 5°, at least 6°, at least 7°, at least 8°, at least 9°, at least 10°, or anywhere in between) as compared to alloys that do not contain V.
- the aluminum alloy may have a T6 yield strength of at least 200 MPa.
- the yield strength is at least 200 MPa, at least 210 MPa, at least 220 MPa, at least 230 MPa, at least 240 MPa, at least 250 MPa, at least 260 MPa, at least 270 MPa, at least 280 MPa, at least 290 MPa, or at least 300 MPa, at least 310 MPa, at least 320 MPa, at least 330 MPa, at least 340 MPa, at least 350 MPa, at least 360 MPa, at least 370 MPa, or at least 375 MPa.
- the yield strength is from 200 MPa to 400 MPa (e.g., 200 MPa, 210 MPa, 220 MPa, 230 MPa, 240 MPa, 250 MPa, 260 MPa, 270 MPa, 280 MPa, 290 MPa, 300 MPa, 310 MPa, 320 MPa, 330 MPa, 340 MPa, 350 MPa, 360 MPa, 370 MPa, or 375 MPa).
- 200 MPa 210 MPa, 220 MPa, 230 MPa, 240 MPa, 250 MPa, 260 MPa, 270 MPa, 280 MPa, 290 MPa, 300 MPa, 310 MPa, 320 MPa, 330 MPa, 340 MPa, 350 MPa, 360 MPa, 370 MPa, or 375 MPa.
- the alloy in the T6 temper provides a uniform elongation of at least 5 % (e.g., from 5 % to 10 % or from 6 % to 9 %).
- the uniform elongation can be 5 %, 6 %, 7 %, 8 %, 9 %, or 10 %.
- the uniform elongation is measured in the longitudinal (L) direction.
- the alloy products also include recrystallization texture components at a surface of the alloy products.
- the alloy products include one or more of the following recrystallization texture components: cube, goss, brass, S, Cu, and rotated cube (referred to as "RC").
- RC rotated cube
- at least 5 volume % of the rotated cube texture component is present in the alloy product (e.g., from 5 vol. % to 20 vol. %, from 6 vol. % to 18 vol. %, from 8 vol. % to 15 vol. %, from 10 vol. % to 13 vol. %, or from 5 vol. % to 6 vol. %).
- Such a rotated cube texture component can result in desirable bending in the alloy product.
- aluminum alloy properties are partially determined by the formation of microstructures during the alloy's preparation.
- the method of preparation for an alloy composition may influence or even determine whether the alloy will have properties adequate for a desired application.
- the alloy described herein can be cast into a cast article using any suitable casting method.
- the casting process can include a direct chill (DC) casting process.
- the casting process can include a continuous casting (CC) process.
- the cast article can then be subjected to further processing steps.
- the processing methods as described herein can include the steps of homogenizing, hot rolling, cold rolling, solutionizing, and pre-aging. In some cases, the processing methods can also include an artificial aging step.
- the homogenization step includes a two-stage heating process.
- a cast article prepared from an alloy composition described herein can be heated to a first stage homogenization temperature (e.g., the dispersoid nucleation temperature).
- the first stage homogenization temperature can be from 470 °C to 530 °C (e.g., 470 °C, 480 °C, 490 °C, 500 °C, 510 °C, 520 °C, 530 °C, or anywhere in between).
- a heating rate to the first stage homogenization temperature can be 100 °C/hour or less, 75 °C/hour or less, 50 °C/hour or less, 40 °C/hour or less, 30 °C/hour or less, 25 °C/hour or less, 20 °C/hour or less, or 15 °C/hour or less.
- the heating rate to the first stage homogenization temperature can be from 10 °C/min to 100 °C/min (e.g., from 15 °C/min to 90 °C/min, from 20 °C/min to 80 °C/min, from 30 °C/min to 80 °C/min, from 40 °C/min to 70 °C/min, or from 45 °C/min to 65 °C/min).
- the cast article is then allowed to soak (i.e., held at the indicated temperature) for a period of time.
- the cast article is allowed to soak for up to 6 hours (e.g., from 30 minutes to 6 hours, inclusively).
- the cast article can be soaked at a temperature of from 470 °C to 530 °C for 30 minutes, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, or anywhere in between.
- the temperature of the cast article is increased to a temperature higher than the temperature used for the first stage of the homogenization process.
- the cast article temperature can be increased, for example, to a temperature at least 5 °C higher than the aluminum alloy cast article temperature during the first stage of the homogenization process.
- the cast article can be further heated to a second stage homogenization temperature (e.g., a dispersoid coarsening temperature) of from 525 °C to 575 °C (e.g., from 530 °C to 570 °C or from 535 °C to 565 °C).
- the second stage homogenization temperature can be 525 °C, 530 °C, 535 °C, 540 °C, 545 °C, 550 °C, 555 °C, 560 °C, 565 °C, 570 °C, 575 °C, or anywhere in between) in a second homogenization step.
- a heating rate to the second stage homogenization temperature can be 50 °C/hour or less, 30 °C/hour or less, or 25 °C/hour or less.
- the cast article is then allowed to soak for a period of time during the second stage.
- the cast article is allowed to soak for up to 5 hours (e.g., from 20 minutes to 5 hours, inclusively).
- the cast article can be soaked at a temperature of from 525 °C to 575 °C for 15 minutes, 20 minutes, 30 minutes, 45 minutes, 1 hour, 1.5 hours, 2 hours, 3 hours, 4 hours, 5 hours, or anywhere in between.
- a hot rolling step is performed.
- the cast articles are laid down and hot-rolled with an entry temperature range of 500 °C to 560 °C (e.g., from 510 °C to 550 °C or from 520 °C to 540 °C).
- the entry temperature can be, for example, 505 °C, 510 °C, 515 °C, 520 °C, 525 °C, 530 °C, 535 °C, 540 °C, 545 °C, 550 °C, 555 °C, 560 °C, or anywhere in between.
- the hot roll exit temperature can range from 250 °C to 380 °C (e.g., from 275 °C to 370 °C or from 300 °C to 360 °C).
- the hot roll exit temperature can be 255 °C, 260 °C, 265 °C, 270 °C, 275 °C, 280 °C, 285 °C, 290 °C, 295 °C, 300 °C, 305 °C, 310 °C, 315 °C, 320 °C, 325 °C, 330 °C, 335 °C, 340 °C, 345 °C, 350 °C, 355 °C, 360 °C, 365 °C, 370 °C, 375 °C, or 380 °C.
- the cast article is hot rolled to a 4 mm to 15 mm gauge (e.g., from 5 mm to 12 mm gauge), which is referred to as a hot band.
- the cast article can be hot rolled to a 15 mm gauge, a 14 mm gauge, a 13 mm gauge, a 12 mm gauge, an 11 mm gauge, a 10 mm gauge, a 9 mm gauge, an 8 mm gauge, a 7 mm gauge, a 6 mm gauge, a 5 mm gauge, or a 4 mm gauge.
- the temper of the as-rolled hot band is referred to as F-temper.
- the hot band is cold rolled to a final gauge aluminum alloy sheet.
- the final gauge aluminum alloy sheet has a thickness of 4 mm or less, 3 mm or less, 2 mm or less, 1 mm or less, 0.9 mm or less, 0.8 mm or less, 0.7 mm or less, 0.6 mm or less, 0.5 mm or less, 0.4 mm or less, 0.3 mm or less, 0.2 mm or less, or 0.1 mm.
- the solutionizing step can include heating the aluminum alloy sheet or other rolled article from room temperature to a peak metal temperature.
- the peak metal temperature can be from 520 °C to 590 °C (e.g., from 520 °C to 580 °C, from 530 °C to 570 °C, from 545 °C to 575 °C, from 550 °C to 570 °C, from 555 °C to 565 °C, from 540 °C to 560 °C, from 560 °C to 580 °C, or from 550 °C to 575 °C).
- the aluminum alloy sheet can soak at the peak metal temperature for a period of time.
- the aluminum alloy sheet is allowed to soak for up to approximately 2 minutes (e.g., from 10 seconds to 120 seconds inclusively).
- the sheet can be soaked at the temperature of from 520 °C to 590 °C for 10 seconds, 15 seconds, 20 seconds, 25 seconds, 30 seconds, 35 seconds, 40 seconds, 45 seconds, 50 seconds, 55 seconds, 60 seconds, 65 seconds, 70 seconds, 75 seconds, 80 seconds, 85 seconds, 90 seconds, 95 seconds, 100 seconds, 105 seconds, 110 seconds, 115 seconds, 120 seconds, or anywhere in between.
- pre-aging can include heating the aluminum alloy sheet to a temperature of from 80 °C to 120 °C (e.g., 80 °C, 85 °C, 90 °C, 95 °C, 100 °C, 105 °C, 110 °C, 115 °C, 120 °C, or anywhere in between) and coiling the aluminum alloy sheet.
- the coiled aluminum alloy sheet can be cooled (i.e., coil cooling is performed) for a period of up to 24 hours (e.g., 1 hour, 2 hours, 6 hours, 12 hours, 18 hours, 24 hours, or anywhere in between).
- the aluminum alloy sheet can then be naturally aged and/or artificially aged.
- the aluminum alloy sheet can be naturally aged for a period of time to result in a T4 temper.
- the aluminum alloy sheet can be naturally aged for 1 week or more, 2 weeks or more, 3 weeks or more, or 4 weeks or more.
- the aluminum alloy sheet in the T4 temper can be artificially aged at a temperature of from 180 °C to 225 °C (e.g., 185 °C, 190 °C, 195 °C, 200 °C, 205 °C, 210 °C, 215 °C, 220 °C, or 225 °C) for a period of time to result in a T6 temper.
- the aluminum alloy sheet can be artificially aged for a period from 15 minutes to 3 hours (e.g., 15 minutes, 30 minutes, 60 minutes, 90 minutes, 105 minutes, 2 hours, 2.5 hours, 3 hours, or anywhere in between) to result in a T6 temper.
- the alloys, products, and methods described herein can be used in automotive, electronics, and transportation applications, such as commercial vehicle, aircraft, or railway applications.
- the alloys can be used for chassis, cross-member, and intra-chassis components (encompassing, but not limited to, all components between the two C channels in a commercial vehicle chassis) to gain strength, serving as a full or partial replacement of high-strength steels.
- the alloys can be used in F, T4, T6, or T8x tempers.
- the alloys are used with a stiffener to provide additional strength.
- the alloys are useful in applications where the processing and operating temperature is approximately 150 °C or lower.
- the alloys and methods can be used to prepare motor vehicle body part products.
- the disclosed alloys and methods can be used to prepare automobile body parts, such as bumpers, side beams, roof beams, cross beams, pillar reinforcements (e.g., A-pillars, B-pillars, and C-pillars), inner panels, side panels, floor panels, tunnels, structure panels, reinforcement panels, inner hoods, or trunk lid panels.
- pillar reinforcements e.g., A-pillars, B-pillars, and C-pillars
- inner panels e.g., side panels, floor panels, tunnels, structure panels, reinforcement panels, inner hoods, or trunk lid panels.
- the disclosed aluminum alloys and methods can also be used in aircraft or railway vehicle applications, to prepare, for example, external and internal panels.
- the described alloys and methods can also be used to prepare housings for electronic devices, including mobile phones and tablet computers.
- the alloy can be used to prepare housings for the outer casing of mobile phones (e.g., smart phones) and tablet bottom chassis, with or without anodizing.
- Exemplary consumer electronic products include mobile phones, audio devices, video devices, cameras, laptop computers, desktop computers, tablet computers, televisions, displays, household appliances, video playback and recording devices, and the like.
- Exemplary consumer electronic product parts include outer housings (e.g., facades) and inner pieces for the consumer electronic products.
- Alloys were prepared for strength and formability testing.
- the compositions for these alloys are provided in Table 4 below.
- the remainder is A Alloys D4, D5 and D6 are according to the present invention, alloys D1, D2 and D3 are not.
- the alloys were prepared by DC casting the components into ingots and homogenizing the ingots in a two-step homogenization step as described herein.
- the first step provided nucleation of a maximum amount of fine dispersoids (e.g., dispersoids having a diameter of less than about 10 nm).
- the second step coarsened the fine dispersoids.
- the homogenized ingots were then laid down and hot rolled according to the methods as described herein to a 10 mm gauge.
- the hot band was coiled and cooled and was then cold rolled to a 2 mm gauge.
- a solution heat treatment step was then performed at 560 °C for 35 seconds.
- a pre-aging step was performed by heating the sheet to 100 °C and soaking for 1 hour (e.g., to simulate coil cooling as described above), followed by natural aging to achieve the T4 temper.
- the T6 temper was then achieved by aging the T4 alloys at 200 °C for 30 minutes.
- the properties of the D1 - D6 alloys in T4 temper were determined.
- Tensile testing was performed according to ASTM B557 in three directions relative to a rolling direction of the alloy sheets to evaluate anisotropic properties that can occur during recrystallization.
- Yield strength (referred to as “YS” and indicated by histograms) and uniform elongation (referred to as "UE” and indicated by points) are shown in Figure 1 for a longitudinal direction along the rolling direction (referred to as "L” and indicated by vertical stripes), a transverse direction 90° to the rolling direction (referred to as "T” and indicated by horizontal stripes), and a diagonal direction 45° to the rolling direction (referred to as "D” and indicated by cross-hatching).
- the alloys exhibited isotropic behavior in all three directions subjected to tensile testing even with elongated recrystallized grain structures as observed in Figure 2 .
- the uniform elongation values ranged from 24 % to 26 % and the yield strengths were from 185 MPa to 195 MPa.
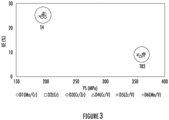
- Figure 3 shows the yield strengths and uniform elongations for alloys D1 - D6 in T4 and T6 tempers.
- the composition had a negligible effect on yield strength and uniform elongation.
- the composition had a negligible effect on uniform elongation and a decrease in yield strength of about 10 MPa for alloys including V in the composition.
- the decrease in yield strength can be attributed to solute loss (e.g., Si, Mg, and/or Cu) during solutionizing by heterogeneous nucleation of solute precipitates on V-containing dispersoids.
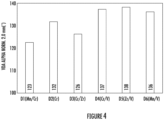
- Figure 4 is a graph showing bend angle test results for alloys D1 - D6 in a T4 temper.
- Addition of Cr and V produced a large number of fine dispersoids which improves bending by diffusing strain distribution during deformation (e.g., bending, forming, stamping, or any suitable deformation process).
- Mn combined with Fe and Si to form and spheroidize Fe- constituents, rather than forming dispersoids, due to the high diffusivity of Mn as compared to Zr, Cr, and/or V.
- Spheroidization of the Fe-constituents improved bending by eliminating elongated (i.e., needle-like) particulates that can initiate cracking during deformation.
- V-containing alloys e.g., alloys D4 - D6
- Figure 5 compares yield strength (YS) and bend angle (VDA) for alloys D1 - D6 in T4 and T6 tempers.
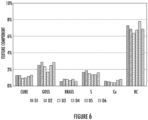
- FIG 6 shows recrystallization texture components for alloys D1 - D6, including cube, goss, brass, S, Cu, and rotated cube (referred to as "RC").
- Each alloy D1 - D6 exhibited a similar distribution of texture components, and composition had a negligible effect on recrystallization texture.
- each alloy exhibited a relatively high amount of rotated cube texture, resulting in the significantly improved bending angles shown in Figure 4 and Figure 5 .
- Figure 7 shows transmission electron microscopy (TEM) images of alloys D1 - D6 in T4 temper. Evident in the TEM images is dispersoid formation (shown as bright white particulates) in each alloy. Alloy D4 (including Cr and V) exhibited a higher dispersoid amount due to the relatively low diffusivities of Cr and V. Likewise, alloys D5 and D6 exhibited a lower number of dispersoids due to the relatively higher diffusivities of Mn and Zr. Accordingly, alloy D6 exhibited a lesser amount of dispersoids attributed to an affinity of Mn to be incorporated in Fe-constituents and to not solely form Mn dispersoids.
- TEM transmission electron microscopy
- Figure 8 shows dispersoid number density (histograms) and area fraction (open circles) for alloys D1 - D6 in T4 temper. Alloys not containing V (alloys D1 - D3) exhibited similar dispersoid number density. Alloy D2 (incorporating only Cr as a transition metal alloying element) exhibited a higher dispersoid area fraction compared to alloys D1 and D3 (incorporating Mn and Cr (D1) and Zr and Cr (D3)). Alloy D4 (incorporating Cr and V) exhibited the highest dispersoid number density and the highest dispersoid area fraction.
- FIG 9 shows scanning electron microscopy (SEM) images of alloys D1 - D6 in a T4 temper.
- SEM scanning electron microscopy
- Fe-constituent formation shown as bright white elongated particulates.
- Each of the alloys D1 - D6 exhibited similar amounts of Fe-constituent formation, and similar Fe-constituent particle size distribution as shown in Figure 10 .
- employing transition metal alloying elements reduced the formation of Fe-constituents (e.g., AlFeSi) by replacing a portion of the Fe, thus forming spherical Al(Fe,X)Si constituents.
- Fe-constituents e.g., AlFeSi
- Each alloy continued to exhibit AlFeSi (elongated particulates) due to the presence of excess Si and processing at a low homogenization temperature (e.g., about 500 °C), with a reduced size and size distribution in alloys not employing the transition metal alloying elements.
- the AlFeSi constituents in alloys not containing the transition metal alloying elements exhibited a larger size than the AlFeSi constituents observed in the alloys containing the transition metal alloying elements.
- Fe-constituent size and size distribution was evaluated at a depth of about 0.5 mm from a surface of the aluminum alloy sheet (referred to as quarter thickness, indicated "QT" in the graph).
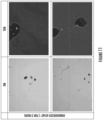
- Figure 11 shows optical microscopy (referred to as "OM") and SEM images of alloy D1.
- Alloy D1 was subjected to a one-step homogenization after casting, including a thermal ramp of 50 °C per hour to 560 °C, soaked for 2 hours, and subsequently hot rolled, cold rolled, solutionized, pre-aged, and naturally aged as described above.
- Evident in the OM images is incipient and/or eutectic melting of Mg 2 Si in alloy D1 (shown as dark areas).
- SEM images show the dark areas are voids that formed in the alloy during homogenization.
- Energy dispersive X-ray spectroscopy (EDXS) showed Fe-constituents present in the voids (shown as bright particulates).
- EDXS Energy dispersive X-ray spectroscopy
Description
- The present disclosure relates to the fields of material science, materials chemistry, metal manufacturing, aluminum alloys, and aluminum manufacturing. In particular, the present disclosure relates to high-strength and highly formable aluminum alloys and methods of making and processing the same.
- Aluminum alloys can exhibit high strength due, in part, to the elemental content of the alloys. For example, high strength 6xxx series aluminum alloys can be prepared by including high concentrations of certain elements, such as magnesium (Mg), silicon (Si), and/or copper (Cu). However, such aluminum alloys containing high concentrations of these elements display poor formability properties. In particular, precipitates can form along grain boundaries in an aluminum matrix. Precipitate formation along grain boundaries can increase strength in the alloy but negatively affect alloy deformation (e.g., reduce bendability, formability, or any suitable desired deformation). In addition, the alloys can exhibit reduced yield strength after artificial aging.
JP H09 202933 A - Covered embodiments of the invention are defined by the claims, not this summary. This summary is a high-level overview of various aspects of the invention and introduces some of the concepts that are further described in the Detailed Description section below. This summary is not intended to identify key or essential features of the claimed subject matter, nor is it intended to be used in isolation to determine the scope of the claimed subject matter. The subject matter should be understood by reference to appropriate portions of the entire specification, any or all drawings, and each claim.
- Described herein are aluminum alloys comprising 0.8 - 1.5 wt. % Si, 0.1 - 0.5 wt. % Fe, 0.5 - 1.0 wt. % Cu, 0.5 - 0.9 wt. % Mg, up to 0.1 wt. % Ti, up to 0.5 wt. % Mn, up to 0.5 wt. % Cr, up to 0.5 wt. % Zr, 0.06 - 0.5 wt. % V, up to 0.15 wt. % impurities, and Al. In some cases, the aluminum alloys can comprise 0.9 - 1.4 wt. % Si, 0.1 - 0.35 wt. % Fe, 0.6 - 0.9 wt. % Cu, 0.6 - 0.9 wt. % Mg, 0.01 - 0.09 wt. % Ti, up to 0.3 wt. % Mn, up to 0.3 wt. % Cr, up to 0.3 wt. % Zr, 0.06 - 0.3 wt. % V, up to 0.15 wt. % impurities, and Al. In some cases, the aluminum alloys can comprise 1.0 - 1.3 wt. % Si, 0.1 - 0.25 wt. % Fe, 0.7 - 0.9 wt. % Cu, 0.6 - 0.8 wt. % Mg, 0.01 - 0.05 wt. % Ti, up to 0.2 wt. % Mn, up to 0.2 wt. % Cr, up to 0.2 wt. % Zr, 0.06 - 0.2 wt. % V, up to 0.15 wt. % impurities, and Al. The aluminum alloy comprises at least one of Mn, Cr, Zr, and V. In some examples, a combined content of Mn, Cr, Zr, and/or V is at least 0.14 wt. % (e.g., from 0.14 wt. % to 0.4 wt. % or from 0.15 wt. % to 0.25 wt. %). Optionally, the aluminum alloy comprises 0.06 - 0.3 wt. % V. In some examples, the aluminum alloy comprises excess Si and the excess Si content is from 0.01 to 1.0.
- Also described herein are aluminum alloy products comprising the aluminum alloy as described herein. Optionally, the aluminum alloy products comprise a rotated cube crystallographic texture at a volume percent of at least 5 %. The aluminum alloy products can comprise dispersoids. Optionally, the dispersoids are present in the aluminum alloy in an amount of at least 1,500,000 dispersoids per mm2. Optionally, the dispersoids occupy an area ranging from 0.5 % to 5 % of the aluminum alloy products. In some cases, the aluminum alloy products comprises Fe-constituents. The Fe-constituents can comprise Al(Fe,X)Si phase particles. Optionally, the average particle size of the Fe-constituents is up to 4 µm. The aluminum alloy products can exhibit a yield strength of at least 300 MPa when in a T6 temper and/or a uniform elongation of at least 20 % and a minimum bend angle of at least 120° when in a T4 temper.
- Further described herein are methods of producing an aluminum alloy product. The methods comprise casting an aluminum alloy as described herein to provide a cast article, homogenizing the cast article in a two-stage homogenization process, hot rolling and cold rolling the cast article to provide a final gauge aluminum alloy product, solution heat treating the final gauge aluminum alloy product, and pre-aging the final gauge aluminum alloy product. The two-stage homogenization process can comprise heating the cast article to a first stage homogenization temperature and holding the cast article at the first stage homogenization temperature for a period of time and further heating the cast article to a second stage homogenization temperature and holding the cast article at the second stage homogenization temperature for a period of time. Optionally, the first stage homogenization temperature is from 470 °C to 530 °C and the second stage homogenization temperature is from 525 °C to 575 °C. In some examples, the second stage homogenization temperature is higher than the first stage homogenization temperature.
- Further aspects, objects, and advantages will become apparent upon consideration of the detailed description and figures that follow.
-
-
Figure 1 is a graph showing tensile properties of aluminum alloys according to certain aspects of the present disclosure. -
Figure 2 is a micrograph showing the grain structure of aluminum alloys according to certain aspects of the present disclosure. -
Figure 3 is a graph showing mechanical properties of aluminum alloys according to certain aspects of the present disclosure. -
Figure 4 is a graph showing mechanical properties of aluminum alloys according to certain aspects of the present disclosure. -
Figure 5 is a graph showing mechanical properties of aluminum alloys according to certain aspects of the present disclosure. -
Figure 6 is a graph showing the distribution of recrystallization textures of aluminum alloys according to certain aspects of the present disclosure. -
Figure 7 is a series of micrographs of aluminum alloys according to certain aspects of the present disclosure. -
Figure 8 is a graph showing dispersoid number density and dispersoid area fraction of aluminum alloys according to certain aspects of the present disclosure. -
Figure 9 is a series of micrographs of aluminum alloys according to certain aspects of the present disclosure. -
Figure 10 is a graph showing size distribution of Fe-constituents of aluminum alloys according to certain aspects of the present disclosure. -
Figure 11 is a series of micrographs of aluminum alloys according to certain aspects of the present disclosure. - Described herein are novel aluminum alloys and products and methods of preparing the same. The alloys exhibit high strength and high formability. As further described herein, solute elements, including Cu, Mg, and Si, are combined with transition elements (e.g., Mn, Cr, Zn, and V) for a synergistic effect of increasing both the strength and formability of the alloys. The transition elements aid in preventing precipitate formation along grain boundaries in the aluminum alloys, as further described below. In addition, the processing methods used to prepare the alloys and products contribute to the high strength and formability exhibited by the alloys and products.
- The terms "invention," "the invention," "this invention" and "the present invention" used herein are intended to refer broadly to all of the subject matter of this patent application and the claims below. Statements containing these terms should be understood not to limit the subject matter described herein or to limit the meaning or scope of the patent claims below.
- In this description, reference is made to alloys identified by aluminum industry designations, such as "series" or "AA6xxx." For an understanding of the number designation system most commonly used in naming and identifying aluminum and its alloys, see "International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys" or "Registration Record of Aluminum Association Alloy Designations and Chemical Compositions Limits for Aluminum Alloys in the Form of Castings and Ingot," both published by The Aluminum Association.
- As used herein, the meaning of "a," "an," or "the" includes singular and plural references unless the context clearly dictates otherwise.
- As used herein, the meaning of "room temperature" can include a temperature of from about 15 °C to about 30 °C, for example about 15 °C, about 16 °C, about 17 °C, about 18 °C, about 19 °C, about 20 °C, about 21 °C, about 22 °C, about 23 °C, about 24 °C, about 25 °C, about 26 °C, about 27 °C, about 28 °C, about 29 °C, or about 30 °C.
- As used herein, a plate generally has a thickness of greater than about 15 mm. For example, a plate may refer to an aluminum product having a thickness of greater than 15 mm, greater than 20 mm, greater than 25 mm, greater than 30 mm, greater than 35 mm, greater than 40 mm, greater than 45 mm, greater than 50 mm, or greater than 100 mm.
- As used herein, a shate (also referred to as a sheet plate) generally has a thickness of from about 4 mm to about 15 mm. For example, a shate may have a thickness of 4 mm, 5 mm, 6 mm, 7 mm, 8 mm, 9 mm, 10 mm, 11 mm, 12 mm, 13 mm, 14 mm, or 15 mm.
- As used herein, a sheet generally refers to an aluminum alloy product having a thickness of less than about 4 mm. For example, a sheet may have a thickness of less than 4 mm, less than 3 mm, less than 2 mm, less than 1 mm, less than 0.5 mm, less than 0.3 mm, or less than 0.1 mm.
- As used herein, terms such as "cast metal article," "cast article," "cast aluminum alloy," and the like are interchangeable and refer to a product produced by direct chill casting (including direct chill co-casting) or semi-continuous casting, continuous casting (including, for example, by use of a twin belt caster, a twin roll caster, a block caster, or any other continuous caster), electromagnetic casting, hot top casting, or any other casting method.
- Reference is made in this application to alloy condition or temper. For an understanding of the alloy temper descriptions most commonly used, see "American National Standards (ANSI) H35 on Alloy and Temper Designation Systems." An F condition or temper refers to an aluminum alloy as fabricated. An O condition or temper refers to an aluminum alloy after annealing. A T1 condition or temper refers to an aluminum alloy cooled from hot working and naturally aged (e.g., at room temperature). A T2 condition or temper refers to an aluminum alloy cooled from hot working, cold worked and naturally aged. A T3 condition or temper refers to an aluminum alloy solution heat treated, cold worked, and naturally aged. A T4 condition or temper refers to an aluminum alloy solution heat treated and naturally aged. A T5 condition or temper refers to an aluminum alloy cooled from hot working and artificially aged (at elevated temperatures). A T6 condition or temper refers to an aluminum alloy solution heat treated and artificially aged. A T7 condition or temper refers to an aluminum alloy solution heat treated and artificially overaged. A T8x condition or temper refers to an aluminum alloy solution heat treated, cold worked, and artificially aged. A T9 condition or temper refers to an aluminum alloy solution heat treated, artificially aged, and cold worked.
- The following aluminum alloys are described in terms of their elemental composition in weight percentage (wt. %) based on the total weight of the alloy. In certain examples of each alloy, the remainder is aluminum, with a maximum wt. % of 0.15 % for the sum of the impurities.
- Described herein are novel aluminum alloys. The alloys exhibit high strength and high formability. In some cases, the properties of the alloys can be achieved due to the elemental composition of the alloys. The aluminum alloys can be precipitation hardened or precipitation hardenable alloys. Optionally, the aluminum alloys can be aluminum alloys classified as 2xxx series aluminum alloys (e.g., wherein copper is a predominant alloying element), 6xxx series aluminum alloys (e.g., wherein magnesium and silicon are predominant alloying elements), or 7xxx series aluminum alloys (e.g., wherein zinc is a predominant alloying element). In some cases, the aluminum alloys can be modified 2xxx series, 6xxx series, or 7xxx series aluminum alloys. As used herein, the term "modified" as related to a series of aluminum alloys refers to an alloy composition that would typically be classified within a particular series, but the modification of one or more elements (types or amounts) results in a different predominant alloying element. For example, a modified 6xxx series aluminum alloy can refer to an aluminum alloy in which copper and silicon are the predominant alloying elements rather than magnesium and silicon.
- In some cases, an aluminum alloy can have the following elemental composition as provided in Table 1:
Table 1 Element Weight Percentage (wt. %) Si 0.8-1.5 Fe 0.1-0.5 Cu 0.5 - 1.0 Mg 0.5-0.9 Ti 0-0.1 Mn 0 - 0.5 Cr 0 - 0.5 Zr 0 - 0.5 V 0.06 - 0.5 Others 0 - 0.05 (each) 0 - 0.15 (total) Al - In other examples, the alloy can have the following elemental composition as provided in Table 2.
Table 2 Element Weight Percentage (wt. %) Si 0.9 - 1.4 Fe 0.1 - 0.3 Cu 0.6 - 0.9 Mg 0.6 - 0.9 Ti 0.01 - 0.09 Mn 0.01 - 0.3 Cr 0.01 - 0.3 Zr 0.01 - 0.3 V 0.06 - 0.3 Others 0 - 0.05 (each) 0 - 0.15 (total) Al - In one example, the alloy can have the following elemental composition as provided in Table 3.
Table 3 Element Weight Percentage (wt. %) Si 1.0 - 1.3 Fe 0.1 - 0.25 Cu 0.7 - 0.9 Mg 0.6 - 0.8 Ti 0.01 - 0.05 Mn 0.05 - 0.2 Cr 0.05 - 0.2 Zr 0.05 - 0.2 V 0.06 - 0.2 Others 0 - 0.05 (each) 0 - 0.15 (total) Al - The alloy described herein includes silicon (Si) in an amount from 0.8 % to 1.5 % (e.g., from 0.9 % to 1.45 %, from 0.9 % to 1.4 %, from 0.9 % to 1.35 %, from 0.9 % to 1.3 %, from 0.9 % to 1.25 %, from 0.9 % to 1.2 %, from 0.95 % to 1.5 %, from 0.95 % to 1.45 %, from 0.95 % to 1.4 %, from 0.95 % to 1.35 %, from 0.95 % to 1.3 %, from 0.95 % to 1.25 %, from 0.95 % to 1.2 %, from 1.0% to 1.5 %, from 1.0% to 1.45 %, from 1.0 % to 1.4 %, from 1.0% to 1.35 %, from 1.0% to 1.3 %, from 1.0% to 1.25 %, or from 1.0 % to 1.2 %) based on the total weight of the alloy. For example, the alloy can include 0.8 %, 0.81 %, 0.82 %, 0.83 %, 0.84 %, 0.85 %, 0.86 %, 0.87 %, 0.88 %, 0.89 %, 0.9 %, 0.91 %, 0.92 %, 0.93 %, 0.94 %, 0.95 %, 0.96 %, 0.97 %, 0.98 %, 0.99 %, 1.0 %, 1.01 %, 1.02 %, 1.03 %, 1.04 %, 1.05 %, 1.06 %, 1.07 %, 1.08 %, 1.09 %, 1.1 %, 1.11 %, 1.12 %, 1.13 %, 1.14 %, 1.15 %, 1.16 %, 1.17 %, 1.18 %, 1.19 %, 1.2 %, 1.21 %, 1.22 %, 1.23 %, 1.24 %, 1.25 %, 1.26 %, 1.27 %, 1.28 %, 1.29 %, 1.3 %, 1.31 %, 1.32 %, 1.33 %, 1.34 %, 1.35 %, 1.36 %, 1.37 %, 1.38 %, 1.39 %, 1.4 %, 1.41 %, 1.42 %, 1.43 %, 1.44 %, 1.45 %, 1.46 %, 1.47 %, 1.48 %, 1.49 %, or 1.5 % Si. All expressed in wt. %.
- The alloy described herein includes iron (Fe) in an amount from 0.1 % to 0.5 % (e.g., from 0.1 % to 0.45 %, from 0.1 % to 0.4 %, from 0.1 % to 0.35 %, from 0.1 % to 0.3 %, from 0.1 % to 0.25 %, from 0.1 % to 0.2 %, from 0.15 % to 0.45 %, from 0.15 % to 0.4 %, from 0.15 % to 0.35 %, from 0.15 % to 0.3 %, from 0.15 % to 0.25 %, from 0.15 % to 0.2 %, from 0.2 % to 0.45 %, from 0.2 % to 0.4 %, from 0.2 % to 0.35 %, from 0.2 % to 0.3 %, from 0.2 % to 0.25 %, from 0.25 % to 0.45 %, from 0.25 % to 0.4 %, from 0.25 % to 0.35 %, from 0.25 % to 0.3 %, from 0.3 % to 0.45 %, from 0.3 % to 0.4 %, or from 0.3 % to 0.35 %) based on the total weight of the alloy. For example, the alloy can include 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2%, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5 % Fe. All expressed in wt. %.
- The alloy described herein includes copper (Cu) in an amount from 0.5 % to 1.0 % (e.g., from 0.55 % to 1.0 %, from 0.6 % to 1.0 %, from 0.65 % to 1.0 %, from 0.7 % to 1.0 %, from 0.75 % to 1.0 %, from 0.8 % to 1.0 %, from 0.5 % to 0.95 %, from 0.55 % to 0.95 %, from 0.6 % to 0.95 %, from 0.65 % to 0.95 %, from 0.7 % to 0.95 %, from 0.75 % to 0.95 %, from 0.8 % to 0.95 %, from 0.5 % to 0.9 %, from 0.55 % to 0.9 %, from 0.6 % to 0.9 %, from 0.65 % to 0.9 %, from 0.7 % to 0.9 %, from 0.75 % to 0.9 %, from 0.8 % to 0.9 %, from 0.5% to 0.85 %, from 0.55 % to 0.85 %, from 0.6 % to 0.85 %, from 0.65 % to 0.85 %, from 0.7 % to 0.85 %, from 0.75 % to 0.85 %, from 0.8 % to 0.85 %, from 0.5 % to 0.8 %, from 0.55 % to 0.8 %, from 0.6% to 0.8 %, from 0.65 % to 0.8 %, from 0.7 % to 0.8 %, or from 0.75 % to 0.8 %) based on the total weight of the alloy. For example, the alloy can include 0.5 %, 0.51 %, 0.52 %, 0.53 %, 0.54 %, 0.55 %, 0.56 %, 0.57 %, 0.58 %, 0.59 %, 0.6 %, 0.61 %, 0.62 %, 0.63 %, 0.64 %, 0.65 %, 0.66 %, 0.67 %, 0.68 %, 0.69 %, 0.7 %, 0.71 %, 0.72 %, 0.73 %, 0.74 %, 0.75 %, 0.76 %, 0.77 %, 0.78 %, 0.79 %, 0.8 %, 0.81 %, 0.82 %, 0.83 %, 0.84 %, 0.85 %, 0.86 %, 0.87 %, 0.88 %, 0.89 %, 0.9 %, 0.91 %, 0.92 %, 0.93 %, 0.94 %, 0.95 %, 0.96 %, 0.97 %, 0.98 %, 0.99 %, or 1.0 % Cu. All expressed in wt. %.
- The alloy described herein includes magnesium (Mg) in an amount from 0.5 % to 0.9 % (e.g., from 0.55 % to 0.9 %, from 0.6 % to 0.9 %, from 0.65 % to 0.9 %, from 0.7 % to 0.9 %, from 0.75 % to 0.9 %, from 0.8 % to 0.9 %, from 0.5% to 0.85 %, from 0.55 % to 0.85 %, from 0.6 % to 0.85 %, from 0.65 % to 0.85 %, from 0.7 % to 0.85 %, from 0.75 % to 0.85 %, from 0.8 % to 0.85 %, from 0.5 % to 0.8 %, from 0.55 % to 0.8 %, from 0.6 % to 0.8 %, from 0.65 % to 0.8 %, from 0.7 % to 0.8 %, or from 0.75 % to 0.8 %) based on the total weight of the alloy. For example, the alloy can include 0.5 %, 0.51 %, 0.52 %, 0.53 %, 0.54 %, 0.55 %, 0.56 %, 0.57 %, 0.58 %, 0.59 %, 0.6 %, 0.61 %, 0.62 %, 0.63 %, 0.64 %, 0.65 %, 0.66 %, 0.67 %, 0.68 %, 0.69 %, 0.7 %, 0.71 %, 0.72 %, 0.73 %, 0.74 %, 0.75 %, 0.76 %, 0.77 %, 0.78 %, 0.79 %, 0.8 %, 0.81 %, 0.82 %, 0.83 %, 0.84 %, 0.85 %, 0.86 %, 0.87 %, 0.88 %, 0.89 %, or 0.9 % Mg. All expressed in wt. %.
- In certain aspects, the alloy described herein includes titanium (Ti) in an amount up to 0.1 % (e.g., from 0.01 % to 0.09 %, from 0.02 % to 0.09 %, from 0.03 % to 0.09 %, from 0.04 % to 0.09 %, from 0.05 % to 0.09 %, from 0.01 % to 0.08 %, from 0.02 % to 0.08 %, from 0.03 % to 0.08 %, from 0.04 % to 0.08 %, from 0.05 % to 0.08 %, from 0.01 % to 0.07 %, from 0.02 % to 0.07 %, from 0.03 % to 0.07 %, from 0.04 % to 0.07 %, from 0.05 % to 0.07 %, from 0.01 % to 0.06 %, from 0.02 % to 0.06 %, from 0.03 % to 0.06 %, from 0.04% to 0.06 %, from 0.05 % to 0.06 %, from 0.01 % to 0.05 %, from 0.02 % to 0.05 %, from 0.03 % to 0.05 %, or from 0.04 % to 0.05 %) based on the total weight of the alloy. For example, the alloy can include 0.01 %, 0.02 %, 0.03 %, 0.04 %, 0.05 %, 0.06 %, 0.07 %, 0.08 %, 0.09 %, or 0.1 % Ti. In some examples, Ti is not present in the alloy (i.e., 0 % Ti). All expressed in wt. %.
- In certain examples, the alloy described herein includes manganese (Mn) in an amount up to 0.5 % (e.g., from 0.01 % to 0.5 %, from 0.01 % to 0.4 %, from 0.01 % to 0.3 %, from 0.01 % to 0.2 %, from 0.01 % to 0.1 %, from 0.06 % to 0.5 %, from 0.06 % to 0.4 %, from 0.06% to 0.3 %, from 0.06% to 0.2 %, from 0.06 % to 0.1 %, from 0.1% to 0.5 %, from 0.1 % to 0.4 %, from 0.1 % to 0.3 %, or from 0.1 % to 0.2 %) based on the total weight of the alloy. For example, the alloy can include 0.01 %, 0.02 %, 0.03 %, 0.04 %, 0.05 %, 0.06 %, 0.07 %, 0.08 %, 0.09 %, 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2 %, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5 % Mn. In some examples, Mn is not present in the alloy (i.e., 0 % Mn). All expressed in wt. %.
- In certain aspects, the alloy described herein includes chromium (Cr) in an amount up to 0.5 % (e.g., from 0.01 % to 0.5 %, from 0.01 % to 0.4 %, from 0.01 % to 0.3 %, from 0.01 % to 0.2 %, from 0.01 % to 0.1 %, from 0.06 % to 0.5 %, from 0.06 % to 0.4 %, from 0.06% to 0.3 %, from 0.06% to 0.2 %, from 0.06 % to 0.1 %, from 0.1% to 0.5 %, from 0.1 % to 0.4 %, from 0.1 % to 0.3 %, or from 0.1 % to 0.2 %) based on the total weight of the alloy. For example, the alloy can include 0.01 %, 0.02 %, 0.03 %, 0.04 %, 0.05 %, 0.06 %, 0.07 %, 0.08 %, 0.09 %, 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2 %, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5 % Cr. In some examples, Cr is not present in the alloy (i.e., 0 % Cr). All expressed in wt. %.
- In certain aspects, the alloy described herein includes zirconium (Zr) in an amount up to 0.5 % (e.g., from 0.01 % to 0.5 %, from 0.01 % to 0.4 %, from 0.01 % to 0.3 %, from 0.01 % to 0.2 %, from 0.01 % to 0.1 %, from 0.06 % to 0.5 %, from 0.06 % to 0.4 %, from 0.06% to 0.3 %, from 0.06% to 0.2 %, from 0.06 % to 0.1 %, from 0.1% to 0.5 %, from 0.1 % to 0.4 %, from 0.1 % to 0.3 %, or from 0.1 % to 0.2 %) based on the total weight of the alloy. For example, the alloy can include 0.01 %, 0.02 %, 0.03 %, 0.04 %, 0.05 %, 0.06 %, 0.07 %, 0.08 %, 0.09 %, 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2 %, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5 % Zr. In certain aspects, Zr is not present in the alloy (i.e., 0 %). All expressed in wt. %.
- The alloy described herein includes vanadium (V) in an amount 0.06 - 0.5 % (e.g., from 0.06 % to 0.5 %, from 0.06 % to 0.4 %, from 0.06 % to 0.3 %, from 0.06 % to 0.2 %, from 0.06 % to 0.1 %, from 0.1% to 0.5 %, from 0.1% to 0.4 %, from 0.1% to 0.3 %, or from 0.1 % to 0.2 %) based on the total weight of the alloy. For example, the alloy can include 0.06 %, 0.07 %, 0.08 %, 0.09 %, 0.1 %, 0.11 %, 0.12 %, 0.13 %, 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2%, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, 0.4 %, 0.41 %, 0.42 %, 0.43 %, 0.44 %, 0.45 %, 0.46 %, 0.47 %, 0.48 %, 0.49 %, or 0.5 % V. All expressed in wt. %.
- Optionally, the alloy compositions can further include other minor elements, sometimes referred to as impurities, in amounts of about 0.05 % or below, 0.04 % or below, 0.03 % or below, 0.02 % or below, or 0.01 % or below each. These impurities may include, but are not limited to, Ni, Sc, Sn, Ga, Ca, Hf, Sr, or combinations thereof. Accordingly, Ni, Sc, Sn, Ga, Ca, Hf, or Sr may be present in an alloy in amounts of 0.05 % or below, 0.04 % or below, 0.03 % or below, 0.02 % or below, or 0.01 % or below. In certain aspects, the sum of all impurities does not exceed 0.15 % (e.g., 0.1 %). All expressed in wt. %. The alloy composition also includes aluminum. In certain aspects, the remaining percentage of the alloy is aluminum.
- One non-limiting example of a suitable alloy includes 1.20 % Si, 0.18 % Fe, 0.80 % Cu, 0.70 % Mg, 0.02 % Ti, 0.08 % Cr, 0.11 % V, and up to 0.15 % total impurities, with the remainder Al. In some cases, another non-limiting example of a suitable alloy includes 1.20 % Si, 0.18 % Fe, 0.80 % Cu, 0.70 % Mg, 0.02 % Ti, 0.09 % Zr, 0.10 % V, and up to 0.15 % total impurities, with the remainder Al. In some cases, another non-limiting example of a suitable alloy includes 1.20 % Si, 0.18 % Fe, 0.80 % Cu, 0.70 % Mg, 0.02 % Ti, 0.09 % Mn, 0.10 % V, and up to 0.15 % total impurities, with the remainder Al.
- In certain aspects, the Si, Mg, and Cu content and ratios are controlled to enhance strength and formability. Optionally, the transition element (e.g., Mn, Cr, Zr, and/or V) content is controlled to enhance strength and formability.
- In some cases, the alloy described herein includes excess Si. Optionally, the Si and Mg content are controlled such that excess Si is present in the alloy as described herein. Excess Si content can be calculated according to the method described in
U.S. Patent No. 4,614,552 , col. 4, lines 49-52. Briefly, Mg and Si combine as Mg2Si, imparting a considerable strength improvement after age-hardening. In addition, Si-containing constituents, such as Al(FeMn)Si, can form. Excess Si is present when the Si content is above the stoichiometric ratio of Mg2Si and above the amount included in Al(FeMn)Si constituents. The excess Si content can be calculated by subtracting from the total Si content the Si needed for Mg2Si (Mg/1.73) and the Fe-containing phase (Fe/3). The excess Si content can be 1.0 or less (e.g., from 0.01 to 1.0, from 0.1 to 0.9, or from 0.5 to 0.8). For example, the excess Si content can be 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, 0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, 0.70, 0.75, 0.80, 0.85, 0.90, 0.95, 1.0, or anywhere in between. - The alloys described herein include at least one transition element (e.g., at least one of Mn, Cr, Zr, and/or V). Optionally, the combined content of the transition elements in the alloys described herein is at least 0.14 wt. %. For example, the combined content of Mn, Cr, Zr, and/or V can be from 0.14 wt. % to 0.40 wt. % (e.g., from 0.15 wt. % to 0.35 wt. % or from 0.25 wt. % to 0.30 wt. %). In some cases, the combined content of Mn, Cr, Zr, and/or V is 0.14 %, 0.15 %, 0.16 %, 0.17 %, 0.18 %, 0.19 %, 0.2%, 0.21 %, 0.22 %, 0.23 %, 0.24 %, 0.25 %, 0.26 %, 0.27 %, 0.28 %, 0.29 %, 0.3 %, 0.31 %, 0.32 %, 0.33 %, 0.34 %, 0.35 %, 0.36 %, 0.37 %, 0.38 %, 0.39 %, or 0.4 %. In some cases, one or more of the transition elements may not be present, as long as the total weight percentage of the present transition elements is at least 0.14 wt. %.
- The presence of one or more of the transition elements, such as Mn, Cr, Zr, and/or V, can advantageously form dispersoids during the processing methods described herein, such as during the homogenization step. The dispersoids can function as heterogeneous nucleation sites for precipitates during processing steps, such as during the solution heat treatment step. In certain aspects, grain boundary (GB) precipitation occurs due to GB misorientation that is favorable for precipitate nucleation. The dispersoids reduce or eliminate GB precipitates and also reduce strain localization, thus diffusing strain distribution during deformation. The reduced or eliminated GB precipitates and/or the diffused strain distribution during deformation result in an improved bendability of the resulting alloys and alloy products.
- In some non-limiting examples, the dispersoids described herein can contain Al and one or more of the alloying elements found in the alloy composition as described above. In some examples, the dispersoids can have a composition according to one or more of the following formulae: AlX, AlXX, AlXSi, Al(Fe,X), Al(Fe,X)Si, or the like, wherein each X is selected from the group consisting of Fe, Si, Mn, Cr, V, or Zr.
- The dispersoid average size and distribution are important factors that result in the desirable strength and formability properties displayed by the alloys and alloy products described herein. The size and distribution are influenced by the presence of transition elements, as described above, and also by the methods of processing the alloys, as further described below. In some examples, the dispersoids can be present in the aluminum alloy in an average amount of at least 1,500,000 dispersoids per square millimeter (mm2). For example, the dispersoids can be present in an amount of at least 1,600,000 dispersoids per mm2, at least 1,700,000 dispersoids per mm2, at least 1,800,000 dispersoids per mm2, at least 1,900,000 dispersoids per mm2, at least 2,000,000 dispersoids per mm2, at least 2,100,000 dispersoids per mm2, at least 2,200,000 dispersoids per mm2, at least 2,300,000 dispersoids per mm2, at least 2,400,000 dispersoids per mm2, at least 2,500,000 dispersoids per mm2, at least 2,600,000 dispersoids per mm2, at least 2,700,000 dispersoids per mm2, at least 2,800,000 dispersoids per mm2, at least 2,900,000 dispersoids per mm2, or at least 3,000,000 dispersoids per mm2. In some examples, the average number of dispersoids present in the aluminum alloy can be from 1,500,000 dispersoids per mm2 to 5,000,000 dispersoids per mm2 (e.g., from 1,750,000 dispersoids per mm2 to 4,750,000 dispersoids per mm2 or from 2,000,000 dispersoids per mm2 to 4,500,000 dispersoids per mm2). The dispersoids in the aluminum alloy can occupy an area ranging from 0.5 % to 5 % of the alloy (e.g., from 1 % to 4 % or from 1.5 % to 2.5 % of the alloy).
- Optionally, the dispersoids can have an average diameter of from 10 nm to 600 nm (e.g., from 50 nm to 500 nm, from 100 nm to 450 nm, from 200 nm to 400 nm, from 10 nm to 200 nm, or from 500 nm to 600 nm). For example, the dispersoids can have a diameter of 10 nm, 20 nm, 30 nm, 40 nm, 50 nm, 60 nm, 70 nm, 80 nm, 90 nm, 100 nm, 110 nm, 120 nm, 130 nm, 140 nm, 150 nm, 160 nm, 170 nm, 180 nm, 190 nm, 200 nm, 210 nm, 220 nm, 230 nm, 240 nm, 250 nm, 260 nm, 270 nm, 280 nm, 290 nm, 300 nm, 310 nm, 320 nm, 330 nm, 340 nm, 350 nm, 360 nm, 370 nm, 380 nm, 390 nm, 400 nm, 410 nm, 420 nm, 430 nm, 440 nm, 450 nm, 460 nm, 470 nm, 480 nm, 490 nm, 500 nm, 510 nm, 520 nm, 530 nm, 540 nm, 550 nm, 560 nm, 570 nm, 580 nm, 590 nm, 600 nm, or anywhere in between.
- The alloys described herein also include Fe-constituents, which are also referred to herein as Fe-containing particles. Optionally, in addition to Fe, the Fe-constituents can include one or more of Al, Mn, Si, Cu, Ti, Zr, Cr, and/or Mg. In some examples, the Fe-constituents can be Al(Fe,X)Si phase particles, wherein X can be Mn, Cr, Zr, and/or V, and/or AlFeSi phase particles. For example, the Fe-constituents can include one or more of Al3Fe, Alx(Fe,Mn), Al3Fe, Al12(Fe,Mn)3Si, Al7Cu2Fe, Al(Fe,Mn)2Si3, Alx(Mn,Fe), and Al12(Mn,Fe)3Si. The presence of the transition elements described herein results in the transformation of AlFeSi particles into Al(Fe,X)Si particles. In some examples, the number of Al(Fe,X)Si phase particles, which are spheroid particles, is greater than the number of AlFeSi phase particles, which are flake or needle type particles. Optionally, at least 50 % of the Fe-constituents are present as Al(Fe,X)Si particles (e.g., at least 55 %, at least 60 %, at least 65 %, at least 70 %, at least 75 %, at least 80 %, at least 85 %, at least 90 %, or at least 95 % of the Fe-constituents are present as Al(Fe,X)Si particles). The Fe-constituents can have an average particle size of up to 4 µm. For example, the Fe-constituents, on average, can range in size from 0.1 µm to 4 µm (e.g., from 0.5 µm to 3.5 µm or from 1 µm to 3 µm).
- Optionally, the Cr, Mn, Zr, and/or V content and ratios are controlled to form the desired size, type, and distribution of dispersoids, which leads to improved formability and strength. In some non-limiting examples, a ratio of Cr to Mn (also referred to herein as the Cr/Mn ratio) can be from 0.15:1 to 0.7:1 (e.g., from 0.3:1 to 0.6:1 or from 0.4:1 to 0.55:1). For example, the Cr/Mn ratio can be 0.15:1, 0.16:1, 0.17:1, 0.18:1, 0.19:1, 0.20:1, 0.21:1, 0.22:1, 0.23:1, 0.24:1, 0.25:1, 0.26:1, 0.27:1, 0.28:1, 0.29:1, 0.30:1, 0.31:1, 0.32:1, 0.33:1, 0.34:1, 0.35:1, 0.36:1, 0.37:1, 0.38:1, 0.39:1, 0.40:1, 0.41:1, 0.42:1, 0.43:1, 0.44:1, 0.45:1, 0.46:1, 0.47:1, 0.48:1, 0.49:1, 0.50:1, 0.51:1, 0.52:1, 0.53:1, 0.54:1, 0.55:1, 0.56:1, 0.57:1, 0.58:1, 0.59:1, 0.60:1, 0.61:1, 0.62:1, 0.63:1, 0.64:1, 0.65:1, 0.66:1, 0.67:1, 0.68:1, 0.69:1, or 0.70:1.
- In some non-limiting examples, a ratio of Cr to V (also referred to herein as the Cr/V ratio) can be from 0.5:1 to 1.5:1 (e.g., from 0.6:1 to 1.4:1 or from 0.7:1 to 1.3:1). For example, the Cr/V ratio can be 0.50:1, 0.51:1, 0.52:1, 0.53:1, 0.54:1, 0.55:1, 0.56:1, 0.57:1, 0.58:1, 0.59:1, 0.60:1, 0.61:1, 0.62:1, 0.63:1, 0.64:1, 0.65:1, 0.66:1, 0.67:1, 0.68:1, 0.69:1, 0.70:1, 0.71:1, 0.72:1, 0.73:1, 0.74:1, 0.75:1, 0.76:1, 0.77:1, 0.78:1, 0.79:1, 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, 1.4:1, or 1.5:1.
- In some non-limiting examples, a ratio of Cr to Zr (also referred to herein as the Cr/Zr ratio) can be from 0.5:1 to 1.5:1 (e.g., from 0.6:1 to 1.4:1 or from 0.7:1 to 1.3:1). For example, the Cr/Zr ratio can be 0.50:1, 0.51:1, 0.52:1, 0.53:1, 0.54:1, 0.55:1, 0.56:1, 0.57:1, 0.58:1, 0.59:1, 0.60:1, 0.61:1, 0.62:1, 0.63:1, 0.64:1, 0.65:1, 0.66:1, 0.67:1, 0.68:1, 0.69:1, 0.70:1, 0.71:1, 0.72:1, 0.73:1, 0.74:1, 0.75:1, 0.76:1, 0.77:1, 0.78:1, 0.79:1, 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, 1.4:1, or 1.5:1.
- In some non-limiting examples, a ratio of V to Mn (also referred to herein as the V/Mn ratio) can be from 0.8:1 to 1.4:1 (e.g., from 0.9:1 to 1.3:1 or from 0.9:1 to 1.2:1). For example, the V/Mn ratio can be 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, or 1.4:1.
- In some non-limiting examples, a ratio of V to Zr (also referred to herein as the V/Zr ratio) can be from 0.8:1 to 1.4:1 (e.g., from 0.9:1 to 1.3:1 or from 0.9:1 to 1.2:1). For example, the V/Zr ratio can be 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, or 1.4:1.
- In some non-limiting examples, a ratio of V to Cr (also referred to herein as the V/Cr ratio) can be from 0.8:1 to 1.4:1 (e.g., from 0.9:1 to 1.3:1 or from 0.9:1 to 1.2:1). For example, the V/Cr ratio can be 0.80:1, 0.81:1, 0.82:1, 0.83:1, 0.84:1, 0.85:1, 0.86:1, 0.87:1, 0.88:1, 0.89:1, 0.90:1, 0.91:1, 0.92:1, 0.93:1, 0.94:1, 0.95:1, 0.96:1, 0.97:1, 0.98:1, 0.99:1, 1.0:1, 1.1:1, 1.2:1, 1.3:1, or 1.4:1.
- The mechanical properties of the aluminum alloy can be controlled by various aging conditions depending on the desired use. As one example, the alloy can be produced (or provided) in a T4 temper or a T6 temper. In some non-limiting examples, the proposed alloy has very high formability and bendability in the T4 temper and very high strength in the T6 temper. In certain aspects, the aluminum alloy may have a T4 yield strength ranging from 150 MPa to 250 MPa (e.g., 150 MPa, 160 MPa, 170 MPa, 180 MPa, 190 MPa, 200 MPa, 210 MPa, 220 MPa, 230 MPa, 240 MPa, or 250 MPa). In some cases, the yield strength is from 185 MPa to 195 MPa.
- In certain aspects, the alloy in the T4 temper provides a uniform elongation of at least 20 % (e.g., from 20% to 30% or from 22 % to 26%). For example, the uniform elongation can be 20 %, 21 %, 22 %, 23 %, 24 %, 25 %, 26 %, 27 %, 28 %, 29 %, or 30 %. Optionally, the uniform elongation is measured in the longitudinal (L) direction.
- Optionally, the alloy in the T4 temper provides a bend angle, as tested according to VDA 238-100, of at least 120°. For example, the bend angle can be from 120° to 140° (e.g., 120°, 121°, 122°, 123°, 124°, 125°, 126°, 127°, 128°, 129°, 130°, 131°, 132°, 133°, 134°, 135°, 136°, 137°,138°, 139°, or 140°). In some non-limiting examples, including V can improve the formability of the alloys. For example, alloys that include V exhibit an increase in bend angle of up to 10° (e.g., showing an improvement of at least 5°, at least 6°, at least 7°, at least 8°, at least 9°, at least 10°, or anywhere in between) as compared to alloys that do not contain V.
- In certain aspects, the aluminum alloy may have a T6 yield strength of at least 200 MPa. In non-limiting examples, the yield strength is at least 200 MPa, at least 210 MPa, at least 220 MPa, at least 230 MPa, at least 240 MPa, at least 250 MPa, at least 260 MPa, at least 270 MPa, at least 280 MPa, at least 290 MPa, or at least 300 MPa, at least 310 MPa, at least 320 MPa, at least 330 MPa, at least 340 MPa, at least 350 MPa, at least 360 MPa, at least 370 MPa, or at least 375 MPa. In some cases, the yield strength is from 200 MPa to 400 MPa (e.g., 200 MPa, 210 MPa, 220 MPa, 230 MPa, 240 MPa, 250 MPa, 260 MPa, 270 MPa, 280 MPa, 290 MPa, 300 MPa, 310 MPa, 320 MPa, 330 MPa, 340 MPa, 350 MPa, 360 MPa, 370 MPa, or 375 MPa).
- In certain aspects, the alloy in the T6 temper provides a uniform elongation of at least 5 % (e.g., from 5 % to 10 % or from 6 % to 9 %). For example, the uniform elongation can be 5 %, 6 %, 7 %, 8 %, 9 %, or 10 %. Optionally, the uniform elongation is measured in the longitudinal (L) direction.
- The alloy products also include recrystallization texture components at a surface of the alloy products. For example, the alloy products include one or more of the following recrystallization texture components: cube, goss, brass, S, Cu, and rotated cube (referred to as "RC"). Optionally, at least 5 volume % of the rotated cube texture component is present in the alloy product (e.g., from 5 vol. % to 20 vol. %, from 6 vol. % to 18 vol. %, from 8 vol. % to 15 vol. %, from 10 vol. % to 13 vol. %, or from 5 vol. % to 6 vol. %). Such a rotated cube texture component can result in desirable bending in the alloy product.
- Without intending to limit the invention, aluminum alloy properties are partially determined by the formation of microstructures during the alloy's preparation. In certain aspects, the method of preparation for an alloy composition may influence or even determine whether the alloy will have properties adequate for a desired application.
- The alloy described herein can be cast into a cast article using any suitable casting method. For example, the casting process can include a direct chill (DC) casting process. Optionally, the casting process can include a continuous casting (CC) process. The cast article can then be subjected to further processing steps. For example, the processing methods as described herein can include the steps of homogenizing, hot rolling, cold rolling, solutionizing, and pre-aging. In some cases, the processing methods can also include an artificial aging step.
- The homogenization step includes a two-stage heating process. In a first stage of the homogenization process, a cast article prepared from an alloy composition described herein can be heated to a first stage homogenization temperature (e.g., the dispersoid nucleation temperature). The first stage homogenization temperature can be from 470 °C to 530 °C (e.g., 470 °C, 480 °C, 490 °C, 500 °C, 510 °C, 520 °C, 530 °C, or anywhere in between). In some cases, a heating rate to the first stage homogenization temperature can be 100 °C/hour or less, 75 °C/hour or less, 50 °C/hour or less, 40 °C/hour or less, 30 °C/hour or less, 25 °C/hour or less, 20 °C/hour or less, or 15 °C/hour or less. In other cases, the heating rate to the first stage homogenization temperature can be from 10 °C/min to 100 °C/min (e.g., from 15 °C/min to 90 °C/min, from 20 °C/min to 80 °C/min, from 30 °C/min to 80 °C/min, from 40 °C/min to 70 °C/min, or from 45 °C/min to 65 °C/min).
- The cast article is then allowed to soak (i.e., held at the indicated temperature) for a period of time. According to one non-limiting example, the cast article is allowed to soak for up to 6 hours (e.g., from 30 minutes to 6 hours, inclusively). For example, the cast article can be soaked at a temperature of from 470 °C to 530 °C for 30 minutes, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, or anywhere in between.
- In the second stage of the homogenization process, the temperature of the cast article is increased to a temperature higher than the temperature used for the first stage of the homogenization process. The cast article temperature can be increased, for example, to a temperature at least 5 °C higher than the aluminum alloy cast article temperature during the first stage of the homogenization process. For example, the cast article can be further heated to a second stage homogenization temperature (e.g., a dispersoid coarsening temperature) of from 525 °C to 575 °C (e.g., from 530 °C to 570 °C or from 535 °C to 565 °C). In some examples, the second stage homogenization temperature can be 525 °C, 530 °C, 535 °C, 540 °C, 545 °C, 550 °C, 555 °C, 560 °C, 565 °C, 570 °C, 575 °C, or anywhere in between) in a second homogenization step. In some cases, a heating rate to the second stage homogenization temperature can be 50 °C/hour or less, 30 °C/hour or less, or 25 °C/hour or less.
- The cast article is then allowed to soak for a period of time during the second stage. According to one non-limiting example, the cast article is allowed to soak for up to 5 hours (e.g., from 20 minutes to 5 hours, inclusively). For example, the cast article can be soaked at a temperature of from 525 °C to 575 °C for 15 minutes, 20 minutes, 30 minutes, 45 minutes, 1 hour, 1.5 hours, 2 hours, 3 hours, 4 hours, 5 hours, or anywhere in between.
- Following the homogenization step, a hot rolling step is performed. In certain cases, the cast articles are laid down and hot-rolled with an entry temperature range of 500 °C to 560 °C (e.g., from 510 °C to 550 °C or from 520 °C to 540 °C). The entry temperature can be, for example, 505 °C, 510 °C, 515 °C, 520 °C, 525 °C, 530 °C, 535 °C, 540 °C, 545 °C, 550 °C, 555 °C, 560 °C, or anywhere in between. In certain cases, the hot roll exit temperature can range from 250 °C to 380 °C (e.g., from 275 °C to 370 °C or from 300 °C to 360 °C). For example, the hot roll exit temperature can be 255 °C, 260 °C, 265 °C, 270 °C, 275 °C, 280 °C, 285 °C, 290 °C, 295 °C, 300 °C, 305 °C, 310 °C, 315 °C, 320 °C, 325 °C, 330 °C, 335 °C, 340 °C, 345 °C, 350 °C, 355 °C, 360 °C, 365 °C, 370 °C, 375 °C, or 380 °C.
- In certain cases, the cast article is hot rolled to a 4 mm to 15 mm gauge (e.g., from 5 mm to 12 mm gauge), which is referred to as a hot band. For example, the cast article can be hot rolled to a 15 mm gauge, a 14 mm gauge, a 13 mm gauge, a 12 mm gauge, an 11 mm gauge, a 10 mm gauge, a 9 mm gauge, an 8 mm gauge, a 7 mm gauge, a 6 mm gauge, a 5 mm gauge, or a 4 mm gauge. The temper of the as-rolled hot band is referred to as F-temper.
- A cold rolling step is performed before the solutionizing step. In certain aspects, the hot band is cold rolled to a final gauge aluminum alloy sheet. In some examples, the final gauge aluminum alloy sheet has a thickness of 4 mm or less, 3 mm or less, 2 mm or less, 1 mm or less, 0.9 mm or less, 0.8 mm or less, 0.7 mm or less, 0.6 mm or less, 0.5 mm or less, 0.4 mm or less, 0.3 mm or less, 0.2 mm or less, or 0.1 mm.
- The solutionizing step can include heating the aluminum alloy sheet or other rolled article from room temperature to a peak metal temperature. Optionally, the peak metal temperature can be from 520 °C to 590 °C (e.g., from 520 °C to 580 °C, from 530 °C to 570 °C, from 545 °C to 575 °C, from 550 °C to 570 °C, from 555 °C to 565 °C, from 540 °C to 560 °C, from 560 °C to 580 °C, or from 550 °C to 575 °C). The aluminum alloy sheet can soak at the peak metal temperature for a period of time. In certain aspects, the aluminum alloy sheet is allowed to soak for up to approximately 2 minutes (e.g., from 10 seconds to 120 seconds inclusively). For example, the sheet can be soaked at the temperature of from 520 °C to 590 °C for 10 seconds, 15 seconds, 20 seconds, 25 seconds, 30 seconds, 35 seconds, 40 seconds, 45 seconds, 50 seconds, 55 seconds, 60 seconds, 65 seconds, 70 seconds, 75 seconds, 80 seconds, 85 seconds, 90 seconds, 95 seconds, 100 seconds, 105 seconds, 110 seconds, 115 seconds, 120 seconds, or anywhere in between.
- The aluminum alloy sheet undergos a pre-aging heat treatment. In some examples, pre-aging can include heating the aluminum alloy sheet to a temperature of from 80 °C to 120 °C (e.g., 80 °C, 85 °C, 90 °C, 95 °C, 100 °C, 105 °C, 110 °C, 115 °C, 120 °C, or anywhere in between) and coiling the aluminum alloy sheet. The coiled aluminum alloy sheet can be cooled (i.e., coil cooling is performed) for a period of up to 24 hours (e.g., 1 hour, 2 hours, 6 hours, 12 hours, 18 hours, 24 hours, or anywhere in between).
- The aluminum alloy sheet can then be naturally aged and/or artificially aged. In some examples, the aluminum alloy sheet can be naturally aged for a period of time to result in a T4 temper. For example, the aluminum alloy sheet can be naturally aged for 1 week or more, 2 weeks or more, 3 weeks or more, or 4 weeks or more.
- In certain aspects, the aluminum alloy sheet in the T4 temper can be artificially aged at a temperature of from 180 °C to 225 °C (e.g., 185 °C, 190 °C, 195 °C, 200 °C, 205 °C, 210 °C, 215 °C, 220 °C, or 225 °C) for a period of time to result in a T6 temper. For example, the aluminum alloy sheet can be artificially aged for a period from 15 minutes to 3 hours (e.g., 15 minutes, 30 minutes, 60 minutes, 90 minutes, 105 minutes, 2 hours, 2.5 hours, 3 hours, or anywhere in between) to result in a T6 temper.
- The alloys, products, and methods described herein can be used in automotive, electronics, and transportation applications, such as commercial vehicle, aircraft, or railway applications. For example, the alloys can be used for chassis, cross-member, and intra-chassis components (encompassing, but not limited to, all components between the two C channels in a commercial vehicle chassis) to gain strength, serving as a full or partial replacement of high-strength steels. In certain embodiments, the alloys can be used in F, T4, T6, or T8x tempers. In certain aspects, the alloys are used with a stiffener to provide additional strength. In certain aspects, the alloys are useful in applications where the processing and operating temperature is approximately 150 °C or lower.
- In certain aspects, the alloys and methods can be used to prepare motor vehicle body part products. For example, the disclosed alloys and methods can be used to prepare automobile body parts, such as bumpers, side beams, roof beams, cross beams, pillar reinforcements (e.g., A-pillars, B-pillars, and C-pillars), inner panels, side panels, floor panels, tunnels, structure panels, reinforcement panels, inner hoods, or trunk lid panels. The disclosed aluminum alloys and methods can also be used in aircraft or railway vehicle applications, to prepare, for example, external and internal panels.
- The described alloys and methods can also be used to prepare housings for electronic devices, including mobile phones and tablet computers. For example, the alloy can be used to prepare housings for the outer casing of mobile phones (e.g., smart phones) and tablet bottom chassis, with or without anodizing. Exemplary consumer electronic products include mobile phones, audio devices, video devices, cameras, laptop computers, desktop computers, tablet computers, televisions, displays, household appliances, video playback and recording devices, and the like. Exemplary consumer electronic product parts include outer housings (e.g., facades) and inner pieces for the consumer electronic products.
- The following examples will serve to further illustrate the present invention without, however, constituting any limitation thereof. On the contrary, it is to be clearly understood that resort may be had to various embodiments, modifications, and equivalents thereof which, after reading the description herein, may suggest themselves to those skilled in the art without departing from the spirit of the invention. During the studies described in the following examples, conventional procedures were followed, unless otherwise stated. Some of the procedures are described below for illustrative purposes.
- Alloys were prepared for strength and formability testing. The compositions for these alloys are provided in Table 4 below. In each of the alloy compositions in Table 4, the remainder is A Alloys D4, D5 and D6 are according to the present invention, alloys D1, D2 and D3 are not.
Table 4 Alloy No. Si Fe Cu Mg Ti Mn Cr Zr V Excess Si* D1 1.20 0.18 0.80 0.70 0.02 0.13 0.07 - - 0.73 D2 1.20 0.18 0.80 0.70 0.02 - 0.14 - - 0.74 D3 1.20 0.18 0.80 0.70 0.02 - 0.07 0.11 - 0.75 D4 1.20 0.18 0.80 0.70 0.02 - 0.08 - 0.11 0.75 D5 1.20 0.18 0.80 0.70 0.02 - - 0.09 0.10 0.77 D6 1.20 0.18 0.80 0.70 0.02 0.09 - - 0.10 0.75 - Elemental values in wt. %. ∗Excess Si values obtained according to calculation methods described herein.
- The alloys were prepared by DC casting the components into ingots and homogenizing the ingots in a two-step homogenization step as described herein. The first step provided nucleation of a maximum amount of fine dispersoids (e.g., dispersoids having a diameter of less than about 10 nm). The second step coarsened the fine dispersoids. The homogenized ingots were then laid down and hot rolled according to the methods as described herein to a 10 mm gauge. The hot band was coiled and cooled and was then cold rolled to a 2 mm gauge. A solution heat treatment step was then performed at 560 °C for 35 seconds. A pre-aging step was performed by heating the sheet to 100 °C and soaking for 1 hour (e.g., to simulate coil cooling as described above), followed by natural aging to achieve the T4 temper. The T6 temper was then achieved by aging the T4 alloys at 200 °C for 30 minutes.
- The properties of the D1 - D6 alloys in T4 temper, including the yield strength, uniform elongation, and bend angle, were determined. Tensile testing was performed according to ASTM B557 in three directions relative to a rolling direction of the alloy sheets to evaluate anisotropic properties that can occur during recrystallization. Yield strength (referred to as "YS" and indicated by histograms) and uniform elongation (referred to as "UE" and indicated by points) are shown in
Figure 1 for a longitudinal direction along the rolling direction (referred to as "L" and indicated by vertical stripes), atransverse direction 90° to the rolling direction (referred to as "T" and indicated by horizontal stripes), and a diagonal direction 45° to the rolling direction (referred to as "D" and indicated by cross-hatching). Evident in the graph based on the yield strength and uniform elongation, the alloys exhibited isotropic behavior in all three directions subjected to tensile testing even with elongated recrystallized grain structures as observed inFigure 2 . The uniform elongation values ranged from 24 % to 26 % and the yield strengths were from 185 MPa to 195 MPa. -
Figure 3 shows the yield strengths and uniform elongations for alloys D1 - D6 in T4 and T6 tempers. For alloys D1 - D6 in T4 temper, the composition had a negligible effect on yield strength and uniform elongation. For alloys D1 - D6 in T6 temper, the composition had a negligible effect on uniform elongation and a decrease in yield strength of about 10 MPa for alloys including V in the composition. The decrease in yield strength can be attributed to solute loss (e.g., Si, Mg, and/or Cu) during solutionizing by heterogeneous nucleation of solute precipitates on V-containing dispersoids. -
Figure 4 is a graph showing bend angle test results for alloys D1 - D6 in a T4 temper. Addition of Cr and V produced a large number of fine dispersoids which improves bending by diffusing strain distribution during deformation (e.g., bending, forming, stamping, or any suitable deformation process). In some cases, Mn combined with Fe and Si to form and spheroidize Fe- constituents, rather than forming dispersoids, due to the high diffusivity of Mn as compared to Zr, Cr, and/or V. Spheroidization of the Fe-constituents improved bending by eliminating elongated (i.e., needle-like) particulates that can initiate cracking during deformation. Additionally, V-containing alloys (e.g., alloys D4 - D6) exhibited improved bending compared to V-free variants due to Fe-constituent spheroidization.Figure 5 compares yield strength (YS) and bend angle (VDA) for alloys D1 - D6 in T4 and T6 tempers. -
Figure 6 shows recrystallization texture components for alloys D1 - D6, including cube, goss, brass, S, Cu, and rotated cube (referred to as "RC"). Each alloy D1 - D6 exhibited a similar distribution of texture components, and composition had a negligible effect on recrystallization texture. Surprisingly, each alloy exhibited a relatively high amount of rotated cube texture, resulting in the significantly improved bending angles shown inFigure 4 andFigure 5 . -
Figure 7 shows transmission electron microscopy (TEM) images of alloys D1 - D6 in T4 temper. Evident in the TEM images is dispersoid formation (shown as bright white particulates) in each alloy. Alloy D4 (including Cr and V) exhibited a higher dispersoid amount due to the relatively low diffusivities of Cr and V. Likewise, alloys D5 and D6 exhibited a lower number of dispersoids due to the relatively higher diffusivities of Mn and Zr. Accordingly, alloy D6 exhibited a lesser amount of dispersoids attributed to an affinity of Mn to be incorporated in Fe-constituents and to not solely form Mn dispersoids.Figure 8 shows dispersoid number density (histograms) and area fraction (open circles) for alloys D1 - D6 in T4 temper. Alloys not containing V (alloys D1 - D3) exhibited similar dispersoid number density. Alloy D2 (incorporating only Cr as a transition metal alloying element) exhibited a higher dispersoid area fraction compared to alloys D1 and D3 (incorporating Mn and Cr (D1) and Zr and Cr (D3)). Alloy D4 (incorporating Cr and V) exhibited the highest dispersoid number density and the highest dispersoid area fraction. -
Figure 9 shows scanning electron microscopy (SEM) images of alloys D1 - D6 in a T4 temper. Evident in the SEM images is Fe-constituent formation (shown as bright white elongated particulates). Each of the alloys D1 - D6 exhibited similar amounts of Fe-constituent formation, and similar Fe-constituent particle size distribution as shown inFigure 10 . As described above, employing transition metal alloying elements reduced the formation of Fe-constituents (e.g., AlFeSi) by replacing a portion of the Fe, thus forming spherical Al(Fe,X)Si constituents. Each alloy continued to exhibit AlFeSi (elongated particulates) due to the presence of excess Si and processing at a low homogenization temperature (e.g., about 500 °C), with a reduced size and size distribution in alloys not employing the transition metal alloying elements. In some aspects, the AlFeSi constituents in alloys not containing the transition metal alloying elements exhibited a larger size than the AlFeSi constituents observed in the alloys containing the transition metal alloying elements. Fe-constituent size and size distribution was evaluated at a depth of about 0.5 mm from a surface of the aluminum alloy sheet (referred to as quarter thickness, indicated "QT" in the graph). -
Figure 11 shows optical microscopy (referred to as "OM") and SEM images of alloy D1. Alloy D1 was subjected to a one-step homogenization after casting, including a thermal ramp of 50 °C per hour to 560 °C, soaked for 2 hours, and subsequently hot rolled, cold rolled, solutionized, pre-aged, and naturally aged as described above. Evident in the OM images is incipient and/or eutectic melting of Mg2Si in alloy D1 (shown as dark areas). SEM images show the dark areas are voids that formed in the alloy during homogenization. Energy dispersive X-ray spectroscopy (EDXS) showed Fe-constituents present in the voids (shown as bright particulates). Employing the exemplary two-step homogenization as described herein can eliminate the incipient and/or eutectic melting when transition metal alloying elements are incorporated in the aluminum alloy compositions. - Various embodiments of the invention have been described in fulfillment of the various objectives of the invention. It should be recognized that these embodiments are merely illustrative of the principles of the present invention. The invention is defined by the appended claims.
Claims (15)
- An aluminum alloy, comprising 0.8 - 1.5 wt. % Si, 0.1 - 0.5 wt. % Fe, 0.5 - 1.0 wt. % Cu, 0.5 - 0.9 wt. % Mg, up to 0.1 wt. % Ti, up to 0.5 wt. % Mn, up to 0.5 wt. % Cr, up to 0.5 wt. % Zr, 0.06 - 0.5 wt. % V, up to 0.15 wt. % impurities, and Al.
- The aluminum alloy of claim 1, comprising 0.9 - 1.4 wt. % Si, 0.1 - 0.35 wt. % Fe, 0.6 - 0.9 wt. % Cu, 0.6 - 0.9 wt. % Mg, 0.01 - 0.09 wt. % Ti, up to 0.3 wt. % Mn, up to 0.3 wt. % Cr, up to 0.3 wt. % Zr, 0.06 - 0.3 wt. % V, up to 0.15 wt. % impurities, and Al.
- The aluminum alloy of claim 1, comprising 1.0 - 1.3 wt. % Si, 0.1 - 0.25 wt. % Fe, 0.7 - 0.9 wt. % Cu, 0.6 - 0.8 wt. % Mg, 0.01 - 0.05 wt. % Ti, up to 0.2 wt. % Mn, up to 0.2 wt. % Cr, up to 0.2 wt. % Zr, 0.06 - 0.2 wt. % V, up to 0.15 wt. % impurities, and Al.
- The aluminum alloy of any of claims 1-3, wherein a combined content of Mn, Cr, Zr, and/or V is at least 0.14 wt. % and preferably,wherein the combined content of Mn, Cr, Zr, and/or V is from 0.14 wt. % to 0.4 wt. % and more preferably,wherein the combined content of Mn, Cr, Zr, and/or V is from 0.15 wt. % to 0.25 wt. %.
- The aluminum alloy of any of claims 1, wherein the aluminum alloy comprises 0.06 - 0.3 wt. % V.
- The aluminum alloy of any of claims 1-5, wherein the aluminum alloy comprises excess Si and wherein an excess Si content is from 0.01 to 1.0.
- An aluminum alloy product, comprising the aluminum alloy according to any of claims 1-6.
- The aluminum alloy product of claim 7, wherein the aluminum alloy product comprises a rotated cube crystallographic texture at a volume percent of at least 5 %.
- The aluminum alloy product of claim 7, wherein the aluminum alloy product comprises dispersoids in an amount of at least 1,500,000 dispersoids per mm2.
- The aluminum alloy product of claim 9, wherein the dispersoids occupy an area ranging from 0.5 % to 5 % of the aluminum alloy product.
- The aluminum alloy product of any of claims 7-10, wherein the aluminum alloy product comprises Fe-constituents and in particular,
wherein the Fe-constituents comprise Al(Fe,X)Si phase particles. - The aluminum alloy product of claim 11, wherein an average particle size of the Fe-constituents is up to 4 µm.
- The aluminum alloy product of any of claims 7-12, wherein the aluminum alloy product comprises a yield strength of at least 300 MPa when in a T6 temper and in particular,
wherein the aluminum alloy product comprises a uniform elongation of at least 20 % and a minimum bend angle of at least 120° when in a T4 temper. - A method of producing an aluminum alloy product, comprising:casting an aluminum alloy according to any of claims 1 to 6 to form a cast article;homogenizing the cast article in a two-stage homogenization process, wherein the two-stage homogenization process comprises heating the cast article to a first stage homogenization temperature and holding the cast article at the first stage homogenization temperature for a period of time and further heating the cast article to a second stage homogenization temperature and holding the cast article at the second stage homogenization temperature for a period of time;hot rolling and cold rolling to provide a final gauge aluminum alloy product;solution heat treating the final gauge aluminum alloy product; andpre-aging the final gauge aluminum alloy product.
- The method of claim 14, wherein the first stage homogenization temperature is from 470 °C to 530 °C and the second stage homogenization temperature is from 525 °C to 575 °C, and wherein the second stage homogenization temperature is higher than the first stage homogenization temperature.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762575573P | 2017-10-23 | 2017-10-23 | |
| PCT/US2018/057054 WO2019083969A1 (en) | 2017-10-23 | 2018-10-23 | High-strength, highly formable aluminum alloys and methods of making the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3676410A1 EP3676410A1 (en) | 2020-07-08 |
| EP3676410B1 true EP3676410B1 (en) | 2023-08-09 |
Family
ID=64110304
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP18797512.3A Active EP3676410B1 (en) | 2017-10-23 | 2018-10-23 | High-strength, highly formable aluminum alloys and methods of making the same |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20190119799A1 (en) |
| EP (1) | EP3676410B1 (en) |
| JP (3) | JP2020537039A (en) |
| CN (1) | CN111247260A (en) |
| ES (1) | ES2955293T3 (en) |
| MX (1) | MX2020003528A (en) |
| WO (1) | WO2019083969A1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BR112022012186A2 (en) * | 2019-12-23 | 2022-09-13 | Alcoa Usa Corp | HIGH STRENGTH 6XXX EXTRUSION ALLOYS |
| CN111876700B (en) * | 2020-06-08 | 2021-12-03 | 中南大学 | Heat treatment process of powder metallurgy aluminum alloy cold-rolled sheet |
| CA3205192A1 (en) * | 2021-01-29 | 2022-08-04 | Lynette M. Karabin | New 6xxx aluminum alloys |
| CN116179904A (en) * | 2022-10-13 | 2023-05-30 | 烟台南山学院 | Aluminum alloy plate with low Sc and low Zn/Mg ratio and aging process thereof |
Family Cites Families (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4614552A (en) | 1983-10-06 | 1986-09-30 | Alcan International Limited | Aluminum alloy sheet product |
| JPH06136478A (en) * | 1992-10-23 | 1994-05-17 | Kobe Steel Ltd | Baking hardening type al alloy sheet excellent in formability and its production |
| JP2626958B2 (en) * | 1993-03-16 | 1997-07-02 | スカイアルミニウム株式会社 | Method for producing aluminum alloy sheet excellent in formability and bake hardenability |
| US5522950A (en) * | 1993-03-22 | 1996-06-04 | Aluminum Company Of America | Substantially lead-free 6XXX aluminum alloy |
| JPH07166285A (en) * | 1993-06-08 | 1995-06-27 | Shinko Alcoa Yuso Kizai Kk | Hardened al alloy sheet by baking and production thereof |
| JPH07252570A (en) * | 1994-03-17 | 1995-10-03 | Kobe Steel Ltd | Al-mg-si alloy sheet for automobile panel |
| JP3351087B2 (en) * | 1994-03-17 | 2002-11-25 | 株式会社神戸製鋼所 | Manufacturing method of Al-Mg-Si alloy plate |
| JPH0978210A (en) * | 1995-09-07 | 1997-03-25 | Mitsubishi Materials Corp | Production of vehicle wheel made of aluminum alloy and vehicle wheel |
| JPH09202933A (en) * | 1996-01-25 | 1997-08-05 | Nippon Steel Corp | High strength aluminum alloy excellent in hardenability |
| JPH11350058A (en) * | 1998-06-12 | 1999-12-21 | Shinko Alcoa Yuso Kizai Kk | Aluminum alloy sheet excellent in formability and baking hardenability and its production |
| JP2000282163A (en) * | 1999-03-30 | 2000-10-10 | Kobe Steel Ltd | Al-Mg-Si ALLOY SHEET EXCELLENT IN BULGE FORMABILITY AND BENDABILITY |
| JP4045326B2 (en) * | 1999-11-09 | 2008-02-13 | 株式会社神戸製鋼所 | Al-Mg-Si Al alloy plate with excellent press formability |
| JP2001262265A (en) * | 2000-03-22 | 2001-09-26 | Kobe Steel Ltd | Hot rolling stock of high formability aluminum alloy sheet |
| JP4603134B2 (en) * | 2000-07-21 | 2010-12-22 | 株式会社神戸製鋼所 | Aluminum alloy sheet for transport panel materials with excellent surface gloss |
| JP4708555B2 (en) * | 2000-12-13 | 2011-06-22 | 株式会社神戸製鋼所 | Continuous solution quenching method for rolled aluminum alloy sheets with excellent formability and flatness |
| JP2003268475A (en) * | 2002-03-12 | 2003-09-25 | Sky Alum Co Ltd | Aluminum alloy sheet for forming, and manufacturing method therefor |
| BR0312098A (en) * | 2002-06-24 | 2005-03-29 | Corus Aluminium Walzprod Gmbh | Method for the production of high strength balanced al-mg-si alloy and weldable alloy product |
| JP2004211177A (en) * | 2003-01-07 | 2004-07-29 | Nippon Steel Corp | Aluminum alloy sheet superior in formability, paint baking hardenability and shape, and manufacturing method therefor |
| JP2004211176A (en) * | 2003-01-07 | 2004-07-29 | Nippon Steel Corp | Aluminum alloy sheet superior in formability, paint baking hardenability and corrosion resistance, and manufacturing method therefor |
| TW200536946A (en) * | 2003-12-11 | 2005-11-16 | Nippon Light Metal Co | Method for producing Al-Mg-Si alloy excellent in bake-hardenability and hemmability |
| JP2007169740A (en) * | 2005-12-22 | 2007-07-05 | Kobe Steel Ltd | Aluminum alloy sheet having excellent formability and its production method |
| JP4939093B2 (en) * | 2006-03-28 | 2012-05-23 | 株式会社神戸製鋼所 | Method for producing 6000 series aluminum alloy plate for automobile panel having excellent hem bendability and bake hardness |
| JP4944474B2 (en) * | 2006-03-30 | 2012-05-30 | 株式会社神戸製鋼所 | Aluminum alloy plate excellent in stretch flangeability and manufacturing method thereof |
| JP2009173973A (en) * | 2008-01-22 | 2009-08-06 | Kobe Steel Ltd | Aluminum alloy sheet having excellent ridging mark property upon forming |
| JP2009173972A (en) * | 2008-01-22 | 2009-08-06 | Kobe Steel Ltd | Aluminum alloy sheet having excellent ridging mark property upon forming |
| JP5203772B2 (en) * | 2008-03-31 | 2013-06-05 | 株式会社神戸製鋼所 | Aluminum alloy sheet excellent in paint bake hardenability and suppressing room temperature aging and method for producing the same |
| JP5385025B2 (en) * | 2009-06-18 | 2014-01-08 | 株式会社神戸製鋼所 | Aluminum alloy wire rod for high-strength bolt excellent in formability and manufacturing method thereof, high-strength flange bolt and manufacturing method thereof |
| JP5400510B2 (en) * | 2009-07-15 | 2014-01-29 | 株式会社Uacj | Aluminum alloy sheet for forming with excellent deep drawability and bending workability |
| CN101643869B (en) * | 2009-09-04 | 2011-04-06 | 河池学院 | High strength automobile aluminium alloy wheel rim |
| JP5758676B2 (en) * | 2011-03-31 | 2015-08-05 | 株式会社神戸製鋼所 | Aluminum alloy plate for forming and method for producing the same |
| CN102337429B (en) * | 2011-08-18 | 2013-12-25 | 苏州有色金属研究院有限公司 | High-strength Al-Mg-Si-Cu alloy and preparation method thereof |
| CN103757507B (en) * | 2014-02-25 | 2016-04-27 | 北京科技大学 | A kind of automobile body outer board high bake hardening aluminum alloy materials and preparation method thereof |
| JP5901738B2 (en) * | 2014-03-27 | 2016-04-13 | 株式会社神戸製鋼所 | Aluminum alloy forging and method for producing the same |
| CN104630556B (en) * | 2015-02-06 | 2016-08-17 | 中南大学 | High anti-corrosion CuNiSiNbSn series elastic copper alloy of a kind of ultra-high-strength/tenacity and preparation method thereof |
| JP6445958B2 (en) * | 2015-12-14 | 2018-12-26 | 株式会社神戸製鋼所 | Aluminum alloy forgings for automobiles |
| CN108474066A (en) * | 2015-12-18 | 2018-08-31 | 诺维尔里斯公司 | High intensity 6XXX aluminium alloys and its manufacturing method |
| JP2017155251A (en) * | 2016-02-29 | 2017-09-07 | 株式会社神戸製鋼所 | Aluminum alloy forging material excellent in strength and ductility and manufacturing method therefor |
| WO2017170835A1 (en) * | 2016-03-30 | 2017-10-05 | 株式会社神戸製鋼所 | Aluminum alloy sheet and aluminum alloy sheet manufacturing method |
-
2018
- 2018-10-23 EP EP18797512.3A patent/EP3676410B1/en active Active
- 2018-10-23 WO PCT/US2018/057054 patent/WO2019083969A1/en unknown
- 2018-10-23 CN CN201880068803.7A patent/CN111247260A/en active Pending
- 2018-10-23 JP JP2020519667A patent/JP2020537039A/en active Pending
- 2018-10-23 ES ES18797512T patent/ES2955293T3/en active Active
- 2018-10-23 US US16/168,146 patent/US20190119799A1/en active Pending
- 2018-10-23 MX MX2020003528A patent/MX2020003528A/en unknown
-
2022
- 2022-08-30 JP JP2022136728A patent/JP2022172234A/en active Pending
-
2023
- 2023-10-23 JP JP2023181583A patent/JP2024010058A/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| JP2024010058A (en) | 2024-01-23 |
| CN111247260A (en) | 2020-06-05 |
| WO2019083969A1 (en) | 2019-05-02 |
| ES2955293T3 (en) | 2023-11-29 |
| EP3676410A1 (en) | 2020-07-08 |
| JP2020537039A (en) | 2020-12-17 |
| JP2022172234A (en) | 2022-11-15 |
| US20190119799A1 (en) | 2019-04-25 |
| MX2020003528A (en) | 2020-07-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3676410B1 (en) | High-strength, highly formable aluminum alloys and methods of making the same | |
| EP2635720B1 (en) | Formed automotive part made from an aluminium alloy product and method of its manufacture | |
| CN102498229B (en) | Almgsi strip for applications having high plasticity requirements | |
| EP3827108B1 (en) | Highly formable, recycled aluminum alloys and methods of making the same | |
| JP2006118047A (en) | Aluminum alloy suitable for use for automotive body and process for fabricating aluminum alloy rolled sheet | |
| CA3068470C (en) | High performance aluminum alloys having high amounts of recycled material and methods of making the same | |
| CA3110293C (en) | Rapidly aged, high strength, heat treatable aluminum alloy products and methods of making the same | |
| EP3662091A1 (en) | 6xxxx-series rolled sheet product with improved formability | |
| JPH0747807B2 (en) | Method for producing rolled aluminum alloy plate for forming | |
| EP3555332B1 (en) | High strength and highly formable aluminum alloys resistant to natural age hardening and methods of making the same | |
| US10704128B2 (en) | High-strength corrosion-resistant aluminum alloys and methods of making the same | |
| US6344096B1 (en) | Method of producing aluminum alloy sheet for automotive applications | |
| WO2018206696A1 (en) | Method of manufacturing an al-si-mg alloy rolled sheet product with excellent formability | |
| WO2021168064A1 (en) | Control of aluminum alloy microstructure for improved corrosion resistance and bonding performance | |
| JP2004238657A (en) | Method of manufacturing aluminum alloy plate for outer panel | |
| EP3652356B1 (en) | High-strength corrosion-resistant aluminum alloy and method of making the same | |
| JP3208234B2 (en) | Aluminum alloy sheet for forming process excellent in formability and method for producing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: UNKNOWN |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20200331 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| TPAC | Observations filed by third parties |
Free format text: ORIGINAL CODE: EPIDOSNTIPA |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20230329 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230524 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602018055088 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20230920 Year of fee payment: 6 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2955293 Country of ref document: ES Kind code of ref document: T3 Effective date: 20231129 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20230809 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231110 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20231117 Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231209 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231211 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231109 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231209 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20231110 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: TR Payment date: 20231013 Year of fee payment: 6 Ref country code: IT Payment date: 20231204 Year of fee payment: 6 Ref country code: FR Payment date: 20231023 Year of fee payment: 6 Ref country code: DE Payment date: 20231018 Year of fee payment: 6 Ref country code: AT Payment date: 20231019 Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230809 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20231023 Year of fee payment: 6 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 602018055088 Country of ref document: DE Representative=s name: WEICKMANN & WEICKMANN PATENT- UND RECHTSANWAEL, DE |