EP3290501B1 - Compositions detergentes contentant des amides d'acides gras alcoxylés - Google Patents
Compositions detergentes contentant des amides d'acides gras alcoxylés Download PDFInfo
- Publication number
- EP3290501B1 EP3290501B1 EP17020387.1A EP17020387A EP3290501B1 EP 3290501 B1 EP3290501 B1 EP 3290501B1 EP 17020387 A EP17020387 A EP 17020387A EP 3290501 B1 EP3290501 B1 EP 3290501B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fatty acid
- rco
- surfactant
- cleaners
- carbon atoms
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/38—Cationic compounds
- C11D1/65—Mixtures of anionic with cationic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/38—Cationic compounds
- C11D1/52—Carboxylic amides, alkylolamides or imides or their condensation products with alkylene oxides
- C11D1/526—Carboxylic amides (R1-CO-NR2R3), where R1, R2 or R3 are polyalkoxylated
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/835—Mixtures of non-ionic with cationic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/86—Mixtures of anionic, cationic, and non-ionic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D10/00—Compositions of detergents, not provided for by one single preceding group
- C11D10/04—Compositions of detergents, not provided for by one single preceding group based on mixtures of surface-active non-soap compounds and soap
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/02—Anionic compounds
- C11D1/04—Carboxylic acids or salts thereof
- C11D1/06—Ether- or thioether carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/662—Carbohydrates or derivatives
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/74—Carboxylates or sulfonates esters of polyoxyalkylene glycols
Definitions
- compositions containing at least one glycolipid biosurfactant and at least one alkoxylated fatty acid amide based on fatty acids from vegetable oils and have an exceptionally high proportion of long-chain ( ⁇ C17), mostly unsaturated hydrocarbon chains.
- Another object is the use as detergents and cleaners.
- the present invention is directed to an agent having improved cleaning performance, especially carbohydrate-containing soil and stains, as well as a method for improving the cleaning performance of a detergent and cleaning agent and the use of the agent.
- animal oils and fats in particular beef tallow, have always been used for cleaning agents. Due to the fatty acid composition, these can only be used to a limited extent in certain applications. From a consumer point of view, animal raw materials are often undesirable for hygienic (e.g., TSE-problematic) and ideological (e.g., vegan-trend) reasons. Furthermore, vegetable soap has been used for millennia for washing and cleaning purposes, whose application is also limited due to the formation of lime soap and binding to an alkaline pH.
- the challenge is to dispense with oil, animal fats and oils, as well as palm oils (ie palm oil, palm kernel oil, coconut oil, babassu oil) as the source of fatty acids and instead to the greatest possible extent surfactants from less problematic sources, such as vegetable oils from European Use cultivation or fermentation.
- palm oils ie palm oil, palm kernel oil, coconut oil, babassu oil
- Glycolipid biosurfactants which can be produced by fermentation from a wide variety of substrates, meet the requirements of sustainability and are also characterized by low skin irritation potential and toxicity.
- synthetic glycolipids e.g. Alkyl polyglycosides, sorbitan esters, methyl glycoside esters or methyl glucamides
- biosurfactant glycolipids are produced via microorganisms and no chemical reaction steps are necessary. However, for many applications it is necessary to combine glycolipid biosurfactants with other surfactants to obtain optimal cleaning performance.
- the state of the art is combined with lauric acid-based surfactants of palm or petrochemical origin; usually with anionic sulfur-containing surfactants such as sodium lauryl sulfate or sodium lauryl ether sulfate.
- the prior art discloses combinations of glycolipid biosurfactants with anionic and nonionic non-glycolipidic surfactants having alkyl chain lengths of C8-C18
- DE19600743 (Henkel) discloses mixtures of glucoselipids and sophorolipids with different surfactants for dishwashing detergents.
- combinations are disclosed with lauric acid-based surfactants for synergistic enhancement in rinse performance, dispersancy and foaming power.
- WO2011051161A1 (Henkel) discloses low-residue hard surface cleaners containing a glycolipid biosurfactant and a solvent.
- the examples disclose surfactant combinations with C12-C14 surfactants.
- EP 0 499434 A1 (Unilever): Combination of at least one glycolipid biosurfactant and at least one non-glycolipidic anionic or nonionic surfactant, one each of the surfactants being in the micellar and one in the lamellar phase.
- the disclosed laundry detergents are characterized by an increased oil-dissolving power of the textiles.
- EP 1 445 302 A1 / US 2014113818 (Ecover): combination of at least one glycolipid biosurfactant and at least one non-glycolipidic surfactant, each in the micellar phase. All solutions presented contain short-chain, saturated hydrocarbon chains as the hydrophobic part of the surfactants. The presence of the biosurfactant and the further surfactant in the micellar phase achieves an increased average cleaning performance on standard or mineral oil with little foaming.
- JP 2009275145 discloses blends for skin cleansing with sophorolipids in combination with soaps. By combining soaps with glycolipids, a better washability of the soap is achieved.
- WO 2012617815 discloses blends based on concentrated sophorolipid blends of 70-99% which are hydrolyzed with other C12 fatty acid based surfactants and mixed with sodium cocoamphoacetate as cosurfactant to reinforce the foam.
- WO 2016050439 discloses biosurfactant formulations having improved foaming and fatliquoring properties containing at least one surfactant selected from the group of betaines, alkoxylated fatty alcohol sulfates and alkylamine oxides.
- WO 2016/066464 discloses detergents containing mannosylerythritol for greasy and oily stains in combination with C12-C14 and C12-C18 ether sulfates and ethoxylated alcohols, respectively.
- US 5520839 is the combination of biosurfactants with anionic surfactants including rape soap for improved oil and fat solubility.
- WO 2013098066 (Evonik) discloses aqueous hair and skin cleansing compositions containing biosurfactants and oleic acid which have a sensory positive effect on the skin. All solutions presented contain short-chain, saturated hydrocarbon chains (C8-C12) as the hydrophobic part of the surfactants.
- C12-C18 surfactants based on sulfur compounds are used as anionic surfactants.
- Examples of disclosed combinations contain, for example, sulfates, sulfonates, isethionates, sulphosuccinates, inter alia, in particular lauryl ether sulphates and lauryl sulphonates.
- the complex technical problem of the invention has been to identify one or more surfactants based on vegetable oil which can be combined with biosurfactant glycolipids.
- the fatty acids of the surfactants should not be obtained from coconut, palm, babassu or palm kernel vegetable oils due to sustainability considerations. This is technically demanding insofar as the desired surfactants instead of lauric a high proportion of unsaturated, long fatty acid residues ⁇ C18, which bring completely new properties such as solubility, stability, wettability, compatibility, etc. with it.
- the novel surfactant combinations should have a good cleaning performance, even beyond grease and oil soiling; ie in particular a high cleaning performance on specific stains such as carbohydrate stains or color stains.
- compositions according to the invention should preferably be based to the greatest possible extent on natural raw materials and be readily biodegradable.
- compositions according to the invention of claims 1-6, 10-11 show a cleaning action on specific soils which can not be foreseen by the person skilled in the art in any way. This allows the production of environmentally friendly agents, even for stubborn soils such as stains or carbohydrate impurities.
- the agents according to the invention act synergistically on the dissolution of carbohydrate spots. It is understood synergistically that the stain-dissolving power of the mixture is higher than the sum of the stain-dissolving power of the individual components. In the enzyme-free embodiment, a comparable cleaning performance is achieved to enzyme-containing market products.
- fatty acid alkyl esters of C-18 plants of the invention are as additional ingredient fatty acid alkyl esters of C-18 plants of the invention.
- washing and cleaning agents also include washing aids which are added to the actual agent during manual or mechanical cleaning.
- detergents within the scope of the invention also include pre- and post-treatment agents, ie those agents which are used before the actual cleaning, for example for dissolving stubborn soiling.
- a special product form solid substrates, such as cloths. These are soaked in a preparation and have the advantage that in them the preparation is already prescribed in the correct dosage. This meets in particular the consumer's desire of convenience, they are easy to handle, to use directly without additional steps and can also on the go, eg when traveling well, even if no water is available.
- Cloths are made of textiles which may be woven, knitted or knitted or present as a composite in nonwoven, paper, wadding or felt, nonwovens being mostly made of polypropylene, polyester or viscose.
- Agent impregnated substrates and wipes can be made in a variety of ways, including the dipping, wiping and spraying processes. The latter is used in particular for non or low foaming preparations.
- the agent according to the invention can be used over the entire pH range, thus allowing a broad range of products.
- compositions according to the invention can be thickened with thickeners on a natural basis, such as, for example, xanthan gum. It is a known technical problem to thicken formulations containing glycolipid biosurfactants ( WO / 2014166796 ).
- compositions according to the invention are distinguished by a high stability.
- agent according to the invention is also disclosed in a preservative-free embodiment.
- a further advantage of the invention is that the agents can be prepared without sulfur surfactants and thus take care of the trend "sulphate-free".
- Another advantage of the invention is that the agent can be prepared without the irritating ingredients cocamide MEA or cocamide DEA.
- Another advantage of the invention is that, depending on the application, the novel compositions low foaming, for example for machine application or in combination with a third surfactant (D) can be produced foaming.
- the vegetable oils from oil palms, babassu, palm kernels, or coconuts clearly differ in the fatty acid composition of the inventive C-18 vegetable oils:
- the following vegetable oils, fats, waxes or resins are referred to as C-18 vegetable oil:
- the C-18 vegetable oils are natural triglycerides.
- C-18 vegetable oils have a mixture of saturated and unsaturated fatty acids, the fatty acid distribution of fatty acids having 18 or more carbon atoms being more than 60% by weight, more preferably more than 72% by weight and most preferably more than 77% by weight. % and wherein the proportion of unsaturated fatty acids over 55 wt .-%, preferably over 65 wt .-% and particularly preferably over 72 wt .-% is.
- the proportion of fatty acids having 16 and fewer carbon atoms is preferably less than 30% by weight, preferably less than 27% by weight and more preferably less than 17% by weight.
- the C-18 vegetable oils contain a proportion of ⁇ 0.5%, particularly preferably> 0.05% fatty acids having 6 carbon atoms.
- the C-18 vegetable oils contain a proportion of ⁇ 75% by weight of hydroxy fatty acids, preferably ⁇ 25% by weight, particularly preferably ⁇ 5% by weight.
- C-18 vegetable oils contain saturated or unsaturated fatty acids having 20 or more carbon atoms, the content of which may be up to 96% by weight.
- the C-18 vegetable oils preferably contain less than 95% by weight of oleic acid, more preferably less than 85% by weight of oleic acid Wt .-% here in each case based on the total content of fatty acids in vegetable oil.
- C-18 vegetable oils can be obtained, which meet the technical characteristics of fatty acid compositions for the inventive compositions, and Amaranth, aniseed, apple, apricot, argan, arnica, avocado, cotton, borage, stinging nettle, broccoli, canola, chia, hemp, hazelnut, beech, boxwood, thistle, spelled, peanut, tigernut, lilac, garden cress , Barley, Pomegranate, Oats, Hemp, Hazelnut, Blueberry, Elderberry, Jasmine, Currant, St.
- the oil is selected from the group: apricot, avocado, cotton, broccoli, beech, thistle, spelled, tigernut, barley, hemp, hazelnut, jojoba, cherry, mullein, Krambe, cross-leaved spurge, pumpkin, Iberian scorpionfish, camelina, linseed , Lupine, alfalfa, macademia, almond, corn, poppy, evening primrose, olive, oil radish, oil raven, peach, rape, rice, marigold, turnip rape, safflower, sage, sea buckthorn, black cumin, sesame, sesame leaf, mustard, sunflower, soy, tobacco , Walnut, grape and wheat, and their combinations.
- apricot avocado, cotton, broccoli, beech, thistle, spelled, tigernut, barley, hemp, hazelnut, jojoba, cherry, mullein, Krambe, cross-leaved spurge
- the oil is selected from the group apricot, thistle, tigernut, hemp, Krambe, Iberian dragon head, camelina, linseed, lupine, alfalfa, corn, almond, olive, oil radish, peach, rapeseed, turnip rape, sesame, sesame leaf, sunflower , Soy, grape and wheat, as well as their combinations.
- oils is used in this invention as representative of fats, waxes and resins.
- fatty acids or fatty alcohol or their derivatives are not stated otherwise - representative of branched or unbranched, saturated, mono- or polyunsaturated carboxylic acids or alcohols or derivatives thereof having preferably 6 to 24 carbon atoms.
- Surfactant in the context of this invention is understood to mean amphiphilic organic substances having surface-active properties which adsorb to the interface between two liquids, such as oil and water, and have the ability to reduce the surface tension of water.
- surfactants tend to self-aggregate and form structures such as micelles, lamellar structures, and the like.
- emulsifiers are included within the concept of surfactants, but not vice versa.
- PEGylated vegetable oils are ethoxylated vegetable oils as defined in " Safety Assessment of PEGylated Oils as Used in Cosmetics ", International Journal of Toxicology November / December 2014, 33 ,
- the terminology used in cosmetic ingredients which describes the etherification and esterification products of glycerides and fatty acids with ethylene oxide is used.
- representatives derived from C-18 plants are preferred; Examples are listed among the surfactants (D).
- PEGylated fatty acid glycerides are mono-, di- and / or triglycerides which have been modified with a specific number of alkylene glycol units, mostly ethylene glycol units, and may contain reaction by-products.
- PEGylated fatty acid glycerides are defined as in " Safety Assessment of PEGylated Alkyl Glycerides as Used in Cosmetics ", Cosmetic Ingredient Review (CIR) 2014 , It should be noted that CIR under "Alkyl” also takes into account unsaturated fatty acids.
- CIR Cosmetic Ingredient Review
- sulfuric surfactants is understood as meaning anionic or amphoteric surfactants having a sulfur-containing hydrophilic radical, such as e.g. Alkyl sulfates, alkyl ether sulfates, (alkoxylated) sulfosuccinates, (alkoxylated) sulfonates, (alkoxylated) isethionates, (alkoxylated) taurates, sulfobetaines and sultaines.
- a sulfur-containing hydrophilic radical such as e.g. Alkyl sulfates, alkyl ether sulfates, (alkoxylated) sulfosuccinates, (alkoxylated) sulfonates, (alkoxylated) isethionates, (alkoxylated) taurates, sulfobetaines and sultaines.
- sulphate-containing surfactants are sodium laureth sulphates, sodium lauryl sulphates, ammonium laureth sulphates, ammonium lauryl sulphates, sodium myreth sulphates, sodium coco sulphates, sodium trideceth sulphates or MIPA laureth sulphates.
- biosurfactant is the biosurfactant glycolipid defined in accordance with the invention.
- Free of sulfuric surfactants, phosphates, phosphonates means that the formulation does not contain significant amounts of sulfur surfactants, phosphates, phosphonates. In particular, this is understood to mean that sulfur surfactants, phosphates, phosphonates in each case in amounts of less than 0.1 wt .-%, preferably less than 0.01 wt .-% based on the total formulation, in particular no detectable amounts are included.
- At least one refers to 1 or more, for example, 1, 2, 3, 4, 5, 6, 7, 8, 9 or more.
- Synergy is understood when the combination of the individual components shows a better effect than any single component alone, each at the same concentration.
- washing and cleaning agent is understood to mean a means for removing undesired soiling or deposits, such as For example, stains, residues, impurities, metabolic products of biological processes of natural or biological surfaces, hard surfaces, as well as textiles, carpets or natural fibers.
- the agents may be applied to the substrate to be cleaned by rubbing, dosing, spraying, foaming and other methods (e.g., anointing, applying, etc.) directly or through an aid such as a wipe, diluted or undiluted.
- cleaning performance or “detergency” is meant in the context of this invention the removal of one or more stains.
- the distance can be metrologically detected or visually assessed by brightening or reducing soiling.
- the HLB (hydrophilic-lipophilic balance) value is a measure of the hydrophilicity or lipophilicity of a substance, usually a nonionic surfactant.
- the value can theoretically be measured as described in relevant literature (e.g., by the Griffin method) or experimentally by comparing the solubility behavior of standard compositions with known HLB.
- a first object of the invention is directed to an agent containing at least one alkoxylated surfactant (A) from the group of alkoxylated fatty acid amides (I) and at least one glycolipid biosurfactant (B) comprising rhamnolipids, sophorolipids, trehaloselipids, mannosylerythritolollipids, and cellobioselipids.
- A alkoxylated surfactant
- B glycolipid biosurfactant
- fatty acid amides of the formula (I) with an HLB> 10.5 and ⁇ 12.0.
- ethoxylated fatty acid amide based on rapeseed oil is highly preferred according to the invention: amides, rape oil, N - (hydroxyethyl), ethoxylated; INCI name: PEG-4 rapeseed amide, or rapeseed amide or PEG-4 rapeseedamide. Trade name: Amidet® N from Kao.
- the alkoxylated surfactant (A) is based on a mixture of fatty acid derivatives based on C18 vegetable oils with different chain length and degree of saturation.
- the mixture preferably follows the fatty acid distribution in the native oil or as obtained in the reaction of naturally occurring vegetable oils or fats.
- fatty acid ester or fatty acid amide mixtures for the synthesis of the surfactant class - as they occur in the conversion of naturally occurring vegetable oils or fats - the surfactants can be produced inexpensively, resource-efficiently and in an environmentally friendly manner. Additional purification methods, such as the separation of the fatty acids, or fatty acid esters by fractional distillation or additional synthesis steps, such. to the fatty alcohol, are not needed here.
- the surfactant mixtures used show an increased cleaning performance.
- compositions preferably contain from 0.1 to 50% by weight of one or more alkoxylated surfactants (A), more preferably from 0.1 to 25% by weight, particularly preferably from 0.1 to 10% by weight and very particularly preferably from 0.25% by weight to 5% by weight. -%. Wt .-% based on the total agent.
- alkoxylated surfactants A
- Biosurfactants are generally surfactants of biological origin. They do not undergo chemical reaction such as the synthetic glycolipids e.g. Alkyl polyglycosides (APG). Biosurfactants can be found in living organisms, or arise in the cultivation of various microorganisms such as e.g. Fungi, yeasts, viruses, bacteria or enzymes.
- APG Alkyl polyglycosides
- glycolipid biosurfactants can be obtained directly by the use of natural raw materials by microbiological processes, while the preparation of synthetic glycolipids usually further chemical steps, such as the reduction of fatty acid to fatty alcohol caused.
- glycolipid biosurfactants By biologically modifying the sugar moiety, glycolipid biosurfactants have specific properties, such as those skilled in the art, of good fat dissolving power.
- the structure of the glycolipid biosurfactants and the chain lengths of the hydrophobic part varies depending on the microorganism or substrate used.
- glycolipid biosurfactants (B) include rhamnolipids, in particular mono-, di- or poly-pyrnolipids, sophorolipids in their acid or lactone form or as their mixtures, diacetylated, acetylated or non-acetylated, trehaloselipids, mannosylerythritolollipids and cellobioselipids.
- Suitable substrates for glycolipid biosurfactants are a wide variety of carbon sources known from the literature, such as vegetable oils and the glycerides or fatty acid methyl esters, fatty acids, fatty alcohols, fatty acid methyl esters or ethyl esters, carbohydrates, eg cellulose, glucose, starch, C4 sources such as succinate, Butane, butyric acid, C1 sources such as CO 2 , CO or methane, oil and / or carbohydrate-containing wastewater from refineries or the food industry and mixtures thereof.
- carbon sources known from the literature, such as vegetable oils and the glycerides or fatty acid methyl esters, fatty acids, fatty alcohols, fatty acid methyl esters or ethyl esters, carbohydrates, eg cellulose, glucose, starch, C4 sources such as succinate, Butane, butyric acid, C1 sources such as CO 2 , CO or methane, oil and / or carbohydrate-containing wastewater from refineries or the food industry
- Preferred microorganisms for producing the biosurfactants are Bacillus, Candida, Pseudomonas, Trichosporan and / or Pseudozyma strains and others as described in the relevant literature, in particular Bacillus licheniformis, Bacillus subtilis, Burkholderia, Candida apicola, Candida antarctica, Candida batistae, Candida bogoriensis, Candida bombicola , Candida sp., Cryptococcus humicola, Gordonia, Mycobacterium tuberculosis, Nocardia cornynebacterium, Pichia anomala, Pseudomonas aeruginosa, Pseudozyma aphidis (MEL), Pseudomonas sp, Pseudozyma antarctica (MEL), Pseudozyma parantarctica, Pseudozyma fusiformata, Rhodococcus,
- the glycolipid biosurfactants can, for. B. as in EP 0 499 434 . US 7,985,722 . WO 03/006146 . JP 60-183032 . DE 19648439 . DE 19600743 . JP 01-304034 . CN 1337439 . JP 2006 - 274233 . KR 2004033376 . JP 2006-083238 . JP 2006-070231 . WO 03/002700 . FR 2740779 . DE 2939519 . US 7,556,654 . FR 2855752 . EP 1445302 . JP 2008-062179 and JP 2007-181789 , Mannosylerythritol: WO 2004/020647 . JP 20042544595 , or the writings cited therein.
- Rhamnolipids are accessible via organisms such as Pseudomonas and Burkholderia, both wild types.
- Commercially available rhamnolipids are available from Evonik under the trade name Rewoferm®, The Gene Biotech or from Jeneil Biosurfactant Co.LLC.
- Particularly preferred in this invention are the rhamnolipids from Evonik Rewoferm® and JBR 425 from Jeneil Biosurfactant, in particular JBR 425.
- sophorolipids may contain impurities from the manufacturing process, such as fatty alcohols, fatty acids, fatty acid esters, triglycerides or oils, sugars, especially glucose, sophorose, or organic acids.
- the biosurfactant can be structurally modified, e.g.
- the ratio of lactone form to free acid can be controlled by the substrate.
- Examples of preferred substrates for sophorolipids from the literature are corn oil ( EP 0282942 ), Oil of C22 fatty acids ( KR20100022289 ), Olive oil ( ES 2018637 . CN 1431312 ), Rapeseed oil or esters ( CN 102250790 . US2008032383 ), Sunflower or rapeseed fatty acid esters ( DE4319540 . FR 2692593 ) or waste oils or fats ( JP 2004254595 . JP 2007252279 . CN 101845468 . CN 101948786 ).
- Sophorolipids are accessible by a variety of different organisms known in the art such as Candida bombicola, Starmerella or Wickerhamiella.
- sophorolipids having the trade names Sophogreen, Sophoclean, Soliance S.
- Mannosylerythritol lipids can be obtained by Pseudozyma Antarctica NBRC 10736 in a nutrient medium with soybean oil, alternatively they are accessible by the fungus Ustilago maydis.
- Tetralose dimycolates, tetralose monomycolates, succinyl trehalose lipids and trehalose tetraesters or mixtures thereof are preferably suitable according to the invention.
- Trehaloselipids can be obtained by literature methods by Rhodococcus erythropolis, Arthobacter spec., Gordonia, Mycobacterium tuberculosis, Nocardia cornynebacterium spec. or Tsukamurella spec. to be obtained.
- the agents preferably contain 0.1 to 50 wt .-% of one or more glycolipid biosurfactants, more preferably 0.1 to 25 wt .-%, particularly preferably 0.1 to 10 wt .-% and most preferably 0.3 to 8 wt .-%, based on the total agent.
- alkoxylated surfactant (s) (A) and glycolipid biosurfactant (s) (B) may be combined in any proportion.
- the surfactants (A) to the glycolipid biosurfactants (B) in a ratio between (A): (B) ⁇ 10: 1 and ⁇ 1: 10 before and very particularly preferably the surfactants (A) are the glycolipid -Biotensiden (B) in a ratio between (A): (B) ⁇ 2: 1 and ⁇ 1: 6 before, most preferred is (A): (B) ⁇ 1: 1 and ⁇ 1: 6; in each case based on the weight percent active content in the entire medium.
- Another object of the invention is directed to an agent additionally containing at least one soap or fatty acid (C), and / or one or more alkoxylated surfactants (D) selected from the group of alkoxylated fatty acid esters, the alkoxylated Fettklareglyceridester, or the alkoxylated vegetable oil esters and mixtures the same.
- C soap or fatty acid
- D alkoxylated surfactants
- the agent additionally contains at least one soap (C) as anionic surfactant.
- Soaps are alkali or ammonium salts of saturated or unsaturated fatty acids of 6 to 24 carbon atoms.
- the surfactant (A) and glycolipid biosurfactant (B) agents may be additionally combined with a soap (C) at neutral or alkaline pH to further improve the overall result, with high carbohydrate or color stain cleaning power.
- the addition of soap has positive effects on the cleaning performance, depending on the embodiment.
- Exemplary representatives of the products useful in the present invention are sodium olive oil soaps, potassium rapeseed oil soaps, ammonium thistle oil soaps, sodium linseed oil soaps, sodium sunflower oil soaps, sodium soybean oil soaps, linseed fatty acids, olive oil fatty acids and others.
- the soaps are present as a mixture according to the fatty acid distribution in the native oil or as obtained in the reaction of naturally occurring vegetable oils or fats.
- the surfactant or surfactants (C) may be combined in any ratio with (A) and (B) and optionally (D).
- the surfactants (C) are present in the surfactant (A) + glycolipid-biosurfactant (B) mixture in a ratio between (C) :( A + B) ⁇ 100: 1 and ⁇ 1: 200; for example, 50: 1, 20: 1, 9: 1, 8: 1, etc
- the surfactants (C) are particularly preferably present in a ratio between (C) :( A + B) ⁇ 50: 1 and ⁇ 1: 20, and very particularly preferably the surfactants (C) are present in a ratio of (C) :( A + B) ⁇ 20: 1 and ⁇ 1: 10 before; in each case based on the weight percent active content in the entire medium.
- Another advantage of the combinations (A), (B) and (D) is a stronger foaming than in the pure combination (A) and (B), in particular it was surprising that the foam also remains stable much longer.
- the foaming embodiment is desirable for some uses and is exemplified in the embodiments.
- the agents consisting of the one or more surfactants (A) and the glycolipid or biosurfactants (B) at any pH value can be additionally combined with a surfactant (D).
- Suitable surfactants are PEG-6 Almond Oil, PEG-8 Almond Oil, PEG-8 Apricot Kernel Oil, PEG-8 Buxus Chinensis Oil, PEG-6 Apricot Kernel Oil, PEG-40 Apricot Kernel Oil, PEG-8 Argan Oil, PEG-8 Avocado Oil, PEG-11 Avocado Oil, PEG-8 Borage seed oil, PEG-8 macademia tenuifolia oil, PEG-6 corn oil, PEG-8 corn oil, PEG-8 grapeseed oil, PEG-8 hazelnut oil, PEG-8 linseed oil, PEG-6 olive oil, PEG-7 olive oil, PEG-7 olive oil, PEG-8 Olea Europaea Oil, PEG-7 Olive Oil, PEG-7 Olive Oil, PEG-8 Olive Oil, PEG-10 Olive Oil, PEG-8 Oryza Sativa Oil, PEG-8 Prunus Dulcis, PEG-8 Persea Gratissma Oil, PEG-8 Passiflora edulis seed
- Ethoxylated rapeseed methyl ester (EO 7-15), ethoxylated rapeseed ethyl ester (EO 7-15), ethoxylated soybean methyl ester (EO 7-15), ethoxylated soya ethyl ester (EO 7-15).
- alkoxylated surfactants having an HLB> 10.5 and ⁇ 12.0.
- these representatives are rapeseed methyl ester oxylate 7 EO, olive oil glycereth-PEG-8 ester, almond oil PEG-7 ester, PEG-10 olive glycerides.
- agents which are characterized in that they contain, based on their weight, from 0.1 to 50% by weight, preferably from 0.1 to 10% by weight and particularly preferably from 0.2 to 5% by weight of one or more additional alkoxylated surfactants, in each case based on the total agent.
- the surfactant or surfactants (D) may be combined in any ratio with (A) and (B) and optionally (C).
- the surfactants (D) are present in the surfactant (A) / glycolipid-biosurfactant (B) mixture in a ratio between (D) :( A / B) ⁇ 50: 1 and ⁇ 1: 50; for example, 20: 1, 9: 1, 8: 1, etc
- the surfactants (D) are particularly preferably present in a ratio of between (D) :( A / B) ⁇ 20: 1 and ⁇ 1: 20, and most preferably the surfactants (D) are present in a ratio of (D) :( A / B) ⁇ 10: 1 and ⁇ 1: 10 before, most preferably (D): (A / B) ⁇ 3: 1 and ⁇ 1: 10; in each case based on the weight percent active content in the entire medium.
- surfactant is understood as meaning amphiphilic organic substances having surface-active properties which are concentrated at the interface adsorb between two liquids, such as oil and water and have the ability to reduce the surface tension of water.
- surfactants tend to self-aggregate and form structures such as micelles, lamellar structures, etc.
- those surfactants (E) having the ability to absorb the surface tension of water at 20 ° C and at a concentration of 0.5% by weight are suitable to reduce the total amount of preparation to below 45 mN / m.
- optional surfactants which can be freely combined by the skilled person with the inventive composition, is based on the relevant specialist literature such as Richard J. Farn, Chemistry and Technology of Surfactants, Blackwell Publishing , referenced. Some examples are mentioned below, wherein the hydrocarbon chains derived from fatty acids or synthetic hydrocarbons comprise saturated or unsaturated, substituted or unsubstituted, linear or branched with 4-24 carbon atoms in the hydrocarbon chain.
- Optional surfactants preferably include, but are not limited to, other surfactants from C18 vegetable oils.
- nonionic surfactants which can be freely combined by the skilled person with the agent according to the invention are, for example, alcohol polyglycol ethers, ie. ethoxylated and / or propoxylated alcohols having 1-40 ethylene oxide (EO) and / or propylene oxide (PO) units, amine oxides, polyethylene glycol mercaptans, glycolipids, such as alkyl polyglycosides having 1-10 glycoside units, polyhydroxy fatty acid amides, polyhydroxy fatty acid esters, carboxylic acid esters, sorbitan esters, and alkoxylated Sorbitan esters, alkanolamine-carboxylic acid condensates, N-alkylpyrrolidones, amidoalkyl-2-pyrrolidones.
- alcohol polyglycol ethers ie. ethoxylated and / or propoxylated alcohols having 1-40 ethylene oxide (EO) and / or propylene oxide (PO) units
- Suitable optional anionic surfactants which can be combined freely by the skilled person with the agent according to the invention are acyl lactylate, fatty alcohol carboxylates, alkyl polyglycol ether carboxylates or mixtures thereof.
- anionic surfactants are used according to the formula RCOX with X the hydrophilic part and RCO derived from a C18 vegetable oil.
- the less preferred sulfur surfactants, phosphates or phosphonates can be used.
- examples are alkylbenzenesulfonates, alkane / alkene sulfonates, alkyl sulfates or fatty alcohol sulfates, alkylpolyglycol ether sulfates having 2 to 6 ethylene oxide units (EO) in the ether part, and also sulfosuccinates, carboxamide ether sulfates, sulfosuccinic acid mono- and di-alkyl esters, ⁇ -olefinsulfonates, alkyl isethionate, acyl isethionate, alkyl sulfoacetates, sulfonated fatty acids, sulfonated Fatty acid esters, such as sulfonated fatty acid glycerol esters and sulfonated fatty acid methyl esters, N-acylaminosulfonic acids
- compositions of the invention show a comparable cleaning performance to conventional agents with sulfur surfactants, even without the use of sulfur surfactants.
- a preferred embodiment is free of all sulfur surfactants.
- the water-polluting phosphates and phosphonates without sacrificing the cleaning performance can be dispensed with.
- Another preferred embodiment is phosphate and phosphonate free.
- amphoteric surfactants which may be freely combined with the inventive agent by the skilled person, may be included, such as N-alkyl betaines, imidazolinium betaines, amine oxides, and less preferably alkylamidobetaines, sulfobetaines, phosphobetaines and sultaines.
- the agent may optionally contain cationic surfactants, for example primary, secondary, tertiary or quaternary alkylammonium salts of the formula (RI) (RII) (RIII) (RIV) N + X - , in which RI to RVI independently of one another are identical or different alkyl radicals, branched and unbranched, saturated or unsaturated, unsubstituted, monosubstituted or polysubstituted, or H, wherein X - represents an anion.
- cationic surfactants for example primary, secondary, tertiary or quaternary alkylammonium salts of the formula (RI) (RII) (RIII) (RIV) N + X - , in which RI to RVI independently of one another are identical or different alkyl radicals, branched and unbranched, saturated or unsaturated, unsubstituted, monosubstituted or polysubstituted, or H, wherein X
- compositions contain surfactants (E) derived from C-18 vegetable oils having the lipophilic residues R derived from fatty acids RCOOH, such as defined under (I).
- the radicals R are preferably present as a mixture according to the fatty acid distribution in the native oil or as they arise in the reaction of native oils.
- the agent contains a proportion of surfactants consisting of surfactant (A) of the formula (I), the surfactants (C) and (D), and optionally surfactants (E), with the proviso that the surfactant or surfactants (E) derived from a C-18 vegetable oil, and biosurfactant glycolipids (B), which is in total ⁇ 30%, preferably ⁇ 60%, more preferably ⁇ 95% and most preferably ⁇ 99%, based on the total content of surfactants in Wt .-% in the agent.
- composition according to the invention may contain all solvents customary in detergents and cleaners. They serve to stabilize the formulation, the solubilization of poorly soluble ingredients and to increase the cleaning performance.
- the agent contains water as solvent, wherein more than 5 wt .-%, preferably more than 15 wt .-% and particularly preferably more than 25 wt.% Water, in each case based on the total amount of the composition ,
- Particularly preferred agents contain - based on their weight - 5 to 98 wt .-%, preferably 10 to 90 wt .-%, particularly preferably 25 to 75 wt.% Water.
- they may be water-poor or anhydrous, with the content of water in a preferred embodiment being less than 10% by weight, and more preferably less than 8% by weight, based in each case on the total liquid agent.
- the agent is anhydrous, the agent containing an organic solvent as the main solvent. It is preferred that the agent contains 5 to 98 wt .-%, preferably 10 to 90 wt .-%, particularly preferably 25 to 75 wt .-% solvent.
- Exemplary solvents are the following compounds named according to INCI: Alcohol denat. (Ethanol), alcohols, buteth-3, butoxy diglycol, butoxyethanol, butoxyisopropanol, butoxypropanol, n-butyl alcohol, t-butyl alcohol, butyl 3-hydroxybutyrate, butylene glycol, butyloctanol, C1-C6 alkanes, C7-C15 alkanes , Diethylene glycol, diethylene glycol monobutyl ether, dimethoxy diglycol, dimethyl ether, dimethyl 2-methylglutarate, dipropylene glycol, dipropylene glycol phenyl ether, ethyl lactate, 2-ethyl lactate, ethyl levulinate glycerol ketal, ethyl levulinate propylene glycol ketal, ethylene glycol ketal, ethoxydiglycol, ethoxyethanol, Ethyl hex
- solvents are selected from the group of solvents derived from vegetable raw materials that are biodegradable. Particular preference is given to solvents which contain no VOCs (volatile organic compounds).
- a particularly preferred embodiment additionally contains fatty acid alkyl esters of the formula R-CO-OR 25 as solvent wherein the fatty acid alkyl ester consists of a mixture of different chain lengths and saturation levels of the fatty acid residue RCO as defined in surfactant (A) and derived from a C18 vegetable oil; and wherein R 25 is a linear or branched hydrocarbon of 1-5 carbon atoms, preferably consisting of a methyl or ethyl group, more preferably methyl.
- Preferred representatives are rapeseed methyl ester, sunflower methyl ester, thistle methyl ester or soybean methyl ester.
- Softeners and complexing agents INCI chelating agents, sequestrants According to the invention, all complexing agents customary in detergents and cleaners are suitable. They increase the detergency and the stability of the agents.
- the complexing agents in the compositions according to the invention additionally increase the foam formation and stability by a factor of 2-4.
- Softeners and complexing agents from the groups of phosphates and phosphonates, phyllosilicates, zeolites, carbonates and polycarboxylates, aminopolycarboxylic acids, such as aminoacetic acids and polyaminoacetic acids and their salts, hydroxycarboxylic acids and their salts, polyglycosides and -gluconic acids and their salts are suitable according to the invention.
- Suitable examples are the following complexing agents: aminotrimethylene phosphonic acid, beta-alanine acetoacetic acid, calcium disodium EDTA, chitosan, citric acid and its salts and hydrates, cyclodextrin, cyclohexanediamine tetraacetic acid, diammonium citrate, diammonium EDTA, diethylenetriamine pentaacetic acid, diethylenetriamine pentamethylene phosphoric acid, dipotassium EDTA, disodium Azacycloheptane diphosphonate, disodium EDTA, disodium pyrophosphate, EDTA, ethylenediamine- N, N'-disuccinic acid (EDDS), etidronic acid, galactaric acid, ⁇ -glucan, gluconic acid, glucuronic acid, glucoheptonic acid, HEDTA, hydroxypropyl cyclodextrin, methyl cyclodextrin, pent
- the agents according to the invention contain complexing agents which are biodegradable.
- the compositions according to the invention therefore preferably contain no phosphates, no phosphonates, no EDTA and no polycarboxylates.
- Very particularly preferred in this invention are the following complexing agents based on renewable raw materials, such as beta-alanines diacetic acid, cyclodextrin, diammonium citrate, galactaric acid, gluconic acid, glucuronic acid, methylcyclodextrin, hydroxypropyl cyclodextrin, polyaspartic acid, alkali salts of gluconates, sodium carbonate, carboxymethyl inulin and sodium carboxymethyl inulin (NaCMI), sodium citrate, sodium dihydroxyethylglycinate, sodium gluconate, sodium glucoheptonate, sodium iminodisuccinate, sodium lactate, sodium lignosulfate, tetrasodium GLDA (I-glutamic acid, N, N-di (acetic acid), tetrasodium salt ), Citric acid and its salts,
- Preferred preparations according to the invention comprise at least one complexing agent in a total amount of 0.1-20% by weight, preferably 0.2-15% by weight, in particular 0.5-10% by weight, based on the total amount of the preparation.
- agents with tetrasodium GLDA, sodium citrate and sodium gluconate as complexing agents are disclosed by way of example. According to the invention, these softeners and complexing agents can be freely combined with other ingredients in a manner well known to those skilled in the art.
- composition according to the invention can contain all preservatives customary in detergents and cleaners, which can be freely combined with other ingredients by the person skilled in the art for the purposes of this application.
- active compounds from the groups of alcohols, aldehydes, antimicrobial acids or their salts, carboxylic esters, acid amides, phenols, phenol derivatives, diphenyls, diphenylalkanes, urea derivatives, oxygen and nitrogen acetals and formals, benzamidines, isothiazoles and derivatives thereof such as Isothiazolinones, phthalimide derivatives, pyridine derivatives, surface active compounds, guanidines, antimicrobial amphoteric compounds, quinolines, 1,2-dibromo-2,4-dicyanobutane, iodo-2-propynyl-butyl-carbamate, iodine, iodophores and peroxides.
- antimicrobial agents selected from antimicrobial peptides, ethanol, benzyl alcohol, dehydroacetic acid and its salts, sorbic acid and potassium sorbate, vegetable organic acids and their salts, formic acid, glycerol, citric acid, lactic acid, salicylic acid, and their salts.
- the embodiment without chemical preservatives as disclosed in the embodiments i. in particular without parabens, without formaldehyde-containing preservatives or formaldehyde releasers, without isothiazoles and their derivatives, without halogen-containing compounds, without phthalimides, without benzalkonium chloride, without benzoic acid, without phenoxyethanol.
- the addition of known strong foaming surfactants to the inventive agent is possible.
- saponins for example saponins from the Indian soapnut (Sapindus mukorossi), Korean ginseng (Panax ginseng), agave plants, Inca cucumber (Cyclanthera pedata), sweet wood (Glycyrrhiza glabra), ivy (Hedera), cowslip ( Primula veris), chickweed (Stellaria media), forest Sanickel (Sanicula europaea), thorny toad (Ononis spinosa), legumes (Leguminosae), Spinach (Spinacia), Asparagus (Asparagaceae), Oats (Avena), (Ononis spinosa), Wegblümchen (Maianthemum bifolium), Soa
- the amount of saponins is usually up to 5 wt .-%, preferably 0.001 to 3 wt .-%, in particular 0.01 to 2 wt .-%. (Wt .-% active based on the total agent)
- the saponins can be freely combined with other ingredients in the inventive composition.
- the pH of the agent according to the invention can be adjusted by means of customary pH regulators, with different pH ranges from acidic (pH 0-4) to neutral (pH 5-7) to basic (pH 8-14), depending on the application. be set.
- the pH-adjusting agents are acids and / or alkalis. Suitable acids are in particular organic acids such as formic acid, acetic acid, citric acid, glycolic acid, lactic acid, succinic acid, adipic acid, malic acid, tartaric acid and gluconic acid or amidosulfonic acid.
- acids which are obtained from vegetable raw materials such as acetic acid, citric acid, lactic acid, malic acid and tartaric acid and to the mineral acids hydrochloric acid, sulfuric acid and nitric acid or mixtures thereof.
- Preferred bases are selected from the group of alkali and alkaline earth metal hydroxides and carbonates.
- the agent may contain ammonia and alkanolamines.
- compositions according to the invention can also contain solubilizers, so-called hydrotropes.
- solubilizers so-called hydrotropes.
- hydrotropes are all commonly used for this purpose in detergents and cleaning agents used.
- Builders which are commonly used in detergents and cleaners, are suitable.
- the builders can be freely combined with other ingredients by those skilled in the inventive composition.
- Especially preferred in the composition according to the invention are builders based on renewable raw materials that can be obtained from plants of the temperate zone, such as polyaspartates, polycarboxylates such as citrates, and gluconates, succinates or malonates.
- fragrances and dyes customary in detergents and cleaners can be added to the composition according to the invention.
- Preferred dyes and fragrances the selection of which presents no difficulty to the skilled person, have a high storage stability and insensitivity to the other ingredients of detergents or cleaners.
- the dyes have no pronounced substantivity to textile fibers or hard surfaces and do not stain them.
- neither color nor fragrances are added.
- the compositions have a satisfactory aesthetics and a pleasant fragrance even without the addition of dyes or fragrances, so as to enable embodiments without dyes and / or fragrances, such as for consumers with allergies and / or sensitive skin.
- the agent may optionally contain enzymes, especially in the embodiments of textile, specialty and dishwashing.
- the enzymes can be combined in the agent according to the invention by the person skilled in the art with all other ingredients mentioned here. Preference is given to using proteases, lipases, amylases, hydrolases and / or cellulases. They can be added to the composition according to the invention in any form established according to the prior art be added. In the case of liquid or gel-containing compositions, these include, in particular, solutions of the enzymes, preferably highly concentrated, low in water and / or mixed with stabilizers. Furthermore, the enzymes can be applied encapsulated.
- enzyme stabilizers can be added to the enzyme-containing agent.
- suitable enzyme stabilizers are, for example: benzamidine hydrochloride, borax, boric acids, boronic acids or their salts or esters, especially derivatives with aromatic groups, for example substituted phenylboronic acids or their salts or esters; Peptide aldehydes, amino alcohols such as mono-, di-, triethanol- and -propanolamine and mixtures thereof, aliphatic carboxylic acids up to C12, such as succinic acid, other dicarboxylic acids or salts of said acids; end-capped fatty acid amide alkoxylates; lower aliphatic alcohols and especially polyols, for example glycerol, ethylene glycol, propylene glycol or sorbi
- biotechnologically produced enzymes with the aid of non-genetically modified organisms (non GMO), stabilizers based on renewable raw materials and / or mineral substances, for example boric acid and / or borax, reducing sugars, succinic acid or other dicarboxylic acids, polyamino compounds in particular based on natural amino acids.
- non GMO non-genetically modified organisms
- stabilizers based on renewable raw materials and / or mineral substances, for example boric acid and / or borax, reducing sugars, succinic acid or other dicarboxylic acids, polyamino compounds in particular based on natural amino acids.
- an outstanding cleaning performance with respect to carbohydrates is found, which is quite comparable with enzyme-containing agents, this is disclosed by way of example in the exemplary embodiments.
- a particularly preferred embodiment of the invention is therefore those without cellulases or amylases, very particularly preferred is the enzyme-free embodiment. This is especially beneficial for consumers with allergies and / or sensitive skin. Enzyme-free embodiments with comparable cleaning power are disclosed in the embodiments.
- the liquid or gel embodiment of the composition according to the invention preferably has a viscosity of from 0.4 to 10000 mPa.s. on.
- the agent may contain viscosity regulators.
- the amount of viscosity regulators is usually up to 1.5 wt .-%, preferably 0.001 to 1.0 wt .-%, in particular 0.01 to 0.5 wt .-%; % By weight of active ingredient based on the total agent.
- Suitable viscosity regulators include organic modified natural products (carboxymethylcellulose and other cellulose ethers, hydroxyethyl and - propylcellulose and the like, core flour ethers), organic fully synthetic thickeners (polyacrylic and polymethacrylic compounds, vinyl polymers, polycarboxylic acids, polyethers, polyimines, polyamides) and inorganic thickeners (polysilicic acids , Phyllosilicates, clay minerals such as montmorillonites, zeolites, silicas), as well as organic natural thickeners (agar-agar, carrageenan, xanthan, tragacanth, gum arabic, alginates, pectins, polyoses, guar flour, locust bean gum, starch, dextrins, gelatin, casein) ,
- the viscosity regulators are natural organic thickening agents from vegetable raw materials - including algae - for example, polysaccharides such as pectins or starch.
- no organic fully synthetic thickeners such as polyacrylic and polymethacrylic compounds, vinyl polymers, polycarboxylic acids, polyethers, polyimines, or polyamides are used.
- inorganic thickeners are also preferred.
- the viscosity regulators can be freely combined by the skilled person with other ingredients mentioned here.
- Another object of the invention relates to a method for cleaning.
- Methods for cleaning are generally distinguished by the fact that different cleaning-active substances are applied to the items to be cleaned and washed off after the contact time, or that the items to be cleaned are otherwise treated with a detergent or a solution of this agent.
- temperatures of up to 90 ° C. and less are used in various embodiments of the invention. Preference is given to temperatures below 60 ° C and very particularly preferred are temperatures that do not require heating the water temperature for energy saving reasons (about 20 ° C). These temperature data refer to the temperatures used in the washing steps.
- the agent is applied to solid substrates, such as cloths of textile, composite, non-woven, nonwoven, paper, wadding or felt, among others. applied. These are impregnated with the agent by a pressing, dipping, wiping or spraying process.
- the invention also relates to the use of the agent for improving the washing or cleaning performance, in particular on dye, pigment or carbohydrate contaminants.
- composition according to the invention can be used as or for the production of detergents and cleaners for surfaces made of natural or manufactured, hard or flexible materials, as well as textiles, carpets or natural fibers.
- the washing and cleaning agents also include washing aids which are added to the actual agent during manual or mechanical cleaning.
- detergents within the scope of the invention also include pre- and post-treatment agents, ie those agents which are used before the actual cleaning, for example for dissolving stubborn soiling.
- the funds can be applied to the items to be cleaned, which can be found in household, industry, trade or institutions, port facilities, as well as industrial and recreational, and sports facilities.
- the agent is used for cleaning hard surfaces or textiles.
- Hard surfaces in the context of this application are windows, mirrors, and other glass surfaces, surfaces made of ceramic, plastic, metal or wood, flat or uneven, painted and unpainted, flexible surfaces are, for example, plastic sheeting, foam, skin, earth or others.
- natural surfaces are surfaces of living beings, humans, animals, plants or soil; such as skin, hair, soil, plants and their fruits or leaves, leather.
- Textiles and fibers are in the sense of the application substances, clothing, upholstery, carpets, yarns, u.a.
- the agent is used at acidic pH between 0 and 7, preferably between 1 and 6, and most preferably at a pH between 2 and 4.
- the combination of the invention shows a very good suitability for acidic cleaners and shows in particular a very good lime release power at acidic pH.
- the agent according to the invention is therefore suitable for use in limesolvent detergents or cleaners, especially as sanitary cleaners, WC cleaners, lime solubilizers, rinse aids, dishwashing detergents, food-grade cleaning agents, such as, for example, detergents. for breweries, bakeries and others.
- the agent is used at an alkaline pH of between 7 and 14, preferably between 8 and 12.
- alkaline pH Typical examples of uses at alkaline pH are detergents, surface cleaners, kitchen cleaners, grill and oven cleaners, rim cleaners, and others.
- the agent is employed at neutral pH between 5 and 8, e.g. when skin-neutral pH is desirable, such as in a dishwashing detergent, neutral detergent, surface cleaner, and others.
- the agent may be present as a liquid, solution, dispersion, emulsion, lotion or gel. It can be used as a spray, foam, Tunknierkeit and adsorbed to powders, granules or tabs. It is suitable for direct application, as well as for use via an aid such. a towel.
- a particular product form are solid substrates, such as cloths. These are soaked with the composition according to the invention, sprayed, coated or used up by another method. Solid substrates have the advantage of being in them the preparation is already prescribed in the correct dosage. This is in particular contrary to the consumer desire of convenience, they are easy to handle, to use directly and without additional steps and can also be used well when traveling, for example, when traveling, even if no water is available.
- compositions according to the invention are used in industry, for example for the purification of food-grade or industrial plants, e.g. in the metalworking industry, the food processing industry, commercial kitchens, chemical and pharmaceutical, paper and textile industries, and the like.
- the agents are used in the household for cleaning surfaces and / or textiles, as well as in the commercial or institutional area, such as in hotels, cleaning companies, clinics, schools or public buildings.
- compositions according to the invention are suitable for cleaning and washing preparations such as, for example, hand soaps, hand dishwashing detergents, machine dishwashing detergents, dishwashing detergents, washing machine cleaners, toilet cleaners or toilet cleaners, universal or all-purpose cleaners, kitchen cleaners, bathroom or sanitary cleaners, floor cleaners, oven and oven cleaners Grill cleaners, glass and window cleaners, metal cleaning agents, upholstery and carpet cleaners, heavy-duty detergents, color detergents, mild detergents, textile auxiliaries, pretreatment agents, special detergents and cleaners, and other agents for industrial & commercial or institutional cleaning, textile and fiber treatment agents, Agent of leather treatment, as well as other forms of preparation.
- the detergents and cleaners are suitable both for dilute application, as well as for direct application to the substrate to be cleaned.
- the liquid or gel embodiment is water or an organic solvent, most preferably the aqueous embodiment.
- surfactants are designated as follows. All concentration data refer to the active content of the ingredients throughout the preparation.
- Comparative washing tests were performed according to the AISE protocol November 2013. Washing temperature: 40 ° C, dosage: 70 ml, washing machine load: 3kg, water hardness: 4.36 mmol CaCO 3 / l (hard water conditions) Representative stain set according to AISE on cotton: Tea, coffee, red wine, fruit juice, tomato puree, carrots baby porridge, French mustard, chocolate, grass, grass / mud, blood, unused motor oil, frying fat, make-up. Standardized soiled test fabric can be purchased from EMPA (Swiss Federal Institute for Materials and Testing Switzerland). Evaluation by statistical evaluation of the washing results.
- the stains were categorized into carbohydrate-rich stains and color stains. It then became the statistical washing performance per stain type, the total washing performance as the arithmetic mean of all stains, the average washing performance on predominantly carbohydrate stains such as cellulose or starch and the averaged washing performance of the predominantly bleachable stains (dye and pigment stains) of the different test formulations.
- compositions according to the invention show surprisingly good cleaning with carbohydrate stains such as starch and cellulose.
- the washing performance of the combination of surfactants A with the glycolipid biosurfactants are shown by way of example with B1 in Table 1 and B4 in Table 2.
- the surfactant combinations A / B1 or A / B4 exceed the washing performance of the formulations with the individual surfactants (Ex 1, 2) on carbohydrate-containing stains. So there is a synergistic surfactant A / B combination.
- carbohydrate-degrading enzymes such as amylases or cellulases, they even surpass the enzyme-containing reference market product (reference A).
- reference B In order to exclude effects due to the washing base, a washing solution is also tested only with soap, as shown in Table 1, reference B.
- Another reference C shows by way of example the combination of the glycolipid biosurfactant with another nonionic ethoxylated surfactant (HLB 14) based on sunflower oil instead of surfactant A.
- HLB 14 nonionic ethoxylated surfactant
- compositions according to the invention also show an unexpected improvement in detergency compared with the comparison formulations in the area of bleachable stains (dyes and pigments). These stains are generally not water soluble. An unusually high washing performance is achieved with pens, coffee and red wine.
- the agent according to the invention therefore additionally exhibits a synergistic detergency increase on color stains (Tables 1 and 2). Despite dispensing with often ecologically questionable bleaching agents, the compositions of the invention show an exceptionally good cleaning performance on bleachable stains.
- pH setting pH setting. water ad 100 ad 100 ad 100 Average washing performance all stains 2:47 1.65 1:47 carbohydrate stains 2.2 1.3 1.2 color spots 3.2 2.4 2.4 Carbohydrates: starch, cellulose (fruit juice, tomato puree, carrot, mustard, grass, gravy) color stains: bleachable stains (tea, coffee, red wine, earth, pens)
- an analogous oleic acid derivative was used as reference D instead of surfactant A.
- Oleic acid is the monounsaturated fatty acid with a chain length of 18 carbon atoms.
- cocamide DEA cocamide DEA, a C12 compound, was chosen.
- Example 3 the inventive combination of surfactant A with biosurfactant B1 shows very good individual and total washing performance, when using the non-inventive combination of biosurfactant with a surfactant based coco fatty acids or oleic acid no synergy on carbohydrate or color spots is found. It is therefore essential for the invention to have a distribution of different fatty acid lengths and degrees of saturation according to C-18 plants.
- synergistic combinations can additionally be combined with soap (C) (see Tables 1 and 2), further ethoxylated surfactants (D) or solvents (here: rapeseed methyl ester) (Table 4). While soap or nonionic alkoxylated surfactants alone combined with biosurfactant show no synergistic effect on carbohydrate or color stains, the wash performance of combination A / B surprisingly remains high, and in some cases is even enhanced.
- Table 4 shows that the synergistic effect on carbohydrate stains and color stains is retained even when adding a third surfactant (D) or when adding a fatty acid alkyl ester, as exemplified here by rape methyl ester. All novel detergents 7-13 are distinguished by very high washing performance, in particular on carbohydrate and color stains.
- the mixtures according to the invention are preferably suitable for the cleaning of textiles, for example as detergents, as bleaching agents, as pretreatment agents, as carpet cleaners, as upholstery cleaners, inter alia
- the synergistic effect of removing carbohydrate dirt or stains can also be used in the cleaning of natural or manufactured, hard or flexible surfaces, even at acidic or neutral pH.
- test series are each standardized to the detergency of a surfactant.
- biosurfactant glycolipid 1 was selected as the standard in Table 6 surfactant A.
- cocamide DEA a surfactant which comes closest to use and behavior surfactant A.
- Cocamide DEA is derived from coconut or palm oil. Contrary to expectations, cocamide DEA and surfactant A show a very different behavior: While cocamide DEA as the sole surfactant shows a comparable detergency to carbohydrates as the biosurfactant, the detergency of the coconut fatty acid analogue in combination with the biosurfactant (negative synergistic) worsens.
- the carbohydrate dissolving power of the test solutions based on the carbohydrate dissolving power of the glycolipid biosurfactant 1 becomes 100%.
- Surfactant A or R combined with biosurfactant 1, each 0.5% by weight Surfactant A 100% 94% 108% Surfactant R 100% 100% 93%
- biosurfactant glycolipid 4 gives a total result of 3.3 as an 8% solution while the 1: 1 mixture with surfactant (A) has a total washing power of 1.32.
- Exemplary are the foam height and stability test results for the addition of alkoxylated surfactants (D) to a 1: 1 mixture of surfactant (A) and biosurfactant glycolipid B1 in Table 10.
- the foam stability is determined by measuring the foam height after 15 min.
- the foam of the biosurfactant is expected to be improved by the binary mixture with surfactant A. Surprisingly, however, it emerges that the tertiary mixtures with surfactant (D) exceed the foam stability of the binary mixture by about twice.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Detergent Compositions (AREA)
- Cosmetics (AREA)
Claims (11)
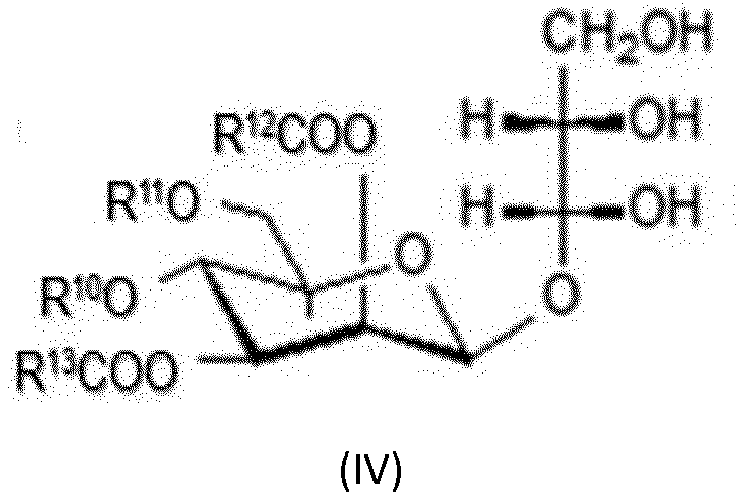
- Composition comprenant au moins un amide d'acide gras alcoxylé (A) de formule (I) et au moins un biotensioactif glycolipidique (B) comprenant des rhamnolipides de formule (II), des sophorolipides de formule (III), des lipides de mannosylérythritol de formule (IV) et des lipides de cellobiose de la formule (V) et des lipides de tréhalose de formule (VI):avec l'amide d'acide gras alcoxylé (I)
(I) R-CO-NH-(CmH2mO)n-H
avecm un nombre entier de 2 ou 3, de préférence 2,n un nombre entre 2 et 10, de préférence entre 2 et 8,R un radical hydrocarburé saturé, mono-insaturé ou polyinsaturé, ayant 5 à 23 atomes de carbone;et dans laquelle le tensioactif (I) est présent sous forme d'un mélange de différents longueurs de chaîne et degrés de saturation des résidus d'acide gras RCO avec une proportion de RCO de 18 atomes de carbone et plus supérieure à 60% en poids, de préférence supérieure à 72% en poids et de manière particulièrement préférée supérieure à 77% en poids;et dans lequel la proportion de résidus d'acide gras insaturés RCO est supérieure à 55% en poids, de préférence supérieure à 65% en poids et de manière particulièrement préférée supérieure à 72% en poids; % en poids chaque fois basé sur la totalité des résidus d'acide gras RCO dans le tensioactif (I);et dans lequel RCO est dérivé d'une huile végétale C-18 du groupe comprenant: amarante, anis, pomme, abricot, argan, arnica, avocat, coton, bourrache, ortie, brocoli, canola, chia, chanvre, noisette, hêtre, buis, chardon, épeautre, arachide, souchet comestible, lilas, cresson alénois, orge, grenade, avoine, chanvre, noisette, bleuet, sureau, jasmin, groseille, millepertuis, jojoba, camélia,camomille, carvi, carotte, cerise, coriandre, molène, crambe, euphorbe épurge, courge, la tête de dragon ibérique, lavande, caméline, graines de lin, troène, lupin, luzerne, macadamia, maïs, amande, marula, mirabelle, melon, pavot, mongongo, moringa, onagre, olive, radis oléagineux, roquette, passiflore, noix de pécan, pêche, prune, pistache, airelle, jatropha, colza, riz, souci, navette, carthame, sauge, argousier, cumin noir, sésame, feuille de sésame, moutarde, tournesol, soja, tabac, noyer, raisin, blé, limnanthe, et rose musquée, ainsi que leurs combinaisons;rhamnolipide selon la formule (II) - Composition selon la revendication 1, caractérisée en ce que le au moins un tensioactif alcoxylé (A) est présent sous forme de mélange de différents longueurs de chaîne et degrés de saturation des résidus d'acide gras RCO, et dans lequel la proportion de résidus d'acide gras saturés et insaturés RCO ayant 20 atomes de carbone ou plus est supérieure à 0,01% en poids, et de préférence supérieure à 0,05% en poids, et de manière particulièrement préférée, supérieure à 0,1% en poids et de manière tout particulièrement préférée égal ou supérieure à 0,2% en poids: basé sur la totalité des résidus d'acide gras RCO dans le tensioactif (I);
- Composition selon l'une des revendications précédentes contenant en outre au moins un savon (C) de formule (VII)
RCOOM (VII)
dans laquelle M est un cation d'un métal alcalin ou d'ammonium,et dans laquelle le savon ou les savons (VII) sont présents sous forme d'un mélange de différents longueurs de chaîne et dégrées de saturation des résidus d'acide gras RCO, et RCO est dérivé d'une huile végétale C-18; avec R et RCO comme formule (I). - Composition selon l'une des revendications précédentes, contenant en outre au moins un autre tensioactif (D) choisi dans le groupe des esters d'acides gras alcoxylés, les esters de glycéride d'acides gras alcoxylés et les esters d'huile végétale alcoxylés de formule (VIII) ou dans chaque cas leurs mélanges;
(VIII) RCO-O(CmH2mO)o-{CH2-CH[O(CmH2mO)pR"]-CH2O}w-(CmH2mO)q-R"'
dans laquellem est 2, 3 ou 4,o, p, q sont, indépendamment l'un de l'autre, des nombres de 0 à 75, où o + p + q ≠ 0, avec un degré total d'alcoxylation x = 2-75,R" est H ou COR,R"' est H, COR ou un radical alkyle linéaire ou ramifié ayant 1 à 8 atomes de carbone,w est 0 ou 1;et dans laquelle le ou les tensioactifs (D) sont présents sous forme d'un mélange de différents longueurs de chaîne et degrés de saturation des résidus d'acide gras RCO, et RCO est dérivé d'une huile végétale C-18; avec R et RCO tels que définis dans la formule (I). - Composition selon l'une des revendications précédentes contenant en outre un ou plusieurs esters alkyliques d'acide gras de formule (X)
(X) R-CO-O-R25
dans laquelle R25 représente un radical hydrocarboné linéaire ou ramifié ayant 1 à 5 atomes de carbone,et dans laquelle l'ester ou les esters alkyliques d'acide gras (X) sont présents sous forme d'un mélange de différents longueurs de chaîne et degrés de saturation des résidus d'acide gras RCO, et RCO est dérivé d'une huile végétale C-18; avec R et RCO tels que définis dans la formule (I). - Composition selon l'une des revendications précédentes, caractérisée en ce que la proportion constituée du tensioactif (A), des tensioactifs (C) et (D), et éventuellement des tensioactifs (E), à condition que le tensioactif ou les tensioactifs (E) sont dérivés d'une huile végétale C-18, et des biotensioactifs glycolipidiques (B), représente au total ≥ 30% en poids, de préférence ≥ 60% en poids, de manière particulièrement préférée ≥ 95% en poids et le plus préférablement ≥ 99% en poids ; % en poids sur la base de la teneur totale en tensioactifs dans la composition.
- Procédé de lavage ou de nettoyage comprenant les étapes du procédéa) fournir une solution de lavage ou de nettoyage comprenant une composition selon l'une des revendications précédentesb) la mise en contact d'un textile ou d'une surface avec l'agent de lavage et de nettoyage selon a).
- Utilisation de la composition selon l'une des revendications précédentes pour renforcer la puissance de nettoyage d'un agent de lavage, d'un agent de nettoyage ou d'un chiffon imbibé pour le nettoyage, en particulier pour les taches de glucides ou de couleur.
- Utilisation de la composition selon l'une des revendications précédentes comme savons pour les mains, détergents pour vaisselle à main, détergents pour lave-vaisselle, nettoyants pour machine à laver la vaisselle, nettoyants pour lave-linge, nettoyants pour toilettes, nettoyant pour WC, nettoyants universels et nettoyants multi-usage, nettoyants pour la cuisine, nettoyants pour salle de bains et nettoyants sanitaires, nettoyants pour plancher, nettoyants pour four et nettoyants pour barbecue, nettoyants pour verre et pour vitres, nettoyants pour métaux, nettoyants pour meubles rembourrés et pour tapis, lessives universelles, détergents pour couleur, détergents pour linges délicats, agents auxiliaires pour les textiles, préparations de prétraitement, détergents et nettoyants spéciaux, produits de nettoyage industriels, commerciaux et institutionnels, agents pour l'industrie alimentaire, produits pour le traitement de textiles et de fibres, produits pour le traitement de cuir.
- Composition selon la revendication 1, caractérisée en ce que le biotensioactif glycolipidique (B) est un sophorolipide ou un rhamnolipide.
- Composition selon la revendication 1, caractérisée en ce que le tensioactif (A) est PEG-4 amide de colza.
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH01114/16A CH712860A2 (de) | 2016-08-29 | 2016-08-29 | Mittel mit alkoxylierten Fettsäureamiden und Glycolipid-Biotensiden. |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3290501A1 EP3290501A1 (fr) | 2018-03-07 |
| EP3290501B1 true EP3290501B1 (fr) | 2019-05-15 |
Family
ID=59738105
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP17020387.1A Active EP3290501B1 (fr) | 2016-08-29 | 2017-08-25 | Compositions detergentes contentant des amides d'acides gras alcoxylés |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP3290501B1 (fr) |
| CH (1) | CH712860A2 (fr) |
| DK (1) | DK3290501T3 (fr) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA3059419A1 (fr) | 2017-04-09 | 2018-10-18 | Locus Ip Company, Llc | Matieres et procedes pour entretenir un equipement industriel, mecanique et de restauration |
| CN109293189B (zh) * | 2018-11-30 | 2021-11-05 | 江门市邑凯环保服务有限公司 | 一种促进污泥水解酸化的方法 |
| EP3686265A1 (fr) | 2019-01-23 | 2020-07-29 | BlueSun Consumer Brands, S.L. | Composition détergente avec sophorolipides |
| CH716229B1 (de) * | 2019-05-26 | 2023-05-15 | Fama Holding Ag | Mittel mit alkoxylierten Fettsäurealkylestern. |
| WO2022011839A1 (fr) * | 2020-09-27 | 2022-01-20 | 北京锐盛明杰知识产权代理有限公司 | Agent de savonnage, procédé de préparation associé et utilisation associée |
| CN111644070A (zh) * | 2020-06-17 | 2020-09-11 | 河南省科学院高新技术研究中心 | 中水回用反渗透膜清洗剂 |
| EP3940049A1 (fr) * | 2020-07-13 | 2022-01-19 | Dalli-Werke GmbH & Co. KG | Lipide de mannosylérythritol comprenant des agents de rinçage liquides |
| EP4083050A1 (fr) | 2021-05-01 | 2022-11-02 | Analyticon Discovery GmbH | Glycolipides microbiens |
| CN114410386B (zh) * | 2021-12-09 | 2024-04-09 | 苏州肽科新材料科技有限公司 | 树脂镜片生产模具清洗用半水性组合物及制备方法和应用 |
| DE102022210849A1 (de) | 2022-10-14 | 2024-04-25 | Henkel Ag & Co. Kgaa | Sophorolipid-Tenside mit oberflächenaktiven Gegenkationen |
| DE102022210850A1 (de) | 2022-10-14 | 2024-04-25 | Henkel Ag & Co. Kgaa | Reinigungsmittelzusammensetzung umfassend Sophorolipid-Tensid und eine antimikrobielle Verbindung |
| DE102023205204A1 (de) | 2023-06-05 | 2024-12-05 | Henkel Ag & Co. Kgaa | Biotenside mit oberflächenaktiven Gegenkationen |
| CN117417789B (zh) * | 2023-10-19 | 2024-08-23 | 太仓立日包装容器有限公司 | 一种再生ibc吨桶绿色清洗溶剂 |
| DE102023212849A1 (de) * | 2023-12-18 | 2025-06-18 | Henkel Ag & Co. Kgaa | Tensidsystem für Waschmittel enthaltend Mannosylerythritollipide (MEL) und mindestens ein weiteres Tensid |
Family Cites Families (42)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6037087B2 (ja) | 1978-09-28 | 1985-08-24 | 花王株式会社 | 化粧料 |
| JPS60183032A (ja) | 1984-03-02 | 1985-09-18 | Shiseido Co Ltd | 乳化組成物 |
| US4933281A (en) | 1987-03-17 | 1990-06-12 | The University Of Iowa Research Foundation | Method for producing rhamnose |
| JPH064665B2 (ja) | 1988-05-30 | 1994-01-19 | 工業技術院長 | 金属セッケン |
| ES2018637A6 (es) | 1989-04-18 | 1991-04-16 | Presas Angeles Manresa | Mejoras introducidas en el metodo para la obtencion de biotensioactivos en su sustrato de aceites y grasa refinados. |
| CA2060698C (fr) | 1991-02-12 | 1997-09-30 | Peter J. Hall | Compositions de detergent |
| FR2692593B1 (fr) | 1992-06-18 | 1995-06-16 | Inst Francais Du Petrole | Procede de production de sophorolipides acetyles sous leur forme acide a partir d'un substrat consistant et une huile ou un ester. |
| US5520839A (en) | 1993-09-10 | 1996-05-28 | Lever Brothers Company, Division Of Conopco, Inc. | Laundry detergent composition containing synergistic combination of sophorose lipid and nonionic surfactant |
| FR2740779B1 (fr) | 1995-11-08 | 1997-12-05 | Rhone Poulenc Chimie | Composition a base d'enzyme et de sophorolipide sous forme lactone et son utilisation dans les formulations detergentes pour le lavage du linge |
| DE19600743A1 (de) | 1996-01-11 | 1997-07-24 | Henkel Kgaa | Verwendung von Mischungen aus Glycolipiden und Tensiden |
| DE19648439A1 (de) | 1996-11-22 | 1998-05-28 | Henkel Kgaa | Verwendung von Mischungen aus Glycolipiden und Tensiden |
| CN1141357C (zh) | 2000-08-09 | 2004-03-10 | 大庆油田有限责任公司勘探开发研究院 | 一种驱油剂及其应用 |
| JP2003013093A (ja) | 2001-06-27 | 2003-01-15 | Saraya Kk | 低泡性洗浄剤組成物 |
| FR2827192B1 (fr) | 2001-07-13 | 2004-06-04 | Cognis France Sa | Preparations contenant des agents tensio-actifs non ioniques comme agents d'extraction |
| GB0219825D0 (en) | 2002-08-24 | 2002-10-02 | Cerestar Holding Bv | Process for producing and recovering mannosylerythritol lipidsfrom culture medium containing the same |
| KR20040033376A (ko) | 2002-10-14 | 2004-04-28 | 주식회사 엘지생활건강 | 소포로리피드를 포함하는 화장료 조성물 |
| CN1219886C (zh) | 2003-01-23 | 2005-09-21 | 湖南大学 | 复合生物表面活性剂及其在堆肥中的应用 |
| DE60305861T2 (de) | 2003-01-28 | 2007-01-04 | Ecover N.V. | Reinigungsmittelzusammensetzungen |
| JP4286558B2 (ja) | 2003-02-26 | 2009-07-01 | 広島県 | マンノシルエリスリトールリピッドの製造方法 |
| US8034605B2 (en) | 2003-03-26 | 2011-10-11 | Kyu-Jin Yum | Microbial materials for degradation of oils and toxic chemicals |
| FR2855752B1 (fr) | 2003-06-03 | 2005-08-26 | Lvmh Rech | Utilisation cosmetique des sophorolipides comme agents regulateurs de la masse adipeuse sous-cutanee et application a l'amincissement |
| JP4548827B2 (ja) | 2004-09-06 | 2010-09-22 | サラヤ株式会社 | 生分解性の液体洗浄剤組成物 |
| JP2006083238A (ja) | 2004-09-14 | 2006-03-30 | Saraya Kk | 洗浄剤組成物 |
| US7556654B1 (en) | 2004-10-15 | 2009-07-07 | Naturell | Methods for cleaning materials |
| JP2006274233A (ja) | 2005-03-29 | 2006-10-12 | Saraya Kk | 漂白剤組成物 |
| JP4858946B2 (ja) | 2006-01-10 | 2012-01-18 | 独立行政法人産業技術総合研究所 | 乳化剤又は可溶化剤 |
| JP4803434B2 (ja) | 2006-03-23 | 2011-10-26 | 独立行政法人産業技術総合研究所 | マンノシルエリスリトールリピッドの高効率生産方法 |
| WO2008013899A2 (fr) | 2006-07-27 | 2008-01-31 | Aurora Advance Beauty Labs | Formulations à base de rhamnolipides |
| JP5294226B2 (ja) | 2006-09-07 | 2013-09-18 | 独立行政法人産業技術総合研究所 | W/o型マイクロエマルジョン |
| JP5649268B2 (ja) | 2008-05-15 | 2015-01-07 | サラヤ株式会社 | ソホロリピッドを含む吸着抑制組成物 |
| KR20100022289A (ko) | 2008-08-19 | 2010-03-02 | 인하대학교 산학협력단 | C-22 소포로리피드 유도체 화합물 및 이의 제조방법 |
| DE102009046169A1 (de) | 2009-10-29 | 2011-05-05 | Henkel Ag & Co. Kgaa | Rückstandsarmer Reiniger für harte Oberflächen |
| CN101845468B (zh) | 2010-03-30 | 2012-11-14 | 湖州紫金生物科技有限公司 | 一种鼠李糖脂的制备方法及其应用 |
| CN101948786B (zh) | 2010-09-03 | 2012-04-04 | 中国石油天然气股份有限公司 | 高产鼠李糖脂的铜绿假单胞菌及其应用 |
| WO2012167815A1 (fr) | 2011-06-06 | 2012-12-13 | Ecover Co-Ordination Center N.V. | Compositions de sophorolactone et leurs utilisations |
| CN102250790B (zh) | 2011-06-14 | 2014-05-07 | 南京农业大学 | 一株生物表面活性剂高效产生菌s2及其发酵培养基 |
| CH705757B1 (de) * | 2011-11-13 | 2016-03-31 | Compad Consulting Gmbh | Nachhaltige Wasch- und Reinigungsmittel. |
| DE102011090030B4 (de) | 2011-12-28 | 2025-11-06 | Evonik Operations Gmbh | Wässrige Haar- und Hautreinigungszusammensetzungen, enthaltend Biotenside |
| EP2950778B1 (fr) | 2013-01-30 | 2018-11-14 | Unilever PLC | Compositions à propriétés esthétiques et sensorielles améliorées |
| DE102013206314A1 (de) | 2013-04-10 | 2014-10-16 | Evonik Industries Ag | Kosmetische Formulierung enthaltend Copolymer sowie Sulfosuccinat und/oder Biotensid |
| EP3002328A1 (fr) | 2014-09-30 | 2016-04-06 | Evonik Degussa GmbH | Formule contenant des bio-tenseurs |
| DE102014221889B4 (de) | 2014-10-28 | 2023-12-21 | Henkel Ag & Co. Kgaa | Waschmittel mit Mannosylerythritollipid, Verstärkung der Reinigungsleistung von Waschmitteln durch Mannosylerythritollipid, und Waschverfahren unter Einsatz von Mannosylerythritollipid |
-
2016
- 2016-08-29 CH CH01114/16A patent/CH712860A2/de not_active Application Discontinuation
-
2017
- 2017-08-25 DK DK17020387.1T patent/DK3290501T3/da active
- 2017-08-25 EP EP17020387.1A patent/EP3290501B1/fr active Active
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3290501A1 (fr) | 2018-03-07 |
| DK3290501T3 (da) | 2019-08-12 |
| CH712860A2 (de) | 2018-03-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3290501B1 (fr) | Compositions detergentes contentant des amides d'acides gras alcoxylés | |
| EP3290500B1 (fr) | Composition de lavage, de nettoyage et de soin contenant de carboxylate polyoxyalkyléné | |
| EP3201302B1 (fr) | Formule contenant des bio-tenseurs | |
| EP1317522B1 (fr) | Agent de lavage et de nettoyage a sechage rapide, notamment liquide de vaisselle a la main | |
| CA2790683C (fr) | Produits nettoyants desinfectants naturels | |
| EP3536769B1 (fr) | Composition d'agent de lavage et de nettoyage comprenant un polyoxyalkylene carboxylate | |
| EP3230428B1 (fr) | Détergent pour lavage manuel de manuel, à action améliorée contre amidon | |
| EP2592134B1 (fr) | Détersifs et nettoyants durables | |
| EP1564283B1 (fr) | combinaison de tensio-actifs | |
| DE102009001559A1 (de) | Kalklösendes Reinigungsmittel | |
| EP4361238A1 (fr) | Compositions comprenant des glycamines | |
| EP3744819A1 (fr) | Composition comprenant des acides gras alcoxylés | |
| WO2007118748A1 (fr) | Détergent aqueux | |
| WO2015120990A1 (fr) | Agent de nettoyage acide pour surfaces dures | |
| CH705767B1 (de) | Milde und nachhaltige Wasch- und Reinigungsmittel. | |
| EP1941020B1 (fr) | Detergents pour vaisselle a la main contenant des agents pour le soin de la peau | |
| DE10248313A1 (de) | Transparentes abrasives Reinigungsmittel, insbesondere Handgeschirrspülmittel | |
| EP1133547A1 (fr) | Detergents encapsules | |
| DE10162648A1 (de) | Sprühbares, schnelltrocknendes Reinigungsmittel | |
| EP3107986A1 (fr) | Lessives ou détergents ayant un comportement de moussage amélioré en cas de fortes salissures | |
| DE102012221021A1 (de) | Wasch- und Reinigungsmittel mit Alkylpolypentosiden | |
| DE102013224454A1 (de) | Handgeschirrspülmittel mit verbesserter Reichweite | |
| WO2026012805A1 (fr) | Détergent, procédés correspondants, kit correspondant et utilisations correspondantes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN PUBLISHED |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20180903 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20181214 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 502017001276 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: GERMAN |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 Effective date: 20190807 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190815 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190915 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190815 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 502017001276 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| 26N | No opposition filed |
Effective date: 20200218 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190825 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20190825 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R081 Ref document number: 502017001276 Country of ref document: DE Owner name: PERFECT IDEAS GMBH, CH Free format text: FORMER OWNER: RICHLI, REMO, HERGISWIL, CH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PUE Owner name: PERFECT IDEAS GMBH, CH Free format text: FORMER OWNER: RICHLI, REMO, CH |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: PD Owner name: PERFECT IDEAS GMBH; CH Free format text: DETAILS ASSIGNMENT: CHANGE OF OWNER(S), ASSIGNMENT; FORMER OWNER NAME: RICHLI, REMO Effective date: 20201028 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20201119 AND 20201125 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: PD Owner name: PERFECT IDEAS GMBH; CH Free format text: DETAILS ASSIGNMENT: CHANGE OF OWNER(S), CESSION Effective date: 20201102 Ref country code: BE Ref legal event code: PD Owner name: PERFECT IDEAS GMBH; CH Free format text: DETAILS ASSIGNMENT: CHANGE OF OWNER(S), CESSION; FORMER OWNER NAME: RICHLI, REMO Effective date: 20201102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190915 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20170825 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: PC Ref document number: 1133432 Country of ref document: AT Kind code of ref document: T Owner name: PERFECT IDEAS GMBH, CH Effective date: 20210824 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190515 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20240809 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20250822 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DK Payment date: 20250818 Year of fee payment: 9 Ref country code: DE Payment date: 20250820 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20250820 Year of fee payment: 9 Ref country code: GB Payment date: 20250818 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20250820 Year of fee payment: 9 Ref country code: FR Payment date: 20250818 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20250901 Year of fee payment: 9 |