EP1992687B1 - Aviäre Zelllinien für die Herstellung von nützlichen Substanzen - Google Patents
Aviäre Zelllinien für die Herstellung von nützlichen Substanzen Download PDFInfo
- Publication number
- EP1992687B1 EP1992687B1 EP08163979.1A EP08163979A EP1992687B1 EP 1992687 B1 EP1992687 B1 EP 1992687B1 EP 08163979 A EP08163979 A EP 08163979A EP 1992687 B1 EP1992687 B1 EP 1992687B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- cells
- cell line
- avian
- virus
- medium
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0603—Embryonic cells ; Embryoid bodies
- C12N5/0606—Pluripotent embryonic cells, e.g. embryonic stem cells [ES]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/90—Serum-free medium, which may still contain naturally-sourced components
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/90—Serum-free medium, which may still contain naturally-sourced components
- C12N2500/92—Medium free of human- or animal-derived components
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/90—Serum-free medium, which may still contain naturally-sourced components
- C12N2500/95—Protein-free medium and culture conditions
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
- C12N2510/02—Cells for production
Definitions
- the present disclosure describes a method for obtaining avian cell lines, including avian stem cells, comprising progressive or total weaning of growth factors, serum and / or feeder. These spontaneously established lines are adherent or non-adherent cells capable of proliferating indefinitely in a basic culture medium.
- the invention relates to cells derived from such lines which are particularly useful for the production of vaccines and substances of interest.
- stem cells In contrast to previously differentiated primary cells, stem cells do not exhibit a readily recognizable characteristic morphological differentiation state (fibroblast, adipocyte, macrophage, etc.), but are rather characterized by a state of proliferation and non-differentiation. This condition results in different behaviors such as rapid in vitro proliferation , characteristic morphology, the presence of different markers, varying growth factor requirements, and ability to respond to particular induction differentiation stimuli. They are not sensitive to replicative senescence, a critical period for a large number of differentiated primary cells, including fibroblasts, for example.
- telomeres are repetitive sequences located at the end of chromosomes. The shortening of these repetitive nucleotide structures is the consequence of DNA replication in a semi conservative mode. In the absence of the enzyme telomerase, in charge of adding the repeated sequences to the end of the chromosomes, a point of no return is reached as to the size of the telomeres, point beyond which is triggered a molecular mechanism yet unknown activation of genes involved in cell cycle control.
- telomere can be seen as a central actor of cellular immortality, because it maintains the length of the telomeres and therefore makes the cell insensitive to this loss generated by successive divisions.
- telomere activity In a developing organism and during the life of this organism, only a few cell types, some of which have a permanent expression of telomerase. This activity also appears to be one of the characteristics of stem cells, both somatic (CSS) and germinal level. This property of expression, expression maintenance or 'wake up' expression of telomerase activity is also often associated with the immortality of a cell maintained in vitro. To date, many cancer cells are also detected positive for telomerase activity. This activity would be partly responsible for the uncontrolled proliferation capacity of tumor cells in vivo.
- telomere activity is, in all cases, an excellent marker of stem cell character as well as germline lineage and the ability of a cell to become immortal. Two criteria are therefore used: the activity of telomerase and the size of telomeres.
- the problem is to be able to maintain cells in culture in an economic environment while avoiding pitfalls such as differentiation and cellular senescence.
- a first step drives the proliferating cell to the Hayflick limit, which according to the cell types is between 10 and 50 passages.
- a first spontaneous mutational event then takes place which allows the cell to cross this first block, an event that often affects the p53, pRb, .... cells.
- the cells therefore continue to proliferate until a second block occurs which is in general raised by new mutations in other genes and by the activation of telomerase, often observed.
- immortalizing genes adenovirus E1A gene, SV40 polyome T ', etc.
- gene fragments also made it possible to obtain lines from cells. already differentiated primary.
- These elements can be introduced into the cells by simple vector transfection allowing the expression of the immortalizing part, but also introduced via genetically modified viruses or retroviruses to express these immortalizing elements.
- the origin of the immortalizers can be avian or not, viral or not.
- the tropism for avian cells may be related to the original virus or also modified.
- the TDF-2A line of duck fibroblasts is thus obtained by introducing a first immortalizing gene and then an anti-apoptotic gene (Guilhot et al., 1993; US6,255,108 ).
- Other methods have been developed, such as over-expression of p53 (Foster et al. U.S. Patent No. 5,830,723 ).
- stem cell populations notably somatic stem cells, which can grow indefinitely in media of basic culture.
- hematopoietic stem cells which are mostly non-adherent cells
- the majority of the cells obtained according to the techniques of the art mentioned above (fibroblasts, hepatocytes, etc.) have an adherent phenotype.
- the industrial use of cells, as a viral replication support favors non-adherent cells. This phenotype is interesting both for reasons of ease of manipulation that avoids the use of proteolytic enzyme dissociation that for the high densities reached by non-adherent cells cultured in vitro.
- the present invention describes obtaining lines that can become spontaneously non-adherent or for which non-adherence has been achieved by weaning the feeder mat. Due to their growth in suspension, these lines are perfectly adapted to an industrial use for the production of substances of interest in bioreactors.
- the cells resulting from the lines obtained in step c) are capable of proliferating for at least 50 days, 100 days, 150 days, 300 days or preferably at least 600 days.
- the 600 days are not a time limit because the resulting cell lines are still alive after much longer periods of time. Therefore, these lines are considered to be able to grow indefinitely in a basic culture medium devoid of exogenous growth factors, serum and / or inactivated feeder.
- line is meant any population of cells capable of indefinitely proliferating in in vitro culture, keeping little or the same morphological and phenotypic characteristics.
- the cells resulting from the lines according to the invention may be avian stem cells, in particular somatic avian stem cells.
- the stem cells according to the invention can be used to derive differentiated cell lines. Indeed, these stem cells have the property of being pluripotent, that is to say they have the potential to be induced in multiple differentiation pathways, characterized by different specific markers.
- These cells may also be precursor cells, which correspond to cells of a partially differentiated adult or embryonic tissue as opposed to a stem cell and which is capable of dividing and giving more differentiated cells.
- differentiated cell is meant any cell of a specialized adult or embryonic tissue, having specific markers or fulfilling specific physiological functions. It is possible, in a particular aspect of the invention, particularly for particular isolates or clones derived from a particular isolate obtained during establishment, that these stem cells can contribute to the germ line. In this case, these stem cells established in lines would be embryonic stem cells.
- the invention is obtained via a method as defined above, and specified hereinafter in which the established lines are adherent stem cells which also proliferate in the absence of the inactivated feeder mat.
- step b) consists of weaning the components of the medium (growth factors only or growth factors then serum or serum and growth factors).
- the invention is obtained via a method as defined above and specified below, in which the established lines are non-adherent stem cells which proliferate in suspension in a medium devoid of exogenous growth factors.
- step b) consists of a gradual or total weaning of the feeder belt in addition to possibly weaning of the other components of the medium (growth factors and serum).
- the invention is obtained via a method as defined above and specified hereinafter, in which the established lines are non-adherent stem cells which proliferate in suspension in a medium also free of serum (aseric medium ).
- the invention is obtained via a method as defined above and specified hereinafter, in which the established lines are non-adherent stem cells which proliferate in suspension in a medium free of exogenous growth factors and of serum.
- step b) consists of progressive or total weaning of the growth factors, optionally followed by progressive weaning of the serum.
- step b) consists of progressive or total weaning of growth factors and serum, optionally followed by weaning of the feeder mat.
- established lines may be cells that proliferate in serum-depleted medium, particularly in serum-free medium.
- depleted serum is meant a gradual decrease in serum concentration spread over time. This method allows a selection of clones that adapt to these new conditions more and more drastic until stable lines are obtained which are able to grow in serum depleted medium or completely serum free.
- the method described above may further comprise a step in which the cells obtained in step c) are selected in culture media used for large scale production so as to obtain clones suitable for production. vaccines for human or animal therapy.
- the cells of the invention have all the characteristics mentioned above.
- the invention is obtained via a method for obtaining avian lines mentioned above and specified hereinafter, in which the cells resulting from the lines obtained in step c) are modified to allow better use. in vitro, such as longer life span or higher growth densities or lower nutrient requirements.
- the cells from the established lines are modified to produce a substance of interest, in particular a polypeptide of interest, an antibody or an attenuated virus.
- Said cells may be modified with any technique within the reach of those skilled in the art, in particular homologous, directed and / or conditional recombination (Cre-Lox system or FLP-FRT), by transformation with any vector, plasmid, especially at the help from retroviruses.
- the medium used in step a) may comprise at least one factor selected from cytokines, in particular LIF, IL-11, IL-6, IL-6R, CNTF, Oncostatin and other factors such as SCF, IGF-1 and bFGF.
- cytokines in particular LIF, IL-11, IL-6, IL-6R, CNTF, Oncostatin and other factors such as SCF, IGF-1 and bFGF.
- the inactivated feeder mat used in step a) preferably consists of fibroblasts, including mouse fibroblasts established in the line.
- fibroblasts are in particular STO cells which may or may not be modified or transfected with expression vectors (Pain et al., 1996).
- the cells used in step a) are cells obtained by suspending cells from fertilized egg blastoderm discs in a culture medium comprising at least one cytokine, b-FGF, and SCF. Said cells are seeded on a carpet of feeder cells, incubated and then removed.
- Step b) consists of a progressive weaning of each growth factor added in the medium of step a), in particular a cytokine, b-FGF, and SCF, comprising a passage in a new medium without at least one said factors and to repeat different successive passages until the medium is deprived of all of said factors.
- Progressive weaning means a factor-by-factor suppression in the culture medium.
- the weaning in step b) may consist in reducing the concentration of one or more factors progressively or in culturing the avian stem cells directly in a medium lacking one or more factors or even without all of said factors.
- Step b) may also include weaning the serum.
- the weaning can be progressive by decreasing the serum concentration during each pass, for example from 10% to 7.5% and then 3.75% and 2% to reach 0% (aseric medium). Alternatively, we can proceed to a drastic weaning.
- Step b) may also include weaning the feeder mat.
- Weaning the feeder mat can also be gradual by decreasing the number of inactivated feeder cells on each pass. Alternatively, we can proceed to a drastic weaning.
- weaning can vary. For example, we can start with the weaning of growth factors and continue with the weaning of the feeder mat.
- the invention relates to established cell lines and cells derived from said lines that can be obtained from the method described above, said cells being capable of proliferating for at least 50 days, 100 days, 150 days, 300 days. days, or preferably at least 600 days in a medium devoid of exogenous growth factor, serum and / or feeder.
- the invention also relates to cell lines and cells derived from such lines described above, characterized in that they are avian stem cells, in particular avian embryonic stem cells.
- stem cells can be adherent while proliferating in the absence of the inactivated feeder pad.
- these stem cells are non-adherent and proliferate in suspension in a basic medium mentioned above.
- these cells are genetically modified so as to produce a substance of interest, in particular a polypeptide of interest, an antibody or an attenuated virus.
- Said cells may, for example, support the replication of live or attenuated viruses, in particular the viruses selected from the group of adenoviruses, hepadnaviruses, herpesviruses, orthomyxoviruses, papovaviruses, paramyxoviruses, picornaviruses, poxviruses, reoviruses and retrovirus.
- viruses selected from the group of adenoviruses, hepadnaviruses, herpesviruses, orthomyxoviruses, papovaviruses, paramyxoviruses, picornaviruses, poxviruses, reoviruses and retrovirus.
- the viruses replicated on these cells belong to the orthomyxovirus family, in particular the influenza virus or to the paramyxovirus family, in particular the measles, mumps and rubella viruses.
- the invention relates to the cell lines described above.
- the invention relates to the cell lines resulting from step c) of the method described above characterized in that they are avian stem cells capable of growing indefinitely in a basal medium devoid of exogenous growth factors, depleted in serum or lacking serum and / or nourishing mats.
- the cells obtained at the end of step c) can be genetically modified.
- the application also describes a cell culture comprising cells derived from the cell lines described above, in particular avian stem cells or avian embryonic stem cells and a basal medium free of exogenous growth factors, depleted in serum or devoid of serum and / or inactivated feeder mat.
- the invention relates to cell lines characterized in that said cells replicate live or attenuated viruses, in particular viruses taken from the group of adenoviruses, hepadnaviruses, herpesviruses, orthomyxoviruses, papovaviruses, paramyxoviruses , picornaviruses, poxviruses, reoviruses and retroviruses.
- viruses taken from the group of adenoviruses, hepadnaviruses, herpesviruses, orthomyxoviruses, papovaviruses, paramyxoviruses , picornaviruses, poxviruses, reoviruses and retroviruses.
- the cell lines and the cells described above are used for the production of viruses belonging to the family of orthomyxoviruses, in particular the influenza virus and for the production of viruses belonging to the family of paramyxoviruses, in particular the viruses of measles, mumps and rubella.
- Example 1 Variable origin of the living material used
- strain S86N a commercial strain intended for the production of Label chicken
- CNRs strain intended for the production of Label chicken
- Marens a genetically and phenotypically well-characterized local strain
- the White Leghorn strain more intended for the production of eggs for consumption and reference strain of research laboratories, etc.
- Valo some eggs
- 'SPF' eggs SPF Specific Pathogen Free
- the eggs are open, the yolk is separated from the white when opened.
- Embryos are taken from the yolk either directly using a Pasteur pipette or using a small filter paper (Whatmann paper 3M), previously cut in the form of a perforated ring at the using a drill. The diameter of the perforation being about 5 mm.
- These small rings are sterilized in dry heat for about 30 minutes in the oven. This small ring of paper is deposited on the surface yellow and centered at the level of the embryo which is thus surrounded by the paper ring. The latter is then cut with small scissors and the whole taken is placed in a petri dish, filled with PBS or a physiological liquid. The embryo thus driven by the ring is cleaned of the excess of yolk in the medium and the embryonic disk, thus rid of excess yolk, is taken with a Pasteur pipette.
- the embryos are put in a tube containing physiological medium (PBSIX, Tris Glucose, medium, ).
- physiological medium PBSIX, Tris Glucose, medium, .
- the embryos are then mechanically dissociated and seeded on a feeder in defined culture medium.
- a mixture of nucleosides may also be added, this mixture being constituted as previously described (Pain et al., 1996).
- the basic media tested under these same conditions and which give similar results are Ham F12, Glasgow MEM and DMEM, the latter supplemented with biotin at a final concentration of 8 mg / l.
- the biotin concentration is 0.2 mg / l in MacCoy medium, 0.0773 mg / l in Ham F12 and 0 in commercial DMEM and GMEM media.
- Growth factors and cytokines added in the culture medium are favorably recombinant factors and cytokines including mouse SCF at a final concentration of 1 ng / ml, IGF-1 at a final concentration of 1 to 5 ng / ml, the CNTF at a final concentration of 1 ng / ml, IL-6 at a final concentration of 1 ng / ml, and the soluble IL-6 receptor at a final concentration of 0.5 ng / ml to 1 ng / ml.
- some other factors may be added during the first runs. For example, until passage 3 or 10, bFGF can be added to the medium at a final concentration of 1 ng / ml and IL-11 at a final concentration of 1 ng / ml.
- Inoculation is carried out in this medium on the inactivated feeder composed of mouse fibroblasts established in line, the STO cells.
- these cells were transfected with simple expression vectors allowing expression of growth factors such as avian SCF, constitutively in STO cells.
- this feeder produces the factor in a soluble and / or attached form in the plasma membrane of the cells.
- the medium After the initial inoculation of the cells directly into this medium, the medium is partially changed the next day, then partially or totally during the following days, depending on the adhesion rate observed for the primary cells. After about 4 to 7 days depending on the case, the initial culture is dissociated and passed into new boxes in the same initial medium on the inactivated feeder. After three to five passages, the cells are cultured on an inactivated feeder of STO cells not transfected or transfected with an expression vector coding for antibiotic resistance, such as the neomycin, hygromycin, puromycin, . After about twenty passages, the cells are gradually weaned in growth factors and cytokines. Progressive weaning means a factor-by-factor suppression in the culture medium.
- the SCF is removed first and then, two or three passages later, the IGF-1. If the cells do not show morphological alterations or variations in their average proliferation rate, the other factors, such as CNTF, IL-6, are then removed. This weaning can be equally drastic. Then all the factors are removed at one time. The cells are then observed and only passed several days later if their proliferation rate is changed. This last solution is generally that which is practiced.
- Very long periods of time are periods of the order of several weeks with a minimum of 50 days, preferably longer than 200 to 400 days, without limitations in time. Times greater than 600 days are observed.
- all cells that are adherent are dissociated with a proteolytic enzyme of dissociation, such as pronase, collagenase, dispase, trypsin, etc.
- a proteolytic enzyme of bacterial origin is used, in order to avoid any potential contaminant of animal origin.
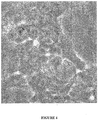
- These cells have the characteristics of embryonic stem cells with a specific morphology illustrated, for example, by the photo of the Figure 4 ie a small size, an important nucleocytoplasmic ratio, a nucleus with at least one well visible nucleolus and a very weak cytoplasm. These cells are characterized by a growth in more or less compact masses.
- Adherent and non-adherent cells exhibit cross-reactivity with a number of antibodies, as previously described in Pain et al., 1996 and in patents US 6,114,168 and EP 787,180 .
- the endogenous telomerase activity component is also present and is an important element of the 'strain' nature of these cells.
- Table 1 illustrates some of the characteristics of these isolates. Name Species beginning "Stop" days Passage Generation S86N 16 Chicken S86N 26-01-2000 05-08-2001 559 207 692 WL3 Chicken WL 28-06-2000 09-08-2001 403 153 333 Valo4 Valo chicken 26-09-2000 07-02-2002 401 135 317 S86N 45 Chicken S86N 29-01-2001 12-11-2001 287 118 329
- stop does not correspond to the end of cell proliferation but to a voluntary stopping of cell cultures by the experimenter.
- stem cells including somatic and embryonic stem cells, are their ability to proliferate in vitro for considerable periods of time.
- the culture medium is changed and replaced by new medium a few hours before their passage.
- the curve presented in figure 1 illustrates a pattern of growth and establishment of cells.
- Example 4 doubling time and average division time
- an average division time can be calculated. For all the independent isolates obtained, the proliferation rate increases slightly during successive passages thus changing the average time of division during the establishment of the cells.
- the cells are initially seeded on an inactivated feeder mat (called 'feeder') and are regularly passed at a constant initial seed density of 1 to 2 x 10 6 cells per 100 mm dish.
- Table 2 shows the doubling time (d) and the mean time of division (TMD in hours) for 3 established cell types as a function of culture time. It can be seen that the average doubling time decreases during establishment.
- the mean division time (TMD) is then obtained in hours by dividing 24 hours by d. * Valo cells are passed during this establishment on plastic support without presence of feeder. The doubling time decreases then re-increases, when the cells become accustomed to this new environment.
- the culture media used are conventional culture media comprising a base (DMEM, GMEM, HamF12, McCoy, etc.) supplemented with various additives such as non-essential amino acids, vitamins, sodium pyruvate.
- This complex medium comprises fetal calf serum, which remains a central element of the culture, although components of different origins of which plant components can be progressively used.
- a process for controlling and habituating cells to relatively low proportions of fetal calf serum is presented. It is thus possible to maintain cells in high proliferation (division time> 1) with low serum percentages (2% for example in the case of S86N 16 cells).
- Example 6 Withdrawal of cells in a feeder belt
- cytokines cytokines and trophic factors.
- Cytokines are mainly cytokines whose action passes through a receptor that associates with the gp130 protein.
- LIF interleukin 11, interleukin 6, CNTF, oncostatin, cardiotrophin
- a similar mode of action with recruitment to the receptor of a specific chain and the association of the latter with the gp130 protein in monomeric or sometimes heterodimeric form.
- the combination of a soluble form of the receptors, a form described inter alia for the interleukin 6 and CNTF receptors makes it possible to increase the proliferative effect observed. It has been previously shown that the addition of at least one of these cytokines seemed necessary for obtaining embryonic stem cells.
- the trophic factors are mainly SCF, IGF-1, bFGF, which are also used at the beginning of the culture, as previously described. Their presence is also necessary to obtain and amplify the cells.
- a high density seeding directly in a bacteriological box makes it possible to obtain, after a few passages, embryonic cells which detach from their substrate and which proliferate in suspension in the form of small regular aggregates.
- This proliferation is encouraged on several passages by simple dilution, mechanical dissociation and non-use of proteolytic enzyme.
- the agitation of the cultures is generally practiced but does not represent a discriminating element for obtaining non-adherent cells.
- these cells have a characteristic morphology of stem cells ie a small size, an important nucleocytoplasmic ratio, a nucleus with at least one well visible nucleolus and a very weak cytoplasm.
- These cells are characterized by growth in small aggregates more or less compact. These non-adherent cells exhibit cross-reactivity with a certain number of antibodies, as previously described in Pain et al., 1996. These cells are also positive for endogenous telomerase activity (as shown in Example 10 for EB1, EB4 and EB5 cells). In the non-adherent phase, the cells exhibit a significant proliferation in different media. The initial density of seeding as well as the supply of fresh medium very regularly ensures high densities that can go beyond 1 x 10 6 cells per ml. Table 5 summarizes the main characteristics of some isolates (parental cells, initial passage of suspension, number of days maintained in suspension culture, number of passages and generations obtained before a voluntary cessation of interviews).
- the passage for suspension can vary from one isolate to another (see isolate EB1 and EB14) as well as the proliferation rate (see isolate EB3 and EB14).
- Table 5 ⁇ / b> Name Parental cells
- Stem cells maintained for long periods of culture are characterized with the same criteria as those described above (Pain et al., 1996). It is thus possible to detect endogenously alkaline phosphatase activity, illustrated by the photo of the figure 5 , endogenous telomerase activity and reactivity with certain specific antibodies such as SSEA-1 (TEC-01) and EMA-1 antibodies.
- telomerase activity is detectable for S86N16 cells, S86N45 cells as well as for EB1, EB4 and EB5 cells derived therefrom in non-adherent form (see Table 6 ).
- Chicken Embryonic Fibroblasts (CEF) maintained in primary culture are considered negative.
- the threshold of an OD ⁇ 0.2 is the threshold recommended by the kit as the negative threshold. All analyzes were performed on an equivalent of 2000 cells.
- Stem cells maintained in long-term growth are transfected with different expression plasmids. It has been shown that avian stem cells can be transfected (Pain et al., 1999). In particular, the non-adherent cells are transfected and various sorting systems make it possible to identify the cells stably transfected (cell sorting, limiting dilution, etc.). These modifications can occur at the undifferentiated stage of the stem cell. This modification obtained, the cell is then induced to differentiate spontaneously or by addition of differentiation inducer.
- retinoic acid can be used at concentrations of 10 -8 M to 10 -6 M, or dimethysulfoxide at concentrations of 1 to 2% final or sodium butyrate at concentrations of 10 -4 to 10 -8 M, or phorbol ester (TPA, PMA, ...) or lipopolysaccharrides (LPS) at concentrations of 1 to 5 ⁇ g / ml final.
- the cells can form embryoid bodies in suspension, embryoid bodies that can be adhered to plastic after dissociation or not the cells that constitute them. These differentiated cells then proliferate but have a more limited capacity for proliferation in the long term. By targeting genetic modification to a gene that influences cell proliferation, these differentiated cells can be made capable of proliferating in the long term.
- Adherent and non-adherent cells are infectable by different viruses and retroviruses including avian viruses and retroviruses. These cells can thus serve as replication support for the production of viral stocks intended for the production of live and attenuated or inactivated human and veterinary vaccines as the case may be.
- viruses of interest mention may be made of those of the adenovirus family (such as Human Adenovirus C, Fowl Adenovirus A, Ovine adenovirus D, Turkey Adenovirus B), circovirids (such as Chicken Anemia Virus, CAV), certain coronaviruses, such as avian infectious bronchitis virus (IBV), flaviviruses (such as Yellow fever virus and hepatitis C virus), hepadnaviruses (such as Hepatitis B virus and Avihepadnavirus such as Duck hepatitis B virus); herpesviruses (such as Gallid herpesvirus, HSV (Herpes simplex virus) and Human herpesviruses 1, 3 and 5), orthomyxoviruses (such as influenza virus: Influenzaavirus A, Influenzavirus B and Influenzavirus C), papovaviruses (such as polyomavirus and more particularly Simian virus 40), paramyxoviruses (such as mea
- Example 13 Protocol of infection of a non-adherent avian cell line (EB1) with a virus
- the EB1 or EB14 cells are seeded in a medium, preferably MacCoy's 5A, HAMF12, DMEM, or any other medium of interest, containing 5% serum at a concentration of 0.2 10 6 cells / ml for an initial volume. 50 ml in general. They are kept in culture at 39 ° C. and 7.5% CO 2 , with stirring. New medium is added daily during the 3 to 4 days of amplification to reach a cell concentration of 1 to 3 x 10 6 cells / ml for a final culture volume of 100 to 250 ml.
- a medium preferably MacCoy's 5A, HAMF12, DMEM, or any other medium of interest, containing 5% serum at a concentration of 0.2 10 6 cells / ml for an initial volume. 50 ml in general. They are kept in culture at 39 ° C. and 7.5% CO 2 , with stirring. New medium is added daily during the 3 to 4 days of amplification to reach a cell concentration of 1 to
- the cells in suspension are removed and centrifuged for 10 minutes at approximately 1000 rpm.
- the pellet is resuspended in 20 to 50 ml of 1X PBS (phosphate buffer salt).
- the cells are then counted, centrifuged and the pelleted cells are taken up in serum-free medium at a final concentration of 3 to 5.10 6 cells / ml.
- Several tubes are then prepared under these conditions containing from 3 to 5 ⁇ 10 6 cells per tube.
- the known virus stock of titer is thawed rapidly at 37 ° C and diluted in serum-free medium at a level of 10x to 1000x the concentration required for final infection.
- the cells are infected with the virus of interest to a me (multiplicity of infection) of 0.01 to 0.5 depending on the types of virus, which makes add between 0.1 and 10% volume / volume of virus suspension at cell pellet. After incubation for 1 hour at the optimum temperature for the virus, usually 33-37 ° C, the cells are centrifuged again and the medium removed with caution. This step is often necessary to limit the effect of the initial virus in the subsequent process.
- One of the Possibilities is to directly dilute the cells without centrifuging them again with medium with serum (5% of serum) at a final concentration of 0.2 to 1.10 6 cells / ml and put back into incubation.
- the medium containing the cells or cellular debris is harvested.
- the viruses only the pellet or the supernatant can be interesting and contain the viral particles.
- the cells are harvested and centrifuged.
- the collected supernatant is centrifuged again for 5 to 10 minutes at 2500 rpm, and stored at -80 ° C. before purification of the particles.
- An aliquot is taken for the realization of the titration.
- the cell pellet is taken up in 5 ml of serum-free medium, sonicated, and centrifuged for 5 to 10 minutes at 2500 rpm.
- the supernatant obtained is stored at -80 ° C. until purification and titration of an aliquot.
- the viral infection and production efficiencies are compared between different conditions.
- titrations are generally carried out by the lysis plaque technique.
- Example 14 Protocol for Infection of an Adhered Avian Cell Lines (S86N45) with a Virus
- the cells are inoculated 48 hours before infection in T150 flasks at a concentration of between 0.03 and 0.06 10 6 cells / cm 2 in a medium, preferably MacCoy's 5A medium, HAMF12, DMEM, or any other interesting medium containing 5% serum. They are maintained at 39 ° C and 7.5% CO 2 .
- the known virus stock of titer is thawed rapidly at 37 ° C and diluted in serum-free medium at a level of 10x to 1000x the concentration required for final infection.
- the cells are infected with the virus of interest to a me (multiplicity of infection) of 0.01 to 0.5 depending on the types of virus, which makes add between 0.1 and 10% volume / volume of virus suspension at cell pellet.
- the infection is usually performed in a minimum of medium (from 5 to 10 ml for a bottle of 75 cm 2 ) in a medium at 0% serum.
- the cells After 1 hour of incubation at the optimal temperature for the virus, generally from 33 to 37 ° C., 20 ml of 5% medium are added to the flasks. In a particular case, the cells can be washed with PBS to remove particles that would not be attached to the cells. In the case of a cytopathic virus, cells are observed daily after infection to follow the appearance of cell lysis range, indicative of the smooth course of infection.
- the medium containing the supernatant, the cells and the cell debris are harvested. According to the viruses, only the pellet or the supernatant can be interesting and contain the viral particles.
- the cells are harvested and centrifuged. The collected supernatant is centrifuged again for 5 to 10 minutes at 2500 rpm, and stored at -80 ° C. before purification of the particles. An aliquot is taken for the realization of the titration.
- the cell pellet is taken up in 5 ml of serum-free medium, sonicated, and centrifuged for 5 to 10 minutes at 2500 rpm. The supernatant obtained is stored at -80 ° C. until purification and titration of an aliquot.
- the viral infection and production efficiencies are compared between different conditions.
- titrations are generally carried out by the technique of lysis plaques.
- the non-adherent EB1 stem cells passing through are amplified and then infected at a pH of 0.1 by a recombinant avipox producing a protein of interest.
- the cells After infection, the cells are kept spinner for the 4 days of infection. An aliquot is taken from the second day and then the next two days to follow the evolution of the viral titre both at the level of the supernatant and at the level of intracellular content, after lysis of cellular content. Titration was performed by the lysis plaque technique.
- Table 7 illustrates the results obtained. These results demonstrate the very satisfactory replication of recombinant avipox on EB1 stem cells. Thus, the infectious titre progresses throughout the culture and the course of the infection to reach a maximum after 4 days of incubation. 7.2 PFU / cell (PFU: Plating Forming Unit). This titre is at least equivalent to that obtained for this same recombinant avipox on primary cells of chicken embryos.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Wood Science & Technology (AREA)
- Chemical & Material Sciences (AREA)
- Gynecology & Obstetrics (AREA)
- Biotechnology (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- Reproductive Health (AREA)
- Developmental Biology & Embryology (AREA)
- Zoology (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Cell Biology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Feeding And Watering For Cattle Raising And Animal Husbandry (AREA)
- Farming Of Fish And Shellfish (AREA)
- Peptides Or Proteins (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
Claims (15)
- Vogelzelllinie, erhältlich durch ein Verfahren, umfassend die Schrittea) Kultivieren von embryonalen Vogelstammzellen in einem Medium, umfassend:- mindestens einen Wachstumsfaktor, ausgewählt aus SCF, IGF-1 und bFGF, und mindestens ein Cytokin, ausgewählt aus LIF, IL-11, IL-6, IL-6R, CNTF, Onkostatin und Cardiotrophin;- eine inaktivierte Feeder-Schicht von STO-Maus-Fibroblastenzellen; und- fötales Kälberserum in einer Konzentration von 12 bis 8%,b) nach etwa zwanzig Passagen Modifizieren des Kulturmediums:- durch fortschreitendes Entziehen des Wachstumsfaktors (der Wachstumsfaktoren) und des Cytokins (der Cytokine); oder- durch fortschreitendes Entziehen der Feeder-Schicht; oder- durch Verringern der Konzentration von fötalem Kälberserum bis zum Erreichen geringer Gehalte von 2%,c) Etablieren adhärenter oder nicht-adhärenter Zelllinien, die zur Proliferation in einem Basismedium in Abwesenheit von exogenen Wachstumsfaktoren und Cytokin, Serum oder inaktivierter Feeder-Schicht im Stande sind, wobei das Etablieren nicht-adhärenter Zellinien erzielt wird durch Animpfen mit mindestens 1x106 Zellen/ml in einer bakteriologischen Schale, gefolgt von mehreren Passagen in einfacher Verdünnung,wobei die besagten Zellen der besagten Linie zum Proliferieren in In-vitro-Kultur in einem Basiskulturmedium, dem exogene Wachstumsfaktoren und Cytokine fehlen, für mindestens 50 Tage im Stande sind und eine Reaktivität mit spezifischen Antikörpern zeigen, die aus der Gruppe SSEA-1 (TEC01) und EMA-1 ausgewählt sind.
- Linie von Vogelstammzellen gemäß Anspruch 1, dadurch gekennzeichnet, dass die Linie mindestens eine der folgenden Eigenschaften aufweist:- ein deutlich sichtbarer Nucleolus und ein sehr schwaches Nucleoplasma;- eine endogene Telomerase-Aktivität;- und eine endogene Alkalische-Phosphatase-Aktivität.
- Zelllinie gemäß einem der Ansprüche 1 oder 2, dadurch gekennzeichnet, dass der Schritt b) auch das fortschreitende Entziehen des fötalen Kälberserums umfasst, wobei die Zellen der besagten Linie zum Proliferieren in Abwesenheit von fötalem Kälberserum und exogenen Wachstumsfaktoren und Cytokinen im Stande sind.
- Zelllinie gemäß einem der Ansprüche 1 bis 3, dadurch gekennzeichnet, dass der Schritt b) auch ein fortschreitendes Entziehen der Feeder-Schicht umfasst, wobei die Zellen der besagten Linie zum Proliferieren in Abwesenheit einer Feeder-Schicht und exogener Wachstumsfaktoren und Cytokinen im Stande sind.
- Vogelzelllinie gemäß einem der Ansprüche 1 bis 4, dadurch gekennzeichnet, dass die Zellen der besagten Linie zum Proliferieren für mindestens 100 Tage im Stande sind.
- Vogelzelllinie gemäß einem der Ansprüche 1 bis 4, dadurch gekennzeichnet, dass die Zellen der besagten Linie zum Proliferieren für mindestens 150 Tage im Stande sind.
- Vogelzelllinie gemäß einem der Ansprüche 1 bis 4, dadurch gekennzeichnet, dass die Zellen der besagten Linie zum Proliferieren für mindestens 300 Tage im Stande sind.
- Vogelzelllinie gemäß einem der Ansprüche 1 bis 4, dadurch gekennzeichnet, dass die Zellen der besagten Linie zum Proliferieren für mindestens 600 Tage im Stande sind.
- Vogelzelllinie gemäß einem der Ansprüche 1 bis 8, dadurch gekennzeichnet, dass das Basiskulturmedium ausgewählt ist aus der Gruppe bestehend aus DMEM, GMEM, HamF12 und McCoy, ergänzt mit nicht-essentiellen Aminosäuren, Vitaminen und Natriumpyruvat.
- Vogelzelllinie gemäß einem der Ansprüche 1 bis 9, dadurch gekennzeichnet, dass die Zelllinie eine Linie adhärenter Zellen ist.
- Vogelzelllinie gemäß einem der Ansprüche 1 bis 9, dadurch gekennzeichnet, dass die Zelllinie eine Linie nicht-adhärenter Zellen ist, die in Suspension proliferieren.
- Vogelzelllinie gemäß einem der Ansprüche 1 bis 11, dadurch gekennzeichnet, dass die Zellen ein lebendes oder abgeschwächtes Virus replizieren, wobei das Virus ausgewählt ist aus der Gruppe von Adenoviren, Hepadnaviren, Herpesviren, Orthomyxoviren, Papovaviren, Paramyxoviren, Picornaviren, Pockenviren, Reoviren und Retroviren.
- Vogelzelllinie gemäß Anspruch 12, dadurch gekennzeichnet, dass das Virus ein Orthomyxovirus ist und das Grippevirus ist.
- Vogelzelllinie gemäß Anspruch 12, dadurch gekennzeichnet, dass das Virus ein Paramyxovirus ist, ausgewählt aus der Gruppe bestehend aus Masern-, Mumps- und Rötelnviren.
- Vogelzelllinie gemäß Anspruch 12, dadurch gekennzeichnet, dass das Virus ein Pockenvirus ist, ausgewählt aus der Gruppe bestehend aus Kanarienpockenvirus und Geflügelpockenvirus.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0202945A FR2836924B1 (fr) | 2002-03-08 | 2002-03-08 | Lignees de cellules aviaires utiles pour la production de substances d'interet |
| EP03725273A EP1483369B1 (de) | 2002-03-08 | 2003-03-07 | Vögelzell-linien und deren verwendung zur herstellung interessanter biologischer substanzen |
| PCT/FR2003/000735 WO2003076601A1 (fr) | 2002-03-08 | 2003-03-07 | Lignées de cellules aviaires utiles pour la production de substances d'intérêt |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP03725273A Division EP1483369B1 (de) | 2002-03-08 | 2003-03-07 | Vögelzell-linien und deren verwendung zur herstellung interessanter biologischer substanzen |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP1992687A2 EP1992687A2 (de) | 2008-11-19 |
| EP1992687A3 EP1992687A3 (de) | 2009-03-25 |
| EP1992687B1 true EP1992687B1 (de) | 2019-06-26 |
Family
ID=27763651
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP08163979.1A Expired - Lifetime EP1992687B1 (de) | 2002-03-08 | 2003-03-07 | Aviäre Zelllinien für die Herstellung von nützlichen Substanzen |
| EP03725273A Expired - Lifetime EP1483369B1 (de) | 2002-03-08 | 2003-03-07 | Vögelzell-linien und deren verwendung zur herstellung interessanter biologischer substanzen |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP03725273A Expired - Lifetime EP1483369B1 (de) | 2002-03-08 | 2003-03-07 | Vögelzell-linien und deren verwendung zur herstellung interessanter biologischer substanzen |
Country Status (15)
| Country | Link |
|---|---|
| US (4) | US20040058441A1 (de) |
| EP (2) | EP1992687B1 (de) |
| JP (2) | JP5025888B2 (de) |
| CN (2) | CN100562567C (de) |
| AT (1) | ATE408004T1 (de) |
| AU (1) | AU2003227820B2 (de) |
| CA (1) | CA2478125C (de) |
| DE (1) | DE60323468D1 (de) |
| DK (1) | DK1483369T3 (de) |
| ES (1) | ES2312775T3 (de) |
| FR (1) | FR2836924B1 (de) |
| HK (1) | HK1142094A1 (de) |
| PT (1) | PT1483369E (de) |
| SI (1) | SI1483369T1 (de) |
| WO (1) | WO2003076601A1 (de) |
Families Citing this family (117)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7445924B2 (en) * | 2000-11-23 | 2008-11-04 | Bavarian Nordic A/S | Modified Vaccinia Ankara virus variant and cultivation method |
| US7145057B2 (en) | 2002-02-01 | 2006-12-05 | Origen Therapeutics, Inc. | Chimeric bird from embryonic stem cells |
| US20060191026A1 (en) | 2005-02-18 | 2006-08-24 | Origen Therapeutics, Inc. | Tissue specific expression of antibodies in chickens |
| FR2836924B1 (fr) | 2002-03-08 | 2005-01-14 | Vivalis | Lignees de cellules aviaires utiles pour la production de substances d'interet |
| NZ538575A (en) * | 2002-09-05 | 2007-03-30 | Bavarian Nordic As | Method for the amplification of a virus wherein primary avian cells are cultivated in a serum-free medium comprising growth factors and attachment factors |
| EP1572985A4 (de) * | 2002-12-13 | 2008-03-19 | Aventis Pasteur Inc | Produktion von alvac auf embryonalenstammzellen von vögeln |
| EP1500699A1 (de) * | 2003-07-22 | 2005-01-26 | Vivalis | Herstellung von Vacciniaviren mit Hilfe adhärenter oder nicht adhärenter Vogelzelllinien |
| CA2531565C (en) * | 2003-07-22 | 2014-02-11 | Vivalis | Production of poxviruses with adherent or non adherent avian cell lines |
| US20070275010A1 (en) * | 2003-09-18 | 2007-11-29 | Mark Feinberg | Mva Vaccines |
| WO2006078294A2 (en) | 2004-05-21 | 2006-07-27 | Novartis Vaccines And Diagnostics Inc. | Alphavirus vectors for respiratory pathogen vaccines |
| DE602005024827D1 (de) | 2004-09-09 | 2010-12-30 | Novartis Vaccines & Diagnostic | Verminderung von potentiellen iatrogenen risiken in verbindung mit influenza impfstoffen |
| JP2008519042A (ja) | 2004-11-03 | 2008-06-05 | ノバルティス ヴァクシンズ アンド ダイアグノスティクス, インコーポレイテッド | インフルエンザワクチン接種 |
| FR2878860A1 (fr) * | 2004-12-08 | 2006-06-09 | Vivalis Sa | Lignees de cellules souches humaines derivees de cellules es et utilisations pour la production de vaccins et de proteines recombinantes |
| AU2011253998B2 (en) * | 2005-04-11 | 2014-04-24 | Valneva | Process of manufacturing viral vaccines in suspension avian embryonic derived stem cell lines |
| FR2884255B1 (fr) | 2005-04-11 | 2010-11-05 | Vivalis | Utilisation de lignees de cellules souches aviaires ebx pour la production de vaccin contre la grippe |
| CN101365480B (zh) | 2005-11-01 | 2014-11-05 | 诺华疫苗和诊断有限两合公司 | 经由β-丙内酯处理的残留细胞DNA水平降低的细胞衍生病毒疫苗 |
| JP2009514844A (ja) | 2005-11-04 | 2009-04-09 | ノバルティス ヴァクシンズ アンド ダイアグノスティクス エスアールエル | スプリットインフルエンザワクチン用のアジュバントとしての遊離水相界面活性剤を含むエマルション |
| WO2007052155A2 (en) | 2005-11-04 | 2007-05-10 | Novartis Vaccines And Diagnostics Srl | Influenza vaccine with reduced amount of oil-in-water emulsion as adjuvant |
| US20110180430A1 (en) | 2005-11-04 | 2011-07-28 | Novartis Vaccines And Diagnostics Srl | Adjuvanted influenza vaccines including cytokine-inducing agents |
| CA2628152C (en) | 2005-11-04 | 2016-02-02 | Novartis Vaccines And Diagnostics S.R.L. | Adjuvanted vaccines with non-virion antigens prepared from influenza viruses grown in cell culture |
| CN102727885A (zh) | 2005-11-04 | 2012-10-17 | 诺华疫苗和诊断有限公司 | 包含颗粒佐剂和免疫增强剂组合的流感疫苗 |
| KR20110110853A (ko) | 2006-01-27 | 2011-10-07 | 노파르티스 파르마 아게 | 적혈구응집소 및 기질 단백질을 함유한 인플루엔자 백신 |
| CA2646349A1 (en) | 2006-03-24 | 2007-10-04 | Novartis Vaccines And Diagnostics Gmbh & Co Kg | Storage of influenza vaccines without refrigeration |
| FR2898909A1 (fr) | 2006-03-24 | 2007-09-28 | Agronomique Inst Nat Rech | Combinaison de marqueurs de cellules aviaires |
| GB0614460D0 (en) | 2006-07-20 | 2006-08-30 | Novartis Ag | Vaccines |
| NZ574678A (en) | 2006-08-09 | 2012-03-30 | Vivalis | Method of production of transgenic avian using embryonic stem cells |
| EP3456348A1 (de) | 2006-09-11 | 2019-03-20 | Seqirus UK Limited | Herstellung von influenza-virus-impfstoffen ohne verwendung von eiern |
| CA2671629C (en) | 2006-12-06 | 2017-08-15 | Novartis Ag | Vaccines including antigen from four strains of influenza virus |
| CN100443588C (zh) * | 2006-12-11 | 2008-12-17 | 中国农业科学院北京畜牧兽医研究所 | 静宁鸡胚成纤维细胞系及其培养方法 |
| EP1985305A1 (de) * | 2007-04-24 | 2008-10-29 | Vivalis | Aus Entenembryonen gewonnene Stammzellkulturen zur Herstellung von Impfstoffen gegen Viren |
| EP1995309A1 (de) * | 2007-05-21 | 2008-11-26 | Vivalis | Rekombinante Proteinproduktion in EBx -Vogelzellen |
| CA2692200A1 (en) | 2007-06-27 | 2008-12-31 | Novartis Ag | Low-additive influenza vaccines |
| KR101528379B1 (ko) * | 2007-07-03 | 2015-06-16 | 트랜스진 에스.에이. | 불멸화된 조류 세포주들 |
| US20090028831A1 (en) * | 2007-07-23 | 2009-01-29 | University Of Kentucky Research Foundation | Stem cell regulator, compositions and methods of use |
| GB0810305D0 (en) | 2008-06-05 | 2008-07-09 | Novartis Ag | Influenza vaccination |
| WO2009081172A1 (en) | 2007-12-24 | 2009-07-02 | Novartis Ag | Assays for adsorbed influenza vaccines |
| US8506966B2 (en) | 2008-02-22 | 2013-08-13 | Novartis Ag | Adjuvanted influenza vaccines for pediatric use |
| EP2257622B1 (de) | 2008-02-25 | 2017-03-22 | Nanotherapeutics, Inc. | Verfahren zur herstellung kontinuierlicher zelllinien |
| AU2009227674C1 (en) | 2008-03-18 | 2015-01-29 | Seqirus UK Limited | Improvements in preparation of influenza virus vaccine antigens |
| WO2009132195A1 (en) * | 2008-04-23 | 2009-10-29 | Michigan State University | Immortal avian cell line and methods of use |
| WO2010081890A1 (en) | 2009-01-19 | 2010-07-22 | Innate Pharma | Anti-kir3d antibodies |
| PL2396032T3 (pl) | 2009-02-10 | 2017-05-31 | Seqirus UK Limited | Szczepionki przeciw grypie o obniżonych zawartościach skwalenu |
| CA2752039A1 (en) | 2009-02-10 | 2010-08-19 | Novartis Ag | Influenza vaccine regimens for pandemic-associated strains |
| CA2752041A1 (en) | 2009-02-10 | 2010-08-19 | Novartis Ag | Influenza vaccines with increased amounts of h3 antigen |
| BE1019643A3 (fr) | 2009-04-27 | 2012-09-04 | Novartis Ag | Vaccins de protection contre la grippe. |
| HUE058971T2 (hu) | 2009-07-15 | 2022-09-28 | Glaxosmithkline Biologicals Sa | RSV F fehérjekészítmények és eljárások azok elõállítására |
| EP2475385A1 (de) | 2009-09-10 | 2012-07-18 | Novartis AG | Kombinationsimpfstoffe gegen atemwegserkrankungen |
| GB0918830D0 (en) | 2009-10-27 | 2009-12-09 | Glaxosmithkline Biolog Niederl | Process |
| GB0919117D0 (en) | 2009-10-30 | 2009-12-16 | Glaxosmithkline Biolog Sa | Process |
| CA2725435C (en) | 2009-12-15 | 2023-01-03 | University Of Saskatchewan | Vaccines for inclusion body hepatitis |
| WO2011095596A1 (en) | 2010-02-04 | 2011-08-11 | Vivalis | Fed-batch process using concentrated cell culture medium for the efficient production of biologics in eb66 cells |
| FR2956409A1 (fr) | 2010-02-15 | 2011-08-19 | Univ Claude Bernard Lyon | Procedes pour optimiser la production virale par l'inactivation ou l'inhibition du gene hipk2 et cellules modifiees pour la production virale |
| CN102791860A (zh) | 2010-03-08 | 2012-11-21 | 诺华有限公司 | 检测胞内病原体的方法 |
| WO2011127316A1 (en) | 2010-04-07 | 2011-10-13 | Novartis Ag | Method for generating a parvovirus b19 virus-like particle |
| WO2011124635A1 (en) | 2010-04-07 | 2011-10-13 | Humalys | Binding molecules against chikungunya virus and uses thereof |
| EP2374816B1 (de) | 2010-04-07 | 2016-09-28 | Agency For Science, Technology And Research | Bindemoleküle gegen Chikungunya-Virus und Verwendungen davon |
| US9426989B2 (en) | 2010-05-06 | 2016-08-30 | Novartis Ag | Organic peroxide compounds for microorganism inactivation |
| WO2011145081A1 (en) | 2010-05-21 | 2011-11-24 | Novartis Ag | Influenza virus reassortment method |
| PL2575872T3 (pl) | 2010-06-01 | 2021-02-22 | Seqirus UK Limited | Zatężanie antygenów szczepionkowych grypy bez liofilizacji |
| PL2575873T3 (pl) | 2010-06-01 | 2016-06-30 | Novartis Ag | Zatężanie i liofilizacja antygenów szczepionkowych grypy |
| WO2012006293A1 (en) | 2010-07-06 | 2012-01-12 | Novartis Ag | Norovirus derived immunogenic compositions and methods |
| GB201011502D0 (en) | 2010-07-08 | 2010-08-25 | Glaxosmithkline Biolog Sa | Novel process |
| EP2605792B1 (de) | 2010-08-20 | 2014-12-10 | Novartis AG | Lösliche nadelfelder zur freisetzung von grippeimpfstoffen |
| SG188411A1 (en) | 2010-09-07 | 2013-04-30 | Technion Res & Dev Foundation | Novel methods and culture media for culturing pluripotent stem cells |
| FR2967072B1 (fr) | 2010-11-05 | 2013-03-29 | Univ Dundee | Procede pour ameliorer la production de virus et semences vaccinales influenza |
| WO2012095514A1 (en) | 2011-01-14 | 2012-07-19 | Vivalis | Recombinant protein production system |
| PL2667892T3 (pl) | 2011-01-26 | 2019-09-30 | Glaxosmithkline Biologicals Sa | Schemat szczepień przeciwko rsv |
| ES2651143T3 (es) | 2011-05-13 | 2018-01-24 | Glaxosmithkline Biologicals Sa | Antígenos de F de prefusión del VRS |
| WO2013006838A1 (en) | 2011-07-06 | 2013-01-10 | Novartis Ag | Immunogenic combination compositions and uses thereof |
| CA2841047A1 (en) | 2011-07-06 | 2013-01-10 | Novartis Ag | Immunogenic compositions and uses thereof |
| TWI575070B (zh) | 2011-07-12 | 2017-03-21 | 傳斯堅公司 | Hbv聚合酶突變體 |
| TW201321016A (zh) | 2011-09-29 | 2013-06-01 | Transgene Sa | 免疫療法組成物及用於治療c型肝炎病毒感染之療程(二) |
| TW201318637A (zh) | 2011-09-29 | 2013-05-16 | Transgene Sa | 免疫療法組成物及用於治療c型肝炎病毒感染之療程(一) |
| WO2013054199A2 (en) | 2011-10-12 | 2013-04-18 | Novartis Ag | Cmv antigens and uses thereof |
| GB201216121D0 (en) | 2012-09-10 | 2012-10-24 | Novartis Ag | Sample quantification by disc centrifugation |
| WO2013057715A1 (en) | 2011-10-20 | 2013-04-25 | Novartis Ag | Adjuvanted influenza b virus vaccines for pediatric priming |
| EP2660316A1 (de) | 2012-05-02 | 2013-11-06 | Helmholtz-Zentrum für Infektionsforschung GmbH | Vogelzelllinie und deren Verwendung bei der Herstellung eines Proteins |
| JP2015522580A (ja) | 2012-07-06 | 2015-08-06 | ノバルティス アーゲー | 免疫学的組成物およびその使用 |
| WO2014009433A1 (en) | 2012-07-10 | 2014-01-16 | Transgene Sa | Mycobacterium resuscitation promoting factor for use as adjuvant |
| TWI638829B (zh) | 2012-07-10 | 2018-10-21 | 法商傳斯堅公司 | 分枝桿菌抗原疫苗 |
| GB201218195D0 (en) | 2012-10-10 | 2012-11-21 | Istituto Zooprofilattico Sperimentale Delle Venezie | Composition |
| MX2015006377A (es) | 2012-11-20 | 2015-07-21 | Glaxosmithkline Biolog Sa | Trimeros prefusion de f de vsr. |
| US20140255447A1 (en) * | 2013-03-05 | 2014-09-11 | Biomune Company | Production of avian embryo cells |
| FR3008992A1 (fr) * | 2013-07-25 | 2015-01-30 | Agronomique Inst Nat Rech | Procede de selection d'une lignee cellulaire permissive pour la replication de virus aviaires |
| US10765731B2 (en) | 2014-01-09 | 2020-09-08 | Transgene Sa | Fusion of heterooligomeric mycobacterial antigens |
| EP2974739A1 (de) | 2014-07-15 | 2016-01-20 | Novartis AG | RSVF-Trimerisierungsdomänen |
| EP3031822A1 (de) | 2014-12-08 | 2016-06-15 | Novartis AG | Cytomegalovirus-Antigene |
| ES2784264T3 (es) | 2014-12-17 | 2020-09-23 | Fundacion Para La Investig Medica Aplicada | Construcciones de ácido nucleico y vectores de terapia génica para su uso en el tratamiento de la enfermedad de Wilson y otras afecciones |
| PL3233130T3 (pl) | 2014-12-17 | 2021-11-22 | Fundacion Para La Investigacion Medica Aplicada | Konstrukty kwasów nukleinowych i wektory do terapii genowej do zastosowania w leczeniu choroby wilsona |
| EP3047856A1 (de) | 2015-01-23 | 2016-07-27 | Novartis AG | Cmv-antigene und ihre verwendungen |
| US11013795B2 (en) | 2015-06-26 | 2021-05-25 | Seqirus UK Limited | Antigenically matched influenza vaccines |
| JP6830070B2 (ja) | 2015-07-07 | 2021-02-17 | セキラス ユーケー リミテッドSeqirus UK Limited | インフルエンザ効力アッセイ |
| WO2018176103A1 (en) | 2017-03-30 | 2018-10-04 | The University Of Queensland | "chimeric molecules and uses thereof" |
| EP4400174A2 (de) | 2017-09-01 | 2024-07-17 | The Francis Crick Institute Limited | Immunregulierende moleküle und deren verwendungen |
| WO2019109051A1 (en) | 2017-12-01 | 2019-06-06 | Encoded Therapeutics, Inc. | Engineered dna binding proteins |
| EP3794127A1 (de) | 2018-05-14 | 2021-03-24 | Vivet Therapeutics | Gentherapeutische vektoren mit s/mar-sequenzen |
| JP2021525517A (ja) | 2018-05-30 | 2021-09-27 | インスティテュート・フォー・リサーチ・イン・バイオメディシンInstitute For Research In Biomedicine | 内在性活性化誘導シチジンデアミナーゼを利用することによるbリンパ球のエンジニアリング |
| WO2020012037A1 (en) | 2018-07-13 | 2020-01-16 | Valneva Se | Method for rescuing and producing a virus in avian cells |
| WO2020074690A1 (en) | 2018-10-12 | 2020-04-16 | Vivet Therapeutics | Codon-optimized transgene for the treatment of progressive familiar intrahepatic cholestasis type 3 (pfic3) |
| BR112021008434A2 (pt) | 2018-11-07 | 2021-09-28 | Vivet Therapeutics | Transgene abcb11 otimizado por códon para o tratamento de colestase intra-hepática familiar progressiva tipo 2 (pfic2) |
| WO2020102723A1 (en) | 2018-11-16 | 2020-05-22 | Encoded Therapeutics, Inc. | Compositions and methods for treating wilson's disease |
| SG11202104869SA (en) | 2018-11-23 | 2021-06-29 | Valneva Se | Food products comprising avian stem cells |
| WO2020234888A1 (en) | 2019-05-22 | 2020-11-26 | Hadasit Medical Research Services And Development Ltd. | Methods of culturing human pluripotent cells |
| AU2020299718A1 (en) | 2019-07-02 | 2022-02-24 | Fundacion Para La Investigacion Medica Aplicada | cPLA2e inducing agents and uses thereof |
| CA3146900A1 (en) | 2019-07-21 | 2021-01-28 | Glaxosmithkline Biologicals Sa | Therapeutic viral vaccine |
| US20230201334A1 (en) | 2019-07-24 | 2023-06-29 | Glaxosmithkline Biologicals Sa | Modified human cytomegalovirus proteins |
| JP2022548179A (ja) * | 2019-09-10 | 2022-11-16 | バイタル ミート | 食品製造のための鳥類幹細胞 |
| JP2023502650A (ja) | 2019-11-18 | 2023-01-25 | セキラス ピーティーワイ リミテッド | 遺伝子再集合インフルエンザウイルスを産生するための方法 |
| MX2023000968A (es) | 2020-08-06 | 2023-03-01 | Fundacion Para La Investig Medica Aplicada | Particulas virales para usarse en el tratamiento contra tauopatias tales como enfermedad de alzheimer mediante terapia genica. |
| US20230265456A1 (en) | 2020-08-10 | 2023-08-24 | Fundacion Para La Investigacion Medica Aplicada | Gene therapy vector expressing cyp27a1 for the treatment of cerebrotendinous xanthomatosis |
| JP2023544264A (ja) | 2020-10-09 | 2023-10-23 | ユーシービー バイオファルマ エスアールエル | 核酸構築物、ウイルスベクター及びウイルス粒子 |
| EP4267197A1 (de) | 2020-12-23 | 2023-11-01 | Vivet Therapeutics | Durch minimale gallensäure induzierbare promotoren zur gentherapie |
| EP4032547A1 (de) | 2021-01-20 | 2022-07-27 | GlaxoSmithKline Biologicals S.A. | Hsv1 fce abgeleitete fragemente zur hsv therapie |
| CA3234666A1 (en) | 2021-10-28 | 2023-05-04 | UCB Biopharma SRL | Nucleic acid constructs, viral vectors and viral particles |
| WO2023144665A1 (en) | 2022-01-28 | 2023-08-03 | Glaxosmithkline Biologicals Sa | Modified human cytomegalovirus proteins |
| CN114480286B (zh) * | 2022-02-16 | 2024-05-31 | 上海健士拜生物科技有限公司 | 无血清悬浮型lmh细胞系及其制备方法和应用 |
| CN116656628A (zh) * | 2023-07-31 | 2023-08-29 | 深圳市卫光生物制品股份有限公司 | 一种痘病毒载体疫苗的制备方法 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5656479A (en) * | 1992-05-15 | 1997-08-12 | North Carolina State University | Avian embryonic stem cells |
Family Cites Families (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4388298A (en) | 1982-07-14 | 1983-06-14 | The United States Of America As Represented By The Secretary Of Agriculture | Propagation of hemorrhagic enteritis virus in a turkey cell line and vaccine produced |
| WO1990001541A1 (en) | 1988-08-04 | 1990-02-22 | Amrad Corporation Limited | In vitro propagation of embryonic stem cells |
| US5162215A (en) * | 1988-09-22 | 1992-11-10 | Amgen Inc. | Method of gene transfer into chickens and other avian species |
| US5388680A (en) | 1990-10-09 | 1995-02-14 | Intellicall, Inc. | Coin handling system with an improved coin chute |
| EP0594743B1 (de) | 1991-07-12 | 1999-09-22 | Pfizer Inc. | Kontinuierliche zellinie sowie impfstoff gegen geflügelinfektion mit kokkidien |
| JPH05227947A (ja) | 1992-01-14 | 1993-09-07 | Rikagaku Kenkyusho | 鳥類原始生殖細胞の分離方法 |
| WO1993015185A1 (en) | 1992-01-27 | 1993-08-05 | North Carolina State University | GENE TRANSFER IN POULTRY BY INTRODUCTION OF EMBRYO CELLS $i(IN OVO) |
| WO1994003585A1 (en) | 1992-08-04 | 1994-02-17 | Commonwealth Scientific And Industrial Research Organisation | A method for maintaining embryonic stem cells and avian factor useful for same |
| US5453357A (en) * | 1992-10-08 | 1995-09-26 | Vanderbilt University | Pluripotential embryonic stem cells and methods of making same |
| US5589458A (en) * | 1992-11-13 | 1996-12-31 | Thomas Jefferson University | Compounds that inhibit T cell proliferation and methods for using the same |
| FR2726003B1 (fr) * | 1994-10-21 | 2002-10-18 | Agronomique Inst Nat Rech | Milieu de culture de cellules embryonnaires totipotentes aviaires, procede de culture de ces cellules, et cellules embryonnaires totipotentes aviaires |
| US5989805A (en) | 1995-10-27 | 1999-11-23 | Board Of Trustees Operating Michigan State University | Immortal avian cell line to grow avian and animal viruses to produce vaccines |
| FR2749022B1 (fr) | 1996-05-23 | 2001-06-01 | Rhone Merieux | Cellules aviaires immortelles |
| US5691200A (en) | 1996-07-03 | 1997-11-25 | The United States Of America As Represented By The Secretary Of Agriculture | Method to produce granulocyte colony stimulating factor from immortalized avian T lymphocytes and method to produce immortalized cells |
| US5672485A (en) | 1996-08-13 | 1997-09-30 | Regents Of The University Of Minnesota | Immortalized cell lines for virus growth |
| US5830723A (en) | 1996-08-13 | 1998-11-03 | Regents Of The University Of Minnesota | Method for immortalizing chicken cells |
| EP1983044B1 (de) | 1996-10-10 | 2016-08-10 | Life Technologies Corporation | Tierzellkulturmedien mit Nährstoffen aus Pflanzen |
| PT841392E (pt) | 1996-11-12 | 2004-11-30 | Univ Auburn | Vacina viva atenuada de neospora |
| BR9811832A (pt) * | 1997-08-04 | 2000-08-15 | Univ Massachusetts | Processo de cultivo, processo de melhorado de produção de aves quiméricas, e, linhagem de células eg de aves |
| ATE307196T1 (de) * | 1997-08-04 | 2005-11-15 | Univ Massachusetts | Primordiale keimzellinie aus vögeln und verfahren zu deren langzeitkultivierung |
| US6406909B1 (en) | 1998-07-10 | 2002-06-18 | Chugai Seiyaku Kabushiki Kaisha | Serum-free medium for culturing animal cells |
| RU2215029C2 (ru) * | 1999-02-11 | 2003-10-27 | Ханми Фарм. Ко., Лтд. | Способ получения установившейся эмбриональной зародышевой клеточной линии птиц, линия куриных эмбриональных зародышевых клеток, способ получения соматических или половых химер, способ трансфекции чужеродного гена в эмбриональные зародышевые клетки |
| EP1210410B1 (de) * | 1999-08-27 | 2008-01-16 | Invitrogen Corporation | Metallbindende verbindungen und deren verwendung in zusammensetzungen für zellkulturmedien |
| DE19947407C2 (de) | 1999-10-01 | 2002-08-01 | Bayerische Motoren Werke Ag | Datenbussystem für Kraftfahrzeuge |
| WO2002086073A2 (en) * | 2001-04-20 | 2002-10-31 | Memorial Sloan-Kettering Cancer Center | Generation of differentiated tissue from nuclear transfer embryonic stem cells and methods of use |
| FR2836924B1 (fr) | 2002-03-08 | 2005-01-14 | Vivalis | Lignees de cellules aviaires utiles pour la production de substances d'interet |
| WO2004005493A1 (en) | 2002-07-09 | 2004-01-15 | Baxter International, Inc. | Animal protein free media for cultivation of cells |
| EP1500699A1 (de) | 2003-07-22 | 2005-01-26 | Vivalis | Herstellung von Vacciniaviren mit Hilfe adhärenter oder nicht adhärenter Vogelzelllinien |
| CA2531565C (en) | 2003-07-22 | 2014-02-11 | Vivalis | Production of poxviruses with adherent or non adherent avian cell lines |
| KR100552844B1 (ko) * | 2003-12-31 | 2006-02-21 | 동부아남반도체 주식회사 | 반도체 소자의 제조 방법 |
| FR2884255B1 (fr) | 2005-04-11 | 2010-11-05 | Vivalis | Utilisation de lignees de cellules souches aviaires ebx pour la production de vaccin contre la grippe |
| WO2007135133A1 (en) | 2006-05-19 | 2007-11-29 | Vivalis | Avian cell lines derived from primordial germ cells useful for the production of substances of interest |
| NZ574678A (en) | 2006-08-09 | 2012-03-30 | Vivalis | Method of production of transgenic avian using embryonic stem cells |
| EP1985305A1 (de) | 2007-04-24 | 2008-10-29 | Vivalis | Aus Entenembryonen gewonnene Stammzellkulturen zur Herstellung von Impfstoffen gegen Viren |
-
2002
- 2002-03-08 FR FR0202945A patent/FR2836924B1/fr not_active Expired - Lifetime
-
2003
- 2003-03-07 AU AU2003227820A patent/AU2003227820B2/en not_active Expired
- 2003-03-07 SI SI200331354T patent/SI1483369T1/sl unknown
- 2003-03-07 EP EP08163979.1A patent/EP1992687B1/de not_active Expired - Lifetime
- 2003-03-07 WO PCT/FR2003/000735 patent/WO2003076601A1/fr active IP Right Grant
- 2003-03-07 CN CNB038101203A patent/CN100562567C/zh not_active Expired - Fee Related
- 2003-03-07 JP JP2003574808A patent/JP5025888B2/ja not_active Expired - Fee Related
- 2003-03-07 AT AT03725273T patent/ATE408004T1/de active
- 2003-03-07 DK DK03725273T patent/DK1483369T3/da active
- 2003-03-07 PT PT03725273T patent/PT1483369E/pt unknown
- 2003-03-07 DE DE60323468T patent/DE60323468D1/de not_active Expired - Lifetime
- 2003-03-07 EP EP03725273A patent/EP1483369B1/de not_active Expired - Lifetime
- 2003-03-07 ES ES03725273T patent/ES2312775T3/es not_active Expired - Lifetime
- 2003-03-07 CN CN2009101796309A patent/CN101676388B/zh not_active Expired - Lifetime
- 2003-03-07 CA CA2478125A patent/CA2478125C/fr not_active Expired - Fee Related
- 2003-07-24 US US10/625,847 patent/US20040058441A1/en not_active Abandoned
-
2009
- 2009-01-08 US US12/350,781 patent/US20090239297A1/en not_active Abandoned
-
2010
- 2010-03-03 US US12/717,096 patent/US9382513B2/en not_active Expired - Fee Related
- 2010-09-08 HK HK10108503.1A patent/HK1142094A1/xx not_active IP Right Cessation
-
2011
- 2011-08-09 US US13/206,056 patent/US20110294209A1/en not_active Abandoned
-
2012
- 2012-02-09 JP JP2012026135A patent/JP5784521B2/ja not_active Expired - Lifetime
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5656479A (en) * | 1992-05-15 | 1997-08-12 | North Carolina State University | Avian embryonic stem cells |
Also Published As
| Publication number | Publication date |
|---|---|
| DK1483369T3 (da) | 2008-12-01 |
| FR2836924A1 (fr) | 2003-09-12 |
| AU2003227820B2 (en) | 2008-04-03 |
| ES2312775T3 (es) | 2009-03-01 |
| SI1483369T1 (sl) | 2008-12-31 |
| CN1649999A (zh) | 2005-08-03 |
| US20110294209A1 (en) | 2011-12-01 |
| ATE408004T1 (de) | 2008-09-15 |
| EP1992687A2 (de) | 2008-11-19 |
| EP1483369B1 (de) | 2008-09-10 |
| JP2012110344A (ja) | 2012-06-14 |
| WO2003076601A1 (fr) | 2003-09-18 |
| FR2836924B1 (fr) | 2005-01-14 |
| EP1483369A1 (de) | 2004-12-08 |
| WO2003076601A8 (fr) | 2005-05-12 |
| DE60323468D1 (de) | 2008-10-23 |
| CA2478125A1 (fr) | 2003-09-18 |
| CA2478125C (fr) | 2013-05-07 |
| US9382513B2 (en) | 2016-07-05 |
| PT1483369E (pt) | 2008-12-17 |
| JP2005525803A (ja) | 2005-09-02 |
| CN101676388B (zh) | 2013-09-04 |
| EP1992687A3 (de) | 2009-03-25 |
| US20100221825A1 (en) | 2010-09-02 |
| CN100562567C (zh) | 2009-11-25 |
| HK1142094A1 (en) | 2010-11-26 |
| US20040058441A1 (en) | 2004-03-25 |
| US20120070893A9 (en) | 2012-03-22 |
| JP5025888B2 (ja) | 2012-09-12 |
| JP5784521B2 (ja) | 2015-09-24 |
| CN101676388A (zh) | 2010-03-24 |
| US20090239297A1 (en) | 2009-09-24 |
| AU2003227820A1 (en) | 2003-09-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1992687B1 (de) | Aviäre Zelllinien für die Herstellung von nützlichen Substanzen | |
| US10017782B2 (en) | Immune cells modified by transient transfection of RNA | |
| ES2345820T3 (es) | Produccion de poxvirus con estirpes de celulas aviares adherentes o no adherentes. | |
| JP2017525351A (ja) | 多能性幹細胞の培養用培地 | |
| EP1149899A1 (de) | Kulturmedium von Vögeln totipotenten embryonalen Zellen | |
| CN1692156A (zh) | 在无血清条件下培养原代细胞和扩增病毒的方法 | |
| WO1997044443A1 (fr) | Cellules aviaires immortelles | |
| EP1500699A1 (de) | Herstellung von Vacciniaviren mit Hilfe adhärenter oder nicht adhärenter Vogelzelllinien | |
| US20050164385A1 (en) | Embryonic stem cells having genetic modifications | |
| EP1824966A1 (de) | Von es-zellen abgeleitete menschliche stammzellinien und verwendungen zur herstellung von impfstoffen und rekombinanten proteinen | |
| EP1363995B1 (de) | Verwendung einer zusammensetzung mit n-acetylcystein zur konditionierung von stammzellen | |
| US20080085554A1 (en) | Culture Medium for Culturing Feeder Cells for Embryonic Stem Cells Culture and the Prepared Feeder Cells | |
| EP3024927B1 (de) | Verfahren zur auswahl einer permissiven zelllinie zur replikation von vogelgrippeviren | |
| CN117957311A (zh) | 视网膜色素上皮细胞的扩增 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 1483369 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: PAIN, BERTRAND Inventor name: GUEHENNEUX, FABIENNE |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: PAIN, BERTRAND Inventor name: GUEHENNEUX, FABIENNE |
|
| 17Q | First examination report despatched |
Effective date: 20090921 |
|
| 17P | Request for examination filed |
Effective date: 20090824 |
|
| AKX | Designation fees paid |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: VALNEVA |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: VALNEVA |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: VALNEVA |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Ref document number: 60352119 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: C12N0005060000 Ipc: C12N0005073500 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C12N 5/10 20060101ALI20181220BHEP Ipc: C12N 5/0735 20100101AFI20181220BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20190122 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 1483369 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 60352119 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1148298 Country of ref document: AT Kind code of ref document: T Effective date: 20190715 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20190626 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190927 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190926 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1148298 Country of ref document: AT Kind code of ref document: T Effective date: 20190626 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20191028 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 60352119 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20200603 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20200331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200307 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200331 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200307 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20200331 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20220321 Year of fee payment: 20 Ref country code: DE Payment date: 20220322 Year of fee payment: 20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20190626 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20220322 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 60352119 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20230306 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20230306 |