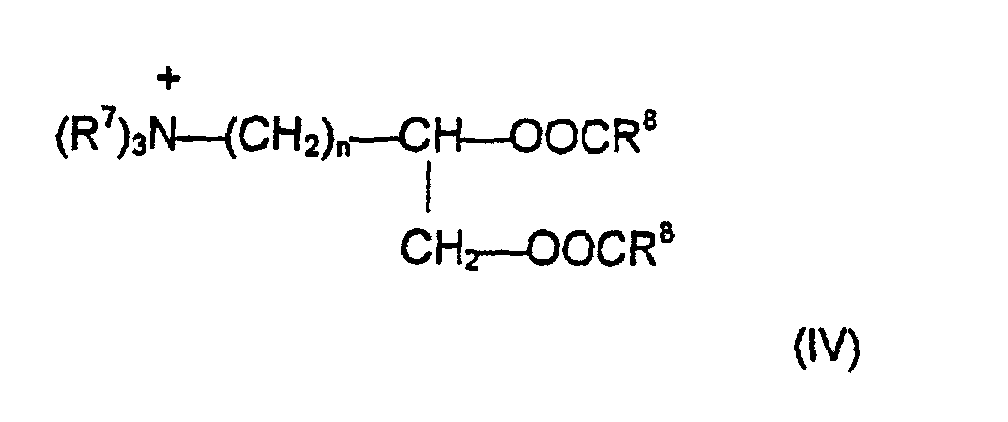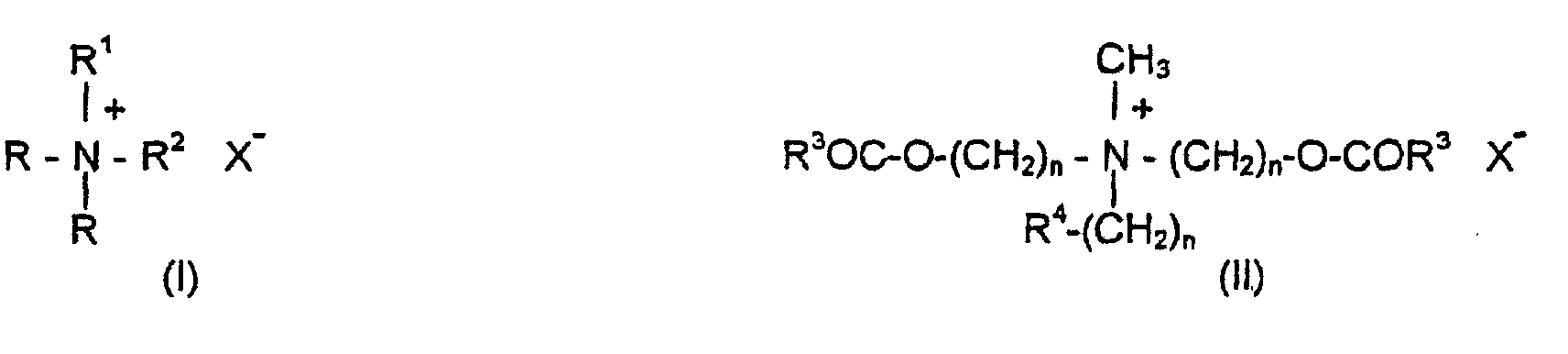EP0920486B2 - Wässriges wäscheweichspülmittel mit hohem zeta-potential - Google Patents
Wässriges wäscheweichspülmittel mit hohem zeta-potential Download PDFInfo
- Publication number
- EP0920486B2 EP0920486B2 EP97927127A EP97927127A EP0920486B2 EP 0920486 B2 EP0920486 B2 EP 0920486B2 EP 97927127 A EP97927127 A EP 97927127A EP 97927127 A EP97927127 A EP 97927127A EP 0920486 B2 EP0920486 B2 EP 0920486B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- fatty
- weight
- carbon atoms
- fatty acids
- fabric softeners
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000004902 Softening Agent Substances 0.000 title 1
- 239000006185 dispersion Substances 0.000 claims abstract description 32
- 239000003995 emulsifying agent Substances 0.000 claims abstract description 22
- 125000002091 cationic group Chemical group 0.000 claims abstract description 12
- 239000012875 nonionic emulsifier Substances 0.000 claims abstract description 8
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 32
- 239000000194 fatty acid Substances 0.000 claims description 32
- 229930195729 fatty acid Natural products 0.000 claims description 32
- -1 fatty acid esters Chemical class 0.000 claims description 26
- 150000004665 fatty acids Chemical class 0.000 claims description 26
- 125000004432 carbon atom Chemical group C* 0.000 claims description 24
- 150000001875 compounds Chemical class 0.000 claims description 22
- 150000002191 fatty alcohols Chemical class 0.000 claims description 20
- QLAJNZSPVITUCQ-UHFFFAOYSA-N 1,3,2-dioxathietane 2,2-dioxide Chemical group O=S1(=O)OCO1 QLAJNZSPVITUCQ-UHFFFAOYSA-N 0.000 claims description 15
- 239000002979 fabric softener Substances 0.000 claims description 15
- 239000000203 mixture Substances 0.000 claims description 13
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 9
- 239000007787 solid Substances 0.000 claims description 7
- 125000000217 alkyl group Chemical group 0.000 claims description 6
- 150000002500 ions Chemical class 0.000 claims description 6
- 150000001298 alcohols Chemical class 0.000 claims description 5
- 150000001412 amines Chemical class 0.000 claims description 4
- 150000005690 diesters Chemical class 0.000 claims description 4
- 229930195733 hydrocarbon Natural products 0.000 claims description 4
- 150000002430 hydrocarbons Chemical class 0.000 claims description 4
- 150000003856 quaternary ammonium compounds Chemical class 0.000 claims description 4
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 claims description 3
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 2
- 125000002768 hydroxyalkyl group Chemical group 0.000 claims description 2
- 239000004215 Carbon black (E152) Substances 0.000 claims 1
- 125000002015 acyclic group Chemical group 0.000 claims 1
- 125000002252 acyl group Chemical group 0.000 claims 1
- 125000001931 aliphatic group Chemical group 0.000 claims 1
- 229930182478 glucoside Natural products 0.000 claims 1
- 125000005843 halogen group Chemical group 0.000 claims 1
- 230000000694 effects Effects 0.000 abstract description 8
- 239000003795 chemical substances by application Substances 0.000 description 12
- UQDUPQYQJKYHQI-UHFFFAOYSA-N methyl laurate Chemical compound CCCCCCCCCCCC(=O)OC UQDUPQYQJKYHQI-UHFFFAOYSA-N 0.000 description 12
- 239000000126 substance Substances 0.000 description 12
- 239000002245 particle Substances 0.000 description 11
- 229910052757 nitrogen Inorganic materials 0.000 description 10
- 239000003760 tallow Substances 0.000 description 8
- 125000000954 2-hydroxyethyl group Chemical group [H]C([*])([H])C([H])([H])O[H] 0.000 description 7
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 7
- 239000003925 fat Substances 0.000 description 7
- 235000019197 fats Nutrition 0.000 description 7
- 239000000835 fiber Substances 0.000 description 7
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 6
- 239000004014 plasticizer Substances 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 239000004615 ingredient Substances 0.000 description 5
- 239000004753 textile Substances 0.000 description 5
- 239000002253 acid Substances 0.000 description 4
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 229940027983 antiseptic and disinfectant quaternary ammonium compound Drugs 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 238000013508 migration Methods 0.000 description 3
- 230000005012 migration Effects 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 238000001179 sorption measurement Methods 0.000 description 3
- XDOFQFKRPWOURC-UHFFFAOYSA-N 16-methylheptadecanoic acid Chemical compound CC(C)CCCCCCCCCCCCCCC(O)=O XDOFQFKRPWOURC-UHFFFAOYSA-N 0.000 description 2
- AOHAPDDBNAPPIN-UHFFFAOYSA-N 3-Methoxy-4,5-methylenedioxybenzoic acid Chemical compound COC1=CC(C(O)=O)=CC2=C1OCO2 AOHAPDDBNAPPIN-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 239000005639 Lauric acid Substances 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 2
- 235000021314 Palmitic acid Nutrition 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 239000004665 cationic fabric softener Substances 0.000 description 2
- WOWHHFRSBJGXCM-UHFFFAOYSA-M cetyltrimethylammonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCC[N+](C)(C)C WOWHHFRSBJGXCM-UHFFFAOYSA-M 0.000 description 2
- MWKFXSUHUHTGQN-UHFFFAOYSA-N decan-1-ol Chemical compound CCCCCCCCCCO MWKFXSUHUHTGQN-UHFFFAOYSA-N 0.000 description 2
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 2
- IQDGSYLLQPDQDV-UHFFFAOYSA-N dimethylazanium;chloride Chemical compound Cl.CNC IQDGSYLLQPDQDV-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- LQZZUXJYWNFBMV-UHFFFAOYSA-N dodecan-1-ol Chemical compound CCCCCCCCCCCCO LQZZUXJYWNFBMV-UHFFFAOYSA-N 0.000 description 2
- ZQPPMHVWECSIRJ-MDZDMXLPSA-N elaidic acid Chemical compound CCCCCCCC\C=C\CCCCCCCC(O)=O ZQPPMHVWECSIRJ-MDZDMXLPSA-N 0.000 description 2
- 239000003792 electrolyte Substances 0.000 description 2
- 239000004744 fabric Substances 0.000 description 2
- LQJBNNIYVWPHFW-QXMHVHEDSA-N gadoleic acid Chemical compound CCCCCCCCCC\C=C/CCCCCCCC(O)=O LQJBNNIYVWPHFW-QXMHVHEDSA-N 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 229930182470 glycoside Natural products 0.000 description 2
- DCAYPVUWAIABOU-UHFFFAOYSA-N hexadecane Chemical compound CCCCCCCCCCCCCCCC DCAYPVUWAIABOU-UHFFFAOYSA-N 0.000 description 2
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 2
- 150000002440 hydroxy compounds Chemical class 0.000 description 2
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000000034 method Methods 0.000 description 2
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 2
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 2
- RZJRJXONCZWCBN-UHFFFAOYSA-N octadecane Chemical compound CCCCCCCCCCCCCCCCCC RZJRJXONCZWCBN-UHFFFAOYSA-N 0.000 description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 2
- REIUXOLGHVXAEO-UHFFFAOYSA-N pentadecan-1-ol Chemical compound CCCCCCCCCCCCCCCO REIUXOLGHVXAEO-UHFFFAOYSA-N 0.000 description 2
- 239000002304 perfume Substances 0.000 description 2
- CNVZJPUDSLNTQU-SEYXRHQNSA-N petroselinic acid Chemical compound CCCCCCCCCCC\C=C/CCCCC(O)=O CNVZJPUDSLNTQU-SEYXRHQNSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- HLZKNKRTKFSKGZ-UHFFFAOYSA-N tetradecan-1-ol Chemical compound CCCCCCCCCCCCCCO HLZKNKRTKFSKGZ-UHFFFAOYSA-N 0.000 description 2
- BGHCVCJVXZWKCC-UHFFFAOYSA-N tetradecane Chemical compound CCCCCCCCCCCCCC BGHCVCJVXZWKCC-UHFFFAOYSA-N 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- OYHQOLUKZRVURQ-NTGFUMLPSA-N (9Z,12Z)-9,10,12,13-tetratritiooctadeca-9,12-dienoic acid Chemical compound C(CCCCCCC\C(=C(/C\C(=C(/CCCCC)\[3H])\[3H])\[3H])\[3H])(=O)O OYHQOLUKZRVURQ-NTGFUMLPSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- DYLIWHYUXAJDOJ-OWOJBTEDSA-N (e)-4-(6-aminopurin-9-yl)but-2-en-1-ol Chemical compound NC1=NC=NC2=C1N=CN2C\C=C\CO DYLIWHYUXAJDOJ-OWOJBTEDSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- OXEDXHIBHVMDST-UHFFFAOYSA-N 12Z-octadecenoic acid Natural products CCCCCC=CCCCCCCCCCCC(O)=O OXEDXHIBHVMDST-UHFFFAOYSA-N 0.000 description 1
- SZWQORDLLFKZQK-UHFFFAOYSA-N 17-[ethyl(2-hydroxyethyl)amino]tritriacontane-16,18-dione Chemical compound CCCCCCCCCCCCCCCC(=O)C(C(=O)CCCCCCCCCCCCCCC)N(CC)CCO SZWQORDLLFKZQK-UHFFFAOYSA-N 0.000 description 1
- FFYRMXUAWVDDQP-UHFFFAOYSA-N 2,2-bis(hydroxymethyl)propane-1,3-diol;octadecanoic acid Chemical class OCC(CO)(CO)CO.CCCCCCCCCCCCCCCCCC(O)=O.CCCCCCCCCCCCCCCCCC(O)=O FFYRMXUAWVDDQP-UHFFFAOYSA-N 0.000 description 1
- ZXIYZDSEASMXPI-UHFFFAOYSA-N 2-(methylamino)ethane-1,1,1-triol Chemical compound CNCC(O)(O)O ZXIYZDSEASMXPI-UHFFFAOYSA-N 0.000 description 1
- UWKDZWSATBBGBN-UHFFFAOYSA-N 2-[ethyl(methyl)amino]ethanol Chemical compound CCN(C)CCO UWKDZWSATBBGBN-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 101150099236 Acly gene Proteins 0.000 description 1
- DPUOLQHDNGRHBS-UHFFFAOYSA-N Brassidinsaeure Natural products CCCCCCCCC=CCCCCCCCCCCCC(O)=O DPUOLQHDNGRHBS-UHFFFAOYSA-N 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 239000005632 Capric acid (CAS 334-48-5) Substances 0.000 description 1
- 239000005635 Caprylic acid (CAS 124-07-2) Substances 0.000 description 1
- 108010059892 Cellulase Proteins 0.000 description 1
- 235000013162 Cocos nucifera Nutrition 0.000 description 1
- 244000060011 Cocos nucifera Species 0.000 description 1
- RUPBZQFQVRMKDG-UHFFFAOYSA-M Didecyldimethylammonium chloride Chemical compound [Cl-].CCCCCCCCCC[N+](C)(C)CCCCCCCCCC RUPBZQFQVRMKDG-UHFFFAOYSA-M 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- URXZXNYJPAJJOQ-UHFFFAOYSA-N Erucic acid Natural products CCCCCCC=CCCCCCCCCCCCC(O)=O URXZXNYJPAJJOQ-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- CNVZJPUDSLNTQU-UHFFFAOYSA-N Petroselaidic acid Natural products CCCCCCCCCCCC=CCCCCC(O)=O CNVZJPUDSLNTQU-UHFFFAOYSA-N 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 239000006096 absorbing agent Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000005210 alkyl ammonium group Chemical group 0.000 description 1
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 1
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 1
- 150000003868 ammonium compounds Chemical class 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 230000003712 anti-aging effect Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 229920006317 cationic polymer Polymers 0.000 description 1
- 239000002752 cationic softener Substances 0.000 description 1
- 239000003093 cationic surfactant Substances 0.000 description 1
- 229940106157 cellulase Drugs 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 239000013065 commercial product Substances 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000005100 correlation spectroscopy Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 229960004670 didecyldimethylammonium chloride Drugs 0.000 description 1
- REZZEXDLIUJMMS-UHFFFAOYSA-M dimethyldioctadecylammonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CCCCCCCCCCCCCCCCCC REZZEXDLIUJMMS-UHFFFAOYSA-M 0.000 description 1
- 239000004664 distearyldimethylammonium chloride (DHTDMAC) Substances 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 238000009713 electroplating Methods 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- DPUOLQHDNGRHBS-KTKRTIGZSA-N erucic acid Chemical compound CCCCCCCC\C=C/CCCCCCCCCCCC(O)=O DPUOLQHDNGRHBS-KTKRTIGZSA-N 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 239000000417 fungicide Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 1
- UBHWBODXJBSFLH-UHFFFAOYSA-N hexadecan-1-ol;octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO.CCCCCCCCCCCCCCCCCCO UBHWBODXJBSFLH-UHFFFAOYSA-N 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- VKOBVWXKNCXXDE-UHFFFAOYSA-N icosanoic acid Chemical compound CCCCCCCCCCCCCCCCCCCC(O)=O VKOBVWXKNCXXDE-UHFFFAOYSA-N 0.000 description 1
- MTNDZQHUAFNZQY-UHFFFAOYSA-N imidazoline Chemical group C1CN=CN1 MTNDZQHUAFNZQY-UHFFFAOYSA-N 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 238000001307 laser spectroscopy Methods 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229960004488 linolenic acid Drugs 0.000 description 1
- KQQKGWQCNNTQJW-UHFFFAOYSA-N linolenic acid Natural products CC=CCCC=CCC=CCCCCCCCC(O)=O KQQKGWQCNNTQJW-UHFFFAOYSA-N 0.000 description 1
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L magnesium chloride Substances [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 229940038384 octadecane Drugs 0.000 description 1
- CKQVRZJOMJRTOY-UHFFFAOYSA-N octadecanoic acid;propane-1,2,3-triol Chemical class OCC(O)CO.CCCCCCCCCCCCCCCCCC(O)=O CKQVRZJOMJRTOY-UHFFFAOYSA-N 0.000 description 1
- CCCMONHAUSKTEQ-UHFFFAOYSA-N octadecene Natural products CCCCCCCCCCCCCCCCC=C CCCMONHAUSKTEQ-UHFFFAOYSA-N 0.000 description 1
- 229960002446 octanoic acid Drugs 0.000 description 1
- 238000006384 oligomerization reaction Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 229940085991 phosphate ion Drugs 0.000 description 1
- 229920000151 polyglycol Polymers 0.000 description 1
- 239000010695 polyglycol Substances 0.000 description 1
- 229920001522 polyglycol ester Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 239000001103 potassium chloride Substances 0.000 description 1
- 235000011164 potassium chloride Nutrition 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 238000005956 quaternization reaction Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000006268 reductive amination reaction Methods 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 230000002940 repellent Effects 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M sodium chloride Inorganic materials [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 235000002316 solid fats Nutrition 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- TUNFSRHWOTWDNC-HKGQFRNVSA-N tetradecanoic acid Chemical compound CCCCCCCCCCCCC[14C](O)=O TUNFSRHWOTWDNC-HKGQFRNVSA-N 0.000 description 1
- AQWHMKSIVLSRNY-UHFFFAOYSA-N trans-Octadec-5-ensaeure Natural products CCCCCCCCCCCCC=CCCCC(O)=O AQWHMKSIVLSRNY-UHFFFAOYSA-N 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 239000001993 wax Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/20—Organic compounds containing oxygen
- C11D3/2003—Alcohols; Phenols
- C11D3/2006—Monohydric alcohols
- C11D3/201—Monohydric alcohols linear
- C11D3/2013—Monohydric alcohols linear fatty or with at least 8 carbon atoms in the alkyl chain
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/38—Cationic compounds
- C11D1/645—Mixtures of compounds all of which are cationic
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/835—Mixtures of non-ionic with cationic compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/0005—Other compounding ingredients characterised by their effect
- C11D3/001—Softening compositions
- C11D3/0015—Softening compositions liquid
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/38—Cationic compounds
- C11D1/42—Amino alcohols or amino ethers
- C11D1/44—Ethers of polyoxyalkylenes with amino alcohols; Condensation products of epoxyalkanes with amines
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/38—Cationic compounds
- C11D1/52—Carboxylic amides, alkylolamides or imides or their condensation products with alkylene oxides
- C11D1/525—Carboxylic amides (R1-CO-NR2R3), where R1, R2 or R3 contain two or more hydroxy groups per alkyl group, e.g. R3 being a reducing sugar rest
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/38—Cationic compounds
- C11D1/62—Quaternary ammonium compounds
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/662—Carbohydrates or derivatives
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/667—Neutral esters, e.g. sorbitan esters
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D1/00—Detergent compositions based essentially on surface-active compounds; Use of these compounds as a detergent
- C11D1/66—Non-ionic compounds
- C11D1/72—Ethers of polyoxyalkylene glycols
Definitions
- the present invention relates to aqueous dispersions which contain fatty substances and emulsifiers as fabric softeners, which are characterized by a high positive zeta potential of the dispersion.
- Textile treatment agents that have a softening effect on laundry are well known. you will be usually used in the last rinse of a machine wash and give the laundry a pleasant, soft feel, as they have a pronounced sorption capacity on a wide variety of fiber surfaces.
- the coating of the fiber with the long-chain molecules leads to a sliding effect between the fibers and prevents the water or dry rigidity that is responsible for the hard grip of the fabric.
- the adsorption mechanism for the drawing up of softening materials consists in the electrostatic attraction between the e.g. due to washing alkali negatively charged fiber surface and positively charged plasticizer particles.
- Water-insoluble quaternary ammonium compounds containing two long chain alkyl or alkenyl radicals are used. Commonly used connections are Ditallow dimethyl ammonium chloride or distearyl dimethyl ammonium chloride. Because such connections but as ecological are of concern, increasingly used difatty acid trialkanolamine ester salts by reaction a trialkanolamine with technical fatty acids and subsequent quaternization can be obtained, such as Methyl-N- (2-hydroxyethyl) -N, N-di (talgacyloxyethyl) ammonium methosulfate.
- fabric softeners are offered in the form of aqueous dispersions.
- EP 043 622 B1 proposed an aqueous stable dispersion which contains 8 to 22% by weight of a water-insoluble cationic fabric softener, and a viscosity-regulating system containing 0.5 to 6% by weight C 10-24 hydrocarbons, C 10-24 fatty acids or C 10-24 fatty acid esters from fatty acids with short-chain alcohols or C 10-24 fatty alcohols and 0.05 to 1% by weight of a water-soluble cationic polymer.
- the addition of polymers for viscosity control often leads to reduced performance of the fabric softener. Agents without polymeric viscosity regulators are described in DE 36 02 089 C2.
- the compositions contain a fatty alcohol with 10 to 24 carbon atoms, the weight ratio between cationic softeners to fatty alcohols being between 3.5: 1 and 6: 1.
- Ethoxylated amines are used as emulsifiers.
- Solid agents are known from German patent application 42 32 448 A1 which contain quaternary difatty acid trialkanolamine ester salts and a hydroxy compound selected from the group of fatty alcohols, fatty alcohol polyglycol ethers, polyol fatty acid partial esters and carbohydrates and which are suitable for the production of liquid, aqueous plasticizer compositions (with 1 to 50% by weight). -% active substance).
- the weight ratio between quaternary esteramine salt and hydroxy compound should be between 9: 1 and 1: 1.
- a disadvantage of these agents is that relatively large amounts of ecologically unsatisfactory nitrogen-containing compounds are still used.
- EP 497 769 A2 proposes acidic, aqueous fabric softeners which contain pentaerythritol esters as a softening component in amounts between 1 and 25% by weight and 0.1 to 10% by weight of a nonionic emulsifier and are therefore ecologically harmless.
- the object of the invention was therefore to provide stable, aqueous textile plasticizer dispersions To produce the basis of largely biodegradable ingredients with advantageous ecotoxicological properties. It has now been found that combinations of biodegradable, water-insoluble fatty substances with emulsifiers in certain quantitative ratios leads to efficient means when the zeta potential of the aqueous Dispersions exceeds a certain value.
- the invention therefore relates to fabric softeners in the form of an aqueous dispersion of an anti-aging component, wherein the finishing component, based on the weight of the fabric softener, is from 0.5 to 20 %
- the finishing component based on the weight of the fabric softener, is from 0.5 to 20 %
- the weight ratio between fat and emulsifiers is between 10: 1 and 0.5: 1 and the proviso that the zeta potential the aqueous dispersion at a pH of 7 and a temperature of 25 ° C at least + 30 mV is.
- zeta potential is a common method for characterizing solid / liquid dispersions (RJ Hunter, Zeta Potential in Colloid Science, pages 150 to 162, Academic Press, New York 1981).
- Dispersed particles can become electrically charged, for example by adsorption of ions on their surface.
- An electrical double layer forms on the surface of these electrically charged particles, which is firmly connected to the particles and causes an apparent increase in volume.
- This solid layer is enveloped by a movable and diffuse ion layer.
- the potential ⁇ 0 on the particle surface now drops linearly within the solid ion layer with the thickness ⁇ to the value ⁇ ⁇ in order to decrease exponentially to the value 0 in the diffuse layer.
- the potential difference between the inner solid ion layer ⁇ ⁇ and the point within the diffuse ion layer at which the potential has decreased to 1 / e • ⁇ ⁇ is called the zeta potential.
- the rate of migration is measured depending on the size of the particles to be examined either by means of light microscopic observation or, especially for smaller particles, by means of laser correlation spectroscopy (W. Demtröder, laser spectroscopy: basics and techniques, 2nd edition, Springer-Verlag, Berlin 1991, chapters 12.7 to 12.7.2).
- the high positive zeta potential of the dispersions means that the dispersed particles completely can pull up the negatively charged fibers and by completely covering the fibers with hydrophobic, long-chain alkyl residues a good softening effect is achieved.
- Means are particularly suitable here have the highest possible zeta potential over a wide pH range, as is present in the wash liquor.
- Dispersions according to the invention which not only have a zeta potential of not only at a pH of 7 are particularly preferred have at least + 30 mV, but also at a pH of 8, which is often in the wash liquor during reached in the rinse cycle, still show at least a zeta potential of + 25 mV (temperature in each case 25 ° C.).
- Dispersions whose zeta potential at a temperature of 25 ° C. and a pH of are particularly preferred 7 show at least + 40 mV.
- the dispersions according to the invention contain at least one fatty substance in amounts between 0.5 and 20 % By weight, preferably between 2 and 12% by weight and in particular between 4 and 6% by weight, based on the Total amount of the agent, and the cationic emulsifier in amounts between 0.2 and 10 wt .-%, preferably between 0.3 and 8% by weight, in particular between 0.4 and 6% by weight and optionally a nonionic Emulsifier in amounts up to 10% by weight. It is essential that the weight ratio between fat and Emulsifier is between 10: 1 and 0.5: 1 and the amounts of the ingredients are adjusted so that the zeta potential the dispersion at a pH of 7 and 25 ° C is at least + 30 mV.
- Particularly preferred dispersions have a weight ratio between fat and emulsifiers between 1: 1 and 8: 1, and in particular between 2: 1 and 6: 1.
- fatty substances are understood to mean solid fats, fatty alcohols, waxes and hydrocarbons at normal temperature (20 ° C.). These include, for example, hardened fats and oils of animal and vegetable origin, as well as non-cyclic, branched and unbranched hydrocarbons with 12 to 30 carbon atoms. Examples of such compounds are tetradecane, hexadecane, octadecane and octadecene.
- the fatty substances are preferably selected from the group of the fatty acid esters of fatty acids with 12 to 22 carbon atoms with mono- or polyhydric alcohols with 1 to 22 carbon atoms, as well as fatty acids or fatty alcohols with 12 to 22 carbon atoms and mixtures of these substances.
- monoesters or diesters of fatty acids with pentaerythritol monoesters and diesters of C 12-18 fatty acids with glycerol or monoesters of C 12-18 fatty acids with C 12-18 fatty alcohols are preferred.
- Examples of such compounds are lauric, myristic, palmitic or stearic acid as well as methyl and Ethyl ester of these acids.
- decanol, dodecanol, tetradecanol, pentadecanol, Hexadecanol or octadecanol and mixtures of these alcohols are used.
- Examples of preferred uses Fat substances are technical pentaerythritol distearic acid esters or glycerol monostearic acid esters as well as technical ones Fatty alcohols.
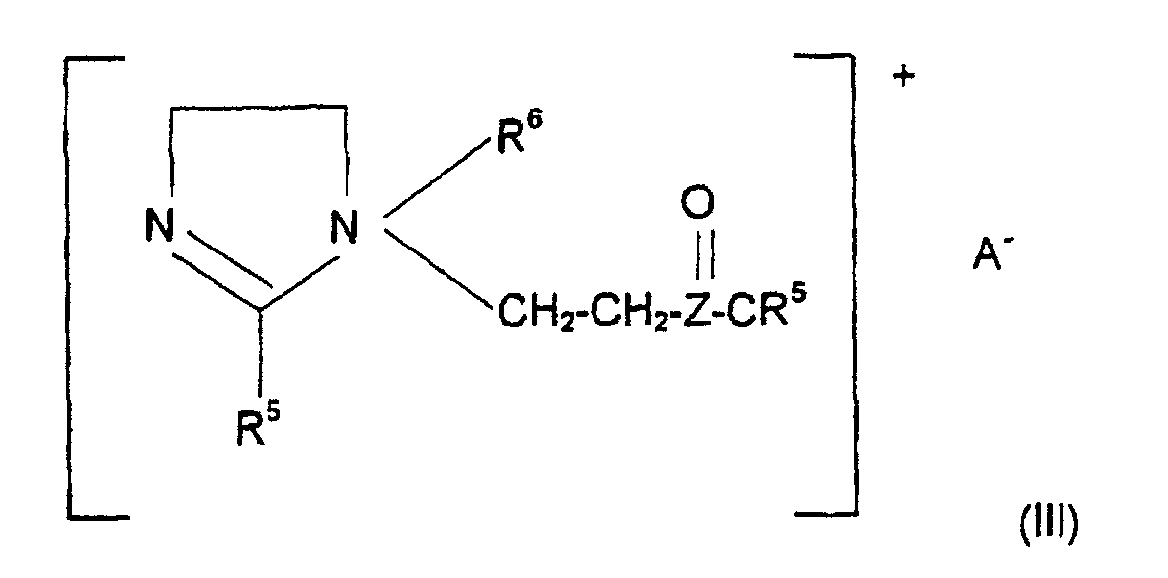
- Cationic emulsifiers are understood in the context of the present application to mean compounds which are selected from the group of the quaternary ammonium compounds of the formulas (I) and (II), where R is an acyclic alkyl radical having 12 to 24 carbon atoms, R 1 is a saturated C 1 -C 4 alkyl or hydroxyalkyl radical, R 2 is either R or R 1 and COR 3 is an aliphatic acyl radical having 12 to 22 carbon atoms 0, 1, 2 or 3 double bonds and R 4 is H or OH, where n is 1, 2 or 3 and X is either a halide, methosulfate, metophosphate or phosphate ion, and mixtures of these compounds. Compounds which contain alkyl radicals having 16 to 18 carbon atoms are particularly preferred.
- Examples of cationic surfactants of the formula (I) are didecyldimethylammonium chloride and ditallow dimethylammonium chloride oderDihexadecylammoniumchlorid.
- Examples of compounds of the formula (II) are methyl-N- (2-hydroxyethyl) -N, N-di (tallow acyl-oxyethyl) ammonium methosulfate, Bis- (palmitoyl) -ethyl hydroxyethyl methyl ammonium methosulfate or methyl-N, N-bis (acyloxyethyl) -N- (2-hydroxyethyl) ammonium methosulfate.
- connection of the Formulas (I) and (II) can also be used in short-chain, water-soluble, quaternary ammonium compounds, such as trihydroxyethyl methyl ammonium methosulfate or cetyl trimethyl ammonium chloride.
- quaternary ammonium compounds such as trihydroxyethyl methyl ammonium methosulfate or cetyl trimethyl ammonium chloride.
- Protonated too Alkylamine compounds that have a softening effect, and the non-quaternized, protonated Precursors of the cationic emulsifiers are suitable.
- quaternized compounds of formula (II) which have unsaturated alkyl chains preference is given to the acly groups whose corresponding fatty acids preferably have an iodine number between 5 and 25 have between 10 and 25 and in particular between 15 and 20 and which have a cis / trans isomer ratio (in % By weight) of 30:70, preferably greater than 50:50 and in particular greater than 70:30.
- R 7 each independently represents a C 1-4 alkyl, alkenyl or hydroxyalkyl group
- R 8 each independently represents a C 8-28 alkyl group
- n is a number between 0 and 5.
- nonionic emulsifiers are understood to mean compounds which come from the group of the alkoxylated fatty acids with 12 to 22 carbon atoms, the alkoxylated fatty acid esters from fatty acids with 12 to 22 carbon atoms with alcohols with 1 to 10 carbon atoms and the alkoxylated fatty alcohols with 12 to 22 carbon atoms , where the alkoxylated compounds have HLB values between 3 and 20, and fatty acid amides and monoalkanolamides from C 12 -C 22 fatty acids with amines or alkanolamines with 1 to 9 carbon atoms, as well as alkyl glycosides or glucamides, are selected.
- Alkoxylated compounds with an HLB value between 3 and 20, preferably between 8 and 14 are preferred.
- nonionic emulsifiers according to the invention are C 12-18 fatty alcohols with 7 EO, cetyl / stearyl alcohol with 20 EO or fatty acid polyglycol esters.
- the alkyl glycosides used are compounds of the general formula RO (G) x in which R is a primary straight-chain or methyl-branched, in particular methyl-branched aliphatic radical having 8 to 22, preferably 12 to 18, carbon atoms and G is the symbol, which stands for a glycose unit with 5 or 6 carbon atoms, preferably for glucose.
- the degree of oligomerization x which indicates the distribution of monoglycosides and oligoglycosides, is any number between 1 and 10; x is preferably 1.2 to 1.4.
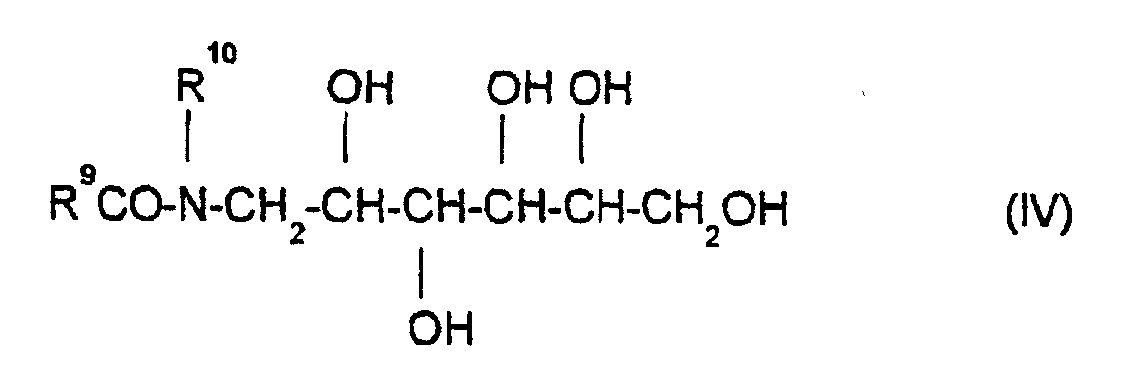
- Fatty acid N-alkylglucamides such as are represented by the formula (IV) are preferably used as glucamides, where R 10 is hydrogen or an alkyl group and R 9 CO is the acyl radical of caproic acid, caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, palmoleic acid, stearic acid, isostearic acid, oleic acid, elaidic acid, petroselinic acid, linoleic acid, linolenic acid, arachic acid, gadoleic acid, gadoleic acid or erucic acid or its technical mixtures.
- Fatty acid N-alkylglucamides of the formula (IV) which are obtained by reductive amination of glucose with methylamine and subsequent acylation with lauric acid or C 12/14 coconut fatty acid or a corresponding derivative are particularly preferred.
- the agents according to the invention can also be used in textile softeners Contain common substances.
- these include, for example, organic solvents such as ethanol or isopropyl alcohol, Fungicides, enzymes, for example cellulase, dyes, optical brighteners, lecithin, UV absorbers, Preservatives, soil repellents, pearlescent agents or fragrances.
- the agents may also contain electrolytes, preferably sodium, magnesium or calcium chloride, as well as pH adjusting agents such as e.g. organic and inorganic Acids.
- the dispersions according to the invention are prepared in a manner known per se by the Ingredients mixed with the necessary amount of water, then heated to a temperature of 60 ° C and Mix for 5 to 30 minutes in a high speed mixer.
- the aqueous white rinse dispersions thus obtained have a pH between 2 and 7, preferably between 3 and 6.
- aqueous dispersions mentioned in Examples 1 to 8 were prepared by the corresponding Fatty substances with the emulsifiers and water were introduced and heated to 80 ° C. with thorough mixing. After this the raw materials were homogeneously dispersed, the cationic emulsifier was added with thorough mixing. The dispersion was cooled to 30 ° C. with moderate stirring and then the remaining constituents, such as perfume oils.
- the zeta potentials of the aqueous dispersions and the assessment of the gripping effect can be found in the table 1 can be removed.
- the determination of the gripping effect was carried out on pre-washed terry towels, which with the investigating agents and then dried in the air.
- the test fabrics were in addition in a glass drum with the agents to be examined (concentration 15 g / kg dry wash, water hardness 16 ° d, liquor ratio 1: 5) treated for 5 minutes, causing the drum to reverse has been.
- the wipes were fingered from a test panel (5 people).
- the zeta potential was measured using a Malvern-Zetazisers® 3 at a temperature of 25 ° C. To determine the zeta potential, the respective dispersion was mixed with 0.001 molar potassium chloride solution 1: 400 diluted and then the pH with hydrochloric acid or sodium hydroxide to the desired Value set. The values given represent mean values from 5 measurements.
- the dispersions according to the invention with a small proportion of quaternary N-containing compounds, good grip properties that are comparable to the performance of commercially available plasticizers, which as Avivage component contain only cationic N-containing salts.
- the missing amounts are up to 100% by weight of water and small amounts other components (electrolytes, perfume oil, auxiliaries, etc.).
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Treatments For Attaching Organic Compounds To Fibrous Goods (AREA)
- Detergent Compositions (AREA)
- Colloid Chemistry (AREA)
Description
| Zeta-Potential [mV] | |||
| Zusammensetzung | pH = 7 | pH = 8 | Note |
| 1 | 52 | 52 | 4,7 |
| 2 | 41 | 38 | 3,9 |
| 3 | 42 | 30 | 4,7 |
| 4 | 45 | 45 | 4,8 |
| 5 | 52 | 41 | 4,7 |
| 6 | 52 | 44 | 4,3 |
| 7 | 24 | 27 | 2,6 |
| 8 | -35 | -32 | 1,5 |
| 9 | 65 | 65 | 5,0 |
| 10 | 27 | 23 | 3,5 |
Claims (8)
- Wäscheweichspülmittel in Form einer wässrigen Dispersion einer Avivagekomponente, dadurch gekennzeichnet, dass die Avivagekomponente, bezogen auf das Gewicht des Wäscheweichspülmittels, aus 0,5 bis 20 Gew.-% mindestens eines bei Normaltemperatur (20° C) festen Fetts, Fettalkohols, Wachses oder Kohlenwasserstoffes als nichtionischen Fettstoff und 0,2 bis 10 Gew.-% eines wasserlöslichen und/oder wasserunlöslichen kationischen Emulgators und 0 bis 10 Gew.-% eines nichtionischen Emulgators besteht, wobei das Gewichtsverhältnis zwischen Fettstoff und Emulgatoren zwischen 10 : 1 und 0,5 : 1 liegt und der Maßgabe, dass das Zeta-Potential der wässrigen Dispersion bei einem pH-Wert von 7 und einer Temperatur von 25 °C mindestens + 30 mV beträgt.
- Wäscheweichspülmittel nach Anspruch 1, dadurch gekennzeichnet, daß der wasserunlösliche nichtionische Fettstoff ausgewählt ist aus der Gruppe der Fettsäureester von Fettsäuren mit 12 bis 22 Kohlenstoffatomen mit ein- oder mehrwertigen Alkoholen mit 1 bis 22 Kohlenstoffatomen, sowie Fettsäuren und Fettalkoholen mit 12 bis 22 Kohlenstoffatomen und Mischungen aus diesen Substanzen.
- Wäscheweichspülmittel nach einem der Ansprüche 1 oder 2, dadurch gekennzeichnet, daß als Fettstoff Monooder Diester von Fettsäuren mit Pentaerythrit, Monoester und Diester von C12-18-Fettsäuren mit Glycerin oder Monoester von C12-18-Fettsäuren mit C12-18-Fettalkoholen enthalten ist.
- Wäscheweichspülmittel nach einem der Ansprüche 1 bis 3, dadurch gekennzeichnet, daß der wasserunlösliche kationische Emulgator ausgewählt ist aus der Gruppe der quaternären Ammoniumverbindungen der Formeln (I) oder (II) wobei R für einen acyclischen Alkylrest mit 12 bis 24 Kohlenstoffatomen, R1 für einen gesättigten C1-C4 Alkyloder Hydroxyalkylrest steht, R2 entweder gleich R oder R1 ist und COR3 für einen aliphatischen Acylrest mit 12 bis 22 Kohlenstoffatomen mit 0, 1, 2 oder 3 Doppelbindungen steht, sowie R4 gleich H oder OH bedeutet, wobei n den Wert 1, 2 oder 3 hat und X entweder ein Halogenid-, Methosulfat- oder Metophosphation ist, sowie Mischungen dieser Verbindungen.
- Wäscheweichspülmittel nach einem der Ansprüche 1 bis 4, dadurch gekennzeichnet, daß der nichtionische Emulgator ausgewählt ist der Gruppe der alkoxylierten Fettsäuren mit 12 bis 22 Kohlenstoffatomen, alkoxylierten Fettsäureester aus Fettsäuren mit 12 bis 22 Kohlenstoffatomen mit Alkoholen mit 1 bis 10 Kohlenstoffatomen, alkoxylierten Fettalkoholen mit 12 bis 22 Kohlenstoffatomen, wobei die alkoxylierten Verbindungen HLB-Werte zwischen 3 und 20 aufweisen, sowie Fettsäureamiden und Monoalkanolamiden aus C12-C22-Fettsäuren mit Aminen oder Alkanolaminen mit 1 bis 9 Kohlenstoffatomen, sowie Alkylglykoside oder Glucamide.
- Wäscheweichspülmittel nach einem der Ansprüche 1 bis 5, dadurch gekennzeichnet, daß die wäßrige Dispersion bei pH = 7 und einer Temperatur von 25 °C ein Zeta-Potential von mindestens + 40 mV aufweist.
- Wäscheweichspülmittel nach einem der Ansprüche 1 bis 6, dadurch gekennzeichnet, daß die wäßrige Dispersion bei pH = 8 und einer Temperatur von 25 °C ein Zeta-Potential von mindestens + 25 mV aufweist.
- Wäscheweichspülmittel nach einem der Ansprüche 1 bis 6, dadurch gekennzeichnet, daß das Gewichtsverhäftnis zwischen Fettstoff und Emulgator zwischen 1 : 1 und 8 : 1, vorzugsweise zwischen 2 : 1 und 6 : 1 liegt.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19623764 | 1996-06-14 | ||
| DE19623764A DE19623764A1 (de) | 1996-06-14 | 1996-06-14 | Wäßriges Wäscheweichspülmittel mit hohem Zeta-Potential |
| PCT/EP1997/002892 WO1997047716A2 (de) | 1996-06-14 | 1997-06-04 | Wässriges wäscheweichspülmittel mit hohem zeta-potential |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0920486A2 EP0920486A2 (de) | 1999-06-09 |
| EP0920486B1 EP0920486B1 (de) | 2002-01-16 |
| EP0920486B2 true EP0920486B2 (de) | 2004-10-13 |
Family
ID=7796957
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP97927127A Expired - Lifetime EP0920486B2 (de) | 1996-06-14 | 1997-06-04 | Wässriges wäscheweichspülmittel mit hohem zeta-potential |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP0920486B2 (de) |
| AT (1) | ATE212050T1 (de) |
| DE (2) | DE19623764A1 (de) |
| ES (1) | ES2171950T5 (de) |
| WO (1) | WO1997047716A2 (de) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8232239B2 (en) | 2010-03-09 | 2012-07-31 | Ecolab Usa Inc. | Liquid concentrated fabric softener composition |
| US8673838B2 (en) | 2011-06-22 | 2014-03-18 | Ecolab Usa Inc. | Solid concentrated fabric softener composition |
| US9150819B2 (en) | 2007-06-15 | 2015-10-06 | Ecolab Usa Inc. | Solid fabric conditioner composition and method of use |
| US9506015B2 (en) | 2014-11-21 | 2016-11-29 | Ecolab Usa Inc. | Compositions to boost fabric softener performance |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0919608A1 (de) * | 1997-11-25 | 1999-06-02 | The Procter & Gamble Company | Verwendung einer Polyhydroyfettsäureamidverbindung als Weichmacherverbindung |
| NZ500399A (en) * | 1998-02-19 | 2000-11-24 | Colgate Palmolive Co | Stable rinse cycle fabric softener composition with glycerol monostearate co-softener |
| US6057285A (en) * | 1998-02-19 | 2000-05-02 | Colgate-Palmolive Co. | Stable rinse cycle fabric softener composition with GMS co-softener |
| GB0014891D0 (en) * | 2000-06-16 | 2000-08-09 | Unilever Plc | Fabric softening compositions |
| US20020187911A1 (en) * | 2001-03-05 | 2002-12-12 | Goldschmist Chemical Company | Viscosity and softening enhancement by low-solids rinse cycle fabric softeners based on quaternary ammonium compounds and amine ethoxylates |
| US10900168B2 (en) | 2002-04-09 | 2021-01-26 | Gregory van Buskirk | Fabric treatment for stain repellency |
| US10822577B2 (en) | 2002-04-09 | 2020-11-03 | Gregory van Buskirk | Fabric treatment method for stain release |
| US7893014B2 (en) | 2006-12-21 | 2011-02-22 | Gregory Van Buskirk | Fabric treatment for stain release |
| GB0213263D0 (en) * | 2002-06-10 | 2002-07-17 | Unilever Plc | Improvements relating to fabric detergent compositions |
| US8470756B2 (en) * | 2009-03-17 | 2013-06-25 | S.C. Johnson & Son, Inc. | Eco-friendly laundry pretreatment compositions |
| US9725679B2 (en) | 2014-11-21 | 2017-08-08 | Ecolab Usa Inc. | Compositions to boost fabric softener performance |
| US9688945B2 (en) | 2014-11-21 | 2017-06-27 | Ecolab Usa Inc. | Compositions to boost fabric softener performance |
| AU2020296116B2 (en) | 2019-06-21 | 2023-09-21 | Ecolab Usa Inc. | Solid nonionic surfactant compositions |
| WO2023105205A1 (en) | 2021-12-06 | 2023-06-15 | Reckitt Benckiser Health Limited | Laundry sanitizing and softening composition |
| WO2025040727A1 (en) | 2023-08-21 | 2025-02-27 | Reckitt Benckiser Health Limited | Laundry sanitizing and softening composition |
| WO2025040730A1 (en) | 2023-08-21 | 2025-02-27 | Reckitt Benckiser Health Limited | Laundry sanitizing and softening composition |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1601359A (en) † | 1977-05-30 | 1981-10-28 | Procter & Gamble | Textile treating composition |
| US4320013A (en) † | 1980-06-10 | 1982-03-16 | The Procter & Gamble Company | Fabric conditioning compositions |
| WO1994019439A1 (en) † | 1993-02-25 | 1994-09-01 | Unilever Plc | Use of fabric softening composition |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2733493C2 (de) * | 1976-05-17 | 1986-11-13 | Henkel KGaA, 4000 Düsseldorf | Glättemittel für Textilfasermaterial |
| GB1601360A (en) * | 1977-07-12 | 1981-10-28 | Procter & Gamble | Textile treatment composition |
| JP2688719B2 (ja) * | 1990-09-25 | 1997-12-10 | ユシロ化学工業株式会社 | 繊維処理用油剤 |
| FI921147A7 (fi) * | 1991-09-06 | 1993-03-07 | Colgate Palmolive Co | Tygmjukgoerande kompositioner baserade pao en pentaerytritolfoerening ochett dispergermedel foer en saodan foerening |
| DE4437032A1 (de) * | 1994-10-17 | 1996-04-18 | Henkel Kgaa | Textile Weichmacher-Konzentrate |
-
1996
- 1996-06-14 DE DE19623764A patent/DE19623764A1/de not_active Withdrawn
-
1997
- 1997-06-04 WO PCT/EP1997/002892 patent/WO1997047716A2/de not_active Ceased
- 1997-06-04 EP EP97927127A patent/EP0920486B2/de not_active Expired - Lifetime
- 1997-06-04 DE DE59706021T patent/DE59706021D1/de not_active Expired - Lifetime
- 1997-06-04 AT AT97927127T patent/ATE212050T1/de active
- 1997-06-04 ES ES97927127T patent/ES2171950T5/es not_active Expired - Lifetime
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1601359A (en) † | 1977-05-30 | 1981-10-28 | Procter & Gamble | Textile treating composition |
| US4320013A (en) † | 1980-06-10 | 1982-03-16 | The Procter & Gamble Company | Fabric conditioning compositions |
| WO1994019439A1 (en) † | 1993-02-25 | 1994-09-01 | Unilever Plc | Use of fabric softening composition |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9150819B2 (en) | 2007-06-15 | 2015-10-06 | Ecolab Usa Inc. | Solid fabric conditioner composition and method of use |
| US8232239B2 (en) | 2010-03-09 | 2012-07-31 | Ecolab Usa Inc. | Liquid concentrated fabric softener composition |
| US8367601B2 (en) | 2010-03-09 | 2013-02-05 | Ecolab Usa Inc. | Liquid concentrated fabric softener composition |
| US8673838B2 (en) | 2011-06-22 | 2014-03-18 | Ecolab Usa Inc. | Solid concentrated fabric softener composition |
| US9388366B2 (en) | 2011-06-22 | 2016-07-12 | Ecolab Usa Inc. | Solid concentrated fabric softener composition |
| US9506015B2 (en) | 2014-11-21 | 2016-11-29 | Ecolab Usa Inc. | Compositions to boost fabric softener performance |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0920486B1 (de) | 2002-01-16 |
| ATE212050T1 (de) | 2002-02-15 |
| DE19623764A1 (de) | 1997-12-18 |
| DE59706021D1 (de) | 2002-02-21 |
| ES2171950T5 (es) | 2005-04-16 |
| WO1997047716A2 (de) | 1997-12-18 |
| ES2171950T3 (es) | 2002-09-16 |
| EP0920486A2 (de) | 1999-06-09 |
| WO1997047716A3 (de) | 1998-03-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0920486B2 (de) | Wässriges wäscheweichspülmittel mit hohem zeta-potential | |
| EP1006176B1 (de) | Niedrigkonzentrierte, hochviskose wässrige Weichspülmittel | |
| DE69618969T2 (de) | Stabile weichspülerzusammensetzungen | |
| DE60022216T2 (de) | Zusammensetzung umfassend quaternäre ammoniumsalze | |
| DE3926740C2 (de) | Wässrige Weichspülmittel und deren Verwendung | |
| DE2631114A1 (de) | Gewebeweichmacher | |
| EP0675941B1 (de) | Wässrige textilweichmacher-dispersionen | |
| EP0625184B1 (de) | Verfahren zur herstellung niedrigviskoser wässriger esterquat-konzentrate | |
| DE602004008217T2 (de) | Einfach dispergierbare konzentrierte Esterquat Zusammensetzungen | |
| DE69532508T2 (de) | Gewebeweichmacherzusammensetzung | |
| DE3588115T2 (de) | Konzentrierte Weichmacherzusammensetzungen auf der Basis von quaternären ammoniumhaltigen kationischen oberflächenaktiven Verbindungen | |
| EP0718275B1 (de) | Quaternierte Triethanolaminfettsäureester | |
| DE69426140T2 (de) | Flüssiges Wäscheweichspülerkonzentrat | |
| DE4004294A1 (de) | Wirkstoff-kombination zur textilbehandlung | |
| DE3730444C2 (de) | ||
| DE2911198C2 (de) | Konzentrierte Wäscheweichspülmittel und Verfahren zu deren Herstellung | |
| DE69935337T3 (de) | Weichmacherzusammensetzung | |
| DE60312204T3 (de) | Esterquathaltiges weichspülmittelkonzentrat mit spezieller esterverteilung und einem elektrolyten | |
| EP2465917A1 (de) | Weichmacher für Textilien | |
| EP0454741B1 (de) | Textilbehandlungsmittel | |
| DE4402527A1 (de) | Wäßrige Lösungen von Esterquats | |
| EP2582780A1 (de) | Verdickter weichspüler | |
| DE4441029A1 (de) | Kationische Wachsdispersionen | |
| EP3818137A1 (de) | Aktivstoffe für hochviskose wasch- und reinigungsformulierungen | |
| DE69918182T2 (de) | Weichmacherzusammensetzung |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19981207 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE DE ES FR GB IT NL |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| 17Q | First examination report despatched |
Effective date: 20010102 |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: COGNIS DEUTSCHLAND GMBH |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE DE ES FR GB IT NL |
|
| REF | Corresponds to: |
Ref document number: 212050 Country of ref document: AT Date of ref document: 20020215 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 59706021 Country of ref document: DE Date of ref document: 20020221 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20020416 |
|
| ET | Fr: translation filed | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2171950 Country of ref document: ES Kind code of ref document: T3 |
|
| PLBQ | Unpublished change to opponent data |
Free format text: ORIGINAL CODE: EPIDOS OPPO |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLBF | Reply of patent proprietor to notice(s) of opposition |
Free format text: ORIGINAL CODE: EPIDOS OBSO |
|
| 26 | Opposition filed |
Opponent name: UNILEVER PLC Effective date: 20021016 |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: UNILEVER PLC |
|
| PLBF | Reply of patent proprietor to notice(s) of opposition |
Free format text: ORIGINAL CODE: EPIDOS OBSO |
|
| PLBF | Reply of patent proprietor to notice(s) of opposition |
Free format text: ORIGINAL CODE: EPIDOS OBSO |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: COGNIS DEUTSCHLAND GMBH & CO. KG |
|
| NLS | Nl: assignments of ep-patents |
Owner name: COGNIS DEUTSCHLAND II GMBH & CO. KG Owner name: COGNIS CHEMIE GMBH & CO. KG |
|
| NLT1 | Nl: modifications of names registered in virtue of documents presented to the patent office pursuant to art. 16 a, paragraph 1 |
Owner name: COGNIS DEUTSCHLAND GMBH & CO. KG |
|
| NLT2 | Nl: modifications (of names), taken from the european patent patent bulletin |
Owner name: COGNIS DEUTSCHLAND GMBH & CO. KG |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20030902 Year of fee payment: 7 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: TP |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040630 |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| 27A | Patent maintained in amended form |
Effective date: 20041013 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): AT BE DE ES FR GB IT NL |
|
| NLR2 | Nl: decision of opposition |
Effective date: 20041013 |
|
| BERE | Be: lapsed |
Owner name: *COGNIS DEUTSCHLAND G.M.B.H. Effective date: 20040630 |
|
| GBTA | Gb: translation of amended ep patent filed (gb section 77(6)(b)/1977) | ||
| NLR3 | Nl: receipt of modified translations in the netherlands language after an opposition procedure | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: DC2A Date of ref document: 20041209 Kind code of ref document: T5 |
|
| ET3 | Fr: translation filed ** decision concerning opposition | ||
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: TP |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E Free format text: REGISTERED BETWEEN 20090514 AND 20090520 |
|
| NLS | Nl: assignments of ep-patents |
Owner name: COGNIS IP MANAGEMENT GMBH Effective date: 20090507 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 20120626 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20130626 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20130902 Year of fee payment: 17 Ref country code: NL Payment date: 20130626 Year of fee payment: 17 Ref country code: ES Payment date: 20130724 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20130722 Year of fee payment: 17 Ref country code: GB Payment date: 20130701 Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 59706021 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: V1 Effective date: 20150101 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 212050 Country of ref document: AT Kind code of ref document: T Effective date: 20140604 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20140604 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20150227 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150101 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 59706021 Country of ref document: DE Effective date: 20150101 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20150101 Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140604 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140604 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140604 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140630 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20150724 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20140605 |