EP0239162A2 - Méthode de la prodution d' un anticorps capable de détecter la présence d'une oncogène humaine. - Google Patents
Méthode de la prodution d' un anticorps capable de détecter la présence d'une oncogène humaine. Download PDFInfo
- Publication number
- EP0239162A2 EP0239162A2 EP87200461A EP87200461A EP0239162A2 EP 0239162 A2 EP0239162 A2 EP 0239162A2 EP 87200461 A EP87200461 A EP 87200461A EP 87200461 A EP87200461 A EP 87200461A EP 0239162 A2 EP0239162 A2 EP 0239162A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- oncogene
- proto
- dna
- protein
- ras
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/577—Immunoassay; Biospecific binding assay; Materials therefor involving monoclonal antibodies binding reaction mechanisms characterised by the use of monoclonal antibodies; monoclonal antibodies per se are classified with their corresponding antigens
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- C07K16/3038—Kidney, bladder
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/32—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against translation products of oncogenes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6813—Hybridisation assays
- C12Q1/6827—Hybridisation assays for detection of mutation or polymorphism
- C12Q1/683—Hybridisation assays for detection of mutation or polymorphism involving restriction enzymes, e.g. restriction fragment length polymorphism [RFLP]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/531—Production of immunochemical test materials
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/156—Polymorphic or mutational markers
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S436/00—Chemistry: analytical and immunological testing
- Y10S436/811—Test for named disease, body condition or organ function
- Y10S436/813—Cancer
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S530/00—Chemistry: natural resins or derivatives; peptides or proteins; lignins or reaction products thereof
- Y10S530/808—Materials and products related to genetic engineering or hybrid or fused cell technology, e.g. hybridoma, monoclonal products
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S530/00—Chemistry: natural resins or derivatives; peptides or proteins; lignins or reaction products thereof
- Y10S530/808—Materials and products related to genetic engineering or hybrid or fused cell technology, e.g. hybridoma, monoclonal products
- Y10S530/809—Fused cells, e.g. hybridoma
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/14—Heterocyclic carbon compound [i.e., O, S, N, Se, Te, as only ring hetero atom]
- Y10T436/142222—Hetero-O [e.g., ascorbic acid, etc.]
- Y10T436/143333—Saccharide [e.g., DNA, etc.]
Definitions
- This invention is in the field of molecular biology and more specifically relates to defining differences between mutant alleles and their corresponding wild type alleles, particularly oncogenes and proto-oncogenes, and to assays which take advantage of such differences.
- DNA isolated from the EJ cell line was serially passed by transfection into NIH3T3 mouse fibroblast cells until a mouse fibroblast cell was selected containing essentially only the human bladder cancer oncogene and a marker.
- the marker used in this work was an Alu DNA sequence, which is repeated about 300,000 times in human DNA, but is not present in mouse fibroblast DNA.
- the i n terspecies transfection thus resulted in the ultimate selection of a cell containing the oncogene of interest and its associated marker. All DNA from this transfected cell was employed in the creation of a genomic library in a lambdaphage and the appropriate chimeric lambdaphage was then selected using a probe specific for the human A lu marker.
- v-Ha-ras This rat sarcoma virus gene, termed v-Ha-ras, had been acquired from the rat genome during the process of formation of the chimeric viral genome. See Scolnick, E.M. and Parks, W.P. J. Virol. 13: 1211-1219 (1974); and, Shih, T.Y., Williams, D.R., Weeks, M.O., Maryak, J.M., Vass, W.C. and Scolnick, E.M. J. Virol. 27: 45-55 (1978). Both the rat and human cellular homologues of the v-Ha-ras have been isolated in the course of studies of this gene.
- This invention relates to an investigation of the differences between the EJ oncogene, previously shown to cause human bladder cancer, and its proto-oncogene.
- the procedures involved can be applied to defining differences between any mutant allele and its corresponding wild type allele; the procedures are particularly useful in defining the differences between oncogenes and proto-oncogenes.
- the area of the 6.6 kb pEJ responsible for cellular transformation in NIH3T3 fibroblasts was narrowed to a 350 kb segment by a series of in vitro recombinations.
- This 350 kb segment was then sequenced for the oncogene and proto-oncogene, and it was found that single base substitutions accounted for the difference at the 60th nucleotide from the XmaI restriction site.
- GTC glycine
- serological reagents such as polyclonal or monoclonal antibodies, can also be developed which are specific for the altered or normal sequence domains in p21 proteins, or for an amino acid sequence not involved in the alteration which occurs during carcinogenesis. Such serological reagents can then be employed in various protocol to provide a very sensitive test for human bladder carcinogenesis. Similarly, these assays could be employed to detect changes in other wild type alleles causing mutant alleles.
- oncogene is used to mean a genetic sequence whose expression within a cell induces that cell to become converted from a normal cell into a tumor cell.
- proto-oncogene is used herein to mean a genetic sequence, residing in the normal genome of a normal, non-tumor cell, which has the potential, when altered in the appropriate manner, of becoming an oncogene.
- a comparison of the expression of the c-Ha-ras proto-oncogene (EC) from a normal human bladder epithelial cell line with the expression of the oncogene in the EJ transformed bladder cell was undertaken.
- the bladder epithelial cells employed, Hbl-5 were a primary tissue culture explant from a five-month.old human bladder grown on inactivated NIH3T3 feeder layers. This culture was grown out from fresh human. bladder tissue and was shown to exhibit several of the properties expected of transitional bladder epithelium. It was free of underlying stromal material, and consequently represented a close counterpart of the cells from which the bladder carcinoma originated.
- RNA was prepared by the technique of Varmus et al. See Varmus, H.E., Quintrell, N. and Ortiz, S. Cell 25: 23-36 (1981). Four micrograms of RNA was then fractionated by electrophoresis through formaldehyde-containing 2 percent agarose gels and transferred to nitrocellulose. A ras-specific probe was prepared by cutting pEJ with BamHI, fractionating the resulting fragments through a 1 percent agarose gel and extracting the 6.6 kb insert with NaI and glass beads. The nick-translated fragment (6.6 x 10 cpm micrograms -1 ) was annealed to the immobilized RNA.
- Figure 1A shows the relative levels of c-Ha-ras specific RNA in the two cell types: Lane 1, RNA from EJ cells; Lane 2, RNA from Hbl-5 cells. As can be seen, similar levels of RNA were detected in the two cultures and the transcripts had a size of 1.2 kb.
- the only known products of the ras genes are proteins of approximately 21,000 daltons mass, referred to as p21.
- p21 proteins of approximately 21,000 daltons mass
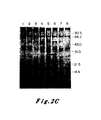
- Experiments were conducted to analyze mobility rates of p21 protein lysates from both EJ and normal bladder cells. Monoclonal antisera against the v-Ha-ras p21 protein were employed. See Furth, M.E., Davis, L.J., Fleurdelys, B. and Scolnick, E.M. J. Virol. 43: 294-304 (1982). Control experiments assured that the amounts of antibody used were in excess of that required to immunoprecipitate the antigen present. The results are shown in Figure 1B.
- cultures were labelled with 35 S-methionine for 12 hours. Lysates were then prepared and immunoprecipitated with non-immune sera (Lanes la and 2a), a monoclonal antisera (Y13-238) which precipitates the p21 encoded by Ha-MuSV but not the p21 encoded by Ki-MuSV (Lanes lb and 2b) or a monoclonal antisera (Y13-259) which detects both the Ha-MuSV and Ki-MuSV p21's (Lane lc and 2c). See Shih, T.Y., Weeks, M.O., Young, H.A. and Scolnick, E.M. Virology 96: 64-79 (1979). 20 x 10 6 cpm of lysate per sample was resolved by electrophoresis through a 12.5 percent SDS-polyacrylamide gel.
- Figure 1B shows a comparison of p21 proteins immunoprecipitated from cell lysates of EJ cell clones (Lanes 1, a-c) and Hbl-5 cells (Lanes 2, a-c). These data indicate that at least two bands of radiolabelled protein were specifically precipitated by the anti-p21 sera from normal bladder cells. Detailed examination of the protein pattern of the bladder carcinoma seen revealed a complex array of bands: two pairs of closely spaced doublets. After comparing the intensities of the p21 bands to intensities pf non-specifically precipitated background bands, it became apparent that the p21 proteins of the normal and the tumor cells were present in comparable amounts.
- bladder epithelial cells were not representative of normal precursors of bladder carcinoma cells. Such a possibility might cloud interpretation since a ras gene could be expressed at a high level in one cell type without inducing transformation, and only achieve this phenotype when inappropriately expressed in a second cell type. Therefore, the levels of transcription and translation of the two genes in the same cellular background were measured.
- a clone of the dominant selectable Ecogpt gene was cotransfected into NIH3T3 cells together with a 10-fold excess of either the cloned EJ oncogene or the cloned proto- oncogene (pEC).
- transfections were carried out employing 75 micrograms NIH3T3 carrier DNA, 500 ng pEJ or pEC DNA, and 50 ng pSVZgpt DNA per 2 x 10 6 cells by known techniques. See Graham, F.L. and van der Eb, A.J. Virology 52: 456-471 (1973); and, Andersson, P., Goldfarb, M.P. and Weinberg, R.A.
- the normal mouse homologue of the ras gene hybridizes only weakly to the pEJ probe. See Parada, L.F., Tabin, C.J., Shih, C. and Weinberg, R.A. Nature 297: 474-479 (1982). Consequently, its presence did not obscure the results.
- the ras-specific sequences were prepared from pEJ and used as a probe. Seventy-five percent of the non-transformed colonies transfected with the proto-oncogene and all of the transformed oncogene-transfected colonies showed the presence of pEJ-homologous sequences migrating at 6.6 kb.
- the positive colonies also had BamHI fragments of other sizes annealing to the probe. These represent copies of the clones that were broken during the transfection process.
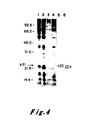
- the data of Figure 4 indicate that the pEJ and pEC transfectants each exhibited two bands of p21.
- the higher molecular weight p21 protein of the pEJ transfectant migrated more slowly than the higher molecular weight protein of the pEC transfectant and the -lower molecular weight p21 of the pEJ transfectant also migrated more slowly than the lower molecular weight p21 of the pEC transfectant.In each case the more slowly migrating band behaved as a kinetic precursor to the more rapidly migrating band.
- Comparable data on the p21 protein of v-Ha-ras previously indicated that the higher band underwent post-translational cleavage to yield its lower, more rapidly migrating partner. See Shih, T.Y. et al. J. Virol. 42: 253-261 (1982).
- Figure 4 may provide an explanation for the complexity of p21 proteins seen in normal and transformed bladder cells (Figure Ib): the normal cells exhibited two bands, reflective of the expression of a proto-oncogene; the carcinoma cells appeared to exhibit four bands, two being specified by the oncogene of these cells, and two by the normal, proto-oncogene of the other homologous chromosome.
- the experimental strategy was to excise a restriction fragment out of the oncogene clone (pEJ) and use it to replace the homologous piece of the proto-oncogene clone (pEC).
- the reciprocal construction would be carried out by splicing the fragment of the proto-oncogene clone into the oncogene clone.
- the assay measured the ability of a fragment of the oncogene to impart transforming activity when placed in the midst of the proto-oncogene clone, and conversely, in the reciprocal recombination, for the loss of activity when the corresponding proto-oncogene fragment was inserted into the oncogene clone was determined.
- Figure 5 presents a diagram of the specific constructions undertaken and a summary of the transfection and transformation data obtained.
- the restriction map shows the cleavage sites for various enzymes within the 6.6 kb BamHI insert. in pBR322. All sites specific for the enzymes are shown except for Xmal which acts in several other places which have not been well characterized. The site shown is the only XmaI site between the first BstEII site and the KpnI site. The solid boxes on the map show the locations of coding exons.
- pEJ/pEC chimeras are shown with segments derived from pEJ shown as solid bars and segments from pEC shown as open bars.
- pEJ and pEC were cleaved with the indicated enzymes either to completion or in a partial digest as required to obtain each indicated fragment.
- the products were. separated by electrophoresis through 1.2 percent agarose and eluted by melting a NaI and absorbing to glass beads.
- the fragment containing pBR322 was then treated with calf intestinal phosphatase.
- the indicated fragments were joined either with the enzyme T4-DNA ligase or in a mock ligation without enzyme. Constructs a-e were made in bimolecular ligations.
- Constructs in f were made by mixing the three fragments simultaneously and in g and h by mixing the four fragments simultaneously.
- the ligation mixtures were directly transformed into the HB101 strain of E. coli. Only when colonies from mock ligations were less than 2 percent of the ligations were colonies analyzed for the presence of clones having appropriate restriction maps. Twenty ng of each clone was transfected into NIH3T3 cells as previously described and then carried without selection until foci were visualized in 10-14 days. Results of the transfections are shown in the first column. The second column shows the number of independent bacterial colonies screened and then transfected into NIH3T3 cells.
- the origin of one parent of a recombinant could be determined by diagnostic restriction digests of the flanking plasmid regions. Since contaminating pEC could itself not give a false positive result, any active clone carrying proto-oncogene flanking sequences must have arisen as a consequence of the acquisition of portions of the transforming gene. Finally, the results were confirmed with several independent clones obtained from a ligation reaction.
- a genetic region 350 nucleotides long was ultimately identified which, when transferred from the oncogene to a corresponding region in the proto-oncogene, was able to impart activity to the latter.
- This region extended from the first XmaI endonuclease site to the Kpnl site. Fifty-five percent of this region consists of the first coding exon, 10 percent is 5' to the exon and 35 percent is part of the first intron.
- NIH3T3 cells transformed with the EJ bladder tumor oncogene, its normal proto-oncogene, or recombinants between the two genes were first biologically cloned in 0.35 percent agar and then metabolically labelled with 35 S-methionine for 18 hours. Lysates were prepared and immunoprecipitated (5 x 10 6 cpm of TCA-precipitable counts) by a monoclonal antibody which detected the p21 encoded by Ha-MuSV but not the p21 encoded by Ki-MuSV (Y13-172). See Furth, M.E., David, L.J., Fleurdelys, B. and Scolnick, E.M. J. Virol.
- Cell lysates were from: NIH3T3 cells (Lane 1); cells transformed with the proto-oncogene [the LTR-activated 3kb SacI fragment described in Payne, G.S., Courtneidge, S.A., Crittendon, L.B., Fadly, A.M., Bishop, J.M. and Varmus, H.E.
- the proto-oncogene the LTR-activated 3kb SacI fragment described in Payne, G.S., Courtneidge, S.A., Crittendon, L.B., Fadly, A.M., Bishop, J.M. and Varmus, H.E.
- the short (350 kb) fragment shown to have biological significance was sequenced for DNA from the oncogene and the proto-oncogene. Sequences were determined by the forward and backward dideoxy DNA sequencing technique of Seif et al. and by the chemical procedure of Maxam and Gilbert. See Seif, I., Khoury, G. and Dhar, R. Nucl. Acid Res. 8: 2225-2238 (1980); and, Maxam, A.H. and Gilbert, W. Proc. Natl. Acad. Sci. USA 74: 560-564 (1977). The results are illustrated in Figure 7 wherein the coding.DNA strand is shown together with the inferred amino acid sequence. Where EJ and the proto-oncogene differ, both codons and amino acids are indicated.
- the only difference between the two DNA segments is in the p21 encoding region of the first known exon, specifically 60 nucleotides from the Xma cleavage site. It occurs in a triplet that encodes glycine in the normal rat and human c-Ha-ras genes. The sequence observed in the EJ oncogene encodes for valine. Thus, this alteration is responsible for the alteration in function of the p21 protein, and for the oncogenic activation of the c-Ha-ras gene that occurs in the EJ bladder carcinoma.
- GCCGGC occurs in the proto-oncogene, and thus represents a recognition site for the endonuclease NaeI.
- This sequence also contains the CCGG recognition site of the endonuclease HpaI. Both of these are changed in the oncogene, whose sequence in the region reads GCCGTC.
- Nael endonuclease was used to independently verify the differences between the two sequences. NaeI was used instead of HpaI because NaeI cleaves DNA less frequently than Hpal. As expected, the pEC clone exhibited one more cleavage site in its inserts than its pEJ counterpart. This also provided retrospective verification of the in vitro recombinant clones. The allele specifying transformation and abnormal p21 migration was seen to precisely co-segregate with the allele disallowing NaeI cleavage at this site.
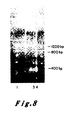
- Figure 8 presents the results of an NaeI restriction enzyme assay of the EJ oncogene and its corresponding proto-oncogene. According to the methods described it was determined that there should exist an NaeI restriction site in the proto-oncogene which should be lost in the alteration which produced the oncogene.
- Molecular clones of the oncogene (pEJ) and proto-oncogene (pEC) and the plasmid into which each was cloned (pBR322) were each purified by known methods. One microgram of each was cut with the enzyme NaeI and the resultant fragments were resolved by electrophoresis through a 15 percent bis-acrylamide gel. The gel was stained by the intercalating dye ethidium bromide and photographed under ultraviolet light.
- the lanes are: Lane 1 is 0 ⁇ x 174 DNA cut with the enzyme HaeIII as a marker lane producing bands of known size; Lane 2 is pBR322 cut with NaeI showing fragments originating in the plasmid vector; Lane 3 is NaeI cleaved pEJ DNA; and, Lane 4 is NaeI cleaved pEC DNA.
- the pEJ DNA contains a band migrating with a molecular weight of 1200 base pairs which is missing in the pEC lane.
- the pEC lane however, has two extra bands, one of 400 bases and the other of 800 bases in length which are missing in pEJ lane.
- the NaeI site in pEC which allows cleavage of the 1200 bp band into two bands of 400 and 800 bases, is lost in the creation of the EJ oncogene.
- valine for glycine might not be expected to have such a profound change in the function of the p21 protein. Nevertheless, several considerations appear to confer importance on this structural alteration.
- the 37 residue long amino acid sequences encoded by the first exons of the two cellular genes are identical, indicating great evolutionary conservation of this region.
- the Kirsten transforming gene is closely related to the v-Ha-ras gene. See Dhar, R., Ellis, R.W., Shih, T.Y., Oroszlan, S., Shapiro, B., Maizel, J., Lowy, D.R. and Scolnick, E.M. Science 217: 934-936 (1982).
- the only difference between the p21 of v-Ki-ras and that of v-Ha-ras in the first 36 amino acids is at position 12 where the residue in Kirsten is serine. See Tsuchida, N., Ryder, T. and Ohtsubo, E. Science 217: 937-939 (1982).
- a second consideration stems from examination of the specific amino acid changes observed.
- glycine is replaced by an amino acid having a relatively bulky side chain.
- Glycine represents an anomaly among the 20 amino acids because it lacks a side chain. Consequently, it is able to participate in extremes of bending and folding of the polypeptide backbone and is the strongest breaker of alpha-helices. See Cantor, C.R. and Schimmel, P.R. Biophysical Chemistrv, Vol. I, p. 303, N.H. Freeman and Co., San Fran- cisco (1980).
- valine or arginine represent abrupt changes in the local stereochemistry of a protein.
- loss of glycine at residue 12 represents a significant change in an essential'domain of the p21 protein.
- a consequence of this change may be a conformational shift of the protein, leading in turn to the aberrant electrophoretic migration or processing of p21 proteins.
- a second, more important consequence is a profound effect on the function of the p21 protein. It is likely that this alteration affects interaction of the p21 with cellular targets.
- Oncogenes of other tumors have also been traced to ras genes.- Specifically, colon and lung carcinomas have been found to carry oncogenes derived from activation of cellular Ki-ras genes. See Der, C., Krontiris, T.G. and Cooper, G.M. Proc. Natl. Acad. Sci. USA 79: 3637-3640 (1980). Therefore, it is likely that activation of many of these oncogenes also depend upon structural alterations similar to those reported above.
- alteration of the Gly 12 codon in the EJ oncogene makes possible a simple diagnostic assay for carcinogenesis or transformation caused by alteration of this codon in the oncogene.
- Any mutation of the Gly 12 codon which occurs during carcinogenesis or related processes alters the cleavage recognition site for the NaeI and HpaI endonucleases, and render the altered DNA of the oncogene resistant to cleavage of this site.
- any test of the cleavability of the DNA at this site by these endonucleases constitutes a diagnostic test for the mutational alteration of this region of the proto-oncogene.
- This test can be performed by treating DNAs of interest with NaeI, for example, resolving the resultant fragments by agarose gel electrophoresis, transferring the resolved fragments to a cellulose nitrate filter, and detecting the transferred fragments by incubation of the filter with a radiolabelled, sequence-specific probe followed by radioautography.
- NaeI NaeI
- the procedures are well known. See Southern, J. Mol. Biol., 98, 503-17 (1975).
- sequence probe used in such experiments can derive from any one of a number of DNA segments which overlap the region of the proto-oncogene, or which lie closely adjacent to this region of the proto-oncogene.
- the sequence probe could be the NaeI fragment of the oncogene beginning at the NaeI site to the left of the altered codon and ending at the NaeI site to the right of it.
- DNA of a cell carrying the normal proto-oncogene would be cleaved into two parts at this site by the NaeI, while DNA of the EJ bladder carcinoma oncogene is unaffected at this site by treatment with a NaeI endonuclease.
- This assay may be made general for the alteration of DNA of a proto-oncogene for its corresponding oncogene. Sensitivity to cleavage by a restriction endonuclease at a DNA sequence of either the proto-oncogene or oncogene, but not the other, is the fundamental concept.
- Another consequence of the change in amino acid sequence of the p21 protein encoded by the proto-oncogene from the p21 protein coded by the oncogene relates to detection of either by specific seralogical reagents.
- the seralogical reagents can be specific for the normal, proto- oncogene-specified amino acid sequence at this site of the protein, or be specific for the altered oncogene-specified amino acid sequence at this site of the protein.
- Other seralogical reagents could be employed that are reacted with a region of the protein that is unaltered, and consequently reactive with either normal or abnormal torms of the p21 protein.
- p21 protein encoded for by the normal site of the proto-oncogene, or by the altered site of the oncogene can be isolated.
- protein segments could be used to produce antibodies by standard antibody production techniques.
- polyclonal antibodies such proteins would be employed to immunize a host, such as a rabbit or-a rat, and antibodies to the protein would be collected from serum obtained from the host.
- monoclonal antibodies could be produced employing cells which produce antibodies to the protein produced by the isolated gene segment in typical fusion techniques for forming hybridoma cells. Basically, these techniques involve the fusing of the antibody producing cell with a cell having immortality, such'as a myeloma cell, to provide a fused cell hybrid which has immortality and is capable of producing the desired antibody; in this case, an antibody to the normal or altered segment of p21 protein coded for by the isolated gene segment. The hybrid cells are then cultured under conditions conducive to the production of antibody after which antibody is collected from the cell culture medium.
- Such techniques for producing monoclonal antibodies have been well described in the literature. See, for example, U.S. Patent Nos. 4,172,124 and 4,196,265, issued to Hilary Koprowski et al., the teachings of which are hereby incorporated by reference.
- seralogical reagents can be developed by the known methods. See Walter, G., Scheidtmann, K.H., Carbone, A., Laudaro, A.P. and Doolittle, R.F., Proc. Nat'1. Acad. Sci. USA, 7.7, 5197-5200 (1980); Lerner, R. A., Green, N., Alexander, H., Liu, F.T., Sut- cliffe, J.G., and Schinnick, T.M., Proc. Nat'1. Acad. Sci. USA, 78, 3403-3407 (1981).
- a peptide segment can be synthesized by a standard organic synthetic technique, the sequence of this peptide corresponding precisely with the amino sequence of the region of interest of the protein to be studied.
- This peptide can then be coupled to a carrier protein and injected into a suitable host (e.g., mouse) to illicit an immune response.
- the serum of the animal immunized in this fashion is then used to immune-precipitate both the immunizing peptide, and more importantly, the protein carrying this amino acid sequence in one of its domains. Consequently, a serum can be made against an oligopeptide sequence (e.g., decapeptide) spanning the amino acid residue site that is altered during the conversion of the normal proto-oncogene to the oncogene.
- Such serum can be made against the normal peptide sequence, or alternatively, against the altered sequence.
- the specificity of the immunoglobulin-antigen interaction will insure that the serum reacting with one oligopeptide will only react with the protein bearing the same, corresponding sequence in one of its domains and not cross-react with a protein bearing an altered version of this sequence in one of its domains.
- P21 protein can be immune-precipitated from a tumor sample or from a tissue homogenate or from fluid released by an autolysing tumor fragment by use of the general, non-specific p21 serum that cross-reacts with domains of the protein (e.g., C-terminal) that are unaffected by the mutation- induced alterations described here.
- the serum with specificity against the N-terminal normal peptides surrounding residue 12 can be used to immune-precipitate protein from the same lysate.
- this N-terminal specific serum which is able to immune-precipitate normal p21 from the non-pathological tissue, is unable to immune-precipitate p21 from a test tissue of interest, then the p21 of this test tissue can be presumed to be altered in a fashion affecting its ability to react with serum reactive with the normal N-terminal sequence.
- the amount of p21 immune-precipitated from this tissue by the general, non-specific serum serves as a control for the amount of p21 which should be precipitable by the serum reactive with the normal N-terminal sequence.
- the above immune-precipitation can be used as a measurement of the presence of altered p21 in a tissue sample.
- a series of peptide specific sera can be developed to diagnose which type of specific alteration has occurred to alter the normal amino acid sequence of this region into an abnormal sequence. For example, a list can be made of the amino acid replacements that can occur by simple point mutation at the codon encoding residue 12. For each of these replacements, a new version of the oligopeptide sequence of this region can be deduced, and a corresponding peptide synthesized for use as described above. Each one of these sera would be specifically reactive with the altered p21 corresponding to the oligopeptide fragment used to induce the serum in question.
- immunoglobulin can be radiolabelled either by direct iodination, or indirectly, by incubation of the immunoglobulin with a second, radiolabelled immunoglobulin that reacts with constant regions of the first immunoglobulin.
- the techniques have particular application and advantage, of course, in detecting differences between oncogenes and proto-oncogenes.
- Members of the ras family of genes have been discussed previously.
- the techniques described herein also lend themselves to finding differences between proto-oncogenes and oncogenes other than members of the ras family. For example, differences between the oncogene present in the HL-60 cell line, known to be responsible for promylo- cytic leukemia, ,certain colon cancers, and Hairy cell leukemia, and its proto-oncogene could be determined using procedures described herein. These differences could then be employed in assays of the type described.
- the invention described herein is useful in defining the differences between proto-oncogenes and their corresponding oncogenes, the proteins coded for by such genes, the preparation of antibodies to such proteins or portions thereof, and the use of such antibodies in assaying for the presence of such proto-oncogenes or oncogenes as a measure of carcinogenesis.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US06/432,337 US4535058A (en) | 1982-10-01 | 1982-10-01 | Characterization of oncogenes and assays based thereon |
| US432337 | 1982-10-01 | ||
| EP83903635A EP0120958B1 (fr) | 1982-10-01 | 1983-09-29 | Caracterisation et analyses d'oncogenes |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP83903635.7 Division | 1983-09-29 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0239162A2 true EP0239162A2 (fr) | 1987-09-30 |
| EP0239162A3 EP0239162A3 (en) | 1989-08-16 |
| EP0239162B1 EP0239162B1 (fr) | 1994-12-14 |
Family
ID=23715723
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP93119195A Ceased EP0605789A1 (fr) | 1982-10-01 | 1983-09-29 | Caractérisation et analyses d'oncogènes |
| EP83903635A Expired - Lifetime EP0120958B1 (fr) | 1982-10-01 | 1983-09-29 | Caracterisation et analyses d'oncogenes |
| EP87200460A Expired - Lifetime EP0241961B1 (fr) | 1982-10-01 | 1983-09-29 | Essai pour la détection de carcinogénèses causées par des oncogènes |
| EP87200461A Expired - Lifetime EP0239162B1 (fr) | 1982-10-01 | 1983-09-29 | Méthode de la prodution d' un anticorps capable de détecter la présence d'une oncogène humaine. |
Family Applications Before (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP93119195A Ceased EP0605789A1 (fr) | 1982-10-01 | 1983-09-29 | Caractérisation et analyses d'oncogènes |
| EP83903635A Expired - Lifetime EP0120958B1 (fr) | 1982-10-01 | 1983-09-29 | Caracterisation et analyses d'oncogenes |
| EP87200460A Expired - Lifetime EP0241961B1 (fr) | 1982-10-01 | 1983-09-29 | Essai pour la détection de carcinogénèses causées par des oncogènes |
Country Status (6)
| Country | Link |
|---|---|
| US (3) | US4535058A (fr) |
| EP (4) | EP0605789A1 (fr) |
| JP (2) | JPH0642840B2 (fr) |
| AT (3) | ATE59412T1 (fr) |
| DE (3) | DE3382765T2 (fr) |
| WO (1) | WO1984001389A1 (fr) |
Families Citing this family (105)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4935341A (en) * | 1986-06-04 | 1990-06-19 | Whitehead Institute For Biomedical Research | Detection of point mutations in neu genes |
| US6713619B1 (en) * | 1980-08-29 | 2004-03-30 | Massachusetts Institute Of Technology | Oncogenes and methods for their detection |
| US4699877A (en) * | 1982-11-04 | 1987-10-13 | The Regents Of The University Of California | Methods and compositions for detecting human tumors |
| USRE35491E (en) * | 1982-11-04 | 1997-04-08 | The Regents Of The University Of California | Methods and compositions for detecting human tumors |
| US5591587A (en) * | 1983-08-17 | 1997-01-07 | The Scripps Research Institute | Polypeptide-induced monoclonal receptors to protein ligands |
| US5030565A (en) * | 1983-08-17 | 1991-07-09 | Scripps Clinic And Research Foundation | Polypeptide-induced monoclonal receptors to protein ligands |
| US5733738A (en) * | 1983-08-17 | 1998-03-31 | Ligand Pharmaceuticals | Polypeptide-induced monoclonal receptors to protein ligands |
| US4681840A (en) * | 1984-01-18 | 1987-07-21 | The United States Of America As Represented By The Secretary Of Commerce | Deoxyribonucleic acid molecules useful as probes for detecting oncogenes incorporated into chromosomal DNA |
| US4725550A (en) * | 1984-01-19 | 1988-02-16 | Research Foundation Of State University Of New York | Novel mutation of the c-K-ras oncogene activated in a human lung carcinoma |
| US4683194A (en) * | 1984-05-29 | 1987-07-28 | Cetus Corporation | Method for detection of polymorphic restriction sites and nucleic acid sequences |
| US5087571A (en) * | 1984-06-22 | 1992-02-11 | President And Fellows Of Harvard College | Method for providing a cell culture from a transgenic non-human mammal |
| US4736866B1 (en) * | 1984-06-22 | 1988-04-12 | Transgenic non-human mammals | |
| US4866166A (en) * | 1984-08-31 | 1989-09-12 | Cold Spring Harbor Laboratory | Bioassay for transforming genes and genes detected thereby |
| US4798787A (en) * | 1984-09-19 | 1989-01-17 | Cetus Corporation | Peptide antibodies and their use in detecting oncogene products |
| US4762706A (en) * | 1984-10-17 | 1988-08-09 | Cetus Corporation | Peptide antibodies and their use in detecting oncogene products |
| EP0177814A3 (fr) * | 1984-09-19 | 1987-12-02 | Cetus Corporation | Anticorps contre les peptides et leurs utilisation dans la détection des produits d'oncogènes |
| US4701409A (en) * | 1984-11-15 | 1987-10-20 | The Wistar Institute | Detection of B-cell neoplasms |
| US4735895A (en) * | 1984-12-28 | 1988-04-05 | Oncotech, Inc. | Cancer susceptibility test |
| US5081230A (en) * | 1987-07-08 | 1992-01-14 | E. I. Dupont Denemours And Company | Monoclonal antibodies reactive with normal and oncogenic forms of the ras p21 protein |
| US5084380A (en) * | 1985-01-29 | 1992-01-28 | Applied Biotechnology | Monoclonal antibodies reactive with activated and oncogenic ras p21 proteins |
| US4898932A (en) * | 1985-01-29 | 1990-02-06 | E. I. Du Pont De Nemours And Company | Monoclonal antibodies reactive with activated and oncogenic ras p21 proteins |
| US6200764B1 (en) | 1985-01-29 | 2001-03-13 | Bayer Corporation | Detection, quantitation and classification of ras proteins in body fluids and tissues |
| US5443956A (en) * | 1985-01-29 | 1995-08-22 | Oncogene Science, Inc. | Detection, quantitation and classification of RAS proteins in body fluids and tissues |
| US5028527A (en) * | 1988-02-22 | 1991-07-02 | Applied Bio Technology | Monoclonal antibodies against activated ras proteins with amino acid mutations at position 13 of the protein |
| CA1296660C (fr) * | 1985-01-29 | 1992-03-03 | Walter P. Carney | Anticorps monoclonal dirige contre un dodecapeptide relie a un oncogene p21 ras |
| US5635389A (en) * | 1985-05-02 | 1997-06-03 | Institut Pasteur | Antibodies which recognize and bind human villin |
| FR2589882B2 (fr) * | 1985-11-13 | 1988-01-15 | Pasteur Institut | Moyens pour le diagnostic in vitro de cellules malignes originaires du tube digestif |
| US5591582A (en) * | 1985-07-23 | 1997-01-07 | The Board Of Rijks Universiteit Leiden | Methods for detecting activated RAS oncogenes |
| US4871838A (en) * | 1985-07-23 | 1989-10-03 | The Board Of Rijks Universiteit Leiden | Probes and methods for detecting activated ras oncogenes |
| US6083709A (en) * | 1985-08-21 | 2000-07-04 | Osi Pharmaceuticals, Inc. | Immunoassay for detection of mutant P53 polypeptide in serum |
| US4946773A (en) * | 1985-12-23 | 1990-08-07 | President And Fellows Of Harvard College | Detection of base pair mismatches using RNAase A |
| US5320941A (en) * | 1986-06-06 | 1994-06-14 | Dallan Young | DNA sequence encoding mas onhcogene, polypeptides encoded therefrom and diagnostic and other methods based therefrom |
| US5015568A (en) * | 1986-07-09 | 1991-05-14 | The Wistar Institute | Diagnostic methods for detecting lymphomas in humans |
| US4820631A (en) * | 1986-07-30 | 1989-04-11 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Deletion mutants and monoclonal antibodies against ras proteins |
| US7223842B1 (en) | 1986-08-11 | 2007-05-29 | Massachusetts Eye And Ear Infirmary | Detection of proteins whose absence is associated with a neoplasm |
| US5853988A (en) * | 1986-08-11 | 1998-12-29 | Massachusetts Eye And Ear Infirmary | Diagnosis of retinoblastoma |
| US7384735B1 (en) | 1986-08-11 | 2008-06-10 | Massachusetts Eye And Ear Infirmary | Retinoblastoma nucleic acids |
| CA1341576C (fr) * | 1986-08-11 | 2008-07-08 | Thaddeus P. Dryja | Diagnostic du retinoblastome |
| WO1988002404A1 (fr) * | 1986-09-30 | 1988-04-07 | Smithkline Beckman Corporation | Transfection cellulaire |
| US4968603A (en) * | 1986-12-31 | 1990-11-06 | The Regents Of The University Of California | Determination of status in neoplastic disease |
| US5789235A (en) * | 1987-03-23 | 1998-08-04 | Tackett; Scott E. | Modifying cell metabolism via extra-cellular nucleases |
| US4870161A (en) * | 1987-09-25 | 1989-09-26 | The United States Of America As Represented By The Department Of Health And Human Services | Reagents and probes for distinguishing and isolating different GTP-binding proteins |
| CA1339069C (fr) * | 1987-11-09 | 1997-07-29 | Henry Lee Niman | Recepteurs monoclonaux induits par des polypeptides pour ligans proteiniques |
| US4957859A (en) * | 1988-02-16 | 1990-09-18 | Hoffmann-La Roche Inc. | Antibodies for transforming ras protein |
| US5112737A (en) * | 1988-02-22 | 1992-05-12 | Applied Biotechnology, Inc. | Monoclonal antibodies against activated ras proteins with amino acid mutations at position 13 of the protein |
| DE3809635C3 (de) * | 1988-03-22 | 1996-06-20 | Niehoff Kg Maschf | Verfahren und Vorrichtung zur Herstellung eines spulenlosen Gebindes sowie ein mit dem Verfahren hergestelltes Gebinde |
| DE68928106T2 (de) | 1988-04-22 | 1997-12-04 | Oncogene Science Inc | Nachweis, quantifizierung und klassifizierung von ras-proteinen in körperflüssigkeiten und geweben |
| WO1989012697A1 (fr) * | 1988-06-22 | 1989-12-28 | The Board Of Regents Of The University Of Washingt | Procede de detection de genes anormaux |
| US5763573A (en) * | 1988-08-10 | 1998-06-09 | Chiron Corporation | GTPase activating protein fragments |
| US5760203A (en) * | 1988-08-10 | 1998-06-02 | Chiron Corporation | Gap gene sequences |
| FR2645877B1 (fr) * | 1989-04-12 | 1991-07-05 | Pasteur Institut | Molecules comportant au moins une sequence peptidique porteuse d'un, ou plusieurs, epitope caracteristique d'une proteine produite par p. falciparum au niveau du stade sporozoite et dans les hepatocytes |
| US6203976B1 (en) | 1989-07-18 | 2001-03-20 | Osi Pharmaceuticals, Inc. | Methods of preparing compositions comprising chemicals capable of transcriptional modulation |
| US5665543A (en) * | 1989-07-18 | 1997-09-09 | Oncogene Science, Inc. | Method of discovering chemicals capable of functioning as gene expression modulators |
| ATE238431T1 (de) * | 1989-07-18 | 2003-05-15 | Osi Pharm Inc | Methode der veränderung der expression von genen bei der transkription und zum nachweis von chemischen substanzen, die wie modulatoren der genexpression wirken |
| US5580722A (en) * | 1989-07-18 | 1996-12-03 | Oncogene Science, Inc. | Methods of determining chemicals that modulate transcriptionally expression of genes associated with cardiovascular disease |
| US5776502A (en) | 1989-07-18 | 1998-07-07 | Oncogene Science, Inc. | Methods of transcriptionally modulating gene expression |
| DE69132771T2 (de) * | 1990-06-27 | 2002-08-01 | Princeton University Princeton | Sonden für die detektion der p53 mutante |
| ATE230024T1 (de) * | 1990-10-25 | 2003-01-15 | Univ Columbia | Entwicklung von dns-sonden und immunologischen reagenzien von menschlichen tumor-assoziierten antigenen |
| US6159751A (en) * | 1990-10-25 | 2000-12-12 | The Trustees Of Columbia University In The City Of New York | Development of DNA probes and immunological reagents of human tumor associated antigens |
| US5851764A (en) | 1990-10-25 | 1998-12-22 | The Trustees Of Columbia University In The City Of New York | Human prostate tumor inducing gene-1 and uses thereof |
| WO1992013091A1 (fr) * | 1991-01-18 | 1992-08-06 | Oncogene Science, Inc. | Procede de modulation par transcription de l'expression genique d'oncogenes et de genes supprimant les tumeurs |
| US6110700A (en) * | 1991-03-11 | 2000-08-29 | The General Hospital Corporation | PRAD1 cyclin and its cDNA |
| US6150398A (en) * | 1991-05-08 | 2000-11-21 | The United States Of America As Represented By The Department Of Health And Human Services | Methods for the treatment of cancer |
| US5468634A (en) * | 1991-06-24 | 1995-11-21 | The University Of North Carolina At Chapel Hill | Axl oncogene |
| US5750335A (en) * | 1992-04-24 | 1998-05-12 | Massachusetts Institute Of Technology | Screening for genetic variation |
| US5726024A (en) * | 1993-08-02 | 1998-03-10 | Health Research, Inc. | p53as protein and antibody therefor |
| US5688918A (en) * | 1993-08-02 | 1997-11-18 | Health Research, Inc. | p53as protein and antibody therefor |
| US5747650A (en) * | 1993-08-02 | 1998-05-05 | Health Research, Inc. | P53AS protein and antibody therefor |
| US6965009B1 (en) | 1993-08-02 | 2005-11-15 | Health Research, Inc. | p53 as protein and antibody therefor |
| AU1094795A (en) * | 1993-11-12 | 1995-05-29 | Oncor, Inc. | Detection of bladder cancer |
| US5670325A (en) * | 1996-08-14 | 1997-09-23 | Exact Laboratories, Inc. | Method for the detection of clonal populations of transformed cells in a genomically heterogeneous cellular sample |
| US5741650A (en) * | 1996-01-30 | 1998-04-21 | Exact Laboratories, Inc. | Methods for detecting colon cancer from stool samples |
| US6524578B1 (en) | 1996-07-10 | 2003-02-25 | Scott E. Tackett | Use of nuclease to reduce wrinkles and discolorations in humans |
| US5928870A (en) * | 1997-06-16 | 1999-07-27 | Exact Laboratories, Inc. | Methods for the detection of loss of heterozygosity |
| US6300077B1 (en) | 1996-08-14 | 2001-10-09 | Exact Sciences Corporation | Methods for the detection of nucleic acids |
| US6020137A (en) * | 1996-08-14 | 2000-02-01 | Exact Laboratories, Inc. | Methods for the detection of loss of heterozygosity |
| US6146828A (en) * | 1996-08-14 | 2000-11-14 | Exact Laboratories, Inc. | Methods for detecting differences in RNA expression levels and uses therefor |
| US5952178A (en) | 1996-08-14 | 1999-09-14 | Exact Laboratories | Methods for disease diagnosis from stool samples |
| US6100029A (en) * | 1996-08-14 | 2000-08-08 | Exact Laboratories, Inc. | Methods for the detection of chromosomal aberrations |
| US6203993B1 (en) | 1996-08-14 | 2001-03-20 | Exact Science Corp. | Methods for the detection of nucleic acids |
| WO1998021585A1 (fr) | 1996-11-15 | 1998-05-22 | Cornell Research Foundation, Inc. | Dosage d'interaction de ras activee |
| US6406857B1 (en) | 1997-06-16 | 2002-06-18 | Exact Sciences Corporation | Methods for stool sample preparation |
| US6268136B1 (en) | 1997-06-16 | 2001-07-31 | Exact Science Corporation | Methods for stool sample preparation |
| US20020064792A1 (en) * | 1997-11-13 | 2002-05-30 | Lincoln Stephen E. | Database for storage and analysis of full-length sequences |
| US6503718B2 (en) | 1999-01-10 | 2003-01-07 | Exact Sciences Corporation | Methods for detecting mutations using primer extension for detecting disease |
| US6280947B1 (en) | 1999-08-11 | 2001-08-28 | Exact Sciences Corporation | Methods for detecting nucleotide insertion or deletion using primer extension |
| WO2000050640A1 (fr) | 1999-02-25 | 2000-08-31 | Exact Laboratories, Inc. | Methodes de conservation de l'integrite de l'adn |
| WO2000061808A2 (fr) * | 1999-04-09 | 2000-10-19 | Exact Laboratories, Inc. | Procedes de detection d'acides nucleiques revelateurs de cancer |
| US6849403B1 (en) | 1999-09-08 | 2005-02-01 | Exact Sciences Corporation | Apparatus and method for drug screening |
| US6586177B1 (en) | 1999-09-08 | 2003-07-01 | Exact Sciences Corporation | Methods for disease detection |
| US6919174B1 (en) | 1999-12-07 | 2005-07-19 | Exact Sciences Corporation | Methods for disease detection |
| ATE458831T1 (de) * | 1999-12-07 | 2010-03-15 | Exact Sciences Corp | Verfahren zum nachweis von lungenneoplasmen in fäkalen proben |
| US7776524B2 (en) | 2002-02-15 | 2010-08-17 | Genzyme Corporation | Methods for analysis of molecular events |
| US20040259101A1 (en) * | 2003-06-20 | 2004-12-23 | Shuber Anthony P. | Methods for disease screening |
| WO2005047266A1 (fr) | 2003-11-14 | 2005-05-26 | Lorus Therapeutics Inc. | Imidazoles d'aryle et leur utilisation comme agents anticancereux |
| US20080241827A1 (en) * | 2004-05-10 | 2008-10-02 | Exact Sciences Corporation | Methods For Detecting A Mutant Nucleic Acid |
| WO2005113769A1 (fr) * | 2004-05-14 | 2005-12-01 | Exact Sciences Corporation | Procédé servant à stabiliser des échantillons biologiques pour une analyse d'acides nucléiques |
| US7981607B2 (en) * | 2004-08-27 | 2011-07-19 | Esoterix Genetic Laboratories LLC | Method for detecting recombinant event |
| WO2006047787A2 (fr) | 2004-10-27 | 2006-05-04 | Exact Sciences Corporation | Methode de surveillance de la progression ou la recurrence d'une maladie |
| DE102005011022A1 (de) * | 2005-03-10 | 2006-09-14 | Häfner & Krullmann Gmbh | Verfahren zum Bewickeln einer Spule mit strangförmigem Wickelgut |
| US9777314B2 (en) * | 2005-04-21 | 2017-10-03 | Esoterix Genetic Laboratories, Llc | Analysis of heterogeneous nucleic acid samples |
| JP2009505658A (ja) * | 2005-08-24 | 2009-02-12 | ブリストル−マイヤーズ スクイブ カンパニー | 上皮増殖因子受容体モデュレーターに対する感受性を決定するためのバイオマーカーおよび方法 |
| US20150104392A1 (en) | 2013-10-04 | 2015-04-16 | Aptose Biosciences Inc. | Compositions, biomarkers and their use in the treatment of cancer |
| KR20190008415A (ko) * | 2016-06-10 | 2019-01-23 | 브록 라이덴 | 상처 패킹을 이용한 상처 치료 시스템 및 방법 |
| WO2019089511A1 (fr) | 2017-10-30 | 2019-05-09 | Aptose Biosciences Inc. | Arylimidazoles pour le traitement du cancer |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4299815A (en) * | 1980-02-08 | 1981-11-10 | Hoffmann-La Roche Inc. | Carcinoembryonic antigen determination |
| US4368262A (en) * | 1981-03-23 | 1983-01-11 | Research Corporation | Diagnostic test for the detection of cancer |
| US4395486A (en) * | 1981-08-19 | 1983-07-26 | Medical College Of Ga. Research Inst., Inc. | Method for the direct analysis of sickle cell anemia |
-
1982
- 1982-10-01 US US06/432,337 patent/US4535058A/en not_active Expired - Lifetime
-
1983
- 1983-09-29 JP JP58503483A patent/JPH0642840B2/ja not_active Expired - Lifetime
- 1983-09-29 AT AT83903635T patent/ATE59412T1/de not_active IP Right Cessation
- 1983-09-29 EP EP93119195A patent/EP0605789A1/fr not_active Ceased
- 1983-09-29 DE DE3382765T patent/DE3382765T2/de not_active Expired - Lifetime
- 1983-09-29 EP EP83903635A patent/EP0120958B1/fr not_active Expired - Lifetime
- 1983-09-29 WO PCT/US1983/001517 patent/WO1984001389A1/fr active IP Right Grant
- 1983-09-29 EP EP87200460A patent/EP0241961B1/fr not_active Expired - Lifetime
- 1983-09-29 AT AT87200461T patent/ATE115588T1/de active
- 1983-09-29 EP EP87200461A patent/EP0239162B1/fr not_active Expired - Lifetime
- 1983-09-29 AT AT87200460T patent/ATE114820T1/de not_active IP Right Cessation
- 1983-09-29 DE DE3382767T patent/DE3382767T2/de not_active Expired - Lifetime
- 1983-09-29 DE DE8383903635T patent/DE3382098D1/de not_active Expired - Lifetime
-
1985
- 1985-08-08 US US06/763,663 patent/US4786718A/en not_active Expired - Lifetime
-
1992
- 1992-01-27 US US07/826,254 patent/US5300631A/en not_active Expired - Lifetime
- 1992-03-10 JP JP4051821A patent/JP2573770B2/ja not_active Expired - Lifetime
Non-Patent Citations (8)
Also Published As
| Publication number | Publication date |
|---|---|
| EP0241961A3 (en) | 1989-01-25 |
| DE3382767D1 (de) | 1995-01-26 |
| EP0239162B1 (fr) | 1994-12-14 |
| JP2573770B2 (ja) | 1997-01-22 |
| EP0120958A1 (fr) | 1984-10-10 |
| EP0239162A3 (en) | 1989-08-16 |
| US5300631A (en) | 1994-04-05 |
| DE3382765T2 (de) | 1995-05-04 |
| DE3382765D1 (de) | 1995-01-12 |
| ATE59412T1 (de) | 1991-01-15 |
| EP0120958B1 (fr) | 1990-12-27 |
| JPH05281228A (ja) | 1993-10-29 |
| DE3382098D1 (de) | 1991-02-07 |
| WO1984001389A1 (fr) | 1984-04-12 |
| US4786718A (en) | 1988-11-22 |
| US4535058A (en) | 1985-08-13 |
| EP0241961B1 (fr) | 1994-11-30 |
| ATE115588T1 (de) | 1994-12-15 |
| JPH0642840B2 (ja) | 1994-06-08 |
| DE3382767T2 (de) | 1995-10-19 |
| JPS60500002A (ja) | 1985-01-10 |
| EP0241961A2 (fr) | 1987-10-21 |
| EP0605789A1 (fr) | 1994-07-13 |
| ATE114820T1 (de) | 1994-12-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4786718A (en) | Method of preparing antibodies to characterize oncogenes | |
| Tabin et al. | Mechanism of activation of a human oncogene | |
| EP0412116B1 (fr) | Detection d'expression de genes neu et produits | |
| US4935341A (en) | Detection of point mutations in neu genes | |
| CA1252046A (fr) | Methodes de depistage du cancer | |
| EP0453560B1 (fr) | Localisation et caracterisation du gene tumoral de wilms | |
| CA1340827C (fr) | Methodes diognostiques detetection de lymphome chez l'etre humain | |
| US5955263A (en) | Sequence specific DNA binding by p53 | |
| EP0608004B1 (fr) | ADN humaine pour le diagnostic du rétinoblastome | |
| US5726288A (en) | Localization and characterization of the Wilms' tumor gene | |
| US7267955B2 (en) | Method for detecting loss of wild-type p53 | |
| WO1992012262A1 (fr) | Sequences d'adn associees au syndrome de chromosome x fragile isole | |
| US6184032B1 (en) | Identification of genes encoding cell surface antigens using CREF-Trans 6 cells | |
| US6087117A (en) | Production and use of human nm23 protein and antibodies therefor | |
| US6713619B1 (en) | Oncogenes and methods for their detection | |
| US6159751A (en) | Development of DNA probes and immunological reagents of human tumor associated antigens | |
| Ucker | The glucocorticoid hormone regulated murine mammary tumor virus transcription unit |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 120958 Country of ref document: EP |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE CH DE FR GB LI LU NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH DE FR GB LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19900130 |
|
| 17Q | First examination report despatched |
Effective date: 19911121 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 120958 Country of ref document: EP |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB LI LU NL SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19941214 Ref country code: LI Effective date: 19941214 Ref country code: CH Effective date: 19941214 Ref country code: BE Effective date: 19941214 Ref country code: AT Effective date: 19941214 |
|
| REF | Corresponds to: |
Ref document number: 115588 Country of ref document: AT Date of ref document: 19941215 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3382767 Country of ref document: DE Date of ref document: 19950126 |
|
| ET | Fr: translation filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19950314 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19950930 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20020830 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20020927 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20020930 Year of fee payment: 20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20030928 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 |