EP0170372B1 - Metallothermische Reduktion seltener Erdoxide mittels Kalzium - Google Patents
Metallothermische Reduktion seltener Erdoxide mittels Kalzium Download PDFInfo
- Publication number
- EP0170372B1 EP0170372B1 EP85304046A EP85304046A EP0170372B1 EP 0170372 B1 EP0170372 B1 EP 0170372B1 EP 85304046 A EP85304046 A EP 85304046A EP 85304046 A EP85304046 A EP 85304046A EP 0170372 B1 EP0170372 B1 EP 0170372B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- rare earth
- bath
- oxide
- metal
- metallothermic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 229910052751 metal Inorganic materials 0.000 title claims abstract description 77
- 239000002184 metal Substances 0.000 title claims abstract description 76
- 239000011575 calcium Substances 0.000 title claims abstract description 35
- 229910001404 rare earth metal oxide Inorganic materials 0.000 title claims abstract description 22
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 title claims abstract description 21
- 229910052791 calcium Inorganic materials 0.000 title claims abstract description 21
- 230000009467 reduction Effects 0.000 title claims description 23
- 229910052761 rare earth metal Inorganic materials 0.000 claims abstract description 73
- 150000002910 rare earth metals Chemical class 0.000 claims abstract description 52
- 238000000034 method Methods 0.000 claims abstract description 45
- 238000006243 chemical reaction Methods 0.000 claims abstract description 38
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 claims abstract description 15
- 239000001110 calcium chloride Substances 0.000 claims abstract description 15
- 229910001628 calcium chloride Inorganic materials 0.000 claims abstract description 15
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 37
- PLDDOISOJJCEMH-UHFFFAOYSA-N neodymium(3+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[Nd+3].[Nd+3] PLDDOISOJJCEMH-UHFFFAOYSA-N 0.000 claims description 33
- 150000003839 salts Chemical class 0.000 claims description 25
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 17
- 229910045601 alloy Inorganic materials 0.000 claims description 15
- 239000000956 alloy Substances 0.000 claims description 15
- 229910052742 iron Inorganic materials 0.000 claims description 15
- -1 rare earth metal ion Chemical class 0.000 claims description 13
- 239000011701 zinc Substances 0.000 claims description 13
- 239000000470 constituent Substances 0.000 claims description 12
- 229910052779 Neodymium Inorganic materials 0.000 claims description 11
- 238000002844 melting Methods 0.000 claims description 11
- 238000003756 stirring Methods 0.000 claims description 11
- 230000008018 melting Effects 0.000 claims description 10
- QEFYFXOXNSNQGX-UHFFFAOYSA-N neodymium atom Chemical compound [Nd] QEFYFXOXNSNQGX-UHFFFAOYSA-N 0.000 claims description 9
- 239000011780 sodium chloride Substances 0.000 claims description 8
- 229910052725 zinc Inorganic materials 0.000 claims description 8
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 claims description 7
- ODINCKMPIJJUCX-UHFFFAOYSA-N calcium oxide Inorganic materials [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 claims description 7
- 239000000292 calcium oxide Substances 0.000 claims description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 5
- BRPQOXSCLDDYGP-UHFFFAOYSA-N calcium oxide Chemical compound [O-2].[Ca+2] BRPQOXSCLDDYGP-UHFFFAOYSA-N 0.000 claims description 5
- 239000001301 oxygen Substances 0.000 claims description 5
- 229910052760 oxygen Inorganic materials 0.000 claims description 5
- MRELNEQAGSRDBK-UHFFFAOYSA-N lanthanum(3+);oxygen(2-) Chemical compound [O-2].[O-2].[O-2].[La+3].[La+3] MRELNEQAGSRDBK-UHFFFAOYSA-N 0.000 claims 4
- 238000013019 agitation Methods 0.000 claims 2
- 229910000420 cerium oxide Inorganic materials 0.000 claims 2
- BMMGVYCKOGBVEV-UHFFFAOYSA-N oxo(oxoceriooxy)cerium Chemical compound [Ce]=O.O=[Ce]=O BMMGVYCKOGBVEV-UHFFFAOYSA-N 0.000 claims 2
- MMKQUGHLEMYQSG-UHFFFAOYSA-N oxygen(2-);praseodymium(3+) Chemical compound [O-2].[O-2].[O-2].[Pr+3].[Pr+3] MMKQUGHLEMYQSG-UHFFFAOYSA-N 0.000 claims 2
- 229910003447 praseodymium oxide Inorganic materials 0.000 claims 2
- 230000008569 process Effects 0.000 abstract description 17
- 150000002739 metals Chemical class 0.000 abstract description 6
- 238000006722 reduction reaction Methods 0.000 description 13
- 239000006023 eutectic alloy Substances 0.000 description 9
- 239000011734 sodium Substances 0.000 description 6
- 230000004907 flux Effects 0.000 description 5
- 230000000717 retained effect Effects 0.000 description 5
- CSDQQAQKBAQLLE-UHFFFAOYSA-N 4-(4-chlorophenyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine Chemical compound C1=CC(Cl)=CC=C1C1C(C=CS2)=C2CCN1 CSDQQAQKBAQLLE-UHFFFAOYSA-N 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 4
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 3
- 229910000640 Fe alloy Inorganic materials 0.000 description 3
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 3
- 229910052777 Praseodymium Inorganic materials 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 238000009835 boiling Methods 0.000 description 3
- 229910052796 boron Inorganic materials 0.000 description 3
- 230000005496 eutectics Effects 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 229910001172 neodymium magnet Inorganic materials 0.000 description 3
- PUDIUYLPXJFUGB-UHFFFAOYSA-N praseodymium atom Chemical compound [Pr] PUDIUYLPXJFUGB-UHFFFAOYSA-N 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 239000000376 reactant Substances 0.000 description 3
- 238000011946 reduction process Methods 0.000 description 3
- 239000010935 stainless steel Substances 0.000 description 3
- 229910001220 stainless steel Inorganic materials 0.000 description 3
- 229910052715 tantalum Inorganic materials 0.000 description 3
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 229910052684 Cerium Inorganic materials 0.000 description 2
- 229910000583 Nd alloy Inorganic materials 0.000 description 2
- GWXLDORMOJMVQZ-UHFFFAOYSA-N cerium Chemical compound [Ce] GWXLDORMOJMVQZ-UHFFFAOYSA-N 0.000 description 2
- 239000003638 chemical reducing agent Substances 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 239000000356 contaminant Substances 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 239000001307 helium Substances 0.000 description 2
- 229910052734 helium Inorganic materials 0.000 description 2
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 2
- 229910052746 lanthanum Inorganic materials 0.000 description 2
- FZLIPJUXYLNCLC-UHFFFAOYSA-N lanthanum atom Chemical compound [La] FZLIPJUXYLNCLC-UHFFFAOYSA-N 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000000155 melt Substances 0.000 description 2
- 230000000737 periodic effect Effects 0.000 description 2
- 238000010992 reflux Methods 0.000 description 2
- 239000011819 refractory material Substances 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 238000007711 solidification Methods 0.000 description 2
- 230000008023 solidification Effects 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 229910052727 yttrium Inorganic materials 0.000 description 2
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 1
- 229910052582 BN Inorganic materials 0.000 description 1
- PZNSFCLAULLKQX-UHFFFAOYSA-N Boron nitride Chemical compound N#B PZNSFCLAULLKQX-UHFFFAOYSA-N 0.000 description 1
- 229910014867 CaCl2—NaCl Inorganic materials 0.000 description 1
- KZBUYRJDOAKODT-UHFFFAOYSA-N Chlorine Chemical compound ClCl KZBUYRJDOAKODT-UHFFFAOYSA-N 0.000 description 1
- 229910000531 Co alloy Inorganic materials 0.000 description 1
- 229910052692 Dysprosium Inorganic materials 0.000 description 1
- 229910052691 Erbium Inorganic materials 0.000 description 1
- 229910052693 Europium Inorganic materials 0.000 description 1
- 229910052688 Gadolinium Inorganic materials 0.000 description 1
- 229910052689 Holmium Inorganic materials 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229910001209 Low-carbon steel Inorganic materials 0.000 description 1
- 229910052765 Lutetium Inorganic materials 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229910000528 Na alloy Inorganic materials 0.000 description 1
- 229910052773 Promethium Inorganic materials 0.000 description 1
- 241000220317 Rosa Species 0.000 description 1
- 229910052772 Samarium Inorganic materials 0.000 description 1
- 229910000612 Sm alloy Inorganic materials 0.000 description 1
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 1
- 229910052771 Terbium Inorganic materials 0.000 description 1
- 229910052775 Thulium Inorganic materials 0.000 description 1
- 229910052769 Ytterbium Inorganic materials 0.000 description 1
- 229910001297 Zn alloy Inorganic materials 0.000 description 1
- QJVKUMXDEUEQLH-UHFFFAOYSA-N [B].[Fe].[Nd] Chemical compound [B].[Fe].[Nd] QJVKUMXDEUEQLH-UHFFFAOYSA-N 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 239000004411 aluminium Substances 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- 238000010923 batch production Methods 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- IKNAJTLCCWPIQD-UHFFFAOYSA-K cerium(3+);lanthanum(3+);neodymium(3+);oxygen(2-);phosphate Chemical compound [O-2].[La+3].[Ce+3].[Nd+3].[O-]P([O-])([O-])=O IKNAJTLCCWPIQD-UHFFFAOYSA-K 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000010924 continuous production Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- RCJVRSBWZCNNQT-UHFFFAOYSA-N dichloridooxygen Chemical compound ClOCl RCJVRSBWZCNNQT-UHFFFAOYSA-N 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- KBQHZAAAGSGFKK-UHFFFAOYSA-N dysprosium atom Chemical compound [Dy] KBQHZAAAGSGFKK-UHFFFAOYSA-N 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 238000005265 energy consumption Methods 0.000 description 1
- UYAHIZSMUZPPFV-UHFFFAOYSA-N erbium Chemical compound [Er] UYAHIZSMUZPPFV-UHFFFAOYSA-N 0.000 description 1
- OGPBJKLSAFTDLK-UHFFFAOYSA-N europium atom Chemical compound [Eu] OGPBJKLSAFTDLK-UHFFFAOYSA-N 0.000 description 1
- 150000004673 fluoride salts Chemical class 0.000 description 1
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 description 1
- 150000004820 halides Chemical class 0.000 description 1
- 231100001261 hazardous Toxicity 0.000 description 1
- KJZYNXUDTRRSPN-UHFFFAOYSA-N holmium atom Chemical compound [Ho] KJZYNXUDTRRSPN-UHFFFAOYSA-N 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 238000009413 insulation Methods 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 238000000622 liquid--liquid extraction Methods 0.000 description 1
- OHSVLFRHMCKCQY-UHFFFAOYSA-N lutetium atom Chemical compound [Lu] OHSVLFRHMCKCQY-UHFFFAOYSA-N 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000002074 melt spinning Methods 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 229910052590 monazite Inorganic materials 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 238000004663 powder metallurgy Methods 0.000 description 1
- VQMWBBYLQSCNPO-UHFFFAOYSA-N promethium atom Chemical compound [Pm] VQMWBBYLQSCNPO-UHFFFAOYSA-N 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 229910052702 rhenium Inorganic materials 0.000 description 1
- 239000011833 salt mixture Substances 0.000 description 1
- KZUNJOHGWZRPMI-UHFFFAOYSA-N samarium atom Chemical compound [Sm] KZUNJOHGWZRPMI-UHFFFAOYSA-N 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 238000001577 simple distillation Methods 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- GZCRRIHWUXGPOV-UHFFFAOYSA-N terbium atom Chemical compound [Tb] GZCRRIHWUXGPOV-UHFFFAOYSA-N 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- 238000011282 treatment Methods 0.000 description 1
- 238000005292 vacuum distillation Methods 0.000 description 1
- NAWDYIZEMPQZHO-UHFFFAOYSA-N ytterbium Chemical compound [Yb] NAWDYIZEMPQZHO-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B5/00—General methods of reducing to metals
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B59/00—Obtaining rare earth metals
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B5/00—General methods of reducing to metals
- C22B5/02—Dry methods smelting of sulfides or formation of mattes
- C22B5/04—Dry methods smelting of sulfides or formation of mattes by aluminium, other metals or silicon
Definitions
- This inveniton relates to a novel metallothermic process for the direct reduction of rare earth oxide, particularly neodymium oxide, to rare earth metal.
- the method has particular application to low cost production of neodymium metal for use in neodymium-iron-boron magnets.
- Sources of the rare earth (RE) elements are bastnaesite and monazite ores. Mixtures of the rear earths can be extracted from the ores by several well known beneficiating techniques. The rear earths can then be separated from one another by such conventional processes as elution and liquid-liquid extraction.
- RE rare earth
- the electrolytic processes include (1) decomposition of anhydrous rare earth chlorides dissolved in molten alkali or alkaline earth salts, and (2) decomposition of rare earth oxides dissolved in molten rare earth fluoride salts.
- Electrolytic processes include the use of expensive electrodes which are eventually consumed, the use of anhydrous chloride or fluoride salts to prevent the formation of undesirable RE-oxy salts (NdOCI, e.g.), high temperature cell operation (generally greater than 1000°C), low current efficiences resulting in high power costs, and low yield of metal from the salt (40% or less of the metal can be recovered).
- the RE-chloride reduction process release corrosive chlorine gas while the fluoride process requires careful control of a temperature gradient in the electrolytic salt cell to cause solidification of rare earth metal nodules.
- An advantage of electrolytic processes is that they can be made to run continuously if provision is made to tap the reduced metal and to refortify the salt bath.
- the metallothermic (non-electrolytic) processes include (1) reduction of RE-fluorides with calcium metal powder (the calciothermic process), and (2) reduction-diffusion of RE-oxide with calcium hydride (CaH 2 ) or calcium metal (Ca).
- both are batch processes, they must be conducted in a non-oxidizing atmosphere, they are energy intensive and the product in the case of reduction-diffusion is a powder which must be hydrated to purify it before use. Both processes involve many steps.
- One advantage of the metallothermic reduction processes is that the yield of metal from the oxide or fluoride is generally better than ninety percent.
- a metallothermic, non-electrolytic method of reducing rare earth oxide to rare earth metal according to the present invention consists in that the reduction takes place in a molten salt bath comprised predominantly of calcium chloride, a volume of rare earth oxide that is less than the salt bath volume is dispersed in the bath, a stoichiometric excess of calcium metal with respect to the amount of rare earth metal ion present is added to the bath, and said bath is agitated so that the oxide is reduced to rare earth metal in accordance with the formula where RE represents one or more rare earth elements having a valency of 2,3 or 4,0 represents oxygen, Ca represents calcium, CaO represents calcium oxide, and n and m are integers such that the valency of the rare earth element multiplied by n equals the valency of oxygen multiplied by m.
- a reaction vessel is provided which can be heated to desired temperatures by electrical resistance heaters or some other heating means.
- the vessel body is preferably made of a metal or refractory material that is either substantially inert or innocuous to the reaction constituents.
- a predetermined amount of RE-oxide is charged into the reaction vessel containing a salt mixture of about 70 weight percent calcium chloride or greater and about 5 to 30 weight percent sodium chloride (NaCI).
- the salt serves as a medium for the reduction reaction.
- a stoichiometric excess of calcium metal, based on the amount of rear earth oxide, is added. It may be advantageous to add an amount of another metal such as iron or zinc to form a eutectic alloy with the reduced rare earth metal to enable the reaction to be carried out at lower temperatures and to obtain the RE metal product in a liquid state.
- the vessel is heated to a temperature above the melting point of the constituents (about 675°C).
- the molten constituents are rapidly stirred in the vessel to keep them in contact with one another as the reaction progresses.
- the bath is replenished with calcium chloride (CaCl 2 ) as necessary to maintain a weight percent of 70% of the combined weights of CaC1 2 and NaCI. While the reaction runs at CaCI 2 concentrations lower than 70%, the yield falls of rapidly.
- n and m are the number of moles of constituent and where the relation of n and m is determined by the oxidation state of the rare earth element.
- the reduced metal has a density of about 7 grams/cc while that of the salt bath is about 1.9 grams/cc.
- the reduced metal forms a clean layer at the bottom of the reaction vessel. This layer may be tapped while molten or separated from the salt layer after it solidifies.
- the method of the invention provides many advantages over prior art methods. It is carried out at a relatively low temperature of about 700°C, particularly where the rare earth metal is recovered as a zinc or iron eutectic alloy. It uses relatively inexpensive RE-oxide, CaCl 2 , NaCI and Ca metal reactants. It does not require pretransformation of RE-oxide to chloride or fluoride, nor the use of expensive pure Ca or CaH 2 reducing agent. Energy consumption is low because the method is not electrolytic and it is preferably carried out at atmospheric pressure at temperatures at about 700°C. The method can be practiced as either a batch or a continuous process, and the by-products of CaC1 2 , NaCI and calcium oxide (CaO) are easily disposed of. Moreover, the rare earth metals may be alloyed with additional iron in the reaction vessel if they are made as a RE-Fe eutectic alloy or may be alloyed later for use in RE-Fe magnets without further expensive purification treatments.
- the rare earth metals include elements 57 to 71 of the periodic table (lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium) and atomic number 39, yttrium.
- the oxides of the rare earths are generally coloured powders produced in the metals separation process.
- the term "light rare earth” refers to the elements lanthanum (La), cerium (ce), praseodymium (Pr) and neodymium (Nd).
- the RE-oxides can generally be used as received from the separator but may be calcined to remove excess absorbed moisture or carbon dioxide.
- the RE-oxides were oven-dried for about two hours at 1000°C prior to use.
- the CaCI 2 and NaCI for the salt baths were reagent grade and dried for about two hours at 500°C prior to use.
- RE-oxychloride is formed by the reaction
- both RE-oxides and RE-oxychlorides are readily reduced by calcuim metal.
- the formation of RE-oxychlorides is advantageous because they float on molten layers of reduced RE metals.
- RE-oxides on the other hand, have densities close to the reduced RE metals so they may be retained as contaminants in the molten layers of reduced RE metals, and may make the RE metals unsuited for use in magnets.
- the RE metals reduced by the method according to the present invention have been substantially oxide-free.
- Unalloyed Nd metal has a melting temperature of about 1025°C.
- the other rare earth metals also have high melting points. If one wanted to run the subject reaction at such temperatures, it would be possible to do so and obtain pure metal at high yields.
- iron forms a low melting eutectic alloy with neodymium (11.5 weight percent Fe; m.p. about 640°C) as does zinc (11.9 weight percent Zn, m.p. about 630°C).
- a Nd-Fe eutectic alloy may be directly alloyed with additional iron and boron to make magnets having the optimum Nd 2 Fe 14 B magnetic phase described in the aforementioned European patent applications.
- a metal with a boiling point much lower than the boiling point of the recovered rare earth can be added to the reaction vessel.
- the low-melting metal can then be readily separated from the rare earth metal by simple distillation.
- Yttria-lined alumina and boron nitride are non-reactive, refractory materials generally acceptable. It is also possible to use a refractory liner made of a substantially inert metal such as tantalum or a consumable but innocuous metal such as iron. An iron liner could be used to contain.reduced RE metal and then be alloyed with the RE for use in magnets.
- a new method has been discovered of using calcium metal to reduce rare earth oxides.
- the method entails bringing together molten calcium and RE-oxide to cause the reaction Unless the reaction vessel is pressurized, it is desirable to keep the temperature at below 910°C to avoid the excessive loss of Na formed by the reaction of Ca with NaCI. It is preferred to run the reactions at atmospheric pressure.
- the most preferred range of operating temperatures is between about 650°C and 750°C. At such temperatures wear on the reaction vessel is not excessive. This temperature range is suitable for reducing Nd 2 0 3 to Nd metal because the Nd-Re and Nd-Zn eutectic melting-point temperatures are below 700°C.
- the solubility of Ca metal in the salt bath is about 1.3 molecular percent. This is sufficient to rapidly reduce RE-oxide to RE metal.
- the reaction temperature must be above the melting point of the reduced RE metal or the melting point of the reduced RE metal alloyed or co-reduced with another metal.
- These relatively dense RE metals and alloys collect at the bottom of the reaction vessel when allowed to settle. There they can be tapped while molten or removed after solidification.
- Table I shows the molecular weight (m.w.), density (p) at 25°C, melting point (m.p.) and boiling point (b.p.) for elements and compounds used in the present invention.
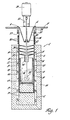
- FIG 1 shows an apparatus suitable for the practice of the invention in which the experiments set out in the several examples were conducted.
- the furnace was heated by means of three tubular, electric, clamshell heating elements 8, 10 and 12 having an inside diameter of 13.3 cm and a total length of 45.7 cm.
- the side and bottom of the furnace well were surrounded with refractory insulation 14.
- Thermocouples 15 were mounted on an outer wall 16 of furnace wall 20 at various locations along its length.
- One of the centrally located thermocouples was used in conjunction with a proportional band temperature controller (not shown) to automatically control centre clamshell heater 10.
- the other three thermocouples were monitored with a digital temperature readout system and top and bottom clamshell heaters 8 and 12 were manually controlled with transformers to maintain a fairly uniform temperature throughout the furnace.
- reaction vessel 22 was carried out in a reaction vessel 22 retained in a stainless steel crucible 18 having a 10.2 cm outer diameter, 12.7 cm deep and 0.15 cm thick retained in stainless steel furnace well 20.
- Reaction vessel 22 was made of tantalum metal unless otherwise noted in the examples.
- a tantalum stirrer 24 was used to agitate the melt during the reduction process. It had a shaft 48.32 cm long and a welded blade 26.
- the stirrer was powered by a 100 W variable speed motor 28 capable of operating at speeds up to 700 revolutions per minute.
- the motor was mounted on a bracket 30 so that the depth of stirrer blade 26 in reaction vessel 22 could be adjusted.
- the shaft was journalled in a bushing 32 carried in an annular support bracket 34. The bracket is retained by collar 35 to which furnace well 20 is fastened by bolts 37.
- Chill water coils 36 were located near the top of well 20 to promote condensation and prevent escape of volatile reaction constituents.
- Cone-shaped stainless steel baffles 38 were used to reflux Na vapors. Reflux products drop through tube 40 on bottom baffle 42.
- Figure 2 is an idealized flow chart for the reduction of Nd 2 0 3 to Nd metal in accordance with this invention.
- the Nd 2 0 3 is added to the reaction vessel along with calcium chloride and sodium chloride in suitable proportions. Calcium metal and enough of a eutectic forming metal such as iron or zinc to form a near eutectic Nd alloy are added.
- the reaction is run, with rapid stirring at about 300-700 revolutions per minute at a temperature of about 700°C for at least one hour.
- a blanket of an inert gas such as helium is maintained over the reaction vessel.
- Nd 2 0 3 After the Nd 2 0 3 has been reduced, stirring is continued at a lower speed of 100 revolutions per minute for one hour and then stirring is stopped to allow the various liquids in the vessel to stratify.
- the reduced Nd eutectic alloy collects at the bottom because it has the highest density.
- the remaining salts and any unreacted Ca collect above the Nd alloy and can be readily broken away after the vessel has cooled and the constituents have solidified.
- Nd-Fe alloys so produced can be alloyed with additional elements to produce permanent magnet compositions. These magnet alloys may be processed by melt-spinning or they can be ground and processed by powder metallurgy to make magnets.
- the furnace temperature was lowered to about 720°C.
- 150 grams of NaCl and 350 grams of CaC1 2 were added to create a salt bath of 70 weight percent CaCl 2 .
- 234 grams (0.7 moles) Nd 2 0 3 were added.
- 104 grams of Ca (2.6 moles) metal were added to the crucible and it was stirred at a rate of 300 revolutions per minute for about two hours and then for another hour at a stirring rate of 60 revolutions per minute.
- the crucible was removed from the furnace and cooled on the floor of the drybox.
- Nd metal of purity greater than 99% was recovered (not including the 265 grams of Nd metal from the original seed pool) by distilling the Nd-Zn alloy collected at the bottom of the vessel.
- the yield of Nd metal from the oxide was about 94%.
- the furnace temperature was lowered to about 720°C.
- 300 grams of NaCl and 700 grams of CaCI 2 were added to create a salt bath of 70 weight percent CaCl 2 .
- 117 grams (0.35 moles) of Nd 2 0 3 were added.
- 46 grams (1.15 moles) of Ca metal and 10.8 grams (0.47 moles) of Na were added to the crucible and they were stirred at a rate of 300 revolutions per minute for about 135 minutes.
- an additional 117 grams (0.35 moles) of Nd 2 0 3 46 grams (1.15 moles) of Ca metal and 10.8 grams (0.47 moles) of Na were added.
- the reactants were stirred for another 114 minutes at 300 rpm and then for another hour at a stirring rate of 60 rpm.
- the reaction vessel was removed from the furnace and cooled on the floor of the drybox. A layer of unreacted Ca-Na alloy formed on top of the salt layer.
- Nd-Fe alloy 594 grams of 97% purity Nd-Fe alloy were recovered. Such alloy could be combined directly as recovered with additional iron and boron to make the ideal Nd-Fe-B alloy for permanent magnet manufacture. 180 grams of Nd metal of purity greater than 99% was recovered as Nd-Fe alloy. This example shows that a calcium and sodium melt is capable of reducing a rare earth oxide in a CaCl 2 -NaCl flux bath.
- Table II sets out the amounts of various constituents used in the metallothermic reduction of about 234 grams Nd 2 O 3 with Ca metal using the process set out in Example except that the reactants were stirred for four hours at 300 revolutions per minute followed by an additional hour of stirring at 60 rpm.
- FIG. 3 is a plot of Nd metal yield from Nd 2 0 3 as a function of the weight percent CaC1 2 in a two component NaCI-CaCI 2 starting salt bath with a Ca metal reductant. Referring to Table II and Figure 3, it has been found that, to obtain high yields, it is necessary to maintain the amount of CaC1 2 in the salt bath above about 70 weight percent.
- the CaC1 2 -containing bath is a significant feature of this invention.
- the resultant alloy was analyzed and was found to be of greater than 99% purity with 0.4% aluminium, 0.1 % silicon, 0.01 % calcium and traces of zinc, magnesium and iron contamination.
- the Nd metal so produced was melted in a vacuum furnace with electrolytic iron and ferroboron to produce an alloy having the nominal composition Nd 0.15 B 0.05 Fe 0.80 .
- the alloy was melt-spun, as described in European patent application No. 0108474 cited above, to produce very finely crystalline ribbon with an as-quenched coercivity of about 10 megaGaussOersteds.
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Mechanical Engineering (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Metallurgy (AREA)
- Environmental & Geological Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Manufacture And Refinement Of Metals (AREA)
- Electrolytic Production Of Metals (AREA)
- Compounds Of Alkaline-Earth Elements, Aluminum Or Rare-Earth Metals (AREA)
- Hard Magnetic Materials (AREA)
- Manufacturing Cores, Coils, And Magnets (AREA)
- Electrical Discharge Machining, Electrochemical Machining, And Combined Machining (AREA)
Claims (11)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT85304046T ATE36560T1 (de) | 1984-07-03 | 1985-06-07 | Metallothermische reduktion seltener erdoxide mittels kalzium. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US62773684A | 1984-07-03 | 1984-07-03 | |
| US627736 | 1984-07-03 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0170372A1 EP0170372A1 (de) | 1986-02-05 |
| EP0170372B1 true EP0170372B1 (de) | 1988-08-17 |
Family
ID=24515917
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP85304046A Expired EP0170372B1 (de) | 1984-07-03 | 1985-06-07 | Metallothermische Reduktion seltener Erdoxide mittels Kalzium |
Country Status (10)
| Country | Link |
|---|---|
| EP (1) | EP0170372B1 (de) |
| JP (1) | JPS6130639A (de) |
| KR (1) | KR910001581B1 (de) |
| AT (1) | ATE36560T1 (de) |
| AU (1) | AU575965B2 (de) |
| BR (1) | BR8503140A (de) |
| DE (1) | DE3564451D1 (de) |
| ES (1) | ES8702508A1 (de) |
| MX (1) | MX173880B (de) |
| ZA (1) | ZA854474B (de) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009139667A1 (ru) * | 2008-05-12 | 2009-11-19 | Volkov Anatoly Evgenievich | Способ и устройство автоклавного производства химически активных металлов |
| US11607734B2 (en) | 2018-05-30 | 2023-03-21 | Hela Novel Metals Llc | Methods for the production of fine metal powders from metal compounds |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4578242A (en) * | 1984-07-03 | 1986-03-25 | General Motors Corporation | Metallothermic reduction of rare earth oxides |
| US4680055A (en) * | 1986-03-18 | 1987-07-14 | General Motors Corporation | Metallothermic reduction of rare earth chlorides |
| US4837109A (en) * | 1986-07-21 | 1989-06-06 | Hitachi Metals, Ltd. | Method of producing neodymium-iron-boron permanent magnet |
| AT389899B (de) * | 1986-08-19 | 1990-02-12 | Treibacher Chemische Werke Ag | Verfahren zur herstellung von se-metallen und se-haltigen legierungen |
| JPH01138119A (ja) * | 1987-11-24 | 1989-05-31 | Mitsubishi Metal Corp | 希土類電解スラグからサマリウムとユーロピウムを回収する方法 |
| DE3817553A1 (de) * | 1988-05-24 | 1989-11-30 | Leybold Ag | Verfahren zum herstellen von titan und zirkonium |
| US4917724A (en) * | 1988-10-11 | 1990-04-17 | General Motors Corporation | Method of decalcifying rare earth metals formed by the reduction-diffusion process |
| KR100373109B1 (ko) * | 1999-09-30 | 2003-02-25 | 해 남 현 | 토양의 치환성 칼리 침출방법 및 이를 이용하여 침출된 토양 치환성 칼리의 정량방법 |
| JP2004052003A (ja) * | 2002-07-16 | 2004-02-19 | Cabot Supermetal Kk | ニオブ粉末またはタンタル粉末の製造方法および製造装置 |
| JP2013517451A (ja) * | 2010-01-12 | 2013-05-16 | シルバン ソース, インコーポレイテッド | 熱伝達インターフェース |
| CN103436718B (zh) * | 2013-08-16 | 2015-06-17 | 宁夏东方钽业股份有限公司 | 一种高纯金属镧的制取方法 |
| KR102153737B1 (ko) * | 2019-12-12 | 2020-09-09 | 한국지질자원연구원 | 철-희토류계 영구자석으로부터 선택염화법에 의한 희토류 원소의 회수 방법 |
| CN111139366B (zh) * | 2020-01-17 | 2024-05-31 | 鹰潭市宇钠科技中心(有限合伙) | 一种钠冷快堆中核纯级冷却材料制备提纯方法及其设备 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB190923215A (en) * | 1909-10-11 | 1910-09-29 | Hans Kuzel | Process for the Production of Zirconium and other Rare Metals. |
| US2950962A (en) * | 1957-03-28 | 1960-08-30 | Carlson Oscar Norman | Reduction of fluoride to metal |
| GB1040468A (en) * | 1964-10-26 | 1966-08-24 | Dow Chemical Co | Preparation of rare earth metal, yttrium, or scandium |
| GB1579978A (en) * | 1977-07-05 | 1980-11-26 | Johnson Matthey Co Ltd | Production of yttrium |
| SU1027232A1 (ru) * | 1982-01-15 | 1983-07-07 | Научно-исследовательский институт металлургии | Способ получени лигатуры |
| DE3379131D1 (en) * | 1982-09-03 | 1989-03-09 | Gen Motors Corp | Re-tm-b alloys, method for their production and permanent magnets containing such alloys |
| JPS59177346A (ja) * | 1983-03-25 | 1984-10-08 | Sumitomo Special Metals Co Ltd | 希土類磁石素材用配合合金 |
| JPS6077943A (ja) * | 1983-10-03 | 1985-05-02 | Sumitomo Special Metals Co Ltd | 希土類磁石用原料合金の製造方法 |
| US4578242A (en) * | 1984-07-03 | 1986-03-25 | General Motors Corporation | Metallothermic reduction of rare earth oxides |
-
1985
- 1985-06-07 AT AT85304046T patent/ATE36560T1/de not_active IP Right Cessation
- 1985-06-07 EP EP85304046A patent/EP0170372B1/de not_active Expired
- 1985-06-07 DE DE8585304046T patent/DE3564451D1/de not_active Expired
- 1985-06-13 ZA ZA854474A patent/ZA854474B/xx unknown
- 1985-06-28 BR BR8503140A patent/BR8503140A/pt not_active IP Right Cessation
- 1985-06-28 MX MX026616A patent/MX173880B/es unknown
- 1985-07-01 KR KR1019850004710A patent/KR910001581B1/ko not_active IP Right Cessation
- 1985-07-02 ES ES544799A patent/ES8702508A1/es not_active Expired
- 1985-07-02 AU AU44488/85A patent/AU575965B2/en not_active Ceased
- 1985-07-03 JP JP14645085A patent/JPS6130639A/ja active Granted
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009139667A1 (ru) * | 2008-05-12 | 2009-11-19 | Volkov Anatoly Evgenievich | Способ и устройство автоклавного производства химически активных металлов |
| US11607734B2 (en) | 2018-05-30 | 2023-03-21 | Hela Novel Metals Llc | Methods for the production of fine metal powders from metal compounds |
Also Published As
| Publication number | Publication date |
|---|---|
| ES8702508A1 (es) | 1987-01-01 |
| ATE36560T1 (de) | 1988-09-15 |
| ES544799A0 (es) | 1987-01-01 |
| ZA854474B (en) | 1986-03-26 |
| KR910001581B1 (ko) | 1991-03-16 |
| DE3564451D1 (en) | 1988-09-22 |
| MX173880B (es) | 1994-04-07 |
| BR8503140A (pt) | 1986-03-18 |
| JPS6137341B2 (de) | 1986-08-23 |
| EP0170372A1 (de) | 1986-02-05 |
| AU4448885A (en) | 1986-01-09 |
| AU575965B2 (en) | 1988-08-11 |
| JPS6130639A (ja) | 1986-02-12 |
| KR860001203A (ko) | 1986-02-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0170373B1 (de) | Metallothermische Reduktion seltener Erdoxide | |
| EP0238185B1 (de) | Metallothermische Reduktion der Chloride der seltenen Erden | |
| EP0170372B1 (de) | Metallothermische Reduktion seltener Erdoxide mittels Kalzium | |
| US6309441B1 (en) | Reduction-melting process to form rare earth-transition metal alloys and the alloys | |
| US4786319A (en) | Proces for the production of rare earth metals and alloys | |
| US4767455A (en) | Process for the preparation of pure alloys based on rare earths and transition metals by metallothermy | |
| Sharma | Neodymium production processes | |
| US5314526A (en) | Metallothermic reduction of rare earth fluorides | |
| Sharma et al. | Metallothermic Reduction of Nd2 O 3 with Ca in CaCl2‐NaCl Melts | |
| CN85100813A (zh) | 稀土氧化物的金属热还原 | |
| US4308245A (en) | Method of purifying metallurgical-grade silicon | |
| JPS6311628A (ja) | 希土類金属の製造法 | |
| KR920007932B1 (ko) | 희토류-철 합금의 제조방법 | |
| AU2021382943A1 (en) | Reduction system and method for high-melting point metal oxides, using liquid metal crucible | |
| Sharma et al. | Metallothermic Reduction of Nd2O3 with Ca in CaCl2-NaCl Melts | |
| JPS61157646A (ja) | 希土類合金の製造方法 | |
| US3951764A (en) | Aluminum-manganese alloy | |
| US20240191321A1 (en) | Semi-continuous rare earth metal production | |
| US3951647A (en) | Reduction method for producing manganese metal | |
| Mehra et al. | Extractive metallurgy of rare earths-developmental work at the Bhabha Atomic Research Centre | |
| WO1994009168A1 (en) | Methods for producing high purity magnesium alloys | |
| RU1791462C (ru) | Шихта дл внепечного получени магнитных сплавов с редкоземельными металлами | |
| PL161378B1 (pl) | Sposób wytwarzania stopu neodym - żelazo | |
| JPH02228433A (ja) | 希土類金属又は希土類金属―遷移金属合金の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19850617 |
|
| AK | Designated contracting states |
Designated state(s): AT DE FR GB IT NL SE |
|
| 17Q | First examination report despatched |
Effective date: 19870506 |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT DE FR GB IT NL SE |
|
| REF | Corresponds to: |
Ref document number: 36560 Country of ref document: AT Date of ref document: 19880915 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3564451 Country of ref document: DE Date of ref document: 19880922 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| ITTA | It: last paid annual fee | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 85304046.7 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: AT Payment date: 19990610 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19990617 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19990630 Year of fee payment: 15 Ref country code: NL Payment date: 19990630 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19990726 Year of fee payment: 15 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000607 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000608 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010101 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 85304046.7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010228 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20010101 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010403 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20010604 Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020607 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20020607 |