EP0094101A2 - Récipient stérile pour usage médical - Google Patents
Récipient stérile pour usage médical Download PDFInfo
- Publication number
- EP0094101A2 EP0094101A2 EP83104752A EP83104752A EP0094101A2 EP 0094101 A2 EP0094101 A2 EP 0094101A2 EP 83104752 A EP83104752 A EP 83104752A EP 83104752 A EP83104752 A EP 83104752A EP 0094101 A2 EP0094101 A2 EP 0094101A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- container according
- closure
- protective cap
- container
- dispensing tube
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 230000001681 protective effect Effects 0.000 claims abstract description 34
- 238000007789 sealing Methods 0.000 claims abstract description 25
- 239000007788 liquid Substances 0.000 claims abstract description 17
- 229920003023 plastic Polymers 0.000 claims abstract description 11
- 239000004033 plastic Substances 0.000 claims abstract description 11
- 230000002093 peripheral effect Effects 0.000 claims abstract description 9
- 239000000463 material Substances 0.000 claims description 10
- 239000011324 bead Substances 0.000 claims description 8
- 230000036512 infertility Effects 0.000 description 6
- 230000000295 complement effect Effects 0.000 description 5
- 239000004698 Polyethylene Substances 0.000 description 4
- 244000052616 bacterial pathogen Species 0.000 description 4
- -1 polyethylene Polymers 0.000 description 4
- 229920000573 polyethylene Polymers 0.000 description 4
- 230000002262 irrigation Effects 0.000 description 3
- 238000003973 irrigation Methods 0.000 description 3
- 238000004806 packaging method and process Methods 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 238000004659 sterilization and disinfection Methods 0.000 description 3
- 210000001015 abdomen Anatomy 0.000 description 2
- 230000003187 abdominal effect Effects 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 239000000645 desinfectant Substances 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 239000003978 infusion fluid Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 230000000149 penetrating effect Effects 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 1
- 206010016717 Fistula Diseases 0.000 description 1
- 206010052428 Wound Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 230000004323 axial length Effects 0.000 description 1
- 238000000071 blow moulding Methods 0.000 description 1
- 230000009172 bursting Effects 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 239000012611 container material Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000013536 elastomeric material Substances 0.000 description 1
- 230000003890 fistula Effects 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 238000001631 haemodialysis Methods 0.000 description 1
- 230000000322 hemodialysis Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000000034 method Methods 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D1/00—Containers having bodies formed in one piece, e.g. by casting metallic material, by moulding plastics, by blowing vitreous material, by throwing ceramic material, by moulding pulped fibrous material, by deep-drawing operations performed on sheet material
- B65D1/02—Bottles or similar containers with necks or like restricted apertures, designed for pouring contents
- B65D1/0223—Bottles or similar containers with necks or like restricted apertures, designed for pouring contents characterised by shape
- B65D1/023—Neck construction
- B65D1/0238—Integral frangible closures
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J1/00—Containers specially adapted for medical or pharmaceutical purposes
- A61J1/05—Containers specially adapted for medical or pharmaceutical purposes for collecting, storing or administering blood, plasma or medical fluids ; Infusion or perfusion containers
- A61J1/06—Ampoules or carpules
- A61J1/067—Flexible ampoules, the contents of which are expelled by squeezing
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D1/00—Containers having bodies formed in one piece, e.g. by casting metallic material, by moulding plastics, by blowing vitreous material, by throwing ceramic material, by moulding pulped fibrous material, by deep-drawing operations performed on sheet material
- B65D1/02—Bottles or similar containers with necks or like restricted apertures, designed for pouring contents
- B65D1/0223—Bottles or similar containers with necks or like restricted apertures, designed for pouring contents characterised by shape
- B65D1/0292—Foldable bottles
Definitions
- the invention relates to a sterile container for medical purposes made of a plastic material, in particular a bottle-pack bottle, for the metered administration of liquids with a bellows-like, compressible container body and a dispensing tube which has a removable closure.
- Sterile containers are used in the medical field for liquids to be kept sterile, in particular for infusion solutions. They consist regularly of a polymeric material, for example polyethylene, and, if they are used as bottle-pack bottles, are both molded and filled and sealed with the liquid to be used in one operation.
- plastic granulate is filled into a corresponding shape and shaped into a bottle according to the usual plastic processing techniques, which is filled with the above-mentioned liquid in a further working step and finally closed with a closure element, for example a cap that is attached.
- Such bottle-shaped containers are suitable for single use, whereby after appropriate sterilization they can dispense the desired liquid, but generally cannot be used for manual dispensing of the liquid within a short period of time, since such conventional bottles with a smooth belly on the one hand are too bulky and on the other hand can only be emptied incompletely.
- syringes are regularly used for the metered manual delivery of such liquids, for example in the daily care and cleaning of inserted bladder catheters, which have to be filled regularly with irrigation solutions.
- previously sterile rinsing liquid is removed from bottles via a catheter with a bladder syringe in order to rinse the bladder catheter on the patient.
- Such handling naturally represents an essential source of danger when the rinsing liquid is kept sterile for repeating the rinsing, so that the rinsing liquid is often contaminated with bacteria by airborne germs in the patient's room.
- a sterile container of the type mentioned is known, which has a bellows-like design of the bottle belly, so that it can be compressed. This bellows bottle is closed with a removable cap that is placed over the end of the outlet pipe.
- this cap is to be placed on the outlet pipe by a press fit, it can be removed by improper use, so that the sterility of the container is often endangered.
- such a container must be accommodated in a further packaging in order to reliably prevent the protective cap from being unintentionally pulled off or from penetrating through the slits between the cap.
- sterile containers are known from FR-PS 22 59 624 and DE-OS 29 00 827.
- the above-mentioned containers can therefore not be regarded as sterile, since the protective sleeves can be removed without further ado, if necessary after removing a security sleeve. If the last-mentioned securing sleeve is present, improper storage cannot prevent germs whirled up by dust or the like from penetrating through slots to the cannula body and thus endanger sterility.
- sterile containers of this type must be provided in a second packaging which is hermetically sealed to the outside in order to ensure sterility safely.
- the invention is therefore based on the object of providing a container of the type mentioned at the outset which is free from further closed packaging, is certainly sterile and can only be opened with certain hand movements.
- closure is integrally connected to the dispensing tube and has a knob-shaped handle.
- the container according to the invention initially has the advantage that it can be manufactured in the factory like a conventional container for infusion solutions and filled with the appropriate solutions.
- Bottlepack containers only have to be modified in the blow mold for the container body in order to achieve the desired shape of a bellows.
- the bottle neck adjoins this bellows, the outlet opening of which is closed with a toggle piece by integral welding.
- the weld seam between the toggle piece and the connection opening is advantageously kept so thin in terms of material that it serves as a line of weakness which can be severed by turning the toggle piece.
- the above container is opened on the bedside similar to a bottle of the prior art and can be connected directly to a catheter tube without further transfer into a large-caliber syringe.
- the bellows-like configuration of the container shell the latter can be pressed together like a syringe, the contents of the bottle being conveyed into the bladder through the bladder catheter sterile connected to the container. Since the folds of the bellows can close together, this bottle is almost completely emptied into the patient's bladder.
- the catheter tube is plugged onto the tubular connection of the container, which may be necessary for reasons of contamination. does not appear harmless, so that the connection should be sprayed with a disinfectant before use
- the inner surface of the connection adjoining the connection opening can be equipped with a female cone into which the male cone of a connector can be inserted in a sealing manner.
- a corresponding locking device for example a thread or the like, is provided on the outer surface of the connection, into which a complementary device of the connector can engage.
- the bellows bottle according to the first embodiment can be provided with a tightly fitting cap.
- This tight fit is achieved in that the cylindrical bottle neck, which is arranged between the bellows area on the one hand and the outlet pipe on the other hand, has a seal in the form of an O-ring which is connected to the Inner surface of the attached cap cooperates sealingly.
- this arrangement is produced by fitting the cap and then sterilized in the autoclave at the appropriate temperature and pressure.
- the entire space located within this cap is sterile, including the outer surface of the outlet pipe, although it should not be particularly pointed out that the toggle-shaped closure itself closes the bottle in a sterile manner.
- the inner surface of the protective cap is provided with at least one radially inwardly directed rib in such a way that it comes into contact with the toggle-like closure during rotation.
- at least two ribs are provided, each of which cooperate with the opposite gag parts, so that the gag can be twisted off without difficulty when the closure cap is turned.
- the area inside the protective cap remains sterile as a result of the seal provided by the O-ring, so that by removing the protective cap after the knob has been unscrewed, a catheter tube can be plugged onto the outlet pipe which is sterile on its outer surface until it is used.
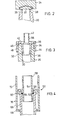
- FIG. 1 shows a sterile container 10, the container body 12 of which is designed as a bellows 14.
- This bellows can be compressed in such a way that, for example, an approximately 200 ml container can be practically 100% emptied by compressing the bellows 14 if the container 10 is not completely filled.
- This bellows 14 is connected to the base 16 and an essentially cylindrical bottle neck 18, which narrows at 19 and merges into an output tube 20, which can be seen in an enlarged form from FIGS. 2-4.
- this dispensing tube 20 has an opening 22 at its tip through which the sterile liquid can be transferred into a catheter connected to the container 10.
- This opening 22 has been closed at the factory with the aid of a toggle-type closure 22 which has been integrally fused to the peripheral edge 26 of the opening 22.
- an annular circumferential line of weakness 28 is advantageously provided between the closure 24 and the peripheral edge 26. which can be easily cut by rotating the closure 24 while holding the container 10 in place.
- a tube or a catheter is pushed over the tubular: dispensing tube 20 until a force fit is achieved.
- the dispensing tube 20 has of course been disinfected using a disinfectant solution before the closure 24 is unscrewed.
- this dispensing tube 20 advantageously has a shape that tapers conically in the direction of the peripheral edge 26, so that the desired frictional connection with the hose can be achieved without difficulty.
- the inner surface of the dispensing tube 20 can taper concentrically inward and thus form a female inner cone 32, which extends from the peripheral edge 26 in the direction of the container body 12.
- This inner cone preferably has 32 standard dimensions and is in particular a Luer cone.
- any customary connector 34 can be inserted into this inner cone 32, provided that it has a complementary outer cone 36.
- a complementary outer cone 36 is described for example in DE-OS 29 07 832, the disclosure of which is expressly incorporated by reference.
- Such a connector 34 essentially consists of a tubular part 38 on which a flange-shaped part 40 is placed.
- the tubular part 38 is on one side of the flange-shaped part as an configured end connector 42, while on the other hand it has the outer cone 36 already mentioned above.
- This outer cone 36 is concentrically surrounded by the tube part 44, on the inner surface of which a fastening device, preferably a screw thread 46, is arranged.
- the inner diameter of the tube part 44 essentially corresponds to the outer diameter of the dispensing tube 20, which according to this embodiment is cylindrical on its outside.
- a complementary fastening element 48 preferably a screw thread, is provided on its outer surface, into which the screw thread of the connector 34 can engage.
- the fastening elements 46 and 48 can of course also be designed differently.
- a suitably designed bayonet lock can also be used.
- at least one pin of a bayonet catch is provided on the outer surface of the dispensing tube 20 and can engage in a complementary bayonet opening in the connector 34.
- connection 20 is cylindrical.
- a connector 52 can be inserted, which in turn has a central tubular part 54 of a cylindrical structure.
- This central tube part 54 is followed by a flange-shaped part 56, against which the peripheral edge 26 bears.
- the central tube part in turn has a connecting piece 58 on one side of the flange 56 and forms a cylindrical connecting tube 60 on the other side, the outer diameter of which corresponds approximately to the inner diameter of the dispensing tube 20.
- annular groove 62 is advantageously provided on the outer circumference of the tube part 60, in which an O-ring 64 is inserted.
- This O-ring 64 lies close to the inner surface 50 when the tube part 60 is inserted into the connector 20.
- the outer surface 66 of the connection 20 has an annular circumferential bead 68. This bead 68 can be engaged behind by at least one fastening device 70 of the connector 52, so that a secure fit of the connector 52 can be achieved.
- the connector 4 is integrally connected to the outer periphery of the flange 50 and is in the form of two radially separable arms 72, one end of which is hook-shaped and the other end serves as a gripping surface for the fingers. Since the connector material is made of plastic, these arms can be moved apart on one side and the other side by compression, so that the connector 52 can be pulled off the dispensing tube 20.
- a deformable plastic material for example polyethylene and the like, is of course used as the material for the container 10. used.
- This plastic material is inserted in the form of a granulate in a blow molding machine and converted into the desired shape, then filled with the sterile liquid and then welded to the tip of the dispensing tube 20 in such a way that a breakable gag-like closure 24 is formed.
- connection is not only conceivable for the container 10.
- plastic bags with a gag-like closure can also be provided on one or more connections.
- connections of such a bag made of a plastic material, such as polyethylene or PVC can be provided with the connection devices shown in FIGS. 2-4, for example with the female inner cone shown in FIGS. 2 and 3 or with the one shown in FIG. 4 shown cylindrical shape of the inner surface of the connector.
- This container 10 in turn has the bellows 14 in the abdominal region, to which the bottle neck 18 connects.
- this bottle neck 18 has a substantially cylindrical region 18a, which is adjoined at 19 by a region 19a which tapers in the direction of the container opening. This area 19a then merges into the dispensing tube 20, which in turn is closed with the toggle-type closure 24 at its end.
- this embodiment does not differ from the first embodiment shown in FIGS. 1 and 2, so that reference is made to the description thereof.
- FIG. 5 advantageously has an annular circumferential sealing device 80 with a raised structure in the cylindrical region 18a.
- This sealing device 80 is advantageously formed by a groove 82 and an O-ring 84 inserted into this groove.
- the groove 82 is already formed during the manufacture of the bottle and runs essentially annularly through the cylindrical region 18a adjacent to the location 19.
- O-ring 84 is not only understood to mean a conventional O-ring made of a plastic material, for example polyethylene, which is inserted into this groove after the container has been removed from the mold, but also a raised elastic sealing arrangement, for example by injection and curing an elastomeric material.
- the raised, ring-shaped sealing device 80 can also be formed directly from the same container material in the form of a raised ring when the container 10 is blown.
- a protective cap 86 is placed on the container 10, which cooperates with the sealing device 80 in an elastically sealing manner.
- the axial length of the protective cap 86 corresponds at least to the distance of the sealing device 80 from the outermost edge of the closure 22, so that a tight fit is ensured.
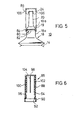
- FIG. 6 shows a sectional view through the protective cap 86 according to a preferred embodiment.
- the protective cap 86 initially has an essentially cylindrical main region 88, to which the cylindrical opening region 90 advantageously adjoins.
- the inner diameter of this cylindrical region 90 corresponds approximately to the diameter of the sealing device 80, both parts being essentially circular in shape. As a result, this opening area 90 can be pushed over the sealing device 80 substantially without exerting pressure.
- the inner diameter of the main area 88 is slightly smaller than the diameter of the sealing device 80, so that when the protective cap 86 is pushed on in the area of the step 92, that is to say the transition from the opening area 90 to the Main area 88 to which sealing device 80 force must be applied in order to overcome the elastic force of sealing device 80.
- the cylindrical main region adjacent to the step 92 is advantageously equipped on its inner surface 94 with an annular circumferential bead 96, the diameter of which is of course smaller than the inner diameter of the main area 88.
- the bead 96 is at such a distance from the cap tip 98 that it can just be pushed over the sealing device 80 with considerable effort and thus, when the protective cap 86 is in the attached state, its surface advantageously bears against the sealing device 80 .
- the circumferential bead 96 with respect to the sealing device 80.
- the protective cap 86 can advantageously be rotated relative to the sealing device 80 without the sealing effect and thus the sterility of the space enclosed by the protective cap 86 being canceled.
- the inner surface 94 of the main region 88 has at least one radially inwardly directed rib.
- 6 shows three ribs 100, 102 and 104, with another rib having been cut away in this illustration.
- two ribs are already sufficient to unscrew the toggle-shaped closure 24 by rotating the protective cap 86 from the outlet pipe 20.
- two of the ribs 100, 102, 104 shown in FIG. 6 and the rib complementary to the rib 104 come into engagement with the toggle-shaped closure 24 and take this along with the further rotation of the protective cap 86, until it is turned off and falls into the space enclosed by the protective cap 86.
- the sterility is basically maintained within this room.
- the distance from the tip edge of a rib 100, 102, 104 to the longitudinal axis of the protective cap 86 is somewhat less than the distance.
- the diameter of the gag-shaped closure corresponds 24, that is the distance from the respective side edges 106, approximately the inner diameter of the cylindrical main region 88.
- the last-mentioned embodiment is produced by first producing the container 10 in the form of a bottle-pack bottle and then filling it with the corresponding sterile liquid.
- the O-ring 84 is then inserted into the groove 82 and the protective cap 86, which preferably has the bead 96 and the ribs 100-104, is pushed onto this sealing device 80.
- the protective cap 86 which preferably has the bead 96 and the ribs 100-104, is pushed onto this sealing device 80. It should also be noted that when two or more ribs are arranged, at least two are each in a plane passing through the longitudinal axis of the protective cap 86.
- the bellows bottle is suitable for internal and external use, such as perfusion of the extracorporeal system during hemodialysis, postoperative bladder irrigation for all urological interventions, irrigation in the gastrointestinal tract and of fistulas and drainage, as well as for wound treatment and for moistening wipes and bandages.
Landscapes
- Engineering & Computer Science (AREA)
- Health & Medical Sciences (AREA)
- Ceramic Engineering (AREA)
- Mechanical Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Hematology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Details Of Rigid Or Semi-Rigid Containers (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT83104752T ATE35773T1 (de) | 1982-05-12 | 1983-05-13 | Steriler behaelter fuer medizinische zwecke. |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE8213816U | 1982-05-12 | ||
| DE8213816U DE8213816U1 (de) | 1982-05-12 | 1982-05-12 | Steriler Behälter für medizinische Zwecke |
| DE3223540 | 1982-06-24 | ||
| DE3223540 | 1982-06-24 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0094101A2 true EP0094101A2 (fr) | 1983-11-16 |
| EP0094101A3 EP0094101A3 (en) | 1984-12-05 |
| EP0094101B1 EP0094101B1 (fr) | 1988-07-20 |
Family
ID=25802590
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP83104752A Expired EP0094101B1 (fr) | 1982-05-12 | 1983-05-13 | Récipient stérile pour usage médical |
Country Status (2)
| Country | Link |
|---|---|
| EP (1) | EP0094101B1 (fr) |
| DE (1) | DE3377399D1 (fr) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3420865C1 (de) * | 1984-06-05 | 1985-08-29 | B. Braun Melsungen Ag, 3508 Melsungen | Infusionsspritzenpumpe |
| FR2576876A1 (fr) * | 1985-02-01 | 1986-08-08 | Mallet Frederic | Recipient souple a volume variable |
| EP0469905A1 (fr) * | 1990-08-01 | 1992-02-05 | Hanshin Kasei Kogyo Kk | Réservoir de liquide pour l'injection de médicaments |
| WO1995030605A1 (fr) * | 1994-05-05 | 1995-11-16 | Allergan Inc | Dispositif de distribution de fluides multiples pour formulations a faible tension superficielle |
| DE4420594A1 (de) * | 1994-06-14 | 1995-12-21 | Bernd Hansen | Behältnis für fließfähige, insbesondere pasteuse Stoffe und Verfahren zu seiner Herstellung |
| EP0788804A3 (fr) * | 1996-02-08 | 1997-12-03 | B. Braun Melsungen Ag | Applicateur médical |
| WO1998033468A1 (fr) * | 1997-01-31 | 1998-08-06 | Wallace Cameron & Company Limited | Flacon ne pouvant etre rebouche et recipient utilise a cet effet |
| GB2331501A (en) * | 1997-11-19 | 1999-05-26 | Simon Feiner | Collapsible containers |
| CN111132647A (zh) * | 2017-09-26 | 2020-05-08 | 科赫尔塑料机械制造有限公司 | 容器以及连接和制造装置 |
| WO2020208160A1 (fr) * | 2019-04-10 | 2020-10-15 | Sartorius Stedim Biotech Gmbh | Récipient pour micro-quantités de liquides |
| CN114616019A (zh) * | 2019-09-04 | 2022-06-10 | 创意气球有限公司 | 用于通过可伸缩的插入辅助件对尿道施加润滑或内腔矫直物质的装置和方法 |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2317420A (en) * | 1940-12-20 | 1943-04-27 | American Can Co | Container |
| FR1241250A (fr) * | 1959-08-05 | 1960-09-16 | Rical Sa | Dispositif obturateur pour récipients à ouverture du type styligoutte |
| FR1426663A (fr) * | 1964-03-12 | 1966-01-28 | Unilever Nv | Conteneur et dispositif de fermeture destiné audit conteneur |
| NL7005068A (fr) * | 1970-04-09 | 1971-10-12 | ||
| US3804282A (en) * | 1971-11-19 | 1974-04-16 | Automatic Liquid Packaging | Container and cap construction |
| FR2268710A1 (fr) * | 1974-04-29 | 1975-11-21 | Abbott Lab | |
| FR2287243A1 (fr) * | 1974-10-09 | 1976-05-07 | Reckitt & Colmann Prod Ltd | Appareil pour introduire des compositions aqueuses dans une cavite du corps |
| CH591855A5 (fr) * | 1974-09-26 | 1977-09-30 | Otsuka Pharma Co Ltd | |
| US4213933A (en) * | 1978-03-15 | 1980-07-22 | Respiratory Care, Inc. | Method of making a blow molded container having an insert molded in situ |
| DE2907832A1 (de) * | 1979-02-28 | 1980-09-04 | Fresenius Chem Pharm Ind | Verbindungseinrichtung zum anschluss von kanuelen, kathetern, infusionsnadeln, schlaeuchen u.dgl. |
| US4258867A (en) * | 1979-03-19 | 1981-03-31 | Automatic Liquid Packaging, Inc. | Hermetically sealed container with twistable overcap |
-
1983
- 1983-05-13 DE DE8383104752T patent/DE3377399D1/de not_active Expired
- 1983-05-13 EP EP83104752A patent/EP0094101B1/fr not_active Expired
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2317420A (en) * | 1940-12-20 | 1943-04-27 | American Can Co | Container |
| FR1241250A (fr) * | 1959-08-05 | 1960-09-16 | Rical Sa | Dispositif obturateur pour récipients à ouverture du type styligoutte |
| FR1426663A (fr) * | 1964-03-12 | 1966-01-28 | Unilever Nv | Conteneur et dispositif de fermeture destiné audit conteneur |
| NL7005068A (fr) * | 1970-04-09 | 1971-10-12 | ||
| US3804282A (en) * | 1971-11-19 | 1974-04-16 | Automatic Liquid Packaging | Container and cap construction |
| FR2268710A1 (fr) * | 1974-04-29 | 1975-11-21 | Abbott Lab | |
| CH591855A5 (fr) * | 1974-09-26 | 1977-09-30 | Otsuka Pharma Co Ltd | |
| FR2287243A1 (fr) * | 1974-10-09 | 1976-05-07 | Reckitt & Colmann Prod Ltd | Appareil pour introduire des compositions aqueuses dans une cavite du corps |
| US4213933A (en) * | 1978-03-15 | 1980-07-22 | Respiratory Care, Inc. | Method of making a blow molded container having an insert molded in situ |
| DE2907832A1 (de) * | 1979-02-28 | 1980-09-04 | Fresenius Chem Pharm Ind | Verbindungseinrichtung zum anschluss von kanuelen, kathetern, infusionsnadeln, schlaeuchen u.dgl. |
| US4258867A (en) * | 1979-03-19 | 1981-03-31 | Automatic Liquid Packaging, Inc. | Hermetically sealed container with twistable overcap |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3420865C1 (de) * | 1984-06-05 | 1985-08-29 | B. Braun Melsungen Ag, 3508 Melsungen | Infusionsspritzenpumpe |
| FR2576876A1 (fr) * | 1985-02-01 | 1986-08-08 | Mallet Frederic | Recipient souple a volume variable |
| EP0469905A1 (fr) * | 1990-08-01 | 1992-02-05 | Hanshin Kasei Kogyo Kk | Réservoir de liquide pour l'injection de médicaments |
| WO1995030605A1 (fr) * | 1994-05-05 | 1995-11-16 | Allergan Inc | Dispositif de distribution de fluides multiples pour formulations a faible tension superficielle |
| US5564596A (en) * | 1994-05-05 | 1996-10-15 | Allergan, Inc. | Multiple fluid dispensing device for low surface tension formulations |
| DE4420594A1 (de) * | 1994-06-14 | 1995-12-21 | Bernd Hansen | Behältnis für fließfähige, insbesondere pasteuse Stoffe und Verfahren zu seiner Herstellung |
| EP0788804A3 (fr) * | 1996-02-08 | 1997-12-03 | B. Braun Melsungen Ag | Applicateur médical |
| WO1998033468A1 (fr) * | 1997-01-31 | 1998-08-06 | Wallace Cameron & Company Limited | Flacon ne pouvant etre rebouche et recipient utilise a cet effet |
| US6164450A (en) * | 1997-01-31 | 2000-12-26 | Benedetti; Giovanni | Non-resealable bottle and container therefor |
| GB2331501A (en) * | 1997-11-19 | 1999-05-26 | Simon Feiner | Collapsible containers |
| CN111132647A (zh) * | 2017-09-26 | 2020-05-08 | 科赫尔塑料机械制造有限公司 | 容器以及连接和制造装置 |
| CN111132647B (zh) * | 2017-09-26 | 2023-12-22 | 科赫尔塑料机械制造有限公司 | 容器以及连接和制造装置 |
| WO2020208160A1 (fr) * | 2019-04-10 | 2020-10-15 | Sartorius Stedim Biotech Gmbh | Récipient pour micro-quantités de liquides |
| CN114616019A (zh) * | 2019-09-04 | 2022-06-10 | 创意气球有限公司 | 用于通过可伸缩的插入辅助件对尿道施加润滑或内腔矫直物质的装置和方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0094101B1 (fr) | 1988-07-20 |
| DE3377399D1 (en) | 1988-08-25 |
| EP0094101A3 (en) | 1984-12-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0975301B1 (fr) | Connecteur sterile | |
| DE69820433T2 (de) | Verbindungsanordnung, fluidsysteme und methode zum erstellen einer verbindung | |
| EP2301622B1 (fr) | Connecteur pour des emballages contenant des fluides médicaux et emballage pour fluides médicaux | |
| DE69302188T2 (de) | Vorrichtung zum Vermischen | |
| EP0250369B1 (fr) | Valve à trois voies | |
| DE69403452T2 (de) | Verschluss für medizinbehälter | |
| DE3687410T2 (de) | Behaelter und verschlussvorrichtung. | |
| DE2947574C2 (de) | Schlauchkupplung für keimfrei zu haltende Leitungsverbinder | |
| EP2332610B1 (fr) | Système de raccordement pour récipients d'aliments destinés à la nutrition entérale | |
| EP2536377B1 (fr) | Clapet de fermeture pour un récipient destiné à la réception de liquides médicaux et récipient | |
| EP1108444A2 (fr) | Système de connection stérile pour sytèmes médicaux ainsi que son utilisation | |
| US20060253103A1 (en) | Removable cap needle access site | |
| WO2011092057A1 (fr) | Raccord pour récipients contenant un principe actif médicinal | |
| DE3238303A1 (de) | Verbindungsstueck fuer medizinische leitungen und damit ausgestattete medizinische loesungsbeutelanordnung | |
| DE69904350T2 (de) | Behaelter für intravenöse applikation | |
| EP0830874B1 (fr) | Dispositif de connecteur en particulier pour usage médical | |
| EP0094101B1 (fr) | Récipient stérile pour usage médical | |
| DE2358128B1 (de) | Vorrichtung und Flaschenkopf zur sterilen Entnahme steriler Flascheninhalte | |
| DE3217913C2 (de) | Steriler Behälter für medizinische Zwecke | |
| WO2009092430A1 (fr) | Système de seringue et son procédé de fabrication | |
| DE69012236T2 (de) | Kappe für medizinisches Verbindungsstück. | |
| DE3716586C2 (de) | Behälter aus Kunststoff zur Aufbewahrung und Applikation eines Kathetergleitmittels und Verfahren zu seiner Herstellung | |
| DE8213816U1 (de) | Steriler Behälter für medizinische Zwecke | |
| WO2002009636A1 (fr) | Capsule a raccorder a une partie de dispositif verseur | |
| DE29612534U1 (de) | Infusionsset |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19850605 |
|
| 17Q | First examination report despatched |
Effective date: 19860714 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: HANSEN, BERND, DIPL.-ING. Owner name: FRESENIUS AG |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 35773 Country of ref document: AT Date of ref document: 19880815 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3377399 Country of ref document: DE Date of ref document: 19880825 |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) | ||
| ET | Fr: translation filed | ||
| ITF | It: translation for a ep patent filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| ITTA | It: last paid annual fee | ||
| EPTA | Lu: last paid annual fee | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 83104752.7 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20020422 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20020517 Year of fee payment: 20 Ref country code: FR Payment date: 20020517 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 20020523 Year of fee payment: 20 Ref country code: AT Payment date: 20020523 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20020524 Year of fee payment: 20 Ref country code: CH Payment date: 20020524 Year of fee payment: 20 Ref country code: BE Payment date: 20020524 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20020717 Year of fee payment: 20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20030512 Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20030512 Ref country code: CH Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20030512 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20030513 Ref country code: LU Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20030513 Ref country code: AT Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20030513 |
|
| BE20 | Be: patent expired |
Owner name: *HANSEN BERND Effective date: 20030513 Owner name: *FRESENIUS A.G. Effective date: 20030513 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| EUG | Se: european patent has lapsed | ||
| NLV7 | Nl: ceased due to reaching the maximum lifetime of a patent |
Effective date: 20030513 |