EP0051189A1 - Procédé pour la fabrication des filaments et fibres en polyacrylonitrile, à section profilée filés à sec - Google Patents
Procédé pour la fabrication des filaments et fibres en polyacrylonitrile, à section profilée filés à sec Download PDFInfo
- Publication number
- EP0051189A1 EP0051189A1 EP81108416A EP81108416A EP0051189A1 EP 0051189 A1 EP0051189 A1 EP 0051189A1 EP 81108416 A EP81108416 A EP 81108416A EP 81108416 A EP81108416 A EP 81108416A EP 0051189 A1 EP0051189 A1 EP 0051189A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- spinning
- cross
- fibers
- dry
- spun
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 238000004519 manufacturing process Methods 0.000 title claims description 12
- 229920002239 polyacrylonitrile Polymers 0.000 title abstract description 15
- 238000009987 spinning Methods 0.000 claims abstract description 81
- 239000000835 fiber Substances 0.000 claims abstract description 59
- 238000000034 method Methods 0.000 claims abstract description 32
- 238000000578 dry spinning Methods 0.000 claims abstract description 15
- 239000002904 solvent Substances 0.000 claims description 40
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 23
- NLHHRLWOUZZQLW-UHFFFAOYSA-N Acrylonitrile Chemical compound C=CC#N NLHHRLWOUZZQLW-UHFFFAOYSA-N 0.000 claims description 13
- 229920000642 polymer Polymers 0.000 claims description 10
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 8
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims description 5
- 229920001223 polyethylene glycol Polymers 0.000 claims description 3
- UWHCKJMYHZGTIT-UHFFFAOYSA-N tetraethylene glycol Chemical compound OCCOCCOCCOCCO UWHCKJMYHZGTIT-UHFFFAOYSA-N 0.000 claims description 3
- 229920001059 synthetic polymer Polymers 0.000 claims description 2
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 42
- 229920001577 copolymer Polymers 0.000 description 21
- 239000007787 solid Substances 0.000 description 18
- 239000000725 suspension Substances 0.000 description 17
- 238000003756 stirring Methods 0.000 description 13
- BAPJBEWLBFYGME-UHFFFAOYSA-N Methyl acrylate Chemical compound COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 description 12
- 238000009835 boiling Methods 0.000 description 12
- 229920002972 Acrylic fiber Polymers 0.000 description 11
- 239000000126 substance Substances 0.000 description 11
- 239000000203 mixture Substances 0.000 description 6
- 238000010438 heat treatment Methods 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- SZHIIIPPJJXYRY-UHFFFAOYSA-M sodium;2-methylprop-2-ene-1-sulfonate Chemical compound [Na+].CC(=C)CS([O-])(=O)=O SZHIIIPPJJXYRY-UHFFFAOYSA-M 0.000 description 5
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 4
- 239000004753 textile Substances 0.000 description 4
- 238000001816 cooling Methods 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000002074 melt spinning Methods 0.000 description 3
- 229920002994 synthetic fiber Polymers 0.000 description 3
- 239000012209 synthetic fiber Substances 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- 210000001520 comb Anatomy 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 239000002657 fibrous material Substances 0.000 description 2
- 230000009969 flowable effect Effects 0.000 description 2
- 235000011187 glycerol Nutrition 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 238000002166 wet spinning Methods 0.000 description 2
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 1
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 1
- 229920002292 Nylon 6 Polymers 0.000 description 1
- 229920002302 Nylon 6,6 Polymers 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000005215 alkyl ethers Chemical class 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 239000011258 core-shell material Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000002932 luster Substances 0.000 description 1
- 125000005394 methallyl group Chemical group 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- D—TEXTILES; PAPER
- D01—NATURAL OR MAN-MADE THREADS OR FIBRES; SPINNING
- D01D—MECHANICAL METHODS OR APPARATUS IN THE MANUFACTURE OF ARTIFICIAL FILAMENTS, THREADS, FIBRES, BRISTLES OR RIBBONS
- D01D5/00—Formation of filaments, threads, or the like
- D01D5/253—Formation of filaments, threads, or the like with a non-circular cross section; Spinnerette packs therefor
-
- D—TEXTILES; PAPER
- D01—NATURAL OR MAN-MADE THREADS OR FIBRES; SPINNING
- D01F—CHEMICAL FEATURES IN THE MANUFACTURE OF ARTIFICIAL FILAMENTS, THREADS, FIBRES, BRISTLES OR RIBBONS; APPARATUS SPECIALLY ADAPTED FOR THE MANUFACTURE OF CARBON FILAMENTS
- D01F6/00—Monocomponent artificial filaments or the like of synthetic polymers; Manufacture thereof
- D01F6/02—Monocomponent artificial filaments or the like of synthetic polymers; Manufacture thereof from homopolymers obtained by reactions only involving carbon-to-carbon unsaturated bonds
- D01F6/18—Monocomponent artificial filaments or the like of synthetic polymers; Manufacture thereof from homopolymers obtained by reactions only involving carbon-to-carbon unsaturated bonds from polymers of unsaturated nitriles, e.g. polyacrylonitrile, polyvinylidene cyanide
Definitions
- polyamide and polyester fibers are preferably produced using the melt spinning process from profiled spinnerets in order to achieve special effects with regard to gloss, grip, luster and loss of goods.
- the effects of changing the thread cross-sectional shape of synthetic fibers in detail on the failure and behavior of finished goods can be seen, for example, from the reports by F. Bolland in Chemiefaser 13 (1963), pages 42-45 and 106-109 and from the article H. Bieser and R. Hesse in Chemiefaser 17 (1967), pages 262-268. H.
- cross-section-modified synthetic fibers for example cross-section-modified acrylic fibers, can also be produced by the wet spinning process.
- acrylic fibers with a triangular fiber cross section are on the market, which are characterized by high color brilliance.
- dumbbell-shaped cross section is always obtained, for example with an acrylonitrile copolymer made of 93.6% by weight acrylonitrile, 5.7% by weight methyl acrylate and 0.7% by weight sodium methallylsulfonate with the K value 81 from a 32% by weight spinning solution in dimethylformamide. If one tries to raise the solids content further, then Such spinning solutions gel on cooling even at temperatures around 50-80 ° C., so that trouble-free spinning becomes impossible.
- the object of the present invention was to provide such a dry spinning process because of the versatile application possibilities of such fibers and threads.
- any predetermined cross-sectional profile can be spun if spinning solutions with a viscosity exceeding a certain value are used and profile nozzles which have certain dimensions are used.
- Fibers with a sharp cross-sectional profile are to be understood as fibers whose cross-section can be used to recognize the geometry of the profile nozzle used, a profile nozzle being understood to mean any nozzle bore with the exception of the simple round nozzle bore. Simple geometric shapes are used in particular.
- the invention therefore relates to dry-spun polyacrylonitrile fibers with a sharp cross-sectional profile.
- Suitable acrylonitrile polymers for the production of threads and fibers are acrylonitrile homopolymers and copolymers, the copolymers containing at least 50% by weight, preferably at least 85% by weight, of copolymerized acrylonitrile units.
- the invention further relates to a method for Manufacture of acrylic fibers and threads with a sharp cross-sectional profile, characterized in that the thread-forming synthetic polymers are spun after a dry spinning process from a solution which has a viscosity of at least 120 ball falling seconds, measured at 80 ° C or at least 75 ball falling seconds, measured at 100 ° C, the nozzle hole area of the profile nozzle being less than 0.2 mm 2 and the leg width being less than 0.17 mm.
- Spinning is followed by the usual further process steps of the acrylonitrile dry spinning process.
- the viscosity in falling ball seconds was determined by the method of K. Jost, Reologica Acta, Vol. 1 (1958), page 303.
- the leg width of a profile nozzle is understood to mean the distance in mm from the outer boundary of the predetermined profile shape, but not the distance to the center of the nozzle hole.
- the distance between two opposite side centers is defined as the middle leg width as the leg width. It has been shown that sharp cross-sectional profiles can always be spun in the sense of the invention if the nozzle hole area is less than 0.2 mm 2 and the leg width is less than 0.17 mm.
- Leg widths are particularly preferred from 0.02 - 0.06 mm and nozzle hole areas up to 0.1 mm 2 are used. For nozzle hole areas larger than 0.2 mm 2 a. Flowing of the cross-sectional shapes found. You get fuzzy, bulbous to formless. deformed playful structures.
- Spinning solutions of the stated viscosity which also contain a higher concentration of the thread-forming polymer, are obtained according to DE-OS 27 06 032 by preparing appropriately concentrated suspensions of the thread-forming polymer, which are easy to convey, in the desired solvent and heating this suspension by briefly heating it up Converted temperatures to just below the boiling point of the spinning solvent used in viscosity-stable spinning solutions.
- the suspensions for the preparation of such spinning solutions are obtained by, if necessary, spinning the solvent with a non-solvent for that to be spun Polymers added and then the polymer is added with stirring. All substances that are non-solvents for the polymer and can be mixed with the spinning solvent within wide limits are suitable as non-solvents in the sense of the invention.
- the boiling points of the non-solvents can be both below and above the boiling point of the spinning solvent used.
- Such substances which may be in solid or liquid state, are, for example, alcohols, esters or ketones and mono- and polysubstituted alkyl ethers and esters of polyhydric alcohols, inorganic or organic acids, salts and the like.
- Water and, on the other hand, glycerin, mono- and tetraethylene glycol and sugar are used as preferred non-solvents because of their easy handling and removal in the spin shaft without residue formation and recovery.
- the water content of such suspensions of polyacrylonitrile and dimethylformamide is in the range between 2 and 10% by weight, based on the total suspension. Below 2% by weight of water, a free-flowing, transportable suspension is no longer obtained, but rather a thick, slurry. On the other hand, if the water content is more than 10% by weight, the threads burst during the spinning process below the nozzle because of the excessive water vapor partial pressure when it emerges from the nozzle holes.
- the percentage of water in the spinning solution influences, as shown in Table II for a 35% spinning solution or for a 36% spinning solution, only to a small extent the profile at the nozzle. It is crucial that the spinning solution has the specified minimum viscosity.
- acrylonitrile copolymer made from 92% acrylonitrile 6% acrylic acid methyl ester and 2% sodium methallylsulfonate with a K value of 60
- a suspension made of 45% copolymer solid, 4% water and 51% dimethylformamide can be produced, which is still flowable at room temperature and heated by heating Spinning solution gives, which has a viscosity of 142 ball falling seconds at 80 ° C.
- the spinning of this spinning solution from profile nozzles results in fibers with a sharp cross-sectional profile in the sense of this invention.
- the required viscosity of the spinning solution can also be achieved with a lower solids concentration.
- the spinning solution has a minimum viscosity.
- non-solvent fractions of 5-10% by weight have proven to be optimal in order to obtain profiled acrylic fibers with a water retention capacity greater than 10%.
- the fibers also have a core-shell structure. The thickness of the fiber sheath can be varied within wide limits by the ratio of polymer solid to non-solvent content.
- the minimum viscosity can be determined at two different temperatures, namely at 80 ° C and 100 ° C.
- This measure takes into account the fact that, on the one hand, the determination of the viscosity in spinning solutions which contain water as the non-solvent is difficult because of the evaporation of the water at 100 ° C., on the other hand the determination of the viscosity in the case of other spinning solutions which contain a substance as the non-solvent whose boiling point is above that of the spinning solvent can become problematic at 80 ° C due to the tendency to gel.
- the viscosity of water-containing spinning solutions can also be determined at 100 ° C when working in a closed system.
- the water retention capacity (WR) is determined based on DIN specification 53 814 (see Melliant "Textile Reports” 4, 1973, page 350).
- the higher boiling solvents such as dimethylacetamide, dimethyl sulfoxide, ethylene carbonate and N-methylpyrrolidone, and the like can also be used as spinning solvents in the production of acrylic fibers with modified fiber cross sections.
- the fibers according to the invention can have individual titers in the stretched state of 1 to 40 dtex.
- DMF dimethylformamide
- 38 kg of an acrylonitrile copolymer composed of 93.6% acrylonitrile, 5.7% methyl acrylate and 0.7% sodium methallylsulfonate with a K value of 81 are then metered in with stirring at room temperature.
- the suspension is pumped via a gear pump into a spinning kettle equipped with an agitator. Then the suspension, which has a solids content of 38% by weight and a water content of 3% by weight, based on the total solution, is heated in a double-walled tube with steam of 4.0 bar.
- the dwell time in the tube is 7 minutes.
- the temperature of the solution at the pipe outlet is 138 ° C.
- the spinning solution which has a viscosity of 176 falling balls at 80 ° C, is filtered after leaving the heating device without intermediate cooling and fed directly to the spinning shaft.
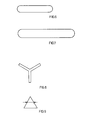
- the spinning solution is spun dry from a 90-hole nozzle with hexalobal nozzle holes (see FIG. 1).
- the nozzle hole area is 0.0696 (mm) 2 and the leg width is 0.04 mm.
- the shaft temperature is 160 ° C and the air temperature is 150 ° C.
- the air flow rate is 30 m 3 / hour.
- the take-off speed is 275 m / min.
- the 750 dtex spinning material is collected on bobbins and closed a volume of 187,000 dtex total titre.
- the fiber cable is then stretched 1: 4 times in boiling water and aftertreated in the usual way to give fibers with a final density of 2.6 dtex.
- the fiber capillaries are embedded in methyl methacrylate and cross-cut.
- the light microscopic images produced in the differential interference contrast method show that the sample cross sections have a completely uniform hexalobal structure.
- the tensile strength is 2.9 cN / dtex and the elongation at break is 27%.
- Table I shows the production of further modified fiber cross-sectional shapes, such as those obtained in dry spinning from profiled nozzles by the process according to the invention.
- an acrylonitrile copolymer with the chemical composition and concentration of Example 1 is used.
- the spinning solution is prepared as described there and spun into fibers from the profiled nozzles given in Table 1 and then aftertreated. It was spun from 90-hole nozzles.
- the thread cross-sectional geometry is determined as stated in Example 1 and is documented with light microscopic images.
- An acrylonitrile copolymer with the chemical composition of Example 1 with a K value of 81 is, as described there, dissolved, filtered and dry spun from a 90-hole nozzle with trilobal nozzle holes (see FIG. 8).
- the nozzle hole area is 0.03 (mm) 2 and the leg width is 0.04 mm.
- the shaft temperature is 150 ° C and the air temperature is 150 ° C.
- the air flow rate is 30 m 3 / h.
- the take-off speed is 125 m / min.
- the spinning material with a titer of 1500 dtex is collected on spools, folded into a ribbon with a total titer of 150,000 dtex and, as described in Example 1, post-treated to fibers with a final titer of 5.0 dtex.
- the following table II shows the limits of the method according to the invention for producing cross-section-modified acrylic fibers according to the dry spinning method using further examples.
- an acrylonitrile copolymer with the chemical composition of Example 1 is used again and transferred to a spinning solution as described there.
- the solids concentration and the type and percentage of non-solvent for PAN are varied. It is spun from one of the 90-hole nozzles described above with trilobal nozzle holes (cf. FIG. 8).
- the spinning and aftertreatment conditions correspond to the information from example 2.
- the viscosities are measured, as described at the beginning, in falling ball seconds at 80 ° C.
- Example 1 67 kg of dimethylformamide are mixed with 3 kg of water in a kettle with stirring. Then 30 kg of an acrylonitrile homopolymer with a K value of 91 according to Fikentscher are metered in with stirring at room temperature.
- the suspension which has a solids concentration of 30%, is again dissolved, as described in Example 1, filtered and from a 90-hole nozzle spun dry with trilobal nozzle holes (see FIG. 8).
- the viscosity measured at 80 ° C was 138 falling seconds.
- the nozzle hole area is 0.03 mm 2 and the leg width is 0.04 mm.
- the other spinning and post-treatment conditions correspond to the explanations of Example 1.
- DMF dimethylformamide
- monoethylene glycol monoethylene glycol
- 37 kg of an acrylonitrile copolymer of 93.6% acrylonitrile, 5.7% methyl acrylate and 0.7% sodium methallylsulfonate with a K value of 81 are then metered in with stirring at room temperature.
- the suspension is pumped via a gear pump in a spinning kettle equipped with an agitator. Then the suspension, which has a solids content of 37% by weight and a non-solvent content of 6% by weight, based on the total solution, is heated in a double-walled tube with steam of 4.0 bar.
- the dwell time in the tube is 7 minutes.
- the temperature of the solution at the pipe outlet is 138 ° C.
- the spinning solution which has a viscosity of 186 falling seconds at 100 ° C, is filtered after leaving the heating device without intermediate cooling and fed directly to the spinning shaft.
- the spinning solution is spun dry from a 90-hole nozzle with hexalobal nozzle holes (see FIG. 1).
- the nozzle hole area is 0.0696 (mm) 2 and the leg width is 0.04 mm.
- the shaft temperature is 160 ° C and the air temperature is 100 ° C.
- the air flow rate is 30 m 3 / hour.
- the take-off speed is 350 m / min.
- the spun material with a titer of 475 dtex is collected on spools and folded into a band with a total titer of 142 500 dtex.
- the fiber cable is then stretched 1: 4 times in boiling water, washed, dried at 110 ° C.
- the fiber capillaries are embedded in methyl methacrylate and cross-cut.
- the light microscopic images produced in the differential interference contrast method show that the sample cross sections have a completely uniform shape with a hexalobal core / shell structure.
- the tear strength is 2.6 cN / dtex and the elongation at break is 34%.
- the surface area is approximately 80%.
- the water retention capacity is 12.6%.
- Table III shows the production of further, modified fiber cross-sectional shapes, as are obtained in dry spinning from profiled nozzles by the process according to the invention.
- an acrylonitrile copolymer with the chemical composition and concentration of Example 5 used.
- the spinning solution is prepared as described there and spun into fibers from the profiled nozzles given in Table III and then aftertreated. It was spun from 90-hole nozzles.
- the thread cross-sectional geometry was determined, as stated in Example 1, and documented with light microscopic images.
- Example 5 55 kg of dimethylformamide are mixed with 7 kg of tetraethylene glycol in a kettle with stirring. Then 38 kg of an acrylonitrile copolymer having the chemical composition of Example 5 with a K value of 81 are metered in with stirring at room temperature.
- the suspension which has a solids concentration of 38%, is again dissolved, as described in Example 5, filtered and spun dry from a 90-hole nozzle with trilobal nozzle holes (cf. FIG. 8).
- the viscosity of the spinning solution measured at 100 ° C is 152 falling seconds.
- the nozzle hole area is 0.03 mm2 and the leg width is 0.04 mm.
- the shaft temperature is 160 ° C and the air temperature is 150 ° C.
- the air flow is 30m J / h.
- the take-off speed is 250 m / min.
- the spinning material with a titer of 2100 dtex is collected on bobbins, folded into a band with a total titer of 210,000 dtex and after-treated as described in Example 5 to give fibers with a final titer of 6.7 dtex.
- the sample cross-sections of the fibers which in turn have a core / sheath structure, show a completely uniform trilobal cross-sectional profile. Fiber strength 2.4 cN / dtex; Elongation at break: 34%; Water retention: 15.2%.
- the following table IV shows the limits of the method according to the invention for producing cross-section-modified acrylic fibers according to the dry spinning method using further examples.
- an acrylonitrile copolymer with the. used chemical composition of Example 5 and transferred to a spinning solution as described therein.
- the solids concentration and the type and percentage of non-solvent for PAN are varied.
- the spinning and post-treatment conditions correspond to the information from Example 2.
- the viscosity in falling ball seconds is determined at 100 ° C.
- Example 5 The other spinning and post-treatment conditions correspond to the explanations of Example 5.
- the sample cross sections of the fibers which have a final titer of 3.1 dtex, show a completely uniform hexalobal cross-sectional profile with a core / shell structure.
- Fiber strength 2.7 cN / dtex; Elongation at break: 31%. Water retention: 10.2%.
- a portion of the spinning solution from Example 5 is fed to another spinning shaft after the filtration and is dry spun from a 90-hole nozzle with hexalobal nozzle holes (see FIG. 1).
- the shaft temperature is 220 ° C and the air temperature is 360 ° C.
- the air flow rate is 40 m 3 / hour.
- the take-off speed is 125 m / min.
- the spun material with a titre of 1770 dtex is collected on bobbins, folded into a band with a total titre of 177,000 dtex and then, as described in Example 5, post-treated into fibers with a final titre of 6.7 dtex.
- the sample cross-sections of the fibers show a completely uniform hexalobal cross-sectional profile. However, they no longer have a core / shell structure, since most of the non-solvent is evaporated out in the spinning shaft.
- the water retention capacity is 4.3%.
- a part of the fiber cable from Example 5 with a total titer of 142,500 dtex was stretched and washed as described there, but then dried at 180 ° C. in a drum dryer with 20% shrinkage and in the usual way to fibers with a final titer of 1.6 dtex aftertreated.
- the sample cross-sections of the fibers show a completely uniform hexalobal cross-sectional profile. However, they no longer have a core / shell structure, since the pore system due to the harsh drying conditions has been eliminated.
- the water retention capacity is 3.9%.
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Health & Medical Sciences (AREA)
- Toxicology (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Mechanical Engineering (AREA)
- Artificial Filaments (AREA)
- Spinning Methods And Devices For Manufacturing Artificial Fibers (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3040970 | 1980-10-30 | ||
| DE19803040970 DE3040970A1 (de) | 1980-10-30 | 1980-10-30 | Trockengesponnene polyacrylnitril-profilfasern und -faeden und ein verfahren zu ihrer herstellung |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0051189A1 true EP0051189A1 (fr) | 1982-05-12 |
| EP0051189B1 EP0051189B1 (fr) | 1985-08-07 |
| EP0051189B2 EP0051189B2 (fr) | 1990-07-04 |
Family
ID=6115591
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP81108416A Expired - Lifetime EP0051189B2 (fr) | 1980-10-30 | 1981-10-16 | Procédé pour la fabrication des filaments et fibres en polyacrylonitrile, à section profilée filés à sec |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US4810448A (fr) |
| EP (1) | EP0051189B2 (fr) |
| JP (1) | JPS57106713A (fr) |
| DE (2) | DE3040970A1 (fr) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5994611A (ja) * | 1982-11-22 | 1984-05-31 | Mitsubishi Rayon Co Ltd | ポリアクリロニトリルフィラメント糸の製造方法 |
| GB8527752D0 (en) * | 1984-11-21 | 1985-12-18 | Mitsubishi Rayon Co | Acrylic fiber |
| SG73992A1 (en) * | 1995-12-18 | 2000-07-18 | Standard Oil Co | Melt spun acrylonitrile olefinically unsaturated fibers and a process to make fibers |
| ATE446395T1 (de) * | 2005-12-06 | 2009-11-15 | Invista Tech Sarl | Im profil sechslappige filamente mit drei grösseren lappen und drei kleineren lappen, tufting-teppich aus garn mit derartigen filamenten sowie kapillare spinndüse zur herstellung derartiger filamente |
| CN105273125A (zh) * | 2014-06-06 | 2016-01-27 | 中国石油化工股份有限公司 | 适用于干法腈纶纺丝的聚丙烯腈干粉及制备方法 |
| WO2021203027A1 (fr) * | 2020-04-02 | 2021-10-07 | Aladdin Manufacturing Corporation | Filaments de type ruban et leurs systèmes et procédés de production |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3169089A (en) * | 1960-04-22 | 1965-02-09 | Celanese Corp | Filaments |
| DE1219165B (de) * | 1958-10-17 | 1966-06-16 | Celanese Corp | Spinnduese |
| DE1435547A1 (de) * | 1961-10-26 | 1969-07-17 | Monsanto Co | Textilfaden mit besonderem Querschnitt und Spinnduese fuer seine Herstellung |
| DE2554124A1 (de) * | 1975-12-02 | 1977-06-08 | Bayer Ag | Hydrophile fasern und faeden aus synthetischen polymeren |
| DE2658179A1 (de) * | 1976-12-22 | 1978-07-06 | Bayer Ag | Trockengesponnene grobtitrige acrylfasern |
| EP0013889A1 (fr) * | 1979-01-18 | 1980-08-06 | Bayer Ag | Procédé pour la fabrication en continu de filaments ou de fibres à partir de polymères synthétiques difficilement solubles |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA729518A (en) * | 1966-03-08 | E. Bishop Clarence | Dry spun y-shaped filaments with bulbous ends | |
| CA750852A (en) * | 1967-01-17 | E. Bishop Clarence | Dry-spinning k-shape filaments | |
| US2843449A (en) * | 1954-04-13 | 1958-07-15 | Eastman Kodak Co | Dry spinning process |
| US3131428A (en) * | 1958-12-19 | 1964-05-05 | Celanese Corp | Spinneret and spinning method |
| US3194002A (en) * | 1962-07-25 | 1965-07-13 | Eastman Kodak Co | Multifilament yarn of non-regular cross section |
| US3340571A (en) * | 1964-04-02 | 1967-09-12 | Celanese Corp | Spinneret for making hollow filaments |
| FR93435E (fr) * | 1966-09-29 | 1969-03-28 | Rhodiaceta | Nouvelle filiere et fils spéciaux obtenus au moyen de cette filiere. |
| JPS5432859B2 (fr) * | 1971-11-15 | 1979-10-17 | ||
| JPS50135323A (fr) * | 1974-04-16 | 1975-10-27 | ||
| JPS546919A (en) * | 1977-06-17 | 1979-01-19 | Mitsubishi Rayon Co Ltd | Production of acrylic noncircular cross-section filament yarns |
| DE2804376A1 (de) * | 1978-02-02 | 1979-08-09 | Bayer Ag | Hydrophile hohlfasern |
-
1980
- 1980-10-30 DE DE19803040970 patent/DE3040970A1/de not_active Withdrawn
-
1981
- 1981-10-16 DE DE8181108416T patent/DE3171719D1/de not_active Expired
- 1981-10-16 EP EP81108416A patent/EP0051189B2/fr not_active Expired - Lifetime
- 1981-10-30 JP JP56173144A patent/JPS57106713A/ja active Granted
-
1987
- 1987-04-22 US US07/041,890 patent/US4810448A/en not_active Expired - Fee Related
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1219165B (de) * | 1958-10-17 | 1966-06-16 | Celanese Corp | Spinnduese |
| US3169089A (en) * | 1960-04-22 | 1965-02-09 | Celanese Corp | Filaments |
| DE1435547A1 (de) * | 1961-10-26 | 1969-07-17 | Monsanto Co | Textilfaden mit besonderem Querschnitt und Spinnduese fuer seine Herstellung |
| DE2554124A1 (de) * | 1975-12-02 | 1977-06-08 | Bayer Ag | Hydrophile fasern und faeden aus synthetischen polymeren |
| DE2658179A1 (de) * | 1976-12-22 | 1978-07-06 | Bayer Ag | Trockengesponnene grobtitrige acrylfasern |
| EP0013889A1 (fr) * | 1979-01-18 | 1980-08-06 | Bayer Ag | Procédé pour la fabrication en continu de filaments ou de fibres à partir de polymères synthétiques difficilement solubles |
Also Published As
| Publication number | Publication date |
|---|---|
| DE3040970A1 (de) | 1982-06-03 |
| JPH0214443B2 (fr) | 1990-04-09 |
| JPS57106713A (en) | 1982-07-02 |
| DE3171719D1 (en) | 1985-09-12 |
| US4810448A (en) | 1989-03-07 |
| EP0051189B2 (fr) | 1990-07-04 |
| EP0051189B1 (fr) | 1985-08-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AT395863B (de) | Verfahren zur herstellung eines cellulosischen formkoerpers | |
| EP0044534B1 (fr) | Filaments et fibres à haut module, en polyacrylonitrile, et leur procédé de fabrication | |
| DE2554124B2 (de) | Hydrophile fasern und faeden aus synthetischen polymeren | |
| DE2607996C2 (de) | Hydrophile Fasern und Fäden aus einem Acrylnitrilpolymerisat | |
| DE2951803C2 (fr) | ||
| EP0051189B1 (fr) | Procédé pour la fabrication des filaments et fibres en polyacrylonitrile, à section profilée filés à sec | |
| DE1256838B (de) | Verfahren zum Herstellen von Faeden durch Nassverspinnen einer Polyvinylidenfluoridloesung | |
| DE2713456C2 (de) | Verfahren zur Herstellung von hydrophilen Fasern | |
| EP0051203B1 (fr) | Procédé pour la fabrication des filaments et fibres creuses en polyacrylonitrile, filés à sec | |
| DE2736302C3 (de) | Verfahren zur Herstellung von Polypyrrolidonfäden | |
| DE2609829C2 (de) | Verfahren zur Herstellung von hydrophilen Fasern und Fäden aus synthetischen Polymeren | |
| DE2657144C2 (de) | Verfahren zur Herstellung hydrophiler Fasern | |
| EP0069268B1 (fr) | Procédé pour la fabrication de filaments et fibres creuses de polyacrylonitrile par filage au fondu | |
| DE2706522C2 (de) | Hydrophile Acrylfasern mit verbesserter Anfärbbarkeit | |
| DE2607659C2 (de) | Hydrophile Fasern und Fäden aus synthetischen Polymeren | |
| EP0029949B1 (fr) | Procédé de fabrication suivant un procédé de filage à sec de fibres et filaments hygroscopiques, à gaine et âme, ayant une section transversale stable | |
| DE3418943A1 (de) | Verfahren zur herstellung von faeden und fasern aus acrylnitrilpolymerisaten | |
| DE2909785A1 (de) | Verfahren zur herstellung von faeden oder fasern aus acrylnitril enthaltenden polymerisaten mit erhoehtem wasserretentionsvermoegen | |
| DE2804376A1 (de) | Hydrophile hohlfasern | |
| DE1771180C3 (de) | Verfahren zur Herstellung von Reyon-Papier oder Vliesstoffen im Naßverfahren | |
| DE2752821A1 (de) | Hydrophile acrylfasern niedriger dichte | |
| DE2737404A1 (de) | Hydrophile fasern und faeden aus synthetischen polymeren | |
| DE2703545A1 (de) | Bikomposit-acrylfasern und verfahren zu ihrer herstellung | |
| DE2759101A1 (de) | Hochschrumpffaehige, hydrophile acrylfasern | |
| DE1494664B (de) | Verfahren zur Herstellung von kontinuier liehen Faden aus Acrylnitrilpolymensaten durch Naßspinnen |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19811016 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB IT |
|
| ITF | It: translation for a ep patent filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): DE FR GB IT |
|
| REF | Corresponds to: |
Ref document number: 3171719 Country of ref document: DE Date of ref document: 19850912 |
|
| ET | Fr: translation filed | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: HOECHST AKTIENGESELLSCHAFT, FRANKFURT Effective date: 19860506 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19881016 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Effective date: 19891031 |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| 27A | Patent maintained in amended form |
Effective date: 19900704 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): DE FR GB IT |
|
| EN3 | Fr: translation not filed ** decision concerning opposition | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19940913 Year of fee payment: 14 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19960702 |
|
| APAH | Appeal reference modified |
Free format text: ORIGINAL CODE: EPIDOSCREFNO |