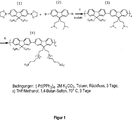
DE112011104427T5 - Organische Halbleiter - Google Patents
Organische Halbleiter Download PDFInfo
- Publication number
- DE112011104427T5 DE112011104427T5 DE112011104427T DE112011104427T DE112011104427T5 DE 112011104427 T5 DE112011104427 T5 DE 112011104427T5 DE 112011104427 T DE112011104427 T DE 112011104427T DE 112011104427 T DE112011104427 T DE 112011104427T DE 112011104427 T5 DE112011104427 T5 DE 112011104427T5
- Authority
- DE
- Germany
- Prior art keywords
- zwitterionic
- polymer
- repeating unit
- opto
- electronic device
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000004065 semiconductor Substances 0.000 title description 8
- 229920000642 polymer Polymers 0.000 claims abstract description 51
- 230000005693 optoelectronics Effects 0.000 claims abstract description 27
- 238000012546 transfer Methods 0.000 claims abstract description 4
- 125000001424 substituent group Chemical group 0.000 claims description 28
- 239000000463 material Substances 0.000 claims description 14
- 239000000178 monomer Substances 0.000 claims description 10
- -1 fluorene radical Chemical class 0.000 claims description 9
- 238000000034 method Methods 0.000 claims description 8
- NIHNNTQXNPWCJQ-UHFFFAOYSA-N o-biphenylenemethane Natural products C1=CC=C2CC3=CC=CC=C3C2=C1 NIHNNTQXNPWCJQ-UHFFFAOYSA-N 0.000 claims description 8
- 238000006116 polymerization reaction Methods 0.000 claims description 8
- 229910052782 aluminium Inorganic materials 0.000 claims description 7
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 7
- 238000002347 injection Methods 0.000 claims description 6
- 239000007924 injection Substances 0.000 claims description 6
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 5
- 229910052799 carbon Inorganic materials 0.000 claims description 5
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 claims description 4
- 125000003342 alkenyl group Chemical group 0.000 claims description 4
- 125000000304 alkynyl group Chemical group 0.000 claims description 4
- 125000003277 amino group Chemical group 0.000 claims description 4
- 239000011575 calcium Substances 0.000 claims description 4
- 229920001940 conductive polymer Polymers 0.000 claims description 4
- 239000002243 precursor Substances 0.000 claims description 4
- 239000000376 reactant Substances 0.000 claims description 4
- 125000001273 sulfonato group Chemical group [O-]S(*)(=O)=O 0.000 claims description 4
- RXACYPFGPNTUNV-UHFFFAOYSA-N 9,9-dioctylfluorene Chemical compound C1=CC=C2C(CCCCCCCC)(CCCCCCCC)C3=CC=CC=C3C2=C1 RXACYPFGPNTUNV-UHFFFAOYSA-N 0.000 claims description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 3
- 229920005603 alternating copolymer Polymers 0.000 claims description 3
- 125000000129 anionic group Chemical group 0.000 claims description 3
- 229910052791 calcium Inorganic materials 0.000 claims description 3
- 125000002091 cationic group Chemical group 0.000 claims description 3
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 230000000379 polymerizing effect Effects 0.000 claims description 2
- 238000006467 substitution reaction Methods 0.000 claims description 2
- 150000003457 sulfones Chemical class 0.000 claims description 2
- 125000001302 tertiary amino group Chemical group 0.000 claims 3
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 claims 1
- 229910052737 gold Inorganic materials 0.000 claims 1
- 239000010931 gold Substances 0.000 claims 1
- 229910052709 silver Inorganic materials 0.000 claims 1
- 239000004332 silver Substances 0.000 claims 1
- 229920000867 polyelectrolyte Polymers 0.000 description 13
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- 230000000052 comparative effect Effects 0.000 description 10
- 150000003512 tertiary amines Chemical group 0.000 description 8
- 239000000243 solution Substances 0.000 description 7
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- 238000010521 absorption reaction Methods 0.000 description 6
- 230000007935 neutral effect Effects 0.000 description 5
- 238000005424 photoluminescence Methods 0.000 description 5
- 230000027756 respiratory electron transport chain Effects 0.000 description 5
- YHEHCZLBPYNMES-UHFFFAOYSA-N 2-[7-(1,3,2-dioxaborolan-2-yl)-9,9-dioctylfluoren-2-yl]-1,3,2-dioxaborolane Chemical compound C1=C2C(CCCCCCCC)(CCCCCCCC)C3=CC(B4OCCO4)=CC=C3C2=CC=C1B1OCCO1 YHEHCZLBPYNMES-UHFFFAOYSA-N 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- 229920000144 PEDOT:PSS Polymers 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 238000004768 lowest unoccupied molecular orbital Methods 0.000 description 2
- 238000004020 luminiscence type Methods 0.000 description 2
- PSBDWGZCVUAZQS-UHFFFAOYSA-N (dimethylsulfonio)acetate Chemical group C[S+](C)CC([O-])=O PSBDWGZCVUAZQS-UHFFFAOYSA-N 0.000 description 1
- XUVUELLZRIIAJG-UHFFFAOYSA-N 2-[2,7-dibromo-9-[2-(dimethylamino)ethyl]fluoren-9-yl]-n,n-dimethylethanamine Chemical compound C1=C(Br)C=C2C(CCN(C)C)(CCN(C)C)C3=CC(Br)=CC=C3C2=C1 XUVUELLZRIIAJG-UHFFFAOYSA-N 0.000 description 1
- XMWRBQBLMFGWIX-UHFFFAOYSA-N C60 fullerene Chemical class C12=C3C(C4=C56)=C7C8=C5C5=C9C%10=C6C6=C4C1=C1C4=C6C6=C%10C%10=C9C9=C%11C5=C8C5=C8C7=C3C3=C7C2=C1C1=C2C4=C6C4=C%10C6=C9C9=C%11C5=C5C8=C3C3=C7C1=C1C2=C4C6=C2C9=C5C3=C12 XMWRBQBLMFGWIX-UHFFFAOYSA-N 0.000 description 1
- 229920000291 Poly(9,9-dioctylfluorene) Polymers 0.000 description 1
- 238000000862 absorption spectrum Methods 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 229920000547 conjugated polymer Polymers 0.000 description 1
- 238000002484 cyclic voltammetry Methods 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000000921 elemental analysis Methods 0.000 description 1
- 230000005669 field effect Effects 0.000 description 1
- 229910003472 fullerene Inorganic materials 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 238000004770 highest occupied molecular orbital Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- MHYFEEDKONKGEB-UHFFFAOYSA-N oxathiane 2,2-dioxide Chemical compound O=S1(=O)CCCCO1 MHYFEEDKONKGEB-UHFFFAOYSA-N 0.000 description 1
- 238000000103 photoluminescence spectrum Methods 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 238000012643 polycondensation polymerization Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 238000004528 spin coating Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K99/00—Subject matter not provided for in other groups of this subclass
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G61/00—Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule
- C08G61/12—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule
- C08G61/122—Macromolecular compounds containing atoms other than carbon in the main chain of the macromolecule derived from five- or six-membered heterocyclic compounds, other than imides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C309/00—Sulfonic acids; Halides, esters, or anhydrides thereof
- C07C309/01—Sulfonic acids
- C07C309/02—Sulfonic acids having sulfo groups bound to acyclic carbon atoms
- C07C309/03—Sulfonic acids having sulfo groups bound to acyclic carbon atoms of an acyclic saturated carbon skeleton
- C07C309/04—Sulfonic acids having sulfo groups bound to acyclic carbon atoms of an acyclic saturated carbon skeleton containing only one sulfo group
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
- H10K85/115—Polyfluorene; Derivatives thereof
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/14—Carrier transporting layers
- H10K50/16—Electron transporting layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/17—Carrier injection layers
- H10K50/171—Electron injection layers
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E10/00—Energy generation through renewable energy sources
- Y02E10/50—Photovoltaic [PV] energy
- Y02E10/549—Organic PV cells
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
Landscapes
- Chemical & Material Sciences (AREA)
- Materials Engineering (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Optics & Photonics (AREA)
- Electroluminescent Light Sources (AREA)
- Polyoxymethylene Polymers And Polymers With Carbon-To-Carbon Bonds (AREA)
- Solid State Image Pick-Up Elements (AREA)
- Photovoltaic Devices (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB1021382.5 | 2010-12-16 | ||
| GB1021382.5A GB2486480A (en) | 2010-12-16 | 2010-12-16 | Organic semiconductor comprising zwitterions |
| PCT/GB2011/052503 WO2012080750A1 (en) | 2010-12-16 | 2011-12-16 | Organic semiconductors |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| DE112011104427T5 true DE112011104427T5 (de) | 2013-09-19 |
Family
ID=43567338
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| DE112011104427T Withdrawn DE112011104427T5 (de) | 2010-12-16 | 2011-12-16 | Organische Halbleiter |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US9755151B2 (enExample) |
| JP (1) | JP6166660B2 (enExample) |
| KR (1) | KR101882538B1 (enExample) |
| CN (1) | CN103262191B (enExample) |
| DE (1) | DE112011104427T5 (enExample) |
| GB (2) | GB2486480A (enExample) |
| WO (1) | WO2012080750A1 (enExample) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2513378A (en) * | 2013-04-25 | 2014-10-29 | Cambridge Display Tech Ltd | Device |
| JP6403108B2 (ja) * | 2014-03-10 | 2018-10-10 | Jxtgエネルギー株式会社 | 有機エレクトロルミネッセンス素子及び該素子を有する発光装置 |
| CN103951813B (zh) * | 2014-03-31 | 2016-08-17 | 南京邮电大学 | 9-芳基取代芴基共轭聚电解质及其制备方法和应用 |
| CN104250364B (zh) * | 2014-08-22 | 2017-08-18 | 武汉工程大学 | 一种两性离子胺基聚芴衍生物及其合成方法和应用 |
| CN105237745A (zh) * | 2015-10-13 | 2016-01-13 | 华南理工大学 | 含季膦盐基团共轭聚电解质及其在有机光电器件中的应用 |
| US11258017B2 (en) | 2016-04-27 | 2022-02-22 | Wuhan Xinqu Chuangrou Optoelectronics Technology Co., Ltd | Semiconducting compositions comprising semiconducting polymers |
| CN106986982A (zh) * | 2017-04-12 | 2017-07-28 | 华南理工大学 | 三键连接的水醇溶共轭聚合物及其在有机光电器件中的应用 |
| CN108559064B (zh) * | 2018-03-13 | 2020-09-29 | 南京邮电大学 | 共轭主链掺杂的两性离子型聚芴乙烯撑及其制备与应用 |
| GB2590920B (en) * | 2020-01-06 | 2024-07-17 | Sumitomo Chemical Co | Light-emitting particles |
| CN113140677B (zh) * | 2020-01-20 | 2025-09-19 | 纳晶科技股份有限公司 | 一种光电器件及其制备方法 |
| CN111564559B (zh) * | 2020-04-09 | 2022-11-08 | 宁波材料所杭州湾研究院 | 电子传输层及其制备方法、钙钛矿电池结构以及太阳能电池 |
| CN112646129B (zh) * | 2020-12-03 | 2023-06-23 | 华南理工大学 | 含苯并双噻二唑的n型水/醇溶共轭聚电解质及其制备与应用 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5641859A (en) * | 1995-07-12 | 1997-06-24 | National Science Council Of Taiwan | Water-soluble self-acid-doped polyaniline, method of preparation thereof, and polymer blends made therefrom |
| JP2001076877A (ja) * | 1999-09-01 | 2001-03-23 | Nippon Telegr & Teleph Corp <Ntt> | 有機エレクトロルミネッセンス素子およびその駆動方法 |
| US6611096B1 (en) | 1999-09-03 | 2003-08-26 | 3M Innovative Properties Company | Organic electronic devices having conducting self-doped polymer buffer layers |
| US6916902B2 (en) * | 2002-12-19 | 2005-07-12 | Dow Global Technologies Inc. | Tricyclic arylamine containing polymers and electronic devices therefrom |
| TWI267545B (en) * | 2003-06-17 | 2006-12-01 | Univ Tsinghua | Electroluminescent conjugated polymers containing phosphorescent moieties and the application thereof in LED |
| JP5909314B2 (ja) * | 2004-09-03 | 2016-04-26 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | 可溶性共役ポリマーを使用する方法及びデバイス |
| GB0507684D0 (en) * | 2005-04-15 | 2005-05-25 | Cambridge Display Tech Ltd | Pulsed driven displays |
| JP4758684B2 (ja) * | 2005-06-14 | 2011-08-31 | セイコーエプソン株式会社 | 光電変換素子および電子機器 |
| JP5540600B2 (ja) * | 2008-08-13 | 2014-07-02 | 三菱化学株式会社 | 電子デバイス、有機電界発光素子、有機el表示装置および有機el照明 |
| GB0907445D0 (en) * | 2009-04-30 | 2009-06-10 | Cambridge Entpr Ltd | Photoresponsive devices |
-
2010
- 2010-12-16 GB GB1021382.5A patent/GB2486480A/en not_active Withdrawn
-
2011
- 2011-12-16 GB GB1309681.3A patent/GB2499350B/en not_active Expired - Fee Related
- 2011-12-16 KR KR1020137018499A patent/KR101882538B1/ko not_active Expired - Fee Related
- 2011-12-16 WO PCT/GB2011/052503 patent/WO2012080750A1/en not_active Ceased
- 2011-12-16 US US13/995,157 patent/US9755151B2/en not_active Expired - Fee Related
- 2011-12-16 JP JP2013543885A patent/JP6166660B2/ja not_active Expired - Fee Related
- 2011-12-16 CN CN201180060055.6A patent/CN103262191B/zh not_active Expired - Fee Related
- 2011-12-16 DE DE112011104427T patent/DE112011104427T5/de not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| CN103262191B (zh) | 2016-10-05 |
| KR20140019778A (ko) | 2014-02-17 |
| US9755151B2 (en) | 2017-09-05 |
| GB2499350A (en) | 2013-08-14 |
| GB2499350B (en) | 2018-05-23 |
| KR101882538B1 (ko) | 2018-07-26 |
| WO2012080750A1 (en) | 2012-06-21 |
| US20130313544A1 (en) | 2013-11-28 |
| JP2014507782A (ja) | 2014-03-27 |
| JP6166660B2 (ja) | 2017-07-19 |
| GB2486480A (en) | 2012-06-20 |
| CN103262191A (zh) | 2013-08-21 |
| GB201021382D0 (en) | 2011-01-26 |
| GB201309681D0 (en) | 2013-07-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE112011104427T5 (de) | Organische Halbleiter | |
| DE69529512T2 (de) | Elektrolumineszente Vorrichtung mit einer Poly-3,4-Ethylen-Dioxythiophen-Schicht | |
| EP1564251B1 (de) | Polythiophenformulierungen zur Verbesserung von organischen Leuchtdioden | |
| DE69625895T2 (de) | Bipolare elektrolumineszierende vorrichtung | |
| EP1798785B1 (de) | Transparente polymere Elektrode für elektro-optische Aufbauten | |
| EP1668058B1 (de) | Konjugierte polymere, deren darstellung und verwendung | |
| DE112009000486T5 (de) | Lösungsmittel für eine Druckzusammensetzung | |
| WO2002010129A2 (de) | Strukturierbare materialien, verfahren zu deren herstellung und deren verwendung | |
| DE102007053251A1 (de) | Leitendes Copolymer, leitende Copolymerzusammensetzung, Film und optoelektronische Vorrichtung unter Verwendung derselben | |
| DE112005002495T5 (de) | Polares halbleitendes Lochtransportmaterial | |
| EP1984964A1 (de) | Elektronisches bauteil, verfahren zu dessen herstellung und dessen verwendung | |
| DE102013101712B4 (de) | Photoaktives organisches Material für optoelektronische Bauelemente | |
| WO2009053088A1 (de) | Optoelektronische vorrichtung | |
| EP2659529B2 (de) | Optoelektronisches bauelement mit dotierten schichten | |
| DE102010012180A1 (de) | Sulfonierte Polyketone als Gegenion leitfähiger Polymere | |
| DE60127568T2 (de) | Lichtemittierende polymermischungen und daraus hergestellte lichtemittierende anordnungen | |
| DE60032744T2 (de) | Aromatische aminoderivate, löslich leitfähige zusammensetzung, und elektroluminescente vorrichtung | |
| DE112006003401T5 (de) | Kationische Zusammensetzungen von elektrisch leitenden Polymeren, dotiert mit vollfluorierten Säurepolymeren | |
| DE102004010811B4 (de) | Polythiophenformulierungen zur Verbesserung von organischen Leuchtdioden | |
| WO2008119338A1 (de) | Organische strahlungsemittierende vorrichtung, deren verwendung sowie ein herstellungsverfahren für die vorrichtung | |
| DE102013206586A1 (de) | Halbleitendes Copolymer sowie Verfahren zu dessen Herstellung, Stoffgemisch, elektrisches oder elektronisches Bauelement sowie Verfahren zu dessen Herstellung | |
| DE102020131756A1 (de) | Schichtsystem für ein organisches elektronisches Bauelement | |
| DE102009031677A1 (de) | Neue Polyelektrolyt-Komplexe und deren Verwendung | |
| DE10349029B4 (de) | Verwendung von Poly(alkoxythiophen) als Funktionsschicht in elektrischen Bauelementen | |
| DE112005003230B4 (de) | Optische Vorrichtungen und ihre Herstellung |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| R005 | Application deemed withdrawn due to failure to request examination |