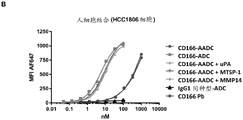
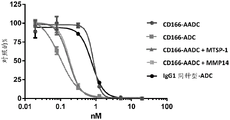
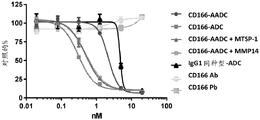
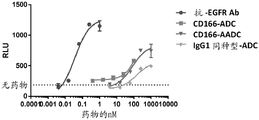
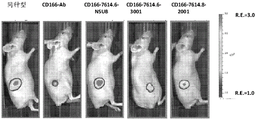
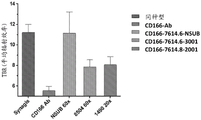
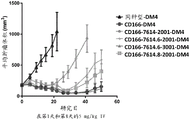
CN107849133B - 抗cd166抗体、可活化抗cd166抗体及其使用方法 - Google Patents
抗cd166抗体、可活化抗cd166抗体及其使用方法 Download PDFInfo
- Publication number
- CN107849133B CN107849133B CN201680038634.3A CN201680038634A CN107849133B CN 107849133 B CN107849133 B CN 107849133B CN 201680038634 A CN201680038634 A CN 201680038634A CN 107849133 B CN107849133 B CN 107849133B
- Authority
- CN
- China
- Prior art keywords
- antibody
- seq
- amino acid
- acid sequence
- conjugated
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/537—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines spiro-condensed or forming part of bridged ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/68033—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a maytansine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6849—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a receptor, a cell surface antigen or a cell surface determinant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6873—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting an immunoglobulin; the antibody being an anti-idiotypic antibody
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6891—Pre-targeting systems involving an antibody for targeting specific cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/50—Fusion polypeptide containing protease site
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/55—Fusion polypeptide containing a fusion with a toxin, e.g. diphteria toxin
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Oncology (AREA)
- Hematology (AREA)
- Cell Biology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202210950237.0A CN115340607A (zh) | 2015-05-04 | 2016-05-04 | 抗cd166抗体、可活化抗cd166抗体及其使用方法 |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562156835P | 2015-05-04 | 2015-05-04 | |
| US62/156835 | 2015-05-04 | ||
| US201562220805P | 2015-09-18 | 2015-09-18 | |
| US62/220805 | 2015-09-18 | ||
| PCT/US2016/030785 WO2016179285A1 (en) | 2015-05-04 | 2016-05-04 | Anti-cd166 antibodies, activatable anti-cd166 antibodies, and methods of use thereof |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210950237.0A Division CN115340607A (zh) | 2015-05-04 | 2016-05-04 | 抗cd166抗体、可活化抗cd166抗体及其使用方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN107849133A CN107849133A (zh) | 2018-03-27 |
| CN107849133B true CN107849133B (zh) | 2022-08-23 |
Family
ID=55967468
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201680038634.3A Expired - Fee Related CN107849133B (zh) | 2015-05-04 | 2016-05-04 | 抗cd166抗体、可活化抗cd166抗体及其使用方法 |
| CN202210950237.0A Pending CN115340607A (zh) | 2015-05-04 | 2016-05-04 | 抗cd166抗体、可活化抗cd166抗体及其使用方法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202210950237.0A Pending CN115340607A (zh) | 2015-05-04 | 2016-05-04 | 抗cd166抗体、可活化抗cd166抗体及其使用方法 |
Country Status (20)
| Country | Link |
|---|---|
| US (3) | US10745481B2 (enExample) |
| EP (2) | EP3708585A1 (enExample) |
| JP (2) | JP7028648B2 (enExample) |
| KR (1) | KR20180010203A (enExample) |
| CN (2) | CN107849133B (enExample) |
| AU (1) | AU2016257929B2 (enExample) |
| BR (1) | BR112017023872A2 (enExample) |
| CA (1) | CA2984948A1 (enExample) |
| DK (1) | DK3292150T3 (enExample) |
| EA (1) | EA201792414A1 (enExample) |
| ES (1) | ES2796698T3 (enExample) |
| HR (1) | HRP20200721T1 (enExample) |
| HU (1) | HUE049866T2 (enExample) |
| IL (2) | IL292798A (enExample) |
| MX (2) | MX388350B (enExample) |
| PL (1) | PL3292150T3 (enExample) |
| PT (1) | PT3292150T (enExample) |
| RS (1) | RS60222B1 (enExample) |
| SG (2) | SG10201913767VA (enExample) |
| WO (1) | WO2016179285A1 (enExample) |
Families Citing this family (77)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9782075B2 (en) | 2013-03-15 | 2017-10-10 | I2Dx, Inc. | Electronic delivery of information in personalized medicine |
| US11089959B2 (en) | 2013-03-15 | 2021-08-17 | I2Dx, Inc. | Electronic delivery of information in personalized medicine |
| RU2727836C2 (ru) * | 2013-07-25 | 2020-07-24 | Сайтомкс Терапьютикс, Инк. | Мультспецифические антитела, мультспецифические активируемые антитела и способы их применения |
| CN118146306A (zh) | 2013-09-25 | 2024-06-07 | 西托姆克斯治疗公司 | 基质金属蛋白酶底物和其它可切割部分及其使用方法 |
| JP6835586B2 (ja) | 2014-01-31 | 2021-02-24 | シトムクス セラピューティクス,インコーポレイティド | マトリプターゼ及びu‐プラスミノーゲン活性化因子の基質及び他の切断可能部分、並びにその使用方法 |
| MA41374A (fr) | 2015-01-20 | 2017-11-28 | Cytomx Therapeutics Inc | Substrats clivables par métalloprotéase matricielle et clivables par sérine protéase et procédés d'utilisation de ceux-ci |
| CN107849133B (zh) | 2015-05-04 | 2022-08-23 | 西托姆克斯治疗公司 | 抗cd166抗体、可活化抗cd166抗体及其使用方法 |
| US12128102B2 (en) | 2016-03-08 | 2024-10-29 | Takeda Pharmaceutical Company Limited | Constrained conditionally activated binding proteins |
| CA3029902A1 (en) | 2016-07-07 | 2018-01-11 | The Board Of Trustees Of The Leland Stanford Junior University | Antibody adjuvant conjugates |
| TWI797097B (zh) | 2016-11-28 | 2023-04-01 | 日商中外製藥股份有限公司 | 包含抗原結合域與運送部分的多胜肽 |
| KR20230073346A (ko) | 2016-11-28 | 2023-05-25 | 추가이 세이야쿠 가부시키가이샤 | 리간드 결합 활성을 조정 가능한 리간드 결합 분자 |
| CN110036034A (zh) | 2016-12-09 | 2019-07-19 | 西雅图遗传学公司 | 卷曲螺旋掩蔽的二价抗体 |
| BR112020000766A2 (pt) * | 2017-07-14 | 2020-07-21 | Cytomx Therapeutics, Inc. | anticorpos anti-cd166 e seus usos |
| JP7368856B2 (ja) | 2017-07-25 | 2023-10-25 | トゥルーバインディング,インコーポレイテッド | Tim-3とそのリガンドとの相互作用の遮断によるがん治療 |
| WO2019036433A2 (en) | 2017-08-16 | 2019-02-21 | Bristol-Myers Squibb Company | ANTIBODIES IN THE FORM OF PRODRUGS, PRODRUGS BASED THEREON, THEIR METHODS OF USE AND PREPARATION |
| CN111511762B (zh) | 2017-08-21 | 2025-05-06 | 天演药业公司 | 抗cd137分子及其用途 |
| US20190117789A1 (en) | 2017-08-30 | 2019-04-25 | Cytomx Therapeutics, Inc. | Activatable anti-cd166 antibodies and methods of use thereof |
| IL302613B2 (en) | 2017-09-08 | 2025-04-01 | Maverick Therapeutics Inc | Binding proteins are activated under limited conditions |
| EP3679068A2 (en) * | 2017-09-08 | 2020-07-15 | Maverick Therapeutics, Inc. | CONDITIONALLY ACTIVATED BINDING MOIETIES CONTAINING Fc REGIONS |
| EP3719035A4 (en) | 2017-11-28 | 2021-09-01 | Chugai Seiyaku Kabushiki Kaisha | POLYPEPTIDE INCLUDING AN ANTIGEN BINDING DOMAIN AND A TRANSPORT SECTION |
| CN111630062A (zh) | 2017-11-28 | 2020-09-04 | 中外制药株式会社 | 具有可调节的配体结合活性的配体结合分子 |
| WO2019148444A1 (en) | 2018-02-02 | 2019-08-08 | Adagene Inc. | Anti-ctla4 antibodies and methods of making and using the same |
| WO2019148445A1 (en) * | 2018-02-02 | 2019-08-08 | Adagene Inc. | Precision/context-dependent activatable antibodies, and methods of making and using the same |
| US20210020264A1 (en) | 2018-03-20 | 2021-01-21 | Cytomx Therapeutics, Inc. | Systems and methods for quantitative pharmacological modeling of activatable antibody species in mammalian subjects |
| FI3794024T3 (fi) | 2018-05-14 | 2023-08-10 | Werewolf Therapeutics Inc | Aktivoitavia interleukiini-2-polypeptidejä ja niiden käyttömenetelmiä |
| JP7497340B2 (ja) | 2018-05-14 | 2024-06-10 | ウェアウルフ セラピューティクス, インコーポレイテッド | 活性化可能なインターロイキン12ポリペプチド及びその使用方法 |
| EP3816182A4 (en) | 2018-05-30 | 2022-07-13 | Chugai Seiyaku Kabushiki Kaisha | LIGAND BINDING MOLECULE WITH SINGLE DOMAIN ANTIBODY |
| US12331320B2 (en) | 2018-10-10 | 2025-06-17 | The Research Foundation For The State University Of New York | Genome edited cancer cell vaccines |
| TWI897855B (zh) | 2018-10-26 | 2025-09-21 | 美商免疫遺傳股份有限公司 | E p C A M 抗體、可活化抗體及免疫偶聯物以及其用途 |
| CN113260383A (zh) * | 2018-11-02 | 2021-08-13 | 西托姆克斯治疗公司 | 可活化的抗cd166抗体及其使用方法 |
| AU2019394972A1 (en) | 2018-12-06 | 2021-06-03 | Cytomx Therapeutics, Inc. | Matrix metalloprotease-cleavable and serine or cysteine protease-cleavable substrates and methods of use thereof |
| WO2020180398A1 (en) * | 2019-01-14 | 2020-09-10 | The Regents Of The University Of California | Compositions and methods for modulating cellular internalization |
| CN116063505B (zh) | 2019-01-30 | 2024-05-03 | 真和制药有限公司 | 抗gal3抗体及其用途 |
| US20220233705A1 (en) * | 2019-02-26 | 2022-07-28 | Cytomx Therapeutics, Inc. | Combined therapies of activatable immune checkpoint inhibitors and conjugated activatable antibodies |
| US11685780B2 (en) | 2019-03-05 | 2023-06-27 | Takeda Pharmaceutical Company Limited | Single domain antigen binding domains that bind human Trop2 |
| JP2022525594A (ja) | 2019-03-15 | 2022-05-18 | ボルト バイオセラピューティクス、インコーポレーテッド | Her2を標的とする免疫結合体 |
| CN113631194A (zh) | 2019-03-21 | 2021-11-09 | 伊缪诺金公司 | 制备细胞结合剂-药物缀合物的方法 |
| KR102212149B1 (ko) * | 2019-03-28 | 2021-02-04 | 부산대학교 산학협력단 | 암 줄기세포에 특이적으로 결합하는 압타머 및 이의 용도 |
| HRP20231619T1 (hr) | 2019-04-26 | 2024-04-26 | Immunogen, Inc. | Derivati kamptotecina |
| EP3969035A4 (en) | 2019-05-14 | 2023-06-21 | Werewolf Therapeutics, Inc. | SEPARATION UNITS AND METHODS AND THEIR USE |
| WO2020236679A1 (en) * | 2019-05-17 | 2020-11-26 | Cytomx Therapeutics, Inc. | Methods and compositions for determining the biodistribution of activatable anti-cd166 antibody conjugates |
| KR20220017430A (ko) | 2019-06-05 | 2022-02-11 | 추가이 세이야쿠 가부시키가이샤 | 항체 절단 부위 결합 분자 |
| US20220251206A1 (en) | 2019-06-11 | 2022-08-11 | Bristol-Myers Squibb Company | Anti-ctla4 antibody prodruggable (probody) at a cdr position |
| WO2021061867A1 (en) * | 2019-09-23 | 2021-04-01 | Cytomx Therapeutics, Inc. | Anti-cd47 antibodies, activatable anti-cd47 antibodies, and methods of use thereof |
| MX2022005666A (es) | 2019-11-14 | 2022-10-07 | Werewolf Therapeutics Inc | Polipeptidos de citocina activables y metodos de uso de los mismos. |
| WO2021207657A1 (en) | 2020-04-09 | 2021-10-14 | Cytomx Therapeutics, Inc. | Compositions containing activatable antibodies |
| US12281166B2 (en) | 2020-05-26 | 2025-04-22 | Truebinding, Inc. | Methods of treating inflammatory diseases by blocking Galectin-3 |
| US20240000960A1 (en) * | 2020-11-16 | 2024-01-04 | The Cleveland Clinic Foundation | Anti-cd6 antibody conjugates for treating t-cell mediated disorders and t-cell lymphoma/leukemia |
| MX2023009100A (es) | 2021-02-03 | 2023-09-25 | Mozart Therapeutics Inc | Agentes aglutinantes y métodos para usar los mismos. |
| WO2023064929A1 (en) | 2021-10-15 | 2023-04-20 | Cytomx Therapeutics, Inc. | Activatable polypeptide complex |
| WO2023076876A1 (en) | 2021-10-26 | 2023-05-04 | Mozart Therapeutics, Inc. | Modulation of immune responses to viral vectors |
| WO2023086897A1 (en) * | 2021-11-12 | 2023-05-19 | University Of Florida Research Foundation, Incorporated | Siglec-6 antibodies, derivative compounds and related uses |
| CN114621348B (zh) * | 2022-01-24 | 2023-09-22 | 深圳市乐土生物医药有限公司 | 一种多肽及其抗磷脂酰肌醇蛋白聚糖3抗体 |
| EP4496816A1 (en) | 2022-03-23 | 2025-01-29 | CytomX Therapeutics, Inc. | Activatable antigen-binding protein constructs and uses of the same |
| WO2023183923A1 (en) | 2022-03-25 | 2023-09-28 | Cytomx Therapeutics, Inc. | Activatable dual-anchored masked molecules and methods of use thereof |
| CN119300859A (zh) | 2022-04-01 | 2025-01-10 | 西托姆克斯治疗公司 | 可激活多特异性分子和其使用方法 |
| CN119278215A (zh) | 2022-04-01 | 2025-01-07 | 西托姆克斯治疗公司 | Cd3结合蛋白和其使用方法 |
| KR20250011922A (ko) | 2022-05-16 | 2025-01-22 | 비온디스 비.브이. | 신규한 차폐된 항체 |
| AU2023307948A1 (en) | 2022-07-12 | 2025-01-09 | Cytomx Therapeutics, Inc. | Epcam immunoconjugates and uses thereof |
| AR130080A1 (es) | 2022-08-01 | 2024-10-30 | Cytomx Therapeutics Inc | Restos escindibles por proteasas, y métodos de uso de los mismos |
| JP2025525754A (ja) | 2022-08-01 | 2025-08-07 | サイトムエックス セラピューティクス,インク. | プロテアーゼ切断性基質及びその使用方法 |
| JP2025525867A (ja) | 2022-08-01 | 2025-08-07 | サイトムエックス セラピューティクス,インク. | プロテアーゼ切断性部分及びその使用方法 |
| WO2024030847A1 (en) | 2022-08-01 | 2024-02-08 | Cytomx Therapeutics, Inc. | Protease-cleavable moieties and methods of use thereof |
| TW202426637A (zh) | 2022-08-01 | 2024-07-01 | 美商Cytomx生物製藥公司 | 蛋白酶可切割受質及其使用方法 |
| WO2024040194A1 (en) | 2022-08-17 | 2024-02-22 | Capstan Therapeutics, Inc. | Conditioning for in vivo immune cell engineering |
| CN120936384A (zh) | 2023-04-12 | 2025-11-11 | 西托姆克斯治疗公司 | 掩蔽多肽、可活化细胞因子构建体以及相关组合物和方法 |
| WO2024216170A2 (en) | 2023-04-12 | 2024-10-17 | Cytomx Therapeutics, Inc. | Activatable cytokine constructs and related compositions and methods |
| CN120958015A (zh) | 2023-04-12 | 2025-11-14 | 西托姆克斯治疗公司 | 掩蔽多肽、可活化细胞因子构建体以及相关组合物和方法 |
| WO2024249954A1 (en) | 2023-05-31 | 2024-12-05 | Capstan Therapeutics, Inc. | Lipid nanoparticle formulations and compositions |
| WO2025047993A1 (en) * | 2023-08-28 | 2025-03-06 | Pharosgen Co., Ltd. | Anti-doppel antibody drug conjugates |
| WO2025076127A1 (en) | 2023-10-05 | 2025-04-10 | Capstan Therapeutics, Inc. | Constrained ionizable cationic lipids and lipid nanoparticles |
| WO2025076113A1 (en) | 2023-10-05 | 2025-04-10 | Capstan Therapeutics, Inc. | Ionizable cationic lipids with conserved spacing and lipid nanoparticles |
| WO2025149667A1 (en) | 2024-01-12 | 2025-07-17 | Pheon Therapeutics Ltd | Antibody drug conjugates and uses thereof |
| US20250302763A1 (en) | 2024-02-22 | 2025-10-02 | Capstan Therapeutics, Inc. | Immune engineering amplification |
| WO2025217452A1 (en) | 2024-04-11 | 2025-10-16 | Capstan Therapeutics, Inc. | Constrained ionizable cationic lipids and lipid nanoparticles |
| WO2025217454A2 (en) | 2024-04-11 | 2025-10-16 | Capstan Therapeutics, Inc. | Ionizable cationic lipids and lipid nanoparticles |
| WO2025240659A2 (en) | 2024-05-14 | 2025-11-20 | Cytomx Therapeutics, Inc. | Activatable constructs, compositions and methods |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009025846A3 (en) * | 2007-08-22 | 2009-05-14 | Univ California | Activatable binding polypeptides and methods of identification and use thereof |
| CN102482347A (zh) * | 2009-01-12 | 2012-05-30 | 希托马克斯医疗有限责任公司 | 修饰抗体组合物及其制备和使用方法 |
| CN104540518A (zh) * | 2012-04-27 | 2015-04-22 | 西托姆克斯治疗公司 | 结合表皮生长因子受体的可活化的抗体其使用方法 |
Family Cites Families (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3773919A (en) | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| US4485045A (en) | 1981-07-06 | 1984-11-27 | Research Corporation | Synthetic phosphatidyl cholines useful in forming liposomes |
| US4522811A (en) | 1982-07-08 | 1985-06-11 | Syntex (U.S.A.) Inc. | Serial injection of muramyldipeptides and liposomes enhances the anti-infective activity of muramyldipeptides |
| US4544545A (en) | 1983-06-20 | 1985-10-01 | Trustees University Of Massachusetts | Liposomes containing modified cholesterol for organ targeting |
| DE3788058T2 (de) | 1986-08-28 | 1994-04-21 | Teijin Ltd | Zelltoxischer antikörperkomplex und verfahren zu seiner herstellung. |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5013556A (en) | 1989-10-20 | 1991-05-07 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5151510A (en) | 1990-04-20 | 1992-09-29 | Applied Biosystems, Inc. | Method of synethesizing sulfurized oligonucleotide analogs |
| EP1005870B1 (en) | 1992-11-13 | 2009-01-21 | Biogen Idec Inc. | Therapeutic application of chimeric antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma |
| US5998172A (en) | 1993-11-02 | 1999-12-07 | Duke University | Anti-CD6 ligand antibodies |
| AU9127098A (en) | 1997-09-04 | 1999-03-22 | Osiris Therapeutics, Inc. | Ligands that modulate differentiation of mesenchymal stem cells |
| US6302855B1 (en) | 1998-05-20 | 2001-10-16 | Novo Nordisk A/S | Medical apparatus for use by a patient for medical self treatment of diabetes |
| WO2001091798A2 (en) | 2000-06-01 | 2001-12-06 | Universite Catholique De Louvain | Tumor activated prodrug compounds |
| JP4334866B2 (ja) | 2000-10-09 | 2009-09-30 | アイシス イノヴェイション リミテッド | 治療用抗体 |
| US7465790B2 (en) | 2000-10-09 | 2008-12-16 | Isis Innovation, Inc. | Therapeutic antibodies |
| EP1501544A4 (en) | 2002-05-03 | 2006-06-21 | Raven Biotechnologies Inc | ALCAM AND ALCAM MODULATORS |
| US20040109855A1 (en) | 2002-07-23 | 2004-06-10 | Herman Waldmann | Therapeutic antibodies with reduced side effect |
| WO2004021861A2 (en) | 2002-09-03 | 2004-03-18 | Vit Lauermann | Targeted release |
| MY144612A (en) | 2003-12-22 | 2011-10-14 | Glaxo Group Ltd | Nogo-a neutralising immunoglobulins for treatment of neurological diseases |
| WO2007026689A1 (ja) | 2005-08-29 | 2007-03-08 | Japan Science And Technology Agency | ダチョウを用いた抗体、及びその作製方法 |
| EP3896076A1 (en) | 2005-08-31 | 2021-10-20 | The Regents of the University of California | Cellular libraries of peptide sequences (clips) and methods of using the same |
| US7582441B1 (en) | 2005-10-17 | 2009-09-01 | Celera Corporation | Methods and compositions for treating and diagnosing disease |
| JP5612247B2 (ja) | 2005-11-29 | 2014-10-22 | 独立行政法人科学技術振興機構 | Cd166に対するモノクローナル抗体およびその産生方法 |
| CA2645347A1 (en) | 2006-03-10 | 2007-09-20 | Diatos | Anticancer drugs conjugated to antibody via an enzyme cleavable linker |
| WO2008007648A1 (fr) | 2006-07-10 | 2008-01-17 | Institute For Antibodies Co., Ltd. | Procédé de classification d'antigène, procédé d'identification d'antigène, procédé d'obtention d' un ensemble d'antigènes ou d'anticorps, procédés de construction d'un panel d'anticorps, anticorps et ens |
| GB0705775D0 (en) | 2007-03-26 | 2007-05-02 | Affitech As | Product |
| JP2009055899A (ja) | 2007-08-07 | 2009-03-19 | Igaku Seibutsugaku Kenkyusho:Kk | Adcc活性を増強させた抗体及びその製造方法 |
| EP2192922A4 (en) | 2007-09-17 | 2010-09-29 | Univ California | INTERNATIONALIZING HUMAN MONOCLONAL ANTIBODIES TO PROMOTE IN SITU ON PROSTATAZELLES |
| RU2010138612A (ru) | 2008-02-20 | 2012-03-27 | Джи2 ИНФЛЕММЕЙШН ПТИ ЛТД (AU) | ГУМАНИЗИРОВАННЫЕ АНТИТЕЛА ПРОТИВ C5аR |
| BRPI0911442A2 (pt) | 2008-04-30 | 2019-03-12 | Immunogen, Inc. | conjugados potentes e ligantes hidrofílicos |
| EP2388320B1 (en) | 2008-12-22 | 2017-02-15 | Chugai Seiyaku Kabushiki Kaisha | Anti-hs6st2 antibodies and uses thereof |
| US9090679B2 (en) | 2009-04-17 | 2015-07-28 | Immunas Pharma, Inc. | Antibodies that specifically bind to A beta oligomers and use thereof |
| US9309325B2 (en) | 2009-05-07 | 2016-04-12 | The Regents Of The University Of California | Antibodies and methods of use thereof |
| CN103269712B (zh) | 2010-11-03 | 2016-09-21 | 伊缪诺金公司 | 包含新型安丝菌素衍生物的细胞毒性剂 |
| US20140199233A1 (en) * | 2011-05-11 | 2014-07-17 | The Regents Of The University Of California | Enhanced Growth Inhibition of Osteosarcoma by Cytotoxic Polymerized Liposomal Nanoparticles Targeting the Alcam Cell Surface Receptor |
| NZ628804A (en) * | 2012-02-24 | 2017-08-25 | Abbvie Stemcentrx Llc | Dll3 modulators and methods of use |
| WO2013192546A1 (en) | 2012-06-22 | 2013-12-27 | Cytomx Therapeutics, Inc. | Activatable antibodies having non-binding steric moieties and mehtods of using the same |
| WO2014026136A2 (en) | 2012-08-10 | 2014-02-13 | Cytomx Therapeutics, Inc. | Protease-resistant systems for polypeptide display and methods of making and using thereof |
| CA2894689A1 (en) | 2012-12-19 | 2014-06-26 | Amplimmune, Inc. | Anti-human b7-h4 antibodies and their uses |
| HK1210831A1 (en) | 2013-01-04 | 2016-05-06 | Cytomx Therapeutics Inc. | Compositions and methods for detecting protease activity in biological systems |
| WO2014134483A2 (en) | 2013-02-28 | 2014-09-04 | Immunogen, Inc. | Conjugates comprising cell-binding agents and cytotoxic agents |
| KR20160018579A (ko) * | 2013-06-04 | 2016-02-17 | 싸이톰스 테라퓨틱스, 인크. | 활성화가능 항체를 접합하기 위한 조성물 및 방법 |
| RU2727836C2 (ru) * | 2013-07-25 | 2020-07-24 | Сайтомкс Терапьютикс, Инк. | Мультспецифические антитела, мультспецифические активируемые антитела и способы их применения |
| CN107849133B (zh) | 2015-05-04 | 2022-08-23 | 西托姆克斯治疗公司 | 抗cd166抗体、可活化抗cd166抗体及其使用方法 |
| EP3325006A4 (en) | 2015-07-17 | 2019-03-06 | The Trustees of Columbia University in the City of New York | METHOD FOR TREATING CD166-EXPRESSIVE CANCER |
| US10858628B2 (en) | 2015-11-04 | 2020-12-08 | Fate Therapeutics, Inc. | Methods and compositions for inducing hematopoietic cell differentiation |
| CN109311992B (zh) | 2016-06-15 | 2022-10-18 | 克利夫兰临床基金会 | 用于治疗t细胞介导疾病的新型抗cd6抗体 |
| JP6183523B2 (ja) | 2016-09-14 | 2017-08-23 | 株式会社アンセイ | 車両用開閉体の開閉装置及び車両用開閉体の開閉装置用リンク機構 |
| WO2018067991A1 (en) | 2016-10-07 | 2018-04-12 | The Brigham And Women's Hospital, Inc. | Modulation of novel immune checkpoint targets |
| WO2018071058A1 (en) | 2016-10-15 | 2018-04-19 | Baylor College Of Medicine | Platform for enhanced targeted cell delivery |
| KR20200016899A (ko) | 2017-06-01 | 2020-02-17 | 싸이톰스 테라퓨틱스, 인크. | 활성화가능 항-pdl1 항체, 및 이의 이용 방법 |
| BR112020000766A2 (pt) | 2017-07-14 | 2020-07-21 | Cytomx Therapeutics, Inc. | anticorpos anti-cd166 e seus usos |
| US20190117789A1 (en) | 2017-08-30 | 2019-04-25 | Cytomx Therapeutics, Inc. | Activatable anti-cd166 antibodies and methods of use thereof |
| CN113260383A (zh) | 2018-11-02 | 2021-08-13 | 西托姆克斯治疗公司 | 可活化的抗cd166抗体及其使用方法 |
| US20220233705A1 (en) | 2019-02-26 | 2022-07-28 | Cytomx Therapeutics, Inc. | Combined therapies of activatable immune checkpoint inhibitors and conjugated activatable antibodies |
-
2016
- 2016-05-04 CN CN201680038634.3A patent/CN107849133B/zh not_active Expired - Fee Related
- 2016-05-04 EP EP20155149.6A patent/EP3708585A1/en not_active Withdrawn
- 2016-05-04 EP EP16722502.8A patent/EP3292150B1/en active Active
- 2016-05-04 ES ES16722502T patent/ES2796698T3/es active Active
- 2016-05-04 WO PCT/US2016/030785 patent/WO2016179285A1/en not_active Ceased
- 2016-05-04 HR HRP20200721TT patent/HRP20200721T1/hr unknown
- 2016-05-04 MX MX2017014136A patent/MX388350B/es unknown
- 2016-05-04 RS RS20200511A patent/RS60222B1/sr unknown
- 2016-05-04 JP JP2017557287A patent/JP7028648B2/ja not_active Expired - Fee Related
- 2016-05-04 EA EA201792414A patent/EA201792414A1/ru unknown
- 2016-05-04 US US15/146,603 patent/US10745481B2/en active Active
- 2016-05-04 IL IL292798A patent/IL292798A/en unknown
- 2016-05-04 HU HUE16722502A patent/HUE049866T2/hu unknown
- 2016-05-04 KR KR1020177034835A patent/KR20180010203A/ko not_active Abandoned
- 2016-05-04 SG SG10201913767VA patent/SG10201913767VA/en unknown
- 2016-05-04 BR BR112017023872A patent/BR112017023872A2/pt not_active IP Right Cessation
- 2016-05-04 CA CA2984948A patent/CA2984948A1/en active Pending
- 2016-05-04 DK DK16722502.8T patent/DK3292150T3/da active
- 2016-05-04 AU AU2016257929A patent/AU2016257929B2/en not_active Expired - Fee Related
- 2016-05-04 CN CN202210950237.0A patent/CN115340607A/zh active Pending
- 2016-05-04 PT PT167225028T patent/PT3292150T/pt unknown
- 2016-05-04 PL PL16722502T patent/PL3292150T3/pl unknown
- 2016-05-04 SG SG10202110908WA patent/SG10202110908WA/en unknown
-
2017
- 2017-11-02 IL IL255414A patent/IL255414B/en unknown
- 2017-11-03 MX MX2021014735A patent/MX2021014735A/es unknown
-
2019
- 2019-12-20 US US16/723,809 patent/US11753466B2/en active Active
-
2022
- 2022-02-17 JP JP2022022910A patent/JP2022065115A/ja not_active Withdrawn
-
2023
- 2023-07-18 US US18/354,353 patent/US20240076374A1/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009025846A3 (en) * | 2007-08-22 | 2009-05-14 | Univ California | Activatable binding polypeptides and methods of identification and use thereof |
| CN102482347A (zh) * | 2009-01-12 | 2012-05-30 | 希托马克斯医疗有限责任公司 | 修饰抗体组合物及其制备和使用方法 |
| CN104540518A (zh) * | 2012-04-27 | 2015-04-22 | 西托姆克斯治疗公司 | 结合表皮生长因子受体的可活化的抗体其使用方法 |
Non-Patent Citations (2)
| Title |
|---|
| Derivation, characterization and gene modification of cynomolgus monkey mesenchymal stem cells;HuiKe et al.;《Differentiation》;20081202;第77卷(第3期);第256-262页 * |
| HuiKe et al..Derivation, characterization and gene modification of cynomolgus monkey mesenchymal stem cells.《Differentiation》.2008,第77卷(第3期),第256-262页. * |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107849133B (zh) | 抗cd166抗体、可活化抗cd166抗体及其使用方法 | |
| US20220306759A1 (en) | Anti-cd71 antibodies, activatable anti-cd71 antibodies, and methods of use thereof | |
| US10233244B2 (en) | Anti-ITGA3 antibodies, activatable anti-ITGA3 antibodies, and methods of use thereof | |
| CN114685611B (zh) | 基质金属蛋白酶可切割且丝氨酸蛋白酶可切割底物及其使用方法 | |
| EP4034171A1 (en) | Anti-cd47 antibodies, activatable anti-cd47 antibodies, and methods of use thereof | |
| CN114702586A (zh) | 抗-pdl1抗体、可活化的抗-pdl1抗体、及其使用方法 | |
| EP3762420A1 (en) | Activatable cd147 antibodies and methods of making and use thereof | |
| HK40038123A (en) | Anti-cd166 antibodies, activatable anti-cd166 antibodies, and methods of use thereof | |
| HK1250036B (en) | Activatable anti-cd166 antibodies, and methods of use thereof | |
| EA041958B1 (ru) | Антитела против cd166, активируемые антитела против cd166 и способы их применения |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20220823 |
|
| CF01 | Termination of patent right due to non-payment of annual fee |