BR112018073017B1 - Forma cristalina i de crisaborol na forma livre, método de preparação e uso da mesma - Google Patents
Forma cristalina i de crisaborol na forma livre, método de preparação e uso da mesma Download PDFInfo
- Publication number
- BR112018073017B1 BR112018073017B1 BR112018073017-3A BR112018073017A BR112018073017B1 BR 112018073017 B1 BR112018073017 B1 BR 112018073017B1 BR 112018073017 A BR112018073017 A BR 112018073017A BR 112018073017 B1 BR112018073017 B1 BR 112018073017B1
- Authority
- BR
- Brazil
- Prior art keywords
- crystalline form
- solvent
- mixed solvent
- chrysaborole
- free form
- Prior art date
Links
- 238000000034 method Methods 0.000 title claims abstract description 29
- 238000002360 preparation method Methods 0.000 title claims description 4
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 3
- 239000007787 solid Substances 0.000 claims description 67
- 239000002904 solvent Substances 0.000 claims description 47
- 239000012046 mixed solvent Substances 0.000 claims description 29
- 238000000634 powder X-ray diffraction Methods 0.000 claims description 27
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 21
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 claims description 21
- 239000000203 mixture Substances 0.000 claims description 21
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 17
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 15
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 claims description 12
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 claims description 12
- 238000000926 separation method Methods 0.000 claims description 12
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 claims description 10
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 claims description 9
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 claims description 9
- 150000008282 halocarbons Chemical class 0.000 claims description 9
- 150000002576 ketones Chemical class 0.000 claims description 9
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 claims description 8
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 8
- 150000004292 cyclic ethers Chemical class 0.000 claims description 7
- 239000003814 drug Substances 0.000 claims description 7
- 150000002148 esters Chemical class 0.000 claims description 7
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 claims description 6
- 150000001298 alcohols Chemical class 0.000 claims description 6
- -1 alkyl nitriles Chemical class 0.000 claims description 6
- 238000002425 crystallisation Methods 0.000 claims description 6
- 230000008025 crystallization Effects 0.000 claims description 6
- 239000000725 suspension Substances 0.000 claims description 6
- 150000004945 aromatic hydrocarbons Chemical class 0.000 claims description 5
- 206010012434 Dermatitis allergic Diseases 0.000 claims description 4
- 150000001408 amides Chemical class 0.000 claims description 4
- 201000008937 atopic dermatitis Diseases 0.000 claims description 4
- 208000010668 atopic eczema Diseases 0.000 claims description 4
- 229920006395 saturated elastomer Polymers 0.000 claims description 4
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 claims description 3
- 150000005215 alkyl ethers Chemical class 0.000 claims description 3
- 229930195733 hydrocarbon Natural products 0.000 claims description 3
- 150000002430 hydrocarbons Chemical class 0.000 claims description 3
- JWUJQDFVADABEY-UHFFFAOYSA-N 2-methyltetrahydrofuran Chemical compound CC1CCCO1 JWUJQDFVADABEY-UHFFFAOYSA-N 0.000 claims description 2
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 claims description 2
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 claims description 2
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 claims description 2
- JMMWKPVZQRWMSS-UHFFFAOYSA-N isopropanol acetate Natural products CC(C)OC(C)=O JMMWKPVZQRWMSS-UHFFFAOYSA-N 0.000 claims description 2
- 229940011051 isopropyl acetate Drugs 0.000 claims description 2
- GWYFCOCPABKNJV-UHFFFAOYSA-N isovaleric acid Chemical compound CC(C)CC(O)=O GWYFCOCPABKNJV-UHFFFAOYSA-N 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
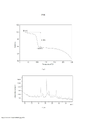
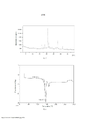
- 238000002441 X-ray diffraction Methods 0.000 description 42
- 239000013078 crystal Substances 0.000 description 29
- 238000010521 absorption reaction Methods 0.000 description 21
- 239000002245 particle Substances 0.000 description 20
- 238000000113 differential scanning calorimetry Methods 0.000 description 18
- 238000002411 thermogravimetry Methods 0.000 description 18
- 238000009826 distribution Methods 0.000 description 14
- 238000005119 centrifugation Methods 0.000 description 10
- 238000001514 detection method Methods 0.000 description 10
- 238000010586 diagram Methods 0.000 description 7
- 238000003756 stirring Methods 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000001035 drying Methods 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 230000007774 longterm Effects 0.000 description 5
- 238000003801 milling Methods 0.000 description 5
- 230000002441 reversible effect Effects 0.000 description 5
- 238000001179 sorption measurement Methods 0.000 description 5
- 238000003860 storage Methods 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical group CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 4
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 230000004584 weight gain Effects 0.000 description 4
- 235000019786 weight gain Nutrition 0.000 description 4
- 201000004681 Psoriasis Diseases 0.000 description 3
- JFDZBHWFFUWGJE-UHFFFAOYSA-N benzonitrile Chemical compound N#CC1=CC=CC=C1 JFDZBHWFFUWGJE-UHFFFAOYSA-N 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 238000004090 dissolution Methods 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical group C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 150000008280 chlorinated hydrocarbons Chemical group 0.000 description 2
- 229910001873 dinitrogen Inorganic materials 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 230000000968 intestinal effect Effects 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- CNPVJWYWYZMPDS-UHFFFAOYSA-N 2-methyldecane Chemical compound CCCCCCCCC(C)C CNPVJWYWYZMPDS-UHFFFAOYSA-N 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 229910002483 Cu Ka Inorganic materials 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 101100296720 Dictyostelium discoideum Pde4 gene Proteins 0.000 description 1
- 101100082610 Plasmodium falciparum (isolate 3D7) PDEdelta gene Proteins 0.000 description 1
- 238000012356 Product development Methods 0.000 description 1
- 238000001237 Raman spectrum Methods 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 239000013583 drug formulation Substances 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- JBTWLSYIZRCDFO-UHFFFAOYSA-N ethyl methyl carbonate Chemical compound CCOC(=O)OC JBTWLSYIZRCDFO-UHFFFAOYSA-N 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000003760 magnetic stirring Methods 0.000 description 1
- 238000010907 mechanical stirring Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000005464 sample preparation method Methods 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 238000000371 solid-state nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 238000004441 surface measurement Methods 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
- C07F5/025—Boronic and borinic acid compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/69—Boron compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/13—Crystalline forms, e.g. polymorphs
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Pulmonology (AREA)
- Immunology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Analysing Materials By The Use Of Radiation (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201610301832 | 2016-05-09 | ||
| CN201610301832.6 | 2016-05-09 | ||
| PCT/CN2017/083631 WO2017193914A1 (zh) | 2016-05-09 | 2017-05-09 | 克立硼罗游离形式的晶型及其制备方法和用途 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| BR112018073017A2 BR112018073017A2 (pt) | 2019-02-19 |
| BR112018073017B1 true BR112018073017B1 (pt) | 2022-06-14 |
Family
ID=60266671
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| BR112018073017-3A BR112018073017B1 (pt) | 2016-05-09 | 2017-05-09 | Forma cristalina i de crisaborol na forma livre, método de preparação e uso da mesma |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US11773113B2 (enExample) |
| EP (1) | EP3456722B1 (enExample) |
| JP (3) | JP2019520321A (enExample) |
| KR (1) | KR102221472B1 (enExample) |
| CN (2) | CN119751489A (enExample) |
| AU (1) | AU2017262235C1 (enExample) |
| BR (1) | BR112018073017B1 (enExample) |
| CA (1) | CA3023851C (enExample) |
| IL (1) | IL262878A (enExample) |
| MX (1) | MX380022B (enExample) |
| SG (1) | SG11201809984PA (enExample) |
| WO (1) | WO2017193914A1 (enExample) |
| ZA (1) | ZA201807892B (enExample) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10329311B1 (en) * | 2017-12-21 | 2019-06-25 | Olon S.P.A. | Process for the preparation of crisaborole |
| PL3737685T3 (pl) * | 2018-01-09 | 2023-10-02 | Halcyon Labs Private Limited | Sposób wytwarzania kryzaborolu i jego związków pośrednich |
| US10597410B2 (en) | 2018-02-02 | 2020-03-24 | Dipharma Francis S.R.L. | Intermediates and process for the preparation of a crystalline form of a topical anti-inflammatory agent |
| CN110780005B (zh) * | 2019-11-14 | 2022-07-29 | 江苏海岸药业有限公司 | 一种克立硼罗原料及其合成中间体的分析方法 |
| CN111983123A (zh) * | 2020-08-14 | 2020-11-24 | 江苏海岸药业有限公司 | 用于治疗皮肤疾病的克立硼罗制剂的体外评价方法 |
| CN112375093A (zh) * | 2020-11-13 | 2021-02-19 | 江苏知原药业股份有限公司 | 一种克立硼罗晶型化合物及其制备方法 |
| CN113087733A (zh) * | 2021-04-06 | 2021-07-09 | 南京科默生物医药有限公司 | 克立硼罗的晶型a、晶型b、晶型c、晶型d、晶型e及其制备方法 |
| WO2024047571A1 (en) * | 2022-09-01 | 2024-03-07 | Savoi Guilherme | Crisaborole cocrystal derivatives |
| CN115417890A (zh) * | 2022-09-24 | 2022-12-02 | 中山万远新药研发有限公司 | 克立硼罗的新晶型及其制备方法与用途 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IN191496B (enExample) * | 1999-07-30 | 2003-12-06 | Ranbaxy Lab Ltd | |
| GB2383042A (en) * | 2001-10-18 | 2003-06-18 | Cipla Ltd | Amorphous alendronate sodium |
| CA2597982C (en) | 2005-02-16 | 2014-07-08 | Anacor Pharmaceuticals, Inc. | Boron-containing small molecules |
| KR20080110984A (ko) | 2005-12-30 | 2008-12-22 | 아나코르 파마슈티칼스 인코포레이티드 | 보론함유 소분자 |
| CN101505603A (zh) * | 2005-12-30 | 2009-08-12 | 安纳考尔医药公司 | 含硼的小分子 |
| RS54108B1 (sr) * | 2006-02-16 | 2015-12-31 | Anacor Pharmaceuticals Inc. | Mali molekuli koji sadrže boron kao agensi protiv zapaljenja |
| WO2007146965A2 (en) * | 2006-06-12 | 2007-12-21 | Anacor Pharmaceuticals, Inc. | Compounds for the treatment of periodontal disease |
| US20070286822A1 (en) | 2006-06-12 | 2007-12-13 | Anacor Pharmaceuticals Inc. | Compounds for the Treatment of Periodontal Disease |
| EP2564857B1 (en) * | 2008-03-06 | 2017-05-03 | Anacor Pharmaceuticals, Inc. | Boron-containing small molecules as anti-inflammatory agents |
| DK3383363T3 (da) * | 2015-11-30 | 2021-01-18 | Anacor Pharmaceuticals Inc | Topiske farmaceutiske formuleringer til behandling af inflammatorisk-relaterede tilstande |
| WO2017203514A1 (en) * | 2016-05-26 | 2017-11-30 | Perrigo Api Ltd | Polymorphs of crisaborole and production processes therefor |
-
2017
- 2017-05-09 MX MX2018013742A patent/MX380022B/es unknown
- 2017-05-09 KR KR1020187035217A patent/KR102221472B1/ko active Active
- 2017-05-09 JP JP2018558377A patent/JP2019520321A/ja not_active Withdrawn
- 2017-05-09 SG SG11201809984PA patent/SG11201809984PA/en unknown
- 2017-05-09 AU AU2017262235A patent/AU2017262235C1/en active Active
- 2017-05-09 BR BR112018073017-3A patent/BR112018073017B1/pt active IP Right Grant
- 2017-05-09 CN CN202411806904.3A patent/CN119751489A/zh active Pending
- 2017-05-09 US US16/099,839 patent/US11773113B2/en active Active
- 2017-05-09 EP EP17795532.5A patent/EP3456722B1/en active Active
- 2017-05-09 WO PCT/CN2017/083631 patent/WO2017193914A1/zh not_active Ceased
- 2017-05-09 CA CA3023851A patent/CA3023851C/en active Active
- 2017-05-09 CN CN201780009362.9A patent/CN108884111A/zh active Pending
-
2018
- 2018-11-08 IL IL262878A patent/IL262878A/en unknown
- 2018-11-22 ZA ZA2018/07892A patent/ZA201807892B/en unknown
-
2021
- 2021-06-29 JP JP2021107201A patent/JP2021167327A/ja active Pending
-
2023
- 2023-06-12 JP JP2023096006A patent/JP2023116645A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| AU2017262235C1 (en) | 2020-08-20 |
| BR112018073017A2 (pt) | 2019-02-19 |
| US11773113B2 (en) | 2023-10-03 |
| EP3456722B1 (en) | 2025-12-10 |
| EP3456722A4 (en) | 2019-12-18 |
| WO2017193914A1 (zh) | 2017-11-16 |
| JP2019520321A (ja) | 2019-07-18 |
| SG11201809984PA (en) | 2018-12-28 |
| JP2021167327A (ja) | 2021-10-21 |
| KR102221472B1 (ko) | 2021-03-02 |
| NZ748385A (en) | 2022-03-25 |
| MX380022B (es) | 2025-03-11 |
| KR20190005195A (ko) | 2019-01-15 |
| CN119751489A (zh) | 2025-04-04 |
| JP2023116645A (ja) | 2023-08-22 |
| IL262878A (en) | 2018-12-31 |
| RU2018142490A (ru) | 2020-06-10 |
| MX2018013742A (es) | 2019-08-01 |
| CA3023851A1 (en) | 2017-11-16 |
| RU2018142490A3 (enExample) | 2020-06-10 |
| NZ786020A (en) | 2025-06-27 |
| EP3456722A1 (en) | 2019-03-20 |
| US20230234974A1 (en) | 2023-07-27 |
| AU2017262235A1 (en) | 2018-12-06 |
| AU2017262235B2 (en) | 2019-09-19 |
| ZA201807892B (en) | 2021-06-30 |
| CA3023851C (en) | 2021-01-26 |
| CN108884111A (zh) | 2018-11-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| BR112018073017B1 (pt) | Forma cristalina i de crisaborol na forma livre, método de preparação e uso da mesma | |
| US9012495B2 (en) | Crystalline forms of genistein | |
| CN109153656A (zh) | 他发米帝司葡甲胺盐的晶型e及其制备方法和用途 | |
| CN109219597A (zh) | 奥扎莫德的晶型、其盐酸盐的晶型及其制备方法 | |
| BR112017007953B1 (pt) | Forma cristalina ii do bissulfato inibidor de jak quinase, seu uso e seu método de preparação, e composição farmacêutica | |
| CN104220431A (zh) | 卡巴他赛的固态形式及其制备方法 | |
| CN105732575A (zh) | 一种治疗前列腺癌的新型抗雄激素类药物的新晶型及其制备方法 | |
| KR20230038229A (ko) | 우파다시티닙의 결정형, 이의 제조 방법 및 이의 용도 | |
| US11447506B2 (en) | Crystal forms of crisaborole in free form and preparation method and use thereof | |
| WO2022170864A1 (zh) | Belumosudil甲磺酸盐的晶型及其制备方法和用途 | |
| BR112021003298A2 (pt) | polimorfo cristalino de cloridrato de 8-bromo-2-(1-metilpiperidin-4-ilamino)-4-(4-fenoiyfenilamino)pirido[4,3-d]pirimidin-5(6h)-ona, processo para a preparação do mesmo e uso terapêutico do dito polimorfo | |
| WO2021000687A1 (zh) | Pac-1晶型的制备方法 | |
| BRPI0608689A2 (pt) | composto, composiÇço, processos para preparar monoidrato e a forma de cristal, e, uso de um monoidrato ou de uma forma de cristal | |
| JP7295025B2 (ja) | (s)-[2-クロロ-4-フルオロ-5-(7-モルホリン-4-イルキナゾリン-4-イル)フェニル]-(6-メトキシ-ピリダジン-3-イル)メタノールの結晶性形態 | |
| WO2022171117A1 (zh) | 含氮稠杂环化合物的盐、晶型及其制备方法、药物组合物和用途 | |
| CN109438372A (zh) | 一种甲基吡嗪衍生物甲醇合物 | |
| RU2845248C2 (ru) | Кристаллические формы кризаборола в свободной форме и способ их получения и применения | |
| CN115124514A (zh) | Kd-025的共晶及其制备方法 | |
| TW202333694A (zh) | 稠環衍生物的晶型、其製備方法及其應用 | |
| WO2018001335A1 (zh) | Nbi-98854的晶型及其制备方法和用途 | |
| CN106794179A (zh) | 马赛替尼甲磺酸盐的新晶型及其制备方法 | |
| BRPI0614634A2 (pt) | novo sal de derivado de piperidina | |
| NZ748385B2 (en) | Crystal forms of crisaborole in free form and preparation method and use thereof | |
| CN115003657A (zh) | (-)-琥珀酸西苯唑啉的多晶型 | |
| HK1259661A1 (en) | Crystal forms of crisaborole in free form and preparation method and use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| B350 | Update of information on the portal [chapter 15.35 patent gazette] | ||
| B06W | Patent application suspended after preliminary examination (for patents with searches from other patent authorities) chapter 6.23 patent gazette] | ||
| B09A | Decision: intention to grant [chapter 9.1 patent gazette] | ||
| B16A | Patent or certificate of addition of invention granted [chapter 16.1 patent gazette] |
Free format text: PRAZO DE VALIDADE: 20 (VINTE) ANOS CONTADOS A PARTIR DE 09/05/2017, OBSERVADAS AS CONDICOES LEGAIS |