WO2022201252A1 - Aiguille d'injection de solution médicamenteuse et dispositif d'aiguille d'injection de solution médicamenteuse - Google Patents
Aiguille d'injection de solution médicamenteuse et dispositif d'aiguille d'injection de solution médicamenteuse Download PDFInfo
- Publication number
- WO2022201252A1 WO2022201252A1 PCT/JP2021/011751 JP2021011751W WO2022201252A1 WO 2022201252 A1 WO2022201252 A1 WO 2022201252A1 JP 2021011751 W JP2021011751 W JP 2021011751W WO 2022201252 A1 WO2022201252 A1 WO 2022201252A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- electrode
- drug solution
- injection needle
- solution injection
- tip
- Prior art date
Links
- 238000002347 injection Methods 0.000 title claims abstract description 86
- 239000007924 injection Substances 0.000 title claims abstract description 86
- 239000003814 drug Substances 0.000 title claims abstract description 82
- 229940079593 drug Drugs 0.000 title claims abstract description 79
- 239000000243 solution Substances 0.000 title claims abstract description 77
- 229910052751 metal Inorganic materials 0.000 claims abstract description 82
- 239000002184 metal Substances 0.000 claims abstract description 82
- 238000000465 moulding Methods 0.000 claims abstract description 9
- 239000004020 conductor Substances 0.000 claims abstract description 7
- 239000011810 insulating material Substances 0.000 claims abstract description 7
- 239000007788 liquid Substances 0.000 claims description 25
- 230000002093 peripheral effect Effects 0.000 claims description 18
- 230000002107 myocardial effect Effects 0.000 claims description 10
- 238000002360 preparation method Methods 0.000 claims description 5
- 238000009413 insulation Methods 0.000 claims description 4
- 239000000919 ceramic Substances 0.000 claims description 3
- 210000000056 organ Anatomy 0.000 claims description 3
- 230000008929 regeneration Effects 0.000 claims 1
- 238000011069 regeneration method Methods 0.000 claims 1
- 210000001124 body fluid Anatomy 0.000 abstract description 9
- 239000011247 coating layer Substances 0.000 description 14
- 239000010410 layer Substances 0.000 description 13
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 8
- 239000010839 body fluid Substances 0.000 description 7
- 210000004165 myocardium Anatomy 0.000 description 6
- 229910052697 platinum Inorganic materials 0.000 description 4
- 229920005989 resin Polymers 0.000 description 4
- 239000011347 resin Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 229910010293 ceramic material Inorganic materials 0.000 description 3
- WABPQHHGFIMREM-UHFFFAOYSA-N lead(0) Chemical compound [Pb] WABPQHHGFIMREM-UHFFFAOYSA-N 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 238000000034 method Methods 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 230000001172 regenerating effect Effects 0.000 description 3
- 229920002614 Polyether block amide Polymers 0.000 description 2
- 238000005452 bending Methods 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 238000000635 electron micrograph Methods 0.000 description 2
- 230000007831 electrophysiology Effects 0.000 description 2
- 238000002001 electrophysiology Methods 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 229920000139 polyethylene terephthalate Polymers 0.000 description 2
- 239000005020 polyethylene terephthalate Substances 0.000 description 2
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 239000010937 tungsten Substances 0.000 description 2
- 238000010146 3D printing Methods 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 210000005242 cardiac chamber Anatomy 0.000 description 1
- 229920006026 co-polymeric resin Polymers 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- -1 polyethylene terephthalate Polymers 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/158—Needles for infusions; Accessories therefor, e.g. for inserting infusion needles, or for holding them on the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/178—Syringes
- A61M5/31—Details
- A61M5/32—Needles; Details of needles pertaining to their connection with syringe or hub; Accessories for bringing the needle into, or holding the needle on, the body; Devices for protection of needles
Definitions
- the present invention relates to a drug solution injection needle for puncturing a patient's organ to inject a drug solution, and a drug solution injection needle device equipped with such a drug solution injection needle.
- a hollow needle (drug injection needle) that punctures the patient's myocardium and injects a drug solution is used to directly administer medication to myocardial cells (see Patent Document 1 below).
- This drug solution injection needle is inserted into a living body cavity (heart chamber) while being inserted into a sheath or a guiding catheter, and the needle tip of the drug solution injection needle protrudes from the tip opening of the sheath or the guiding catheter to reach the target site. (Myocardium) is punctured, and a drug solution is administered to the myocardial cells at the target site.
- Patent document 2 discloses a sharp metal tube (12) as a hollow needle for puncturing the myocardium of a patient and injecting a drug solution, and a first electrode for potential measurement comprising a tip portion of the metal tube. (18), an insulating coating layer (22) covering the outer peripheral surface of the base end portion of the metal tube, and a second electrode (28) arranged on the outer peripheral surface of the insulating coating layer. (210) is described.
- Patent Document 3 describes a puncture needle provided with a plurality of electrodes along the axial direction of the needle. It describes that the second electrode (511) is connected to the second electrode (511) via an electrically insulating coupling pipe (591).
- the first electrode (18) and the second electrode (28) that constitute the liquid injection needle described in Patent Document 2 are insulated from each other by an insulating coating layer (22).
- these electrodes (18, 22) and the insulating coating layer (22) are not sufficiently bonded.
- a minute gap inevitably exists between the insulating coating layer (22) and the ring-shaped second electrode (28) mounted on the outer periphery thereof.
- the patient's bodily fluid or the like may enter the joint surface between the first electrode (18) and the insulating coating layer (22) and/or the joint surface between the second electrode (28) and the insulating coating layer (22).
- drift baseline fluctuation
- noise occur in the potential measured by the electrode, making it impossible to measure the potential accurately.
- a first electrode (521) and a second electrode (511) that constitute the puncture needle described in Patent Document 3 are connected to each other by an insulating connecting tube (591).
- these electrodes (521, 511) and the connecting pipe (591) are only fitted and not sufficiently joined. Therefore, the body fluid of the patient does not enter the contact surface between the first electrode (521) and the connecting pipe (591) and/or the contact surface between the second electrode (511) and the connecting pipe (591). In this case, drift and noise occur in the potential measured by the electrode, and accurate potential cannot be measured.
- the electrode lead extends into the lumen of the needle.
- the lead comes into contact with the drug solution flowing through the lumen of the needle, which is not preferable from the viewpoint of biological safety, and limits the coating resin and the like that constitute the lead. It is also conceivable that the lead extending into the lumen of the needle impedes the flow of the drug solution.
- a first object of the present invention is to firmly bond a conductive portion and an insulating portion such as an electrode, and to provide a distal end that prevents body fluids from entering the bonding surface between the conductive portion and the insulating portion.
- a second object of the present invention is to provide a drug solution injection needle in which the electrode lead does not come into contact with the drug solution at least at the tip.
- the drug solution injection needle of the present invention is a hollow needle for puncturing an organ of a patient to inject a drug solution, comprising a sharp tip having an interior space and a metal tube having a lumen communicating with the interior space of the tip;
- the distal end portion includes a circular tube portion, a sharp distal end portion continuous to the distal end of the circular tubular portion, and a proximal end continuous to the circular tubular portion, and is inserted into the lumen to connect with the metal tube.
- At least one side hole (outflow path for the chemical solution) communicating with the internal space and opening to the outer surface is formed in the circular tube portion and/or the sharp tip portion,
- the tip portion is formed by integrally molding a conductive portion made of a conductive material and an insulating portion made of an insulating material, As the conductive portion, a first electrode is formed on the sharp tip portion, a second electrode is formed on the outer periphery of the cylindrical portion, and a first electrode land is formed on the outer periphery of the small-diameter cylindrical portion.
- a second electrode land are formed apart from each other, and a first lead for electrically connecting the first electrode and the first electrode land is provided inside the pipe wall of the tip portion; A second lead for electrically connecting the second electrode and the second electrode land is embedded in each of the second electrodes.
- portion in the insulating portion and the conductive portion means a portion of each of the sharp tip portion, the circular tube portion, and the small-diameter circular tube portion, or by straddling these boundaries to form the tip. It refers to the part made of the same material that constitutes the part.
- integralally molded means that the insulating portion and the conductive portion are joined together with the molding.
- the conductive portion and the insulating portion are formed integrally to form the tip portion.
- the first electrode land, the second electrode land, and the insulating portion of the distal end portion are firmly joined to each other, so that body fluids and the like can be prevented from entering the joining surfaces of the two.
- a first lead connecting the first electrode and the first electrode land, and a second lead connecting the second electrode and the second electrode land are embedded in the tube wall at the distal end. Moreover, since these leads are not exposed to the inner peripheral surface of the distal end portion, it is possible to avoid contact of these leads with the chemical solution.
- the conductive portion constituting the tip portion is made of metal, and the insulating portion is made of ceramic.
- a bonding layer in which the conductive material and the insulating material are mixed is formed on the bonding surface between the conductive portion and the insulating portion forming the tip portion. It is preferable that
- the insulating material forming the insulating portion and the conductive material forming the conductive portion are placed on the joint surface between the conductive portion forming the tip portion and the insulating portion. are mixed (mutually diffused) between the first electrode, the second electrode, the first electrode land and the second electrode land, which are conductive portions, and the insulating portion. A strong bonding force is exhibited between them, and it is possible to reliably prevent body fluids and the like from entering the bonding surfaces of the two.
- the tip region of the sharp tip portion is made of only the metal.
- the toughness of the distal end region of the sharp tip portion can be increased, and the sharp tip portion can be prevented from being damaged.
- the drug solution injection needle having such a configuration, it is possible to prevent the edge of the opening of the side hole from being damaged.
- either one of the first electrode land and the second electrode land is electrically connected to the metal tube,
- the tip end is connected to the other of the first electrode land and the second electrode land, and extends in the proximal direction on the outer peripheral surface of the metal tube while ensuring insulation with respect to the metal tube. It is preferable to have a conductive layer extending to the proximal end of the metal tube.
- the metal tube can be used as one lead of the first electrode land and the second electrode land, and the conductive layer can be used as the other lead. can be done.
- the conductive layer can be used as the other lead.
- a spiral slit is formed in the tip region of the metal tube.
- the strength of the tip region of the metal tube can be lowered to some extent, and the injection needle can be made flexible.
- the drug solution injection needle of the present invention is preferably myocardial regenerative cell preparation.
- the drug solution injection needle device of the present invention comprises the drug solution injection needle of the present invention, a grip portion connected to the proximal end side of the drug solution injection needle, and the above-described device for supplying the drug solution to the inside of the drug solution injection needle. It is characterized by comprising an injection port attached to a grip portion, and a connector attached to the grip portion and electrically connected to the first electrode and the second electrode.
- the first electrode, the second electrode, the land for the first electrode, and the land for the second electrode which are the conductive portions at the tip, are firmly joined to the insulating portion at the tip. Since it is possible to prevent body fluids and the like from entering the joint surfaces of both, drift and noise do not occur in the potential measured by the first electrode and the second electrode, and accurate potential can be measured by these electrodes. can do.
- a first lead connecting the first electrode and the first electrode land, and a second lead connecting the second electrode and the second electrode land are embedded in the tube wall at the distal end. , so these leads can be avoided from coming into contact with the chemical solution.
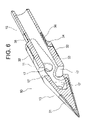
- FIG. 1 is a plan view showing a drug solution injection needle according to one embodiment of the present invention
- FIG. FIG. 2 is a front view showing the drug solution injection needle shown in FIG. 1
- FIG. 3 is a detailed view of part III of FIG. 2
- FIG. 4 is a sectional view along IV-IV in FIG. 3
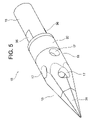
- FIG. 2 is a perspective view showing the tip of the liquid injection needle shown in FIG. 1
- 2 is a perspective view showing a cross-sectional configuration of the tip portion of the drug solution injection needle shown in FIG. 1
- FIG. FIG. 2 is a vertical cross-sectional view of the tip portion of the liquid injection needle shown in FIG. 1
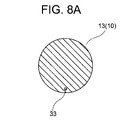
- FIG. 8 is a cross-sectional view taken along line VIIIA-VIIIA of FIG. 7;
- FIG. 8 is a cross-sectional view taken along line VIIIB-VIIIB of FIG. 7;
- FIG. 8 is a cross-sectional view taken along line VIIIC-VIIIC of FIG. 7;
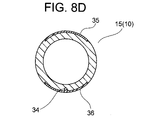
- FIG. 8 is a cross-sectional view taken along line VIIID-VIIID in FIG. 7;
- FIG. 4 is a cross-sectional view schematically showing a state of electrical connection from each of a first electrode land and a second electrode land to a connector; It is a longitudinal cross-sectional view showing a preferred modification of the present invention. It is a longitudinal cross-sectional view showing a preferred modification of the present invention. 4 is an electron micrograph showing a boundary between an insulating portion and a conductive portion;
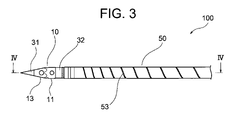
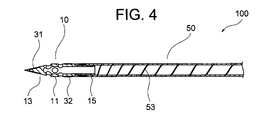
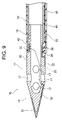
- ⁇ Medicine injection needle> 1 to 8 is a hollow needle for puncturing a patient's myocardium and injecting a drug solution into myocardial cells. It comprises a sharp distal end portion 10 having an internal space and a metal tube 50 having a lumen communicating with the internal space of the distal end portion 10, and the distal end portion 10 of the drug solution injection needle 100 is a circular tube portion. 11, a sharp distal end portion 13 that continues to the distal end of the circular tube portion 11, and a small diameter circular tube that continues to the proximal end of the circular tube portion 11 and is inserted into the lumen of the metal tube 50 to connect with the metal tube 50.
- the circular tube portion 11 and the sharp tip portion 13 are formed with six side holes 17 that communicate with the inner space of the tip portion 10 and open to the outer surface as outflow paths for the liquid medicine.
- 10 is formed by integrally molding a conductive portion made of a conductive material and an insulating portion made of an insulating material.
- a ring-shaped second electrode 32 is formed on the outer periphery of the circular tube portion 11, and a first electrode land 35 and a second electrode land 36 are spaced apart from each other on the outer periphery of the small-diameter circular tube portion 15.
- a first lead 33 for electrically connecting a first electrode 31 and a first electrode land 35, a second electrode 32 and a second electrode land 36 are formed inside the tube wall of the distal end portion 10. and a second lead 34 for electrically connecting the .
- a drug solution injection needle 100 of this embodiment includes a tip portion 10 and a metal tube 50 . As shown in FIGS. 1 and 2 , a grasping portion 60 is connected to the proximal end of the metal tube 50 that constitutes the liquid injection needle 100 . An injection port 70 and a connector 80 are attached to the grasping portion 60, and the liquid injection needle 100, the grasping portion 60, the injection port 70, and the connector 80 constitute a liquid injection needle device.
- the grasping portion 60 that constitutes the liquid injection needle device is made of resin, rubber, elastomer, or the like.
- An injection port 70 attached to the grasping portion 60 is a port for supplying a liquid medicine to the lumen of the liquid medicine injection needle 100 .
- a connector 80 attached to the grip portion 60 is electrically connected to the first electrode 31 and the second electrode 32, respectively.
- the effective length of the liquid injection needle 100 protruding from the tip of the grasping portion 60 is usually 800-2500 mm, preferably 900-2300 mm.
- the drug solution injection needle 100 of this embodiment is a hollow needle for puncturing a patient's myocardium and injecting a drug solution into myocardial cells.
- the "drug solution” includes cell preparations such as myocardial regenerative cell preparations, gene introduction drugs, and the like.
- the tip portion 10 of the liquid injection needle 100 is composed of a sharp tip portion 13 , a circular tube portion 11 , and a small diameter circular tube portion 15 .
- a side hole 17 that communicates with the internal space of the tip portion 10 and opens to the outer surface of the tip portion 10 is formed in the circular tube portion 11 and the tip portion 13 .
- the drug solution supplied from the injection port 70 passes through the lumen of the metal tube 50, the internal space of the distal end portion 10 and the side hole 17, flows out from the opening, and is injected into the patient's myocardial cells.
- the small-diameter circular tube portion 15 of the tip portion 10 is inserted from the tip opening of the metal tube 50 into the lumen of the metal tube 50 , thereby connecting the tip portion 10 and the metal tube 50 . Therefore, the small-diameter circular tube portion 15 does not appear as an external appearance of the liquid injection needle 100 .
- the tip portion 10 (sharp tip portion 13, circular tube portion 11, small-diameter circular tube portion 15) is formed by integrally forming a conductive portion made of conductive metal and an insulating portion made of ceramic.
- integral molding means that the bonding of the insulating part and the conductive part is performed at the same time as the tip part 10 is formed, instead of bonding or mechanically joining the insulating part and the conductive part. means to do A preferred specific example of “integral molding” is molding by 3D printing.
- the tip of the tip portion 13 of the tip portion 10 is closed, and two side holes 17 are formed in the tube wall of the tip tip portion 13 .
- a first electrode 31 is formed on the sharp tip portion 13 as a conductive portion that constitutes the tip portion 10 . There is no step between the first electrode 31 and the insulating portion located on the proximal side thereof, and the tip sharp portion 13 has a smooth outer surface (conical surface).
- a ring-shaped second electrode 32 is formed on the circular tube portion 11 as a conductive portion of the distal end portion 10 . There is no level difference between the second electrode 32 and the insulating portions positioned on both end sides thereof, and the cylindrical portion 11 has a smooth outer peripheral surface.
- a first electrode land 35 and a second electrode land 36 are formed apart from each other in the small-diameter circular tube portion 15 as conductive portions that constitute the distal end portion 10 .
- a first electrode for electrically connecting the first electrode 31 and the first electrode land 35 is provided inside the tube wall of the distal end portion 10 (the sharp distal end portion 13, the circular tube portion 11, the small diameter circular tube portion 15). Leads 33 are embedded. A second lead 34 for electrically connecting the second electrode 32 and the second electrode land 36 is provided inside the tube wall of the distal end portion 10 (the circular tube portion 11 and the small-diameter circular tube portion 15). embedded.
- Each of the first lead 33 and the second lead 34 is embedded inside the tube wall of the distal end portion 10 (covered with an insulating portion constituting the distal end portion 10), and the inner peripheral surface of the distal end portion 10 not exposed to
- the length of the tip portion 10 (the length of the sharp tip portion 13 + the length of the circular tube portion 11) is usually 1.0 to 4.2 mm, preferably 1.5 to 3.0 mm. Also, the length of the sharp tip portion 13 is usually 0.3 to 3.0 mm, preferably 0.8 to 2.2 mm.
- the outer diameter of the tip portion 10 is usually 0.34 to 1.0 mm, preferably 0.5 to 0.8 mm.
- the inner diameter of the tip portion 10 (circular tube portion 11) is usually 0.24 to 0.90 mm, preferably 0.32 to 0.70 mm.
- the conductive metal forming the first electrode 31, the second electrode 32, the first lead 33, the second lead 34, the first electrode land 35, and the second electrode land 36 which are the conductive portions of the distal end portion 10, all conventionally known metals constituting the liquid injection needle can be used.
- the conductive metal forming the first electrode 31, the second electrode 32, the first electrode land 35, and the second electrode land 36 radiation-resistant metals such as platinum, platinum-based alloys, gold, tungsten, and tantalum may be used. Transparent metals are preferred.
- the conductive metal forming the first lead 33 and the second lead 34 copper, gold, silver, platinum, tungsten, titanium, stainless steel, etc. are preferable.
- FIG. 12 is an electron micrograph showing the bonding state between the insulating portion and the conductive portion that constitute the tip portion 10 of the liquid injection needle 100 .
- the upper right side of this photograph is the insulating part, and the gray material is the ceramic material.
- the lower left side of this photograph is the conductive part, and the conductive metal (platinum) is visible in white.
- a bonding layer in which a ceramic material and a conductive metal are mixed is formed from the upper left to the lower right of this photograph.
- the small-diameter circular tube portion 15 of the distal end portion 10 is inserted into the inner cavity of the metal tube 50 through the distal end opening of the metal tube 50, thereby connecting the distal end portion 10 and the metal tube 50, so that the inner space of the distal end portion 10 and the metal tube 50 are connected. are communicated with each other, thereby constituting the liquid injection needle 100 of the present embodiment.
- the metal tube 50 is required to have the strength (especially bending strength) and elasticity (especially bending elasticity) required for an ordinary liquid injection needle.
- the metal forming the metal tube 50 the same metal as the conductive metal forming the conductive portion can be used. Also, part or all of the distal end portion of the metal tube 50 may be made of a radiopaque metal.
- a spiral slit 53 is formed in the tip region of the metal tube 50 .
- the slit 53 is a through slit extending from the outer peripheral surface to the inner peripheral surface of the metal pipe, but the slit may be formed so as not to extend to the inner peripheral surface.
- the pitch of the slits 53 is formed so as to continuously narrow toward the distal direction. As a result, the strength of the distal end region can be continuously (smoothly) reduced in the distal direction, thereby improving the operability when introducing the drug solution injection needle 100 to the target site.
- the length of the metal tube 50 is usually 600-2200 mm, preferably 700-1800 mm.
- the outer diameter of the metal tube 50 is usually 0.34-1.0 mm, preferably 0.4-0.9 mm.
- the inner diameter of the metal tube 50 is usually 0.3-0.9 mm, preferably 0.34-0.80 mm.
- the length of the slit 53 formed in the tip region of the metal tube 50 is normally 590-2100 mm, preferably 650-1000 mm.
- the first electrode 31 forming the distal end portion 10 is electrically connected to a connector 80 forming the liquid injection needle device via the first lead 33 and the first electrode land 35 .
- the second electrode 32 forming the distal end portion 10 is electrically connected to a connector 80 forming a liquid injection needle device via a second lead 34 and a second electrode land 36 .
- the method of electrically connecting each of the first electrode lands 35 and the second electrode lands 36 to the connector 80 is not particularly limited, but for example, the following method can be adopted.
- the first electrode land 35 of the tip portion 10 is joined to the inner peripheral surface of the metal tube 50 to electrically connect the one electrode land 35 and the metal tube 50 . Also, although not shown, the proximal end of the metal tube 50 and the connector 80 are connected by a lead wire. Thereby, the first electrode 31 and the connector 80 can be electrically connected.
- the tip is joined to the second electrode land 36 of the distal end portion 10, and extends in the proximal direction on the outer peripheral surface of the metal tube 50 while ensuring insulation with respect to the metal tube 50.
- a strip-shaped conductive layer 55 is formed to reach the base end of the .
- the proximal end of the conductive layer 55 and the connector 80 are connected by a lead wire. Thereby, the second electrode 32 and the connector 80 can be electrically connected.
- the insulation between the metal tube 50 and the conductive layer 55 is achieved by forming an insulating coating layer 40 covering the outer peripheral surface of the metal tube 50 and disposing the conductive layer 55 on the outer peripheral surface, as shown in FIG. can be secured by
- 45 is a strip-shaped insulating coating layer that covers the conductive layer 55 .
- the insulating coating layer 40 can close the slit (not shown in FIG. 9) formed in the tip region of the metal tube 50, and the liquid-tightness of the liquid injection needle 100 can be ensured.
- the insulating coating layer 40 can be formed by shrinking a heat-shrinkable resin tube in which the metal tube 50 is inserted.
- the heat-shrinkable resin tube for forming the insulating coating layer 40 include polyethylene terephthalate (PET) and polyether block amide copolymer resin (PEBAX (registered trademark)).
- the first electrode land 35 and the connector 80 are electrically connected using the metal tube 50, and the second electrode land 36 and the connector 80 are connected by a conductive layer insulated from the metal tube 50.
- electrically connecting via 55 there is no need to provide lead wires for these electrodes extending inside or outside the metal tube 50, so the diameter of the base end (metal tube 50) of the liquid injection needle 100 can be reduced. can be achieved, and a sufficient space can be secured for the lumen of the needle.
- the drug solution injection needle 100 of the present embodiment and the grip portion 60 constitute a drug solution injection needle device, and the drug solution is injected into the patient's myocardium with this drug solution injection needle device.
- the injection port 70 is connected to a syringe filled with the drug solution to be supplied to the lumen of the drug solution injection needle 100, and the connector 80 is connected to the electrocardiograph.
- the tip portion 10 is configured by integrally molding the conductive portion and the insulating portion, and the joint surface between the conductive portion and the insulating portion of the tip portion 10
- a bonding layer in which a ceramic material and a conductive metal are mixed
- the first electrode 31, the second electrode 32, the first electrode land 35, and the conductive portion of the tip portion 10 are formed.
- a strong bonding force is developed between the second electrode land 36 and the insulating portion of the distal end portion 10, and it is possible to reliably prevent body fluids from entering the bonding surfaces of the two.
- the potentials measured by the first electrode 31 and the second electrode 32 are free from drift and noise, and the potentials can be accurately measured by these electrodes.
- a first lead 33 connecting the first electrode 31 and the first electrode land 35 and a second lead 34 connecting the second electrode 32 and the second electrode land 36 are connected to the tube of the distal end portion 10. By embedding them inside the wall and not exposing them to the inner peripheral surface of the tip portion 10 , it is possible to avoid contact of these leads 33 and 34 with the chemical liquid flowing in the internal space of the tip portion 10 . can.
- the present invention is not limited to this, and various modifications are possible.
- the tip region of the sharp tip portion 13 may be made of only conductive metal, and the first electrode 31A may be made of pure metal.
- the toughness of the tip region of the tip sharp portion can be increased, and the tip tip portion can be prevented from being chipped.
- the inner peripheral surface of the side hole 17A formed in the sharp tip portion 13 and the cylindrical portion 11 may be coated with a conductive metal. Thereby, it is possible to prevent the edge of the opening of the side hole 17A from being damaged.
- the second electrode may have a shape other than the ring shape.
Landscapes
- Health & Medical Sciences (AREA)
- Vascular Medicine (AREA)
- Engineering & Computer Science (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Abstract
Le but de la présente invention est de fournir une aiguille d'injection de solution médicamenteuse équipée d'une partie d'extrémité avant qui empêche un fluide corporel ou similaire d'infiltrer un plan de jonction entre une partie isolante et une partie conductrice d'une électrode ou similaire. Une aiguille d'injection de solution médicamenteuse (100) selon la présente invention comprend une partie d'extrémité avant pointue (10) et un tube métallique (50). La partie d'extrémité avant comprend une section de tube circulaire (11), une section pointue d'extrémité avant (13) et une section de tube circulaire de petit diamètre (15). La section de tube circulaire et la section pointue d'extrémité avant ont chacune des trous latéraux (17) formés à l'intérieur de celles-ci. La partie d'extrémité avant est formée par moulage d'un seul tenant d'une partie conductrice constituée d'un matériau conducteur et d'une partie isolante constituée d'un matériau isolant. En tant que partie conductrice, une première électrode (31) est formée dans la section pointue d'extrémité avant. Une seconde électrode (32) est formée dans la circonférence externe de la section de tube circulaire. La section de tube circulaire de petit diamètre comporte, au niveau de sa circonférence externe, une première plage d'électrode (35) et une seconde plage d'électrode (36). Un premier fil (33) qui connecte électriquement la première électrode et la première plage d'électrode et un second fil (34) qui connecte électriquement la seconde électrode et la seconde plage d'électrode sont formés et incorporés dans la paroi tubulaire de la partie d'extrémité avant.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/JP2021/011751 WO2022201252A1 (fr) | 2021-03-22 | 2021-03-22 | Aiguille d'injection de solution médicamenteuse et dispositif d'aiguille d'injection de solution médicamenteuse |
| JP2023508172A JPWO2022201252A1 (fr) | 2021-03-22 | 2021-03-22 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/JP2021/011751 WO2022201252A1 (fr) | 2021-03-22 | 2021-03-22 | Aiguille d'injection de solution médicamenteuse et dispositif d'aiguille d'injection de solution médicamenteuse |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022201252A1 true WO2022201252A1 (fr) | 2022-09-29 |
Family
ID=83395377
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/011751 WO2022201252A1 (fr) | 2021-03-22 | 2021-03-22 | Aiguille d'injection de solution médicamenteuse et dispositif d'aiguille d'injection de solution médicamenteuse |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JPWO2022201252A1 (fr) |
| WO (1) | WO2022201252A1 (fr) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09140802A (ja) * | 1995-11-21 | 1997-06-03 | Nippon Zeon Co Ltd | 電極カテーテル |
| JP2016513520A (ja) * | 2013-03-15 | 2016-05-16 | ベイリス メディカル カンパニー インコーポレイテッドBaylis Medical Company Inc. | 遠位開口部を有する電気外科手術デバイス |
| JP2017127498A (ja) * | 2016-01-20 | 2017-07-27 | 日本ライフライン株式会社 | 焼灼用針装置、高周波焼灼治療システムおよび化学焼灼治療システム |
-
2021
- 2021-03-22 WO PCT/JP2021/011751 patent/WO2022201252A1/fr active Application Filing
- 2021-03-22 JP JP2023508172A patent/JPWO2022201252A1/ja active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09140802A (ja) * | 1995-11-21 | 1997-06-03 | Nippon Zeon Co Ltd | 電極カテーテル |
| JP2016513520A (ja) * | 2013-03-15 | 2016-05-16 | ベイリス メディカル カンパニー インコーポレイテッドBaylis Medical Company Inc. | 遠位開口部を有する電気外科手術デバイス |
| JP2017127498A (ja) * | 2016-01-20 | 2017-07-27 | 日本ライフライン株式会社 | 焼灼用針装置、高周波焼灼治療システムおよび化学焼灼治療システム |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2022201252A1 (fr) | 2022-09-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2138200B1 (fr) | Cathéter d'aspiration d'un thrombus | |
| US7657324B2 (en) | Seal for use with cardiac lead | |
| US7691086B2 (en) | Catheter for introduction of medications to the tissues of a heart or other organ | |
| KR101997372B1 (ko) | 케미컬 어블레이션 장치 및 케미컬 어블레이션 시스템 | |
| EP1462142B1 (fr) | Cathéter avec capteur de ponction | |
| WO2008112870A2 (fr) | Cathéter d'ablation épicardique et procédé d'utilisation | |
| US20040049158A1 (en) | Hemostasis valve for use with a left ventricular pacing lead | |
| JP2000024120A (ja) | 治療用および診断用の薬剤注射用カテ―テル | |
| CN217067424U (zh) | 一种临时起搏器漂浮电极组件 | |
| WO2022201252A1 (fr) | Aiguille d'injection de solution médicamenteuse et dispositif d'aiguille d'injection de solution médicamenteuse | |
| JPH0647089A (ja) | 医療−外科用装置 | |
| CN115379868B (zh) | 药液注入针以及药液注入针系统 | |
| WO2018174251A1 (fr) | Cathéter et procédé de fabrication d'un cathéter | |
| JP7373055B2 (ja) | 薬液注入針システム | |
| JP7426263B2 (ja) | 穿刺針、針カテーテル、及び、カテーテルシステム | |
| JP7373056B2 (ja) | 薬液注入針および薬液注入針システム | |
| WO2024090235A1 (fr) | Système d'aiguille | |
| JP2019017501A (ja) | 医療器具 | |
| JP2023158448A (ja) | 針システム | |
| JP7286670B2 (ja) | 電気治療用具 | |
| JP7440541B2 (ja) | 薬液注入装置 | |
| CN214105533U (zh) | 一种医用导管及导引鞘 | |
| EP4295886A1 (fr) | Dispositif de perforation | |
| PL237439B1 (pl) | Elektroda wewnątrzsercowa/cewnik do stymulacji serca i podawania leków | |
| JP2000202031A (ja) | 抗凝血性イントロデュ―サ―シ―ス |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21932865 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2023508172 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21932865 Country of ref document: EP Kind code of ref document: A1 |