WO2022009883A1 - 熱活性化遅延蛍光材料、及び有機電界発光素子 - Google Patents
熱活性化遅延蛍光材料、及び有機電界発光素子 Download PDFInfo
- Publication number
- WO2022009883A1 WO2022009883A1 PCT/JP2021/025469 JP2021025469W WO2022009883A1 WO 2022009883 A1 WO2022009883 A1 WO 2022009883A1 JP 2021025469 W JP2021025469 W JP 2021025469W WO 2022009883 A1 WO2022009883 A1 WO 2022009883A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ring
- activated delayed
- light emitting
- carbon atoms
- fluorescent material
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- BWVNHVQXEHRNDX-UHFFFAOYSA-N CC(C)c1cc(C(C)C)c(B(c(c(Sc2c3)c4)ccc4-[n](c4ccccc44)c5c4c(cccc4)c4cc5)c2ccc3-[n]2c(cc(c(c3ccccc33)c4)[n]3-c3ccccc3)c4c3c2cccc3)c(C(C)C)c1 Chemical compound CC(C)c1cc(C(C)C)c(B(c(c(Sc2c3)c4)ccc4-[n](c4ccccc44)c5c4c(cccc4)c4cc5)c2ccc3-[n]2c(cc(c(c3ccccc33)c4)[n]3-c3ccccc3)c4c3c2cccc3)c(C(C)C)c1 BWVNHVQXEHRNDX-UHFFFAOYSA-N 0.000 description 1
- FZGOMOIHPGOOCK-UHFFFAOYSA-N CC(C)c1cc(C(C)C)c(B(c(ccc(-[n]2c(c(c3ccccc33)c(cc4)[n]3-c3ccccc3)c4c3ccccc23)c2)c2Sc2c3)c2ccc3-[n]2c(c(c3c4cccc3)c(cc3)[n]4-c4ccccc4)c3c3ccccc23)c(C(C)C)c1 Chemical compound CC(C)c1cc(C(C)C)c(B(c(ccc(-[n]2c(c(c3ccccc33)c(cc4)[n]3-c3ccccc3)c4c3ccccc23)c2)c2Sc2c3)c2ccc3-[n]2c(c(c3c4cccc3)c(cc3)[n]4-c4ccccc4)c3c3ccccc23)c(C(C)C)c1 FZGOMOIHPGOOCK-UHFFFAOYSA-N 0.000 description 1
- BDLMNJFGTTWGFR-UHFFFAOYSA-N CC(C)c1cc(C(C)C)c(B2c(ccc(-[n]3c(c(cccc4)c4cc4)c4c4ccccc34)c3)c3Sc3cc(-[n](c4ccccc4c4c5c6ccccc66)c4ccc5[n]6-c4ccccc4)ccc23)c(C(C)C)c1 Chemical compound CC(C)c1cc(C(C)C)c(B2c(ccc(-[n]3c(c(cccc4)c4cc4)c4c4ccccc34)c3)c3Sc3cc(-[n](c4ccccc4c4c5c6ccccc66)c4ccc5[n]6-c4ccccc4)ccc23)c(C(C)C)c1 BDLMNJFGTTWGFR-UHFFFAOYSA-N 0.000 description 1
- JVOUCXCUYMJUEN-UHFFFAOYSA-N CC(C)c1cc(C(C)C)c(B2c(ccc(-[n]3c4cc5ccccc5cc4c4ccccc34)c3)c3Sc3cc(-[n]4c5ccc(c(cccc6)c6[n]6-c7ccccc7)c6c5c5ccccc45)ccc23)c(C(C)C)c1 Chemical compound CC(C)c1cc(C(C)C)c(B2c(ccc(-[n]3c4cc5ccccc5cc4c4ccccc34)c3)c3Sc3cc(-[n]4c5ccc(c(cccc6)c6[n]6-c7ccccc7)c6c5c5ccccc45)ccc23)c(C(C)C)c1 JVOUCXCUYMJUEN-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F5/00—Compounds containing elements of Groups 3 or 13 of the Periodic Table
- C07F5/02—Boron compounds
- C07F5/027—Organoboranes and organoborohydrides
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
- H10K50/115—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers comprising active inorganic nanostructures, e.g. luminescent quantum dots
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/658—Organoboranes
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
- C09K2211/1033—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom with oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
- C09K2211/1037—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom with sulfur
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
- C09K2211/104—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom with other heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1044—Heterocyclic compounds characterised by ligands containing two nitrogen atoms as heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1096—Heterocyclic compounds characterised by ligands containing other heteroatoms
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2101/00—Properties of the organic materials covered by group H10K85/00
- H10K2101/20—Delayed fluorescence emission
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
- H10K50/12—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers comprising dopants
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/18—Carrier blocking layers
- H10K50/181—Electron blocking layers
Definitions
- the present invention relates to a thermally activated delayed fluorescent material and an organic electroluminescent device (referred to as an organic EL device) using the material as a light emitting layer.
- Patent Document 1 discloses an organic EL device using a TTF (Triplet-Triplet Fusion) mechanism, which is one of the mechanisms of delayed fluorescence.
- TTF Triplet-Triplet Fusion
- the TTF mechanism utilizes the phenomenon that singlet excitons are generated by the collision of two triplet excitons, and it is theoretically thought that the internal quantum efficiency can be increased to 40%.
- the efficiency is lower than that of the phosphorescent light emitting type organic EL element, further improvement in efficiency is required.
- Patent Document 2 discloses an organic EL device using a Thermally Activated Delayed Fluorescence (TADF) mechanism.
- the TADF mechanism utilizes the phenomenon that reverse intersystem crossing from a triplet exciter to a singlet exciter occurs in a material with a small energy difference between the singlet level and the triplet level, and theoretically determines the internal quantum efficiency. It is believed that it can be increased to 100%.
- Patent Document 2 discloses a thermally activated delayed fluorescent material composed of an indolocarbazole compound as shown below.
- Patent Document 3 discloses an organic electroluminescent device using an indrocarbazole compound as shown below for a delayed fluorescent light emitting layer.
- Patent Document 4 discloses a thermally activated delayed fluorescent material as shown below.
- the organic EL element with thermal activated delayed fluorescence is also required to have further improved life characteristics as in the case of the phosphorescent light emitting type element. That is, a delayed fluorescent organic EL device using such a thermally activated delayed fluorescent light emitting material is characterized by high luminous efficiency, but further improvement is required.
- the present invention has been made in view of such a situation, and is a thermally activated delayed fluorescent material capable of obtaining a practically useful organic EL device having high efficiency of light emission and high drive stability. , And an organic EL device using the same.
- the present invention is a thermally activated delayed material represented by the following general formula (1), and the present invention is an organic EL element including one or more light emitting layers between the opposing anode and cathode.
- the organic EL element characterized in that at least one light emitting layer contains the thermal activated delayed fluorescent material.
- X 1 represents O or S.
- D is independently a nitrogen-containing heterocycle represented by Eq. (1a). * In the formula (1a) represents a position to be bonded to the ring of the general formula (1), and the ring Y is the position of the formula (1a-1), (1a-2), or (1a-3). Either.
- the ring Y is condensed at either the position of d or e.
- the ring Y is condensed at the position of g, and X 2 represents O, S, or N-Ar 2 .
- the ring Y is of the above formula (1a-3)
- the ring Y is condensed at any of the positions i, j, or k.
- Ar 1 to Ar 3 are substituted or unsubstituted aromatic hydrocarbon groups having 6 to 18 carbon atoms, substituted or unsubstituted aromatic heterocyclic groups having 3 to 17 carbon atoms, or 2 to 2 of these aromatic rings.
- R 1 to R 4 are heavy hydrogens, aliphatic hydrocarbon groups having 1 to 10 carbon atoms, aromatic hydrocarbon groups having 6 to 18 substituted or unsubstituted carbon atoms, or substituted or unsubstituted aromatic hydrocarbon groups having 3 to 17 carbon atoms.
- a and b represent the number of permutations and represent an integer of 0 or 1, but a and b cannot be 0 at the same time.
- c, f, h, and l represent the number of permutations and represent integers from 0 to 4.
- the ring Y is represented by the formula (1a-2) or (1a-3).
- X 1 in the general formula (1) is S.
- the difference between the singlet excitation energy (S1) and the triplet excitation energy (T1) of the thermally activated delayed fluorescent material represented by the general formula (1) is preferably smaller than 0.20 eV, preferably. It should be less than 0.10 eV.
- thermally activated delayed fluorescent material of the present invention by incorporating this into the light emitting layer, it becomes possible to provide an organic EL device having high luminous efficiency and long life.
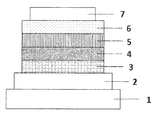
- FIG. 1 is a schematic cross-sectional view showing a structural example of the organic EL element used in the present invention.
- the organic EL element of the present invention has one or more light emitting layers between the facing anode and the cathode, and at least one of the light emitting layers is the thermally activated delayed fluorescence represented by the above general formula (1).
- Contains materials referred to as TADF materials.
- This organic EL device has a plurality of layers between the opposite anode and the cathode, and at least one of the plurality of layers is a light emitting layer, and the light emitting layer can contain a host material if necessary. Further, it can contain a luminescent dopant other than the thermally activated delayed fluorescent material represented by the general formula (1).
- the above general formula (1) will be described below.
- X 1 represents O or S. It preferably represents S.
- D is independently a nitrogen-containing heterocycle represented by the above formula (1a), and the ring Y in the above formula (1a) is the above formula (1a-1), (1a-2), or (1a-). It is any of 3), preferably the formula (1a-2) or (1a-3), and more preferably the formula (1a-3).
- the ring Y is of the above formula (1a-1)
- the ring Y is condensed at either the d or e position.
- the ring Y is of the above formula (1a-2
- the ring Y is condensed at the position of g.
- X 2 represents O, S, or N-Ar 2 , preferably N-Ar 2 .
- the ring Y is condensed at any position of i, j, or k, preferably at the position of i.
- a and b represent the number of permutations and represent an integer of 0 or 1, but a and b cannot be 0 at the same time.
- Ar 1 to Ar 3 are independently substituted or unsubstituted aromatic hydrocarbon groups having 6 to 18 carbon atoms, substituted or unsubstituted aromatic heterocyclic groups having 3 to 17 carbon atoms, or aromatic rings thereof.
- an unsubstituted aromatic hydrocarbon group having 6 to 15 carbon atoms, or an substituted or unsubstituted aromatic heterocyclic group having 3 to 15 carbon atoms, or 2 to 4 aromatic rings thereof are linked to each other.
- it is composed of an unsubstituted aromatic hydrocarbon group having 6 to 12 carbon atoms, an substituted or unsubstituted aromatic heterocyclic group having 3 to 12 carbon atoms, or a chain of 2 to 3 aromatic rings thereof. Represents a substituted or unsubstituted linked aromatic group to be substituted.
- unsubstituted Ar 1 to Ar 3 include benzene, naphthalene, acenaften, acenaphtylene, azulene, anthracene, chrysene, pyrene, phenanthrene, triphenylene, fluorene, benzo [a] anthracene, pyridine, pyrimidine, triazine, thiophene, Isothiazole, thiazole, pyridazine, pyrrol, pyrazole, imidazole, triazole, thiazylazole, pyrazine, furan, isoxazole, quinoline, isoquinoline, quinoxalin, quinazoline, thiazilazole, phthalazine, tetrazole, indol, benzofuran, benzothiophene, benzoxazole, benzothiazole, Indazole, benzimidazo
- a group produced by taking one hydrogen from the above can be mentioned.
- Each of these aromatic hydrocarbon groups, aromatic heterocyclic groups or linked aromatic groups may have a substituent.
- the substituents are a cyano group, an aliphatic hydrocarbon group having 1 to 10 carbon atoms, a diarylamino group having 12 to 44 carbon atoms, an alkoxy group having 1 to 10 carbon atoms, and 6 to 18 carbon atoms. It is an aryloxy group, an alkylthio group having 1 to 10 carbon atoms, and an arylthio group having 6 to 18 carbon atoms.
- the number of substituents is preferably 0 to 5, preferably 0 to 2.
- the carbon number calculation does not include the carbon number of the substituent. However, it is preferable that the total number of carbon atoms including the number of carbon atoms of the substituent satisfies the above range.
- substituents include cyano, methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, diphenylamino, naphthylphenylamino, dinaphthylamino, dianthranylamino and diphenan.
- substituents include cyano, methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, diphenylamino, naphthylphenylamino, dinaphthylamino, dianthranylamino and diphenan.
- examples thereof include threnylamino, dipyrenylamino, methoxy, ethoxy, phenol, diphenyloxy, methylthio, ethylthio, thi
- Preferred examples include cyano, methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, diphenylamino, naphthylphenylamino, dinaphthylamino, phenol, or thiophenol.
- R 1 to R 4 are independently each of a heavy hydrogen, an aliphatic hydrocarbon group having 1 to 10 carbon atoms, an substituted or unsubstituted aromatic hydrocarbon group having 6 to 18 carbon atoms, or an aromatic hydrocarbon group having 3 to 17 carbon atoms. Represents an aromatic heterocyclic group.
- a heavy hydrogen, an aliphatic hydrocarbon group having 1 to 8 carbon atoms, a substituted or unsubstituted aromatic hydrocarbon group having 6 to 15 carbon atoms, or an aromatic heterocyclic group having 3 to 15 carbon atoms is preferable. show.
- c, f, h, and l represent integers from 0 to 4.
- R 1 to R 4 are aliphatic hydrocarbon groups having 1 to 10 carbon atoms
- R 1 to R 4 are aliphatic hydrocarbon groups having 1 to 10 carbon atoms
- Preferred examples include methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl and octyl.
- R 1 to R 4 are an substituted aromatic hydrocarbon group having 6 to 18 carbon atoms or an unsubstituted aromatic heterocyclic group having 3 to 17 carbon atoms include benzene, naphthalene, and acenaphene.
- Dibenzoselenovene a group produced by taking one hydrogen from carbazole. More preferably, benzene, naphthalene, azulene, pyridine, pyrimidine, triazine, thiophene, isothiazole, thiazole, pyridazine, pyrrole, pyrazole, imidazole, triazole, thiazylazole, pyrazine, furan, isoxazole, quinoline, isothiazole, quinoxalin, quinazoline, thiazylazole.
- Each of these aromatic hydrocarbon groups or aromatic heterocyclic groups may have a substituent.
- the substituent is a cyano group, an aliphatic hydrocarbon group having 1 to 10 carbon atoms, and a diarylamino group having 12 to 44 carbon atoms.
- the number of substituents is preferably 0 to 5, preferably 0 to 2.
- the carbon number calculation does not include the carbon number of the substituent. However, it is preferable that the total number of carbon atoms including the number of carbon atoms of the substituent satisfies the above range.
- substituents include cyano, methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, diphenylamino, naphthylphenylamino, dinaphthylamino, dianthranylamino and diphenan. Examples thereof include threnylamino and dipyrenylamino.
- Preferred examples include cyano, methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl, octyl, diphenylamino, naphthylphenylamino, or dinaphthylamino.
- thermally activated delayed fluorescent material represented by the general formula (1) Specific examples of the thermally activated delayed fluorescent material represented by the general formula (1) are shown below, but the present invention is not limited to these exemplified compounds.
- S1 and T1 are measured as follows.
- a sample compound thermalally activated delayed fluorescent material
- a quartz substrate by a vacuum vapor deposition method under the conditions of a vacuum degree of 10 -4 Pa or less to form a thin-film vapor deposition film with a thickness of 100 nm.
- S1 measures the emission spectrum of this vapor-deposited film, draws a tangent to the rising edge of the emission spectrum on the short wavelength side, and shows the wavelength value ⁇ edge [nm] at the intersection of the tangent and the horizontal axis in the following equation (i). Substitute in to calculate S1.
- S1 [eV] 1239.85 / ⁇ edge (i)
- T1 measures the phosphorescence spectrum of the vapor deposition film, draws a tangent to the rising edge of the phosphorescence spectrum on the short wavelength side, and formulates the wavelength value ⁇ edge [nm] at the intersection of the tangent and the horizontal axis (ii). Substitute in to calculate T1.
- T1 [eV] 1239.85 / ⁇ edge (ii)
- thermally activated delayed fluorescent material represented by the general formula (1) into the light emitting layer as a light emitting dopant or TADF material, an excellent delayed fluorescent organic EL element can be obtained.
- the light emitting layer can contain a host material together with the thermal activated delayed fluorescent material represented by the general formula (1). By containing the host material, it becomes an excellent organic EL element.
- the light emitting layer contains the thermally activated delayed fluorescent material represented by the general formula (1) and a fluorescent light emitting material other than the one represented by the general formula (1) as a light emitting dopant. Can be done. By containing a fluorescent light emitting material, it becomes an excellent organic EL element. In this case, the light emitting layer can further contain a host material.
- FIG. 1 is a cross-sectional view showing a structural example of a general organic EL device used in the present invention, in which 1 is a substrate, 2 is an anode, 3 is a hole injection layer, 4 is a hole transport layer, and 5 is a light emitting layer. , 6 represent an electron transport layer, and 7 represents a cathode.
- the organic EL device of the present invention may have an exciton blocking layer adjacent to the light emitting layer, or may have an electron blocking layer between the light emitting layer and the hole injection layer.
- the exciton blocking layer can be inserted into either the cathode side or the anode side of the light emitting layer, and both can be inserted at the same time.
- the organic EL device of the present invention has an anode, a light emitting layer, and a cathode as essential layers, but it is preferable to have a hole injection transport layer and an electron injection transport layer in addition to the essential layers, and further, a light emitting layer and an electron injection. It is preferable to have a hole blocking layer between the transport layers.
- the hole injection transport layer means either or both of the hole injection layer and the hole transport layer
- the electron injection transport layer means either or both of the electron injection layer and the electron transport layer.
- the structure opposite to that of FIG. 1, that is, the cathode 7, the electron transport layer 6, the light emitting layer 5, the hole transport layer 4, the hole injection layer 3, and the anode 2 can be laminated in this order on the substrate 1. In some cases, layers can be added or omitted as needed.
- the organic EL element as described above in addition to the electrodes such as the anode and the cathode, the layers constituting the laminated structure on the substrate may be collectively referred to as an organic layer.
- the organic EL element of the present invention is preferably supported by a substrate.
- the substrate is not particularly limited as long as it is conventionally used for an organic EL element, and for example, a substrate made of glass, transparent plastic, quartz or the like can be used.
- anode material in the organic EL element a material having a large work function (4 eV or more), an alloy, an electrically conductive compound, or a mixture thereof is preferably used.
- electrode materials include metals such as Au, and conductive transparent materials such as CuI, indium tin oxide (ITO), SnO 2, and ZnO.
- conductive transparent materials such as CuI, indium tin oxide (ITO), SnO 2, and ZnO.
- an amorphous material such as IDIXO (In 2 O 3- ZnO) capable of producing a transparent conductive film may be used.
- a thin film may be formed by forming a thin film of these electrode materials by a method such as thin film deposition or sputtering, and a pattern of a desired shape may be formed by a photolithography method, or when pattern accuracy is not required so much (about 100 ⁇ m or more).
- the pattern may be formed through a mask having a desired shape during vapor deposition or sputtering of the electrode material.
- a coatable substance such as an organic conductive compound
- a wet film forming method such as a printing method or a coating method can also be used.
- the sheet resistance as the anode is preferably several hundred ⁇ / ⁇ or less.
- the film thickness depends on the material, but is usually selected in the range of 10 to 1000 nm, preferably 10 to 200 nm.
- the cathode material a material consisting of a metal having a small work function (4 eV or less) (referred to as an electron-injectable metal), an alloy, an electrically conductive compound, or a mixture thereof is used.
- electrode materials include sodium, sodium-potassium alloy, magnesium, lithium, magnesium / copper mixture, magnesium / silver mixture, magnesium / aluminum mixture, magnesium / indium mixture, aluminum / aluminum oxide (Al 2 O). 3 ) Examples include mixtures, indium, lithium / aluminum mixtures, rare earth metals and the like.
- a mixture of an electron injectable metal and a second metal which is a stable metal having a larger work function value than this, for example, a magnesium / silver mixture, magnesium.
- a magnesium / silver mixture for example, magnesium.
- Aluminum mixture, magnesium / indium mixture, aluminum / aluminum oxide (Al 2 O 3 ) mixture, lithium / aluminum mixture, aluminum and the like are suitable.
- the cathode can be produced by forming a thin film of these cathode materials by a method such as vapor deposition or sputtering.
- the sheet resistance of the cathode is preferably several hundred ⁇ / ⁇ or less, and the film thickness is usually selected in the range of 10 nm to 5 ⁇ m, preferably 50 to 200 nm.
- the emission brightness is improved, which is convenient.
- a transparent or translucent cathode can be produced. By applying this, it is possible to manufacture an element in which both the anode and the cathode have transparency.
- the light emitting layer is a layer that emits light after excitons are generated by recombination of holes and electrons injected from the anode and the cathode, respectively.
- the TADF material represented by the general formula (1) may be used alone, or this TADF material may be used together with the host material. When a TADF material is used with a host material, the TADF material becomes a luminescent dopant. Further, the TADF material represented by the general formula (1) may be used together with a fluorescent light emitting material other than the material represented by the general formula (1). When used with a fluorescent material, it may be further used with a host material. When the TADF material represented by the general formula (1) is used together with the fluorescent light emitting material, the fluorescent light emitting material becomes a light emitting dopant.
- the content of the luminescent dopant is preferably 0.1 to 50 wt%, more preferably 0.1 to 40 wt% with respect to the host material.
- a known host material used in a phosphorescent light emitting element or a fluorescent light emitting element can be used.
- Known host materials that can be used are compounds having hole transporting ability and electron transporting ability and having a high glass transition temperature, and T1 is larger than T1 of the TADF material represented by the general formula (1). It is preferable to have.
- a TADF active compound may be used as the host material, in which case a compound having ⁇ EST of 0.20 eV or less is preferable.
- Anthracene derivatives fluorenone derivatives, stylben derivatives, triphenylene derivatives, carborane derivatives, porphyrin derivatives, phthalocyanine derivatives, metal complexes of 8-quinolinol derivatives and metal phthalocyanine, various metal complexes represented by metal complexes of benzoxazole and benzothiazole derivatives, poly Examples thereof include polymer compounds such as (N-vinylcarbazole) derivatives, aniline-based copolymers, thiophene oligomers, polythiophene derivatives, polyphenylene derivatives, polyphenylene vinylene derivatives, and polyfluorene derivatives.
- polymer compounds such as (N-vinylcarbazole) derivatives, aniline-based copolymers, thiophene oligomers, polythiophene derivatives, polyphenylene derivatives, polyphenylene vinylene derivatives, and polyfluorene derivatives.
- each host can be vapor-deposited from different vapor deposition sources, or multiple types of hosts can be simultaneously vapor-deposited from one vapor deposition source by premixing them before vapor deposition to form a premixture. ..
- a method capable of mixing as uniformly as possible is desirable, and examples thereof include pulverizing and mixing, heating and melting under reduced pressure or in an atmosphere of an inert gas such as nitrogen, sublimation, and the like. It is not limited to the method.
- the form of the host and its premixture may be powder, stick or granular.
- the preferred host is not particularly limited, but specific examples include the following.
- examples of the fluorescent light emitting material include a fused polycyclic aromatic derivative, a styrylamine derivative, a condensed ring amine derivative, and boron.
- examples thereof include compounds, pyrrole derivatives, indole derivatives, carbazole derivatives, indolecarbazole derivatives and the like.
- a fused ring amine derivative, a boron-containing compound, a carbazole derivative, and an indolocarbazole derivative are preferable.
- fused ring amine derivative examples include a diaminepyrene derivative, a diaminochrysene derivative, a diaminoanthracene derivative, a diaminofluorenone derivative, and a diaminofluorene derivative in which one or more benzoflo skeletons are fused.
- boron-containing compound examples include a pyrromethene derivative and a triphenylborane derivative.
- the preferable fluorescent light emitting material other than the one represented by the general formula (1) is not particularly limited, but specific examples thereof include the following.
- the injection layer is a layer provided between the electrode and the organic layer in order to reduce the driving voltage and improve the emission brightness.
- the injection layer includes a hole injection layer and an electron injection layer, and is located between the anode and the light emitting layer or the hole transport layer. And may be present between the cathode and the light emitting layer or the electron transporting layer.
- the injection layer can be provided as needed.
- the hole blocking layer has the function of an electron transporting layer in a broad sense, and is made of a hole blocking material having a function of transporting electrons and a significantly small ability to transport holes, and is composed of a hole blocking material while transporting electrons. It is possible to improve the recombination probability of electrons and holes in the light emitting layer by blocking the above.
- a known hole blocking material can be used for the hole blocking layer. Further, a plurality of types of hole blocking materials may be used in combination.
- the electron blocking layer has a function of a hole transporting layer in a broad sense, and by blocking electrons while transporting holes, the probability of recombination of electrons and holes in the light emitting layer can be improved. ..
- As the material of the electron blocking layer a known electron blocking layer material can be used.
- the exciton blocking layer is a layer for blocking excitons generated by recombination of holes and electrons in the light emitting layer from diffusing into the charge transport layer, and excitons are inserted by inserting this layer. It is possible to efficiently confine it in the light emitting layer, and it is possible to improve the light emitting efficiency of the element.
- the exciton blocking layer can be inserted between two adjacent light emitting layers in an element in which two or more light emitting layers are adjacent to each other. As the material of such an exciton blocking layer, a known exciton blocking layer material can be used.
- the layers adjacent to the light emitting layer include a hole blocking layer, an electron blocking layer, an exciton blocking layer, and the like, but if these layers are not provided, the hole transport layer, the electron transport layer, and the like are adjacent layers. Become.
- the hole transport layer is made of a hole transport material having a function of transporting holes, and the hole transport layer may be provided with a single layer or a plurality of layers.
- the hole transport material has any of hole injection, transport, and electron barrier properties, and may be either an organic substance or an inorganic substance. Any compound can be selected and used for the hole transport layer from conventionally known compounds. Examples of such hole transport materials include porphyrin derivatives, arylamine derivatives, triazole derivatives, oxadiazole derivatives, imidazole derivatives, polyarylalkane derivatives, phenylenediamine derivatives, arylamine derivatives, amino-substituted chalcone derivatives, oxazole derivatives, and styrylanthracene.
- Examples thereof include derivatives, fluorenone derivatives, hydrazone derivatives, stylben derivatives, silazane derivatives, aniline-based copolymers, and conductive polymer oligomers, especially thiophene oligomers, but porphyrin derivatives, arylamine derivatives and styrylamine derivatives may be used. It is preferable to use an arylamine compound, and it is more preferable to use an arylamine compound.
- the electron transport layer is made of a material having a function of transporting electrons, and the electron transport layer may be provided with a single layer or a plurality of layers.
- the electron transport material (which may also serve as a hole blocking material) may have a function of transmitting electrons injected from the cathode to the light emitting layer.
- any conventionally known compound can be selected and used, for example, a polycyclic aromatic derivative such as naphthalene, anthracene, phenanthroline, tris (8-quinolinolate) aluminum (III).
- Derivatives phosphine oxide derivatives, nitro-substituted fluorene derivatives, diphenylquinone derivatives, thiopyrandioxide derivatives, carbodiimide, fleolenilidene methane derivatives, anthracinodimethane and antron derivatives, bipyridine derivatives, quinoline derivatives, oxadiazole derivatives, benzoimidazole Derivatives, benzothiazole derivatives, indrocarbazole derivatives and the like can be mentioned. Further, a polymer material in which these materials are introduced into a polymer chain or these materials are used as a polymer main chain can also be used.
- the film forming method for each layer is not particularly limited, and it may be manufactured by either a dry process or a wet process.
- P (t-Bu) 3 / HBF 4 tri-tert-butylphosphonium tetrafluoroborate
- tert-BuONa sodium tert-butoxide
- toluene 42 ml in a three-necked flask, stir at 80 ° C for 15 minutes, and then bis (dibenzylideneacetone) palladium (0) [Pd (dba) 2 ] 0.12 g. was added, and the mixture was stirred at 110 ° C. for 30 minutes.
- Example 1 Each of the thin films shown below was laminated with a vacuum degree of 4.0 ⁇ 10 -5 Pa on a glass substrate having an anode made of ITO with a film thickness of 70 nm formed by a vacuum vapor deposition method.
- HAT-CN shown above as a hole injection layer was formed on ITO to a thickness of 10 nm, and then HT-1 was formed to a thickness of 25 nm as a hole transport layer.
- BH-1 was formed to a thickness of 5 nm as an electron blocking layer.
- BH-1 as a host and compound (1-15) as a dopant were co-deposited from different vapor deposition sources to form a light emitting layer having a thickness of 30 nm.
- HBL-1 was formed to a thickness of 5 nm as a hole blocking layer.
- ET-1 was formed to a thickness of 40 nm as an electron transport layer.
- lithium fluoride (LiF) was formed on the electron transport layer as an electron injection layer to a thickness of 1 nm.
- Al aluminum
- Example 2 An organic EL device was produced in the same manner as in Example 1 except that the dopant was compound (1-11).
- Example 3 An organic EL device was produced in the same manner as in Example 1 except that the dopant was compound (1-81).
- Example 4 Each of the thin films shown below was laminated with a vacuum degree of 4.0 ⁇ 10 -5 Pa on a glass substrate on which an anode made of ITO with a film thickness of 70 nm was formed by a vacuum vapor deposition method.
- HAT-CN shown above as a hole injection layer was formed on ITO to a thickness of 10 nm, and then HT-1 was formed to a thickness of 25 nm as a hole transport layer.
- BH-1 was formed to a thickness of 5 nm as an electron blocking layer.
- BH-1 as a host, compound (1-11) as a TADF material, and BD-1 as a dopant were co-deposited from different vapor deposition sources to form a light emitting layer having a thickness of 30 nm.
- co-deposited under the vapor deposition conditions where the concentration of BH-1 was 69% by mass, the concentration of compound (1-11) was 29%, and the concentration of BD-1 was 2% by mass.
- HBL-1 was formed to a thickness of 5 nm as a hole blocking layer.
- ET-1 was formed to a thickness of 40 nm as an electron transport layer.
- lithium fluoride (LiF) was formed on the electron transport layer as an electron injection layer to a thickness of 1 nm.
- aluminum (Al) was formed on the electron injection layer as a cathode to a thickness of 70 nm to produce an organic EL device according to Example 4.
- Comparative Example 1 An organic EL device was produced in the same manner as in Example 1 except that the dopant was TD-1.
- Table 2 shows the maximum emission wavelength, external quantum efficiency, and lifetime of the emission spectrum of the manufactured organic EL device.
- the maximum emission wavelength and external quantum efficiency are the values when the drive current density is 2.5 mA / cm 2, which are the initial characteristics.
- the life was measured by measuring the time until the brightness attenuated to 50% of the initial brightness when the initial brightness was 500 cd / m 2.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Organic Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Nanotechnology (AREA)
- Optics & Photonics (AREA)
- Electroluminescent Light Sources (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202180044870.7A CN115943193A (zh) | 2020-07-08 | 2021-07-06 | 热活化延迟荧光材料、及有机电场发光元件 |
| KR1020227044920A KR20230035528A (ko) | 2020-07-08 | 2021-07-06 | 열활성화 지연 형광재료, 및 유기 전계 발광소자 |
| US18/011,403 US20230309400A1 (en) | 2020-07-08 | 2021-07-06 | Thermally activated delayed fluorescence material and organic electroluminescent element |
| EP21837328.0A EP4180500A1 (en) | 2020-07-08 | 2021-07-06 | Heat-activated delayed fluorescence material and organic electroluminescent element |
| JP2022535348A JPWO2022009883A1 (enExample) | 2020-07-08 | 2021-07-06 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020118001 | 2020-07-08 | ||
| JP2020-118001 | 2020-07-08 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022009883A1 true WO2022009883A1 (ja) | 2022-01-13 |
Family
ID=79553141
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/025469 Ceased WO2022009883A1 (ja) | 2020-07-08 | 2021-07-06 | 熱活性化遅延蛍光材料、及び有機電界発光素子 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20230309400A1 (enExample) |
| EP (1) | EP4180500A1 (enExample) |
| JP (1) | JPWO2022009883A1 (enExample) |
| KR (1) | KR20230035528A (enExample) |
| CN (1) | CN115943193A (enExample) |
| WO (1) | WO2022009883A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024228382A1 (ja) | 2023-05-02 | 2024-11-07 | キヤノン株式会社 | 有機化合物および有機発光素子 |
| WO2025041677A1 (ja) * | 2023-08-23 | 2025-02-27 | 日鉄ケミカル&マテリアル株式会社 | 有機電界発光素子用混合材料及び有機電界発光素子 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010134350A1 (ja) | 2009-05-22 | 2010-11-25 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子 |
| WO2011070963A1 (ja) | 2009-12-07 | 2011-06-16 | 新日鐵化学株式会社 | 有機発光材料及び有機発光素子 |
| WO2014050904A1 (ja) | 2012-09-28 | 2014-04-03 | 新日鉄住金化学株式会社 | 有機電界発光素子用化合物及び有機電界発光素子 |
| WO2015072537A1 (ja) | 2013-11-18 | 2015-05-21 | 国立大学法人九州大学 | 発光材料、有機発光素子および化合物 |
| US20180366653A1 (en) * | 2015-12-04 | 2018-12-20 | Guangzhou Chinaray Optoelectronic Materials Ltd. | D-a type compound and application thereof |
| CN110698504A (zh) * | 2019-10-31 | 2020-01-17 | 上海天马有机发光显示技术有限公司 | 硼杂环化合物、显示面板以及显示装置 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107602601B (zh) * | 2017-09-29 | 2020-05-05 | 中节能万润股份有限公司 | 一种含硼多元杂环有机化合物及其在有机电致发光器件中的应用 |
| CN109575056A (zh) * | 2017-09-29 | 2019-04-05 | 江苏三月光电科技有限公司 | 一种含硼有机化合物及其在有机电致发光器件中的应用 |
-
2021
- 2021-07-06 EP EP21837328.0A patent/EP4180500A1/en not_active Withdrawn
- 2021-07-06 US US18/011,403 patent/US20230309400A1/en active Pending
- 2021-07-06 JP JP2022535348A patent/JPWO2022009883A1/ja active Pending
- 2021-07-06 WO PCT/JP2021/025469 patent/WO2022009883A1/ja not_active Ceased
- 2021-07-06 CN CN202180044870.7A patent/CN115943193A/zh active Pending
- 2021-07-06 KR KR1020227044920A patent/KR20230035528A/ko not_active Withdrawn
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010134350A1 (ja) | 2009-05-22 | 2010-11-25 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子 |
| WO2011070963A1 (ja) | 2009-12-07 | 2011-06-16 | 新日鐵化学株式会社 | 有機発光材料及び有機発光素子 |
| WO2014050904A1 (ja) | 2012-09-28 | 2014-04-03 | 新日鉄住金化学株式会社 | 有機電界発光素子用化合物及び有機電界発光素子 |
| WO2015072537A1 (ja) | 2013-11-18 | 2015-05-21 | 国立大学法人九州大学 | 発光材料、有機発光素子および化合物 |
| US20180366653A1 (en) * | 2015-12-04 | 2018-12-20 | Guangzhou Chinaray Optoelectronic Materials Ltd. | D-a type compound and application thereof |
| CN110698504A (zh) * | 2019-10-31 | 2020-01-17 | 上海天马有机发光显示技术有限公司 | 硼杂环化合物、显示面板以及显示装置 |
Non-Patent Citations (1)
| Title |
|---|
| AHN DAE HYUN, LEE HYUNA, KIM SI WOO, KARTHIK DURAI, LEE JUNGSUB, JEONG HYEIN, LEE JU YOUNG, KWON JANG HYUK: "Highly Twisted Donor–Acceptor Boron Emitter and High Triplet Host Material for Highly Efficient Blue Thermally Activated Delayed Fluorescent Device", APPLIED MATERIALS & INTERFACES, AMERICAN CHEMICAL SOCIETY, US, vol. 11, no. 16, 24 April 2019 (2019-04-24), US , pages 14909 - 14916, XP055886593, ISSN: 1944-8244, DOI: 10.1021/acsami.9b00931 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024228382A1 (ja) | 2023-05-02 | 2024-11-07 | キヤノン株式会社 | 有機化合物および有機発光素子 |
| KR20250168432A (ko) | 2023-05-02 | 2025-12-02 | 캐논 가부시끼가이샤 | 유기 화합물, 유기 발광 소자, 표시장치, 전자기기, 및 조명장치 |
| WO2025041677A1 (ja) * | 2023-08-23 | 2025-02-27 | 日鉄ケミカル&マテリアル株式会社 | 有機電界発光素子用混合材料及び有機電界発光素子 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP4180500A1 (en) | 2023-05-17 |
| US20230309400A1 (en) | 2023-09-28 |
| CN115943193A (zh) | 2023-04-07 |
| KR20230035528A (ko) | 2023-03-14 |
| JPWO2022009883A1 (enExample) | 2022-01-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7456997B2 (ja) | 有機電界発光素子用溶融混合物、及び有機電界発光素子 | |
| JP5723764B2 (ja) | 有機電界発光素子 | |
| JP7037543B2 (ja) | 有機電界発光素子 | |
| WO2011049063A1 (ja) | 有機電界発光素子 | |
| EP4083033B1 (en) | Organic electroluminescent element | |
| JPWO2019171891A1 (ja) | 有機電界発光素子 | |
| KR20180099713A (ko) | 유기 전계 발광 소자 | |
| WO2022131123A1 (ja) | 有機電界発光素子及びその製造方法 | |
| WO2022149493A1 (ja) | 有機電界発光素子及びその製造方法 | |
| WO2021200250A1 (ja) | 有機電界発光素子 | |
| WO2022009883A1 (ja) | 熱活性化遅延蛍光材料、及び有機電界発光素子 | |
| JP7094215B2 (ja) | 熱活性化遅延蛍光発光材料、及び有機電界発光素子 | |
| WO2022045272A1 (ja) | 有機電界発光素子 | |
| KR20190111687A (ko) | 다환 화합물 및 이를 포함하는 유기 발광 소자 | |
| EP4486097A1 (en) | Material for organic electroluminescent element, and organic electroluminescent element | |
| EP4234562B1 (en) | Light-emitting material, and organic electroluminescent element | |
| WO2022215580A1 (ja) | 発光材料、及び有機電界発光素子 | |
| WO2022124366A1 (ja) | 有機電界発光素子 | |
| KR102870351B1 (ko) | 유기 전계 발광 소자용 재료 및 유기 전계 발광 소자 | |
| WO2024058146A1 (ja) | 発光材料、及び有機電界発光素子 | |
| WO2023157629A1 (ja) | 発光材料、及び有機電界発光素子 | |
| WO2022085776A1 (ja) | 有機電界発光素子用材料及び有機電界発光素子 | |
| CN119678677A (zh) | 有机电场发光元件用主体材料及预混合物以及有机电场发光元件 | |
| WO2022085777A1 (ja) | 有機電界発光素子用材料及び有機電界発光素子 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21837328 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2022535348 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2021837328 Country of ref document: EP Effective date: 20230208 |