WO2020196801A1 - 皮膚用組成物 - Google Patents
皮膚用組成物 Download PDFInfo
- Publication number
- WO2020196801A1 WO2020196801A1 PCT/JP2020/013856 JP2020013856W WO2020196801A1 WO 2020196801 A1 WO2020196801 A1 WO 2020196801A1 JP 2020013856 W JP2020013856 W JP 2020013856W WO 2020196801 A1 WO2020196801 A1 WO 2020196801A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cells
- col17a1
- substance
- extract
- skin
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/005—Enzyme inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/105—Plant extracts, their artificial duplicates or their derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/095—Sulfur, selenium, or tellurium compounds, e.g. thiols
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/12—Ketones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/155—Amidines (), e.g. guanidine (H2N—C(=NH)—NH2), isourea (N=C(OH)—NH2), isothiourea (—N=C(SH)—NH2)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
- A61K31/17—Amides, e.g. hydroxamic acids having the group >N—C(O)—N< or >N—C(S)—N<, e.g. urea, thiourea, carmustine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/21—Esters, e.g. nitroglycerine, selenocyanates
- A61K31/215—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids
- A61K31/216—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids of acids having aromatic rings, e.g. benactizyne, clofibrate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/21—Esters, e.g. nitroglycerine, selenocyanates
- A61K31/215—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids
- A61K31/235—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids having an aromatic ring attached to a carboxyl group
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/415—1,2-Diazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4409—Non condensed pyridines; Hydrogenated derivatives thereof only substituted in position 4, e.g. isoniazid, iproniazid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4468—Non condensed piperidines, e.g. piperocaine having a nitrogen directly attached in position 4, e.g. clebopride, fentanyl
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/517—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with carbocyclic ring systems, e.g. quinazoline, perimidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/551—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having two nitrogen atoms, e.g. dilazep
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/63—Compounds containing para-N-benzenesulfonyl-N-groups, e.g. sulfanilamide, p-nitrobenzenesulfonyl hydrazide
- A61K31/635—Compounds containing para-N-benzenesulfonyl-N-groups, e.g. sulfanilamide, p-nitrobenzenesulfonyl hydrazide having a heterocyclic ring, e.g. sulfadiazine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/661—Phosphorus acids or esters thereof not having P—C bonds, e.g. fosfosal, dichlorvos, malathion or mevinphos
- A61K31/6615—Compounds having two or more esterified phosphorus acid groups, e.g. inositol triphosphate, phytic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/30—Nerves; Brain; Eyes; Corneal cells; Cerebrospinal fluid; Neuronal stem cells; Neuronal precursor cells; Glial cells; Oligodendrocytes; Schwann cells; Astroglia; Astrocytes; Choroid plexus; Spinal cord tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/36—Skin; Hair; Nails; Sebaceous glands; Cerumen; Epidermis; Epithelial cells; Keratinocytes; Langerhans cells; Ectodermal cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/48—Reproductive organs
- A61K35/54—Ovaries; Ova; Ovules; Embryos; Foetal cells; Germ cells
- A61K35/545—Embryonic stem cells; Pluripotent stem cells; Induced pluripotent stem cells; Uncharacterised stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/24—Apocynaceae (Dogbane family), e.g. plumeria or periwinkle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/27—Asclepiadaceae (Milkweed family), e.g. hoya
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/28—Asteraceae or Compositae (Aster or Sunflower family), e.g. chamomile, feverfew, yarrow or echinacea
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/48—Fabaceae or Leguminosae (Pea or Legume family); Caesalpiniaceae; Mimosaceae; Papilionaceae
- A61K36/489—Sophora, e.g. necklacepod or mamani
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/53—Lamiaceae or Labiatae (Mint family), e.g. thyme, rosemary or lavender
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/58—Meliaceae (Chinaberry or Mahogany family), e.g. Azadirachta (neem)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/60—Moraceae (Mulberry family), e.g. breadfruit or fig
- A61K36/605—Morus (mulberry)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/61—Myrtaceae (Myrtle family), e.g. teatree or eucalyptus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/68—Plantaginaceae (Plantain Family)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/73—Rosaceae (Rose family), e.g. strawberry, chokeberry, blackberry, pear or firethorn
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/73—Rosaceae (Rose family), e.g. strawberry, chokeberry, blackberry, pear or firethorn
- A61K36/734—Crataegus (hawthorn)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/73—Rosaceae (Rose family), e.g. strawberry, chokeberry, blackberry, pear or firethorn
- A61K36/736—Prunus, e.g. plum, cherry, peach, apricot or almond
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/73—Rosaceae (Rose family), e.g. strawberry, chokeberry, blackberry, pear or firethorn
- A61K36/738—Rosa (rose)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/74—Rubiaceae (Madder family)
- A61K36/742—Coffea, e.g. coffee
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/75—Rutaceae (Rue family)
- A61K36/752—Citrus, e.g. lime, orange or lemon
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/82—Theaceae (Tea family), e.g. camellia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/88—Liliopsida (monocotyledons)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/88—Liliopsida (monocotyledons)
- A61K36/906—Zingiberaceae (Ginger family)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/12—Cyclic peptides, e.g. bacitracins; Polymyxins; Gramicidins S, C; Tyrocidins A, B or C
- A61K38/13—Cyclosporins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
- A61K38/1808—Epidermal growth factor [EGF] urogastrone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/35—Ketones, e.g. benzophenone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/40—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing nitrogen
- A61K8/43—Guanidines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/40—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing nitrogen
- A61K8/44—Aminocarboxylic acids or derivatives thereof, e.g. aminocarboxylic acids containing sulfur; Salts; Esters or N-acylated derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/494—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with more than one nitrogen as the only hetero atom
- A61K8/4946—Imidazoles or their condensed derivatives, e.g. benzimidazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/494—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with more than one nitrogen as the only hetero atom
- A61K8/4953—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with more than one nitrogen as the only hetero atom containing pyrimidine ring derivatives, e.g. minoxidil
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/4973—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with oxygen as the only hetero atom
- A61K8/498—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with oxygen as the only hetero atom having 6-membered rings or their condensed derivatives, e.g. coumarin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/55—Phosphorus compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/60—Sugars; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/64—Proteins; Peptides; Derivatives or degradation products thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/69—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing fluorine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/69—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing fluorine
- A61K8/70—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing fluorine containing perfluoro groups, e.g. perfluoroethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9789—Magnoliopsida [dicotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9794—Liliopsida [monocotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/14—Drugs for dermatological disorders for baldness or alopecia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/16—Emollients or protectives, e.g. against radiation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/18—Antioxidants, e.g. antiradicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
- A61P39/06—Free radical scavengers or antioxidants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/02—Preparations for care of the skin for chemically bleaching or whitening the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/08—Anti-ageing preparations
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/78—Connective tissue peptides, e.g. collagen, elastin, laminin, fibronectin, vitronectin or cold insoluble globulin [CIG]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/502—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing non-proliferative effects
- G01N33/5023—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing non-proliferative effects on expression patterns
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6881—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids from skin
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2121/00—Preparations for use in therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/136—Screening for pharmacological compounds
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/158—Expression markers
Definitions
- the present invention relates to a composition having a beneficial effect on the skin. More specifically, the present invention relates to a composition having an effect of promoting skin wound healing, an effect of suppressing skin aging, and / or an effect of enhancing the ability of epidermal stem cells to regenerate.
- the present invention also relates to the areas of pharmaceutical compositions, cosmetic compositions and beauty supplements in more detail.
- Animal organ / organ aging is a multi-step process that results in its structural and functional decline.
- the exact fate of depleted / stressed cells has been linked to cell senescence and / or stem cell depletion as cell kinetics, but is not known in detail. In general, little is known about the in vivo dynamics of cells that make up aging tissues / organs.
- Non-Patent Documents 1 and 2 This raises a new fundamental question as to whether large solid organs such as the skin have a unique aging program that governs the cellular dynamics of organ aging.
- Human skin exhibits skin atrophy (thinning), weakening, dryness, hyperpigmentation abnormality, delayed skin wound healing, and irritability with age.
- the skin loses its epidermal and dermal thickness, epidermal protrusions, epidermal keratinocytes, and a reserve of functional skin fibroblasts.
- Weakness of aged human skin is partly attributed to the remodeling of the dermis-epidermal junction, especially the hemidesmosome (HD) component, the structure that connects epithelial cells to the extracellular matrix of the basement membrane.
- HD hemidesmosome
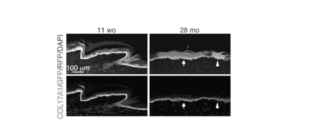
- COL17A1 gene deficiency is a rare form of junction epidermolysis bullosa (JEB) characterized by relatively mild skin weakening, atrophy, abnormal skin pigmentation (poikiloderma) and alopecia. causes benign generalized atrophic epidermolysis bullosa (GABEB). Consistent with its phenotype, previous studies have shown that Col17a1 gene deficiency in mice causes hair follicle aging with loss of hair follicle cells (Non-Patent Documents 2 and 1).
- One of the objects of the present invention is to provide a method and a composition for preventing / ameliorating skin ulcers, that is, promoting skin wound healing.
- One of the objectives is a method and composition for reducing or preventing skin disorders associated with cancer treatment.
- Another object of the present invention is to provide a method for suppressing skin aging, a composition for use in suppressing skin aging, and a composition for suppressing hair follicle aging, which is an appendage to the skin. Make one.
- the present invention provides a method for enhancing the regenerative capacity of epidermal stem cells, a composition for enhancing the regenerative capacity of epidermal stem cells (which may also include hair follicle stem cells), and compositions and methods for controlling stem cell competition. It is also one of the purposes to do.
- the present invention provides substances that are effective in reducing or preventing skin disorders associated with cancer treatment, promoting skin wound healing, suppressing skin aging, controlling cell competition, and / or improving the ability of epidermal stem cells to regenerate.
- One of the purposes is to provide a method for screening.
- mice with the same genetic and environmental background age-related skin atrophy, weakening, and hyperpigmentation abnormalities, as seen in aged human skin.
- the tail skin of C57BL6 inbred mice exhibiting impaired wound healing.
- the present inventor has found that skin homeostasis is maintained by epidermal cell competitive dynamics linked to COL17A1-mediated proliferation of epidermal stem cells, thereby controlling aging of skin organs.
- epidermal stem cell competitive dynamics mediate the epidermal aging process with decreased skin regeneration capacity and decreased epidermal melanocytes and dermal mesenchymal cells, thereby regulating the aging of the skin organs themselves. It was.
- COL17A1 is used in competition of epidermal stem cells and heterologous cells. It has been shown to regulate the aging of skin organs through maintenance.
- epidermal stem cells are cell compatible / stressed through the stability of the HD component, type XVII collagen (COL17A1). It was revealed that it senses.
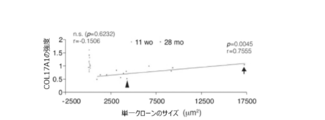
- COL17A1 high epidermal stem cells proliferate clonally through COL17A1-dependent symmetric cell division, thereby maintaining homeostasis from the skin. It was revealed that COL17A1 low / -depleted cells were eliminated.
- a composition for promoting skin wound healing or preventing or ameliorating skin ulcers or decubitus a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, and epidermal stem cells.
- a composition for use in suppressing skin aging a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, a substance that maintains the self-renewal ability of epidermal stem cells, and a genome in cells.
- a composition comprising, as an active ingredient, a substance selected from the group consisting of a substance that suppresses stress or oxidative stress, and a substance that suppresses DNA damage in cells.
- a composition for enhancing the regenerative ability of epidermal stem cells which induces or maintains the expression of COL17A1 in cells, suppresses the degradation of COL17A1 in cells, maintains the self-renewal ability of epidermal stem cells, and in cells.
- a composition comprising a substance selected from the group consisting of a substance that suppresses genomic stress or oxidative stress and a substance that suppresses DNA damage in cells as an active ingredient.
- a composition for use in the prevention or amelioration of skin disorders caused by anticancer agents which induces or maintains the expression of COL17A1 in cells, suppresses the degradation of COL17A1 in cells, and exhibits the self-renewal ability of epidermal stem cells.
- a composition comprising, as an active ingredient, a substance selected from the group consisting of a substance that maintains, a substance that suppresses genomic stress or oxidative stress in cells, and a substance that suppresses DNA damage in cells.
- a composition for use in anti-wrinkle a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, a substance that maintains the self-renewal ability of epidermal stem cells, genomic stress in cells, or A composition comprising, as an active ingredient, a substance selected from the group consisting of a substance that suppresses oxidative stress and a substance that suppresses DNA damage in cells.
- a composition for use in anti-staining which induces or maintains the expression of COL17A1 in cells, suppresses the degradation of COL17A1 in cells, maintains the self-renewal ability of epidermal stem cells, genomic stress in cells, or A composition comprising, as an active ingredient, a substance selected from the group consisting of a substance that suppresses oxidative stress and a substance that suppresses DNA damage in cells.
- a composition for use in improving rough skin a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, a substance that maintains the self-renewal ability of epidermal stem cells, and genomic stress in cells.
- a composition comprising, as an active ingredient, a substance selected from the group consisting of a substance that suppresses oxidative stress and a substance that suppresses DNA damage in cells.
- the composition according to any one of aspects 1 to 8, wherein the substance that induces or maintains the expression of COL17A1 in cells further maintains the self-renewal ability of epidermal stem cells.
- any one of aspects 1-9, wherein the substance that induces or maintains the expression of COL17A1 in cells is selected from the group consisting of NADPH oxidase inhibitors, COX inhibitors, iNOS inhibitors, ROCK inhibitors and estrogen-like substances.
- the NADPH oxidase inhibitor is selected from the group consisting of apocynin, ebselen, Diphenyleneiodonium (DPI), GKT137831, AEBSF, GK-136901, ML171, Coenzyme Q10 (CoQ10), VAS2870 and VAS3947.
- COX inhibitors are celecoxib, delacoxib, tolfenamic acid, nifluic acid, FR122047, LM-1685, SC-791, BTB02472, nimesulide, SPB04674, curcumin, diclofenac, 4'-hydroxydiclofenac, DuP-697, ebuserene, ETYA, fluvi Profen, ibuprofen, indomethacin, meloxicam, NPPB, NS-398, pterostilben, resveratrol, SC-560, SKF-86002, TXA (tranexamic acid), TXC (cetyl tranexamic acid hydrochloride), carrot extract, and sulfide
- the composition according to aspect 10 selected from the group consisting of sulindac.
- iNOS inhibitors are 1400W, L-NIL, aminoguanidine, BYK190123, S-ethylisothiourea, S-methylisothiourea, S-aminoethylisothiourea, 2-iminopiperidine, butylamine, ONO-1714 ((1S, 5S,) 6R, 7R) -7-Chloro-3-imino-5-methyl-2-azabicyclo [4.1.0] heptanehydrochloride), AMT hydrochloride (2-amino-5,6-dihydro-6-methyl- 4H-1,3-thiazine hydrochloride), AR-C102222, L-NG-nitroarginine, L-NG-monomethylarginine, L-nitroarginine methyl ester, L-NIO, dexamethasone, estrogen, astaxanthin, and apigenin.
- ROCK inhibitors are Y-27632, Ripasudil (K-115), Thiazovivin, Faszil (HA-1077), GSK429286A, RKI-1447, GSK269962, Netalsudil (AR-13324), Y-39983, ZINC00881524, KD025 , Hydroxyfasudil (HA-1100), GSK180736A and AT13148, the composition of embodiment 10.
- composition according to aspect 10 wherein the estrogen-like substance is selected from the group consisting of estrogen, ethinyl estradiol, biochanin A, soybean extract, isoflavone, barley extract, and Pueraria mirifica root extract.
- Substances that induce or maintain the expression of COL17A1 in cells are aposinine, evselene, selecoxib, apigenin, Y-27632, lipasyl, 1400W, ethynyl estradiol, biochanin A, necrostatin 1, rafuma extract, mulberry extract, gymnema extract.
- composition according to any one of aspects 1 to 9, wherein the substance that suppresses the degradation of COL17A1 in cells is selected from the group consisting of a neutrophil elastase (ELANE) inhibitor and a matrix metalloproteinase (MMP) inhibitor. ..
- ELANE neutrophil elastase
- MMP matrix metalloproteinase
- ELANE inhibitors are Sibelestat sodium hydrate (Monosodium N- ⁇ 2- [4- (2,2-dimethylpropanoyloxy) -phenylsulfonylamino] benzoyl ⁇ aminoacetate tetrahydrate), ONO-6818 (2- (5-Amino-6-) oxo-2-phenylhydropyrimidinyl) -N- [2- (5-tert-butyl-1,3,4-oxadiazol-2-yl) -1- (methylethyl) -2-oxoethyl] acetamide), ⁇ 1 antitrypsin ( ⁇ 1) -AT), Deperestat, Neilwan (isopropyloxopropylaminocarbonylpyrrolidin carbonylmethylpropylaminocarbonylbenzoylaminoacetate Na), perilla leaf fermented product, parsley fermented product, pepper fermented product, antibody against ELANE, ELANE The composition according to give the following
- composition according to embodiment 17, wherein the MMP inhibitor is selected from the group consisting of Marimastat, Batimastat, PD166793, Ro32-3555, WAY170523, UK370106, TIMP1, TIMP2, TIMP3, and TIMP4.
- the genomic stress is either UV, radiation or DNA damage stress due to an anticancer agent, or replication stress associated with cell division.
- composition according to aspect 2 wherein the skin aging is at least one selected from the group consisting of the following (a) to (f).
- composition according to aspect 26 which contains the active ingredient in an amount of 0.01 to 15% by weight.
- [Aspect 30] Promotes skin wound healing or prevents or improves skin ulcers, suppresses skin aging, controls cell competition, improves the ability of epidermal stem cells to regenerate, prevents or improves skin disorders caused by anticancer agents, anti-wrinkles, and / or rough skin It is a method for screening substances that are effective in improving the skin.
- i) In vitro contact between cells and test material A method comprising ii) measuring the expression of COL17A1 in cells, and iii) determining whether the test substance increases the expression of COL17A1.
- a kit for use in an evaluation method of skin aging which comprises an antibody against COL17A1, a probe for a gene encoding COL17A1, or a primer for amplifying the gene.
- a kit for evaluation of epidermal stem cells which comprises an antibody against COL17A1, a probe for a gene encoding COL17A1, or a primer for amplifying the gene.
- a kit for use in a method for selecting epidermal stem cells which comprises an antibody against COL17A1, a probe for a gene encoding COL17A1, or a primer for amplifying the gene.
- Epidermal stem cells and / or epidermis including the step of measuring the expression level of COL17A1 using an antibody against COL17A1, a probe for a gene encoding COL17A1 or a primer for amplifying the gene, and selecting epidermal stem cells having a high expression level of COL17A1. Cell amplification method.
- a kit for use in a method for amplifying epidermal stem cells and / or epidermal cells which comprises an antibody against COL17A1, a probe for a gene encoding COL17A1, or a primer for amplifying the gene.
- Substances that induce or maintain the expression of COL17A1 in cells substances that suppress the degradation of COL17A1 in cells, substances that maintain the self-renewal ability of epidermal stem cells, substances that suppress genomic or oxidative stress in cells, and DNA damage in cells.
- a functional food or beauty supplement comprising, as an active ingredient, a substance selected from the group consisting of substances that suppress the disease.
- the functional food or cosmetic according to embodiment 39, wherein the substance that induces or maintains the expression of COL17A1 in cells is selected from the group consisting of NADPH oxidase inhibitors, COX inhibitors, iNOS inhibitors, ROCK inhibitors and estrogen-like substances. supplement.
- Substances that induce or maintain the expression of COL17A1 in cells are aposinine, evselene, selecoxib, apigenin, Y-27632, lipasyl, 1400W, ethynyl estradiol, biochanin A, necrostatin 1, rafuma extract, mulberry extract, gymnema extract.
- Aspect 43 The functional food or beauty supplement according to any one of aspects 39-42, which is in the form of tablets, powders, semi-solids, jellies or liquids.
- the functional food or beauty supplement according to any one of aspects 39 to 43 further comprising at least one of a skin anti-aging agent, a vitamin preparation, collagen and a mineral.
- a cell composition comprising isolated cells expressing COL17A1.
- the cell composition according to aspect 45 wherein the cells are epidermal keratinocytes or mucosal epithelial keratinocytes.
- Aspect 48 The cell composition according to any one of aspects 45 to 47 for use in the treatment of skin diseases or injuries.
- the cell composition according to any one of aspects 45 to 48 comprising at least 1 ⁇ 10 3 cells.
- the cell composition according to any one of aspects 45 to 49 wherein at least 50% of the cells express COL17A1.
- the cell composition according to any one of aspects 45 to 50, wherein the cell is a cell derived from a stem cell.
- the cell composition according to any one of aspects 45 to 51, wherein the cells are frozen.
- Aspect 53 The cell composition according to any one of aspects 45 to 52, wherein the cells are contained in one container.
- the substance that induces or maintains the expression of COL17A1 in cells is selected from the group consisting of NADPH oxidase inhibitors, COX inhibitors, iNOS inhibitors, ROCK inhibitors and estrogen-like substances.
- Substances that induce or maintain the expression of COL17A1 in cells are aposinine, evselene, selecoxib, apigenin, Y-27632, lipasyl, 1400W, ethynyl estradiol, biochanin A, necrostatin 1, rafuma extract, mulberry extract, gymnema extract.
- a cell sheet comprising the cell composition according to any one of aspects 45 to 56.
- a composition for use in controlling cell competition a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, a substance that maintains the self-renewal ability of epidermal stem cells, and a genome in cells.
- a composition comprising, as an active ingredient, a substance selected from the group consisting of a substance that suppresses stress or oxidative stress and a substance that suppresses DNA damage in cells.
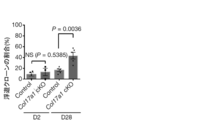
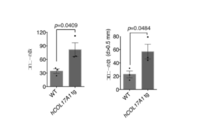
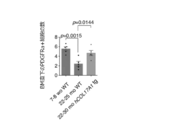
- Graph of HCS analysis of COL17A1 expression (corresponding to Step 1 of screening for competitive self-renewal promoter mediated by COL17A1). Treatment with apocynin or ebselen in HCS significantly increased the amount of COL17A1 compared to the control. A graph analyzing photographs and results of a colony forming assay (corresponding to Step 2 of screening for a competitive self-renewal promoter mediated by COL17A1 expression). Treatment with apocynin or ebselen significantly increased the number and area of colonies per well compared to controls. Decreased COL17A1 expression due to anticancer drugs and various stresses.
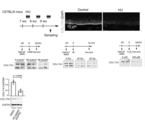
- Middle left figure Western blotting image of HaCaT cells examined for the amount of COL17A1 24 hours after irradiation with ultraviolet rays.
- Middle figure Western blotting image of HaCaT cells examined for the amount of COL17A1 72 hours after irradiation.
- Middle right figure Western blotting image of HaCaT cells treated with hydrogen peroxide and examined for the amount of COL17A1 24 hours later.
- the figure which shows the prevention and therapeutic effect of apocynin in the skin disorder (dry eczema / hair loss) model induced in the cervical-dorsal upper skin of aged mouse.
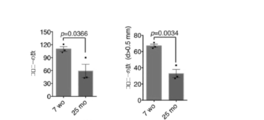
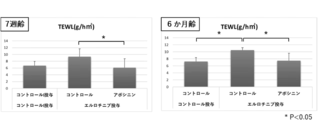
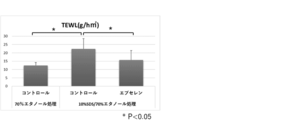
- a bar graph showing the effect of retaining water content in the stratum corneum (top) and the effect of suppressing the increase in TEWL (bottom) by a substance that promotes the cell competitiveness of epidermal stem cells.
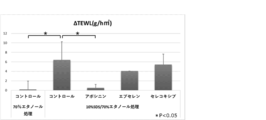
- a bar graph (top) showing the effect of suppressing the increase in TEWL caused by ultraviolet irradiation of epidermal stem cells by a competitive amplification agent for epidermal stem cells and a bar graph (bottom) showing the effect of improving the decrease in skin elasticity.
- the graph which shows the effect of improving skin elasticity by long-term continuous application of a competitive amplification agent and a comparative agent of epidermal stem cells (top).
- a bar graph showing TEWL after long-term continuous application of a competitive amplification agent and a comparative agent for epidermal stem cells (bottom).
- Retinol which is known as a wrinkle improver, significantly increases TEWL with long-term application.
- the TEWL value of the substance that promotes the cell competitive ability of epidermal stem cells is not much different from that of the control, and it is considered that it has an action of improving skin elasticity while maintaining the barrier function of the skin.
- High-resolution photograph showing the effect of improving the wrinkles caused by UV irradiation and the colored image of wrinkles (top), and the total wrinkle volume (mm 3 ) and total wrinkles by the competitive amplification agent and comparison agent of epidermal stem cells.
- Retinol which is known as a wrinkle improver, significantly increases TEWL with long-term application.
- the TEWL value of the substance that promotes the cell competitive ability of epidermal stem cells is not much different from that of the control, and is considered to have an effect of improving wrinkles while maintaining the barrier function of the skin.
- the graph shows "Ra (arithmetic mean roughness)” and "Rz (ten-point average roughness)", which are indicators of skin surface roughness.
- the graph which shows the action which suppresses the increase of TEWL associated with the rough human skin caused by SDS by the competitive amplification agent (apocynin, ebselen, celecoxib) of epidermal stem cells.
- the error bar in the graph indicates S.D. (standard deviation).
- COL17A1 type XVII collagen
- COL17A1 type XVII collagen
- Skin Wound Healing Skin wounds occur for a variety of reasons, including trauma, cuts, burns, poor blood circulation, bedsore ulcers, and diabetes, which temporarily impair normal function and skin structure. If the body is unable to heal these wounds, it becomes chronic and eventually becomes suppurated. Skin wounds are classified into acute skin wounds and chronic skin wounds. Acute skin wounds are wounds with normal wound healing mechanisms, such as fresh trauma and surgical wounds, and chronic skin wounds are wounds with some cause for which normal wound healing mechanisms do not work. The causes of prolonged healing of chronic skin wounds can be broadly divided into systemic factors such as underlying diseases and local factors. 1% of patients develop chronic skin wounds, but half of these wounds are not completely healed.
- the skin is an organ that occupies the largest area of the human body, acts as a natural barrier to the outside world, and protects the tissues inside from wear and infection.
- the skin is generally classified into three layers: epidermis, dermis, and subcutaneous tissue.
- the epidermis is the outermost layer and contains melanocytes (pigment cells) as well as keratinocytes (keratinocytes).
- the keratinocytes form the epidermis, line up in multiple layers, and cover the outer surface of the body.
- Melanocytes are distributed in the basal layer of the epidermis, give melanin pigment to the surrounding keratinocytes, and determine the skin tone by the distribution of pigment cells, the amount of melanin pigment, and the type of melanin.
- the epidermis is further classified into four layers from the deep part: the basal layer (basal cell layer), the spinous layer (spinned cell layer), the granular layer (granule cell layer), and the stratum corneum (keratinocyte layer).
- the basal layer consists of a single layer of basal cells, including keratinocyte stem cells.
- the basal layer has desmosomes, gap junctions, and hemidesmosomes as structures for binding to adjacent cells and the basement membrane under the basal cells.
- the stratum spinosum consists of 5 to 10 layers. In this layer, cells appear to be connected to each other by spines, hence the name spinous cells.
- the granular layer consists of 2 to 3 layers.
- the stratum corneum also called the stratum corneum, consists of about 10 layers. Enucleated and dead keratinocytes become membranous and layered as if they were covered with fallen leaves. The stratum lucidum is extremely thick on the sole of the palm and foot, and has a transparent layer (stratum lucidum) just below it. The cytoplasm of keratinocytes is filled with aggregated keratin fibers. In addition, hair follicles and sweat glands are continuously present in the epidermis, and they grow hair and discharge sweat as an appendage to the skin.
- Epidermal stem cells existing in the basal layer of the epidermis become the driving force for epidermal regeneration, and perform even division that divides horizontally with respect to the basement membrane or uneven division due to vertical division in a well-balanced manner. Then, the structure of the epidermis is maintained by supplying the differentiated epidermal keratinocytes toward the skin surface while performing self-replication. Keratinocytes make up most of the cells in the epidermis. Almost all of the cells found in the stratum corneum on the surface of the skin are dead cells, and when these dead cells are peeled off, new keratinocytes are raised from the lower layer and replaced. The process of lifting from below is constantly repetitive, with the entire skin surface being renewed in 15 to 30 days.
- the dermis layer is composed of fibroblasts, blood vessels, immune cells, skin appendages (hair follicles, sweat glands), and the extracellular matrix that supports these cells. Blood vessels nourish the skin, immune cells protect the skin, fibroblasts produce collagen proteins to give them strength, and they also produce elastin proteins to give them elasticity.
- the subcutaneous tissue is the layer of fat beneath the dermis layer that absorbs shock to the underlying muscles and bones and acts as another barrier to protect against infection.
- Endothelial cells create new blood vessels around the wound. These cell activities create a tissue called “granulation tissue” just below the blood clot.
- Granulation tissue refers to new tissue created as a repair / inflammatory reaction to tissue damage, and is composed of new blood vessels, connective tissue, fibroblasts, inflammatory cells, and the like.
- epidermis regeneration occurs from the epidermis around the defect and the skin appendages, and keratinocytes move from the wound edge to the wound and also from the hair follicle base. In deep skin defects with no residual appendages, the epidermis extends from the surroundings after the wound surface is replaced with granulation tissue. The keratinocytes divide on the granulation tissue and bring the epidermis together.
- this process occurs continuously, with epidermal keratinocytes migrating from around the wound or from the remaining appendages to restore the epidermis, and keratinocytes and fibroblasts are newly formed basement membrane matrix proteins. Is produced, and the interface between the epidermis and the dermis is regenerated.
- Hair follicles and sweat glands may also be destroyed if damage is made from the deep part of the dermis to the adipose tissue, such as in the case of severe deep burns or cuts, and the number of keratinocytes that can be used to repair the epidermis is reduced. As a result, full-thickness wounds take longer to heal and may require skin grafting (skin grafting), treatment with cultured skin such as epidermal sheets, or spraying of epidermal keratinocytes.
- compositions for Promotion of Skin Wound Healing or Prevention or Improvement of Skin Ulcers or Decubitus One of the embodiments of the present invention is for promotion of skin wound healing or prevention or amelioration of skin ulcers or decubitus.
- Compositions that induce or maintain the expression of COL17A1 in cells suppress the degradation of COL17A1 in cells, suppress genomic or oxidative stress in cells, DNA damage in cells (epidermal stem cells) (DNA) It relates to a composition comprising a substance as an active ingredient that suppresses a damage response or DNA damage itself).
- Substances that induce or maintain the expression of COL17A1 in cells As described above, the present inventor forcibly maintains the expression of COL17A1 in epidermal stem cells to prevent skin aging and improve or promote wound healing, or skin ulcers. Alternatively, they succeeded in preventing or ameliorating pressure ulcers, and showed that COL17A1 regulates aging of skin organs through competition of epidermal stem cells and maintenance of heterologous cells. The inventor has also shown that the matrix metalloproteinase (MMP) inhibitor marimastat stabilizes COL17A1 and blocks UV-induced downward inhibition of COL17A1.
- MMP matrix metalloproteinase
- the present inventor has used a ROCK inhibitor (Y27632) and a NADPH oxidase inhibitor (apocynin) as chemical substances that maintain or induce COL17A1 expression in keratinocytes in vitro and maintain and promote self-renewal ability in cultured keratinocytes. Identified.
- Y27632 (ROCK inhibitor) and apocynin (NADPH oxidase inhibitor) significantly promote the wound repair process, similar to transgenic mice that overexpress human COL17A1. Shown both in vitro and in vivo. Therefore, those skilled in the art may use substances that induce or maintain the expression of COL17A1 in cells, or substances that suppress the degradation of COL17A1 in cells, to promote or improve skin wound healing, or to prevent or improve skin ulcers or pressure ulcers. It is understood that it can be done.
- a substance that maintains the self-renewal ability of epidermal stem cells a substance that suppresses genomic stress or oxidative stress in cells, and a substance that suppresses DNA damage in cells are used to promote and improve skin wound healing. , Or it is understood that it can prevent or ameliorate skin ulcers or pressure ulcers.
- COL17A1 (XVII type collagen / BP180 / BPAG2) is a transmembrane protein type cell adhesion molecule and is a protein of hemidesmosome (hemidesmosome), which is one of the cell bonds of epithelial tissue.
- Substances that induce or maintain the expression of COL17A1 in cells include, but are not limited to, ROCK inhibitors, NADPH oxidase inhibitors, iNOS inhibitors, and COX inhibitors.
- Substances that induce or maintain COL17A1 expression in cells also include DNA or RNA encoding COL17A1.
- the DNA or RNA encoding COL17A1 may be introduced into the cell using a suitable vector.
- ROCK inhibitors examples include Y27632, Thiazovivin, Faszil (HA-1077), GSK429286A, RKI-1447, GSK269962, Netalszil (AR-13324), Y-39983, ZINC00881524, KD025, Ripasudil (K-115). ), Hydroxyfasudil (HA-1100), GSK180736A, AT13148, but not limited to these.
- NADPH oxidase inhibitors include, but are not limited to, apocynin, ebselen, Diphenyleneiodonium (DPI), GKT137831, AEBSF, GK-136901, ML171, Coenzyme Q10 (CoQ10), VAS2870, and VAS3947. It also contains plant extracts such as Rafuma extract containing apocynins.
- Rafuma extract (trade name: Venetron), mulberry extract (trade name: mulberry leaf extract powder), gymnema extract (trade name: gymnema extract powder), tea extract (trade name: Tiacaron 90), Biwa leaf extract (trade name: Biwa leaf extract powder), Maria thistle extract (trade name: Maria thistle extract powder), black corn extract (trade name: Sartmax) are Joban Botanical Chemicals Co., Ltd. It is made in the laboratory and is available.
- Seiyo Obako Seed Extract (Product Name: Absolute), Coffee Seed Extract (Product Name: cafe Noage), Somei Yoshino Leaf Extract (Product Name: Sakura Extract B), Melia Azajirakuta Leaf Extract (Product Name: Neem Leaf Liquid B), Mandarin orange peel extract (brand name: Mandarinkria), lavender flower extract (brand name: Ecofarm lavender B), clara root extract (brand name: Falcolex Clara B), rice bran extract (brand name: Falcorex rice bran BK), canina Rose extract (brand name: Falcolex Novara B), Hamemaris leaf extract (brand name: Falcolex Hamemaris B), Eucalyptus leaf extract (brand name: Falcolex Eucalyptus B), Sanzashi extract (brand name: Falcolex Sanzashi B) It is listed in the Ichimaru Falcos Co., Ltd. Product Guide and can be easily obtained.
- iNOS inhibitor either a selective iNOS inhibitor or a non-selective iNOS inhibitor can be used, but for example, a selective iNOS inhibitor is preferable from the viewpoint of side effects.
- iNOS inhibitors include 1400W, L-NIL, aminoguanidine, BYK190123, S-ethylisothiourea, S-methylisothiourea, S-aminoethylisothiourea, 2-iminopiperidine, butylamine, ONO-1714 (fusion piperidine).
- COX inhibitors include, for example, celecoxib, delacoxib, tolfenamic acid, FR122047, LM-1685, SC-791, BTB02472, nimesulide, SPB04674, curcumin, diclofenac, 4'-hydroxydiclofenac, DuP-697, ebuserene, ETYA, fluvi Profen, ibuprofen, indomethacin, meloxicam, nifluic acid, NPPB, NS-398, pterostilben, resveratrol, SC-560, SKF-86002, TXA (tranexamic acid), TXC (cetyl tranexamic acid hydrochloride), carrot extract , Astaxanthin, sulindac sulfide, but not limited to these.
- the COX inhibitor either a selective COX inhibitor or a non-selective COX inhibitor can be used, but for example, a selective COX-2 inhibitor is prefer
- estrogen-like substances include, but are not limited to, estrogen, ethinyl estradiol, biochanin A, soybean extract, isoflavone, barley extract, and Pueraria mirifica root extract.
- ELANE inhibitors include, for example, Sibelestat sodium hydrate (Monosodium N- ⁇ 2- [4- (2,2-dimethylpropanoyloxy) -phenylsulfonylamino] benzoyl ⁇ aminoacetate tetrahydrate; also called elastole), ONO-6818 (2-( 5-Amino-6-oxo-2-phenylhydropyrimidinyl) -N- [2- (5-tert-butyl-1,3,4-oxadiazol-2-yl) -1- (methylethyl) -2-oxoethyl] acetamide) , ⁇ 1 antitrypsin ( ⁇ 1-AT; A1AT), Deperestat (also called EPI-hNE4 or D
- the ELANE inhibitor may be an antibody against ELANE, siRNA against a gene encoding ELANE, an antisense oligonucleotide against a gene encoding ELANE, or the like.
- MMP inhibitors include, but are not limited to, Marimastat, Batimastat, PD166793, Ro32-3555, WAY170523, UK370106, TIMP1, TIMP2, TIMP3, TIMP4, and the like.
- a substance that maintains the self-renewal ability of epidermal stem cells The present inventor has found that maintenance of epidermal stem cells is made possible by the expression and maintenance of COL17A1 and is based on the self-renewal ability of epidermal stem cells. Therefore, in the present invention, substances that maintain the self-renewal ability of epidermal stem cells include ROCK inhibitors, NADPH oxidase inhibitors, iNOS inhibitors, and COX inhibitors, as well as growth factors and plant extracts.
- Maria thistle extract black turmeric extract, blue-green algae seed extract, coffee seed extract, someiyoshino leaf extract, melia azalea lacta leaf extract, mandarin orange peel extract, lavender flower extract, clara root extract, rice bran extract, canina rose fruit extract, Examples include Hamemaris leaf extract, Eucalyptus leaf extract, Sanzashi extract, and carrot extract.
- epidermal stem cells In vivo or in three-dimensional cultured epidermis, epidermal stem cells expressing a sufficient amount of COL17A1 competitively occupy and amplify the epidermis while competing with surrounding epidermal stem cells expressing low COL17A1. I will do it. Therefore, the self-renewal ability based on the expression of COL17A1 is called "competitive self-renewal ability".
- the self-renewal ability of epidermal stem cells includes, for example, the competitive self-renewal ability of epidermal stem cells, and the above-mentioned substances can also be called “competitive amplification agents for epidermal stem cells”.
- a substance that suppresses genomic stress or oxidative stress in cells The present inventor has also found that the expression of COL17A1 is reduced by various stresses.
- the stresses to be suppressed are genomic stress (genetic toxic stress) and oxidative stress
- the genomic stress includes genetic toxic stress such as ultraviolet rays, radiation stress, or DNA damage stress caused by anticancer agents.
- substances that suppress genomic or oxidative stress in cells, or substances that suppress DNA damage in cells can be used to promote and improve skin wound healing.
- Substances that suppress DNA damage in cells include Sarcandra glabra extract, Saraca dives extract, Cudrania pubescens extract, and Taki. Sodium distichum extract, Ludwigia octovalis extract, Deutzianthus tonkinensis extract, Alchornea trewioides extract, Berchemia polyphila. polyphylla) extract, Glochidion puberum extract, Sassafras tzumu extract (see Japanese Patent Laid-Open No.
- kina extract confrey extract
- coffee tree extract cucumber extract Thing
- Gobo extract Ouren extract
- Clara extract Chlorella extract
- Lavender extract Mematsuyoigusa extract
- Rose extract Mematsuyoigusa extract
- Amachazuru extract Mematsuyoigusa extract
- Enmeisou extract Odorikosou extract
- Carrot extract Shinanoki extract
- Jiyu extract lotus extract
- tea extract or okura extract
- the ability of a substance that suppresses DNA damage to suppress DNA damage is evaluated using any method known to those skilled in the art, such as a comet assay (COMET assay) or an assay that detects DNA damage focus induced by a DNA damage response. be able to.
- a substance that suppresses DNA damage a substance that promotes DNA damage repair may be used.
- an agent that promotes DNA damage repair for example, Suginori (Gigartinatenella) extract (see JP-A-2006-273761) or Kina extract (see WO2013 / 031003) can be used.
- the DNA damage repair ability of an agent that promotes DNA damage repair can be evaluated using any method known to those skilled in the art, such as a host-cell reactivation assay. Examples of substances that suppress genomic stress or oxidative stress in cells include, but are not limited to, antioxidants, UV absorbers, UV scatterers, radioprotectors, and DNA damage repair promoters.
- composition for use in suppressing skin aging is a composition for use in suppressing skin aging, which is a substance that induces or maintains the expression of COL17A1 in cells, COL17A1 in cells. It is characterized by containing as an active ingredient a substance that suppresses degradation, a substance that maintains the self-renewal ability of epidermal stem cells, a substance that suppresses genomic stress or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells. With respect to the composition.
- One of the embodiments of the present invention may be, in particular, a composition for use in suppressing epidermal aging.
- Some of the embodiments of the present invention may be compositions for use, in particular, for suppressing skin aging, including hair follicles.
- a part of the embodiment of the present invention may be a composition for use in suppressing aging of the skin excluding hair follicles.
- composition for use in suppressing skin aging contains the active ingredient described in the above-mentioned "Composition for promoting skin wound healing or preventing or ameliorating skin ulcer or pressure ulcer".
- active ingredient of the composition can be applied in the same manner as described above.
- Skin function is maintained by epidermal stem cells present in the basal layer of the epidermis and their normal differentiation.
- Progeny cells of epidermal stem cells adhere vertically to the skin surface and are arranged to form a layer with a certain thickness or more to form a normal keratinization, thereby maintaining the skin's ability to regenerate and barrier to the outside world. It retains the strength to prevent erosion and ulceration.
- they have come to show common characteristics as changes that cannot play their role due to aging or various stresses, and these are collectively called skin aging.
- the appearance of the skin loses its fineness and becomes uneven, causing crepe wrinkles and fine wrinkles.
- the epidermis, dermis and / or fatty tissue becomes thinner or weaker, which makes it more likely to cause redness, eczema, sores, erosions and ulcers.
- the shade of pigment on the skin becomes conspicuous.
- wrinkles are formed due to a decrease in collagen in the dermis, thinning (atrophy) of the epidermis, dermis, and adipose tissue, decreased tension and weakness, as well as spots, senile pigmented spots, or depigmented spots.
- the present inventor has histologically atrophy of the epidermis, a decrease in pigment cells, a decrease or immaturity of hemidesmosome components in the basement membrane, especially a decrease in COL17A1 and hemisome, as common items in aged skin of mice and humans. Decrease in desmosomes, disappearance of fibroblasts in the upper dermis, or wrinkles and fine wrinkles due to dry skin were observed, and PDGFR ⁇ + mesenchymal cells in the upper dermis were also decreased.
- COL17A1 deficiency makes the skin fragile, but the above findings can explain that wrinkles and erosions are likely to occur in aged skin, and ulcers are likely to occur.
- those skilled in the art may have substances that induce or maintain the expression of COL17A1 in cells, substances that suppress the degradation of COL17A1 in cells, substances that suppress genomic or oxidative stress in cells, and / or suppress DNA damage in cells. It is understood that skin aging can be suppressed by using substances that produce.
- One of the embodiments of the present invention is a composition for use against the following skin aging.
- (a) Thinning, weakening, atrophy or reduced tension of the epidermis, dermis and / or fatty fabric (b) Decreased epidermal barrier function or dry skin (c) Decrease or immaturity of basement membrane hemidesmosomes (d) Crepe wrinkles or fine wrinkles due to disappearance of fibroblasts in the upper dermis or dry skin (e) Wrinkle formation due to decreased collagen in the dermis (f) Spots, senile pigment spots, or depigmented spots
- One of the embodiments of the present invention is a composition for use in suppressing wrinkles in particular.
- One of the embodiments of the present invention is a composition for maintaining the regenerative ability or competitive self-renewal ability of epidermal stem cells, which is a substance that induces or maintains the expression of COL17A1 in cells, and decomposes COL17A1 in cells.
- the present invention relates to a composition comprising a substance that suppresses genomic stress or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells as an active ingredient.
- skin aging includes thinning of the epidermis, decreased barrier function, dryness, weakening of the basement membrane, wrinkle formation due to collagen reduction of the dermis, thinning of the epidermis, dermis and / or fatty tissue, With one or more of atrophy, reduced tension, weakening, age spots or senile pigment spots.
- Epidermal stem cells are stem cells that exist in the basal layer of the epidermis and supply new epidermal keratinocytes, play an important role in skin metabolism, and have a function of keeping the skin surface in a healthy state. When epidermal stem cells age due to aging, various genomic stresses, oxidative stress, and blockade of rapid self-renewal signals by molecular targets, the original self-renewal ability of epidermal stem cells decreases and is easily lost by final differentiation.
- epidermal stem cells with high expression of COL17A1 have high regenerative ability.
- those skilled in the art may have substances that induce or maintain the expression of COL17A1 in cells, substances that suppress the degradation of COL17A1 in cells, substances that suppress genomic or oxidative stress in cells, and / or suppress DNA damage in cells.
- the regenerative capacity of epidermal stem cells can be enhanced by using the substances that produce.
- Anti-wrinkle composition One of the embodiments of the present invention is a composition for use in anti-wrinkle, which is a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the decomposition of COL17A1 in cells, and cells. It is a composition characterized by containing a substance selected from the group consisting of a substance that suppresses genomic stress or oxidative stress in cells and a substance that suppresses DNA damage in cells as an active ingredient.
- Anti-wrinkle includes at least one selected from the group consisting of improved skin elasticity, improved stratum corneum function, improved skin moisturizing ability, and improved skin barrier function.
- skin elasticity refers to mechanical properties such as flexibility (softness) and elasticity (the degree to which stretched skin returns to its original state like a spring within a certain period of time).
- Skin elasticity is regarded as an index of skin age, and moisturized and elastic spring-like skin is felt to be "soft" and "tight”.
- Ultraviolet rays are thought to affect the decrease in skin elasticity, and the decrease in elasticity is noticeable in exposed areas such as the face exposed to ultraviolet rays.
- Skin elasticity is also called viscoelasticity, and by measuring the process from when the skin is deformed by applying force to the skin returning to its original position, Cutometer MPA580 (Courage + Khazaka, Germany, Integral Co., Ltd.) is used.
- the Cutometer is a device commonly used in the fields of dermatology and cosmetics science, and the principle is that negative pressure is applied to the skin using a probe with a hole with a diameter of 2 mm, and then negative pressure is applied. This is to measure the degree of return of the skin and measure the viscoelasticity.
- the following values are used for the analysis, and it is generally considered that the larger the value, the better the elasticity, and it decreases with aging.
- Ur / Uf value (R7) An index of elasticity expressed using the maximum elongation value (Uf or R0) and the height of elastic elongation during relaxation (Ur), which indicates the instantaneous return rate of the skin to maximum elongation. Shown. Ur / Ue (R5): An index of elasticity expressed using the height of elastic elongation during relaxation (Ur) and the height of elastic elongation when sucked into the probe (Ue). It shows an instantaneous return rate and is considered to be net elasticity.
- Ua / Uf (R2) An index of elasticity expressed using maximum elongation (Uf) and Ua, which indicates the instantaneous return rate of the skin to maximum elongation and is also called total elasticity. Ua is the difference between Uf and R1 (the height of residual strain after the first measurement cycle).
- Improvement of stratum corneum function Improvement of stratum corneum function is improvement of moisturizing ability to increase or retain the amount of water in the stratum corneum when applied to the skin, and improvement of the barrier function to suppress the invasion of foreign substances from the outside world.
- the function improving agent refers to one that can exert any of these effects.
- moisture moisturizing ability refers to retention of keratin water content, and the larger the keratin water content, the better the moisturizing ability.
- An agent for improving skin moisturizing ability refers to an agent capable of retaining these keratin water contents.
- Barrier function refers to suppression of transepidermal water loss, and the smaller the transdermal water evaporation amount (TEWL), the better the barrier function.
- the skin barrier function has the function of preventing water from evaporating from the body and the invasion of foreign substances from outside the body, and its function is reduced by ultraviolet irradiation.
- the water content of the stratum corneum, TEWL, and the thickness of the epidermis are used as indicators of the skin barrier function, and are indicators of the degree of skin damage caused by ultraviolet rays.
- An agent for improving the skin barrier function is one that can exert either an effect of preventing water evaporation and preventing foreign matter from entering from outside the body.
- Rough skin generally refers to a condition in which smoothness is lost from the surface of the skin and dryness and troubles appear on healthy skin that is well-textured and moisturized. Itching may occur, including superficial problems such as rough skin, redness, and unevenness. Rough skin is known to be closely related to the deterioration of skin barrier function. For this reason, a method of quantitatively analyzing and evaluating rough skin symptoms has been performed by measuring TEWL, which is an index of skin barrier function. Furthermore, since the surface roughness (unevenness) of the stratum corneum occurs with rough skin, the arithmetic average roughness (Ra), maximum height (Ry), and ten-point average roughness, which are the ISO standard surface roughness parameters of the skin, are generated.
- Rough skin can be quantitatively analyzed from numerical values such as (Rz).
- the skin surface can be photographed using a three-dimensional high-resolution imaging device PRIMOS-CR, and the surface analysis can be performed using dedicated software (Primos OMC3_22).
- the present inventor uses a rough skin model, which is a standard for skin disorders caused by chemical substances in humans, and suppresses the increase in TEWL associated with artificially induced rough skin and improves the roughness of the skin surface, thereby allowing the epidermal stem cells to self. We have found that substances that maintain the ability to replicate improve rough skin.
- one of the embodiments of the present invention is a composition for use in improving rough skin, which is a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, and epidermal stem cells.
- a composition characterized by containing, as an active ingredient, a substance selected from the group consisting of a substance that maintains self-renewal ability, a substance that suppresses genomic stress or oxidative stress in cells, and a substance that suppresses DNA damage in cells.
- Rough skin includes a condition in which the smoothness, humidity, smooth appearance and texture of the skin are lost, often accompanied by dryness, itchiness, redness, erosion, etc., and the composition of the present invention has the above-mentioned symptoms or The condition can be improved.
- Anti-spot composition Spots mean those with a pigment called melanin produced in the skin.
- "Nikko Kuroko” stress pigment spots
- the uniformity of pigments such as freckles, post-inflammatory hyperpigmentation, and chloasma has collapsed. Broadly means state. Therefore, "anti-staining” means maintaining the uniformity of the dye.
- a composition for use in anti-staining a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, a substance that maintains the self-renewal ability of epidermal stem cells,
- a composition characterized by containing, as an active ingredient, a substance selected from the group consisting of a substance that suppresses genomic stress or oxidative stress in cells and a substance that suppresses DNA damage in cells.
- Anti-cancer agent-induced skin disorder prevention / ameliorating agent One of the embodiments of the present invention relates to a method and a composition for preventing or ameliorating a skin disorder caused by anti-cancer agent treatment.
- Skin disorders frequently appear as side effects of treatment with anticancer drugs.
- hand-foot syndrome palm / sole redness dyssensitivity syndrome
- pigmentation pigmentation
- nail changes and aging-like skin disorders
- Conventionally used metabolic antagonists, alkylating agents, and platinum preparations cause dryness, atrophy, fragility, decreased barrier function, dyschromia, hair loss, and the like.
- molecular-targeted drugs that selectively attack molecules specifically expressed in cancer cells have been developed and used in various cancers such as breast cancer and lung cancer. It is replacing the anti-cancer drug of.
- molecular target drugs (various multityrosine kinase inhibitors, EGF receptor inhibitors, etc.) ) causes erythematous rash, acne-like rash, seborrheic dermatitis, pigmentation of hair and nails, peritonitis, dry / cracked skin, xeroderma, itching, strong tingling sensation, etc. Seen at. In addition, the appearance of skin disorders is also observed in new immunotherapeutic agents (immune checkpoint inhibitors).
- Skin disorders caused by these molecular-targeted drugs are not easily regarded as harmful, and are evaluated as a manifestation of pharmacological action on skin cells expressing molecules common to cancer cells or as an index of the effect of anticancer drugs, and moisturize.
- Anti-cancer treatment is continued while continuing symptomatic treatment with drugs. However, it causes great mental distress and burden on patients, and there are many cases in which they give up treatment by themselves.
- administration of anticancer drugs chemotherapy and molecular-targeted drugs
- is discontinued due to severe skin disorders but there is no appropriate preventive method or local treatment method.
- the present invention is a composition for use in preventing or ameliorating skin disorders caused by anticancer agents, a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, and epidermal stem cells.
- the active ingredient is a substance selected from the group consisting of a substance that maintains the self-renewal ability of the cell, a substance that suppresses genomic stress or oxidative stress in the cell, and a substance that suppresses DNA damage in the cell.
- the composition is provided.
- the preventive / ameliorating agent for skin disorders according to the present invention may be characterized in that, in one of the embodiments, a substance that maintains the expression of COL17A1 in cells and the self-renewal ability is contained as an active ingredient. .. More specifically, the preventive / ameliorating agent for skin disorders according to the present invention, as a substance that induces or maintains the expression of COL17A1 in cells, is a ROCK inhibitor, NADPH oxidase inhibitor, iNOS inhibitor, and / or COX inhibitor. May include agents.
- the preventive / ameliorating agent for skin disorders is a substance that maintains the expression of COL17A1 in cells and maintains the self-renewal ability, such as aposinine, ebselene, selecoxib, apigenin, Y-27632, lipasyl, 1400W.
- Plant Extracts in the present invention, various plant extracts can be used for the above-mentioned uses (below). ⁇ Promotion of skin wound healing or prevention / or improvement of skin ulcer ⁇ Suppression of skin aging ⁇ Enhancement of regenerative ability of epidermal stem cells ⁇ Prevention or improvement of skin disorders by anticancer agents ⁇ Anti-wrinkle (improvement of skin elasticity, improvement of skin elasticity, Improvement of stratum corneum function, improvement of skin moisturizing ability, and improvement of skin barrier function) -Anti-staining Further, in the present invention, the plant extract can also be used as a COL17A1 accelerator and a hair loss / gray hair inhibitor.
- Rafuma As raw materials for the plant extract used in the present invention, Rafuma (Apocynum venetum), Mulberry (Morus alba), Gymnema (Gymnema sylvestre), Tea (Camellia sinensis), Milk thistle (Silybum marianum), Biwa leaf (Eriobotrya japonica) , Black thistle (Kaempferia parviflora), Otanen carrot (Panax ginseng), Ashwagandha (Indian ginseng), etc.
- the sites of use of these plants are not limited as long as the above effects can be obtained.
- each plant extract is not particularly limited, and can be performed according to a method well known to those skilled in the art.
- the extraction solvent water, an alcohol solvent, and an organic solvent such as acetone, esters, polyhydric alcohols, and ethers can be used. Therefore, for example, the above-mentioned plant-based ethanol extract, hot water extract, 1,3-butylene glycol extract and the like can be prepared. These solvents may be used alone or in combination.
- the amount of extraction solvent, extraction temperature, extraction time and extraction method are not limited as long as the present composition exhibiting the above effects can be obtained.
- the extract may be the filtrate itself obtained by filtering the obtained extract, the concentrated solution obtained by concentrating the filtrate, the dried product obtained by drying the concentrated solution, or a crude or purified product thereof.
- the concentration method and the drying method can be performed by any method. Excipients such as dextrin may be added if necessary. Purification can be carried out according to means known to those skilled in the art such as synthetic adsorption resin, activated carbon, ion exchange resin, column chromatography, and recrystallization.
- the present composition takes a form as a food composition, a pharmaceutical composition, or a cosmetic composition, and particularly in the case of a food composition, it may take a form as a food with functional claims or a health food.
- compositions according to the present invention may be a pharmaceutical composition.
- One of the embodiments of the present invention is a pharmaceutical composition for use in promoting skin wound healing or preventing or ameliorating skin ulcers or decubitus, which is a substance that induces or maintains the expression of COL17A1 in cells, in cells.
- a pharmaceutical composition for use in promoting skin wound healing or preventing or ameliorating skin ulcers or decubitus which is a substance that induces or maintains the expression of COL17A1 in cells, in cells.
- Contains as active ingredients a substance that suppresses the degradation of COL17A1, a substance that maintains the self-renewal ability of epidermal stem cells, a substance that suppresses genomic or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells. It relates to a characteristic pharmaceutical composition.
- the pharmaceutical composition according to the present invention can be used for the treatment of acute skin wounds or the prevention or treatment of chronic skin wounds.
- one of the embodiments of the present invention induces or maintains the expression of COL17A1 in cells in the manufacture of a medicament for promoting skin wound healing or preventing or ameliorating skin ulcers or decubitus.
- substances that suppress the degradation of COL17A1 in cells substances that maintain the self-renewal ability of epidermal stem cells, substances that suppress genomic or oxidative stress in cells, and / or substances that suppress DNA damage in cells. ..
- one of the embodiments of the present invention is a pharmaceutical composition for use in suppressing skin aging, which is a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses decomposition of COL17A1 in cells, and epidermis.
- the present invention relates to a pharmaceutical composition, which comprises, as an active ingredient, a substance that maintains the self-renewal ability of stem cells, a substance that suppresses genomic stress or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells.
- the pharmaceutical composition according to the present invention can be used for the prevention or treatment of skin symptoms of diseases associated with the progression of skin aging.
- one of the embodiments of the present invention is a substance that induces or maintains the expression of COL17A1 in cells in the manufacture of a medicament for use in suppressing skin aging, and suppresses the degradation of COL17A1 in cells.
- one of the embodiments of the present invention is a pharmaceutical composition for enhancing the regenerative ability of epidermal stem cells, which is a substance that induces or maintains the expression of COL17A1 in cells, and a substance that suppresses the degradation of COL17A1 in cells.
- the present invention relates to a pharmaceutical composition comprising a substance that maintains the self-renewal ability of epidermal stem cells, a substance that suppresses genomic stress or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells as an active ingredient. ..
- the pharmaceutical composition according to the present invention can be used for the prevention or treatment of skin symptoms of diseases associated with a decrease in the ability of epidermal stem cells to regenerate.
- one of the embodiments of the present invention is in the manufacture of a drug for enhancing the regenerative ability (self-renewal ability) of epidermal stem cells or a regenerative medical product (processed cell, processed cell sheet, etc.).
- Substances that induce or maintain the expression of COL17A1 in cells substances that suppress the degradation of COL17A1 in cells, substances that maintain the self-renewal ability of epidermal stem cells, substances that suppress genomic or oxidative stress in cells, and / or in cells.
- substances that suppress DNA damage are examples of substances that suppress DNA damage.
- one of the embodiments of the present invention is a substance that resists a molecular target drug that impairs the regenerative ability (self-renewal and maintenance) of epidermal stem cells, and is a substance that induces or maintains the expression of COL17A1 in the cell, in the cell.
- the active ingredient is a substance that suppresses the degradation of COL17A1, a substance that maintains the self-renewal ability of epidermal stem cells, a substance that suppresses genomic stress or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells. It relates to a characteristic pharmaceutical composition.
- one of the embodiments of the present invention is a pharmaceutical product for use in preventing or ameliorating skin disorders caused by anticancer agents, which is a substance that induces or maintains the expression of COL17A1 in cells, COL17A1 in cells. It is characterized by containing as an active ingredient a substance that suppresses degradation, a substance that maintains the self-renewal ability of epidermal stem cells, a substance that suppresses genomic stress or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells. With respect to pharmaceutical compositions.
- the pharmaceutical composition according to the present invention can be used for prevention or amelioration of skin disorders caused by anticancer agents.
- one of the embodiments of the present invention is a substance, cell, that induces or maintains the expression of COL17A1 in cells in the manufacture of a medicament for use in preventing or ameliorating skin damage caused by an anticancer agent.
- substances that suppress the degradation of COL17A1 substances that maintain the self-renewal ability of epidermal stem cells, substances that suppress genomic or oxidative stress in cells, and / or substances that suppress DNA damage in cells.
- one of the embodiments of the present invention is a pharmaceutical product for use in anti-wrinkle, which is a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, and epidermal stem cells.
- the present invention relates to a pharmaceutical composition, which comprises, as an active ingredient, a substance that maintains self-renewal ability, a substance that suppresses genomic stress or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells.
- the pharmaceutical composition according to the present invention can be used for anti-wrinkle.
- one of the embodiments of the present invention is a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, in the production of a medicament for use in anti-wrinkle.
- one of the embodiments of the present invention is a pharmaceutical product for use in improving rough skin, which is a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, and epidermal stem cells.
- the present invention relates to a pharmaceutical composition comprising a substance that maintains the self-renewal ability of the cell, a substance that suppresses genomic stress or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells as an active ingredient.
- the pharmaceutical composition according to the present invention can be used for improving rough skin.
- one of the embodiments of the present invention is a substance that induces or maintains the expression of COL17A1 in cells and a substance that suppresses the decomposition of COL17A1 in cells in the manufacture of a medicine for use in improving rough skin.
- substances that maintain the self-renewal ability of epidermal stem cells substances that suppress genomic or oxidative stress in cells, and / or substances that suppress DNA damage in cells.
- the pharmaceutical composition according to the present invention can be used for anti-staining.
- one of the embodiments of the present invention is a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, in the manufacture of a drug for use in anti-staining.
- substances that act as active ingredients include, for example, ROCK inhibitors, NADPH oxidase inhibitors, iNOS inhibitors, COX inhibitors, and ELANE as described in other parts of the present specification.
- Inhibitors and the like more specifically, include, but are not limited to, Y27632, apocynin, and sivelestat.
- the pharmaceutical composition according to the present invention can take any form such as tablets, powders, liquids and semi-solids.
- the pharmaceutical composition according to the present invention may be an external preparation such as a coating agent used for topical treatment applied through the skin or directly to a skin lesion, and may be prepared by blending various main agents with a base.
- the pharmaceutical composition according to the present invention contains pharmaceutically acceptable excipients, additives, buffers, salts for adjusting isotonicity, antioxidants, preservatives, and drug stabilizers. May include agents and the like.
- Excipients include, but are not limited to, water, purified water, alcohol, glycerin, lactose, starch, dextrin, sucrose, precipitated silica, honey, rice starch, and tragant.
- other active ingredients may be blended in the pharmaceutical composition according to the present invention.
- the blending amount of each component can be appropriately determined within a range permitted as a medicine.
- the dose of the composition can be appropriately determined according to the type of the drug to be used and the subject to be administered.
- the active ingredient can be 0.01 to 15% by weight, for example 0.1 to 5% by weight.
- the route of administration can also be appropriately determined according to the type of drug to be used and the subject to be administered.
- the pharmaceutical composition according to the present invention may be contained in a wound dressing such as a dressing material.
- Dressings are medical materials that can maintain a moist environment and provide an optimal environment for wound healing.
- One of the embodiments of the present invention is a method for promoting skin wound healing in a mammal, a method for preventing or ameliorating skin ulcer or pressure ulcer, a method for suppressing skin aging, and an ability to regenerate epidermal stem cells. It relates to a method for enhancing skin damage, a method for preventing or ameliorating skin disorders caused by anticancer agents, an anti-wrinkle method, an anti-spot method, and / or a method for improving rough skin.
- Such methods induce or maintain expression of COL17A1 in cells, suppress degradation of COL17A1 in cells, suppress genomic or oxidative stress in cells, and / or suppress DNA damage in cells. May include steps. More specifically, one of the embodiments of the present invention may include administering to a mammal, for example, a ROCK inhibitor, a NADPH oxidase inhibitor, an iNOS inhibitor, and / or a COX inhibitor.
- a mammal for example, a ROCK inhibitor, a NADPH oxidase inhibitor, an iNOS inhibitor, and / or a COX inhibitor.
- Mammals include, for example, humans, monkeys, mice, rats, rabbits, dogs, cats, sheep, goats, alpaca, horses, cows, pigs, minks, foxes, ten, raccoon dogs, chinchillas, sea otters, otters, beavers, seals. Etc. are included.
- the dose can be appropriately determined according to the type of the drug to be used and the subject to be administered (similar to the above).
- the route of administration can also be appropriately determined according to the type of drug to be used and the subject to be administered.
- Preferred routes of administration include, for example, application or spraying of liquids, lotions and creams to the skin, subcutaneous injection of liquids, and oral administration of solids and liquids.
- a patch containing the composition according to the present disclosure may be prepared and applied to the skin.
- Dyschromia Prevention and Improvement Agent One of the embodiments of the present invention relates to a method and a composition for preventing or ameliorating dyschromia in aged skin. Such methods induce or maintain expression of COL17A1 in cells, suppress degradation of COL17A1 in cells, suppress genomic or oxidative stress in cells, and / or suppress DNA damage in cells. May include steps. Such compositions also include substances that induce or maintain the expression of COL17A1 in cells, substances that suppress the degradation of COL17A1 in cells, substances that suppress genomic or oxidative stress in cells, and / or DNA damage in cells. May contain substances that suppress.
- One of the embodiments of the present invention relates to a method and composition for preventing or ameliorating crepe wrinkles, fine wrinkles, dryness and sores due to age-related changes in the skin.
- Such methods induce or maintain expression of COL17A1 in cells, suppress degradation of COL17A1 in cells, suppress genomic or oxidative stress in cells, and / or suppress DNA damage in cells. May include steps.
- Such compositions also include substances that induce or maintain the expression of COL17A1 in cells, substances that suppress the degradation of COL17A1 in cells, substances that suppress genomic or oxidative stress in cells, and / or DNA damage in cells. May contain substances that suppress.
- the method according to the present invention is a step of inducing or maintaining the expression of COL17A1 in cells, a step of suppressing degradation of COL17A1 in cells, a step of suppressing genomic stress or oxidative stress in cells, and / or DNA damage in cells. May include a step of suppressing.
- composition according to the present invention includes a substance that induces or maintains the expression of COL17A1 in cells, a substance that suppresses the degradation of COL17A1 in cells, a substance that suppresses genomic stress or oxidative stress in cells, and / or DNA in cells. It may contain substances that suppress damage. It will also be appreciated by those skilled in the art that inhibition of COL17A1 can negatively control cell competition.
- Beauty supplements or functional foods One of the embodiments of the present invention is a substance capable of maintaining the expression of COL17A1 in cells and a competitive self-renewal ability based on the expression, a substance suppressing the degradation of COL17A1 in cells, a substance maintaining the self-renewal ability of epidermal stem cells, and the like. Concerning cosmetic supplements or functional foods containing substances that suppress genomic or oxidative stress in cells and / or substances that suppress DNA damage in cells.
- the beauty supplement or functional food according to the present invention may be characterized in that, in one of the embodiments, a substance that induces or maintains the expression of COL17A1 in cells is contained as an active ingredient.
- the beauty supplement or functional food according to the present invention is a ROCK inhibitor, NADPH oxidase inhibitor, iNOS inhibitor, and / or COX inhibitor as a substance that induces or maintains the expression of COL17A1 in cells.
- the beauty supplement or functional food according to the present invention is a substance that maintains the expression of COL17A1 in cells and maintains the self-renewal ability, such as aposinine, ebselene, selecoxib, apigenin, Y-27632, lipasyl, 1400W.
- Etynyl estradiol Biochanin A, Necrostatin 1, Rafuma extract, Mulberry extract, Gymnema extract, Tea extract, Biwa leaf extract, Maria thistle extract, Black corn extract, Blue-green algae seed extract, Coffee seed extract , Someiyoshino leaf extract, Melia azajirakuta leaf extract, mandarin orange peel extract, lavender flower extract, clara root extract, rice bran extract, canina rose fruit extract, hamemaris leaf extract, eucalyptus leaf extract, sanzashi extract, carrot extract.
- the beauty supplement or functional food according to the present invention may take any form such as tablets, powders, semi-solids, jellies or liquids.
- the beauty supplement according to the present invention may further contain at least one of skin anti-aging agents, vitamins, collagen and minerals.
- the beauty supplement or functional food according to the present invention can be prepared, for example, to be orally ingested 1 to 3 times a day before, during, and after a meal.
- Cosmetic composition is a cosmetic composition, which is a substance capable of maintaining the expression of COL17A1 in cells and the competitive self-renewal ability based on the expression, a substance that suppresses the decomposition of COL17A1 in cells, and the epidermis.
- the present invention relates to a cosmetic composition, which comprises, as an active ingredient, a substance that maintains the self-renewal ability of stem cells, a substance that suppresses genomic stress or oxidative stress in cells, and / or a substance that suppresses DNA damage in cells.
- Substances that induce or maintain the expression of COL17A1 in cells include, for example, ROCK inhibitors, NADPH oxidase inhibitors, iNOS inhibitors, and COX inhibitors, as described elsewhere herein. Not limited to. Substances that induce or maintain the expression of COL17A1 in cells include, for example, aposinine, ebuserene, selecoxib, apigenin, Y-27632, lipasdil, 1400W, astaxanthin, ethynyl estradiol, biochanin A, necrostatin 1, rafuma extract, mulberry extract.
- Thing Gymnema extract, Tea extract, Biwa leaf extract, Maria thistle extract, Black corn extract, Blue-green algae seed extract, Coffee seed extract, Someiyoshino leaf extract, Melia azalea lacta leaf extract, Mandarin orange peel extract, Lavender flower extract, clara root extract, rice bran extract, canina rose fruit extract, hamemaris leaf extract, eucalyptus leaf extract, and sanzashi extract can be used.
- the cosmetic composition according to the present disclosure is a composition for use in skin care.
- the cosmetic composition according to the present disclosure relates to a composition for application to a skin surface.
- the composition includes, but is not limited to, solutions, suspensions, lotions, creams, gels, lotions, sticks, pencils, sprays, aerosols, ointments, cleansing detergents and cleansing sticks, shampoos and hair conditioners, pasta.
- the cosmetic composition according to the present disclosure may contain a substance (Y27632, apocynin, etc.) that induces or maintains the expression of COL17A1 in 0.01 to 15% by weight, for example, 0.1 to 5% by weight of cells. ..
- the cosmetic composition according to the present disclosure may further contain at least one of a skin anti-aging agent, a skin color-adjusting agent, an anti-inflammatory agent, and a sunscreen agent.
- a skin anti-aging agent include, but are not limited to, a peptide having an anti-aging effect (WO2014157485A1).
- Suitable skin tone preparations include, but are not limited to, sugar amines, arbutins, deoxyalbutin, hexylresorcinol, kodiic acid, hexamidine compounds, salicylic acid, and retinoids including retinol propionate and retinyl propionate.
- Suitable anti-inflammatory agents include non-steroidal anti-inflammatory agents (NSAIDS such as ibuprofen, naproxene), glycyrrhizinic acid (also known as glycyrrhetinic acid glycoside), and salts such as dipotassium glycyrrhizinate, glycyrrhetenic acid.
- NSAIDS non-steroidal anti-inflammatory agents
- ibuprofen, naproxene glycyrrhizinic acid
- glycyrrhizinic acid also known as glycyrrhetinic acid glycoside
- salts such as dipotassium glycyrrhizinate, glycyrrhetenic acid.
- Glico extract bisabolol (eg, alpha bisabolol), mandista (extracted from Akane plants, especially Rubia cordifolia), and guggal (Commiphora plants, especially Commiphora) (Extracted from mukul)), cola tree extract, chamomile, murasaki tsumekusa extract, and whip coral extract (extract from goat eyes), derivatives of any of the above, and mixtures thereof. Not limited.
- Suitable sunscreens are 2-ethylhexyl-p-methoxycinnamate, 4,4'-t-butylmethoxydibenzoylmethane, 2-hydroxy-4-methoxybenzophenone, octyldimethyl-p-aminobenzoic acid, digalloyl.
- Trioleate 2,2-dihydroxy-4-methoxybenzophenone, ethyl-4- (bis (hydroxypropyl) aminobenzoate, 2-ethylhexyl-2-cyano-3,3-diphenylacrylate, 2-ethylhexyl salicylate, glyceryl) -P-Aminobenzoate, 3,3,5-tri-methylcyclohexylsalicylate, methyl anthranilic acid, p-dimethyl-aminobenzoic acid or aminobenzoate, 2-ethylhexyl-p-dimethylaminobenzoate, 2-phenylbenzoimidazole Included are -5-sulfonic acid, 2- (p-dimethylaminophenyl) -5-sulfonic benzooxazoic acid, octocrylene, zinc oxide, benzylidene camphor and derivatives thereof, titanium dioxide, and mixtures thereof. There is no limitation.
- the cosmetic compositions according to the present disclosure are conventionally used in the cosmetics field and do not affect the properties of the compositions according to the present disclosure, such as at least one additive such as a thickener, a fragrance, a pearl brightener.
- Fatty acids such as fatty acids, gelling agents, antioxidants, solvents, filters, screening agents, odor absorbers, colorants, abrasives, absorbents, beauty ingredients such as fragrances, pigments, pigments / colorants, essential oils, anti-caking Agents, antifoaming agents, antibacterial agents, binders, biological additives, buffers, fillers, chelating agents, chemical additives, beauty astringents, beauty abatements, modifiers, drug astringents,
- additives are, but are not limited to, preferably or advantageously in proportions ranging from 0 to 50% by weight, 5 to 40% by weight or 30 to 50% by weight, with respect to the total weight of the composition. It may be present in such compositions.
- compositions according to the present disclosure are specifically for topical application to the skin, in particular aqueous or oily solutions, lotions or serum-type dispersions, dispersion of the fatty phase in the aqueous phase (O / W) or vice versa.
- Emulsions with milk-type liquid or semi-liquid consistency, aqueous or anhydrous gel or cream-type softness suspensions or emulsions obtained by (W / O), as well as microcapsules or microparticles, ions and / Or may have a nonionic vesicle dispersion, or a foamy morphology.
- These compositions are prepared according to conventional methods.
- the composition preferably has an aqueous phase between 5 and 99.5%.
- the pH of the cosmetic composition according to the present disclosure is not limited, but is generally between 2 and 12, preferably between 3 and 9.
- the pH is, for example, ammonia, sodium hydroxide, potassium hydroxide, or primary, secondary or tertiary (poly) such as monoethanolamine, diethanolamine, triethanolamine, isopropanolamine or 1,3-propanediamine.
- an amine base organic or inorganic
- a basic or polyamino acid such as lysine or arginine
- an inorganic or organic acid preferably such as citric acid, etc. It can be adjusted to the target value by adding the carboxylic acid of.
- the cosmetic compositions according to the present disclosure are creams for washing, protecting, treating or caring for, in particular, the face, hands, feet, major structural folds or body (eg, day creams, night creams, etc.).
- the cosmetic composition according to the present disclosure may be applied at least once a day, twice a day, or more frequently during the treatment period.
- the first and second applications are, for example, spaced at least 1-12 hours apart.
- the composition can be applied in the morning and / or in the evening before bedtime.
- Screening method Substances effective in promoting skin wound healing or prevention or improvement of skin ulcers, substances effective in suppressing skin aging, control of cell competition, improvement (improvement, enhancement) of epidermal stem cell regeneration ability, anticancer agents Concerning methods for screening for substances effective in preventing or ameliorating skin disorders caused by skin disorders, anti-wrinkles, anti-blemishes, and / or amelioration of rough skin.
- the suppression of skin aging may be the suppression of aging of hair follicles, which are appendages of the skin.
- Such a screening method may include the following steps i-iii: i) In vitro contact between cells and test material, ii) A step of measuring the expression of COL17A1 in cells, and iii) a step of determining whether or not the test substance increases the expression of COL17A1.
- the expression of COL17A1 can be measured by any method known to those skilled in the art.
- the substances identified by the above screening method are the components used for promoting skin wound healing, the components used for suppressing skin aging, the components used for controlling cell competition, and the epidermal stem cells according to the present invention.
- a component for enhancing regenerative ability a component for preventing or ameliorating skin disorders caused by anticancer agents, an anti-wrinkle component, an anti-spot component, and / or a component for improving rough skin.
- a method for promoting skin wound healing in mammals a method for suppressing skin aging, a method for enhancing the regenerative ability of epidermal stem cells, prevention of skin damage caused by anticancer agents, or It is also useful as a substance to be administered to the mammal in methods for amelioration, anti-wrinkle methods, anti-spot methods, and / or methods for ameliorating rough skin.
- the screening method described above may further include a step of determining whether the test substance promotes colony formation.
- agents that promote "stem cell amplification through stem cell competition" can be screened by assessing self-renewal ability-maintaining agents by maintaining continuous expression of COL17A1-. That is, in one of the embodiments, the screening method may include the following steps i-iv: i) In vitro contact between cells and test material, ii) Step of measuring the expression of COL17A1 in cells, iii) A step of determining whether the test substance increases the expression of COL17A1 and iv) a step of determining whether the test substance promotes colony formation.
- One of the embodiments of the present invention relates to an evaluation method for skin aging using the expression of COL17A1 in cells as an index.
- the present inventor has found that the expression of COL17A1 is reduced in aged epidermal cells. Therefore, COL17A1 can be used as a marker for evaluation of skin aging, and skin aging can be evaluated by measuring the expression of COL17A1 in epidermal cells.
- the expression of COL17A1 in epidermal cells can be measured by any method known to those skilled in the art, such as Western blotting and antibody staining using an antibody against COL17A1 (anti-COL17A1 antibody), and measurement of mRNA by RT-PCR of a gene encoding COL17A1. Can be done by.
- Skin aging can be assessed by comparing the measurement results with controls or by comparing over time. For example, as a result of comparison, when the expression level of COL17A1 is decreased, it is judged that skin aging is progressing.
- kits for use in an evaluation method of skin aging using the expression of COL17A1 in cells as an index contains, for example, an antibody that specifically recognizes COL17A1. Such antibodies may be directly labeled or may be recognized by the labeled secondary antibody. The label can be, for example, based on color development or chemiluminescence due to enzymatic activity.
- the kit may also include, for example, oligonucleotides (probes, primers) for detecting transcripts of COL17A1. Such oligonucleotides can be used for detection by hybridization with transcripts and for amplification reactions such as PCR.
- one of the embodiments of the present invention relates to a kit for use in an evaluation method of skin aging, which comprises an antibody for detecting COL17A1 (anti-COL17A1 antibody), a probe or a primer for a gene encoding COL17A1.
- the kit may include components (antibodies, probes, primers, etc.) for measuring the expression levels of other marker proteins such as p16Ink4a.
- One of the embodiments of the present invention relates to an evaluation method for epidermal stem cells using the expression of COL17A1 in cells as an index.
- the present inventor has found that there is a correlation between the proliferative capacity of epidermal stem cells and the expression level of COL17A1. Therefore, COL17A1 can be used as a marker for evaluation of epidermal stem cells, and epidermal stem cells can be evaluated by measuring the expression of COL17A1 in epidermal stem cells.
- Epidermal stem cells expressing high levels of COL17A1 have high proliferative potential and can be evaluated as suitable cells for use in culture.
- the expression of COL17A1 in epidermal stem cells can be measured by any method known to those skilled in the art, such as Western blotting using an anti-COL17A1 antibody, antibody staining, ELISA, and FACS.
- the present inventor has found that the expression of COL17A1 is decreased in aged epidermal stem cells. Therefore, the aging of epidermal stem cells can be evaluated by measuring the expression level of COL17A1 in epidermal stem cells.
- aging of epidermal stem cells refers to a phenomenon in which the original self-renewal ability of epidermal stem cells decreases due to aging and various genomic stresses and oxidative stresses, and is easily lost by final differentiation. For example, as a result of comparison with the value set as a standard, when the expression level of COL17A1 is considered to be high, it is judged that the cell is suitable for use in culture or regenerative medicine products.
- one of the embodiments of the present invention relates to a kit for use in an evaluation method of epidermal stem cells using the expression of COL17A1 in cells as an index.
- the kit can include, for example, an antibody that specifically recognizes COL17A1, a probe for the gene encoding COL17A1, or an amplification primer for the gene encoding COL17A1. Therefore, one of the embodiments of the present invention relates to a kit for use in a method for evaluating epidermal stem cells, which comprises an antibody, probe or primer for detecting COL17A1.
- Selection method and selection kit for epidermal stem cells One of the embodiments of the present invention relates to a method for selecting epidermal stem cells using the expression of COL17A1 in cells as an index.
- the present inventor has found that there is a correlation between the proliferative capacity of epidermal stem cells and the expression level of COL17A1.
- Epidermal stem cells with a high expression level of COL17A1 can be said to be suitable cells for use in culture because of their high proliferative capacity.
- COL17A1 can be used as a marker for evaluating the proliferative capacity of epidermal stem cells, and the expression of COL17A1 in epidermal stem cells can be measured, and epidermal stem cells having a high expression level of COL17A1 can be selected afterwards by culturing or transplanting. Can be beneficial in doing so. Selection of cells highly expressing COL17A1 can be performed by any method known to those skilled in the art, for example, a method using FACS.
- one of the embodiments of the present invention relates to a kit for use in a method for selecting epidermal stem cells, which uses the expression of COL17A1 in cells as an index.
- the kit can include, for example, an antibody, probe or primer that specifically recognizes COL17A1 as described above. Therefore, one of the embodiments of the present invention relates to a kit for use in a method for selecting epidermal stem cells, which comprises an antibody, probe or primer for detecting COL17A1.
- Epidermal stem cell and / or epidermal cell amplification method and evaluation kit One of the embodiments of the present invention is also a method for amplifying epidermal stem cells and / or epidermal cells, which comprises a step of selecting epidermal stem cells having a high expression level of COL17A1.
- epidermal stem cells having a high expression level of COL17A1 have high proliferative ability, it is possible to efficiently amplify epidermal stem cells and / or epidermal cells by selecting such cells.
- Epidermal stem cells with a high expression level of COL17A1 mean that the expression level is quantitatively higher than that in aged cells. For example, it can be isolated by a method using FACS or a method using beads. Cell sheets can be made ex vivo using such amplification methods.
- one of the embodiments of the present invention relates to a kit for use in a method for amplifying epidermal stem cells and / or epidermal cells, which comprises a step of selecting epidermal stem cells having a high expression level of COL17A1.
- the kit can include, for example, an antibody, probe or primer that specifically recognizes COL17A1 as described above. Therefore, one of the embodiments of the present invention relates to a kit for use in a method for amplifying epidermal stem cells and / or epidermal cells, which comprises an antibody, probe or primer for detecting COL17A1.
- One of the embodiments of the present invention is involved in cell competition by changing the expression level of a molecule that modifies the expression of a molecule involved in cell competition such as COL17A1 in some cells. It relates to a method for evaluating a molecule, a substance that causes changes such as promoting or attenuating cell competition, and a substance that changes the efficiency or rate of competition.
- the present inventor has found that the expression level of COL17A1 correlates with the cell competition of epidermal stem cells. Epidermal stem cells with a high expression level of COL17A1 are advantageous in cell competition.
- evaluation of cell competition can be performed using a three-dimensional culture of epithelial cells forming stratified squamous epithelium such as keratinocytes (including cultured keratinocytes, HaCat cells of keratinocytes and Pam212 cells).
- stratified squamous epithelium such as keratinocytes (including cultured keratinocytes, HaCat cells of keratinocytes and Pam212 cells).
- a cell having a high expression level of COL17A1 proliferates predominantly over a cell having a low expression level of COL17A1 as compared with the cell, and thus can be evaluated as having a high cell competitive ability.
- cell competition can be evaluated by three-dimensional culture of keratinocytes, and compounds, extracts, nucleic acids, etc. that control this can be searched for. Therefore, one of the embodiments of the present invention relates to a method for evaluating cell competition in keratinocytes, and in particular, a method for screening a substance that controls cell competition using the cell competition in a three-dimensional culture of keratinocytes as an index. Also related to.
- one of the embodiments of the present invention relates to a kit for use in a method for evaluating cell competition in keratinocytes (keratinocytes).
- This kit includes, for example, nucleic acids that change the expression of cell competition-related molecules such as COL17A1 and cancer-related genes, such as nucleic acids for use in RNA interference (siRNA for genes encoding COL17A1, shRNA, etc.), as well as gene transfer reagents.
- siRNA RNA interference
- shRNA shRNA interference
- gene transfer reagents RNA interference
- Lentivirus, retrovirus, antibodies used for immunostaining such as antibodies that specifically recognize COL17A1, and devices for three-dimensional culture can be included.
- one of the embodiments of the present invention relates to a kit for use in a method for evaluating cell competition, which comprises a cell line, a virus, an antibody, and a device involved in the evaluation of cell competition in keratinocytes.
- the kit may also include a keratinocyte line (eg, HaCaT cell) for 3D culture.
- a keratinocyte line eg, HaCaT cell
- Cell Composition One of the embodiments of the present invention relates to a cell composition (cell population) comprising isolated cells expressing COL17A1. Isolation of cells expressing COL17A1 can be performed by any method known to those skilled in the art, for example, a method using FACS or beads. Further, the cell composition of the present invention may contain a cell composition having a three-dimensional structure such as an epithelial sheet or an organoid.
- the cell compositions of the present invention include those treated with substances that induce or maintain the expression of COL17A1 in cells.
- Cells expressing high levels of COL17A1 can be isolated using, for example, FACS.
- the terms “high” and “hi” indicating the expression level are well known in the art, and "COL17A1 high " is a comparison in the cell population to be analyzed, and the expression level of COL17A1 is high. Indicates a high level.
- the cells in the present cell composition may be, for example, epidermal keratinocytes or mucosal epithelial keratinocytes, but may contain other cells.
- the cells in the cell composition can be, for example, at least 50%, 60%, 70%, 80%, 90%, 95%, 98%, or 99% of cells expressing COL17A1.
- the cell composition may contain at least 1 ⁇ 10 3 cells, 1 ⁇ 10 4 cells, 1 ⁇ 10 5 cells, 1 ⁇ 10 6 cells, 1 ⁇ 10 7 cells, or 1 ⁇ 10 8 cells. ..
- This cell composition can be a cell composition for use in transplantation.
- the cell composition can be a cell composition for use in the treatment of skin disorders or injuries.
- the cells in the cell composition can be stem cell-derived cells.
- the cells in the present cell composition may be cells in which the expression of COL17A1 is induced or maintained by using a substance that induces or maintains the expression of COL17A1 in the cells.
- the substance that induces or maintains the expression of COL17A1 in cells can be at least one of a ROCK inhibitor, a NADPH oxidase inhibitor, an iNOS inhibitor, and a COX inhibitor.
- the substance that induces or maintains the expression of COL17A1 in cells may be Y27632 or apocynin.
- the cells in the cell composition may be frozen.
- the cell composition may be contained in one container.
- Cell Sheet One of the embodiments of the present invention relates to a cell sheet containing cells expressing COL17A1. Such cell sheets can be made by isolating and culturing cells expressing COL17A1. Such cell sheets can be transplanted into a patient for use in treating a patient's disease or injury. The cell sheet can be for autologous or allogeneic transplantation.
- Example 1 Decreased COL17A1 protein expression in the skin with aging
- the skin in the tail of the mouse unlike the skin in the back, was observed in the histological analysis of the human epidermis of the layered epidermal cell layer and epidermal melanosite.
- the tail skin of aged mice is also characterized by atrophy with a reduced number of spinous cell layers in the scaly epidermis.
- the basal cells of young mice have a rectangular and longitudinal shape with respect to the basement membrane, whereas the aged basal cells have a more rounded or flatter morphology.
- the present inventor hypothesized that the HD component is directly involved in the skin aging process.
- the expression of various HD components and the major cell adhesion molecule, integrin ⁇ 1 (ITGB1) was analyzed in the tail epidermis of young and old (weekly) mice.
- IGB1 major cell adhesion molecule
- Fig. 1 immunoelectron microscopy revealed a significant reduction in the COL17A1 signal at the dermis-epidermal junction of aged skin.
- systemic immunostaining revealed a heterogeneous distribution of COL17A1 and ITGA6 in aged skin.
- COL17A1-negative cell region expressing ITGA6 was found in addition to the double-negative cell region of the aged tail epidermis. This indicates that the expression of the COL17A1 protein is the most unstable, and as a result, when COL17A1 is inadequate, other HD components are finally destabilized with aging.
- COL17A1 in the stability of HD components, we analyzed the number of HD in the skin of young and aged mice (Fig. 2).
- COL17A1 proteolysis is also induced by sustained DNA damage responses such as X-ray irradiation in mice and genomic instability in TTD progeria mice.
- UV irradiation also shows down-regulation of COL17A1 expression in some basal keratinocytes in vitro and in vivo. As a result, UV irradiation of the skin caused HD instability.
- COL17A1 acts as a "stem cell checkpoint molecule" as a stress / compatibility sensor that monitors the DNA damage response, determining whether stem cells self-renew or differentiate. ing.
- This stress-sensing mechanism can make a difference in the self-renewal ability of epidermal stem cell clones with different levels of COL17A1 expression present in the aged basal layer of the epidermis.
- Example 2 Clone proliferation of COL17A1 + epidermal stem cells during skin aging To visualize the dynamics of epidermal stem cell clones appearing with aging and to track the fate of COL17A1-deficient clones, K14-CreERT2; R26R Brainbow 2.1 A basal layer keratinocyte lineage trace system was generated by drug-induced multicolor labeling using mice. This system allows stochastic multicolor labeling of basal stem / progenitor cells by Cre-LoxP-mediated recombination. After administration of tamoxifen (TAM), GFP, RFP, YFP or CFP-expressing cells were stochastically found in the tail skin of young (4.5 months).
- TAM tamoxifen
- basal keratinocyte clone size was positively correlated with COL17A1 expression level in aged mouse skin (Fig. 5). This indicates that stem cell clones that maintain higher levels of COL17A1 expression exhibit higher clonogenicity and dominate the basal epidermis during the physiological aging process. In addition, no significant induction of apoptotic cell death or so-called "cellular senescence" was observed in the basal cells of aged skin. These data suggest that COL17A1 high epidermal stem cells compete with COL17A1 low cells to tolerate epidermal aging.
- Example 3 Differential expression of COL17A1 that causes competition for epidermal stem cells
- COL17A1 low / -clones directly causes cell competition with neighboring surrounding COL17A1 high clones in vivo.
- the present inventors have generated drug-induced Col17a1 gene knockout (cKO) mice in combination with a multicolor labeling system specific for basal layer keratinocytes.
- cKO drug-induced Col17a1 gene knockout
- EmGFP + COL17A1 - stable HaCaT cells expressing shCOL17A1 and control EmGFP + COL17A1 + HaCaT cells expressing shScramble were prepared and cultured in a 3D culture system. Neither shScramble nor shCOL17A1-expressing HaCaT cells showed a clear difference in structure, stratification, proliferation number or apoptotic cells in 3D cultured epidermis.
- Example 4 Maintenance of epidermal homeostasis by COL17A1-mediated SCD
- COL17A1 promotes SCD, which theoretically maintains COL17A1 + epidermal stem cell clone proliferation. did.
- the basal cell division axis was analyzed in vivo. While normal basal cells showed mostly parallel cell division to the basal membrane, Col17a1-deficient basal keratinocytes abnormally induce vertical cell division and are located above the basal cells and basal layer. Produced a layer of descendants.
- An in vitro assay that can be said to be a gold standard for assessing the self-renewal ability of epithelial stem cells, including cells, in the tail epidermis to test whether COL17A1-mediated SCD maintains cloned proliferation of epidermal stem cell clones.
- Colony formation assay was used. Aged epidermal keratinocytes expressing low levels of COL17A1 had reduced colony forming ability and produced smaller colonies compared to younger tail keratinocytes (Fig. 7). In contrast, forced expression of hCOL17A1 in basal keratinocytes of mice promoted the ability of epidermal stem cells to clone with significantly larger colony sizes and numbers than control mice (Fig. 8).
- Example 5 Maintenance of heterotyped cell lineage by COL17A1-expressing epidermal stem cells Skin aging is characterized not only by epidermal aging, but also by melanocyte-related hyperpigmentation abnormalities and changes in the dermis.
- skin aging was characterized not only by epidermal aging, but also by melanocyte-related hyperpigmentation abnormalities and changes in the dermis.
- physiological aging induces epidermal pigmentation abnormalities in the epidermis of the tail.
- Physiological aging is observed to gradually induce abnormal skin pigmentation (uneven skin pigmentation with areas of low and high pigmentation) and ultimately hypopigmentation in the tail skin. It was.
- Detailed microscopic observation of the tail hole mount revealed that the pigmentation pattern became non-uniform with age.
- Chronic UVB irradiation induces abnormal skin pigmentation and HD damage in the epidermis of mice and humans.
- the inventor investigated whether chronic UVB exposure induces abnormal skin pigmentation and HD damage, as well as its associated phenotype in the tail epidermis (Fig. 9).
- Fig. 9 Following repeated skin pigmentation induced by repeated UVB irradiation, epidermal pigmentation abnormalities were induced within 1 month after the final UVB irradiation, similar to Col17a1 or ITGA6cKO mice.
- Ultramicrostructure analysis using TEM and immune TEM revealed that UVB irradiation inhibited the formation of mature HD and reduced the signal of COL17A1 as well as Col17a1cKO.
- Example 6 To test the above hypothesis that HD is the key to skin organ regeneration, we performed a full-thickness wound healing experiment using the tail skin of aged wild-type mice. Measurement of the wound area showed that physiological aging significantly delayed the wound healing process. The inventor then analyzed Col17a1 or ITGA6-deficient skin and found that both showed significantly delayed wound repair (Fig. 11), where HD instability is the wound in physiological aging. It has been shown to lead to poor healing. In addition, forced expression of hCOL17A1 by basal keratinocytes in mice promoted wound healing (Fig. 12). These data indicate that COL17A1-mediated clonal proliferation of epidermal stem cells is essential not only for stem cell competition in skin homeostasis, but also for regeneration of full-thickness skin wound healing.
- the present inventor searched for a novel compound that induces COL17A1 expression in keratinocytes in vitro. As shown in Table 1 and FIG. 15, the inventor identified two chemicals, Y27632 and Apocynin, that induce the expression of COL17A1 in cultured keratinocytes. To confirm in vitro that the effects of these compounds are mediated by increased competitive self-renewal capacity of SCD-presenting epidermal stem cells, we performed a colony forming assay using human epidermal keratinocytes. .. Consistent with this, colony number was significantly increased by Y-27632 and colony size was significantly increased by both agents (Table 1, FIG. 13 and FIG. 14).
- these drugs were administered to full-thickness wounds on the tail skin of the rat. Both agents significantly promoted the wound repair process, similar to tg mice overexpressing human COL17A1 (Fig. 12). These results indicate that COL17A1-inducible agents promote skin wound healing through promotion of reepithelialization of the wound edge by proliferation of epidermal stem cells, and these findings promote skin regeneration. It can be said that it opens a new way to lead to a new drug for the purpose.
- Example 7 Search for substances that maintain and promote the competitive self-renewal ability of epidermal stem cells Further search for substances that induce or maintain the expression of COL17A1 was performed.
- Human epidermal keratinocytes were seeded on a 384-well plate, cultured for 48 hours after adding a reagent, and subjected to fluorescent immunostaining with an anti-COL17A1 antibody and a fluorescently labeled secondary antibody, and then the cells were subjected to a high-content imaging system. Fluorescent intensity per area was detected (HCS: high content screening).
- HCS high content screening
- some substances were analyzed by Western blotting (WB).
- WB Western blotting
- Table 3 summarizes the anticancer agents and molecular-targeted agents that have been found to decrease the expression level of COL17A1.
- the expression of COL17A1 fluctuates depending on conventional chemotherapeutic agents such as metabolic antagonists and alkylating agents.
- molecular-targeted drugs targeting cancer cells significantly reduce the expression of COL17A1 (Table 3).
- Example 8 Decrease in COL17A1 due to anticancer drug and various stresses
- the anticancer drug hydroxyurea was administered to 7-week-old C57BL6 mice three times a month, and after 3 months, the tissue was collected and the antibody of COL17A1 was used. Immunostaining was performed. As a result, the expression of COL17A1 in the basal layer was significantly reduced in the mice treated with hydroxyurea (Fig. 17).
- HaCaT cells which are human epidermal keratinocytes, are irradiated with radiation (20 Gy, 30 Gy) and ultraviolet rays (20 mJ, 30 mJ), and the cells are collected after 24 hours and 72 hours, respectively, and an antibody against COL17A1 is used.
- Example 9 Improvement test by overexpression of COL17A1 in skin disorders caused by anticancer agents
- Transgenic mice (COL17A1-Tg) and control mice (about 18 months old) overexpressing human COL17A1 were put into an experimental environment. After acclimatization, the hair on the back was removed under anesthesia and divided into the following 6 groups.
- Erlotinib or gefitinib a type of anticancer drug and EGF receptor molecular target drug, is dissolved in 0.5% methylcellulose solution at a concentration of 5 mg / ml and injected intraperitoneally to 50 mg / kg / day daily from the start of the test. Was administered by. A 0.5% methylcellulose solution of solvent was similarly administered to the control non-administered group. Transepidermal water loss (TEWL) was measured for each group of mice on day 7 of erlotinib or gefitinib administration. The measurement was performed using a Tewameter TN300 (Courage + Khazaka, Germany, Integral Co., Ltd.) for a specific part of the back of the mouse (two places symmetrically from the center of the spine to the left and right).
- Tewameter TN300 Cosmetic + Khazaka, Germany, Integral Co., Ltd.
- FIG. 18 shows the test results. From the results shown in FIG. 18, TEWL was significantly increased in Control mice treated with erlotinib or gefitinib, but it was significantly lower in Tg mice overexpressing COL17A1 even under the administration of erlotinib or gefitinib. It showed a TEWL value, suggesting that skin disorders were alleviated. From this, it can be seen that the skin disorders caused by the administration of anticancer drugs are remarkably improved by promoting the self-renewal ability of epidermal stem cells by overexpression of COL17A1.
- Erlotinib was dissolved in a 0.5% methylcellulose solution at a concentration of 5 mg / ml, and was administered by intraperitoneal injection to 50 mg / kg / day daily from the start of the test. A 0.5% methylcellulose solution of solvent was similarly administered to the control non-administered group. An aqueous solution of each preparation was applied to the control solvent application group and the apocynin 200 ⁇ M application group once a day and 5 times a week on the back. Apocynin solvent (50% ethanol / PBS) was similarly applied to the control solvent application group. TEWL was measured on 7 days after the start of erlotinib administration for 7-week-old mice and on 12 days for 6-month-old mice in each group of mice. The measurement was performed using a Tewameter TN300 (Courage + Khazaka, Germany, Integral Co., Ltd.) for a specific part of the back of the mouse (two places symmetrically from the center of the spine to the left and right).
- Figure 19 shows the test results. From the results shown in FIG. 19, TEWL was significantly increased in the mice to which erlotinib was administered, but TEWL was significantly decreased and the skin disorder was alleviated in the group to which apocynin was applied even under the administration of erlotinib. From this, it can be seen that the skin disorders caused by the administration of molecular-targeted drugs are remarkably improved by substances that promote the cell competitive ability of epidermal stem cells.
- Example 11 Improvement test of skin disorders in aged mice by a substance that promotes the self-renewal ability of epidermal stem cells Irritation skin disorders induced by high concentration ethanol (100%) in the cervical-dorsal upper skin of aged mice (for the dry eczema / hair loss model, a substance (aposinin) that promotes the cell competitive ability of epidermal stem cells was continuously applied. As a result, as shown in FIG. 20, the skin disorder was remarkably reduced, and the preventive and therapeutic effects were remarkably obtained.
- Example 12 Barrier function improvement test
- the skin barrier function is a main function performed by the skin, and has a function of preventing water from evaporating from the body and invasion of foreign substances from outside the body, but the epidermis is reactively thickened by ultraviolet irradiation. At the same time, the barrier function deteriorates.
- the water content of the stratum corneum and TEWL are used for evaluating the skin barrier function, and are used as an index that reflects the degree of skin damage caused by ultraviolet rays, anticancer agents, etc., together with the thickness of the reactive epidermis.
- the aqueous solution of each preparation was applied to the hairless mice once a day from the day before the ultraviolet irradiation, and daily on the back for 5 days. Further, in the "ultraviolet + solvent coating group", the solvent (50% ethanol / PBS) of the preparation of Example 1 was similarly coated.
- an ultraviolet irradiation device (Y-UV-Lab-K-D5 lamp type) manufactured by Yayoi Co., Ltd. was used, and irradiation was performed once a day at an intensity of 150 mJ / cm 2 .
- FIG. 21 shows the action of retaining the water content of the stratum corneum against ultraviolet irradiation by a substance that promotes the self-renewal ability of epidermal stem cells.
- the water content of the stratum corneum after UV irradiation was significantly lower than that of the control group without UV irradiation, but was significantly higher in the apocynin 200 ⁇ M administration group, Y-27632 2 ⁇ M, and 20 ⁇ M administration group. The amount was shown. From this, it was clarified that the substance that promotes the cell competitive ability of epidermal stem cells has an action of retaining the water content of the stratum corneum against ultraviolet irradiation.
- the TEWL inhibitory effect of substances that promote the self-renewal ability of epidermal stem cells on ultraviolet irradiation As shown in FIG. 21, the TEWL after UV irradiation showed a significantly higher value than the control group without UV irradiation, but the TEWL was significantly lower in the apocynin 200 ⁇ M administration group and the Y-27632 20 ⁇ M administration group. showed that. From this, it was clarified that the substance that promotes the cell competitive ability of epidermal stem cells has an action of suppressing the increase of TEWL due to ultraviolet irradiation.
- Example 13 Evaluation of improvement of skin elasticity (skin elasticity)
- skin elasticity skin elasticity
- the indexes for confirming the elasticity of the skin include stretching when the skin is pulled and returning when the skin is released, and that a plurality of elasticity indexes decrease with aging. It is considered that the increase in the elasticity of the skin leads to the effect of improving the wrinkles, sagging or firmness of the skin.
- the aqueous solution of each preparation was applied to the solution application group once a day from the day before UV irradiation and on the back three times a week for 5 weeks.
- the solvent of apocynin (50% ethanol / PBS) was similarly coated.
- UVB irradiation an ultraviolet irradiation device (Y-UV-Lab-K-D5 lamp type) manufactured by Yayoi Co., Ltd. was used, and UVA was irradiated at an intensity of 2 J / cm 2 and UVB was irradiated at an intensity of 150 mJ / cm 2 three times a week for 5 weeks.
- the skin elasticity of the back of each group was measured 5 weeks after the start of ultraviolet irradiation.
- the elasticity of the skin was measured using Cutometer MPA580 (Courage + Khazaka, Inc., Integral Co., Ltd.) (parameter setting: 300 mba, on time 2 seconds, off time 2 seconds), and the average value of 3 measurements was taken. Used for analysis. By sucking the skin on the back of the mouse for 2 seconds and then releasing the suction 2 seconds later, the length of the skin stretched immediately after suction, immediately before the release of suction, and immediately after the release of suction is measured, and the length of the skin stretched is measured at the initial stage of suction.
- Rapid extensibility (Ue: unit mm), maximum skin extensibility immediately before suction release (Uf: unit mm), and rapid recovery amount immediately after suction release (Ur: unit mm) were taken.
- R5 Ur / Ue was used as an index of net elasticity
- R7 Ur / Uf was used as an index of elasticity.
- the value of skin elasticity is significantly reduced by ultraviolet irradiation, but the skin elasticity is significantly reduced in the test group coated with a substance that promotes the self-renewal ability of epidermal stem cells as compared with the comparative group.
- the increase in skin elasticity is considered to lead to the effect of improving wrinkles, sagging or firmness of the skin. From this, it was suggested that the effect of improving wrinkles, sagging or firmness of the skin can be obtained by applying a substance that promotes the cell competitive ability of epidermal stem cells.
- ⁇ Control (solvent) coating group ⁇ Apocynin 200 ⁇ M coating group ⁇ Retinol 0.1% application group
- the aqueous solution of each preparation was applied to the solution application group once a day, three times a week for 7 weeks. Seven weeks after the start of application (53rd day), TEWL measurement and skin elasticity measurement were performed on the back of each group (two places symmetrically with respect to the central part of the spine). TEWL measurement was performed using Tewameter TN300 (Courage + Khazaka, Germany, Integral Co., Ltd.) on specific parts of the back of the mouse (two places symmetrically from the center of the spine to the left and right).
- the elasticity of the skin was measured using Cutometer MPA580 (Courage + Khazaka, Inc., Integral Co., Ltd.) (parameter setting: 300 mba, on time 2 seconds, off time 2 seconds), and the average value of 3 measurements was taken. Used for analysis. By sucking the skin on the back of the mouse for 2 seconds and then releasing the suction 2 seconds later, the length of the stretched skin is measured immediately after suction, immediately before the release of suction, and immediately after the release of suction, and the suction is released.
- Example 15 Wrinkle improvement test In a mouse model in which the formation of wrinkles was induced by long-term irradiation with ultraviolet rays (UVA), the effect of improving wrinkles by a substance that promotes the self-renewal ability of epidermal stem cells was observed.
- UVA ultraviolet rays
- the aqueous solution of each preparation was applied to the hairless mice once a day from the day before UV irradiation and on the back three times a week for 5 weeks.
- the solvent of apocynin 50% ethanol / PBS
- Retinol which is said to be effective in improving wrinkles, was similarly applied as a comparative control group at a concentration of 0.1%.
- an ultraviolet irradiation device (Y-UV-Lab-K-D5 lamp type) manufactured by Yayoi Co., Ltd.
- UVA was irradiated at an intensity of 20 J / cm 2
- UVB was irradiated at an intensity of 100 mJ / cm 2 three times a week for 4 weeks.
- PRIMOS-CR Integral Co., Ltd., manufactured by Canfield Scientific Co., Ltd.
- the skin flexibility was measured using a Cutometer MPA580 (Courage + Khazaka, Germany, Integral Co., Ltd.), and the value of R0 was taken as the skin flexibility.
- the value of R0 reflects the condition of keratin and is an index of skin softness.
- TEWL was measured using the Tewameter TN300 (Courage + Khazaka, Germany, Integral Co., Ltd.) for the back of each group (two places symmetrically with respect to the central part of the spine).
- wrinkles on the skin are significantly increased by UV irradiation, but wrinkle formation is suppressed in the test group coated with a substance that promotes the cell competitiveness of epidermal stem cells as compared with the control group.
- the degree was more remarkable than that of retinol.
- the wrinkles on the skin were significantly increased by ultraviolet irradiation, but the cell competitive ability of epidermal stem cells.
- Example 16 Rough skin improvement test (apocynin) Using a rough skin model induced by a 10% sodium lauryl sulfate (SDS) solution (a standard test for chemical skin disorders), a substance that promotes competitive self-renewal ability of epidermal stem cells (aposinin) was administered. The effect of preventing or improving rough skin was evaluated.
- SDS sodium lauryl sulfate
- Example 17 Rough skin improvement test (ebselen) Using a rough skin model induced by a 10% sodium lauryl sulfate (SDS) solution, a substance (ebselen) that promotes the self-renewal ability of epidermal stem cells was administered, and the rough skin improving effect of the rough skin preventing or improving agent was evaluated.
- SDS sodium lauryl sulfate
- Example 18 Rough skin improvement test for humans Two subjects were subjected to a rough skin test induced by a 10% sodium lauryl sulfate (SDS) solution (a standard test for skin damage caused by chemical substances in humans), and self-renewal of epidermal stem cells was used. Substances that promote the ability (aposinine, evselene, selecoxib) were administered, and the effect of the rough skin prevention or improving agent on the rough skin was evaluated.
- SDS sodium lauryl sulfate
- test site was photographed with a digital camera and with a high-resolution three-dimensional image analyzer PRIMOS-CR (manufactured by Canfield Scientific, Inc., Integral Co., Ltd.).
- PRIMOS-CR manufactured by Canfield Scientific, Inc., Integral Co., Ltd.
- the degree of rough skin was measured using Primos OMC3_22 software with height parameters called "Ry (maximum height)” and "Rz (ten-point average roughness)” that represent surface roughness.
- Rz maximum height
- Rz ten-point average roughness
- Figures 31-33 show the test results. As shown in FIG. 31, redness and rough skin were clearly observed in the affected area to which the control and each solution were applied after the SDS treatment, but on the 7th day, redness and rough skin continued in the affected area to which the control solution was applied. Remarkable reduction was observed in the application areas of apocynin, ebselen, and celecoxib. Further, on the 16th day, the control showed pigmentation on the affected area, but the affected area coated with apocynin showed almost no pigmentation. Further, as shown in FIG.
- the ⁇ Rz value and ⁇ Ry value which indicate the roughness of the skin surface and are indicators of rough skin, are significantly increased under the control of SDS treatment, but these ⁇ Rz value and ⁇ Ry value are significant in the affected area to which apocynin, ebselen, and celecoxib are applied.
- these ⁇ Rz value and ⁇ Ry value are significant in the affected area to which apocynin, ebselen, and celecoxib are applied.
- the rough skin and pigmentation significantly induced by the SDS solution have a remarkable improving effect by the substance that promotes the competitive self-renewal ability of epidermal stem cells.
- Rafuma (Apocynum venetum) extract The Rafuma extract used was manufactured by Joban Institute of Plant Chemistry Co., Ltd. The dried leaves of Apocynum venetum were crushed, 60% ethanol was added to the crushed product, and the mixture was heated and recirculated and filtered to obtain an extract. The extraction residue was recirculated by heating with 60% ethanol and then filtered to obtain an extract. The first extract and the second extract were combined, concentrated under reduced pressure, adjusted to pH 3, and stirred overnight.
- the insoluble material is removed by filtration, the obtained filtrate is passed through a synthetic adsorption resin, washed with water, and then the fractions desorbed with 70% ethanol are collected, concentrated under reduced pressure, and spray-dried to obtain a Rafuma extract. It was.
- Mulberry (Morus alba) extract The mulberry extract used was manufactured by Joban Institute of Plant Chemistry. 50% ethanol was added to the leaves of mulberry (Morus alba), and the mixture was heated under reflux and filtered to obtain an extract. After the extract was concentrated under reduced pressure, an excipient was added and dried to obtain a mulberry extract.
- Gymnema sylvestre extract used was manufactured by Joban Plant Chemistry Research Institute Co., Ltd. A pulverized product of Gymnema sylvestre leaves was extracted by heating under reflux with 70% ethanol and filtered to obtain an extract. The obtained extract was concentrated under reduced pressure and then dried under reduced pressure to obtain a Gymnema extract.
- Tea (Camellia sinensis) extract The tea extract used was manufactured by Joban Institute of Plant Chemistry. Tea (Camellia sinensis) leaves were extracted with hot water and filtered to obtain an extract. The obtained extract was purified with an adsorption resin, concentrated under reduced pressure, and dried to obtain a tea extract.
- Milk thistle (Silybum marianum) extract The milk thistle extract used was manufactured by Joban Institute of Plant Chemistry. Milk thistle (Silybum marianum) saw fruit (defatted slag) was extracted by heating under reflux with 90 to 95% ethanol and filtered to obtain an extract. The extract was concentrated under reduced pressure, purified with ethanol, concentrated under reduced pressure, and dried with warm air to obtain a milk thistle extract.
- Biwa (Eriobotrya japonica) leaf extract The Biwa leaf extract used was manufactured by Joban Institute of Plant Chemistry Co., Ltd. The crushed leaf of Eriobotrya japonica was extracted by heating under reflux with 80% ethanol and filtered to obtain an extract. Activated carbon was added to the obtained extract to carry out a decolorization step, and then the decolorizing solution was concentrated under reduced pressure and dried under reduced pressure to obtain a biwa leaf extract.
- the black turmeric extract used was manufactured by Joban Plant Chemistry Research Institute Co., Ltd.
- the dried chips of Kaempferia parviflora rhizome were pulverized by a mixer, 80% ethanol was added to the pulverized product, and the mixture was heated under reflux and filtered to obtain an extract.
- the extraction residue was heated under reflux with 80% ethanol again and then filtered to obtain an extract.
- the first extract and the second extract were combined, concentrated under reduced pressure, an excipient was added, and the mixture was dried under reduced pressure to obtain a black turmeric extract.
- Manufacturing example 2 The plant extracts shown in Table 4 were extracted from each part of various plants using a predetermined extraction solvent.
- compositions that are useful for inhibition, control of cell competition, and improvement of the regenerative capacity of epidermal stem cells Continuation of treatment due to skin disorders caused by anticancer drugs has become a major issue, and has become a problem related to prognosis. The number of cancer patients who continue to work while continuing cancer treatment is increasing year by year, and the need for appearance care is extremely high.
- the compositions, cells and methods disclosed in the present application can be effectively used in the fields of continuation of anticancer drug treatment, surgical medicine, regenerative medicine, beauty and the like.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Botany (AREA)
- Medical Informatics (AREA)
- Alternative & Traditional Medicine (AREA)
- Birds (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Dermatology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Cell Biology (AREA)
- Biomedical Technology (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Developmental Biology & Embryology (AREA)
- Food Science & Technology (AREA)
- Emergency Medicine (AREA)
- Toxicology (AREA)
- Gastroenterology & Hepatology (AREA)
- Analytical Chemistry (AREA)
- Urology & Nephrology (AREA)
- Wood Science & Technology (AREA)
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021509620A JP7214268B2 (ja) | 2019-03-27 | 2020-03-26 | 皮膚用組成物 |
| KR1020217034883A KR20210144835A (ko) | 2019-03-27 | 2020-03-26 | 피부용 조성물 |
| CN202410273876.7A CN118079006A (zh) | 2019-03-27 | 2020-03-26 | 皮肤组合物 |
| US17/593,810 US20220331389A1 (en) | 2019-03-27 | 2020-03-26 | Skin composition |
| EP20778483.6A EP3978020A4 (en) | 2019-03-27 | 2020-03-26 | SKIN COMPOSITION |
| CN202080038284.7A CN113874044B (zh) | 2019-03-27 | 2020-03-26 | 皮肤组合物 |
| JP2023002222A JP7564568B2 (ja) | 2019-03-27 | 2023-01-11 | 皮膚用組成物 |
| JP2024161580A JP2024175085A (ja) | 2019-03-27 | 2024-09-19 | 皮膚用組成物 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019-059616 | 2019-03-27 | ||
| JP2019059616 | 2019-03-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020196801A1 true WO2020196801A1 (ja) | 2020-10-01 |
Family
ID=72608843
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2020/013856 Ceased WO2020196801A1 (ja) | 2019-03-27 | 2020-03-26 | 皮膚用組成物 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20220331389A1 (enExample) |
| EP (1) | EP3978020A4 (enExample) |
| JP (3) | JP7214268B2 (enExample) |
| KR (1) | KR20210144835A (enExample) |
| CN (2) | CN118079006A (enExample) |
| WO (1) | WO2020196801A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023276942A1 (ja) * | 2021-06-28 | 2023-01-05 | 株式会社 資生堂 | 血管内皮増殖因子の増加の抑制剤およびそのスクリーニング方法 |
| JP2023054511A (ja) * | 2021-10-04 | 2023-04-14 | 日本メナード化粧品株式会社 | 皮膚幹細胞増殖促進剤及び皮膚再生促進剤 |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102749211B1 (ko) * | 2022-03-03 | 2025-01-07 | 코스맥스 주식회사 | 한방 약재의 미생물 발효 오일을 포함하는 조성물 및 그의 피부 상태 개선 용도 |
| CN115040430B (zh) * | 2022-06-15 | 2024-03-12 | 和讯科技(吉林省)集团有限公司 | 一种含ebselen的防晒化妆品及其制备方法 |
| KR102853873B1 (ko) * | 2022-11-28 | 2025-09-04 | 주식회사 하람 | 왕벚나무 추출물을 유효성분으로 포함하는 자외선에 의한 피부 손상 개선용 조성물 |
| CN117327660A (zh) * | 2023-10-17 | 2024-01-02 | 安可来(重庆)生物医药科技有限公司 | 一种同时表达il1-rn和col17a蛋白永生化间充质干细胞系及其制备方法与应用 |
| CN117253543B (zh) * | 2023-10-20 | 2024-06-21 | 广东丸美生物技术股份有限公司 | 一种皮肤表皮细胞抗衰基因库及其构建方法和应用 |
| JP2025084198A (ja) * | 2023-11-22 | 2025-06-03 | 克昭 團 | 血管修復機能向上用組成物、男性機能向上用組成物、医薬品、化粧品および食品または飲料 |
| CN117815149B (zh) * | 2024-03-04 | 2024-06-04 | 广州汇芬生物科技有限公司 | 一种具有除螨作用的组合物及其在制备除螨乳液中的应用 |
| US12357725B1 (en) | 2024-05-30 | 2025-07-15 | King Saud University | Clinical dressing loaded with coffee extract |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006061091A (ja) | 2004-08-27 | 2006-03-09 | Toyo Shinyaku:Kk | シソの葉から得られる発酵物 |
| JP2006075085A (ja) | 2004-09-09 | 2006-03-23 | Toyo Shinyaku:Kk | パセリから得られる発酵物 |
| JP2006076926A (ja) | 2004-09-09 | 2006-03-23 | Toyo Shinyaku:Kk | ピーマンから得られる発酵物 |
| JP2006273761A (ja) | 2005-03-29 | 2006-10-12 | Kanebo Cosmetics Inc | Dna修復促進剤及び皮膚外用剤 |
| JP2008247854A (ja) | 2007-03-30 | 2008-10-16 | Shiseido Co Ltd | 抗酸化剤、dna損傷抑制剤および皮膚外用剤 |
| JP2009161509A (ja) | 2008-05-21 | 2009-07-23 | Kanazawa Univ | Xvii型コラーゲンに関する脱毛抑制剤、毛髪の脱色素化抑制剤 |
| WO2013031003A1 (ja) | 2011-09-01 | 2013-03-07 | 株式会社マンダム | Dna修復促進剤およびdna損傷抑制剤 |
| JP2014504155A (ja) * | 2010-11-30 | 2014-02-20 | 株式會社アモーレパシフィック | 皮膚老化と関連した遺伝子及び皮膚老化を防止する物質をスクリーニングする方法 |
| WO2014157485A1 (ja) | 2013-03-29 | 2014-10-02 | 国立大学法人大阪大学 | 抗老化作用を有するペプチドおよびその利用 |
| WO2017122668A1 (ja) | 2016-01-12 | 2017-07-20 | 国立大学法人 東京医科歯科大学 | 脱毛および白毛化を抑制もしくは改善するための組成物ならびにその使用 |
| JP2018108973A (ja) * | 2017-01-05 | 2018-07-12 | 丸善製薬株式会社 | Xvii型コラーゲン産生促進剤 |
| JP2018193336A (ja) * | 2017-05-18 | 2018-12-06 | 共栄化学工業株式会社 | 皮膚外用剤 |
| JP2019059616A (ja) | 2017-09-28 | 2019-04-18 | セイコーエプソン株式会社 | 媒体搬送装置、画像読取装置、記録装置 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002076456A1 (en) * | 2001-03-27 | 2002-10-03 | Nutricia, N.V. | Method for inhibiting apoptosis of stem cells |
| US20050232957A1 (en) * | 2004-04-14 | 2005-10-20 | Katz Kenneth A | Compositions and methods for moisturizing skin |
| DE602006006995D1 (de) * | 2005-05-04 | 2009-07-09 | Coty Prestige Lancaster Group | Verwendung von freien radikalfängern zum schutz und zur behandlung von durch chemotherapie verursachten haut- und haarschäden |
| JP6545926B2 (ja) * | 2012-11-30 | 2019-07-17 | 株式会社ナノエッグ | 手足症候群治療用組成物 |
| CN109045001A (zh) * | 2018-09-13 | 2018-12-21 | 潘治忠 | p300活化剂CTPB及其衍生物在提高胶原蛋白Col17A1表达的用途 |
-
2020
- 2020-03-26 WO PCT/JP2020/013856 patent/WO2020196801A1/ja not_active Ceased
- 2020-03-26 CN CN202410273876.7A patent/CN118079006A/zh active Pending
- 2020-03-26 CN CN202080038284.7A patent/CN113874044B/zh active Active
- 2020-03-26 JP JP2021509620A patent/JP7214268B2/ja active Active
- 2020-03-26 EP EP20778483.6A patent/EP3978020A4/en active Pending
- 2020-03-26 KR KR1020217034883A patent/KR20210144835A/ko active Pending
- 2020-03-26 US US17/593,810 patent/US20220331389A1/en active Pending
-
2023
- 2023-01-11 JP JP2023002222A patent/JP7564568B2/ja active Active
-
2024
- 2024-09-19 JP JP2024161580A patent/JP2024175085A/ja active Pending
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006061091A (ja) | 2004-08-27 | 2006-03-09 | Toyo Shinyaku:Kk | シソの葉から得られる発酵物 |
| JP2006075085A (ja) | 2004-09-09 | 2006-03-23 | Toyo Shinyaku:Kk | パセリから得られる発酵物 |
| JP2006076926A (ja) | 2004-09-09 | 2006-03-23 | Toyo Shinyaku:Kk | ピーマンから得られる発酵物 |
| JP2006273761A (ja) | 2005-03-29 | 2006-10-12 | Kanebo Cosmetics Inc | Dna修復促進剤及び皮膚外用剤 |
| JP2008247854A (ja) | 2007-03-30 | 2008-10-16 | Shiseido Co Ltd | 抗酸化剤、dna損傷抑制剤および皮膚外用剤 |
| JP2009161509A (ja) | 2008-05-21 | 2009-07-23 | Kanazawa Univ | Xvii型コラーゲンに関する脱毛抑制剤、毛髪の脱色素化抑制剤 |
| JP2014504155A (ja) * | 2010-11-30 | 2014-02-20 | 株式會社アモーレパシフィック | 皮膚老化と関連した遺伝子及び皮膚老化を防止する物質をスクリーニングする方法 |
| WO2013031003A1 (ja) | 2011-09-01 | 2013-03-07 | 株式会社マンダム | Dna修復促進剤およびdna損傷抑制剤 |
| WO2014157485A1 (ja) | 2013-03-29 | 2014-10-02 | 国立大学法人大阪大学 | 抗老化作用を有するペプチドおよびその利用 |
| WO2017122668A1 (ja) | 2016-01-12 | 2017-07-20 | 国立大学法人 東京医科歯科大学 | 脱毛および白毛化を抑制もしくは改善するための組成物ならびにその使用 |
| JP2018108973A (ja) * | 2017-01-05 | 2018-07-12 | 丸善製薬株式会社 | Xvii型コラーゲン産生促進剤 |
| JP2018193336A (ja) * | 2017-05-18 | 2018-12-06 | 共栄化学工業株式会社 | 皮膚外用剤 |
| JP2019059616A (ja) | 2017-09-28 | 2019-04-18 | セイコーエプソン株式会社 | 媒体搬送装置、画像読取装置、記録装置 |
Non-Patent Citations (3)
| Title |
|---|
| MATSUMURA, H ET AL.: "Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis", SCIENCE, vol. 351, 2016, pages aad4395, XP055754974, DOI: 10.1126/science.aad4395 |
| NATSUGA KEN : "Elucidation of the control mechanism of stem cell quiescence cycle by an epidermal basement membrane", RESEARCH REPORTS OF THE UEHARA MEMORIAL FOUNDATION, vol. 32, no. 178, 2018, JP, pages 178, XP009531127, ISSN: 2433-3441 * |
| TANIMURA ET AL., CELL STEM CELL, vol. 8, February 2011 (2011-02-01), pages 177 - 187 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023276942A1 (ja) * | 2021-06-28 | 2023-01-05 | 株式会社 資生堂 | 血管内皮増殖因子の増加の抑制剤およびそのスクリーニング方法 |
| JP2023054511A (ja) * | 2021-10-04 | 2023-04-14 | 日本メナード化粧品株式会社 | 皮膚幹細胞増殖促進剤及び皮膚再生促進剤 |
| JP7754483B2 (ja) | 2021-10-04 | 2025-10-15 | 日本メナード化粧品株式会社 | 皮膚幹細胞増殖促進剤及び皮膚再生促進剤 |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2020196801A1 (enExample) | 2020-10-01 |
| CN113874044A (zh) | 2021-12-31 |
| KR20210144835A (ko) | 2021-11-30 |
| JP2023052310A (ja) | 2023-04-11 |
| JP2024175085A (ja) | 2024-12-17 |
| EP3978020A4 (en) | 2023-08-09 |
| CN118079006A (zh) | 2024-05-28 |
| CN113874044B (zh) | 2024-03-29 |
| EP3978020A1 (en) | 2022-04-06 |
| JP7214268B2 (ja) | 2023-01-30 |
| US20220331389A1 (en) | 2022-10-20 |
| JP7564568B2 (ja) | 2024-10-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7564568B2 (ja) | 皮膚用組成物 | |
| CN101068525B (zh) | 用于改善皮肤情况和外观的组合物及其应用方法 | |
| TWI581809B (zh) | 用於刺激magp-1以改善皮膚外觀之組合物及方法 | |
| JP5184094B2 (ja) | 化粧品組成物における植物抽出物の使用 | |
| JP3843943B2 (ja) | N−アシルアミノ−アミドファミリーの新規な化合物、それを含有する組成物及び用途 | |
| JP2010514774A (ja) | 肌の状態および外観を改善するための組成物およびその使用方法 | |
| TW201206494A (en) | Use of Tiliacora triandra in cosmetics and compositions thereof | |
| KR102771814B1 (ko) | Pgc-1알파의 발현을 증가시키는 조성물 | |
| JP2018150262A (ja) | 抗老化剤 | |
| KR20230172226A (ko) | 라벤더잎세포추출물을 유효성분으로 포함하는 외부 자극에 의한 피부 장벽개선 및 피부 진정(붉은기)에 효과적인 조성물 | |
| CN115400129A (zh) | 细胞衰老表型改变用或皮肤老化防止用组合物 | |
| KR102511527B1 (ko) | 칠엽수 추출물을 포함하는 조성물 | |
| KR20180108255A (ko) | 파리신 비를 포함하는 화장료 조성물 | |
| JP7411196B2 (ja) | Xvii型コラーゲン維持強化剤 | |
| CH706016A2 (de) | Auf Stammzellen der Epidermis und Dermis und auf deren Mikro-Umgebung wirkende kosmetische Zusammensetzung. | |
| KR20120032366A (ko) | 말태반을 포함하는 줄기세포 증식 촉진용 조성물 | |
| JP2022135960A (ja) | 組成物 | |
| JP2018108973A (ja) | Xvii型コラーゲン産生促進剤 | |
| KR20180108254A (ko) | 파리신 a를 포함하는 화장료 조성물 | |
| RU2352322C1 (ru) | Дерматологическое и/или косметологическое средство, защищающее кожу от последствий воздействия повреждающих факторов, средство, обладающее способностью элиминировать клетки с выраженной хромосомной нестабильностью | |
| CN119730842A (zh) | 用于诱导视黄醇样作用的试剂及其用途 | |
| HK1110508B (en) | Compositions and methods of their use for improving the condition and appearance of skin |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2021509620 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20217034883 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2020778483 Country of ref document: EP Effective date: 20211027 |