WO2019080898A1 - COMPOSITIONS AND METHODS FOR MODULATION OF IMMUNE RESPONSE BY ACTIVATION OF PROTEIN KINASE ALPHA 1 - Google Patents
COMPOSITIONS AND METHODS FOR MODULATION OF IMMUNE RESPONSE BY ACTIVATION OF PROTEIN KINASE ALPHA 1Info
- Publication number
- WO2019080898A1 WO2019080898A1 PCT/CN2018/111885 CN2018111885W WO2019080898A1 WO 2019080898 A1 WO2019080898 A1 WO 2019080898A1 CN 2018111885 W CN2018111885 W CN 2018111885W WO 2019080898 A1 WO2019080898 A1 WO 2019080898A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alpk1
- compound
- virus
- adp
- agonist
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 CCCC(C*P(C*P(O)(OC(*)C1(C)OC(*)(*)*1)=*)(O)=*)C(*)C(C)C(C)C(C)N(C*)N Chemical compound CCCC(C*P(C*P(O)(OC(*)C1(C)OC(*)(*)*1)=*)(O)=*)C(*)C(C)C(C)C(C)N(C*)N 0.000 description 17
- YNAVUWVOSKDBBP-UHFFFAOYSA-N C1NCCOC1 Chemical compound C1NCCOC1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- PAFZNILMFXTMIY-UHFFFAOYSA-N NC1CCCCC1 Chemical compound NC1CCCCC1 PAFZNILMFXTMIY-UHFFFAOYSA-N 0.000 description 1
- ZYTJUQJJOZWHSZ-KUEPYMCMSA-N Nc1ncnc2c1nc[n]2[C@@H](C1F)O[C@H](COP(N2CCOCC2)(O)=O)C1O Chemical compound Nc1ncnc2c1nc[n]2[C@@H](C1F)O[C@H](COP(N2CCOCC2)(O)=O)C1O ZYTJUQJJOZWHSZ-KUEPYMCMSA-N 0.000 description 1
- HHBMCLRSXJLPGZ-SBXXRYSUSA-N OC1C(O)=C(COP(N2CCOCC2)(O)=O)O[C@H]1[n]1c(ncnc2O)c2nc1 Chemical compound OC1C(O)=C(COP(N2CCOCC2)(O)=O)O[C@H]1[n]1c(ncnc2O)c2nc1 HHBMCLRSXJLPGZ-SBXXRYSUSA-N 0.000 description 1
- NTXAXZOCYGFDQQ-OMNKOJBGSA-N OC1C(O)=C(COP(O)(O)=O)O[C@H]1[n]1c(ncnc2O)c2nc1 Chemical compound OC1C(O)=C(COP(O)(O)=O)O[C@H]1[n]1c(ncnc2O)c2nc1 NTXAXZOCYGFDQQ-OMNKOJBGSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H19/00—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof
- C07H19/02—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof sharing nitrogen
- C07H19/04—Heterocyclic radicals containing only nitrogen atoms as ring hetero atom
- C07H19/16—Purine radicals
- C07H19/20—Purine radicals with the saccharide radical esterified by phosphoric or polyphosphoric acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H19/00—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof
- C07H19/02—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof sharing nitrogen
- C07H19/04—Heterocyclic radicals containing only nitrogen atoms as ring hetero atom
- C07H19/06—Pyrimidine radicals
- C07H19/10—Pyrimidine radicals with the saccharide radical esterified by phosphoric or polyphosphoric acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
- A61K31/7072—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid having two oxo groups directly attached to the pyrimidine ring, e.g. uridine, uridylic acid, thymidine, zidovudine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7076—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines containing purines, e.g. adenosine, adenylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7076—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines containing purines, e.g. adenosine, adenylic acid
- A61K31/708—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines containing purines, e.g. adenosine, adenylic acid having oxo groups directly attached to the purine ring system, e.g. guanosine, guanylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H19/00—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof
- C07H19/02—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof sharing nitrogen
- C07H19/04—Heterocyclic radicals containing only nitrogen atoms as ring hetero atom
- C07H19/16—Purine radicals
- C07H19/20—Purine radicals with the saccharide radical esterified by phosphoric or polyphosphoric acids
- C07H19/207—Purine radicals with the saccharide radical esterified by phosphoric or polyphosphoric acids the phosphoric or polyphosphoric acids being esterified by a further hydroxylic compound, e.g. flavine adenine dinucleotide or nicotinamide-adenine dinucleotide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- the present invention relates to compositions and methods for therapy by activating alpha protein kinase 1 (ALPK1) .
- APK1 alpha protein kinase 1
- Alpha-kinases are a unique protein kinase superfamily, displaying little sequence similarity to typical protein kinases.
- a total of six alpha kinase members including alpha-protein kinase 1 (ALPK1) , ALPK2, ALPK3, elongated factor-2 kinase (eEF2K) , and transient receptor potential cation channel M6 and M7 (TRPM6 and TRPM7) have been identified (Ryazanov AG et al., Curr Biol 1999 9 (2) : R43-45; Ryazanov AG et al., Proc Natl Acad Sci USA 1997 94 (10) : 4884-4889) .
- ALPK1 was identified as a new component of raft-containing sucrose-isomerase (SI) vesicles in epithelial cells (Heinet M et al., J. Biol. Chem. 2005 280 (27) : 25637-43) . It was shown that ALPK1 phosphorylates myosin 1 and plays an essential role in the exocytic transport to the apical plasma membrane. A transposon-inserted homozygous inactivating mutation of ALPK1 in mice resulted in motor coordination deficits which could be rescued by overexpressing full-length ALPK1 (Chen M et al., BMC Neurosci. 2011 12: 1) .
- SI sucrose-isomerase
- ALPK1 activation has also been implicated as playing a role in cancer, including lung, colorectal, and breast cancers (Liao HF et al. Scientific Reports 2016 6: 27350; Strietz J et al., Oncotarget 2016 1-16) .
- ALPK1 is an important regulator of the innate immune response activated by certain bacteria.
- APLK1 was suggested to be a key regulator of innate immunity against bacteria through its promotion of TIFA oligomerization and interleukin 8 (IL-8) expression in response to infection with S. flexneri, S. typhimurium, and Neisseria meningitides (Milivojevic M et al., PLoS Pathog 2017 13 (2) : e1006224) .
- IL-8 interleukin 8
- Zimmerman et al. describe an ALPK1 and TIFA dependent innate immune response triggered by the Helicobacter pylori Type IV Secretion System.
- the present invention is based, in part, on the discovery that certain bacterial metabolites, including D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate (heptose 1, 7 bisphosphate or “HBP” ) as well as D-glycero- ⁇ -D-manno-heptose-1-phosphate (HMP-1bP) , L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) and D-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) , and derivatives thereof represented by formula IA, IB, or IC described herein, induce ALPK1-dependent activation of downstream signaling, including increased expression of proinflammatory cytokines such as IL-8 and TNF ⁇ .
- HBP D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate
- HMP-1bP
- HMP-1bP biological activity of HMP-1bP, its downstream product H1b-ADP-6L, and particularly H1b-ADP
- H1b-ADP biological activity of HMP-1bP
- H1b-ADP-6L downstream product of innate immunity by bacterial metabolites.
- present disclosure also provides evidence of anti-tumor activity by H1b-ADP and H1b-ADP derviatives and demonstrates that co-administration of H1b-ADP with immune checkpoint inhibitors and immune modulators, including anti-PD-L1 and anti-PD-1 antibodies, anti-CTLA4 antibody, and anti-CD4 antibody, has a synergistic anti-tumor effect.
- the present disclosure also shows that co-administration of H1b-ADP with immune modulators including each of interferon alpha (INF ⁇ ) , a stimulator of interferon genes ( “STING” ) agonist, and a TLR agonist (resquimod) has a synergistic anti-tumor effect.
- immune modulators including each of interferon alpha (INF ⁇ ) , a stimulator of interferon genes ( “STING” ) agonist, and a TLR agonist (resquimod) has a synergistic anti-tumor effect.
- compositions and methods related to modulating an immune response treating cancer, potentiating an immune response to a target antigen, treating a disease or disorder amendable to treatment by activation of NFkB, p38, and JNK cell signaling pathways, and treating or preventing a disease or disorder caused by an infectious agent through activation of ALPK1.
- ALPK1 activation is achieved via the administration of an ALPK1 agonist selected from HBP, HMP-1bP, H1b-ADP-6L, and H1b-ADP, preferably HMP-1bP, H1b-ADP-6L, and H1b-ADP, and most preferably H1b-ADP-6L and H1b-ADP, or a derivative thereof represented by formula IA, IB, or IC described herein.
- the disclosure provides methods of modulating an immune response in a subject, the methods comprising administering to the subject a composition comprising any one of an ALPK1 agonist represented by formula I, IA, IB, or IC described herein.
- the present invention discloses novel heterocyclic compounds as agonists of ALPK1.
- the compounds are represented by formula (I) :
- a 1 , A 2 , L 1 , L 2 , L 3 , Z 1 , Z 2 , W 1 , W 2 , R 1 , R 2 , R 3 , R 4 , R 5 , R 6 and R 7 are as defined herein.
- stereoisomers, tautomers, stable isotopes, prodrugs, and pharmaceutically acceptable salts of the compounds of Formula I are also included within the scope of the disclosure.
- L 1 and L 2 are independently selected from O, CH 2 , CHF and CF 2 ;
- L 3 is O, S, CH 2 or CH (OH) ;
- Z 1 and Z 2 are independently selected from O and S;
- W 1 is -C (R 10 R 11 ) -, wherein R 10 and R 11 are independently selected from H, D, -OH, halogen, and optionally substituted groups selected from C1-C4 alkyl, C1-C4 alkoxyl, C1-C4 haloalkyl, C1-C4-haloalkoxyl, C1-C4 alkenyloxyl, aralkyloxyl and R 12 CO 2 -, wherein R 12 is selected from C1-C4 alkyl, C1-C4 alkoxyl, C1-C4 alkenyloxyl, C1-C4 alkylamino, C3-C6 cycloalkyl, cycloheteroalkyl containing 3 to 6 ring members and having 1-3 heteroatoms selected from N, O and S as ring members, C6-C10 aryl, and heteroaryl containing 5 to 10 ring atoms and having 1-3 heteroatoms selected from N, O and S as
- R 2 , R 3 and R 4 are independently selected from H, D, halogen, C1-C4 alkyl and C1-C4 haloalkyl;
- R 5 , R 6 and R 7 are selected from H, D, halogen and -OH, R 12 CO 2 -, wherein R 12 is
- the disclosure provides a method for modulating an immune response in a subject in need of such treatment, the method comprising administering to the subject a composition comprising any one of an ALPK1 agonist, including an ALPK1 agonist represented by formula I, IA, IB, or IC described herein, a polynucleotide encoding ALPK1 or a constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the method for modulating an immune response is selected from activation of innate immunity and activation of adaptive immunity.
- the disclosure provides a method for treating cancer in a subject in need of such treatment, the method comprising administering to the subject a composition comprising any one of an agonist of ALPK1, including an ALPK1 agonist represented by formula I, IA, IB, or IC described herein, a polynucleotide encoding ALPK1 or a constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- a composition comprising any one of an agonist of ALPK1, including an ALPK1 agonist represented by formula I, IA, IB, or IC described herein, a polynucleotide encoding ALPK1 or a constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the composition comprises an an ALPK1 agonist selected from a compound represented by formula I, IA, IB, or IC described herein, or selected from HBP, HMP-1bP, H1b-ADP-6L, and H1b-ADP, preferably HMP-1bP, H1b-ADP-6L, and H1b-ADP, and most preferably H1b-ADP-6L and H1b-ADP.
- the ALPK1 agonist is H1b-ADP.
- the ALPK1 agonist is selected from any one of Compounds 1-3, 9-17, 19, 20-22, and 26-32.
- the ALPK1 agonist is Compound 15.
- the cancer is selected from soft tissue sarcoma, breast cancer, head and neck cancer, melanoma, cervical cancer, bladder cancer, hematologic malignancy, glioblastoma, pancreatic cancer, prostate cancer, colon cancer, breast cancer, renal cancer, lung cancer, merkel cell carcinoma, small intestine cancer, thyroid cancer, acute myelogenous leukemia (AML) , acute lymphocytic leukemia (ALL) , chronic lymphocytic leukemia (CLL) , chronic myelogenous leukemia (CML) , gastric cancer, gastrointestinal stromal tumors, non-Hodgkins lymphoma, Hodgkins lymphoma, liver cancer, leukemia, lymphoma, T-cell lymphoma, brain cancer, and multiple myeloma.
- the cancer is selected from breast cancer, head and neck cancer, melanoma, renal cancer, lung cancer, merkel cell carcinoma, and lymphoma.
- the disclosure provides a method for potentiating an immune response to a target antigen in a subject, the method comprising administering to the subject a composition comprising any one of an agonist of ALPK1, including an ALPK1 agonist represented by formula I, IA, IB, or IC described herein, a polynucleotide encoding ALPK1 or a constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein, as a vaccine or immunologic adjuvant that acts to potentiate an immune response to the target antigen.
- a composition comprising any one of an agonist of ALPK1, including an ALPK1 agonist represented by formula I, IA, IB, or IC described herein, a polynucleotide encoding ALPK1 or a constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein, as a vaccine or immunologic adjuvant that acts to potentiate an immune response to the target antigen.
- the target antigen is an antigen of an infectious agent selected from the group consisting of adenovirus, Coxsackie B virus, cytomegalovirus, eastern equine encephalitis virus, ebola virus, enterovirus 71, Epstein–Barr virus, Haemophilus influenzae type b (Hib) , hepatitis C virus (HCV) , herpes virus, human immunodeficiency virus (HIV) , human papillomavirus (HPV) , hookworm, Marburg virus, norovirus, respiratory syncytial virus (RSV) , rotavirus, salmonella typhi, Staphylococcus aureus, Streptococcus pyogenes, varicella, West Nile virus, Yersinia pestis, and Zika virus.
- an infectious agent selected from the group consisting of adenovirus, Coxsackie B virus, cytomegalovirus, eastern equine encepha
- the agonist of ALPK1, including an ALPK1 agonist represented by formula I, IA, IB, or IC described herein, the polynucleotide encoding ALPK1 or a constitutively active mutant thereof, or the ALPK1 protein or constitutively active mutant of said protein acts as a vaccine adjuvant for a vaccine in the treatment or prevention of anthrax, caries, Chagas disease, dengue, diphtheria, ehrlichiosis, hepatits A or B, herpes, seasonal influenza, Japanese encephalitis, leprosy, lyme disease, malaria, measles, mumps, meningococcal disease, including meningitis and septicemia, Onchocerciasis river blindness, pertussis (whooping cough) , pneumococcal disease, polio, rabies, rubella, schistosomiasis, severe acute respiratory syndrome (SARS) , shingles, smallpox, syphilis,
- the disclosure provides a method for treating a disease or disorder amendable to treatment by activation of NFkB, p38, and JNK cell signaling pathways in cells of a subject, the method comprising administering to the subject a composition comprising any one of an agonist of ALPK1, a polynucleotide encoding ALPK1 or constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the disease or disorder is selected from tuberculosis, meningitis, pneumonia, ulcer, sepsis, rhinitis, asthma, allergy, COPD, inflammatory bowel disease, arthritis, obesity, radiation-induced inflammation, psoriasis, atopic dermatitis, non-alcoholic steatohepatitis (NASH) , Alzheimer’s disease, systemic lupus, erythematosus (SLE) , autoimmune thyroiditis (Grave’s disease) , multiple sclerosis, ankylosing spondylitis bullous diseases, and diseases and disorders caused by the hepatitis C virus (HCV) , the hepatitis B virus (HBV) , or the human immunodeficiency virus (HIV) .
- HCV hepatitis C virus
- HBV hepatitis B virus
- HMV human immunodeficiency virus
- the disclosure provides a method for treating or preventing a disease or disorder caused by an infectious agent selected from a bacteria, virus, or parasite in a subject in need thereof, the methods comprising administering to the subject a composition comprising any one of an agonist of ALPK1, including an ALPK1 agonist represented by formula I, IA, IB, or IC described herein, a polynucleotide encoding ALPK1 or constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the infectious agent is a bacteria.
- the infectious agent is a virus.
- the infectious agent is a parasite.
- the bacteria is a Gram-negative or a Gram-positive bacteria.
- the Gram-negative bacteria is selected from the group consisting of Acinetobacter baumanii, Aggregatobacter actinomycetemcomitans, Bartonella bacilliformis, Bartonella henselae, Bartonella quintana, Bifidobacterium, Borrelia, Bortadella pertussis, Brucella sp, Burkholderia cepacis, Burkholderia psedomallei, Campylobacter jejuni, Cardiobacterium hominis, Campylobacter fetus, Chlamydia pneumonia, Chlymydia trachomatis, Clostridium difficile, Cyanobacteria, Eikennella corrodens, Enterobacter, Enterococcus faccium, Escherichia coli, Escherichia coli 0157, Franceilla tularensis, Fusobacterium nucleatum, Haemophilus influenza, Haemophilus a
- the Gram-positive bacteria selected from the group consisting of Actinomycetes, Bacillus anthracis, Bacillus subtilis, Clostridium tetani, Clostridium perfingens, Clostridium botulinum, Clostridium tetani.
- MRSA methicillin-resistant Staphylococcus aure
- the virus is selected from the group consisting of ebolavirus, hepatitis B virus, hepatitis C virus, herpes simplex virus, human immunodeficiency virus (HIV) , human papillomavirus (HPV-6, HPV-11) , human SARS coronavirus, influenza A virus, influenza B virus, influenza C virus, measles virus, rabies virus, poliovirus, SARS corona virus, and yellow fever virus.
- ebolavirus hepatitis B virus, hepatitis C virus, herpes simplex virus, human immunodeficiency virus (HIV) , human papillomavirus (HPV-6, HPV-11) , human SARS coronavirus, influenza A virus, influenza B virus, influenza C virus, measles virus, rabies virus, poliovirus, SARS corona virus, and yellow fever virus.
- the parasite is selected from the group consisting of Acanthamoeba spp, American trypanosomiasis, Balamuthia mandnillanis, Babesia divergenes, Babesia bigemina, Babesia equi, Babesia microfti, Babesia duncani, Balantidium coli, Blastocystis spp Cryptosporidium spp, Cyclospora cayetanensis, Dientamoeba fragilis, Diphyllobothrium latum, Leishmania amazonesis, Naegleria fowderi, Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale curtisi, Plasmodium malariae, Rhinosporidium seeberi, Sarcocystis bovihominis, Sarcocystiss suihominis, Toxoplasma gondii, Trichmon
- the method may further comprise administering to the subject one or more additional therapeutic agents or immune modulators, and combinations thereof.
- the one or more additional therapeutic agents is selected from an anti-microbial agent, such as an anti-bacterial agent, an anti-viral agent, or an anti-parasitic agent, an anti-cancer agent, or a therapeutic agent for the treatment of tuberculosis, meningitis, pneumonia, ulcer, sepsis, rhinitis, asthma, allergy, COPD, inflammatory bowel disease, arthritis, obesity, radiation-induced inflammation, psoriasis, atopic dermatitis, non-alcoholic steatohepatitis (NASH) , Alzheimer’s disease, systemic lupus, erythematosus (SLE) , autoimmune thyroiditis (Grave’s disease) , multiple sclerosis, and ankylosing spondylitis bullous diseases.
- an anti-microbial agent such as an anti-bacterial agent, an anti-viral agent, or an anti-paras
- the one or more additional therapeutic agents is an immune modulator.
- the immune modulator is selected from one or more of an inhibitor or antagonist of an immune checkpoint regulator, an immune stimulatory molecule, and an agonist of an immune co-stimulatory molecule.
- the inhibitor or antagonist of an immune checkpoint regulator is a PD-1/PD-L1 inhibitor.
- the PD-1/PD-L1 inhibitor is selected from the group consisting of nivolumab, pembrolizumab, pidilizumab, BMS-936559, atezolizumab, durvalumab, and avelumab.
- the immune modulator is selected from interferon alpha (INF ⁇ ) , a stimulator of interferon genes ( “STING” ) agonist, a TLR agonist (e.g., resquimod) , and an anti-OX40 (CD134) agonist antibody.
- the agonist of an immune co-stimulatory molecule is an anti-OX40 (CD134) agonist antibody.
- the cancer is selected from advanced melanoma, non-small cell lung cancer, renal cell carcinoma, bladder cancer, Hodgkin’s lymphoma, liver cancer, gastric cancer, colon cancer, breast cancer, non-Hodgkin’s lymphoma, prostate cancer, head and neck cancer, thyroid cancer, brain cancer, acute myeloid leukemia (AML) , merkel cell carcinoma, multiple myeloma, cervical cancer, and sarcoma.
- AML acute myeloid leukemia
- the one or more additional immune modulators is an inhibitor or antagonist of an immune checkpoint regulator, or a vaccine against an immune checkpoint regulator.
- the one or more additional immune modulators is an agonist of an immune an immune checkpoint regulator, such as a co-stimulatory molecule, for example an agonist of OX40 (CD134) .
- the immune checkpoint regulator is selected from the programed cell death 1 (PD-1) receptor (CD279) , a ligand of PD-1 (e.g., PD-L1) , cytotoxic T-lymphocyte associated protein 4 (CTLA4) , tumor necrosis factor receptor superfamily member 9 (alternatively TNFRSF9, 4-1BB) and 4-1BB ligands, tumor necrosis factor receptor superfamily member 4 (alternatively TNFRSF4, OX40) and OX40 ligands, glucocorticoid-induced TNFR-related protein (GITR) , Tumor Necrosis Factor Receptor Superfamily Member 7 (alternatively TNFRSF7, cluster of differentiation 27, CD27) , TNFRSF25 and TNF-like ligand 1A (TL1A) , TNF Receptor Superfamily Member 5 (alternatively TNFRSF5, CD40) and CD40 ligand, Herpesvirus entry mediator (HVEM)
- the one or more additional immune modulators is a vaccine.
- the vaccine is a vaccine against a tumor antigen.
- the tumor antigen is selected from glycoprotein 100 (gp100) , mucin 1 (MUC1) , and melanoma-associated antigen 3 (MAGEA3) .
- the one or more additional immune modulators is a T cell, preferably a chimeric antigen receptor T cell.
- the one or more additional immune modulators is a recombinant protein, preferably selected from granulocyte-macrophage colony-stimulating factor (GM-CSF) , interleukin 7 (IL-7) , IL-12, IL-15, IL-18, and IL-21.
- GM-CSF granulocyte-macrophage colony-stimulating factor
- IL-7 interleukin 7
- IL-12 interleukin-12
- IL-15 interleukin-15
- IL-18 interleukin-21
- the composition may comprise an ALPK1 agonist represented by formula I, IA, IB, or IC described herein, or an ALPK1 agonist selected from D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate (HBP) , and prodrugs, analogues and derivatives thereof.
- the ALPK1 agonist is HBP.
- the ALPK1 agonist is selected from any one of Compounds 1-3, 9-17, 19, 20-22, and 26-32.
- the ALPK1 agonist is Compound 15.
- the ALPK1 agonist is a prodrug of HBP.
- the prodrug comprises a protecting group selected from the group consisting of a carbonyloxymethyl, a cyclosaligenyl, a cyclic 1-aryl-1, 3-propanyl ester, an aryloxy phosphoramidate or phosphonamidate, and a methylaryl haloalkylamdiate.
- the prodrug is a compound of Formula 3a, 3b, 3c, 3d, or 3e.
- the prodrug is selected from a compound of Table 1.
- the composition may comprise a polynucleotide encoding ALPK1, or a constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the composition comprises a polynucleotide encoding ALPK1, or a constitutively active mutant thereof.
- the composition is adapted for administration to the subject using a viral or non-viral gene delivery system.
- the composition is adapted for administration to the subject using a viral gene delivery system.
- the composition further comprises viral particles.
- the composition is adapted for administration to the subject using a non-viral gene delivery system.
- the composition further comprises one or more of liposomal particles, nanoparticles, minicircles, minivectors, and polymeric carriers.
- the non-viral gene delivery system comprises a gene editing technique.
- the gene editing technique utilizes a meganuclease, zinc finger nucleases (ZFNs) , transcription activator-like effector nucleases (TALENs) , or CRISPR/Cas-9.
- the disclosure provides a method for treating a liver disease or disorder in a subject in need of such treatment, the method comprising administering a low dose of H1b-ADP, or a derivative thereof, to the subject.
- the low dose is in the range of from 1 nanogram to 1 milligram per kilogram body weight (1 ng/kg to 1 mg/kg) , preferably 1 microgram to 100 micrograms per kilogram body weight (1 ug/kg to 100 ug /kg) .
- the liver disease or disorder is selected from liver cancer, non-alcoholic steatohepatitis (NASH) , and a disease or disorder caused by infection with the hepatitis C virus (HCV) or the hepatitis B virus (HBV) .
- NASH non-alcoholic steatohepatitis
- HCV hepatitis C virus
- HBV hepatitis B virus
- the disclosure provides a method for treating cancer, the method comprising administering to the subject in need of such treatment a composition comprising bacteria producing H1b-ADP or H1b-ADP-6L.
- the composition is administered via intratumoral injection.
- the subject may be a vertebrate. In embodiments, the subject is a human.
- the disclosure also provides a vaccine composition or vaccine adjuvant composition comprising an agonist of ALPK1 and a pharmaceutical composition comprising an agonist of ALPK1, and a carrier.
- the agonist of ALPK1 is a compound represented by formula I, IA, IB, or IC described herein, HBP, or a prodrug, analogue, or derivative thereof.
- the ALPK1 agonist is selected from any one of Compounds 1-3, 9-17, 19, 20-22, and 26-32.
- the ALPK1 agonist is Compound 15.
- the ALPK1 agonist is a prodrug of HBP.
- the prodrug comprises a protecting group selected from the group consisting of a carbonyloxymethyl, a cyclosaligenyl, a cyclic 1-aryl-1, 3-propanyl ester, an aryloxy phosphoramidate or phosphonamidate, and a methylaryl haloalkylamdiate.
- the prodrug is a compound of Formula 3a, 3b, 3c, 3d, or 3e.
- the prodrug is selected from a compound of Table 1.
- the disclosure also provides methods of selecting a compound capable of modulating an immune response in a mammalian subject, the method comprising contacting ALPK1 with the test compound in the presence of ATP and, separately but concurrently, in the absence of ATP, followed by performing an assay to detect ALPK1 phosphorylation and/or activation of one or more downstream targets of ALPK1 signaling.
- the contacting of ALPK1 with the test compound is performed in a cell-free system or in a cellular system.
- the assay to detect ALPK1 phosphorylation and/or activation of one or more downstream targets of ALPK1 signaling comprises a radiometric based kinase assay, a fluorescence-based kinase assay, a time-resolved fluorescence energy transfer (TR-FRET) based assay, an alpha-technology based assay, an enzyme-linked immunosorbent assay, luminescence detection, a mobility shift based kinase assay, a Western based kinase assay, and a ligand-kinase binding assay.
- a radiometric based kinase assay e assay
- a fluorescence-based kinase assay e.g., a time-resolved fluorescence energy transfer (TR-FRET) based assay
- TR-FRET time-resolved fluorescence energy transfer
- the ALPK1 agonist may be selected from a compound represented by formula I, IA, IB, or IC described herein, D-glycero-b-D-manno-heptose 1, 7-bisphosphate (HBP) , D-glycero-b-D-manno-heptose-1-phosphate (HMP-1bP) , L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) and D- glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) , and prodrugs, analogues and derivatives of any of the foregoing molecules.
- HBP D-glycero-b-D-manno-heptose 1, 7-bisphosphate
- HMP-1bP D-glycero-b-D-manno-heptose-1-phosphate
- the ALPK1 agonist is selected from HMP-1bP, H1b-ADP, and H1b-ADP-6L. In embodiments, the ALPK1 agonist is H1b-ADP or H1b-ADP-6L, and derivatives thereof as described herein. In embodiments, the ALPK1 agonist is H1b-ADP. In embodiments, the ALPK1 agonist is selected from any one of Compounds 1-3, 9-17, 19, 20-22, and 26-32. In embodiments, the ALPK1 agonist is Compound 15.
- the disclosure provides a vaccine composition or vaccine adjuvant composition comprising an agonist of ALPK1 selected from a compound represented by formula I, IA, IB, or IC described herein, HBP, HMP-1bP, H1b-ADP, and H1b-ADP-6L.
- the ALPK1 agonist is selected from HMP-1bP, H1b-ADP, and H1b-ADP-6L.
- the ALPK1 agonist is H1b-ADP or H1b-ADP-6L, and derivatives thereof as described herein.
- the ALPK1 agonist is H1b-ADP.
- the ALPK1 agonist is a compound represented by formula I, IA, IB, or IC described herein. In embodiments, the ALPK1 agonist is selected from any one of Compounds 1-3, 9-17, 19, 20-22, and 26-32. In embodiments, the ALPK1 agonist is Compound 15.
- the disclosure provides a pharmaceutical composition comprising an agonist of ALPK1 selected from a compound represented by formula I, IA, IB, or IC described herein, HBP, HMP-1bP, H1b-ADP, and H1b-ADP-6L.
- the ALPK1 agonist is selected from HMP-1bP, H1b-ADP, and H1b-ADP-6L.
- the ALPK1 agonist is H1b-ADP or H1b-ADP-6L, and derivatives thereof as described herein.
- the ALPK1 agonist is H1b-ADP.
- the ALPK1 agonist is a compound represented by formula I, IA, IB, or IC described herein.
- the ALPK1 agonist is selected from any one of Compounds 1-3, 9-17, 19, 20-22, and 26-32.
- the ALPK1 agonist is Compound 15.
- the disclosure provides a method of treating cancer in a subject in need of such treatment, comprising administering to the subject a composition comprising an agonist of ALPK1 selected from the group consisting of a compound represented by formula I, IA, IB, or IC described herein, HBP, HMP-1bP, H1b-ADP and H1b-ADP-6L.
- the ALPK1 agonist is selected from HMP-1bP, H1b-ADP, and H1b-ADP-6L.
- the ALPK1 agonist is H1b-ADP or H1b-ADP-6L, and derivatives thereof as described herein.
- the ALPK1 agonist is H1b-ADP.
- the ALPK1 agonist is selected from any one of Compounds 1-3, 9-17, 19, 20-22, and 26-32. In embodiments, the ALPK1 agonist is Compound 15. In embodiments, the method further comprises administering to the subject a PD-1/PD-L1 inhibitor or an agonist of an immune co-stimulatory molecule. In embodiments, the ALPK1 agonist is H1b-ADP and the PD-1/PD-L1 inhibitor is selected from the group consisting of nivolumab, pembrolizumab, pidilizumab, BMS-936559, atezolizumab, durvalumab, and avelumab.
- the ALPK1 agonist is H1b-ADP and the agonist of an immune co-stimulatory molecule is an anti-OX40 (CD134) agonist antibody.
- the subject may be a human subject and the cancer may be a cancer as described hereinabove.
- the cancer is a solid tumor.
- the cancer is refractory.
- the disclosure further provides a composition for use in therapy, the composition comprising an ALPK1 agonist selected from D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate (HBP) , D-glycero- ⁇ -D-manno-heptose-1-phosphate (HMP-1bP) , L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) and D-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) , and prodrugs, analogues and derivatives thereof; or the composition comprising an ALPK1 agonist selected from H1b-ADP-6L, H1b-ADP, or a derivative thereof selected from a compound of any one of claims 1 to 22 and any one of Compounds 1-33 of Table 1.
- HBP D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate
- the disclosure also provides a composition for use in a method for modulating an immune response in a subject in need of such treatment, the composition comprising an ALPK1 agonist selected from D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate (HBP) , D-glycero- ⁇ -D-manno-heptose-1-phosphate (HMP-1bP) , L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) and D-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) , and prodrugs, analogues and derivatives thereof; or the composition comprising an ALPK1 agonist selected from H1b-ADP-6L, H1b-ADP, or a derivative thereof selected from a compound of any one of claims 1 to 22 and any one of Compounds 1-33 of Table 1.
- an ALPK1 agonist selected from D-
- the disclosure also provides a composition for use in a method for treating cancer in a subject in need of such treatment, the composition comprising an ALPK1 agonist selected from D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate (HBP) , D-glycero- ⁇ -D-manno-heptose-1-phosphate (HMP-1bP) , L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) and D-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) , and prodrugs, analogues and derivatives thereof; or the composition comprising an ALPK1 agonist selected from H1b-ADP-6L, H1b-ADP, or a derivative thereof selected from a compound of any one of claims 1 to 22 and any one of Compounds 1-33 of Table 1.
- HBP D-glycero- ⁇ -D-
- the disclosure also provides a composition for use in a method for potentiating an immune response in a subject in need of such treatment, the composition comprising an ALPK1 agonist selected from D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate (HBP) , D-glycero- ⁇ -D-manno-heptose-1-phosphate (HMP-1bP) , L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) and D-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) , and prodrugs, analogues and derivatives thereof; or the composition comprising an ALPK1 agonist selected from H1b-ADP-6L, H1b-ADP, or a derivative thereof selected from a compound of any one of claims 1 to 22 and any one of Compounds 1-33 of Table 1.
- an ALPK1 agonist selected from
- the disclosure also provides a composition for use in a method for treating a disease or disorder amendable to treatment by activation of NFkB, p38, and JNK cell signaling pathways in cells of a subject in a subject in need of such treatment, the composition comprising an ALPK1 agonist selected from D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate (HBP) , D-glycero- ⁇ -D-manno-heptose-1-phosphate (HMP-1bP) , L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) and D-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) , and prodrugs, analogues and derivatives thereof; or the composition comprising an ALPK1 agonist selected from H1b-ADP-6L, H1b-ADP, or a derivative thereof selected from a compound of
- the disclosure also provides a composition for use in treating or preventing a disease or disorder caused by an infectious agent selected from a bacteria, virus, or parasite in a subject in need thereof, the composition comprising an ALPK1 agonist selected from D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate (HBP) , D-glycero- ⁇ -D-manno-heptose-1-phosphate (HMP-1bP) , L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) and D-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) , and prodrugs, analogues and derivatives thereof; or the composition comprising an ALPK1 agonist selected from H1b-ADP-6L, H1b-ADP, or a derivative thereof selected from a compound of any one of claims 1 to 22 and any one of Compounds 1-33 of
- the disclosure also provides a composition for use in a method for treating cancer in a subject in need of such treatment, the composition comprising an agonist of ALPK1 selected from the group consisting of H1b-ADP-6L, H1b-ADP, or a derivative thereof selected from a compound of any one of claims 1 to 22 and any one of Compounds 1-33 of Table 1, and the method comprising combination therapy of the ALPK1 agonist with an immune modulator selected from one or more of an inhibitor or antagonist of an immune checkpoint regulator, an immune stimulatory molecule, and an agonist of an immune co-stimulatory molecule.
- an immune modulator selected from one or more of an inhibitor or antagonist of an immune checkpoint regulator, an immune stimulatory molecule, and an agonist of an immune co-stimulatory molecule.
- the disclosure also provides a composition for use in a method for treating a liver disease or disorder in a subject in need of such treatment, the composition comprising a low dose of H1b-ADP, or a derivative thereof, wherein the liver disease or disorder is optionally selected from liver cancer, non-alcoholic steatohepatitis (NASH) , and a disease or disorder caused by infection with the hepatitis C virus (HCV) or the hepatitis B virus (HBV) .
- NASH non-alcoholic steatohepatitis
- HCV hepatitis C virus
- HBV hepatitis B virus
- the disclosure also provides a composition for use in a method for treating cancer, the composition comprising bacteria producing H1b-ADP or H1b-ADP-6L, wherein the composition is optionally adapted for intratumoral injection.
- FIG. 1A-B Protein sequences for ALPK1 isoform 1 (A) and isoform 2 (B) .
- FIG. 2A-B IL-8 (A) and TNF ⁇ (B) mRNA expression were both increased by HBP (chemically-synthesized) in an ALPK1-dependent manner.
- FIG. 3A-B IL-8 (A) and TNF ⁇ (B) mRNA expression were both increased by HMP-1bP (chemically-synthesized) in an ALPK1-dependent manner.
- FIG. 4A-B IL-8 (A) and TNF ⁇ (B) mRNA expression were both increased by H1b-ADP (chemically-synthesized) in an ALPK1-dependent manner.
- FIG. 5A-B IL-8 (A) and TNF ⁇ (B) mRNA expression induced by each of HBP, HMP-1bP, and H1b-ADP.
- FIG 6 Thermal-shift assay showing binding to ALPK1 in the presence of chemically-synthesized HBP, HMP-1bP, and H1b-ADP (only H1b-ADP binds) .
- FIG 7 Cell-free kinase assay showing phosphorylation of the ALPK1 substrate TIFA in the presence of chemically-synthesized HBP, HMP-1bP and H1b-ADP (TIFA is phosphorylated only in the presence of H1b-ADP) .
- FIG 8 Cell-free kinase assay of auto-phosphorylation of ALPK1 in the presence of H1b-ADP (chemically-synthesized) .
- FIG 9 Cell-free kinase assay of ALPK1-dependent phosphorylation of I ⁇ B in the presence of H1b-ADP (chemically-synthesized) .
- FIG 10 Cell-free kinase assay showing phosphorylation of the ALPK1 substrate TIFA in the presence of chemically-synthesized H1b-ADP and H1b-ADP-6L.
- FIG 11 Intratumoral injection of H1b-ADP, but not HMP-1bP, inhibits tumor growth in mouse CT26 xenograft model.
- FIG 12 Intratumoral injection of H1b-ADP results in increased expression of cytokines and PD-1, PD-L1.
- FIG. 13A-B Intratumoral injection of H1b-ADP and anti-PD-1 antibody (RMP1-14) synergistically inhibit tumor growth in the injected tumor (A) and the distant tumor (B) in mouse CT26 xenograft model.
- RMP1-14 anti-PD-1 antibody
- FIG 14 Intratumoral injection of H1b-ADP and anti-PD-1 antibody (OX40) synergistically inhibit tumor growth in mouse CT26 xenograft model.
- FIG 15 Western analysis of phospho-TIFA following in vitro kinase reaction for ALPK1-dependent TIFA phosphorylation in the presence of either HBP+ HIda purified from HIdE mutant E. coli (left) or HBP+ HIda purified from wild-type E. coli (right) .
- FIG. 16A-B Intratumoral injection of H1b-ADP and anti-PD-L1 antibody synergistically inhibit tumor growth in the injected tumor (A) and the distant tumor (B) in mouse CT26 xenograft model.
- FIG. 17A-B Intratumoral injection of H1b-ADP and IFN-asynergistically inhibit tumor growth in the injected tumor (A) and the distant tumor (B) in mouse CT26 xenograft model.
- FIG. 18 Intratumoral injection of H1b-ADP and anti-CTLA-4 antibody synergistically inhibit tumor growth in mouse CT26 xenograft model. Pair-wise p values were determined by T test and are shown by bars on the right, *p ⁇ 0.05, **p ⁇ 0.01, ***p ⁇ 0.001.
- FIG. 19 Intratumoral injection of H1b-ADP and STING agonist c-di-AM (PS) 2 synergistically inhibit tumor growth in mouse CT26 xenograft model. Pair-wise p values were determined by T test and are shown by bars on the right, *p ⁇ 0.05, **p ⁇ 0.01, ***p ⁇ 0.001.
- FIG. 20 Intratumoral injection of H1b-ADP and anti-CD4 antibody synergistically inhibit tumor growth in mouse CT26 xenograft model.
- FIG. 21 Intratumoral injection of H1b-ADP and TLR agonist resquimod synergistically inhibit tumor growth in mouse CT26 xenograft model.
- FIG. 22 Fetal bovine, human, and mouse serum decrease H1b-ADP’s activity in inducing IL8 secretion in HEK293 cells.
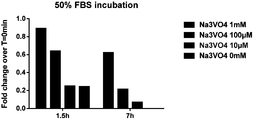
- FIG. 23 Na 3 VO 4 protects H1b-ADP from degradation by fetal bovine serum.
- FIG. 24 Na 3 VO 4 protects H1b-ADP from degradation by fetal bovine serum and retains its activity of inducing IL8 secretion in HEK293 cells.
- FIG. 25 AMP protects H1b-ADP from degradation by fetal bovine serum and retains its activity of inducing IL8 secretion in HEK293 cells.
- FIG. 26 Compounds of Formula I (1, 2, 9-12) activate IL8 secretion in HEK293 cells through activating ALPK1. HEK293 cells were cultured without FBS.
- FIGS. 27A-B Compounds of Formula I (3, 15, 19, 20) activate IL8 secretion in HEK293 without FBS (A) and with 10%FBS (B) cells through activating ALPK1.
- Compound 15 is resistant to FBS degradation.
- FIGS. 28A-B Compounds of Formula I (5, 13, 14, 17, 21, 22) activate ALPK1 as demonstrated by increased IL8 secretion in HEK293 cells without FBS (A) and with 10%FBS (B) .
- FIGS. 29A-B Compounds of Formula I (16, 26-32) activate ALPK1 as demonstrated by increased IL8 secretion in HEK293 cells without FBS (A) and with 10%FBS (B) .
- FIG. 30 Compounds of Formula I (1, 2) inhibit tumor growth in CT26 syngeneic mouse tumor model.
- FIG. 31 Bone marrow-derived mouse macrophages can be activated by a very low concentration of H1b-ADP.
- FIG. 32A-B Compound 1 activates an inflammatory response in liver tissue (A) at a dose as low as 2 nmole (1.2 ⁇ g) and in lung tissue (B) at a dose of 200 nmole.
- FIG. 33 Schematic of bacterial H1b-ADP-biosynthetic pathway.
- ALPK1 may refer to either one of two splice variants, isoform 1 or isoform 2, of the human ALPK1 gene. Each isoform shares the same kinase domain.
- the human ALPK1 gene is identified by Entrez Gene ID 80216.
- the term “activation of ALPK1” refers to the activation of ALPK1 kinase activity.
- the disclosure provides methods of activating ALPK1 by providing an ALPK1 agonist which may be, for example, an ALPK1 activating ligand, such as HBP, or a prodrug, analog or derivative thereof.
- an ALPK1 agonist which may be, for example, an ALPK1 activating ligand, such as HBP, or a prodrug, analog or derivative thereof.
- Methods for making synthetic HBP are known, for example, as described in Inuki S et al. Organic Letter 2017 19 (12) : 3079-82.
- the ALPK1 agonist is selected from HMP-1bP and H1b-ADP and prodrugs, analogs and derivatives thereof.
- the ALPK1 agonist is H1b-ADP, or a prodrug, analog or derivative thereof.
- the disclosure provides methods of activating ALPK1 by providing an ALPK1 agonist represented by formula I, IA, IB, or IC.
- alkyl refers to a straight or branched, saturated, aliphatic radical having the number of carbon atoms indicated. Alkyl can include any number of carbons, such as C 1-2 , C 1-3 , C 1-4 , C 1-5 , C 1-6 , C 1-7 , C 1-8 , C 1-9 , C 1-10 , C 2-3 , C 2-4 , C 2-5 , C 2-6 , C 3-4 , C 3-5 , C 3-6 , C 4-5 , C 4-6 and C 5-6 .
- C 1-6 alkyl includes, but is not limited to, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, hexyl, etc.
- Alkyl can also refer to alkyl groups having up to 20 carbons atoms, such as, but not limited to heptyl, octyl, nonyl, decyl, etc. Alkyl groups can be substituted or unsubstituted. In some embodiments, alkyl groups are substituted with 1-2 substituents. As a non-limiting example, suitable substituents include halogen and hydroxyl.
- alkenyl refers to a straight chain or branched hydrocarbon having at least 2 carbon atoms and at least one double bond. Alkenyl can include any number of carbons, such as C 2 , C 2-3 , C 2-4 , C 2-5 , C 2-6 , C 2-7 , C 2-8 , C 2-9 , C 2-10 , C 3 , C 3-4 , C 3-5 , C 3-6 , C 4 , C 4-5 , C 4-6 , C 5 , C 5-6 , and C 6 . Alkenyl groups can have any suitable number of double bonds, including, but not limited to, 1, 2, 3, 4, 5 or more. Alkenyl groups can be substituted or unsubstituted.
- alkylene refers to a straight or branched, saturated, aliphatic radical having the number of carbon atoms indicated, and linking at least two other groups, i.e., a divalent hydrocarbon radical.
- the two moieties linked to the alkylene can be linked to the same atom or different atoms of the alkylene group.
- a straight chain alkylene can be the bivalent radical of - (CH 2 ) n-, where n is 1, 2, 3, 4, 5 or 6.
- Representative alkylene groups include, but are not limited to, methylene, ethylene, propylene, isopropylene, butylene, isobutylene, sec-butylene, pentylene and hexylene.
- Alkylene groups can be substituted or unsubstituted. In some embodiments, alkylene groups are substituted with 1-2 substituents. As a non-limiting example, suitable substituents include halogen and hydroxyl.
- alkoxy refers to an alkyl group having an oxygen atom that connects the alkyl group to the point of attachment: alkyl-O-.
- alkyl group alkoxyl groups can have any suitable number of carbon atoms, such as C1-6.
- Alkoxyl groups include, for example, methoxy, ethoxy, propoxy, iso-propoxy, butoxy, 2-butoxy, iso-butoxy, sec-butoxy, tert-butoxy, pentoxy, hexoxy, etc.
- the alkoxy groups can be substituted or unsubstituted.
- alkenyloxy refers to an alkenyl group, as defined above, having an oxygen atom that connects the alkenyl group to the point of attachment: alkenyl-O-.
- Alkenyloxyl groups can have any suitable number of carbon atoms, such as C1-6. Alkenyloxyl groups can be further substituted with a variety of substituents described within. Alkenyloxyl groups can be substituted or unsubstituted.
- alkylamine or “alkylamino” refers to an alkyl group having a nitrogen atom that connects the alkyl group to the point of attachment: alkyl-N-.
- alkyl group alkoxyl groups can have any suitable number of carbon atoms, such as C1-6.
- halogen refers to fluorine, chlorine, bromine and iodine.
- haloalkyl refers to alkyl, as defined above, where some or all of the hydrogen atoms are replaced with halogen atoms.
- alkyl group haloalkyl groups can have any suitable number of carbon atoms, such as C 1-6 .
- haloalkyl includes trifluoromethyl, fluoromethyl, etc.
- haloalkoxyl refers to an alkoxyl group where some or all of the hydrogen atoms are substituted with halogen atoms.
- haloalkoxy groups can have any suitable number of carbon atoms, such as C 1-6 .
- the alkoxy groups can be substituted with 1, 2, 3, or more halogens.
- alkanoyl refers to an alkyl group having a carbonyl group that connects the alkyl group to the point of attachment: alkyl-C (O) -.
- alkyl group alkanoyloxyl groups can have any suitable number of carbon atoms, such as C1-4.
- an alkanoyl groups include acetyl, propinoyl, butyryl, etc.
- alkanoyloxyl refers to an alkanoyl group having a an oxygen atom that connects the alkanoyl group to the point of attachment: alkyl-C (O) -O-.
- alkyl group alkanoyloxyl groups can have any suitable number of carbon atoms, such as C1-4.
- Exemplary alkanoyloxyl groups include acetoxy, propionyloxy, butryloxy, etc.
- aryl refers to an aromatic ring system having any suitable number of ring atoms and any suitable number of rings.
- Aryl groups can include any suitable number of ring atoms, such as, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 or 16 ring atoms, as well as from 6 to 10, 6 to 12, or 6 to 14 ring members.
- Aryl groups can be monocyclic, fused to form bicyclic or tricyclic groups, or linked by a bond to form a biaryl group.
- Representative aryl groups include phenyl, naphthyl and biphenyl.
- Other aryl groups include benzyl, having a methylene linking group.
- aryl groups have from 6 to 12 ring members, such as phenyl, naphthyl or biphenyl. Other aryl groups have from 6 to 10 ring members, such as phenyl or naphthyl. Some other aryl groups have 6 ring members, such as phenyl.
- Aryl groups can be substituted or unsubstituted. In some embodiments, aryl groups are substituted with 1-2 substituents. As a non-limiting example, suitable substituents include halogen, hydroxyl, -NO2, C1-8 alkyl, C1-8 alkoxy.
- aralkyloxyl refers to an aryl group, as defined above, having an alkyl and oxygen atom that connects the aryl group to the point of attachment: aryl-alkyl-O-.
- alkyl group aralkyloxyl groups can have any suitable number of carbon atoms, such as C1-4.
- heteroaryl refers to a monocyclic or fused bicyclic aromatic ring assembly containing 5 to 12 ring atoms, where from 1 to 5 of the ring atoms are a heteroatom such as N, O or S. Additional heteroatoms can also be useful, including, but not limited to, B, Al, Si and P. The heteroatoms can also be oxidized, such as, but not limited to, -S (O) -and -S (O) 2 -. Heteroaryl groups can include any number of ring atoms, such as, 3 to 6, 4 to 6, 5 to 6, 3 to 8, 4 to 8, 5 to 8, 6 to 8, 3 to 9, 3 to 10, 3 to 11, or 3 to 12 ring members.
- heteroaryl groups can have from 5 to 9 ring members and from 1 to 4 heteroatoms, or from 5 to 9 ring members and from 1 to 3 heteroatoms, or from 5 to 6 ring members and from 1 to 4 heteroatoms, or from 5 to 6 ring members and from 1 to 3 heteroatoms.
- the heteroaryl group can include groups such as pyrrole, pyridine, imidazole, pyrazole, triazole, tetrazole, pyrazine, pyrimidine, pyridazine, triazine (1, 2, 3-, 1, 2, 4-and 1, 3, 5-isomers) , purine.
- heteroaryl groups can also be fused to aromatic ring systems, such as a phenyl ring, to form members including, but not limited to, benzopyrroles such as indole and isoindole, benzopyridines such as quinoline and isoquinoline, benzopyrazine (quinoxaline) , benzopyrimidine (quinazoline) , benzopyridazines such as phthalazine and cinnoline, benzothiophene, and benzofuran.
- Other heteroaryl groups include heteroaryl rings linked by a bond, such as bipyridine. Heteroaryl groups can be substituted or unsubstituted.
- cycloalkyl refers to a saturated ring assembly containing from 3 to 8 ring atoms, or the number of atoms indicated. Cycloalkyl can include any number of carbons, such as C 3-6 , C 4-6 , C 5-6 , C 3-8 , C 4-8 , C 5-8 , C 6-8 . Cycloalkyl rings include, for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and cyclooctyl. Cycloalkyl groups can be substituted or unsubstituted.
- cycloheteroalkyl refers to a saturated ring system having from 3 to 12 ring members and from 1 to 4 heteroatoms of N, O and S. Additional heteroatoms can also be useful, including, but not limited to, B, Al, Si and P. The heteroatoms can also be oxidized, such as, but not limited to, -S (O) -and -S (O) 2 -. Heterocycloalkyl groups can include any number of ring atoms, such as, 3 to 6, 4 to 6, 5 to 6, 3 to 8, 4 to 8, 5 to 8, 6 to 8, 3 to 9, 3 to 10, 3 to 11, or 3 to 12 ring members.
- heterocycloalkyl groups can include groups such as aziridine, azetidine, pyrrolidine, piperidine, azepane, azocane, quinuclidine, pyrazolidine, imidazolidine, piperazine (1, 2-, 1, 3-and 1, 4-isomers) , oxirane, tetrahydrofuran, oxane (tetrahydropyran) , oxepane, thiolane (tetrahydrothiophene) , thiane (tetrahydrothiopyran) , oxazolidine, isoxazolidine, thiazolidine, isothiazolidine, dioxolane, dithiolane, morpholine, etc.
- Heterocycloalkyl groups can be any suitable number of heteroatoms.
- Certain compounds of the present invention possess asymmetric carbon atoms (optical centers) or double bonds; the racemates, diastereomer, geometric isomers, regioisomers and individual isomers (e.g., separate enantiomers) are all intended to be encompassed within the scope of the present invention.
- the compounds of the present invention are a particular enantiomer, anomer, or diastereomer substantially free of other forms.
- the term “substantially free” refers to an amount of 10%or less of another form, preferably 8%, 5%, 4%, 3%, 2%, 1%, 0.5%, or less of another form.
- the isomer is a stereoisomer.
- the disclosure provides an ALPK1 agonist represented by formula (I)
- L 1 and L 2 are independently selected from O, CH 2 , CHF and CF 2 ;
- L 3 is O, S, CH 2 or CH (OH) ;
- Z 1 and Z 2 are independently selected from O and S;
- W 1 is -C (R 10 R 11 ) -, wherein R 10 and R 11 are independently selected from H, D, -OH, halogen, and optionally substituted groups selected from C1-C4 alkyl, C1-C4 alkoxyl, C1-C4 haloalkyl , C1-C4-haloalkoxyl, C1-C4 alkenyloxyl, aralkyloxyl and R 12 CO 2 -, wherein R 12 is selected from C1-C4 alkyl, C1-C4 alkoxyl, C1-C4 alkenyloxyl, C1-C4 alkylamino, C3-C6 cycloalkyl, cycloheteroalkyl containing 3 to 6 ring members and having 1-3 heteroatoms selected from N, O and S as ring members, C6-C10 aryl, and heteroaryl containing 5 to 10 ring atoms and having 1-3 heteroatoms selected from N, O and S as
- R 2 , R 3 and R 4 are independently selected from H, D, halogen, C1-C4 alkyl and C1-C4 haloalkyl;
- R 5 , R 6 and R 7 are selected from H, D, halogen and -OH, R 12 CO 2 -, wherein R 12 is selected from C1-C4 alkyl, C1-C4 alkoxyl, C1-C4 alkenyloxyl, C1-C4 alkylamino, C3-C6 cycloalkyl, cycloheteroalkyl containing 3 to 6 ring members and having 1-3 heteroatoms selected from N, O and S as ring members, C6-C10 aryl, and heteroaryl containing 5 to 10 ring atoms and having 1-3 heteroatoms selected from N, O and S as ring members; wherein any two of the adjacent groups of R 5 , R 6 and R 7 can cyclize to form cycloheteroalkyl containing 5 to 9 ring members and having 1-3 heteroatoms selected from N, O and S as ring members, each optionally substituted by 1-3 substituents independently selected from D, halogen, -OH,
- the compound of formula I is represented by the compound of formula IA and/or a stereoisomer, a stable isotope, prodrug or a pharmaceutically acceptable salt thereof
- R 1 -R 7 , L 1 -L 3 , Z 1 , Z 2 , W 1 and W 2 are defined above.
- Y 1 and Y 2 in the compound of formula IA are independently selected from H, D, -OH, halogen, C1-C4 alkyl, C1-C4 alkoxyl, C1-C4 haloalkyl, C1-C4 haloalkoxyl, C1-C4 alkanoyloxyl, and C1-C4 alkenyloxyl; and R 1 -R 7 , L 1 -L 3 , Z 1 , Z 2 , W 1 and W 2 are as defined above.
- Y 1 and Y 2 in the compound of formula IA are independently selected from -OH, halogen, C1-C4 alkyl, and C1-C4 alkanoyloxyl; and R 1 -R 7 , L 1 -L 3 , Z 1 , Z 2 , W 1 and W 2 are defined above.
- the compound of formula I or formula IA does not include D-glycero-D-manno-heptose-1 ⁇ -ADP (also referred to herein as H1b-ADP or H1b-D-ADP) , the compound shown below:
- L-glycero-D-manno-heptose-1 ⁇ -ADP also referred to herein as H1b-ADP-6L or H1b-L-ADP
- the compound of formula I is represented by the compound of formula IB and/or a stereoisomer, a stable isotope, prodrug or a pharmaceutically acceptable salt thereof
- n 1 and n 2 are each an integer independently selected from the group consisting of 0-2;
- R 1 -R 7 , L 1 -L 3 , Z 1 , Z 2 , W 1 and W 2 are defined above.
- n 1 and n 2 of formula IB are each 0.
- X 1 and X 2 of formula IB are independently selected from H, D, C1-C4 alkoxyl and C1-C4 alkyl; and R 1 -R 7 , L 1 -L 3 , Z 1 , Z 2 , W 1 and W 2 are defined above.
- the compound of Formula I is represented by the compound of Formula IC and/or a stereoisomer, a stable isotope, prodrug or a pharmaceutically acceptable salt thereof
- a 1 is -C (R 10 R 11 ) -, O or S;
- R 1 -R 9 , L 1 -L 3 , Z 1 , Z 2 , W 1 and W 2 are defined above.
- R 2 , R 3 , and R 4 in formulas I, IA, IB, and IC are each H.
- R 5 , R 6 , and R 7 in formulas I, IA, IB, and IC are each independently selected from the group consisting of -OH, and C1-C4 alkanoyloxyl -.
- L 3 in formulas I, IA, IB, and IC is O.
- L 2 in formulas I, IA, IB, and IC is O.
- L 1 in formulas I, IA, IB, and IC is O or S.
- W 1 in formulas I, IA, IB, and IC is -C (R 10 R 11 ) -, wherein R 10 and R 11 are independently selected from H, D, -OH, halogen, C1-C4 alkyl, C1-C4 alkoxyl, C1-C4 haloalkyl , C1-C4-haloalkoxyl, C1-C4 alkanoyloxyl, C1-C4 alkenyloxyl, R 12 CO 2 -, wherein R 12 is selected from C1-C4 alkyl, C1-C4 alkoxyl, C1-C4 alkanoyloxyl and C1-C4 alkenyloxyl.
- W 1 in formulas I, IA, IB, and IC is -C (R 10 R 11 ) -, wherein R 10 and R 11 are independently selected from H, D, -OH, halogen and C1-C4 alkanoyloxyl.
- W 2 in formulas I, IA, IB, and IC is C1-C3 alkyl optionally substituted with 1-3 substituents independently selected from D, halogen, -OH and R 12 CO 2 -, wherein R 12 is C1-C3 alkyl .
- W 2 in formulas I, IA, IB, and IC is C1 alkyl optionally substituted with 1 substituent selected from -OH and R 12 CO 2 -, wherein R 12 is C1-C3 alkyl.
- R 1 in formulas I, IA, IB and IC is
- R 1 in formulas I, IA, IB and IC is
- R 1 in formulas I, IA, IB and IC is
- the compound of Formula I is N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-N-phenyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl
- the compound of formula I is a compound described in the Examples of this application.
- the compound of the present disclosure can be prepared using the general processes describes in Schemes I, II, III, and IV as well as the techniques described in the exemplary embodiments.
- the disclosure provides an ALPK1 agonist in the form of a small organic molecule, such as D-glycero- ⁇ -D-manno-heptose 1, 7-bisphosphate (heptose 1, 7 bisphosphate or “HBP” ) , D-glycero- ⁇ -D-manno-heptose-1-phosphate (HMP-1bP) , D-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) , and L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) and prodrugs, analogs and derivatives thereof, or in the form of a large biomolecule such as a protein (e.g., ALPK1 itself, or an ALPK1-directed antibody or Fc fragment thereof that activates ALPK1 kinase activity) or a polynucleotide (e.g., a polynucleotide (
- the disclosure provides methods of treating cancer by administering an ALPK1 agonist selected from HBP, HMP-1bP, H1b-ADP-6L, and H1b-ADP, preferably HMP-1bP, H1b-ADP-6L, and H1b-ADP, and most preferably H1b-ADP-6L and H1b-ADP.
- an ALPK1 agonist selected from HBP, HMP-1bP, H1b-ADP-6L, and H1b-ADP, preferably HMP-1bP, H1b-ADP-6L, and H1b-ADP, and most preferably H1b-ADP-6L and H1b-ADP.
- the disclosure provides a combination therapy comprising administering an ALPK1 agonist selected from H1b-ADP-6L and H1b-ADP and an immune checkpoint modulator selected from a checkpoint inhibitor, such as an anti-PD-1/PD-L1 antibody, and an agonist of an immune co-stimulatory molecule, such as an anti-OX40 (CD134) agonist antibody.
- an ALPK1 agonist selected from H1b-ADP-6L and H1b-ADP and an immune checkpoint modulator selected from a checkpoint inhibitor, such as an anti-PD-1/PD-L1 antibody, and an agonist of an immune co-stimulatory molecule, such as an anti-OX40 (CD134) agonist antibody.
- H1b-ADP and similar molecules such as H1b-ADP-6L may promote the antigen-presenting functions of tumor infiltrating antigen presenting cells (APC) and tumor-specific T cell proliferation and differentiation.
- APC tumor infiltrating antigen presenting cells
- the disclosure provides methods of activating ALPK1 by administering ALPK1 to a subject, or introducing ALPK1 into a cell, for example, cells or tissues of a subject, in the form of a recombinant protein or in the form of a polynucleotide encoding ALPK1, or in the form of a composition comprising a recombinant ALPK1 protein or polynucleotide encoding same.
- a polynucleotide encoding ALPK1 is one which is transcribed and translated into the ALPK1 protein when placed under the control of appropriate regulatory sequences, for example a promoter sequence.
- Such polynucleotides may include sequences from prokaryotic or eukaryotic DNA, or synthetic DNA sequences, and combinations of any of the foregoing
- the ALPK1 administered or introduced is a constitutively active ALPK1 (or polynucleotide encoding same) .
- constitutively active refers to an ALPK1 protein whose kinase activity is active in the absence of ligand.
- a constitutively active ALPK1 carries an activating mutation in its N-terminal domain that promotes ligand-independent oligomerization and kinase activation.
- a polynucleotide encoding ALPK1 may in the form of a nucleic acid vector or other vehicle suitable for gene transfer into living cells.
- a plasmid is a common type of nucleic acid vector that is an extra-chromosomal DNA molecule capable of replicating independently of the chromosomal DNA. Plasmids may be single stranded or double stranded and are often circular. Other useful vehicles may include DNA or RNA minicircles and minivectors. Minicircles are formed by deleting most of the bacterial DNA from the parent plasmid using site-specific recombination. The resulting circular DNA molecules contain the desired gene sequence to be transferred, e.g., an ALPK1 sequence, and only small amounts of bacterial DNA.
- Minivectors are similar except they include short integration sequences. These and other suitable non-viral DNA vectors for gene transfer are described, for example, in Hardee et al., “Advances in Non-Viral DNA Vectors for Gene Therapy” , Genes 2017 8: 65.
- nucleic acid vectors for gene transfer of ALPK1 may include, for example, viral vectors such as adenovirus vectors, adeno-associated virus vectors, retrovirus vectors, and lentivirus vectors.
- viral vectors such as adenovirus vectors, adeno-associated virus vectors, retrovirus vectors, and lentivirus vectors.
- a nucleic acid vector encoding ALPK1 can be introduced into target cells using a suitable technique, for example, a viral delivery system, direct injection such as using a gene gun, or non-viral delivery system including for example, liposomes, nanoparticles, polymers, electroporation, cell squeezing, sonoporation, optical transfection, impalefection, and hydrodynamic delivery.
- a suitable technique for example, a viral delivery system, direct injection such as using a gene gun, or non-viral delivery system including for example, liposomes, nanoparticles, polymers, electroporation, cell squeezing, sonoporation, optical transfection, impalefection, and hydrodynamic delivery.
- exemplary non-viral delivery systems and their use are described, for example, in Jones et al., “Contemporary approaches for nonviral gene therapy, ” Discov. Med. 2015; 19: 447–454.
- ALPK1 may be administered in a suitable formulation including, for example, in the form of viral particles, liposomal particles, nanoparticles, as complexes with polymeric carriers, including for example polylysine, polyarginine, polyornithine, protamine, spermine, spermidine, and putrescine.
- Liposomal particles may be used to deliver ALPK1 in various forms including DNA, RNA, and plasmid forms.
- the ALPK1 polynucleotide may administered as plasmid DNA in the absence of another particle or carrier.
- the polynucleotide encoding ALPK1, or an active mutant thereof is inserted into cells using a gene editing technique.
- Gene editing techniques include those based on meganucleases, zinc finger nucleases (ZFNs) , transcription activator-like effector nucleases (TALENs) , and CRISPR/Cas-9.
- the disclosure provides methods of modulating an immune response in a subject, the methods comprising administering to the subject a composition comprising any one of an ALPK1 agonist, a polynucleotide encoding ALPK1 or constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the disclosure provides methods of potentiating an immune response to a target antigen in a subject, the methods comprising administering to the subject a composition comprising any one of an ALPK1 agonist, a polynucleotide encoding ALPK1 or constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the target antigen may be an antigen of an infectious agent, such as a bacterial antigen, a viral antigen, or an antigen of a parasite.
- the antigen is a tumor antigen.
- the ALPK1 agonist, polynucleotide, or protein, as described herein may serve as an adjuvant to a vaccine composition for the treatment or prevention of a disease or disorder caused by an infectious agent, or for the treatment of cancer, or for the treatment of another disease or disorder that may be treated with a vaccine composition, including, for example, Alzheimer’s disease.
- the antigen is selected from amyloid protein in the treatment of Alzheimer’s disease.
- the antigen is selected from glycoprotein 100 (gp100) , mucin 1 (MUC1) , and melanoma-associated antigen 3 (MAGEA3) in the treatment of cancer.
- the cancer is selected from breast, ovarian, or prostate cancer.
- the cancer is HTLV-1 T-lymphotropic leukemia.
- the cancer is melanoma and the ALPK1 agonist, polynucleotide, or protein, as described herein, may serve as an adjuvant to treatment with Talimogene laherparepvec (T-VEC) , or may be used in a combination therapy regimen with T-VEC.
- T-VEC Talimogene laherparepvec
- the ALPK1 agonist, polynucleotide, or protein, as described herein may serve as an adjuvant to a vaccine composition for the treatment or prevention of anthrax, caries, Chagas disease, dengue, diphtheria, ehrlichiosis, hepatitis A or B, herpes, seasonal influenza, Japanese encephalitis, leprosy, lyme disease, malaria, measles, mumps, meningococcal disease, including meningitis and septicemia, Onchocerciasis river blindness, pertussis (whooping cough) , pneumococcal disease, polio, rabies, rubella, schistosomiasis, severe acute respiratory syndrome (SARS) , shingles, smallpox, syphilis, tetanus, tuberculosis, tularemia, tick-borne encephalitis virus, typhoid fever, trypanos
- SARS severe acute respiratory syndrome
- the ALPK1 agonist, polynucleotide, or protein, as described herein may serve as an adjuvant to a vaccine composition for the treatment or prevention of a disease or disorder caused by adenovirus, Coxsackie B virus, cytomegalovirus, eastern equine encephalitis virus, ebola virus, enterovirus 71, Epstein–Barr virus, Haemophilus influenzae type b (Hib) , hepatitis C virus (HCV) , herpes virus, human immunodeficiency virus (HIV) , human papillomavirus (HPV) , hookworm, Marburg virus, norovirus, respiratory syncytial virus (RSV) , rotavirus, Salmonella typhi, Staphylococcus aureus, Streptococcus pyogenes, varicella, West Nile virus, Yersinia pestis, and Zika virus.
- adenovirus Coxsackie B
- the method may comprise administering a vaccine composition or adjuvant comprising any one of an ALPK1 agonist, preferably an ALPK1 agonist selected from HBP, HMP-1bP, H1b-ADP-6L and H1b-ADP, or selected from HMP-1bP, H1b-ADP-6L, and H1b-ADP, and most preferably an ALPK1 agonist selected from H1b-ADP-6L and H1b-ADP, a polynucleotide encoding ALPK1 or constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- an ALPK1 agonist preferably an ALPK1 agonist selected from HBP, HMP-1bP, H1b-ADP-6L and H1b-ADP, or selected from HMP-1bP, H1b-ADP-6L, and H1b-ADP, and most preferably an ALPK1 agonist selected from H1b-ADP-6L and H1b-ADP, a poly
- the disclosure provides methods of treating a disease or disorder amendable to treatment by activation of NFkB, p38, and JNK cell signaling pathways in cells of a subject, the method comprising administering to the subject a composition comprising any one of an agonist of ALPK1, a polynucleotide encoding ALPK1 or constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the disease or disorder is caused by a bacterial, viral, or parasitic infection, as described in more detail below, and including for example diseases and disorders caused by the hepatitis C virus (HCV) , the hepatitis B virus (HBV) , and the human immunodeficiency virus (HIV) .
- the disease or disorder is selected from tuberculosis, meningitis, pneumonia, ulcer, and sepsis.
- the disease or disorder is selected from rhinitis, asthma, allergy, COPD, inflammatory bowel disease, arthritis, obesity, radiation-induced inflammation, psoriasis, atopic dermatitis, non-alcoholic steatohepatitis (NASH) , Alzheimer’s disease, systemic lupus, erythematosus (SLE) , autoimmune thyroiditis (Grave’s disease) , multiple sclerosis, ankylosing spondylitis and bullous diseases.
- the disease or disorder is selected from actinic keratoses, ulcerative colitis, Crohn’s disease, and alopecia areata.
- the disclosure provides methods of treating or preventing a bacterial, viral, or parasitic infection in a subject in need thereof, the methods comprising administering to the subject a composition comprising any one of an ALPK1 agonist, a polynucleotide encoding ALPK1 or constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the method is a method of treating or preventing a bacterial infection.
- the bacterial infection is caused by a Gram-negative or a Gram-positive bacteria.
- the bacteria is a Gram-negative bacteria selected from the group consisting of Acinetobacter baumanii, Aggregatobacter actinomycetemcomitans, Bartonella bacilliformis, Bartonella henselae, Bartonella quintana, Bifidobacterium Borrelia, Bortadella pertussis, Brucella sp, Burkholderia cepacis, Burkholderia pseudomallei, Campylobacter jejuni, Cardiobacterium hominis, Campylobacter fetus, Chlamydia pneumonia, Chlymydia trachomatis, Clostridium difficile, Cyanobacteria, Eikennella corrodens, Enterobacter, Enterococcus faccium, Escherichia coli, Es
- the bacteria is a Gram-positive bacteria selected from the group consisting of Actinomycetes, Bacillus anthracis, Bacillus subtilis, Clostridium tetani, Clostridium perfingens, Clostridium botulinum, Clostridium tetani.
- MRSA methicillin resistant Staphylococcus aureus
- the method is a method of treating or preventing a viral infection.
- the viral infection is caused by a virus selected from the group consisting of Adeno-associated virus, Aichi virus, Alpha virus, Arena virus, Arobovirus, Australian bat lyssavirus, BK polyomavirus, Banna virus, Birnavirus, Bornavirus, bunyamwera virus, Bunyavirus La Crosse, Bunyavirus snowshoe hare, Valicivirus, Cercopithecine herpesvirus, Chandipura virus, Chikugunya virus, Cosavirus A, Coxpox virus, Coxsakievirus, Crimean-Congo hemorrhagic fever virus, Dengue virus, Dhori virus, Dugbe virus, Devenhage virus, Eastern equine encephalitis virus, Ebolavirus, Echovirus, Encephalomyocarditis virus, Epstein-Barr virus, European bat lyssavirus, Flavivirus, GB virus/Hepatit
- louis encephalitis virus Tick-borne powassan virus, togavirus, Torque virus, Toscana virus, Uukuniemi virus, Vaccina virus, Varicella-zoster virus, Variola virus, Venezuelan equine encephalitis virus, Vesicular stomatitits virus, Western equine encephalitis virus, UU polyomavirus, West Nile virus, Yaba monkey tumor virus, Yaba-like disease virus, Yellow fever virus, and Zika virus.
- the method is a method of treating or preventing a parasitic infection.
- the parasitic infection is caused by parasite selected from the group consisting of Acanthamoeba spp, American tryppanosomiasis, Balamuthia mandnillanis, Babesia divergenes, Babesia bigemina, Babesia equi, Babesia microfti, Babesia duncani, Balantidium coli, Blastocystis spp Cryptosporidium spp, Cyclospora cayetanensis, transphyllobothrium latum, Leishmania amazonesis, Naegleria fowderi, Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale curtisi, Plasmodium malariae, Rhinosporidium seeberi, Sarcocystis bovihominis, Sar
- the disclosure provides methods of treating cancer in a subject, the methods comprising administering to the subject a composition comprising any one of an ALPK1 agonist, a polynucleotide encoding ALPK1 or constitutively active mutant thereof, or an ALPK1 protein or constitutively active mutant of said protein.
- the ALPK1 agonist is selected from HBP, HMP-1bP, H1b-ADP-6L and H1b-ADP, preferably selected from HMP-1bP, H1b-ADP-6L, and H1b-ADP, and most preferably an ALPK1 agonist selected from H1b-ADP-6L and H1b-ADP, and prodrugs, analogs and derivatives thereof.
- the ALPK1 agonist is HMP-1bP, H1b-ADP-6L or H1b-ADP, or a prodrug, analog or derivative thereof. In further embodiments of the methods for treating cancer, the ALPK1 agonist is H1b-ADP-6L or H1b-ADP, or a prodrug, analog or derivative thereof.
- the cancer is selected from soft tissue sarcoma, breast cancer, head and neck cancer, melanoma, cervical cancer, bladder cancer, hematologic malignancy, glioblastoma, pancreatic cancer, prostate cancer, colon cancer, breast cancer, renal cancer, lung cancer, merkel cell carcinoma, small intestine cancer, thyroid cancer, acute myelogenous leukemia (AML) , acute lymphocytic leukemia (ALL) , chronic lymphocytic leukemia (CLL) , chronic myelogenous leukemia (CML) , gastric cancer, gastrointestinal stromal tumors, non-Hodgkins lymphoma, Hodgkins lymphoma, liver cancer, leukemia, lymphoma, T-cell lymphoma.
- AML acute myelogenous leukemia
- ALL acute lymphocytic leukemia
- CLL chronic lymphocytic leukemia
- CML chronic myelogenous leukemia
- the ALPK1 agonist preferably selected from H1b-ADP-6L and H1b-ADP, may be administered in combination with one or more additional therapeutic agents or immune modulators, including for example in combination with a vaccine or vaccine adjuvant.
- the one or more additional therapeutic agents is an inhibitor or antagonist of, or a vaccine against, an immune checkpoint molecule including, for example, the programed cell death 1 (PD-1) receptor (CD279) , a ligand of PD-1 (e.g., PD-L1) , cytotoxic T-lymphocyte associated protein 4 (CTLA4) , tumor necrosis factor receptor superfamily member 9 (alternatively TNFRSF9, 4-1BB) and 4-1BB ligands, tumor necrosis factor receptor superfamily member 4 (alternatively TNFRSF4, OX40) and OX40 ligands, glucocorticoid-induced TNFR-related protein (GITR) , Tumor Necrosis Factor Receptor Superfamily Member 7 (alternatively TNFRSF7, cluster of differentiation 27, CD27) , TNFRSF25 and TNF-like ligand 1A (TL1A) , TNF Receptor Superfamily Member 5 (alternatively TNFR
- the ALPK1 agonist may be administered in combination with checkpoint inhibitor or an agonist of an immune co-stimulatory molecule, such as an anti-OX40 (CD134) agonist antibody.
- the checkpoint inhibitor is a PD-1/PD-L1 inhibitor, such as an anti-PD1 antibody or an anti-PD-L1 antibody
- the ALPK1 agonist is selected from H1b-ADP-6L and H1b-ADP, and prodrugs, analogs and derivatives thereof.
- the ALPK1 agonist may be administered in combination with one or more immune modulators.
- the immune modulator may be a vaccine.
- the vaccine is a vaccine against an infectious agent, as described above.
- the vaccine is a cancer vaccine.
- the cancer vaccine targets a tumor antigen selected from glycoprotein 100 (gp100) , mucin 1 (MUC1) , and melanoma-associated antigen 3 (MAGEA3) .
- the one or more immune modulators may be a recombinant protein, for example, granulocyte-macrophage colony-stimulating factor (GM-CSF) , interleukin 7 (IL-7) , IL-12, IL-15, IL-18, or IL-21.
- GM-CSF granulocyte-macrophage colony-stimulating factor
- IL-7 interleukin 7
- IL-12 interleukin 7
- IL-15 interleukin 15
- IL-18 IL-21
- the ALPK1 agonist may be administered in combination with a T cell therapy, such as chimeric antigen receptor (CAR) T cell therapy,
- a T cell therapy such as chimeric antigen receptor (CAR) T cell therapy
- the ALPK1 agonist may be administered in combination with a PD-1/PD-L1 inhibitor or an agonist of an immune co-stimulatory molecule, such as an anti-OX40 (CD134) agonist antibody.
- the ALPK1 agonist adiminstered in combination with a PD-1/PD-L1 inhibitor or an agonist of an immune co-stimulatory molecule is selected from H1b-ADP-6L and H1b-ADP.
- the ALPK1 agonist is H1b-ADP, or a prodrug, analog or derivative thereof.
- the cancer is selected from advanced melanoma, non-small cell lung cancer, renal cell carcinoma, bladder cancer, liver cancer, gastric cancer, colon cancer, breast cancer, non-Hodgkin’s lymphoma, prostate cancer, head and neck cancer, thyroid cancer, brain cancer, acute myeloid leukemia (AML) , merkel cell carcinoma, multiple myeloma, cervical cancer, and sarcoma and the method further comprises administering a PD-1/PD-L1 inhibitor or an agonist of an immune co-stimulatory molecule to the subject.
- AML acute myeloid leukemia
- the one or more additional therapeutic agents may be an immune modulator, for example, an inhibitor or antagonist of immune checkpoint molecule.
- immune modulator for example, an inhibitor or antagonist of immune checkpoint molecule.
- Such molecules generally act as key regulators of the immune system, for example, as co-stimulators of the immune response.
- the disclosure also provides a vaccine composition or vaccine adjuvant comprising an ALPK1 agonist.
- a vaccine composition described here may further comprise one or more adjuvants.
- the disclosure also provides a pharmaceutical composition comprising an ALPK1 agonist.
- the ALPK1 agonist may be in the form of a small organic molecule, such as HBP, or in the form of a large biomolecule such as a protein (e.g., ALPK1 itself, or an ALPK1-directed antibody or Fc fragment thereof that activates ALPK1 kinase activity) or a polynucleotide (e.g., a polynucleotide encoding ALPK1) , as discussed above.
- the ALPK1 agonist is selected from HMP-1bP and H1b-ADP and prodrugs, analogs and derivatives thereof.
- the ALPK1 agonist is H1b-ADP, or a prodrug, analog or derivative thereof.
- the disclosure also provides methods of selecting a compound capable of modulating an immune response by measuring the effect of a test compound on ALPK1 autophosphorylation and/or the activation of downstream targets of ALPK1 signaling, the method comprising contacting ALPK1 with the test compound in the presence of ATP and, separately, in the absence of ATP, followed by performing an assay to detect ALPK1 autophosphorylation and/or activation of one or more downstream targets of ALPK1 signaling.
- the contacting of ALPK1 with the test compound is performed in a cell-free system or in a cell-based system.
- the term “treating” may refer to the amelioration or stabilization of one or more symptoms associated with the disease, disorder or condition being treated.
- the term “treating” may also encompass the management of disease, disorder or condition, referring to the beneficial effects that a subject derives from a therapy but which does not result in a cure of the underlying disease, disorder, or condition.
- the term “prevention” refers to preventing the recurrence, development, progression or onset of one or more symptoms of the disease, disorder, or condition.
- the therapeutically effective amount is the amount sufficient to achieve a desired therapeutic outcome, for example the amelioration or stabilization of one or more symptoms of the disease, disorder or condition being treated, or in the context of prevention, the amount sufficient to achieve prevention of the recurrence, development, progression or onset of one or more symptoms of the disease, disorder, or condition.
- a therapeutically effective amount is the amount required to achieve at least an equivalent therapeutic effect compared to a standard therapy.
- a standard therapy is an FDA-approved drug indicated for treating the same disease, disorder or condition.
- the subject is preferably a human but may be a non-human vertebrate.
- the non-human vertebrate may be, for example, a dog, cat, a rodent (e.g., a mouse, a rat, a rabbit) , a horse, a cow, a sheep, a goat, a chicken, a duck, or any other non-human vertebrate.
- the human subject is selected from an adult human, a pediatric human, or a geriatric human, as those terms are understood by the medical practitioner, for example as defined by the U.S. Food and Drug Administration.
- the disclosure provides a composition comprising an ALPK1 agonist, or a composition comprising a polynucleotide encoding ALPK1, or a composition comprising ALPK1 protein, and one or more excipients or carriers, preferably pharmaceutically acceptable excipients or carriers.
- pharmaceutically acceptable refers to those compounds, materials, compositions, carriers, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio.
- Excipients for preparing a pharmaceutical composition are generally those that are known to be safe and non-toxic when administered to a human or animal body.
- pharmaceutically acceptable excipients include, without limitation, sterile liquids, water, buffered saline, ethanol, polyol (for example, glycerol, propylene glycol, liquid polyethylene glycol and the like) , oils, detergents, suspending agents, carbohydrates (e.g., glucose, lactose, sucrose or dextran) , antioxidants (e.g., ascorbic acid or glutathione) , chelating agents, low molecular weight proteins, and suitable mixtures of any of the foregoing.
- the particular excipients utilized in a composition will depend upon various factors, including chemical stability and solubility of the compound being formulated and the intended route of administration.
- a pharmaceutical composition can be provided in bulk or unit dosage form. It is especially advantageous to formulate pharmaceutical compositions in unit dosage form for ease of administration and uniformity of dosage.
- unit dosage form refers to physically discrete units suited as unitary dosages for the subject to be treated; each unit containing a predetermined quantity of an active compound calculated to produce the desired therapeutic effect in association with the required pharmaceutical carrier.
- a unit dosage form can be an ampoule, a vial, a suppository, a dragee, a tablet, a capsule, an IV bag, or a single pump on an aerosol inhaler.
- dose may vary depending on the chemical and physical properties of the active compound as well as clinical characteristics of the subject, including e.g., age, weight, and co-morbidities. Generally, the dose should be a therapeutically effective amount.
- An effective amount of a pharmaceutical composition is that which provides an objectively identifiable improvement as noted by the clinician or other qualified observer. For example, alleviating a symptom of a disorder, disease or condition.
- a pharmaceutical compositions may take any suitable form (e.g. liquids, aerosols, solutions, inhalants, mists, sprays; or solids, powders, ointments, pastes, creams, lotions, gels, patches and the like) for administration by any desired route (e.g. pulmonary, inhalation, intranasal, oral, buccal, sublingual, parenteral, subcutaneous, intravenous, intramuscular, intraperitoneal, intrapleural, intrathecal, transdermal, transmucosal, rectal, and the like) .
- pulmonary, inhalation intranasal, oral, buccal, sublingual, parenteral, subcutaneous, intravenous, intramuscular, intraperitoneal, intrapleural, intrathecal, transdermal, transmucosal, rectal, and the like
- the pharmaceutical composition is in the form of an orally acceptable dosage form including, but not limited to, capsules, tablets, buccal forms, troches, lozenges, and oral liquids in the form of emulsions, aqueous suspensions, dispersions or solutions.
- Capsules may contain excipients such as inert fillers and/or diluents including starches (e.g., corn, potato or tapioca starch) , sugars, artificial sweetening agents, powdered celluloses, such as crystalline and microcrystalline celluloses, flours, gelatins, gums, etc.
- carriers which are commonly used include lactose and corn starch.
- Lubricating agents such as magnesium stearate, can also be added.
- the pharmaceutical composition is in the form of a tablet.
- the tablet can comprise a unit dose of a compound described here together with an inert diluent or carrier such as a sugar or sugar alcohol, for example lactose, sucrose, sorbitol or mannitol.
- the tablet can further comprise a non-sugar derived diluent such as sodium carbonate, calcium phosphate, calcium carbonate, or a cellulose or derivative thereof such as methyl cellulose, ethyl cellulose, hydroxypropyl methyl cellulose, and starches such as corn starch.
- the tablet can further comprise binding and granulating agents such as polyvinylpyrrolidone, disintegrants (e.g.
- the tablet may be a coated tablet.
- the coating can be a protective film coating (e.g. a wax or varnish) or a coating designed to control the release of the active compound, for example a delayed release (release of the active after a predetermined lag time following ingestion) or release at a particular location in the gastrointestinal tract. The latter can be achieved, for example, using enteric film coatings such as those sold under the brand name
- Tablet formulations may be made by conventional compression, wet granulation or dry granulation methods and utilize pharmaceutically acceptable diluents, binding agents, lubricants, disintegrants, surface modifying agents (including surfactants) , suspending or stabilizing agents, including, but not limited to, magnesium stearate, stearic acid, talc, sodium lauryl sulfate, microcrystalline cellulose, carboxymethylcellulose calcium, polyvinylpyrrolidone, gelatin, alginic acid, acacia gum, xanthan gum, sodium citrate, complex silicates, calcium carbonate, glycine, dextrin, sucrose, sorbitol, dicalcium phosphate, calcium sulfate, lactose, kaolin, mannitol, sodium chloride, talc, dry starches and powdered sugar.
- pharmaceutically acceptable diluents including, but not limited to, magnesium stearate, stearic acid, talc, sodium lau
- Preferred surface modifying agents include nonionic and anionic surface modifying agents.
- Representative examples of surface modifying agents include, but are not limited to, poloxamer 188, benzalkonium chloride, calcium stearate, cetostearyl alcohol, cetomacrogol emulsifying wax, sorbitan esters, colloidal silicon dioxide, phosphates, sodium dodecyl sulfate, magnesium aluminum silicate, and triethanolamine.
- the pharmaceutical composition is in the form of a hard or soft gelatin capsule.
- the compound of the present invention may be in a solid, semi-solid, or liquid form.
- the pharmaceutical composition is in the form of a sterile aqueous solution or dispersion suitable for parenteral administration.
- parenteral as used herein includes subcutaneous, intracutaneous, intravenous, intramuscular, intra-articular, intraarterial, intrasynovial, intrasternal, intrathecal, intralesional and intracranial injection or infusion techniques.
- the pharmaceutical composition is in the form of a sterile aqueous solution or dispersion suitable for administration by either direct injection or by addition to sterile infusion fluids for intravenous infusion, and comprises a solvent or dispersion medium containing, water, ethanol, a polyol (e.g., glycerol, propylene glycol and liquid polyethylene glycol) , suitable mixtures thereof, or one or more vegetable oils. Solutions or suspensions can be prepared in water with the aid of co-solvent or a surfactant.
- a solvent or dispersion medium containing, water, ethanol, a polyol (e.g., glycerol, propylene glycol and liquid polyethylene glycol) , suitable mixtures thereof, or one or more vegetable oils.
- Solutions or suspensions can be prepared in water with the aid of co-solvent or a surfactant.
- surfactants include polyethylene glycol (PEG) -fatty acids and PEG-fatty acid mono and diesters, PEG glycerol esters, alcohol-oil transesterification products, polyglyceryl fatty acids, propylene glycol fatty acid esters, sterol and sterol derivatives, polyethylene glycol sorbitan fatty acid esters, polyethylene glycol alkyl ethers, sugar and its derivatives, polyethylene glycol alkyl phenols, polyoxyethylene-polyoxypropylene (POE-POP) block copolymers, sorbitan fatty acid esters, ionic surfactants, fat-soluble vitamins and their salts, water-soluble vitamins and their amphiphilic derivatives, amino acids and their salts, and organic acids and their esters and anhydrides. Dispersions can also be prepared, for example, in glycerol, liquid polyethylene glycols and mixtures of the same in oils.
- a compound or composition described here may be administered as monotherapy or adjunctive therapy.
- a compound or composition described here may be administered alone or in combination with one or more additional therapeutic agents (i.e., additional APIs) or therapies, for example as part of a therapeutic regimen that includes, e.g., aspects of diet and exercise) .
- the methods described here include administration of an ALPK1 agonist as the primary therapy.
- the administration of an ALPK1 agonist is an adjuvant therapy.
- the methods of the invention contemplate the administration of an ALPK1 agonist in combination with one or more additional therapeutic agents and/or therapies for the treatment or prevention of a disease, disorder, or condition as described here.
- the terms “therapy” and “therapies” refer to any method, protocol and/or agent that can be used in the prevention, treatment, management or amelioration of a disease, disorder, or condition, one or more symptoms thereof.
- the present disclosure also provides packaging and kits comprising pharmaceutical compositions for use in the methods described here.
- the kit can comprise one or more containers selected from the group consisting of a bottle, a vial, an ampoule, a blister pack, and a syringe.
- the kit can further include one or more of instructions for use, one or more syringes, one or more applicators, or a sterile solution suitable for reconstituting a compound or composition described here.
- the compounds of formula I in which L 1 is O can be made by general synthetic method as illustrated in Scheme 1.
- Compound II “PG” refers to a protection group
- PG refers to a protection group
- Compound II can be obtained by compound I (when M is OH) with protected phosphorochloridate under basic condition or appropriate protected phosphate under Mitsunobu reaction condition.
- Compound II can be obtained as a mixture of alpha and beta isomers which can be separated on silica gel chromatography.
- the beta isomer of compound II is deprotected under 1-4 atm of H 2 catalyzed by Pd/C or PtO 2 to give compound III.
- the compounds of formula I in which Z 2 is S (L 1 is O, compound XV) can be synthesized by the alternatively method as illustrated in Scheme III.
- the compound III can be activated by forming imidazole salt under 10 to 40 °C in a suitable solvent like DMF under inert gas system.
- Compound VII is introduced phosphate by reaction with phenoxyphosphonoyloxybenzene.
- compound XIV can be obtained.
- the coupling of compound XII and XIV under mild condition, like 0-40°C in a suitable solvent like DMF inert gas system with the catalyst of Lewis acid provides the final compound XV.
- the compounds of formula I in which L 1 is CF 2 can be made by general synthetic method as illustrated in Scheme V.
- Compound XIX ( “PG” refers to a protection group) is converted to the protected di-fluoromethyl diphosphate compound XX by using N-fluorobenzenesulfonimide (NFSI) under basic NaH conditions in a suitable solvent starting from a low reaction temperature of -20°C to 0°C. Selective removal of one of the protection groups in compound XX yields compound XXI.
- Compound VII is converted to compound XXII by converting the hydroxyl group into a leaving group, such as OTs, OMs or halogen.
- Table 1 lists exemplary compounds prepared according to the procedures as described herein.
- (2S, 3S, 4S, 5S, 6S) -2- ( (S) -2-acetoxy-1-fluoroethyl) -6- ( ( ( ( ( ( ( (2R, 3S, 4R, 5R) -5- (6- amino-9H-purin-9-yl) -3, 4-dihydroxytetrahydrofuran-2- yl) methoxy) (hydroxy) phosphoryl) oxy) (hydroxy) phosphoryl) oxy) tetrahydro-2H-pyran-3, 4, 5- triyl triacetate
- Step 8 Preparation of compound (3S, 4S, 5S, 6S) -6- ( (S) -2-acetoxy-1-fluoroethyl) tetrahydro-2H-pyran-2, 3, 4, 5-tetrayl tetraacetate
- (2R, 3R, 4S, 5S, 6S) -2- (acetoxymethyl) -6- ( ( ( ( ( ( ( ( (2R, 3S, 4R, 5R) -5- (6-amino-9H- purin-9-yl) -3, 4-dihydroxytetrahydrofuran-2- yl) methoxy) (hydroxy) phosphoryl) oxy) (hydroxy) phosphoryl) oxy) tetrahydro-2H-pyran-3, 4, 5- triyl triacetate
- PPh3 (360.0 mg, 1.4 mmol) and DEAD (239.5 mg, 1.4 mmol, 250 ⁇ L) were added sequentially to a solution of the product of Step 2 above (200 mg, 448.1 ⁇ mol) and (2R, 3R, 4S, 5S) -2- (acetoxymethyl) -6-hydroxytetrahydro-2H-pyran-3, 4, 5-triyl triacetate (225 mg, 574.9 ⁇ mol) in THF (5 mL) . The resulting mixture was stirred at 40 °C for 2 h.
- Step 8 Preparation of 2R, 3R, 4S, 5S) -2- (acetoxymethyl) -6- ( ( ( benzyloxy) ( ( (benzyloxy) ( ( (2R, 3R, 4R, 5R) -3, 4-diacetoxy-5- (6- (tritylamino) -9H-purin-9- yl) tetrahydrofuran-2-yl) methoxy) phosphoryl) methyl) phosphoryl) oxy) tetrahydro-2H-pyran- 3, 4, 5-triyl triacetate
- Step 10 Preparation of benzyl ( (3S, 4S, 5S, 6R) -3, 4, 5-trihydroxy-6- (hydroxymethyl) tetrahydro-2H-pyran-2-yl) ( ( ( ( (2R, 3S, 4R, 5R) -5- (6-amino-9H-purin-9-yl) - 3, 4-dihydroxytetrahydrofuran-2-yl) methoxy) (benzyloxy) phosphoryl) methyl) phosphonate
- Phenoxyphosphonoyloxybenzene (3.41 g, 14.6 mmol) was added to a solution of product of Step 1 above (2 g, 3.64 mmol) in pyridine (20 mL) . The resulting mixture was stirred at 25 °C for 2 h. Then Et 3 N (2.21 g, 21.8 mmol, 3.04 mL) and H 2 O (786.9 mg, 43.7 mmol) was added. The resulting mixture was stirred at 25 °C for 0.5 h.
- CDI (945 mg, 5.83 mmol) was added to a solution of the compound of the product of Step 14 in the preparation of Compound 1 above (350 mg, 0.58 mmol, ) in anhydrous DMF (15 mL) under N 2 atmosphere. The resulting mixture was stirred at 25 °C for 3 h. After completion of the reaction, MeOH (0.2 mL) was added to quench the reaction, the mixture was concentrated under reduced pressure to give crude desired product (1 g, crude) , which was used directly in next step.
- (2S, 3S, 4S, 5R, 6R) -2- ( ( ( ( ( ( ( ( ( ( ( ( ( ( (2R, 3R, 4S, 5R) -5- (6-amino-9H-purin-9-yl) -3-fluoro-4-hydroxytetrahydrofuran-2-yl) methoxy) (hydroxy) phosphoryl) oxy) (hydroxy) phosphoryl) oxy) -6- ( (R) -1, 2-diacetoxyethyl) tetrahydro-2H-pyran-3, 4, 5-triyl triacetate
- Step 8 (2R, 3R, 4S, 5R) -5- (6-amino-9H-purin-9-yl) -3-fluoro-4-hydroxytetrahydrofuran-2-yl) methyl hydrogen morpholinophosphonate (4’-morpholine-N, N’-dicyclohexylcarboxamidinium salt)
- Step 1 Preparation of compound 3'- (s) -fluoro-adenosine-5'- (D-glycero- ⁇ -D-manno-heptopyranosyl) diphosphate
- reaction mixture was diluted with ethyl acetate (250 mL) and the milky aqueous layer was extracted with ethyl acetate (250 mL x 2) .
- the combined organic layers were washed with brine (250 mL) , dried, concentrated in vacuum to give the desired compound (25.0 g, crude) as light yellow solid.
- Step 4 Preparation of two isomers of compound N- (9- ( (3aR, 4R, 6R, 6aS) -6-fluoro-6- (iodomethyl) -2, 2-dimethyltetrahydrofuro [3, 4-d] [1, 3] dioxol-4-yl) -9H-purin-6-yl) benzamide
- Step 7 Preparation of two isomers of ( (3aS, 4S, 6R, 6aR) -6- (6-amino-9H-purin-9-yl) -4- fluoro-2, 2-dimethyltetrahydrofuro [3, 4-d] [1, 3] dioxol-4-yl) methyl dibenzyl phosphate
- Step 8 Preparation of the two isomers of ( (3aS, 4S, 6R, 6aR) -6- (6-amino-9H-purin-9-yl) -4-fluoro-2, 2-dimethyltetrahydrofuro [3, 4-d] [1, 3] dioxol-4-yl) methyl dihydrogen phosphate
- Step 10 Preparation of two isomers of (2S, 3S, 4S, 5R, 6R) -2- ( ( ( ( ( ( (3aS, 6R, 6aR) -6- (6-amino-9H-purin-9-yl) -4-fluoro-2, 2-dimethyltetrahydrofuro [3, 4-d] [1, 3] dioxol-4-yl) methoxy) (hydroxy) phosphoryl) oxy) (hydroxy) phosphoryl) oxy) -6- ( (R) -1, 2-diacetoxyethyl) tetrahydro-2H-pyran-3, 4, 5-triyl triacetate
- the crude product (60 mg) was purified by Pre-HPLC (column: Waters Xbridge 150*25 5u; mobile phase: [water (10mM NH 4 HCO 3 ) -ACN] ; B%: 0%-35%, 10 min) to give isomer 1 (17 mg) and isomer 2 (7 mg) .
- (2S, 3S, 4S, 5R, 6R) -2- ( ( ( ( ( ( ( ( ( ( ( ( (2S, 3S, 4R, 5R) -5- (6-amino-9H-purin-9-yl) -2-fluoro-3, 4- dihydroxytetrahydrofuran-2- yl) methoxy) (hydroxy) phosphoryl) oxy) (hydroxy) phosphoryl) oxy) -6- ( (R) -1, 2- diacetoxyethyl) tetrahydro-2H-pyran-3, 4, 5-triyl triacetate
- (2R, 3R, 4S, 5S, 6S) -2- (acetoxymethyl) -6- ( ( ( ( ( ( ( ( (2R, 3R, 4R, 5R) -3, 4-diacetoxy-5- (6-amino-9H-purin-9-yl) tetrahydrofuran-2- yl) methoxy) (hydroxy) phosphorothioyl) oxy) (hydroxy) phosphoryl) oxy) tetrahydro-2H-pyran- 3, 4, 5-triyl triacetate
- PH (OPh) 2 (1.35 g, 5.75 mmol) was added to a solution of compound of product of Step 2 in Example 13 (1 g, 1.86 mmol) in pyridine (10 mL) . The resulting mixture was stirred at 25°C for 2 h. Then Et 3 N (1.33 mL, 9.52 mmol) and H 2 O (0.37 mL, 20.42 mmol) was added. The resulting mixture was stirred at 25°C for 0.5 h.
- TMSCl (2.00 mL, 15.7 mmol) was added to a solution of compound of product of Step 1 above (1.3 g, 1.85 mmol, Et 3 N) in pyridine (15 mL) and Et 3 N (15 mL) .
- the mixture was stirred at 25°C for 0.5 h and then sulfur (758 mg, 23.6 mmol) was added. The resulting mixture was stirred for another 1 h.
- H 2 O (3.79 mL, 210 mmol) was added. The mixture was stirred for another 0.5 h.
- HBP shown below in Formula 1b, is very hydrophilic, making it difficult for the molecule to penetrate cell membranes to reach ALPK1, which is a cytosolic protein.
- the disclosure provides various prodrugs of HBP adapted to enable penetration of the plasma membrane.
- the prodrug comprises one or more biolabile protecting groups at one or more of the phosphate moieties of HBP.
- the one or more biolabile protecting groups is linked via an ester linkage to the one or more phosphate moieties of HBP.
- Exemplary protecting groups that may be attached in this manner include, for example, a carbonyloxymethyl (e.g., POM, POC) , a cyclosaligenyl (e.g., cycloSal) , a cyclic 1-aryl-1, 3-propanyl ester (e.g., HepDirect) , an aryloxy amino acid phosphoramidate or phosphonamidate (e.g., ProTide) , and a methylaryl haloalkylamidate.
- a carbonyloxymethyl e.g., POM, POC
- a cyclosaligenyl e.g., cycloSal
- a cyclic 1-aryl-1, 3-propanyl ester e.g., HepDirect
- an aryloxy amino acid phosphoramidate or phosphonamidate e.g., ProTide
- a methylaryl haloalkylamidate e.g
- SATE S-acyl-2-thioethyl
- DTE (2- hydroxyethyl) sulfidyl] -2-thioethyl
- alkyloxyalkyl e.g., HDP, ODE
- an amino acid phosphoramidate or phosphonamidate monoester e.g., a bis (amino acid) phosphoramidate or phosphonamidate, and a di-or tri-phosphonate.
- Carbonyloxymethyl is a class of phosphate protecting groups.
- carbonyloxymethyl protecting groups have the generic Formula 2a
- R 1a and R 1b are each independently C 1-12 alkyl or C 1-12 alkoxy, and the wavy line indicates the point of attachment to the rest of the molecule.
- R 1a and R 1b are each independently C 1-8 alkyl or C 1-8 alkoxy.
- the prodrug of HBP has a Formula 3a
- R 1a , R 1b , R 1c , and R 1d are each independently C 1-12 alkyl or C 1-12 alkoxy. In some embodiments, R 1a , R 1b , R 1c , and R 1d are each independently C 1-8 alkyl or C 1-8 alkoxy.
- Carbonyloxymethyl prodrugs of HBP can be prepared using the methods described in Hwang, Y. and Cole, P.A. Organic Letters 2004, 6, 1555; Inuki, S. et al., Fujimoto, Y. Organic Letters 2017, 19, 3079, or similar.
- Cyclosaligenyl are a class of phosphate protecting groups.
- cycloSal protecting groups have the generic Formula 2b
- R 2 is H, C 1-8 alkyl, or halogen, ;
- R 3 is H, C 1-8 alkyl, or halogen;
- the subscript n is an integer from 1 to 3; and the wavy line indicates the point of attachment to the rest of the molecule.
- R 2 is H or C 1-8 alkyl.
- R 3 is C 1-8 alkyl.
- the subscript n is 1.
- the prodrug of HBP has a Formula 3b
- R 2a and R 2b are each independently H, C 1-8 alkyl, or halogen; R 3a and R 3b are each independently is H, C 1-8 alkyl, or halogen; and the subscripts n1 and n2 are each independently an integer from 1 to 3.
- R 2a and R 2b are each independently H or C 1-8 alkyl.
- R 3a and R 3b are each independently C 1- 8 alkyl.
- the subscripts n1 and n2 are each 1.
- CycloSal prodrugs of HBP can be prepared using the methods described in P. et al., ChemMedChem 2010, 5, 1386; Inuki, S. et al., Organic Letters 2017, 19, 3079, or similar.
- HepDirects are a class of phosphate protecting groups.
- HepDirect protecting groups have the generic Formula 2c
- R 4 is aryl or 5-or 6-membered heteroaryl, wherein the heteroaryl group has 1-3 heteroatom ring vertices selected from the group consisting of O, N, and S; and the wavy line indicates the point of attachment to the rest of the molecule.
- R 4 is aryl or 6-membered heteroaryl.
- R 4 is phenyl or pyridyl.
- the prodrug of HBP has a Formula 3c
- R 4a and R 4b are each independently aryl or 5-or 6-membered heteroaryl, the heteroaryl group having 1-3 heteroatom ring vertices selected from the group consisting of O, N, and S.
- R 4a and R 4b are each independently aryl or 6-membered heteroaryl.
- R 4a and R 4b are each independently phenyl or pyridyl.
- HepDirect prodrugs of HBP can be prepared using the methods described in Reddy, K. R. et al., Tetrahedron Letters 2005, 46, 4321; Inuki, S. et al., Organic Letters 2017, 19, 3079, or similar.
- Aryloxy amino acid amidates are a class of phosphate protecting group.
- protide protecting groups have the generic Formula 2d
- R 5 and R 6 are each independently H or C 1-8 alkyl; R 7 is C 1-8 alkyl; R 8 is aryl; and the wavy line indicates the point of attachment to the rest of the molecule.
- R 7 is methyl or isopropyl.
- R 8 is phenyl.
- the prodrug of HBP has a Formula 3d
- R 5a , R 5b , R 6a , and R 6 are each independently H or C 1-8 alkyl; R 7a and R 7b are each independently C 1-8 alkyl; R 8a and R 8b are each independently aryl. In some embodiments, 7a and R 7b are each independently is methyl or isopropyl. In some embodiments, R 8a and R 8b are each phenyl.
- Protide prodrugs of HBP can be prepared using the methods described in van Boom, J. H. et al., Tetrahedron 1975, 31, 2953; Inoue, J. -i. and Fujimoto, Y. Organic Letters 2017, 19, 3079, or similar.
- Methylaryl haloalkylamidates are a class of phosphate protecting groups.
- methylaryl haloalkylamdiate protecting groups have the generic Formula 2e
- R 9 is C 1-8 alkyl; X 1 is C 3-5 alkylene; and R 10 is aryl, heteroaryl, arylC 1-4 alkylene, or heteroarylC 1-4 alkylene, the heteroaryl group is a 5 or 6 membered ring having 1-3 heteroatom ring vertices selected from the group consisting of O, N, and S.
- R 9 is C 1-4 alkyl.
- X 1 is C 4 alkylene.
- R 10 is aryl or arylC 1-4 alkylene.
- R 10 is phenyl.
- R 10 is benzyl.
- the prodrug of HBP has a Formula 3e
- R 9a and R 9b are each independently C 1-8 alkyl; X 1a and X 1b are each independently C 3-5 alkylene; and R 10a and R 10b are each independently aryl, heteroaryl, arylC 1-4 alkylene, or heteroarylC 1-4 alkylene, the heteroaryl group is a 5 or 6 membered ring having 1-3 heteroatom ring vertices selected from the group consisting of O, N, and S.
- R 9a and R 9b are each independently C 1-4 alkyl.
- X 1a and X 1b are each independently C 4 alkylene.
- R 10a and R 10b are each independently aryl or arylC 1-4 alkylene. In some embodiments, R 10a and R 10b are each independently is phenyl. In some embodiments, R 10a and R 10b are each independently are benzyl.
- Methylaryl haloalkylamdiate prodrugs of HBP can be prepared using the methods described in Wu, W. et al., Journal of Medicinal Chemistry 2007, 50, 3743; Inoue, J. -i. and Fujimoto, Y. Organic Letters 2017, 19, 3079, or similar.
- prodrug of HBP is represented by Formula 3
- Y 1 and Y 2 are independently each a phosphate, Formula 2a, Formula 2b, Formula 2c, Formula 2d, or Formula 2e, provided that Y 1 and Y 2 are not both phosphate.
- the prodrug of HBP is a compound of Table 1
- H1b-ADP The synthesis of H1b-ADP proceeded from Compound I, which was synthesized according to Inuki, et al., Organic Letters (2017) , 19: 3079-3082.
- Compound IX below was synthesized according to Zamyatina et al., Angewandte Chemie, Int’l Ed. (2000) , 39 (22) : 4150-4153.
- the compound VIII (28.6 mg, 0.058 mmol) was dissolved in anhydrous pyridine and concentrated under vacuum. This azeotropic was repeated three times to remove of residual water.
- AMP-morpholidate (97 mg, 0.233 mmol) and 1H-tetrazole (32 mg, 0.453 mmol) was added to the dried compound VIII.
- the resulting solid was purified by preparative HPLC (RP-C18) using as the mobile phase 10 Mm NH 4 COOCH 3 in water (solvent A) and acetonitrile (solvent B) , and as an elution gradient 5%-85% (solvent B) over 3 minutes followed by 85%-95% (solvent B) over 0.5 minutes and holding at 95%for 1 minute at a flow rate of 0.4 ml/min.; Column: HSS T3 1.8 um, 2.1*100mm, Column 40 C) to give a mixture of compound IX and compound VIII. The mixture was purified again on a Sephadex G-15 column to give compound IX as a white solid (3.5 mg, 10%) .
- the 1 H NMR and 31 P NMR data were consistent with that reported in the literature.
- HBP is a metabolic intermediate in the bacterial ADP heptose biosynthetic pathway.
- HBP is generated from D-glycero-D-manno-heptose-7-phosphate by either the HIdA enzyme or the kinase domain of the HIdE enzyme, depending on bacterial strain, and converted into D-glycero- ⁇ -D-manno-heptose-1-phosphate (HMP-1bP) by the bacterial enzyme GmhB.
- HMP-1bP is in turn converted into D-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP) by the bacterial enzyme HIdC or in some bacterial cells by the ADP transferase domain of HIdE.
- H1b-ADP is then converted to L-glycero-D-manno-heptose-1 ⁇ -ADP (H1b-ADP-6L) by HIdD (GmhD) .
- Fig. 16 for a schematic of the pathway and associated enzymes.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Molecular Biology (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Pulmonology (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Saccharide Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Enzymes And Modification Thereof (AREA)
Priority Applications (12)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA3078267A CA3078267C (en) | 2017-10-27 | 2018-10-25 | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
| KR1020207012233A KR102733555B1 (ko) | 2017-10-27 | 2018-10-25 | 알파 단백질 키나제 1의 활성화에 의해 면역 반응을 조절하는 조성물 및 방법 |
| US16/759,426 US11186606B2 (en) | 2017-10-27 | 2018-10-25 | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
| JP2020523748A JP2021500394A (ja) | 2017-10-27 | 2018-10-25 | αタンパク質キナーゼ1を活性化することによって免疫応答をモジュレートするための組成物および方法 |
| CN201880081664.1A CN111565726A (zh) | 2017-10-27 | 2018-10-25 | 通过激活α蛋白激酶1调节免疫应答的组合物和方法 |
| AU2018355487A AU2018355487B2 (en) | 2017-10-27 | 2018-10-25 | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
| EP18869754.4A EP3700536A4 (en) | 2017-10-27 | 2018-10-25 | COMPOSITIONS AND METHODS FOR MODULATING THE IMMUNE RESPONSE BY ACTIVATION OF KINASE ALPHA 1 PROTEIN |
| US16/395,463 US11149051B2 (en) | 2017-10-27 | 2019-04-26 | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
| US17/445,122 US11840551B2 (en) | 2017-10-27 | 2021-08-16 | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
| US17/445,113 US11773131B2 (en) | 2017-10-27 | 2021-08-16 | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
| JP2023169347A JP2023182695A (ja) | 2017-10-27 | 2023-09-29 | αタンパク質キナーゼ1を活性化することによって免疫応答をモジュレートするための組成物および方法 |
| US18/497,384 US20240124511A1 (en) | 2017-10-27 | 2023-10-30 | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNPCT/CN2017/107962 | 2017-10-27 | ||
| CNPCT/CN2017/107962 | 2017-10-27 | ||
| CNPCT/CN2018/083153 | 2018-04-16 | ||
| CNPCT/CN2018/083153 | 2018-04-16 | ||
| CNPCT/CN2018/100871 | 2018-08-16 | ||
| CNPCT/CN2018/100871 | 2018-08-16 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNPCT/CN2018/100871 Continuation | 2017-10-27 | 2018-08-16 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/395,463 Continuation-In-Part US11149051B2 (en) | 2017-10-27 | 2019-04-26 | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019080898A1 true WO2019080898A1 (en) | 2019-05-02 |
Family
ID=66246202
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2018/111885 Ceased WO2019080898A1 (en) | 2017-10-27 | 2018-10-25 | COMPOSITIONS AND METHODS FOR MODULATION OF IMMUNE RESPONSE BY ACTIVATION OF PROTEIN KINASE ALPHA 1 |
Country Status (8)
| Country | Link |
|---|---|
| US (5) | US11186606B2 (enExample) |
| EP (1) | EP3700536A4 (enExample) |
| JP (2) | JP2021500394A (enExample) |
| KR (1) | KR102733555B1 (enExample) |
| CN (1) | CN111565726A (enExample) |
| AU (1) | AU2018355487B2 (enExample) |
| CA (1) | CA3078267C (enExample) |
| WO (1) | WO2019080898A1 (enExample) |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10704021B2 (en) | 2012-03-15 | 2020-07-07 | Flodesign Sonics, Inc. | Acoustic perfusion devices |
| WO2020216327A1 (en) * | 2019-04-26 | 2020-10-29 | Shanghai Yao Yuan Biotechnology Co., Ltd. | Derivatives of glycero-manno-heptose adp for use in modulating immune response |
| EP3752163A4 (en) * | 2018-06-14 | 2021-04-07 | National Institute Of Biological Sciences, Beijing | PROMOTING IMMUNE RESPONSES |
| US10975368B2 (en) | 2014-01-08 | 2021-04-13 | Flodesign Sonics, Inc. | Acoustophoresis device with dual acoustophoretic chamber |
| WO2021231436A1 (en) * | 2020-05-11 | 2021-11-18 | The Board Of Trustees Of The Leland Stanford Junior University | Therapeutic methods for treating covid-19 infections |
| WO2022127914A1 (en) | 2020-12-18 | 2022-06-23 | Pyrotech (Beijing) Biotechnology Co., Ltd. | Nucleoside-thiodiphosphate-heptose compounds for treating conditions associated with alpk1 activity |
| US11377651B2 (en) | 2016-10-19 | 2022-07-05 | Flodesign Sonics, Inc. | Cell therapy processes utilizing acoustophoresis |
| WO2023051675A1 (en) | 2021-09-30 | 2023-04-06 | Pyrotech (Beijing) Biotechnology Co., Ltd. | Nucleoside-diphosphate-heptose compounds for treating conditions associated with alpk1 activity |
| US11708572B2 (en) | 2015-04-29 | 2023-07-25 | Flodesign Sonics, Inc. | Acoustic cell separation techniques and processes |
| US11773131B2 (en) | 2017-10-27 | 2023-10-03 | Shanghai Yao Yuan Biotechnology Co., Ltd. | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
| US11939367B2 (en) | 2015-06-30 | 2024-03-26 | Sanford Burnham Prebys Medical Discovery Institute | BTLA fusion protein agonists and uses thereof |
| WO2025113626A2 (en) | 2023-11-30 | 2025-06-05 | Pyrotech (Beijing) Biotechnology Co., Ltd. | Salt forms of alpk1 activators |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4196131A1 (en) * | 2020-08-14 | 2023-06-21 | Merck Sharp & Dohme LLC | Synthesis of fluorinated nucleotides |
| CN116808216A (zh) * | 2022-07-06 | 2023-09-29 | 四川大学 | Alpk1基因作为脑缺血导致的中枢神经系统疾病的防治靶点的应用 |
| CN117517657B (zh) * | 2024-01-08 | 2024-04-09 | 中国农业科学院北京畜牧兽医研究所 | Lnx1基因或蛋白在调控禽类先天免疫应答反应中的应用 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100016250A1 (en) | 2006-04-14 | 2010-01-21 | Kyowa Hakko Kirin Co., Ltd. | Toll-like receptor 9 agonists |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE7611930U1 (de) | 1976-04-15 | 1977-01-13 | Filterwerk Mann & Hummel Gmbh, 7140 Ludwigsburg | Vorrichtung zum mischen von koernigem oder pulverfoermigem material mit einem oder mehreren zusatzstoffen |
| ES2389203T3 (es) | 2004-01-12 | 2012-10-24 | Ym Biosciences Australia Pty Ltd | Inhibidores de quinasa selectivos |
| US20070167408A1 (en) * | 2005-12-19 | 2007-07-19 | Zentaris Gmbh | Novel alkyl phospholipid derivatives with reduced cytotoxicity and uses thereof |
| US20090098056A1 (en) | 2007-09-10 | 2009-04-16 | National Health Research Institute | Alpk1 gene variants in diagnosis risk of gout |
| US8071636B2 (en) | 2008-06-09 | 2011-12-06 | Allergan, Inc. | Methods of treating alpha adrenergic mediated conditions |
| CN201735264U (zh) | 2010-05-27 | 2011-02-09 | 蓝星化工有限责任公司 | 一种尘降器 |
| CN202590724U (zh) | 2010-12-20 | 2012-12-12 | 西安磁林电气有限公司 | 一种加料装置 |
| WO2013023084A2 (en) | 2011-08-09 | 2013-02-14 | Fred Hutchinson Cancer Research Center | Methods and compositions for inhibiting the growth and/or proliferation of myc-driven tumor cells |
| JP6272846B2 (ja) | 2012-06-27 | 2018-01-31 | 4エスツェー ディスカバリー ゲゼルシャフト ミット ベシュレンクテル ハフツング | 癌、自己免疫性炎症及びcns疾患の処置のためのビフルオロジオキサラン−アミノ−ベンゾイミダゾールキナーゼ阻害剤 |
| GB201408100D0 (en) | 2014-05-07 | 2014-06-18 | Sec Dep For Health The | Detection method |
| CN204182373U (zh) | 2014-09-30 | 2015-03-04 | 天津博弘化工有限责任公司 | 流化床下料斗排气装置 |
| EP3204014B1 (en) | 2014-10-10 | 2020-07-01 | The Governing Council of the University of Toronto | Methods of modulating immune system responses |
| CN204973750U (zh) | 2015-05-26 | 2016-01-20 | 柳州钢铁股份有限公司 | 圆筒混合机刮料器 |
| EP3190062A1 (en) * | 2016-01-07 | 2017-07-12 | Pont Packaging B.V. | A cap construction with a storage space and a container provided therewith as well as a method of using same |
| CN105536635A (zh) | 2016-02-02 | 2016-05-04 | 珠海格力智能装备有限公司 | 一种车用尿素加料装置 |
| CN106582336B (zh) | 2016-12-13 | 2022-09-13 | 珠海格力智能装备有限公司 | 吸料装置及具有其的尿素机 |
| CN206366365U (zh) | 2016-12-13 | 2017-08-01 | 珠海格力智能装备有限公司 | 吸料装置及具有其的尿素机 |
| CN111565726A (zh) | 2017-10-27 | 2020-08-21 | 上海药苑生物科技有限公司 | 通过激活α蛋白激酶1调节免疫应答的组合物和方法 |
-
2018
- 2018-10-25 CN CN201880081664.1A patent/CN111565726A/zh active Pending
- 2018-10-25 CA CA3078267A patent/CA3078267C/en active Active
- 2018-10-25 EP EP18869754.4A patent/EP3700536A4/en active Pending
- 2018-10-25 AU AU2018355487A patent/AU2018355487B2/en active Active
- 2018-10-25 KR KR1020207012233A patent/KR102733555B1/ko active Active
- 2018-10-25 JP JP2020523748A patent/JP2021500394A/ja active Pending
- 2018-10-25 US US16/759,426 patent/US11186606B2/en active Active
- 2018-10-25 WO PCT/CN2018/111885 patent/WO2019080898A1/en not_active Ceased
-
2019
- 2019-04-26 US US16/395,463 patent/US11149051B2/en active Active
-
2021
- 2021-08-16 US US17/445,122 patent/US11840551B2/en active Active
- 2021-08-16 US US17/445,113 patent/US11773131B2/en active Active
-
2023
- 2023-09-29 JP JP2023169347A patent/JP2023182695A/ja active Pending
- 2023-10-30 US US18/497,384 patent/US20240124511A1/en not_active Abandoned
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100016250A1 (en) | 2006-04-14 | 2010-01-21 | Kyowa Hakko Kirin Co., Ltd. | Toll-like receptor 9 agonists |
Non-Patent Citations (39)
| Title |
|---|
| ALLA ZAMYATINA ET AL., , CARBOHYDRATE RESEARCH, vol. 338, 2003, pages 2571 - 2589 |
| BOOM, J. H. ET AL., TETRAHEDRON, vol. 31, 1975, pages 2953 |
| CHEN M ET AL., , BMC NEUROSCI., vol. 12, 2011, pages 1 |
| CHIBA T ET AL., , HUMAN CELL, vol. 28, 2015, pages 1 - 4 |
| FUJIMAKI T ET AL., , BIOMED REPORT, vol. 2, 2014, pages 127 - 131 |
| GAUDET ET AL., SCIENCE, vol. 348, pages 1251 - 2015 |
| HARDEE ET AL.: "Advances in Non-Viral DNA Vectors for Gene Therapy", GENES, vol. 8, 2017, pages 65 |
| HEINET M ET AL., J. BIOL. CHEM., vol. 280, no. 27, 2005, pages 25637 - 43 |
| HWANG, Y.COLE, P. A., ORGANIC LETTERS, vol. 6, 2004, pages 1555 |
| INOUE, J.-I.FUJIMOTO, Y., ORGANIC LETTERS, vol. 19, 2017, pages 3079 - 3082 |
| INUKI S ET AL., ORGANIC LETTER, vol. 19, no. 12, 2017, pages 3079 - 82 |
| INUKI, S. ET AL., , FUJIMOTO, Y. ORGANIC LETTERS, vol. 19, 2017, pages 3079 |
| INUKI, S. ET AL., , ORGANIC LETTERS, vol. 19, 2017, pages 3079 |
| JONES ET AL.: "Contemporary approaches for nonviral gene therapy", DISCOV. MED., vol. 19, 2015, pages 447 - 454 |
| KO AM ET AL., J. INTL. EPIDEMIOL., vol. 42, 2013, pages 466 - 474 |
| KUO TM ET AL., J STEROID BIOCHEM MOL BIOL, vol. 154, 2015, pages 150 - 158 |
| LEE T.W. ET AL., J. MED.CHEM., vol. 56, 2013, pages 1405 - 17 |
| LIAO HF ET AL., SCIENTIFIC REPORTS, vol. 6, 2016, pages 27350 |
| LIAO, H.F. ET AL.: "Down-regulated and Commonly mutated ALPK1 in Lung and Colorectal Cancers", SCIENTIFIC REPORTS., vol. 6, 10 June 2016 (2016-06-10), pages 27350, XP055594962, ISSN: 2045-2322 * |
| MILIVOJEVIC ET AL., , PLOS PATHOGENS, vol. 13, no. 2, 2017, pages e1006224 |
| MILIVOJEVIC ET AL., PLOS PATHOGENS, vol. 13, no. 2, 2017, pages e1006224 |
| MILIVOJEVIC M ET AL., , PLOS PATHOG, vol. 13, no. 2, 2017, pages e1006224 |
| MILIVOJEVIC, M. ET AL.: "ALPK1 controls TIFA/TRAF6-dependent innate immunity against heptose-1, 7-bisphosphate of gram-negative bacteria", PLOS PATHOGENS., vol. 13, no. 2, 21 February 2017 (2017-02-21), pages e1006224, XP055364679, ISSN: 1553-7366, DOI: doi:10.1371/journal.ppat.1006224 * |
| REDDY, K. R ET AL., TETRAHEDRON LETTERS, vol. 46, 2005, pages 4321 |
| RYAZANOV AG ET AL., CURR BIOL, vol. 9, no. 2, 1999, pages R43 - 45 |
| RYAZANOV AG ET AL., PROC NATL ACAD SCI USA, vol. 94, no. 10, 1997, pages 4884 - 4889 |
| See also references of EP3700536A4 |
| SHIMOTAKA S ET AL., , BIOMED REPORT, vol. 1, 2013, pages 940 - 44 |
| SHINSUKE INUKI ET AL., , ORG. LETT., vol. 19, 2017, pages 3079 - 3082 |
| SHINSUKE INUKI ET AL., ORG. LETT, vol. 19, 2017, pages 3079 - 3082 |
| SPÁČILOVÁ, P. ET AL., CHEMMEDCHEM, vol. 5, 2010, pages 1386 |
| STRIETZ J ET AL., ONCOTARGET, 2016, pages 1 - 16 |
| TIEHAI LI ET AL., BIOORG. MED. CHEM., vol. 22, 2014, pages 1139 - 1147 |
| WANG SJ ET AL., J. MOL. MED., vol. 89, 2011, pages 1241 - 51 |
| WU, W ET AL., JOURNAL OF MEDICINAL CHEMISTRY, vol. 50, 2007, pages 3743 |
| YAMADA Y ET AL., , BIOMED. REPORT, 2015 |
| YAMADA Y ET AL., J MED GENET, vol. 50, 2013, pages 410 - 418 |
| ZAMYATINA ET AL., ANGEWANDTE CHEMIE, INT'L ED., vol. 39, no. 22, 2000, pages 4150 - 4153 |
| ZIMMERMANN S ET AL., CELL REPORTS, vol. 20, no. 10, 2017, pages 2384 - 95 |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10704021B2 (en) | 2012-03-15 | 2020-07-07 | Flodesign Sonics, Inc. | Acoustic perfusion devices |
| US10975368B2 (en) | 2014-01-08 | 2021-04-13 | Flodesign Sonics, Inc. | Acoustophoresis device with dual acoustophoretic chamber |
| US11708572B2 (en) | 2015-04-29 | 2023-07-25 | Flodesign Sonics, Inc. | Acoustic cell separation techniques and processes |
| US11939367B2 (en) | 2015-06-30 | 2024-03-26 | Sanford Burnham Prebys Medical Discovery Institute | BTLA fusion protein agonists and uses thereof |
| US11377651B2 (en) | 2016-10-19 | 2022-07-05 | Flodesign Sonics, Inc. | Cell therapy processes utilizing acoustophoresis |
| US11773131B2 (en) | 2017-10-27 | 2023-10-03 | Shanghai Yao Yuan Biotechnology Co., Ltd. | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
| US11840551B2 (en) | 2017-10-27 | 2023-12-12 | Shanghai Yao Yuan Biotechnology Co., Ltd. | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 |
| EP3752163A4 (en) * | 2018-06-14 | 2021-04-07 | National Institute Of Biological Sciences, Beijing | PROMOTING IMMUNE RESPONSES |
| US11229707B2 (en) | 2018-06-14 | 2022-01-25 | National Institute Of Biological Sciences | Promoting immune responses |
| WO2020216327A1 (en) * | 2019-04-26 | 2020-10-29 | Shanghai Yao Yuan Biotechnology Co., Ltd. | Derivatives of glycero-manno-heptose adp for use in modulating immune response |
| WO2021231436A1 (en) * | 2020-05-11 | 2021-11-18 | The Board Of Trustees Of The Leland Stanford Junior University | Therapeutic methods for treating covid-19 infections |
| WO2022127914A1 (en) | 2020-12-18 | 2022-06-23 | Pyrotech (Beijing) Biotechnology Co., Ltd. | Nucleoside-thiodiphosphate-heptose compounds for treating conditions associated with alpk1 activity |
| TWI819820B (zh) * | 2021-09-30 | 2023-10-21 | 大陸商北京炎明生物科技有限公司 | 用於治療與alpk1活性相關的病症的化合物和組合物 |
| WO2023051675A1 (en) | 2021-09-30 | 2023-04-06 | Pyrotech (Beijing) Biotechnology Co., Ltd. | Nucleoside-diphosphate-heptose compounds for treating conditions associated with alpk1 activity |
| JP2024538847A (ja) * | 2021-09-30 | 2024-10-23 | パイロテック(ベイジン)バイオテクノロジー カンパニー リミテッド | Alpk1活性に関連する状態を治療するためのヌクレオシド-ジホスフェート-ヘプトース化合物 |
| WO2025113626A2 (en) | 2023-11-30 | 2025-06-05 | Pyrotech (Beijing) Biotechnology Co., Ltd. | Salt forms of alpk1 activators |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2018355487A1 (en) | 2020-04-23 |
| US11186606B2 (en) | 2021-11-30 |
| US11773131B2 (en) | 2023-10-03 |
| US11840551B2 (en) | 2023-12-12 |
| EP3700536A1 (en) | 2020-09-02 |
| CN111565726A (zh) | 2020-08-21 |
| US20240124511A1 (en) | 2024-04-18 |
| CA3078267A1 (en) | 2019-05-02 |
| KR102733555B1 (ko) | 2024-11-25 |
| CA3078267C (en) | 2024-06-18 |
| JP2023182695A (ja) | 2023-12-26 |
| AU2018355487A2 (en) | 2020-05-21 |
| JP2021500394A (ja) | 2021-01-07 |
| KR20200090154A (ko) | 2020-07-28 |
| US11149051B2 (en) | 2021-10-19 |
| EP3700536A4 (en) | 2021-11-17 |
| US20210403501A1 (en) | 2021-12-30 |
| US20200283468A1 (en) | 2020-09-10 |
| AU2018355487B2 (en) | 2023-09-21 |
| US20190367553A1 (en) | 2019-12-05 |
| US20220017560A1 (en) | 2022-01-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11840551B2 (en) | Compositions and methods of modulating the immune response by activating alpha protein kinase 1 | |
| JP7483975B2 (ja) | 新規環状ジヌクレオチド誘導体及びその抗体薬物コンジュゲート | |
| US11559542B2 (en) | Phosphoramidate derivatives of 5-fluoro-2′-deoxyuridine for use in the treatment of cancer | |
| AU2025234261A1 (en) | Antibody-drug conjugate including novel cyclic dinucleotide derivative | |
| WO2020216327A1 (en) | Derivatives of glycero-manno-heptose adp for use in modulating immune response | |
| WO2020216326A1 (en) | Derivatives of glycero-manno-heptose phosphate and their use in modulating an immune response | |
| RU2809547C2 (ru) | Новое производное циклического динуклеотида и его конъюгат антитело-лекарственное средство | |
| JP2025037980A (ja) | 炭素環ヌクレオシド類似体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18869754 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 3078267 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2018355487 Country of ref document: AU Date of ref document: 20181025 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2020523748 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2018869754 Country of ref document: EP Effective date: 20200527 |