WO2018230109A1 - Composition de résine conductrice et procédé de production d'un boîtier blindé l'utilisant - Google Patents
Composition de résine conductrice et procédé de production d'un boîtier blindé l'utilisant Download PDFInfo
- Publication number
- WO2018230109A1 WO2018230109A1 PCT/JP2018/014287 JP2018014287W WO2018230109A1 WO 2018230109 A1 WO2018230109 A1 WO 2018230109A1 JP 2018014287 W JP2018014287 W JP 2018014287W WO 2018230109 A1 WO2018230109 A1 WO 2018230109A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- resin composition
- conductive resin
- package
- compound
- conductive
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G59/00—Polycondensates containing more than one epoxy group per molecule; Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups
- C08G59/18—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing
- C08G59/40—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing characterised by the curing agents used
- C08G59/4007—Curing agents not provided for by the groups C08G59/42 - C08G59/66
- C08G59/4014—Nitrogen containing compounds
- C08G59/4042—Imines; Imides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G59/00—Polycondensates containing more than one epoxy group per molecule; Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups
- C08G59/18—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing
- C08G59/40—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing characterised by the curing agents used
- C08G59/50—Amines
- C08G59/5046—Amines heterocyclic
- C08G59/5053—Amines heterocyclic containing only nitrogen as a heteroatom
- C08G59/5073—Amines heterocyclic containing only nitrogen as a heteroatom having two nitrogen atoms in the ring
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G59/00—Polycondensates containing more than one epoxy group per molecule; Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups
- C08G59/18—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing
- C08G59/40—Macromolecules obtained by polymerising compounds containing more than one epoxy group per molecule using curing agents or catalysts which react with the epoxy groups ; e.g. general methods of curing characterised by the curing agents used
- C08G59/50—Amines
- C08G59/56—Amines together with other curing agents
- C08G59/58—Amines together with other curing agents with polycarboxylic acids or with anhydrides, halides, or low-molecular-weight esters thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/02—Elements
- C08K3/08—Metals
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3442—Heterocyclic compounds having nitrogen in the ring having two nitrogen atoms in the ring
- C08K5/3445—Five-membered rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L101/00—Compositions of unspecified macromolecular compounds
- C08L101/12—Compositions of unspecified macromolecular compounds characterised by physical features, e.g. anisotropy, viscosity or electrical conductivity
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, which oxygen atoms are present only as part of the carboxyl radical
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, which oxygen atoms are present only as part of the carboxyl radical
- C08L33/10—Homopolymers or copolymers of methacrylic acid esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L35/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical, and containing at least one other carboxyl radical in the molecule, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L63/00—Compositions of epoxy resins; Compositions of derivatives of epoxy resins
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/28—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection
Definitions
- the present invention relates to a conductive resin composition and a method for producing a shield package using the same.
- Patent Document 1 an electromagnetic shielding member having a high shielding effect is easily obtained by spraying (coating) a conductive resin composition containing conductive particles on the surface of a package, coating, and heat-curing. It is stated that it can.
- the color of the shield layer changes and it is difficult to obtain a preferable appearance.
- the shield layer is formed using high-purity silver particles, discoloration can be suppressed, but it is expensive and therefore lacks versatility.
- the conductive resin composition according to the present invention includes (A) a compound represented by the following general formula (I), a molecular weight of 500 to 2000, and having two or more maleimide groups in one molecule, (B) thermosetting A conductive compound, (C) a compound having an imidazole group, and (D) a conductive filler.
- A a compound represented by the following general formula (I), a molecular weight of 500 to 2000, and having two or more maleimide groups in one molecule
- thermosetting A conductive compound thermosetting A conductive compound
- C a compound having an imidazole group
- D a conductive filler
- X represents an aliphatic, alicyclic or aromatic hydrocarbon group which is a hydrocarbon group having 10 to 30 carbon atoms in the main chain, and these groups are , A hetero atom, a substituent, or a siloxane skeleton.
- thermosetting compound (B) may be at least one selected from the group consisting of an epoxy resin that is solid at room temperature, an epoxy resin that is liquid at room temperature, and a (meth) acrylate compound. .
- the content of the compound (C) having an imidazole group is 0.5 to 50 parts by mass with respect to 100 parts by mass of the total amount of the compound (A) having the maleimide group and the thermosetting compound (B). It can be assumed that
- the content of the conductive filler (D) is 200 to 1800 parts by mass with respect to 100 parts by mass of the total amount of the maleimide group-containing compound (A) and the thermosetting compound (B). can do.
- the conductive filler (D) may be copper powder, silver-coated copper powder, or silver-coated copper alloy powder.
- the conductive resin composition according to the present invention can be used for shielding electronic component packages.
- a method of manufacturing a shield package according to the present invention is a method of manufacturing a shield package in which an electronic component is mounted on a substrate, and the package in which the electronic component is sealed with a sealing material is covered with a shield layer.
- a step of sealing the electronic component by mounting a plurality of electronic components on the substrate, filling the substrate with a sealing material, and curing, and cutting the sealing material between the plurality of electronic components Forming a package of each electronic component on the substrate by these groove portions, applying the conductive resin composition to the surface of the individualized package by spraying, and the surface of the package Heating the substrate coated with the conductive resin composition to form a shield layer by curing the conductive resin composition, and extending the substrate along the groove It can be assumed to have at least a step of obtaining a shielding package singulation by cutting.
- a coating film having a uniform thickness can be formed by a spray method, and the obtained coating film can suppress discoloration even under severe heating conditions. Therefore, by applying the conductive resin composition of the present invention to the package surface by spraying, it becomes possible to easily form a shield layer having excellent shielding effect and appearance and excellent adhesion to the package.
- a shield package of the present invention it is possible to efficiently manufacture a shield package having excellent shielding properties, discoloration resistance and adhesion to the package as described above without using a large-scale apparatus. it can.
- the conductive resin composition according to the present invention is (A) a compound represented by the following general formula (I), having a molecular weight of 500 to 2000, and having two or more maleimide groups in one molecule, B) contains at least a thermosetting compound, (C) a compound having an imidazole group, and (D) a conductive filler.
- the use of the conductive resin composition is not particularly limited, but a shield layer is formed by spraying spray or the like on the surface of the package before being singulated or on the surface of the singulated package. Therefore, it is preferably used for obtaining a shield package.
- X represents an aliphatic, alicyclic, or aromatic hydrocarbon group, which is a hydrocarbon group having 10 to 30 carbon atoms in the main chain. May have a heteroatom, a substituent, or a siloxane skeleton.
- X is preferably an aliphatic or alicyclic hydrocarbon group, or an aliphatic hydrocarbon group modified with an alicyclic hydrocarbon group, and an aliphatic hydrocarbon group modified with an alicyclic hydrocarbon group Is particularly preferred.
- the main chain of X preferably has 10 to 30 carbon atoms, more preferably 15 to 25 carbon atoms.
- the shield layer obtained by heat curing is blackened by using together with the compound (C) having an imidazole group. Even if it is added, the discoloration of the shield layer becomes inconspicuous.
- the aliphatic hydrocarbon group including the side chain preferably has 10 to 55 carbon atoms, and more preferably 10 to 40 carbon atoms.
- the molecular weight of the compound (A) is preferably 500 or more, and more preferably 550 or more.
- the shield layer obtained by heat-curing is blackened, so that the discoloration of the shield layer is not noticeable even if it is put into the solder reflow process.
- the conductive filler is less likely to settle during storage of the conductive resin composition. Furthermore, after applying the conductive resin composition to the package, it can be prevented from dripping on the wall surface of the package, and a uniform coating film can be easily formed.
- the molecular weight of the compound (A) is preferably 2000 or less, more preferably 1000 or less, and still more preferably 900 or less.
- the shield layer obtained by heat curing is blackened. Therefore, even if it is put into the solder reflow process, discoloration of the shield layer is not noticeable. Moreover, it becomes easy to form a uniform coating film after the conductive resin composition is applied to the package.
- the method for producing the compound (A) is not particularly limited, and for example, it can be produced by a known method in which an acid anhydride and a diamine are subjected to a condensation reaction and then dehydrated and cyclized (imidized).
- acid anhydrides examples include polybutadiene-graft-maleic anhydride; polyethylene-graft-maleic anhydride; polyethylene-maleic anhydride alternating copolymer; polymaleic anhydride-1-octadecene alternating copolymer Polypropylene-graft-maleic anhydride; poly (styrene-maleic anhydride) copolymer; pyromellitic anhydride; maleic anhydride, succinic anhydride; 1,2,3,4-cyclobutanetetracarboxylic dianhydride 1,4,5,8-naphthalenetetracarboxylic dianhydride; 3,4,9,10-perylenetetracarboxylic dianhydride; bicyclo (2.2.2) oct-7-ene-2,3 , 5,6-tetracarboxylic dianhydride; diethylenetriaminepentaacetic acid dianhydride; ethylenediaminetetraace
- diamines examples include 1,10-diaminodecane; 1,12-diaminododecane; dimer diamine; 1,2-diamino-2-methylpropane; 1,2-diaminocyclohexane; 1,2-diaminopropane; 1,4-diaminobutane; 1,5-diaminopentane; 1,7-diaminoheptane; 1,8-diaminomentane; 1,8-diaminooctane; 1,9-diaminononane; '-Diamino-N-methyldipropylamine;diaminomaleonitrile;1,3-diaminopentane;9,10-diaminophenanthrene;4,4'-diaminooctafluorobiphenyl; 3,5-diaminobenzoic acid; -Diamino-2-methoxyfluorene; 4,4'-d
- BMI-689 (synthesized from dimer diamine and maleic anhydride) and the like can be suitably used.
- BMI-689 is represented by the following structural formula.
- thermosetting compound (B) The compound which has an epoxy group, the compound which has a (meth) acryloyl group, a phenol resin, a melamine resin, a silicon resin, an alkyd resin, a xylene resin etc. are used. be able to. These may be used alone or in combination of two or more. Among these, it is preferable to contain a compound having an epoxy group or a compound having a (meth) acryloyl group.
- thermosetting compound (B) As described above, it does not soften even when exposed to the solder reflow process, and it is easy to maintain the function as a package.
- the content of the compound (B) is preferably 30 to 90 parts by mass, and more preferably 50 to 85 parts by mass, in 100 parts by mass in total with the compound (A).
- a mixture of the compound (A) and the compound (B) is used as a binder component.
- an epoxy resin that is solid at room temperature hereinafter sometimes referred to as “solid epoxy resin”
- an epoxy resin that is liquid at room temperature hereinafter referred to as “liquid”. Any of the “epoxy resins” may be used.
- solid at normal temperature for an epoxy resin means a state that does not have fluidity in a solvent-free state at 25 ° C.
- liquid at normal temperature has fluidity under the same conditions. It means to be in a state.
- the solid epoxy resin can be used by dissolving in a solvent.
- the solvent to be used is not particularly limited, and can be appropriately selected from those described below.
- the solid epoxy resin include, but are not limited to, bisphenol type epoxy resin such as bisphenol A type epoxy resin, bisphenol F type epoxy resin, bisphenol S type epoxy resin, spiro ring type epoxy resin, naphthalene type epoxy resin. , Biphenyl type epoxy resin, terpene type epoxy resin, glycidyl ether type epoxy resin such as tris (glycidyloxyphenyl) methane, tetrakis (glycidyloxyphenyl) ethane, glycidylamine type epoxy resin such as tetraglycidyldiaminodiphenylmethane, tetrabromobisphenol A Type epoxy resin, cresol novolac type epoxy resin, phenol novolac type epoxy resin, ⁇ -naphthol novolak type epoxy resin, brominated pheno Novolac type novolak epoxy resin having an epoxy resin, rubber-modified epoxy resins. These can be used alone or in combination of two or more.
- the epoxy resin that is liquid at normal temperature include, but are not particularly limited to, liquid glycidylamine epoxy resins and liquid glycidyl ether epoxy resins, and are preferably liquid glycidylamine epoxy resins.
- the compound having the (meth) acryloyl group is not particularly limited as long as it has an acryloyl group or a methacryloyl group.
- isoamyl acrylate, neopentyl glycol diacrylate, trimethylolpropane triacrylate, ditrimethylolpropane tetraacrylate examples include 2-hydroxy-3-acryloyloxypropyl methacrylate, phenylglycidyl ether acrylate hexamethylene diisocyanate urethane prepolymer, bisphenol A diglycidyl ether acrylic acid adduct, ethylene glycol dimethacrylate, and diethylene glycol dimethacrylate. These can be used alone or in combination of two or more.
- the content ratio of the compound having a (meth) acryloyl group is the compound having an epoxy group and (meth)
- the total amount with the compound having an acrylate group is preferably 5 to 95% by mass, more preferably 20 to 80% by mass.
- the compound having a (meth) acrylate group is 5% by mass or more, the storage stability of the conductive resin composition is excellent, the conductive resin composition can be quickly cured, and further, the coating dripping at the time of curing can be prevented. Can be prevented.
- the compound which has a (meth) acrylate group is 95 mass% or less, the adhesiveness of a package and a shield layer tends to become favorable.
- a compound (C) having an imidazole group is used to cure the binder component.
- the compound (C) having an imidazole group as a curing agent, when the coating layer made of the conductive resin composition of the present invention is heated and cured to form a shielding layer, the resulting shielding layer is blackened. For this reason, even if the solder reflow process is performed, discoloration of the shield layer is less noticeable.
- the compound (C) having an imidazole group used in the present invention is not particularly limited, and examples thereof include imidazole, 2-undecylimidazole, 2-heptadecylimidazole, 2-methylimidazole, 2-ethylimidazole, and 2-phenyl. Examples include imidazole, 2-ethyl-4-methyl-imidazole, and 1-cyanoethyl-2-undecylimidazole. These may be used alone or in combination of two or more.
- the content of the compound (C) having an imidazole group is not particularly limited, but usually it is preferably 0.5 to 50 parts by mass and preferably 5 to 50 parts by mass with respect to 100 parts by mass of the binder component. More preferred.
- the content of the compound (C) having an imidazole group is 0.5 parts by mass or more, the adhesion between the shield layer and the package surface and the conductivity of the shield layer become good, and the shield layer has an excellent shielding effect. Is easily obtained, and when it is 50 parts by mass or less, the storage stability of the conductive resin composition is easily maintained.
- a radical curing agent can be used as a curing agent, and the content thereof is 0.3 to 8 parts by mass with respect to 100 parts by mass of the binder component. It is preferable that When the content of the radical curing agent is 0.3 parts by mass or more, the adhesion between the conductive coating film and the surface of the coating object and the conductivity of the conductive coating film become good, and the conductivity is excellent in the shielding effect. If the amount is 8 parts by mass or less, the storage stability of the conductive resin composition is improved.
- the conductive filler (D) in the conductive resin composition of the present invention is not particularly limited, but is preferably copper powder, silver powder, silver nanopowder, silver-coated copper powder, or silver-coated copper alloy powder. More preferably, silver-coated copper powder or silver-coated copper alloy powder.
- the silver-coated copper alloy powder may have a copper alloy powder and a silver-containing layer that covers the copper alloy powder. Further, the copper alloy powder may have a nickel content of 0.5 to 20% by mass and a zinc content of 1 to 20% by mass. Nickel and zinc are included within the above-described range, and the balance is made of copper, and the balance of copper may contain unavoidable impurities.
- the shield package excellent in shielding property can be obtained by using the copper alloy powder which has a silver coating layer.
- the silver coating amount is preferably 3 to 30% by mass and more preferably 5 to 20% by mass in the ratio of silver-coated copper powder or silver-coated copper alloy powder.
- the silver coating amount is 3% by mass or more, it is easy to suppress discoloration of the shield package even under severe heating conditions, and good conductivity can be obtained.
- the silver coating layer is 30% by mass or less, a package having excellent shielding properties can be obtained at low cost.
- Examples of the shape of the conductive filler (D) include flakes (scales), dendrites, spheres, fibers, irregular shapes (polyhedrons), etc., but the resistance value is lower and the shielding property is more. From the viewpoint of obtaining an improved shield layer, a flake shape is preferred.
- the tap density of the conductive filler (D) is preferably 4.0 to 6.5 g / cm 3 .
- the conductivity of the shield layer becomes better.
- the conductive filler (D) when the conductive filler (D) is flaky, the conductive filler (D) preferably has an aspect ratio of 2 to 10. When the aspect ratio is within the above range, the conductivity of the shield layer becomes better.
- the average particle diameter of the conductive filler (D) is preferably 1 to 30 ⁇ m. When the average particle diameter of the conductive filler (D) is 1 ⁇ m or more, the dispersibility of the conductive filler (D) is good and aggregation can be prevented, and it is difficult to oxidize. Good connectivity.
- the content of the conductive filler (D) is not particularly limited, but is preferably 200 to 1800 parts by mass with respect to 100 parts by mass of the binder component.
- the content is 200 parts by mass or more, the conductivity of the shield layer is good, and when it is 1800 parts by mass or less, the adhesion between the shield layer and the package and the physical properties of the conductive resin composition after curing are good.
- the shield layer is less likely to be chipped when cut with a dicing saw described later. Further, even if the conductive coating is heat-cured and then left undisturbed due to the blackening of the resin, the discoloration becomes inconspicuous.
- additives such as antifoaming agents, thickeners, pressure-sensitive adhesives, fillers, flame retardants, and colorants are added to the conductive resin composition of the present invention within a range that does not impair the purpose of the invention. be able to.
- the conductive resin composition of the present invention preferably has a lower viscosity than the so-called conductive paste so that the conductive resin composition can be uniformly applied to the package surface by spraying.
- the viscosity of the conductive resin composition of the present invention is preferably adjusted as appropriate according to the application and the equipment used for coating, and is not particularly limited, but general guidelines are as described below.
- the method for measuring the viscosity is not limited, but if the conductive resin composition has a low viscosity, it can be measured with a cone-plate rotational viscometer (so-called cone-plate viscometer). For example, it can be measured with a single cylindrical rotational viscometer (so-called B-type or BH-type viscometer).
- the viscosity measured at 0.5 rpm using a cone field CP40 (Cone angle: 0.8 °, cone radius: 24 mm) manufactured by Brookfield is 100 mPa. ⁇ It is preferably at least s, more preferably at least 150 mPa ⁇ s. When the viscosity is 100 mPa ⁇ s or more, it is easy to form a conductive coating film without unevenness by preventing dripping when the coated surface is not horizontal.
- a thin film is formed by reducing the coating amount once, and a thin film is formed thereon.
- a so-called overcoating method that repeats the operation is effective. If the viscosity is measurable with a conical plate type rotational viscometer, there is no problem even if it is high.
- Rotator No. when measuring with a single cylindrical rotational viscometer.
- the viscosity measured at 10 rpm using 5 is preferably 30 dPa ⁇ s or less, and more preferably 25 dPa ⁇ s or less. When it is 30 dPa ⁇ s or less, the spray nozzle is prevented from being clogged, and a conductive coating film is easily formed without unevenness. If the viscosity is measurable with a single cylindrical rotational viscometer, there is no problem even if it is low.
- a solvent can be used so as to be within the above range.
- the solvent that can be used in the present invention is not particularly limited.
- the content of the solvent is appropriately adjusted according to the use of the conductive resin composition and the equipment used for coating. Accordingly, although it varies depending on the viscosity of the binder component, the content of the conductive filler, and the like, the standard is about 10 to 60% by mass with respect to the total amount of the components (excluding the solvent) of the conductive resin composition.
- the shield layer obtained by the conductive resin composition of the present invention is excellent in adhesion with a ground circuit formed of copper foil or the like. Specifically, since the adhesion between the copper foil of the ground circuit exposed from a part of the shield package and the shield layer is good, after the conductive resin composition is applied to the shield package surface and the shield layer is formed When the package is cut into individual pieces, it is possible to prevent the shield layer from being peeled off from the ground circuit due to an impact at the time of cutting.
- the coating film formed from the conductive resin composition of the present invention preferably has a specific resistance of 2 ⁇ 10 ⁇ 4 ⁇ ⁇ cm or less from the viewpoint of obtaining excellent shielding properties. .
- a plurality of electronic components (IC or the like) 2 are mounted on a substrate 1 and a ground circuit pattern (copper foil) 3 is provided between the plurality of electronic components 2.
- the electronic component 2 is sealed by filling the electronic component 2 and the ground circuit pattern 3 with a sealing material 4 and curing it.
- the sealing material 4 is cut between the plurality of electronic components 2 to form grooves, and the packages of the electronic components on the substrate 1 are individualized by these grooves.
- Reference symbol A indicates an individual package. At least a part of the ground circuit is exposed from the wall surface constituting the groove, and the bottom of the groove does not completely penetrate the substrate.
- a predetermined amount of the binder component, the conductive filler and the curing agent described above and a solvent used as necessary are mixed to prepare a conductive resin composition.
- the conductive resin composition is sprayed in the form of a mist with a known spray gun or the like and applied evenly on the package surface.
- the spray pressure and spray flow rate at this time, and the distance between the spray gun spray port and the package surface are appropriately set as necessary.
- FIG. 2 is a plan view showing the substrate in this state.
- Reference numerals B 1 , B 2 ,... B 9 denote shield packages before being separated into individual pieces, and reference numerals 11 to 19 denote grooves between these shield packages, respectively.
- an individual package B is obtained by cutting the substrate with a dicing saw or the like along the bottom of the groove of the package before the individualization.
- the individual package B thus obtained has a uniform shield layer formed on the package surface (all of the upper surface portion, the side surface portion, and the corner portion of the boundary between the upper surface portion and the side surface portion). Good shielding characteristics can be obtained.
- the adhesion between the shield layer and the package surface and the ground circuit is excellent, it is possible to prevent the shield layer from being peeled off from the package surface or the ground circuit due to an impact when the package is separated into pieces by a dicing saw or the like. .
- Compound (b1) Liquid glycidylamine epoxy resin, manufactured by ADEKA Corporation, trade name “EP-3905S”
- Compound (b2) Liquid glycidyl ether epoxy resin, manufactured by ADEKA Corporation, trade name “EP-4400”
- Compound (b3) (meth) acrylate compound, 2-hydroxy-3-acryloyloxypropyl methacrylate (manufactured by Kyoeisha Chemical Co., Ltd., trade name “Light Ester G-201P”)
- ⁇ Curing agent> Compound having imidazole group (c1): 2-ethyl-4-methylimidazole (manufactured by Shikoku Kasei Kogyo Co., Ltd., trade name “2E4MZ”)
- Solvent Methyl ethyl ketone (MEK)
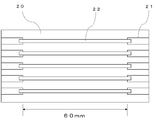
- Conductivity of conductive coating film The conductivity of the conductive coating film obtained from the conductive resin composition of Example 1 was evaluated by specific resistance. Specifically, as shown in FIG. 3, the electrode pad 21 formed of copper foil has a thickness of 55 ⁇ m provided with a slit having a width of 5 mm on the glass epoxy substrate 20 provided at both ends with an interval of 60 mm. The polyimide film was affixed so that the ends of the slits overlapped with the electrode pads 21 at both ends to form a printing plate. On top of that, the conductive resin compositions obtained in the examples and comparative examples were printed using a metal squeegee. The conductive resin composition is cured by heating at 190 ° C.
- the polyimide film is peeled off, and a cured product 22 having a length of 70 mm, a width of 5 mm, and a thickness of about 40 ⁇ m is connected between the electrode pads 21 at both ends.
- the substrate 20 formed in the above was obtained.
- the resistance value ((ohm)) between electrode pads was measured using a tester, and specific resistance ((omega
- the cross-sectional area, length, and specific resistance of the sample a total of 15 lines were formed by printing 5 lines each on 3 glass epoxy substrates, and the average value was obtained. If the specific resistance is 2 ⁇ 10 ⁇ 4 ⁇ ⁇ cm or less, it can be suitably used as a conductive resin composition used for the shield layer.
- Adhesiveness of conductive coating (comparison before and after solder dip) The adhesion between the shield layer and the package surface or the ground circuit was evaluated based on JIS K 5600-5-6: 1999 (cross cut method). Specifically, a copper foil was prepared for evaluation of adhesion to the ground circuit, and a mold resin for evaluation of adhesion to the package surface was prepared. Each is masked with a polyimide tape so as to form an opening having a width of 5 cm and a length of 10 cm, and after spray application of the conductive resin composition using a spray coating apparatus SL-940 (manufactured by Nordson Asymtek) at 190 ° C.
- SL-940 manufactured by Nordson Asymtek
- the evaluation of adhesion was performed according to the following criteria. 0: The edge of the cut is completely smooth and there is no peeling to the eyes of any lattice. 1: Small peeling of the coating film occurs at the intersection of cuts. The cross-cut portion is clearly not affected by more than 5%. 2: The coating film is peeled along the edge of the cut and / or at the intersection. The cross-cut part is clearly affected by more than 5% but not more than 15%. 3: The coating film is partially or completely peeled along the edge of the cut, and / or various parts of the eye are partially or completely peeled off. The cross-cut part is clearly affected by more than 15% but not more than 35%.
- the coating film is partially or completely peeled along the edge of the cut, and / or some eyes are partially or completely peeled off. It is clearly not more than 35% that the cross-cut is affected. 5: Any of the degree of peeling that cannot be classified even with classification 4.
- the color (hue H, lightness V, and saturation C) of the obtained cured coating film was examined according to JIS Z 8721 (1993). Next, these cured coating films were further heated at 200 ° C. for 60 minutes, and the colors of the cured coating films were examined in the same manner as described above. The color after further heating the cured coating film at 200 ° C. for 60 minutes is almost the same as the color of the cured coating film after the heat cycle test (200 cycles of ⁇ 65 ° C. for 30 minutes and 150 ° C. for 30 minutes). It has been confirmed in advance. The color change was evaluated according to the following criteria.
- Pass (O) The amount of change in viscosity before and after freezing was less than 20%.
- Fail (x) The amount of change in viscosity before and after freezing storage was 20% or more.
Abstract
L'invention concerne : une composition de résine conductrice qui présente de bonnes propriétés de blindage, une bonne adhérence à un boîtier, et qui peut, par revêtement par pulvérisation, former une couche de blindage qui est moins susceptible de changer de couleur, même dans des conditions de chaleur intense ; et un procédé de production d'un boîtier blindé utilisant la composition de résine conductrice. La composition de résine conductrice contient au moins (A) un composé qui est représenté par la formule générale (I), qui présente une masse moléculaire de 500 à 2000 et qui comprend deux groupes maléimide ou plus dans une molécule, (B) un composé thermodurcissable, (C) un composé comportant un groupe imidazole, et (D) une charge conductrice.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020197025414A KR20200019593A (ko) | 2017-06-15 | 2018-04-03 | 도전성 수지 조성물 및 그것을 사용한 차폐 패키지의 제조 방법 |
| CN201880017919.8A CN110382620A (zh) | 2017-06-15 | 2018-04-03 | 导电性树脂组合物及使用该导电性树脂组合物的屏蔽封装体的制造方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017117814A JP2019001912A (ja) | 2017-06-15 | 2017-06-15 | 導電性樹脂組成物及びそれを用いたシールドパッケージの製造方法 |
| JP2017-117814 | 2017-06-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018230109A1 true WO2018230109A1 (fr) | 2018-12-20 |
Family
ID=64660003
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/014287 WO2018230109A1 (fr) | 2017-06-15 | 2018-04-03 | Composition de résine conductrice et procédé de production d'un boîtier blindé l'utilisant |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP2019001912A (fr) |
| KR (1) | KR20200019593A (fr) |
| CN (1) | CN110382620A (fr) |
| TW (1) | TW201904764A (fr) |
| WO (1) | WO2018230109A1 (fr) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7272284B2 (ja) * | 2020-01-15 | 2023-05-12 | 信越化学工業株式会社 | 低誘電樹脂組成物 |
| CN111508911B (zh) * | 2020-04-30 | 2022-03-25 | 青岛歌尔微电子研究院有限公司 | 分腔电磁屏蔽封装方法及封装结构 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003258137A (ja) * | 2002-02-28 | 2003-09-12 | Mitsubishi Electric Corp | 半導体装置 |
| JP2008063449A (ja) * | 2006-09-07 | 2008-03-21 | Hitachi Ltd | ポリマーナノコンポジット材料、その製造方法電子部品装置およびその製造方法 |
| JP2009179725A (ja) * | 2008-01-31 | 2009-08-13 | Sumitomo Bakelite Co Ltd | 樹脂組成物およびそれを用いて作製した半導体装置または回路基板 |
| WO2016051700A1 (fr) * | 2014-09-30 | 2016-04-07 | タツタ電線株式会社 | Matériau de revêtement conducteur et procédé de production d'un emballage de protection l'utilisant |
| JP2017008160A (ja) * | 2015-06-18 | 2017-01-12 | 京セラ株式会社 | ダイボンディングペーストの製造方法およびダイボンディングペースト |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5266719B2 (ja) * | 2007-10-29 | 2013-08-21 | 住友ベークライト株式会社 | 樹脂組成物及び樹脂組成物を使用して作製した半導体装置 |
-
2017
- 2017-06-15 JP JP2017117814A patent/JP2019001912A/ja active Pending
-
2018
- 2018-04-03 WO PCT/JP2018/014287 patent/WO2018230109A1/fr active Application Filing
- 2018-04-03 CN CN201880017919.8A patent/CN110382620A/zh active Pending
- 2018-04-03 KR KR1020197025414A patent/KR20200019593A/ko not_active Application Discontinuation
- 2018-04-17 TW TW107113043A patent/TW201904764A/zh unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2003258137A (ja) * | 2002-02-28 | 2003-09-12 | Mitsubishi Electric Corp | 半導体装置 |
| JP2008063449A (ja) * | 2006-09-07 | 2008-03-21 | Hitachi Ltd | ポリマーナノコンポジット材料、その製造方法電子部品装置およびその製造方法 |
| JP2009179725A (ja) * | 2008-01-31 | 2009-08-13 | Sumitomo Bakelite Co Ltd | 樹脂組成物およびそれを用いて作製した半導体装置または回路基板 |
| WO2016051700A1 (fr) * | 2014-09-30 | 2016-04-07 | タツタ電線株式会社 | Matériau de revêtement conducteur et procédé de production d'un emballage de protection l'utilisant |
| JP2017008160A (ja) * | 2015-06-18 | 2017-01-12 | 京セラ株式会社 | ダイボンディングペーストの製造方法およびダイボンディングペースト |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019001912A (ja) | 2019-01-10 |
| TW201904764A (zh) | 2019-02-01 |
| CN110382620A (zh) | 2019-10-25 |
| KR20200019593A (ko) | 2020-02-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9236169B2 (en) | Electromagnetic wave shielding structure and method for fabricating the same | |
| US10259952B2 (en) | Conductive coating material for shielding electronic component package and method for producing shielded package | |
| TWI722136B (zh) | 導電性塗料及使用其之屏蔽封裝體之製造方法 | |
| WO2017017923A1 (fr) | Feuille de cuivre revêtue de résine, et panneau de câblage imprimé | |
| US20180079856A1 (en) | Resin composition, conductive resin conposition, adhesive, conductive adhesive, paste for forming electrodes, and semiconductor device | |
| WO2019073809A1 (fr) | Boîtier de blindage | |
| WO2018230109A1 (fr) | Composition de résine conductrice et procédé de production d'un boîtier blindé l'utilisant | |
| JP2019134024A (ja) | 電磁波シールドフィルム | |
| JP6831731B2 (ja) | 導電性塗料及びそれを用いたシールドパッケージの製造方法 | |
| WO2018012017A1 (fr) | Matériau de revêtement conducteur, et procédé de fabrication d'enveloppe de blindage mettant en œuvre celui-ci | |
| JP2022179517A (ja) | 樹脂組成物 | |
| JP2012255147A (ja) | 半導体モジュール部品及び液状封止用樹脂組成物 | |
| JP2020055977A (ja) | 導電性塗料 | |
| KR102014583B1 (ko) | 커버레이용 필름 | |
| JP7464044B2 (ja) | 磁性粉体を含む樹脂組成物 | |
| JP2017120879A (ja) | 保護素子、保護素子の製造方法、実装基板および電子機器 | |
| JP2022149589A (ja) | フレキシブルプリント配線板(fpc)用接着剤組成物、並びに該組成物を含む熱硬化性樹脂フィルム、プリプレグ、及びfpc基板 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18818213 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20197025414 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18818213 Country of ref document: EP Kind code of ref document: A1 |