WO2018159957A1 - 에쿠올 유도체를 생산하는 재조합 대장균 및 이를 이용한 에쿠올 유도체 합성 방법 - Google Patents
에쿠올 유도체를 생산하는 재조합 대장균 및 이를 이용한 에쿠올 유도체 합성 방법 Download PDFInfo
- Publication number
- WO2018159957A1 WO2018159957A1 PCT/KR2018/002201 KR2018002201W WO2018159957A1 WO 2018159957 A1 WO2018159957 A1 WO 2018159957A1 KR 2018002201 W KR2018002201 W KR 2018002201W WO 2018159957 A1 WO2018159957 A1 WO 2018159957A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- hydroxy
- reductase
- equol
- recombinant
- coli
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/70—Vectors or expression systems specially adapted for E. coli
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/0004—Oxidoreductases (1.)
- C12N9/001—Oxidoreductases (1.) acting on the CH-CH group of donors (1.3)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/90—Isomerases (5.)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P17/00—Preparation of heterocyclic carbon compounds with only O, N, S, Se or Te as ring hetero atoms
- C12P17/02—Oxygen as only ring hetero atoms
- C12P17/06—Oxygen as only ring hetero atoms containing a six-membered hetero ring, e.g. fluorescein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y103/00—Oxidoreductases acting on the CH-CH group of donors (1.3)
- C12Y103/01—Oxidoreductases acting on the CH-CH group of donors (1.3) with NAD+ or NADP+ as acceptor (1.3.1)
Definitions
- the present invention relates to a recombinant Escherichia coli producing an equol derivative and a method for synthesizing the equol derivative using the same.
- Some anaerobic microorganisms in human intestines are known to metabolize isoflavones that humans consume through legumes and convert them into equol.
- the equol synthesizing microorganisms found so far are all bacteria, typically slaves in Ischia (Slackia sp.), Egg telra in Stavanger (Eggerthella sp.) and Adlercreutizia sp., most of which belong to the genus Coriobacteria, with the exception of Lactococcus .
- the equol produced by the enzymatic reaction of the intestinal microorganisms is synthesized only with the optical isomer of the S structure, which is known to have a phytoestrogen effect is absorbed by the human body. Therefore, it has been proved that steadily ingesting equol alleviates the onset and symptoms of menopausal symptoms, especially osteoporosis, in women without the side effects of conventional treatment for menopausal symptoms such as breast cancer. This effect is due to the fact that the molecular structure of equol has similarity to human estrogen, and the selectivity of ⁇ among the estrogen receptors ⁇ and ⁇ is high.
- (S) -Ecuol has been shown to prevent cardiovascular diseases and to reduce the prostate cancer (Non-Patent Document 1).
- 5-Hydroxy-Equol an analog of equol, is derived from genistein, an isoflavone, and has been reported to be a phytoestrogens with relatively less biological efficacy than equol, but with better antioxidant activity than equol.
- Non Patent Literature 2 it has been reported that dehydroequol inhibits the growth of prostate and ovarian cancer cells, and has been reported to be effective in suppressing ovarian cancer that has become resistant to chemocancer drugs as a result of the second stage of clinical trials (non-patent). Document 3).
- 5-hydroxy equol production using these strains has a limitation on the growth of microorganisms under anaerobic conditions, 5-hydroxy equol is produced at least 15 hours after the inoculation of microorganisms, and the productivity is low. There is a limitation that it is difficult to accurately predict the final yield due to absence.
- Genistein one of the isoflavones, is bioconverted to 5-hydroxy-equol by the microorganism Coriobacteriacae Strain Mt1B8 (16), Slackia isoflavoniconvertens (17), strain AUH-JLC159. This has been reported, but the reaction and incubation time is 2 to 3 days long, requires anaerobic reaction conditions, there is a problem that the conversion rate falls below 80% when the amount of substrate genistein is more than 0.6 mmol / L (Patent Document 1 ).
- Non Patent Literature 6 The enzyme is composed of three reductases and one isomerase enzyme, a diidzein reductase, a dihydrodaidzediase (dihydrodaidzein racemase), a dihydrodide dihydrodaidzein reductase) and tetrahydrodaidzein reductase.
- Patent Document 1 Chinese Patent Publication No. 103275884
- Non-Patent Document 1 Nutrition Reviews, 69 (8), 432-448, 2011
- Non-Patent Document 2 J. Chin. Pharm. Sci., 23 (6), 378-384, 2014
- Non-Patent Document 3 Int. J. Gynecol. Cancer, 21 (4), 633-639, 2011
- Non-Patent Document 4 Applied and Environmental Microbiology, 74, 4847 (2008) / Applied and Environmental Microbiology, 75, 1740 (2009)
- Non-Patent Document 5 Conghui Zhang et al., J. Chin. Pharm. Sci., 23 (6): 378-384, 2014
- Non-Patent Document 6 Appl. Environ. Microbiol., 79 (11), 3494, 2013
- An object of the present invention is to provide a recombinant Escherichia coli expressing four enzymes derived from Slackia isoflavoneconductance.
- Another object of the present invention is to provide a novel compound, 5-hydroxy-dihydroequol, there is no synthesis method for this.
- another object of the present invention is to synthesize a method for synthesizing equol, dihydroecuol, 5-hydroxy-ecuol or 5-hydroxy-dihydroecuol from dyedzein or genistein using the recombinant E. coli. To provide.
- another object of the present invention is 5-hydroxy-ecuol, dihydroequiol using recombinant E. coli expressing a combination of two or three of the four enzymes derived from Sciakia isoflavoneconductance And a method for selectively and efficiently synthesizing 5-hydroxy-dihydroequol.
- the inventors of the present invention are Slackia isoflavoneconductance ( Slackia) which is a human intestinal microorganism. isoflavoniconvertens ), a diedzein reductase derived from a died agent, a dihydrodaidzein racemase derived from a dihydrodide, a dihydrodaidzein reductase derived from a dihydrodide, and a tetrahydrodaidzein reductase derived from a tetrahydrodide.
- Recombinant Escherichia coli expressing a recombinant E. coli and two or three of the above four enzymes in combination was prepared.
- the recombinant E. coli of the present invention can be used as a whole cell biocatalyst for bioconversion of equol or dehydroequol from daidzein. It can be used as a whole-cell catalyst that selectively bioconverts to hydroxy-equol or 5-hydroxy-dihydroecuol.
- the present invention biosynthesizes equol, dihydroecuol, 5-hydroxy-ecuol or 5-hydroxy-dihydroecuol at 95% substrate conversion at a concentration of about 0.2 to about 1 mmol / L of substrate in aerobic conditions. can do.
- the present invention can selectively synthesize 5-hydroxy-ecuol, dihydroecuol or 5-hydroxy-dihydroecuol.
- the present invention can be carried out by the selective synthesis of 5-hydroxy- equol, dihydroekuol and 5-hydroxy-dihydroekuol in a few hours in aerobic conditions, reducing the production cost and high Value added can be obtained and can be utilized for various applications such as food and medicine through efficient mass production.
- the compounds synthesized by the present invention can be used for preventing and treating menopausal diseases, and can be used as anticancer agents for treating prostate cancer and ovarian cancer. It can also be used as an antioxidant in the form of cosmetics or food additives.
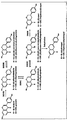
- FIG. 2 shows equol, dehydroequol, 5-hydroxy-equol and 5-hydroxy-dihydro using recombinant E. coli expressing all four enzymes. Schematic of the 5-hydroxy-dehydroequol synthesis reaction.
- FIG. 3 is a synthetic reaction of FIG. 2, genistein (GSN), dihydrogenistein (DHG), 5-hydroxy-equol (5-hydroxy-equol, 5OH-EQ) according to the reaction time, The concentration ( ⁇ M) in the reaction system of 5-hydroxy-dehydroequol (5-OH-DEQ) is shown.
- FIG. 4 is a schematic of a synthetic reaction compartmentalized using recombinant E. coli 1 and 2 expressing two enzymes.
- 5 is a synthetic reaction of FIG. 4, genistein (GSN), dihydrogenistein (DHG), 5-hydroxy-equol (5-hydroxy-equol, 5OH-EQ) according to the reaction time, The concentration ( ⁇ M) in the reaction system of 5-hydroxy-dehydroequol (5-OH-DEQ) is shown.
- Figure 6 shows the amount of protein in the cell extract of the recombinant E. coli mentioned in Figures 2 and 4 by SDS-PAGE.
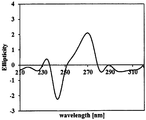
- FIG. 7 is a chart of measuring ellipticity using a dichroic dispersometer in the 210-320 nm wavelength range.
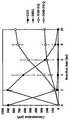
- FIG. 9 is a chart showing substrate conversion and yield of 5-hydroxy-dihydroequol depending on the initial concentration of genistein.
- Figure 10 (a) is the mass spectrum and molecular structure analyzed by EI-MS of 5-hydroxy-ecuol, (b) is the mass spectrum and molecular structure of 5-hydroxy-dihydroequol.
- FIG. 11 shows the concentration in the reaction system of (S) -equol in the reaction solution to which a water-soluble polymer, polyethylene glycol (PEG) or polyvinylpyrrolidone (PVP), is added. It is a chart.
- Expression The production of recombinant protein in a microorganism including a vector containing the recombinant gene.
- Vector- refers to a polynucleotide consisting of single-stranded, double-stranded, circular or ultra-stranded DNA or RNA.
- the vector consists of a replication origin, promoter, ribosomal binding site, nucleic acid cassette, termination and the like, which are operably linked at appropriate distances to produce a recombinant protein.
- the gene encoding the recombinant protein to be expressed is inserted at the nucleic acid cassette position.
- Cell extract- refers to the supernatant after crushing the microorganism expressing the recombinant protein and then removing the solid cell debris by centrifugation. In the present invention, it means a microbial extract in which some or all of the diase reductase, the dihydrodide racemase, the dihydrodide reductase, and the tetrahydrodide reductase are expressed.
- Whole cell reaction refers to the reaction using whole cell without breaking down the cell containing specific enzyme and using cell extract or separating and purifying enzyme.
- the present invention is a diidzein reductase (diaidzein reductase), a dihydrodaidzein racemase (dihydrodaidzein racemase), a dihydrodaidzein reductase (dihydrodaidzein reductase) and a tetrahydrodide reductase (tetrahydrodaidzein reductase) It relates to a recombinant E. coli expressing.
- a reductase which is a dide agent derived from Slackia isoflavoniconvertens , a racemase which is a dihydrodide agent, a reductase which is a dihydrodide agent, and a reductase which is a tetrahydrodide agent.
- DNA sequences were amplified by polymerase chain reaction (PCR), and then placed in pRSFDuet or pCDFDuet vectors, respectively, and expressed as his-tag (6 histidine) in E. coli .
- the recombinant E. coli can be used to synthesize equol or dihydroecuol from the substrate Dydzein.
- the recombinant E. coli can be used to synthesize 5-hydroxy- equol or 5-hydroxy-dihydroequol from the substrate, Genistein.
- the equol or 5-hydroxy-equol synthesis method can be used to synthesize (S) -equol or 5-hydroxy- (S) -equol.
- the recombinant E. coli can be used as a whole cell biocatalyst that bioconverts equol and dehydroequol from daidzein.
- 5-hydroxy-equol and 5-hydroxy-dehyroequol can be used as a whole-cell catalyst for bioconversion.
- a reducing agent is added to inhibit oxidation of the product, and as a reducing agent, NAD (P) H, L-ascorbic acid, glutathione, and ditaio Dithiothreitol, cysteine and the like can be used.
- the concentration of the added reducing agent is preferably about 10 to about 100 times the initial substrate concentration.
- the reaction solution includes potassium phosphate buffer for pH buffering, and the concentration of potassium phosphate is preferably about 50 to about 400 mM.
- the reaction temperature is preferably about 18 to about 37 ° C, more preferably about 25 to about 30 ° C.
- the pH of the reaction solution is preferably about 5 to about 10, more preferably about 6 to about 9, still more preferably about 7 to about 8.
- the stirring speed of the reactor is preferably about 50 to about 400 rpm, more preferably about 80 to about 250 rpm, more preferably about 100 to about 200 rpm.
- Glucose and glycerol may be used as the carbon source in the equol synthesis reactor, and the concentration of glucose or glycerol is preferably about 1 to about 5 (w / v)%, and about 1.5 to about 4 (w / v)% More preferred, about 2 to about 3 (w / v)% is more preferred.
- the concentration of the recombinant microorganism of the present invention is preferably about 1 to 100, more preferably about 5 to about 50, and more preferably about 10 to about 30, optical density (O.D.).
- genistein, glycidin, diaidzein, ortho- As a substrate of the equol synthesis reaction system, genistein, glycidin, diaidzein, ortho-, depending on the type of isoflavan or isoflavene to be synthesized hydroxy-daidzein (ortho -hydroxy-daidzein), ortho-can be used genistein (ortho -hydroxy-genistein) such as -hydroxy.
- Polyethylene glycol, polyvinyl pyrrolidone, polyvinyl alcohol and beta cyclodextrin, and methyl beta cyclodextrin to improve the solubility of the substrate in the reaction solution methyl- ⁇ -cyclodextrin), 2-hydroxypropyl beta cyclodextrin (2- (Hydroxypropyl) - ⁇ -cyclodextrin) and the like can be used.
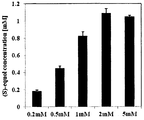
- FIG. 1 is a diagram showing the yield of (S) -equiol according to the concentration of the initial daidzein (D), as shown in Figure 1, as the concentration of the initial dyedzein increases the (S) -equol Yield (%) decreases, but the produced (S) -equol (mM) tends to increase.
- Figure 2 is a didezein reductase (DZNR), dihydrodaidzein racemase (DDRC), dihydrodaidzein reductase (DHDR), from diedzein and genistein, Schematic diagram of the synthesis of equol, dihydroequiol, 5-hydroxy-ecuol and 5-hydroxy-dihydroecuol using recombinant E. coli expressing tetrahydrodaidzein reductase (THDR) to be.
- DZNR didezein reductase
- DDRC dihydrodaidzein racemase
- DHDR dihydrodaidzein reductase
- daidzein is converted to (R) -dihydrodidezein by diedzein reductase (DZNR), which is a racemase (DDRC) which is dihydrodide It is converted into (S) -dihydroidane.
- DZNR diedzein reductase
- DDRC racemase
- S -dihydroidane
- S) -dihydrodide is converted to (3S, 4R) -trans-tetrahydrodide by dihydrodide, a reductase (DHDR), which is a tetrahydrodide, reductase (THDR) To equol or to dehydroequiol by dehydration.
- DHDR reductase
- THDR tetrahydrodide
- genistein is converted to (R) -dihydrogenysteine by didase reductase (DZNR), which is racemase (DDRC) which is a dihydrodide agent.
- DZNR didase reductase
- DDRC racemase
- S) -dihydrogenysteine is converted to (3S, 4R) -trans-tetrahydrogenysteine by dihydrodide reductase (DHDR), which is 5-folded by tetrahydrodide reductase (THDR) Conversion to hydroxy-equiol or, by dehydration, to 5-hydroxy-dihydroecuol.
- DHDR dihydrodide reductase
- THDR tetrahydrodide reductase
- the present invention is a diidzein reductase (diaidzein reductase), a dihydrodaidzein racemase (dihydrodaidzein racemase), a dihydrodaidzein reductase (dihydrodaidzein reductase) and a tetrahydrodide reductase (tetrahydrodaidzein reductase) It expresses and selectively selects 5-hydroxy-ecuol from the substrate genistein using recombinant E. coli overexpressing five times or more of the tetrahydrodaidzein reductase among the four enzymes. It relates to a method of synthesis.
- 5-hydroxy-ecuol can be selectively synthesized using a recombinant Escherichia coli overexpressed by at least 5 times, preferably 10 times, or more than the other three enzymes.
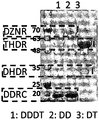
- Figure 6 shows the amount of protein in the cell extract of the recombinant E. coli referred to in Figures 2 and 4 in SDS-PAGE, the expression of the four enzymes, compared with the expression of four recombinant enzymes in one E. coli
- recombinant E. coli expressing a didase reductase and a dihydrodide racemase in one vector and a recombinant E. coli expressing a dihydrodide reductase and a tetrahydrodide reductase in one vector
- DDDT is the case when the four recombinant enzymes mentioned in Figure 2 in one E.
- DD is a diase diase and dihydrodase racemase in Figure 4
- 3 DT in the cell extract of recombinant E. coli expressing the dihydroreductase reductase and tetrahydrodide reductase as one vector in FIG. Corresponding.
- Figure 5 shows the concentration in the reaction system ( ⁇ M) of genistein, dihydrogenistein, 5-hydroxy-equol with the reaction time, the expression of the tetrahydrodide reductase 5 compared to the other three enzymes Using the system expressing recombinant E. coli more than doubled (2 and 3 in FIG. 6), the yield and productivity of 5-hydroxy-equol and 5- It indicates that selectivity to 5-hydroxy-equol relative to hydroxy-dihydroequol can be increased.
- the present invention is recombinant Escherichia coli 1 and tetrahydrodaidzete reductase (tetrahydrodaidzein reductase) and tetrahydrodide expressing a diidzein reductase and dihydrodaidase dihydrodaidzein racemase
- the present invention relates to a method for selectively synthesizing 5-hydroxy-equol from the substrate genistein using recombinant E. coli 2 expressing tetrahydrodaidzein reductase.
- 5-hydroxy-ecuol can be selectively synthesized from genistein by a biotransformation reaction system in which the recombinant E. coli 1 and 2 are mixed with a whole cell catalyst.
- Figure 4 is a recombinant E. coli and dihydrodaidzein reductase (dihydrodaidzein reductase (DZNR), dihydrodaidzein racemase (DDRC) expressed as a single vector) DHDR) and tetrahydrodaidzein reductase (THDR), which represents a whole cell reaction system in which the reaction is partitioned with other recombinant E. coli expressing one vector.
- DZNR dihydrodaidzein reductase
- DDRC dihydrodaidzein racemase
- THDR tetrahydrodaidzein reductase
- genistein is converted to (S) -dihydrogenysteine by the recombinant E. coli 1
- (S) -dihydrogenysteine is 5-hydroxy- (S) -equiol by the recombinant E. coli 2.
- the method for selectively synthesizing the 5-hydroxy-equol may selectively synthesize 5-hydroxy- (S) -equol.
- 5-hydroxy- (S) -equol may be selectively synthesized using recombinant E. coli overexpressing at least five times the tetrahydrodaidzein reductase, or the recombinant E. coli 1 and 2. have.
- Figure 7 is a chart measuring the ellipticity using a dichroic dispersion system in the wavelength range of 210 ⁇ 320 nm to know the optical structure of 5-hydroxy-Equol synthesized using the recombinant E. coli, It can be seen that 5-hydroxy-equol biosynthesized by the recombinant E. coli of the invention has the S-form at carbon 3.
- the present invention expresses a diidzein reductase, a dihydrodide diacedidezein racemase, and a dihydrodide direzeid reductase, and a tetrahydrodide reductase.
- the present invention relates to a method for selectively synthesizing dihydroequiol from the substrate dyedzein using recombinant E. coli that does not express (tetrahydrodaidzein reductase).
- E. coli as a whole-cell catalyst can be selectively synthesized dihydroekuol from Dyzezein,
- the amount of boric acid added is preferably about 50 mM to about 200 mM.
- the present invention expresses a diidzein reductase (diaidzein reductase), a dihydrodide agent (dihydrodaidzein racemase) and a dihydrodide agent reductase (dihydrodaidzein reductase), a tetrahydrodide reductase
- the present invention relates to a method for selectively synthesizing 5-hydroxy-dihydroecuol from the substrate genistein using recombinant E. coli that does not express tetrahydrodaidzein reductase.
- 5-hydroxy-dihydroechool can be selectively synthesized from genistein using the recombinant E. coli as a whole cell catalyst.
- the expression method of the recombinant E. coli of the present invention and the method for synthesizing dihydroecuol or 5-hydroxy-dihydroecuol using the same, recombination expressing all four Slakia isoflavone convergence-derived enzymes described above It is the same as the expression method of an enzyme and the equol synthesis method using the same.
- FIG. 8 shows recombinant E. coli (DZNR + DHDR) expressing diedzein reductase (DZNR), dihydrodaidzein reductase (DHDR) and diedzein reductase (DZNR).
- DZNR + DRDR + DHDR diedzein reductase
- DHDR dihydrodaidzein reductase
- DCRC dihydrodaidzein racemase
- DHDR dihydrodaidzein reductase
- coli can be selectively synthesized dihydroekuol from Dyzeze without producing equol, using the recombinant E. coli (DZNR + DRDR + DHDR) expressing three enzymes In this case, the concentration of dihydroequiol produced is higher than that of recombinant E. coli (DZNR + DHDR) expressing two enzymes.
- Figure 9 is a substrate according to the concentration of the initial genistein when the whole cell reaction to Genistein using a recombinant E. coli expressing a direzease, a dihydrodase racemase, a dihydrodide reducing enzyme It is a table showing the conversion rate and the yield of 5-hydroxy-dihydroecuol, and as shown in FIG. 9, 5-hydroxy-dihydroecuol can be selectively synthesized from Genistein using the recombinant E. coli.
- the recombinant E. coli of the present invention can be used as a whole cell catalyst.
- Whole-cell catalysts are used in many biocatalytic reactions, and the reaction proceeds under mild conditions at room temperature and atmospheric pressure, and has the advantage of excellent reaction specificity. It is more advantageous to perform the biocatalyst process using whole cells in the case where a crude enzyme is necessary or a process involving several stages of reaction or when the activity of the enzyme is significantly reduced during purification.
- the recombinant E. coli used as whole cells can be selectively separated using a centrifuge, even when the product is purified after completion of the reaction.
- the synthesized equol, dihydroecuol, 5-hydroxy-ecuol or 5-hydroxy-dihydroecuol synthesis method may proceed under aerobic conditions, compared to the prior art for synthesizing equol under anaerobic conditions. It is possible to synthesize equol under mild conditions. Thus, the synthesis method of the present invention does not require the apparatus or techniques used to provide anaerobic conditions in the prior art.
- the synthesized equol, dihydroequol, 5-hydroxy-equol or 5-hydroxy-dihydroequol synthesis method can use a substrate of about 0.2 to about 5 mmol / L.
- concentration of the substrate dyedzein or genistein is less than 0.2 mmol / L, the amount of equol, dihydroecuol, 5-hydroxy-ecuol and 5-hydroxy-dihydroecuol synthesized is not sufficient. Purification costs may also be high, and above 5 mmol / L, the conversion of the substrate may be reduced, resulting in additional purification costs.
- the concentration of the substrate is about 0.2 to 1 mmol / L, more preferably, the concentration of the substrate is about 0.8 to about 1 mmol / L.
- the concentration of the substrate is preferably about 3 to 5 mmol / L.
- the method for synthesizing equol or dihydroecuol has a conversion rate of dyzezein of at least 95%, and the method for synthesizing 5-hydroxy-ecuol or 5-hydroxy-dihydroecuol has at least 95% conversion of genistein. to be.
- the conversion represents (reduced substrate concentration / initial substrate concentration) X 100 (%).
- the conversion of the substrate may be 95% or more.
- 3 and 5 show the concentration ( ⁇ M) in the reaction system of genistein (GSN), dihydrogenistein (DHG), and 5-hydroxy-equol (5OH-EQ) according to the reaction time. It is shown that after 10 hours the conversion of the substrate is greater than 95%.
- the equol, dihydroecuol, 5-hydroxy-ecuol or 5-hydroxy-dihydroecuol synthesis method may further include a water-soluble polymer in the reaction system.
- the water-soluble polymer may be used a food-soluble water-soluble polymer.
- water-soluble polymers for example, polyethylene glycol, polyvinyl pyrrolidone, polyvinyl alcohol and beta cyclodextrin, beta cyclodextrin, methyl beta cyclodextrin -cyclodextrin), 2-hydroxypropyl beta cyclodextrin (2- (Hydroxypropyl) - ⁇ -cyclodextrin) and the like can be used.
- polyethylene glycol, polyvinyl alcohol or polyvinylpyrrolidone can be used.
- the solubility of the substrate in the reaction system increases, so that the final yield of the prepared equol, dihydroequol, 5-hydroxy-equol or 5-hydroxy-dihydroequol is Can be increased.
- FIG. 11 is a chart showing the concentration in the reaction system of (S) -Equol in the reaction solution of the reaction solution of Example 1, in which polyethylene glycol or polyvinylpyrrolidone, which is a water-soluble polymer, is further added.
- the reaction solution further includes polyethylene glycol (PEG) or polyvinylpyrrolidone (PVP)
- equol may be synthesized in a high yield even by using a 5 mmol / L substrate. .
- the present invention also relates to equols, dihydroequols, 5-hydroxy-equols or 5-hydroxy-dihydroequols prepared by the above-mentioned synthetic methods of the present invention.
- 5-hydroxy-dihydroequol prepared by the synthesis method of the present invention is a novel compound which is not known in the art, and may be represented by the following formula.
- the present invention is capable of synthesizing (S) -equol with a high conversion of substrate in aerobic conditions, and since the synthesized equol has a molecular structure similar to estrogen, it can be used in women without side effects of conventional menopausal treatments such as breast cancer. It can alleviate the onset and symptoms of menopausal symptoms, especially osteoporosis, prevent cardiovascular disease and alleviate prostate cancer.
- dihydroequiol synthesized in the present invention can be used as an anticancer agent for the treatment of prostate cancer and ovarian cancer.
- the present invention can be carried out by the selective synthesis of 5-hydroxy- equol, dihydroekuol and 5-hydroxy-dihydroekuol in a few hours in aerobic conditions, reducing the production cost and high Value added can be obtained and can be utilized for various applications such as food and medicine through efficient mass production.
- tyrosinase cytochrome P450 or flavin monooxygenase may be used as the oxidoreductase, and 3'-hydroxy-equol, 6-hydroxy-equol, 8-hydroxy-equu Hydroxy-isoflavonoids such as all, 6,3'-dihydroxy-equol can be synthesized.
- 5-hydroxy-equol or 5-hydroxy-dihydroequol synthesized by the present invention may be used for preventing and treating menopausal diseases, and may be used as an anticancer agent for treating prostate cancer and ovarian cancer. It can also be used as an antioxidant in the form of cosmetics or food additives.
- DZNR Diidzein reductase
- DDRC dihydrodaidzein racemase
- DHDR dihydrodaidzein reductase
- tetrahydrodide reductase tetrahydrodaidzein
- the plasmid into which the nucleotide sequence of the enzyme was inserted was transformed into BL21 competent cells and incubated for 12 hours in a 37 ° C incubator in LB solid medium containing antibiotics suitable for each plasmid.
- One colony was inoculated in 3 mL LB liquid medium containing antibiotics and incubated at 200 rpm in a 37 ° C. incubator for 12 hours. 2 v% (1 mL) of this culture was inoculated into fresh LB liquid medium containing 50 mL of antibiotic. O.D. of cultures.
- reaction method 1 Whole cell In the reaction system Equol Production method (reaction method 1)
- L-ascorbic acid was added at an initial substrate concentration of 10 times as a reducing agent, and the reaction solution contained a 200 mmol / L potassium phosphate buffer solution for pH buffering, and the reaction temperature was 30 ° C.
- the pH of the reaction solution is 8, and the stirring speed of the reactor is 150 rpm.
- glucose or glycerol was used, the concentration of glucose or glycerol was 2 (w / v)%, and the concentration of the recombinant microorganism was O.D. 10 or O.D. 20.
- Example 2 Equol Using recombinant E. coli expressing all four enzymes involved in the transformation From Genistein 5- Hydroxy - Equol Or 5- Hydroxy - Dehydroequol synthesis.
- 500 ⁇ M of genistein (FIG. The reaction was carried out as in Reaction 1 using recombinant E. coli expressing the four enzymes of 10. Substrates, intermediates, and products were extracted with at least four times ethyl acetate (EA), the solvent was removed using a centrifugal decompressor, and redissolved in a certain amount of methanol to confirm the reactivity and concentration of the material by high performance liquid chromatography. After 6 hours of reaction, 500 ⁇ M of genistein was converted to 99% or more, and it was confirmed that 97 mg / L of 5-hydroxy-equiol and 22 mg / L of 5-hydroxy-dihydroecuol were produced.
- EA ethyl acetate
- E. coli 1 expressing a diase reducing agent and a racemizing enzyme dihydrating agent and a recombinant E. coli 2 expressing a reducing enzyme which is a dihydrodide agent and a tetrahydrodide agent are transferred.
- a bioconversion reaction system was constructed to mix with the cell catalyst.
- 1 mM Genistein reacted according to Reaction Method 1, as shown in FIG. 5, the concentrations of the substrate and the product according to the reaction time were confirmed by liquid chromatography.
- the reaction system produced a substrate conversion of more than 99%, 5-hydroxy-equol 231 mg / L, and 5-hydroxy-dihydroequol 12 mg / L in 6 hours of reaction time.
- the productivity of 5-hydroxy-equol increased 1.6-fold, which is defined as the concentration of 5-hydroxyequol concentration / 5-hydroxy-dihydroequol.
- Selectivity to 5-hydroxy-equol increased by 5 times.
- the recombinant E. coli in Example 2 and the cell extracts of Recombinant E. coli 1 and 2 in Example 3 were prepared by crushing by sonication, and the cell extract was 13000 rpm for 30 minutes. After centrifugation at 4 ° C to obtain a supernatant was analyzed by SDS-PAGE as shown in FIG.
- the expression levels of the four enzymes were increased in both recombinant E. coli used in Example 3 than that of E. coli used in Example 2.
- the expression amount of the reductase, a tetrahydrodide agent was remarkably increased, more than 20 times compared to the E. coli crushing solution of Example 1, and overexpressed 5 times or more than the expression amounts of the remaining three enzymes.
- Dichroic dispersion spectrum analysis was performed to determine the optical structure of 5-hydroxy-equol synthesized by the recombinant E. coli of the present invention.
- the measurement wavelength ranged from 210 nm to 320 nm and a dichroic dispersion system ChirascanTM plus CD spectrometer (Applied Photophysics, UK) was used.
- the sample was dissolved in pure methanol at a concentration of 1.5 mg / ml and then measured.
- the recombinant E. coli of Example 1 lacks a tetrahydrodide reductase and contains a dihydrodide racemase (DZNR + DDRC + DHDR, hereinafter DDD strain).
- DZNR + DDRC + DHDR dihydrodide racemase
- Recombinant Escherichia coli (DZNR + DHDR, DD strain hereinafter) was prepared.
- the reaction was carried out using Dyzezein as a substrate according to Reaction Method 1, and 200 mM boric acid was further added to the reaction solution. As a result, it was confirmed that only dihydroequol was produced without producing equol (FIG. 8).
- EI-MS used TRACE GC Ultra gas chromatograph / ITQ1100 from Thermo. In the positive mode, a nonpolar capillary column (TR-5 ms) was used, the helium gas flow rate was 1 ml / min, and the inlet, mass transfer line, and ion source temperatures were 250, 275, and 230 ° C, respectively. The oven temperature of the gas chromatograph is held at 65 ° C. for 5 minutes and then increased to 250 ° C. at a rate of 3 ° C./min. The ionization voltage was 70 eV and the measured mass range was 50-600 amu.
- EI-MS analysis showed the same spectrum of DHD as previously reported (Chang, YC, and MG Nair. J. Natr. Proc. 58: 1892-1896, 1995; Wahala, K., A. Salakka, and H. Adlercreutz. Proc. Soc. Exp. Biol. Med. 217: 293-29, 1998).
- the EI-MS of the synthesized compound showed a molecular ion peak of [M + H] +, consistent with C 15 H 12 O 4 (DHD) at m / z 256 as expected. Other ion peaks of the synthesized compounds were 137 (100), 120 (52), 91 (36) and 65 (16).
- the reaction was carried out in a reactor further comprising 5% (w / v) of water-soluble polymer PEG or PVP.
- 5 mM equol was synthesized (yield 99% or more, 1.16 g / L), and for the reaction with PEG, 3.7 mM equol was synthesized in 24 hours of reaction time (yield 74). %, 0.9 g / L).
- Example 8 it was confirmed that the addition of the water-soluble polymer in the reaction system, the yield of equol was superior to the reaction system (Example 1) containing no water-soluble polymer.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Medicinal Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Plant Pathology (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019546813A JP7002557B2 (ja) | 2017-02-28 | 2018-02-22 | エクオール誘導体を生産する組換え大腸菌およびこれを利用したエクオール誘導体の合成方法 |
| CN201880014592.9A CN110382701A (zh) | 2017-02-28 | 2018-02-22 | 生产雌马酚衍生物的重组大肠杆菌及利用其的雌马酚衍生物合成方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020170026774A KR102005237B1 (ko) | 2017-02-28 | 2017-02-28 | 에쿠올 유도체를 생산하는 재조합 대장균 및 이를 이용한 에쿠올 유도체 합성 방법 |
| KR10-2017-0026774 | 2017-02-28 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018159957A1 true WO2018159957A1 (ko) | 2018-09-07 |
Family
ID=63370438
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2018/002201 Ceased WO2018159957A1 (ko) | 2017-02-28 | 2018-02-22 | 에쿠올 유도체를 생산하는 재조합 대장균 및 이를 이용한 에쿠올 유도체 합성 방법 |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP7002557B2 (enExample) |
| KR (1) | KR102005237B1 (enExample) |
| CN (1) | CN110382701A (enExample) |
| WO (1) | WO2018159957A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020092690A (ja) * | 2018-12-07 | 2020-06-18 | 学校法人東京理科大学 | エクオール誘導体の産生のための組成物 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102079003B1 (ko) * | 2018-08-10 | 2020-02-19 | 서울대학교산학협력단 | 활성이 증대된 테트라하이드로다이드제인 환원효소 및 에쿠올 유도체 생산에의 응용 |
| KR102222517B1 (ko) | 2019-03-26 | 2021-03-03 | 서울대학교산학협력단 | 모노옥시게나아제를 포함하는 생촉매 조성물 및 이를 이용한 에쿠올 수산화 유도체의 제조 방법 |
| CN114806999B (zh) * | 2022-06-30 | 2022-09-27 | 华熙生物科技股份有限公司 | 一种基因工程菌及其在制备二氢大豆苷元中的应用 |
| CN115725614B (zh) * | 2022-08-05 | 2025-06-06 | 华熙生物科技股份有限公司 | 一种生产雌马酚的菌株及其应用 |
| WO2025181921A1 (ja) * | 2024-02-27 | 2025-09-04 | 株式会社ダイセル | エクオールおよび5-ヒドロキシエクオールの製造方法 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002074307A1 (en) * | 2001-03-16 | 2002-09-26 | Novogen Research Pty Ltd | Treatment of restenosis |
| US20060167037A1 (en) * | 2003-11-19 | 2006-07-27 | Kelly Graham E | Combinational radiotherapy and chemotherapy compositions and methods |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK2404995T3 (da) * | 2007-12-27 | 2013-10-14 | Otsuka Pharma Co Ltd | Enzym associeret med equolsyntese |
| CN103275884B (zh) | 2013-02-04 | 2014-08-20 | 河北农业大学 | 史雷克氏菌auh-jlc159及其转化制备(-)-5-oh-雌马酚的方法 |
| CN104031875B (zh) * | 2014-05-30 | 2016-04-20 | 浙江省农业科学院 | 一种s-雌马酚产生工程菌及应用 |
-
2017
- 2017-02-28 KR KR1020170026774A patent/KR102005237B1/ko active Active
-
2018
- 2018-02-22 CN CN201880014592.9A patent/CN110382701A/zh active Pending
- 2018-02-22 JP JP2019546813A patent/JP7002557B2/ja active Active
- 2018-02-22 WO PCT/KR2018/002201 patent/WO2018159957A1/ko not_active Ceased
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002074307A1 (en) * | 2001-03-16 | 2002-09-26 | Novogen Research Pty Ltd | Treatment of restenosis |
| US20060167037A1 (en) * | 2003-11-19 | 2006-07-27 | Kelly Graham E | Combinational radiotherapy and chemotherapy compositions and methods |
Non-Patent Citations (5)
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020092690A (ja) * | 2018-12-07 | 2020-06-18 | 学校法人東京理科大学 | エクオール誘導体の産生のための組成物 |
| JP7449526B2 (ja) | 2018-12-07 | 2024-03-14 | 学校法人東京理科大学 | エクオール誘導体の産生のための組成物 |
| JP2024059796A (ja) * | 2018-12-07 | 2024-05-01 | 学校法人東京理科大学 | エクオール誘導体の産生のための組成物 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20180099380A (ko) | 2018-09-05 |
| JP2020508671A (ja) | 2020-03-26 |
| JP7002557B2 (ja) | 2022-02-10 |
| KR102005237B1 (ko) | 2019-07-30 |
| CN110382701A (zh) | 2019-10-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2018159957A1 (ko) | 에쿠올 유도체를 생산하는 재조합 대장균 및 이를 이용한 에쿠올 유도체 합성 방법 | |
| JP4791664B2 (ja) | 新規のシトクロムp450モノオキシゲナーゼ及び有機化合物の酸化のためのその使用 | |
| WO2014208847A1 (ko) | 티로시나아제를 이용하여 제조된 카테콜형 구조 물질, 그의 제조 방법 및 그의 응용 | |
| US7745198B2 (en) | Method of producing macrolide compound | |
| Liu et al. | Efficient microbial synthesis of key steroidal intermediates from bio-renewable phytosterols by genetically modified Mycobacterium fortuitum strains | |
| WO2019132510A2 (ko) | 세포 소기관이 변이된 재조합 효모 및 이를 이용한 아이소프레노이드 생산 방법 | |
| WO2015093831A1 (ko) | D(-)2,3-부탄디올의 생성능이 증강된 재조합 미생물 및 이를 이용한 d(-)2,3-부탄디올의 생산 방법 | |
| CN112094797B (zh) | 基因工程菌及其制备9α,22-二羟基-23,24-双降胆甾-4-烯-3-酮的应用 | |
| CN101805742A (zh) | 透明颤菌血红蛋白vgbS核苷酸序列及其质粒和制备方法 | |
| CN111253408B (zh) | 抗生素pactamide G及其制备方法和在制备抗菌药物中的应用 | |
| CA2546614A1 (en) | Dna participating in hydroxylation of macrolide compound | |
| Horinouchi et al. | ORF18-disrupted mutant of Comamonas testosteroni TA441 accumulates significant amounts of 9, 17-dioxo-1, 2, 3, 4, 10, 19-hexanorandrostan-5-oic acid and its derivatives after incubation with steroids | |
| WO2015093832A1 (ko) | 1,3-프로판디올 생성능이 개선된 재조합 미생물 및 이를 이용한 1,3-프로판디올의 생산 방법 | |
| WO2014133291A1 (ko) | (2rs)-아미노-(3s)-히드록시-부티르산 또는 이의 유도체의 제조방법 | |
| TW200846473A (en) | Method for preparing (4S)-3,4-dihydroxy-2,6,6-trimethylcyclohex-2-enone and derivatives thereof | |
| HU204574B (en) | Process for producing 4-androstene-3,17-dion and 1,4-androstadiene-3,17- - -dion with microbiological method | |
| JPH10130269A (ja) | カルボリン誘導体 | |
| Sasamura et al. | Bioconversion of FR901459, a novel derivative of cyclosporin A, by Lentzea sp. 7887 | |
| Fosse et al. | Parameters and mechanistic studies on the oxidative ring cleavage of synthetic heterocyclic naphthoquinones by Streptomyces strains | |
| WO2014171635A1 (ko) | 신규 d-솔비톨 탈수소화효소 및 이를 이용한 l-소르보스의 생산방법 | |
| KR102079003B1 (ko) | 활성이 증대된 테트라하이드로다이드제인 환원효소 및 에쿠올 유도체 생산에의 응용 | |
| WO2011068341A2 (ko) | 삼원환 화합물의 생합성을 담당하는 유전자와 단백질 | |
| Shindo et al. | Hydroxylation of ionized aromatics including carboxylic acid or amine using recombinant Streptomyces lividans cells expressing modified biphenyl dioxygenase genes | |
| CN110835639B (zh) | 一种制备(s)-1,2,3,4-四氢异喹啉-1-甲酸及其衍生物的方法 | |
| EP0466901B1 (fr) | Segments d'adn et micro-organismes transformes comprenant le gene de la delta-1 -deshydrogenase, et leurs applications |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18760830 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2019546813 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18760830 Country of ref document: EP Kind code of ref document: A1 |