WO2016158513A1 - 液晶パネル及び画像表示装置 - Google Patents
液晶パネル及び画像表示装置 Download PDFInfo
- Publication number
- WO2016158513A1 WO2016158513A1 PCT/JP2016/058794 JP2016058794W WO2016158513A1 WO 2016158513 A1 WO2016158513 A1 WO 2016158513A1 JP 2016058794 W JP2016058794 W JP 2016058794W WO 2016158513 A1 WO2016158513 A1 WO 2016158513A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- liquid crystal
- meth
- sensitive adhesive
- pressure
- weight
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J11/00—Features of adhesives not provided for in group C09J9/00, e.g. additives
- C09J11/02—Non-macromolecular additives
- C09J11/06—Non-macromolecular additives organic
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J133/00—Adhesives based on homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides, or nitriles thereof; Adhesives based on derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09J—ADHESIVES; NON-MECHANICAL ASPECTS OF ADHESIVE PROCESSES IN GENERAL; ADHESIVE PROCESSES NOT PROVIDED FOR ELSEWHERE; USE OF MATERIALS AS ADHESIVES
- C09J175/00—Adhesives based on polyureas or polyurethanes; Adhesives based on derivatives of such polymers
- C09J175/04—Polyurethanes
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/30—Polarising elements
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/13—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on liquid crystals, e.g. single liquid crystal display cells
- G02F1/133—Constructional arrangements; Operation of liquid crystal cells; Circuit arrangements
- G02F1/1333—Constructional arrangements; Manufacturing methods
-
- G—PHYSICS
- G02—OPTICS
- G02F—OPTICAL DEVICES OR ARRANGEMENTS FOR THE CONTROL OF LIGHT BY MODIFICATION OF THE OPTICAL PROPERTIES OF THE MEDIA OF THE ELEMENTS INVOLVED THEREIN; NON-LINEAR OPTICS; FREQUENCY-CHANGING OF LIGHT; OPTICAL LOGIC ELEMENTS; OPTICAL ANALOGUE/DIGITAL CONVERTERS
- G02F1/00—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics
- G02F1/01—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour
- G02F1/13—Devices or arrangements for the control of the intensity, colour, phase, polarisation or direction of light arriving from an independent light source, e.g. switching, gating or modulating; Non-linear optics for the control of the intensity, phase, polarisation or colour based on liquid crystals, e.g. single liquid crystal display cells
- G02F1/133—Constructional arrangements; Operation of liquid crystal cells; Circuit arrangements
- G02F1/1333—Constructional arrangements; Manufacturing methods
- G02F1/1335—Structural association of cells with optical devices, e.g. polarisers or reflectors
Definitions
- the present invention relates to a liquid crystal panel and an image display device using the liquid crystal panel.
- a liquid crystal panel used for a liquid crystal display device or the like usually has a polarizing film laminated on both sides of a liquid crystal cell formed of a liquid crystal layer disposed between a pair of transparent substrates via an adhesive layer.
- Such an adhesive layer is required to have high durability. For example, in an endurance test such as heating and humidification that is usually performed as an environmental promotion test, defects such as peeling or floating due to the adhesive layer may not occur. Desired.
- a transparent conductive film such as an indium tin oxide (ITO) thin film is formed on one transparent substrate of the liquid crystal cell constituting the liquid crystal panel, and the adhesive layer in contact with the transparent conductive film is a glass substrate. It was found that, compared with the pressure-sensitive adhesive layer in contact with the transparent substrate, etc., peeling and floating are likely to occur, and the durability tends to be low.
- the pressure-sensitive adhesive layer formed from the pressure-sensitive adhesive composition of Patent Document 1 has poor adhesion to an indium tin oxide (ITO) layer, and is not sufficient as a pressure-sensitive adhesive composition for a liquid crystal panel having a transparent conductive layer. .
- a transparent conductive film such as an ITO thin film is formed on one transparent substrate of the liquid crystal cell, and the other is a transparent substrate such as a glass substrate, and the properties of both surfaces of the liquid crystal cell are often different.
- the durability of the liquid crystal panel is difficult to maintain the durability of the liquid crystal panel as a whole by maintaining durability that does not cause foaming or peeling on both surfaces of such a liquid crystal cell.
- Patent Document 1 nothing is defined in relation to an adherend such as a liquid crystal cell, and it is not sufficient from the viewpoint of durability as a whole liquid crystal panel.
- the liquid crystal panel In recent years, thinning of the liquid crystal panel is required. However, when the liquid crystal panel is thinned, the panel may be warped. When the warpage of the panel becomes large, the display image becomes non-uniform due to the contact between the liquid crystal panel and the display casing, and the double-sided tape that fixes the liquid crystal panel and the backlight peels off. Further, although the area of the peripheral portion of the liquid crystal display device is further narrowed to increase the area ratio of the display region in the display device (narrowing the frame), the light-shielded peripheral portion outside the display region has progressed.
- the polarizing film end may enter the inside of the display area due to the contraction of the polarizing film, and light leakage may occur in the peripheral area of the display area.
- the required level for preventing warpage of the panel and light leakage is increasing.
- the present invention can improve the durability of the liquid crystal panel as a whole in a liquid crystal panel including a liquid crystal cell having a transparent conductive layer on one surface, and can further suppress panel warpage and light leakage.
- An object is to provide a liquid crystal panel.
- Another object of the present invention is to provide an image display device using the liquid crystal panel.
- a transparent conductive layer is formed on one surface of the liquid crystal cell, and the first polarization is formed on the transparent conductive layer via the first pressure-sensitive adhesive layer formed from the pressure-sensitive adhesive composition (A1).
- a liquid crystal panel in which a film is bonded and a second polarizing film is bonded to the other surface of the liquid crystal cell via a second pressure-sensitive adhesive layer formed from the pressure-sensitive adhesive composition (A2),
- the pressure-sensitive adhesive composition (A1) includes a (meth) acrylic polymer (a1) and an epoxy group-containing silane coupling agent
- the pressure-sensitive adhesive composition (A2) includes a (meth) acrylic polymer (a2) and a thiol group-containing silane coupling agent
- the liquid crystal panel is characterized in that the gel fraction of the first pressure-sensitive adhesive layer and the second pressure-sensitive adhesive layer is 65% or more and 90% or less and further satisfies the following formula (1).
- the epoxy group-containing silane coupling agent is preferably an oligomer type epoxy group-containing silane coupling agent having two or more alkoxysilyl groups in the molecule.
- the thiol group-containing silane coupling agent is preferably an oligomer type thiol group-containing silane coupling agent having two or more alkoxysilyl groups in the molecule.
- the compounding amount of the epoxy group-containing silane coupling agent is preferably 0.001 to 5 parts by weight with respect to 100 parts by weight of the (meth) acrylic polymer (a1).
- the blending amount of the thiol group-containing silane coupling agent is preferably 0.001 to 5 parts by weight with respect to 100 parts by weight of the (meth) acrylic polymer (a2).
- the (meth) acrylic polymer (a1) and / or (a2) preferably contains 0.01 to 2% by weight of a carboxyl group-containing monomer as a monomer unit.
- the (meth) acrylic polymer (a1) and / or (a2) preferably contains 0.1 to 8% by weight of an amide group-containing monomer as a monomer unit.
- the (meth) acrylic polymer (a1) and / or (a2) preferably contains 0.01 to 7% by weight of a hydroxyl group-containing monomer as a monomer unit.
- the pressure-sensitive adhesive composition (A1) and / or (A2) contains at least one crosslinking agent selected from the group consisting of an isocyanate crosslinking agent and a peroxide crosslinking agent.
- the present invention also relates to an image display device using the liquid crystal panel.
- the first polarizing film is disposed on the transparent conductive layer surface on one side of the liquid crystal cell via the first pressure-sensitive adhesive layer formed from the specific pressure-sensitive adhesive composition (A1), and the other side of the liquid crystal cell.
- the second polarizing film is disposed on the surface of the first polarizing layer via the second pressure-sensitive adhesive layer formed from the specific pressure-sensitive adhesive composition (A2), and the gel fraction of the first pressure-sensitive adhesive layer and the second pressure-sensitive adhesive layer
- a transparent conductive layer is formed on one surface of a liquid crystal cell, and a first pressure-sensitive adhesive layer formed from the pressure-sensitive adhesive composition (A1) is formed on the transparent conductive layer.
- the pressure-sensitive adhesive composition (A1) includes a (meth) acrylic polymer (a1) and an epoxy group-containing silane coupling agent
- the pressure-sensitive adhesive composition (A2) includes a (meth) acrylic polymer (a2) and a thiol group-containing silane coupling agent
- the gel fraction of the first pressure-sensitive adhesive layer and the second pressure-sensitive adhesive layer is 65% or more and 90% or less, and further satisfies the following formula (1).
- Adhesive composition (A1) The pressure-sensitive adhesive composition (A1) used in the present invention contains a (meth) acrylic polymer (a1) and an epoxy group-containing silane coupling agent, and contains a (meth) acrylic polymer (a1) as a main component. It is preferable.
- a main component refers to a component with the largest content ratio among the total solids contained in the pressure-sensitive adhesive composition (A1), for example, 50 of the total solids contained in the pressure-sensitive adhesive composition (A1). It is a component that occupies more than% by weight, and further refers to a component that occupies more than 70% by weight.
- the (meth) acrylic polymer (a1) usually contains alkyl (meth) acrylate as a main component as a monomer unit.
- (meth) acrylate refers to acrylate and / or methacrylate, and (meth) of the present invention has the same meaning.
- alkyl (meth) acrylate constituting the main skeleton of the (meth) acrylic polymer (a1) examples include linear or branched alkyl groups having 1 to 18 carbon atoms.
- the alkyl group includes methyl group, ethyl group, propyl group, isopropyl group, butyl group, isobutyl group, amyl group, hexyl group, cyclohexyl group, heptyl group, 2-ethylhexyl group, isooctyl group, nonyl group, decyl group.
- the average carbon number of these alkyl groups is preferably 3 to 9.
- the monomer constituting the (meth) acrylic polymer (a1) in addition to the alkyl (meth) acrylate, a carboxyl group-containing monomer, a hydroxyl group-containing monomer, an amide group-containing monomer, an aromatic ring-containing (meth) acrylate, and the like. Can be mentioned.
- the carboxyl group-containing monomer is a compound containing a carboxyl group in its structure and a polymerizable unsaturated double bond such as a (meth) acryloyl group or a vinyl group.
- Specific examples of the carboxyl group-containing monomer include (meth) acrylic acid, carboxyethyl (meth) acrylate, carboxypentyl (meth) acrylate, itaconic acid, maleic acid, fumaric acid, crotonic acid and the like.
- acrylic acid is preferable from the viewpoints of copolymerizability, cost, and adhesive properties.
- the hydroxyl group-containing monomer is a compound containing a hydroxyl group in its structure and a polymerizable unsaturated double bond such as a (meth) acryloyl group or a vinyl group.
- Specific examples of the hydroxyl group-containing monomer include 2-hydroxyethyl (meth) acrylate, 3-hydroxypropyl (meth) acrylate, 4-hydroxybutyl (meth) acrylate, 6-hydroxyhexyl (meth) acrylate, 8- Examples thereof include hydroxyalkyl (meth) acrylate and (4-hydroxymethylcyclohexyl) -methyl acrylate, such as hydroxyoctyl (meth) acrylate, 10-hydroxydecyl (meth) acrylate, and 12-hydroxylauryl (meth) acrylate.
- hydroxyl group-containing monomers 2-hydroxyethyl (meth) acrylate and 4-hydroxybutyl (meth) acrylate are preferable from the viewpoint of durability, and 4-hydroxybutyl (meth) acrylate is particularly preferable.
- the amide group-containing monomer is a compound containing an amide group in its structure and a polymerizable unsaturated double bond such as a (meth) acryloyl group or a vinyl group.
- Specific examples of the amide group-containing monomer include (meth) acrylamide, N, N-dimethyl (meth) acrylamide, N, N-diethyl (meth) acrylamide, N-isopropylacrylamide, N-methyl (meth) acrylamide, N- Butyl (meth) acrylamide, N-hexyl (meth) acrylamide, N-methylol (meth) acrylamide, N-methylol-N-propane (meth) acrylamide, aminomethyl (meth) acrylamide, aminoethyl (meth) acrylamide, mercaptomethyl Acrylamide monomers such as (meth) acrylamide and mercaptoethyl (meth) acrylamide; N-actyl such as N-
- the aromatic ring-containing (meth) acrylate is a compound containing an aromatic ring structure in its structure and a (meth) acryloyl group.
- the aromatic ring include a benzene ring, a naphthalene ring, and a biphenyl ring.
- the aromatic ring-containing (meth) acrylate can satisfy durability (particularly durability against the transparent conductive layer).
- aromatic ring-containing (meth) acrylate examples include, for example, benzyl (meth) acrylate, phenyl (meth) acrylate, o-phenylphenol (meth) acrylate, phenoxyethyl (meth) acrylate, phenoxypropyl (meth) acrylate, Phenoxydiethylene glycol (meth) acrylate, ethylene oxide modified nonylphenol (meth) acrylate, ethylene oxide modified cresol (meth) acrylate, phenol ethylene oxide modified (meth) acrylate, 2-hydroxy-3-phenoxypropyl (meth) acrylate, methoxybenzyl ( Having a benzene ring such as (meth) acrylate, chlorobenzyl (meth) acrylate, and polystyryl (meth) acrylate; Having a naphthalene ring such as fluorinated ⁇ -naphthol acrylate, 2-nap
- the aromatic ring-containing (meth) acrylate is preferably benzyl (meth) acrylate or phenoxyethyl (meth) acrylate, particularly preferably phenoxyethyl (meth) acrylate, from the viewpoint of adhesive properties and durability.
- the carboxyl group-containing monomer, hydroxyl group-containing monomer, amide group-containing monomer, and aromatic ring-containing (meth) acrylate serve as a reaction point with the crosslinking agent when the pressure-sensitive adhesive composition (A1) contains a crosslinking agent.
- the carboxyl group-containing monomer and the hydroxyl group-containing monomer are preferably used for improving the cohesiveness and heat resistance of the resulting pressure-sensitive adhesive layer because they are highly reactive with the intermolecular crosslinking agent.
- the (meth) acrylic polymer (a1) used in the present invention preferably contains the above monomers as monomer units in the following amounts in the weight ratio of all constituent monomers (100% by weight).
- the weight ratio of the alkyl (meth) acrylate can be set as the remainder of the monomer other than the alkyl (meth) acrylate, and specifically, it is preferably 70% by weight or more. Setting the weight ratio of the alkyl (meth) acrylate within the above range is preferable for securing adhesiveness.
- the weight ratio of the carboxyl group-containing monomer is preferably 2% by weight or less, more preferably 0.01 to 2% by weight, still more preferably 0.05 to 1.5% by weight, ⁇ 1% by weight is particularly preferred.
- the weight ratio of the carboxyl group-containing monomer is less than 0.01% by weight, the durability tends not to be satisfied.
- the transparent conductive layer may be corroded, which tends to be unsatisfactory in terms of durability, which is not preferable.
- the weight ratio of the hydroxyl group-containing monomer is preferably 0.01 to 7% by weight, more preferably 0.01 to 5% by weight, still more preferably 0.1 to 3% by weight, ⁇ 2% by weight is particularly preferred.
- the weight ratio of the hydroxyl group-containing monomer is less than 0.01% by weight, the pressure-sensitive adhesive layer is insufficiently crosslinked, and there is a tendency that durability and pressure-sensitive adhesive properties cannot be satisfied.
- it exceeds 5% by weight the durability tends not to be satisfied.
- the weight ratio of the amide group-containing monomer is preferably 8% by weight or less, more preferably 0.1 to 8% by weight, further preferably 0.3 to 5% by weight, and 0.3 to 4% by weight. % Is more preferable, and 0.7 to 2.5% by weight is particularly preferable.
- the weight ratio of the amide group-containing monomer is less than 0.1% by weight, the durability particularly with respect to the transparent conductive layer tends not to be satisfied. On the other hand, if it exceeds 8% by weight, the durability tends to decrease, which is not preferable.
- the weight ratio of the aromatic ring-containing (meth) acrylate is preferably 25% by weight or less, more preferably 0 to 22% by weight, and further preferably 0 to 18% by weight. When the weight ratio of the aromatic ring-containing (meth) acrylate exceeds 25% by weight, durability tends to decrease.
- the (meth) acrylic polymer (a1) is not required to contain other monomer units in addition to the monomer units, but for the purpose of improving adhesiveness and heat resistance, )
- One or more copolymerization monomers having a polymerizable functional group having an unsaturated double bond such as acryloyl group or vinyl group can be introduced by copolymerization.
- Such a copolymerization monomer include acid anhydride group-containing monomers such as maleic anhydride and itaconic anhydride; caprolactone adducts of acrylic acid; allyl sulfonic acid, 2- (meth) acrylamide-2-methylpropane
- acid anhydride group-containing monomers such as maleic anhydride and itaconic anhydride
- caprolactone adducts of acrylic acid such as maleic anhydride and itaconic anhydride
- caprolactone adducts of acrylic acid allyl sulfonic acid, 2- (meth) acrylamide-2-methylpropane
- examples include sulfonic acid group-containing monomers such as sulfonic acid, (meth) acrylamide propanesulfonic acid, and sulfopropyl (meth) acrylate; and phosphoric acid group-containing monomers such as 2-hydroxyethylacryloyl phosphate.
- alkylaminoalkyl (meth) acrylates such as aminoethyl (meth) acrylate, N, N-dimethylaminoethyl (meth) acrylate, and t-butylaminoethyl (meth) acrylate; methoxyethyl (meth) acrylate, ethoxyethyl ( Alkoxyalkyl (meth) acrylates such as meth) acrylate; N- (meth) acryloyloxymethylene succinimide, N- (meth) acryloyl-6-oxyhexamethylene succinimide, N- (meth) acryloyl-8-oxyoctamethylene succinimide, etc.
- Succinimide monomers N-cyclohexylmaleimide, N-isopropylmaleimide, N-laurylmaleimide, N-phenylmaleimide and other maleimide monomers; N-methylitaconimide, Examples of monomers for modification include itaconic imide monomers such as -ethyl itaconimide, N-butyl itaconimide, N-octyl itaconimide, N-2-ethylhexyl itaconimide, N-cyclohexyl leuconconimide, and N-lauryl itaconimide. Can be mentioned.
- vinyl monomers such as vinyl acetate and vinyl propionate; cyanoacrylate monomers such as acrylonitrile and methacrylonitrile; epoxy group-containing (meth) acrylates such as glycidyl (meth) acrylate; polyethylene glycol (meth) Glycol-based (meth) acrylates such as acrylate, polypropylene glycol (meth) acrylate, methoxyethylene glycol (meth) acrylate, methoxypolypropylene glycol (meth) acrylate; tetrahydrofurfuryl (meth) acrylate, fluorine (meth) acrylate, silicone (meta (Meth) acrylate monomers such as acrylate and 2-methoxyethyl acrylate can also be used.
- isoprene, butadiene, isobutylene, vinyl ether and the like can be mentioned.
- examples of copolymerizable monomers other than the above include silane-based monomers containing silicon atoms.
- examples of the silane monomer include 3-acryloxypropyltriethoxysilane, vinyltrimethoxysilane, vinyltriethoxysilane, 4-vinylbutyltrimethoxysilane, 4-vinylbutyltriethoxysilane, and 8-vinyloctyltrimethoxysilane.
- copolymer monomers examples include tripropylene glycol di (meth) acrylate, tetraethylene glycol di (meth) acrylate, 1,6-hexanediol di (meth) acrylate, bisphenol A diglycidyl ether di (meth) acrylate, neo Pentyl glycol di (meth) acrylate, trimethylolpropane tri (meth) acrylate, pentaerythritol tri (meth) acrylate, pentaerythritol tetra (meth) acrylate, dipentaerythritol penta (meth) acrylate, dipentaerythritol hexa (meth) acrylate (Meth) acryloyl such as esterified product of (meth) acrylic acid and polyhydric alcohol such as caprolactone-modified dipentaerythritol hexa (meth) acrylate Groups such as polyfunctional
- polyester (meth) acrylate, epoxy (meth) acrylate, urethane (meth) acrylate, or the like to which two or more saturated double bonds have been added can also be used.
- the ratio of the copolymerization monomer in the (meth) acrylic polymer (a1) is about 0 to 10% by weight in the weight ratio of all the constituent monomers (100% by weight) of the (meth) acrylic polymer (a1). It is preferably about 0 to 7% by weight, more preferably about 0 to 5% by weight.
- the (meth) acrylic polymer (a1) of the present invention usually has a weight average molecular weight of 1 million to 2.5 million. Considering durability, particularly heat resistance, the weight average molecular weight is preferably 1.2 million to 2 million. When the weight average molecular weight is less than 1,000,000, it is not preferable from the viewpoint of heat resistance. On the other hand, when the weight average molecular weight is larger than 2.5 million, the pressure-sensitive adhesive tends to be hard and peeling is likely to occur. Further, the weight average molecular weight (Mw) / number average molecular weight (Mn) indicating the molecular weight distribution is preferably 1.8 or more and 10 or less, more preferably 1.8 to 7, more preferably 1.8 to More preferably, it is 5.
- the weight average molecular weight and molecular weight distribution (Mw / Mn) are determined by GPC (gel permeation chromatography) and calculated from polystyrene.
- the (meth) acrylic polymer (a1) For the production of such a (meth) acrylic polymer (a1), known production methods such as solution polymerization, bulk polymerization, emulsion polymerization and various radical polymerizations can be appropriately selected. Further, the (meth) acrylic polymer (a1) obtained may be any of a random copolymer, a block copolymer, a graft copolymer, and the like.
- solution polymerization for example, ethyl acetate, toluene or the like is used as a polymerization solvent.
- the reaction is carried out in an inert gas stream such as nitrogen and a polymerization initiator is added, and the reaction is usually performed at about 50 to 70 ° C. under reaction conditions for about 5 to 30 hours.
- the polymerization initiator, chain transfer agent, emulsifier and the like used for radical polymerization are not particularly limited and can be appropriately selected and used.
- the weight average molecular weight of the (meth) acrylic polymer (a1) can be controlled by the amount of polymerization initiator, the amount of chain transfer agent used, and the reaction conditions, and the amount used is appropriately adjusted according to these types.
- polymerization initiator examples include 2,2′-azobisisobutyronitrile, 2,2′-azobis (2-amidinopropane) dihydrochloride, 2,2′-azobis [2- (5-methyl-2 -Imidazolin-2-yl) propane] dihydrochloride, 2,2'-azobis (2-methylpropionamidine) disulfate, 2,2'-azobis (N, N'-dimethyleneisobutylamidine), 2,2 Azo initiators such as' -azobis [N- (2-carboxyethyl) -2-methylpropionamidine] hydrate (trade name: VA-057, manufactured by Wako Pure Chemical Industries, Ltd.), potassium persulfate, Persulfates such as ammonium sulfate, di (2-ethylhexyl) peroxydicarbonate, di (4-tert-butylcyclohexyl) peroxydicarbonate, di- ec-butyl peroxydicarbon
- the polymerization initiator may be used alone or in combination of two or more, but the total content is 0.005 with respect to 100 parts by weight of the total amount of monomer components. It is preferably about 1 to 1 part by weight, more preferably about 0.02 to 0.5 part by weight.
- the (meth) acrylic polymer (a1) having the weight average molecular weight using, for example, 2,2′-azobisisobutyronitrile as the polymerization initiator, use of the polymerization initiator is used.
- the amount is preferably about 0.06 to 0.2 parts by weight, and more preferably about 0.08 to 0.175 parts by weight with respect to 100 parts by weight of the total amount of monomer components.
- the pressure-sensitive adhesive composition (A1) includes an epoxy group-containing silane coupling agent.
- an epoxy group-containing silane coupling agent included in the pressure-sensitive adhesive composition (A1), it is possible to improve the durability of the liquid crystal panel as a whole, and to suppress panel warpage and light leakage.
- epoxy group-containing silane coupling agent examples include 3-glycidoxypropyltrimethoxysilane, 3-glycidoxypropyltriethoxysilane, 3-glycidoxypropylmethyldiethoxysilane, 2- (3,4 -Epoxycyclohexyl) ethyltrimethoxysilane and the like.
- an oligomer type epoxy group-containing silane coupling agent having two or more alkoxysilyl groups in the molecule as the epoxy group-containing silane coupling agent.
- Specific examples include X-41-1053, X-41-1059A, and X-41-1056 manufactured by Shin-Etsu Chemical Co., Ltd. These coupling agents are preferable because they are difficult to volatilize and are effective in improving durability because they have a plurality of alkoxysilyl groups.
- the oligomer type refers to a polymer of monomer dimer or more and less than about 100 mer, and the weight average molecular weight of the oligomer type silane coupling agent is preferably about 300 to 30,000.
- the number of alkoxysilyl groups in the oligomer type epoxy group-containing silane coupling agent may be two or more in the molecule, and the number is not limited.
- the amount of alkoxy groups in the oligomer type epoxy group-containing silane coupling agent is preferably 10 to 60% by weight, more preferably 15 to 50% by weight in the silane coupling agent, More preferably, it is ⁇ 40% by weight.
- alkoxy group is not limited, and examples thereof include alkoxy groups having 1 to 6 carbon atoms such as methoxy, ethoxy, propoxy, butoxy, pentyloxy, hexyloxy and the like. Among these, methoxy and ethoxy are preferable, and methoxy is more preferable. It is also preferred that both methoxy and ethoxy are contained in one molecule.
- the epoxy equivalent of the epoxy group-containing silane coupling agent is preferably 1000 g / mol or less, more preferably 500 g / mol or less, and more preferably 300 g / mol or less.
- the lower limit of an epoxy equivalent is not specifically limited, For example, it is preferable that it is 200 g / mol or more.
- the epoxy group-containing silane coupling agent may be used alone or in combination of two or more, but the total content is the (meth) acrylic polymer (a1) 100.
- the amount is preferably 0.001 to 5 parts by weight, more preferably 0.01 to 3 parts by weight, still more preferably 0.02 to 2 parts by weight, and particularly preferably 0.05 to 1 part by weight with respect to parts by weight.
- silane coupling agents other than the said epoxy group containing silane coupling agent can also be added to the adhesive composition (A1) used by this invention.
- Other coupling agents include 3-aminopropyltrimethoxysilane, N-2- (aminoethyl) -3-aminopropylmethyldimethoxysilane, 3-triethoxysilyl-N- (1,3-dimethylbutylidene)
- Amino group-containing silane coupling agents such as propylamine and N-phenyl- ⁇ -aminopropyltrimethoxysilane, (meth) acryl group-containing silanes such as 3-acryloxypropyltrimethoxysilane and 3-methacryloxypropyltriethoxysilane
- Examples thereof include coupling agents and isocyanate group-containing silane coupling agents such as 3-isocyanatopropyltriethoxysilane.
- the silane coupling agent other than the epoxy group-containing silane coupling agent can be added within a range that does not impair the effects of the present invention, and the addition amount is not particularly limited.
- the pressure-sensitive adhesive composition (A1) used in the present invention preferably contains a crosslinking agent.
- a crosslinking agent an organic crosslinking agent or a polyfunctional metal chelate can be used.
- the organic crosslinking agent include an isocyanate crosslinking agent, a peroxide crosslinking agent, an epoxy crosslinking agent, and an imine crosslinking agent.
- a polyfunctional metal chelate is one in which a polyvalent metal is covalently or coordinately bonded to an organic compound.
- polyvalent metal atoms include Al, Cr, Zr, Co, Cu, Fe, Ni, V, Zn, In, Ca, Mg, Mn, Y, Ce, Sr, Ba, Mo, La, Sn, Ti, and the like.
- Examples of the atom in the organic compound to be covalently bonded or coordinated include an oxygen atom, and examples of the organic compound include alkyl esters, alcohol compounds, carboxylic acid compounds, ether compounds, and ketone compounds.
- crosslinking agent an isocyanate-based crosslinking agent and / or a peroxide-based crosslinking agent is preferable.
- isocyanate crosslinking agent a compound having at least two isocyanate groups can be used.
- known aliphatic polyisocyanate, alicyclic polyisocyanate, aromatic polyisocyanate and the like generally used for urethanization reaction are used.
- aliphatic polyisocyanate examples include trimethylene diisocyanate, tetramethylene diisocyanate, hexamethylene diisocyanate, pentamethylene diisocyanate, 1,2-propylene diisocyanate, 1,3-butylene diisocyanate, dodecamethylene diisocyanate, 2,4,4-trimethyl. And hexamethylene diisocyanate.
- Examples of the alicyclic isocyanate include 1,3-cyclopentene diisocyanate, 1,3-cyclohexane diisocyanate, 1,4-cyclohexane diisocyanate, isophorone diisocyanate, hydrogenated diphenylmethane diisocyanate, hydrogenated xylylene diisocyanate, and hydrogenated tolylene diisocyanate hydrogen. Examples include added tetramethylxylylene diisocyanate.
- aromatic diisocyanate for example, phenylene diisocyanate, 2,4-tolylene diisocyanate, 2,6-tolylene diisocyanate, 2,2′-diphenylmethane diisocyanate, 4,4′-diphenylmethane diisocyanate, 4,4 Examples include '-toluidine diisocyanate, 4,4'-diphenyl ether diisocyanate, 4,4'-diphenyl diisocyanate, 1,5-naphthalene diisocyanate, and xylylene diisocyanate.
- isocyanate-based crosslinking agent examples include the above-mentioned diisocyanate multimers (dimers, trimers, pentamers, etc.), urethane-modified products reacted with polyhydric alcohols such as trimethylolpropane, urea-modified products, and biurets.
- examples include modified products, alphanate modified products, isocyanurate modified products, and carbodiimide modified products.
- isocyanate-based crosslinking agents include, for example, trade names “Millionate MT”, “Millionate MTL”, “Millionate MR-200”, “Millionate MR-400”, “Coronate L” manufactured by Nippon Polyurethane Industry Co., Ltd. ”,“ Coronate HL ”,“ Coronate HX ”, trade names“ Takenate D-110N ”,“ Takenate D-120N ”,“ Takenate D-140N ”,“ Takenate D-160N ”, manufactured by Mitsui Chemicals, Inc. “Takenate D-165N”, “Takenate D-170HN”, “Takenate D-178N”, “Takenate 500”, “Takenate 600” and the like. These compounds may be used alone or in combination of two or more.
- an aliphatic polyisocyanate and an aliphatic polyisocyanate-based compound which is a modified product thereof are preferable.
- Aliphatic polyisocyanate compounds are more flexible in cross-linking structures than other isocyanate cross-linking agents, tend to relieve stress associated with the expansion / contraction of optical films, and do not easily peel off in durability tests.
- the aliphatic polyisocyanate compound hexamethylene diisocyanate and modified products thereof are particularly preferable.
- Any peroxide can be used as long as it generates radical active species by heating or light irradiation to promote crosslinking of the base polymer ((meth) acrylic polymer) of the pressure-sensitive adhesive composition.
- a peroxide having a half-life temperature of 80 ° C. to 160 ° C. for 1 minute it is preferable to use a peroxide having a temperature of 90 ° C. to 140 ° C.
- peroxides examples include di (2-ethylhexyl) peroxydicarbonate (1 minute half-life temperature: 90.6 ° C.), di (4-tert-butylcyclohexyl) peroxydicarbonate (1 Minute half-life temperature: 92.1 ° C.), di-sec-butyl peroxydicarbonate (1 minute half-life temperature: 92.4 ° C.), t-butyl peroxyneodecanoate (1 minute half-life temperature: 103 0.5 ° C.), t-hexyl peroxypivalate (1 minute half-life temperature: 109.1 ° C.), t-butyl peroxypivalate (1 minute half-life temperature: 110.3 ° C.), dilauroyl peroxide ( 1 minute half-life temperature: 116.4 ° C.), di-n-octanoyl peroxide (1 minute half-life temperature: 117.4 ° C.), 1,1,3,3-tetramethylbuty
- di (4-t-butylcyclohexyl) peroxydicarbonate (1 minute half-life temperature: 92.1 ° C.)
- dilauroyl peroxide (1 minute half-life temperature: 116. 4 ° C.
- dibenzoyl peroxide (1 minute half-life temperature: 130.0 ° C.) and the like are preferably used.
- the peroxide half-life is an index representing the decomposition rate of the peroxide, and means the time until the remaining amount of peroxide is reduced to half.
- the decomposition temperature for obtaining a half-life at an arbitrary time and the half-life time at an arbitrary temperature are described in the manufacturer catalog, for example, “Organic Peroxide Catalog No. 9 of Nippon Oil & Fats Co., Ltd.” Edition (May 2003) ".
- the amount of the crosslinking agent used is preferably 0.01 to 2 parts by weight, more preferably 0.02 to 1 part by weight, and more preferably 0.03 to 0.03 parts by weight per 100 parts by weight of the (meth) acrylic polymer (a1). 5 parts by weight is more preferable. If the cross-linking agent is less than 0.01 parts by weight, the pressure-sensitive adhesive layer may be insufficiently cross-linked and the durability and adhesive properties may not be satisfied. On the other hand, if it exceeds 2 parts by weight, the pressure-sensitive adhesive layer becomes too hard. The durability tends to decrease.
- the isocyanate-based crosslinking agent may be used singly or in combination of two or more, but the total content is the (meth) acrylic polymer (a1) 100
- the content is preferably 0.01 to 2 parts by weight, more preferably 0.02 to 1 part by weight, and more preferably 0.03 to 0.5 parts by weight with respect to parts by weight. Is more preferable. It can be appropriately contained in consideration of cohesive force, prevention of peeling in a durability test, and the like.
- the peroxide may be used alone or as a mixture of two or more, but the total content is 100 weight of the (meth) acrylic polymer (a1).
- the content is preferably 0.01 to 2 parts by weight, more preferably 0.04 to 1.5 parts by weight, and still more preferably 0.05 to 1 part by weight. .
- processability, reworkability, cross-linking stability, releasability, etc. it is appropriately selected within this range.
- the pressure-sensitive adhesive composition (A1) used in the present invention can further contain an ionic compound.
- an ionic compound those that can be usually used in this field can be suitably used, and the addition amount can be appropriately determined within a range not impairing the effects of the present invention.
- a polyether compound having a reactive silyl group can be blended with the pressure-sensitive adhesive composition (A1) used in the present invention.
- Polyether compounds are preferred because they can improve reworkability.
- the polyether compound for example, those disclosed in JP 2010-275522 A can be used.
- the pressure-sensitive adhesive composition (A1) used in the present invention may contain other known additives, for example, a polyalkylene glycol polyether compound such as polypropylene glycol, a colorant, a powder such as a pigment.
- a polyalkylene glycol polyether compound such as polypropylene glycol
- a colorant such as a pigment.
- the filler, metal powder, particles, foil and the like can be appropriately added depending on the use.
- These additives are preferably used in an amount of 5 parts by weight or less, further 3 parts by weight or less, and further 1 part by weight or less with respect to 100 parts by weight of the (meth) acrylic polymer (a1).
- Adhesive composition (A2) The pressure-sensitive adhesive composition (A2) used in the present invention contains a (meth) acrylic polymer (a2) and a thiol group-containing silane coupling agent, and contains a (meth) acrylic polymer (a2) as a main component. It is preferable.
- the main component is as described above.
- the pressure-sensitive adhesive composition (A2) includes a thiol group-containing silane coupling agent.
- a thiol group-containing silane coupling agent included in the pressure-sensitive adhesive composition (A2), it is possible to improve the durability of the liquid crystal panel as a whole, and to suppress panel warpage and light leakage.
- Examples of the thiol group-containing silane coupling agent include 3-mercaptopropyltrimethoxysilane, 3-mercaptopropylmethyldimethoxysilane, 3-mercaptopropyltriethoxysilane, 3-mercaptopropylmethyldiethoxysilane, and ⁇ -mercaptomethylphenylethyl.
- Examples include compounds having a mercapto group such as trimethoxysilane, mercaptomethyltrimethoxysilane, 6-mercaptohexyltrimethoxysilane, and 10-mercaptodecyltrimethoxysilane.
- an oligomer type thiol group-containing silane coupling agent having two or more alkoxysilyl groups in the molecule is preferable.
- Specific examples include X-41-1805, X-41-1818, and X-41-1810 manufactured by Shin-Etsu Chemical Co., Ltd. These thiol group-containing silane coupling agents are preferred because they are less likely to volatilize and are effective in improving durability because they have a plurality of alkoxysilyl groups.
- the oligomer type refers to a polymer of monomer dimer or more and less than about 100 mer, and the weight average molecular weight of the oligomer type silane coupling agent is preferably about 300 to 30,000.
- the number of alkoxysilyl groups in the oligomer type thiol group-containing silane coupling agent may be two or more in the molecule, and the number is not limited.
- the amount of the alkoxy group in the oligomer type thiol group-containing silane coupling agent is preferably 10 to 60% by weight, more preferably 20 to 50% by weight, more preferably 20 to 40% in the silane coupling agent. More preferably, it is% by weight.
- alkoxy group is not limited, and examples thereof include alkoxy groups having 1 to 6 carbon atoms such as methoxy, ethoxy, propoxy, butoxy, pentyloxy, hexyloxy and the like. Among these, methoxy and ethoxy are preferable, and methoxy is more preferable. It is also preferred that both methoxy and ethoxy are contained in one molecule.
- the content of the thiol group in the thiol group-containing silane coupling agent is preferably 1000 g / mol or less, more preferably 800 g / mol or less, and more preferably 500 g / mol or less. It is more preferable that Moreover, the lower limit of the mercapto equivalent is not particularly limited, but for example, it is preferably 200 g / mol or more.
- the thiol group-containing silane coupling agent may be used alone or in combination of two or more, but the total content is the (meth) acrylic polymer (a2) 100.
- the amount is preferably 0.001 to 5 parts by weight, more preferably 0.01 to 3 parts by weight, still more preferably 0.02 to 2 parts by weight, and particularly preferably 0.05 to 1 part by weight with respect to parts by weight.
- a silane coupling agent other than the thiol group-containing silane coupling agent can be added to the pressure-sensitive adhesive composition (A2) used in the present invention.
- Other coupling agents include 3-aminopropyltrimethoxysilane, N-2- (aminoethyl) -3-aminopropylmethyldimethoxysilane, 3-triethoxysilyl-N- (1,3-dimethylbutylidene)
- Amino group-containing silane coupling agents such as propylamine and N-phenyl- ⁇ -aminopropyltrimethoxysilane, (meth) acryl group-containing silanes such as 3-acryloxypropyltrimethoxysilane and 3-methacryloxypropyltriethoxysilane
- Examples thereof include coupling agents and isocyanate group-containing silane coupling agents such as 3-isocyanatopropyltriethoxysilane.
- the silane coupling agent other than the thiol group-containing silane coupling agent can be added within a range that does not impair the effects of the present invention, and the addition amount is not particularly limited.
- a (meth) acrylic polymer having any composition exemplified for the (meth) acrylic polymer (a1) used in the pressure-sensitive adhesive composition (A1) can be suitably used.
- a crosslinking agent, a polyether compound, other additives, etc. can be added to an adhesive composition (A2) similarly to an adhesive composition (A1), about the composition and addition amount, This is the same as the pressure-sensitive adhesive composition (A1).
- the composition and addition amount of the (meth) acrylic polymer may be the same or different, and the crosslinking agent, the polyether compound, and others
- the type and amount of additive may be the same or different.
- Pressure-sensitive adhesive layer First and second pressure-sensitive adhesive layers are formed from the pressure-sensitive adhesive compositions (A1) and (A2).
- the gel fraction of the first pressure-sensitive adhesive layer formed from the pressure-sensitive adhesive composition (A1) is 65 to 90%, preferably 70 to 85%.
- the pressure-sensitive adhesive layer is hard.
- the pressure-sensitive adhesive layer is too hard, it is not preferable from the viewpoint of durability when bonded to the display cell.
- the gel fraction of the first pressure-sensitive adhesive layer is 65% or more, light leakage can be suppressed, and when the gel fraction is 90% or less, It is preferable because of its excellent durability.
- the gel fraction of the second pressure-sensitive adhesive layer formed from the pressure-sensitive adhesive composition (A2) is also 65 to 90%, preferably 70 to 85%, for the same reason as the first pressure-sensitive adhesive layer. .
- the gel fraction of the second pressure-sensitive adhesive layer is 65% or more, light leakage can be suppressed.
- it is excellent in durability with respect to glass when a gel fraction is 90% or less, it is preferable.
- the gel fraction of a said 1st adhesive layer and a 2nd adhesive layer is following formula (1): It satisfies.
- the first adhesive layer formed on the transparent conductive layer on one surface of the liquid crystal cell and the second adhesive layer formed on the other surface of the liquid crystal cell can suppress the warpage of the panel when the hardness is closer And the curvature of a panel can be suppressed because a gel fraction satisfy
- the range is preferably 0.1 or less, and more preferably 0.05 or less.
- first and second pressure-sensitive adhesive layers it is preferable to fully consider the influence of the crosslinking treatment temperature and the crosslinking treatment time as well as adjusting the addition amount of the entire crosslinking agent.
- the crosslinking treatment temperature and crosslinking treatment time can be adjusted depending on the crosslinking agent used.
- the crosslinking treatment temperature is preferably 170 ° C. or lower.
- this crosslinking process may be performed at the temperature at the time of the drying process of an adhesive layer, and you may carry out by providing a crosslinking process process separately after a drying process.
- the crosslinking treatment time can be set in consideration of productivity and workability, but is usually about 0.2 to 20 minutes, preferably about 0.5 to 10 minutes.
- the method for forming the pressure-sensitive adhesive layer is not particularly limited, but the pressure-sensitive adhesive composition is applied on various substrates, dried with a dryer such as a heat oven to volatilize the solvent, etc.
- the pressure-sensitive adhesive layer may be formed by performing a crosslinking treatment, and the pressure-sensitive adhesive layer may be transferred onto a polarizing film or a liquid crystal cell substrate, which will be described later, or directly on the polarizing film or the liquid crystal cell.
- the composition may be applied to form an adhesive layer.
- the method of producing the polarizing film with an adhesive layer which formed the adhesive layer on the polarizing film previously, and sticking the said polarizing film with an adhesive layer to a liquid crystal cell is preferable.
- the substrate is not particularly limited, and examples thereof include various substrates such as a release film and a transparent resin film substrate.
- Various methods are used as a method of applying the pressure-sensitive adhesive composition to the substrate or polarizing film.
- fountain coater roll coat, kiss roll coat, gravure coat, reverse coat, roll brush, spray coat, dip roll coat, bar coat, knife coat, air knife coat, curtain coat, lip coat, Daiko
- the method include an extrusion coating method using a catalyst.
- the drying conditions are not particularly limited and can be appropriately set depending on the composition, concentration, etc. of the pressure-sensitive adhesive composition, and are, for example, about 80 to 170 ° C., preferably 90 to 200 ° C. 1 to 60 minutes, preferably 2 to 30 minutes.
- a crosslinking treatment can be performed as necessary, and the conditions are as described above.
- the thickness of the pressure-sensitive adhesive layer (after drying) is, for example, preferably 5 to 100 ⁇ m, more preferably 7 to 70 ⁇ m, and even more preferably 10 to 50 ⁇ m.
- the thickness of the pressure-sensitive adhesive layer is less than 5 ⁇ m, the adhesion to the adherend becomes poor, and the durability under high temperature and high temperature and humidity tends to be insufficient.
- the thickness of the pressure-sensitive adhesive layer exceeds 100 ⁇ m, the pressure-sensitive adhesive composition is not sufficiently dried during the application and drying of the pressure-sensitive adhesive layer, and bubbles remain or the surface of the pressure-sensitive adhesive layer. There is a tendency that unevenness in thickness occurs and problems in appearance tend to become apparent.
- constituent material of the release film examples include resin films such as polyethylene, polypropylene, polyethylene terephthalate, and polyester films, porous materials such as paper, cloth, and nonwoven fabric, nets, foam sheets, metal foils, and laminates thereof.
- resin films such as polyethylene, polypropylene, polyethylene terephthalate, and polyester films
- porous materials such as paper, cloth, and nonwoven fabric, nets, foam sheets, metal foils, and laminates thereof.
- An appropriate thin leaf body can be used, but a resin film is preferably used from the viewpoint of excellent surface smoothness.
- the resin film examples include polyethylene film, polypropylene film, polybutene film, polybutadiene film, polymethylpentene film, polyvinyl chloride film, vinyl chloride copolymer film, polyethylene terephthalate film, polybutylene terephthalate film, polyurethane film, ethylene- Examples thereof include a vinyl acetate copolymer film.
- the thickness of the release film is usually 5 to 200 ⁇ m, preferably about 5 to 100 ⁇ m.
- release agent and antifouling treatment with a silicone-based, fluorine-based, long-chain alkyl-based or fatty acid amide-based release agent, silica powder, etc., coating type, kneading type, An antistatic treatment such as a vapor deposition type can also be performed.
- the release property from the pressure-sensitive adhesive layer can be further improved by appropriately performing a release treatment such as silicone treatment, long-chain alkyl treatment, or fluorine treatment on the surface of the release film.
- the transparent resin film substrate is not particularly limited, and various resin films having transparency are used.
- the resin film is formed of a single layer film.
- the materials include polyester resins such as polyethylene terephthalate and polyethylene naphthalate, acetate resins, polyethersulfone resins, polycarbonate resins, polyamide resins, polyimide resins, polyolefin resins, (meth) acrylic resins.
- polyester resins, polyimide resins and polyethersulfone resins are particularly preferable.
- the thickness of the film substrate is preferably 15 to 200 ⁇ m.
- an anchor layer may be provided between the polarizing film and the pressure-sensitive adhesive layer.
- the material for forming the anchor layer is not particularly limited, and examples thereof include various polymers, metal oxide sols, and silica sols. Among these, polymers are particularly preferably used.
- the polymer may be used in any of a solvent-soluble type, a water-dispersed type, and a water-soluble type.
- polymers examples include polyurethane resins, polyester resins, acrylic resins, polyether resins, cellulose resins, polyvinyl alcohol resins, polyvinyl pyrrolidone, polystyrene resins, and the like.
- polyurethane resins, polyester resins, and acrylic resins are particularly preferable. These resins can be appropriately mixed with a crosslinking agent. These other binder components can be used singly or in combination of two or more as appropriate.
- the thickness of the anchor layer is not particularly limited, but is preferably 5 to 300 nm.
- the method for forming the anchor layer is not particularly limited and can be performed by a generally known method.
- the iodine polarizing film can be activated.
- Various methods can be employed for the activation treatment, such as corona treatment, low-pressure UV treatment, plasma treatment, and the like.
- the method for forming the pressure-sensitive adhesive layer on the anchor layer on the polarizing film is as described above.
- the pressure-sensitive adhesive layer of the polarizing film with the pressure-sensitive adhesive layer and the pressure-sensitive adhesive layer on the liquid crystal cell are exposed, the pressure-sensitive adhesive layer may be protected with a release film (separator) until practical use. .
- a release film is used as a base material in the production of the above-mentioned pressure-sensitive adhesive layer, the release film is bonded to the pressure-sensitive adhesive layer on the release film and a polarizing film or a liquid crystal cell. It can be used as a release film for a pressure-sensitive adhesive layer of a polarizing film with a layer or a liquid crystal cell with a pressure-sensitive adhesive layer, and the process can be simplified.
- first polarizing film and the second polarizing film are not particularly limited, but those having a transparent protective film on one side or both sides of the polarizer are generally used.
- the polarizer is not particularly limited, and various types can be used.
- polarizers include dichroic iodine and dichroic dyes on hydrophilic polymer films such as polyvinyl alcohol films, partially formalized polyvinyl alcohol films, and ethylene / vinyl acetate copolymer partially saponified films.
- hydrophilic polymer films such as polyvinyl alcohol films, partially formalized polyvinyl alcohol films, and ethylene / vinyl acetate copolymer partially saponified films.
- polyene-based oriented films such as those obtained by adsorbing substances and uniaxially stretched, polyvinyl alcohol dehydrated products and polyvinyl chloride dehydrochlorinated products.
- a polarizer composed of a polyvinyl alcohol film and a dichroic substance such as iodine is preferable, and an iodine polarizer containing iodine and / or iodine ions is more preferable.
- the thickness of these polarizers is not particularly limited, but is generally about 5 to 80 ⁇ m.
- a polarizer obtained by dyeing a polyvinyl alcohol film with iodine and uniaxially stretching it can be produced, for example, by dyeing polyvinyl alcohol in an aqueous iodine solution and stretching it 3 to 7 times the original length. If necessary, it can be immersed in an aqueous solution such as potassium iodide which may contain boric acid, zinc sulfate, zinc chloride or the like. Further, if necessary, the polyvinyl alcohol film may be immersed in water and washed before dyeing.
- Stretching may be performed after dyeing with iodine, may be performed while dyeing, or may be dyed with iodine after stretching.
- the film can be stretched even in an aqueous solution such as boric acid or potassium iodide or in a water bath.
- thermoplastic resins excellent in transparency, mechanical strength, thermal stability, moisture barrier property, isotropy, and the like are used.
- thermoplastic resins include cellulose resins such as triacetyl cellulose, polyester resins, polyethersulfone resins, polysulfone resins, polycarbonate resins, polyamide resins, polyimide resins, polyolefin resins, (meth) acrylic resins, cyclic Examples thereof include polyolefin resins (norbornene resins), polyarylate resins, polystyrene resins, polyvinyl alcohol resins, and mixtures thereof.
- a transparent protective film is bonded to one side of the polarizer by an adhesive layer.
- a transparent protective film (meth) acrylic, urethane-based, acrylurethane-based, epoxy-based, silicone
- a thermosetting resin such as a system or an ultraviolet curable resin can be used.
- One or more kinds of arbitrary appropriate additives may be contained in the transparent protective film. Examples of the additive include an ultraviolet absorber, an antioxidant, a lubricant, a plasticizer, a mold release agent, a coloring inhibitor, a flame retardant, a nucleating agent, an antistatic agent, a pigment, and a coloring agent.
- the content of the thermoplastic resin in the transparent protective film is preferably 50 to 100% by weight, more preferably 50 to 99% by weight, still more preferably 60 to 98% by weight, and particularly preferably 70 to 97% by weight. .
- content of the said thermoplastic resin in a transparent protective film is 50 weight% or less, there exists a possibility that the high transparency etc. which a thermoplastic resin originally has cannot fully be expressed.
- the thickness of the protective film can be determined as appropriate, but is generally about 1 to 500 ⁇ m from the viewpoints of workability such as strength and handleability, and thin film properties.
- the polarizer and the protective film are usually in close contact with each other through an aqueous adhesive or the like.
- the water-based adhesive include an isocyanate-based adhesive, a polyvinyl alcohol-based adhesive, a gelatin-based adhesive, a vinyl-based latex, a water-based polyurethane, and a water-based polyester.
- examples of the adhesive between the polarizer and the transparent protective film include an ultraviolet curable adhesive and an electron beam curable adhesive.
- the electron beam curable polarizing film adhesive exhibits suitable adhesiveness to the various transparent protective films.
- the adhesive used in the present invention can contain a metal compound filler.
- a retardation film or the like can be formed on the polarizer instead of the transparent protective film of the polarizing film. Further, another transparent protective film or a retardation film can be provided on the transparent protective film.
- the surface of the transparent protective film that does not adhere to the opposite side (viewing side) of the liquid crystal cell may be subjected to a hard coat layer, antireflection treatment, anti-sticking treatment, or treatment for diffusion or anti-glare. good.
- a transparent conductive layer is formed on one surface of a liquid crystal cell, and the adhesive formed from the pressure-sensitive adhesive composition (A1) on the transparent conductive layer.
- the first polarizing film is bonded through the adhesive layer, and the second polarizing film is bonded to the other surface of the liquid crystal cell through the adhesive layer formed from the adhesive composition (A2).
- the pressure-sensitive adhesive composition (A1) includes a (meth) acrylic polymer (a1) and an epoxy group-containing silane coupling agent
- the pressure-sensitive adhesive composition (B2) includes a (meth) acrylic polymer (a2) and a thiol group-containing silane coupling agent, and controls the gel fraction of the first pressure-sensitive adhesive layer and the second pressure-sensitive adhesive layer.
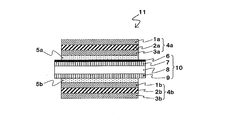
- the other configurations are not particularly limited. One embodiment of a specific configuration of the liquid crystal panel of the present invention will be described with reference to FIG. 1, but the present invention is not limited to this.
- the mode of the liquid crystal panel 11 of the present invention shown in FIG. 1 is as follows.
- the liquid crystal cell 10 includes a liquid crystal layer 8, a first transparent substrate 7 (viewing side) disposed on one side of the liquid crystal layer 8, and a second transparent substrate disposed on the other side of the liquid crystal layer. 9 (light source side), and the transparent conductive layer 6 is formed on the first transparent substrate 7.
- the 1st polarizing film 4a is laminated
- the 1st polarizing film 4a has the polarizer 2a between the visual recognition side transparent protective film 1a and the liquid crystal cell side transparent protective film 3a.
- the 2nd polarizing film 4b is laminated
- the 2nd polarizing film 4b has the polarizer 2b between the light source side transparent protective film 3b and the liquid crystal cell side transparent protective film 1b.
- both the first polarizing film 4a and the second polarizing film 4b use a double-sided protective polarizing film having transparent protective films on both sides of the polarizer.
- a single-sided protective polarizing film having a transparent protective film only on one side of the polarizer can also be used.
- a retardation layer can be used instead of the liquid crystal cell side transparent protective films (3a, 1b) of the polarizing films 4a, 4b, and an adhesive layer is further provided on the liquid crystal cell side transparent protective films (3a, 1b).
- a retardation layer can also be formed via
- the liquid crystal panel 11 can be appropriately provided with an optical film such as a retardation film, a viewing angle compensation film, and a brightness enhancement film.
- an optical film such as a retardation film, a viewing angle compensation film, and a brightness enhancement film.
- the liquid crystal layer 8 is not particularly limited, and for example, an arbitrary type such as an arbitrary type such as a TN type, an STN type, a ⁇ type, a VA type, and an IPS type can be used. Further, the first transparent substrate 7 (viewing side) constituting the liquid crystal cell 10 and the second transparent substrate 9 (light source side) disposed on the other side of the liquid crystal layer may be transparent substrates.
- the material is not particularly limited, and examples thereof include glass and a transparent resin film substrate. Examples of the transparent resin film substrate include those described above.
- the first and second pressure-sensitive adhesive layers formed from the first polarizing film 4a, the second polarizing film 4b, and the pressure-sensitive adhesive composition (A1) or (A2) are as described above.
- the constituent material of the transparent conductive layer 6 is not particularly limited, and is selected from the group consisting of indium, tin, zinc, gallium, antimony, titanium, silicon, zirconium, magnesium, aluminum, gold, silver, copper, palladium, tungsten.
- a metal oxide of at least one metal is used.
- the metal oxide may further contain a metal atom shown in the above group, if necessary.
- indium oxide (ITO) containing tin oxide and tin oxide containing antimony are preferably used, and ITO is particularly preferably used.
- ITO preferably contains 80 to 99% by weight of indium oxide and 1 to 20% by weight of tin oxide.
- examples of the ITO include crystalline ITO and non-crystalline (amorphous) ITO, both of which can be suitably used.
- the thickness of the transparent conductive layer 6 is not particularly limited, but is preferably 10 nm or more, more preferably 15 to 40 nm, and further preferably 20 to 30 nm.
- the method for forming the transparent conductive layer 6 is not particularly limited, and a conventionally known method can be employed. Specifically, for example, a vacuum deposition method, a sputtering method, and an ion plating method can be exemplified. In addition, an appropriate method can be adopted depending on the required film thickness.
- an undercoat layer, an oligomer prevention layer, or the like can be provided between the transparent conductive layer 6 and the first transparent substrate 7 as necessary.
- the first polarizing film is arranged on the transparent conductive layer on one side of the liquid crystal cell via the first pressure-sensitive adhesive layer formed from the specific pressure-sensitive adhesive composition, and the other liquid crystal cell
- the durability of the entire liquid crystal panel is improved by arranging the second polarizing film on the surface (surface on the light source side, glass surface) via the second pressure-sensitive adhesive layer formed from the specific pressure-sensitive adhesive composition. Further, panel warpage and light leakage can be suppressed.
- the image display device of the present invention includes the liquid crystal panel of the present invention.
- a liquid crystal display device will be described as an example, but the present invention can be applied to any display device that requires a liquid crystal panel.
- the image display device to which the liquid crystal panel of the present invention can be applied include a liquid crystal display device, an electroluminescence (EL) display, a plasma display (PD), a field emission display (FED: Field Emission Display), and the like. it can.
- EL electroluminescence
- PD plasma display
- FED Field Emission Display
- the image display device of the present invention only needs to include the liquid crystal panel of the present invention, and other configurations are the same as those of the conventional image display device.
- room temperature standing conditions not particularly specified are all 23 ° C. and 65% R.S. H. It is.
- Production Example 1 (Preparation of polarizing film) A 80 ⁇ m-thick polyvinyl alcohol film was stretched up to 3 times while being dyed in an iodine solution of 0.3 wt% concentration at 30 ° C. between rolls having different speed ratios. Thereafter, the total draw ratio was stretched up to 6 times while being immersed in an aqueous solution containing boric acid at 4% by weight at 60 ° C. and 10% by weight potassium iodide for 0.5 minutes. Next, after washing by immersing in an aqueous solution containing potassium iodide at 30 ° C. and 1.5 wt% concentration for 10 seconds, drying was performed at 50 ° C. for 4 minutes to obtain a polarizer having a thickness of 30 ⁇ m. A saponified 80 ⁇ m thick triacetyl cellulose film was bonded to both sides of the polarizer with a polyvinyl alcohol adhesive to produce a polarizing film.
- Production Example 2 (Preparation of acrylic polymer (a-1) solution) A monomer mixture containing 99 parts by weight of butyl acrylate and 1 part by weight of 4-hydroxybutyl acrylate was charged into a four-necked flask equipped with a stirring blade, a thermometer, a nitrogen gas inlet tube, and a condenser. Furthermore, with respect to 100 parts by weight of the monomer mixture (solid content), 0.1 part by weight of 2,2′-azobisisobutyronitrile as a polymerization initiator was charged together with 100 parts by weight of ethyl acetate, while gently stirring. After introducing nitrogen gas and substituting with nitrogen, the temperature of the liquid in the flask was kept at around 55 ° C. for 8 hours to conduct a polymerization reaction, and an acrylic polymer having a weight average molecular weight (Mw) of 1560,000 and Mw / Mn3.2 ( A solution of a-1) was prepared.
- Mw weight average molecular weight
- Production Examples 3 and 4 In Production Example 2, as shown in Table 1, the acrylic polymer (a-2) was prepared in the same manner as in Production Example 2, except that the type of monomer used for the preparation of the acrylic polymer and the use ratio thereof were changed. , (A-3) was prepared.
- Example 1 Adjustment of acrylic pressure-sensitive adhesive composition (A1)
- an isocyanate crosslinking agent trade name: Takenate D160N, trimethylolpropane hexamethylene diisocyanate, manufactured by Mitsui Chemicals, Inc.
- benzoyl peroxide Niper BMT 40SV, manufactured by NOF Corporation
- ⁇ -glycidoxypropylmethoxysilane trade name: KBM-403, manufactured by Shin-Etsu Chemical Co., Ltd.
- Isocyanate crosslinking agent (trade name: Takenate D160N, trimethylolpropane hexamethylene diisocyanate, manufactured by Mitsui Chemicals, Inc.) 0 with respect to 100 parts by weight of the solid content of the solution of the acrylic polymer (a1) obtained in Production Example 2 .1 part, 0.3 part of benzoyl peroxide (Niper BMT 40SV, manufactured by NOF Corporation), and 3-mercaptopropyltrimethoxysilane (trade name: KBM-803, manufactured by Shin-Etsu Chemical Co., Ltd.) 3 parts was blended to prepare an acrylic pressure-sensitive adhesive composition (A2) solution.
- the acrylic adhesive composition (A1) solution was dried on one side of a polyethylene terephthalate film (separator film, trade name: MRF38, manufactured by Mitsubishi Chemical Polyester Film Co., Ltd.) treated with a silicone release agent. It applied so that the thickness of an agent layer might be set to 23 micrometers, and it dried at 155 degreeC for 1 minute, and formed the adhesive layer on the surface of a separator film.
- the pressure-sensitive adhesive layer formed on the separator film was transferred to the polarizing film produced in Production Example 1 to produce a pressure-sensitive adhesive layer-attached polarizing film (A1).
- the polarizing film (A2) with an adhesive layer was produced by the same method using the solution of an acrylic adhesive composition (A2).
- Example 2 Comparative Examples 1 to 10
- Example 1 as shown in Table 2, except for changing the type of acrylic polymer (a1), (a2), the type of silane coupling agent, and the amount of addition thereof, the same method as in Example 1, Solutions of acrylic pressure-sensitive adhesive compositions (A1) and (A2) were prepared. Using the obtained acrylic pressure-sensitive adhesive composition solution, a polarizing film with a pressure-sensitive adhesive layer was produced in the same manner as in Example 1.
- Isocyanate type Trade name: Takenate D160N, trimethylolpropane hexamethylene diisocyanate, peroxide manufactured by Mitsui Chemicals, Inc .: Product name: Niper BMT 40SV, benzoyl peroxide, KBM403 manufactured by Nippon Oil & Fats Co., Ltd .: ⁇ -glycid Xipropylmethoxysilane, trade name: KBM-403, Shin-Etsu Chemical Co., Ltd. KBM803: 3-mercaptopropyltrimethoxysilane, trade name: KBM-803, Shin-Etsu Chemical Co., Ltd.
- X-41-1056 oligomer Type epoxy group-containing silane coupling agent, alkoxy group amount: 17% by weight, epoxy equivalent: 280 g / mol, Shin-Etsu Chemical Co., Ltd.
- X-41-1810 oligomer type mercapto group-containing silane coupling agent, alkoxy Base amount: 30% by weight, mercapto equivalent 450 g / mol, manufactured by Shin-Etsu Chemical Co., Ltd.
- KBM-5103 3- acryloxypropyltrimethoxysilane, Shin-Etsu Chemical Co., Ltd.
- KBE-9007 3- isocyanatopropyltriethoxysilane, manufactured by Shin-Etsu Chemical Co.,
- a polarizing film with an adhesive layer (A1) was attached to an ITO surface of a liquid crystal panel having a size of 110 mm ⁇ 60 mm and a thickness of 0.32 mm, and a polarizing film with an adhesive layer (A2) was attached to a glass surface using a laminator.
- autoclaving was performed at 50 ° C. and 0.5 MPa for 15 minutes, and the polarizing film was completely adhered to the liquid crystal panel to obtain an evaluation liquid crystal panel.
- the polarizing film with an adhesive layer (A1) and (A2) stuck on the ITO surface and glass surface of a liquid crystal panel are the polarizing film with an adhesive layer (A1) produced by each Example and a comparative example, ( It is as the combination of A2).
- ⁇ Panel warpage> The prepared liquid crystal panel for evaluation was exposed to an atmosphere at 80 ° C. for 24 hours. Place the exposed LCD panel at room temperature for 1 hour, and place it on a flat table so that the four corners rise according to the direction of warping.
- Laser displacement meter (Product name: LK-G35, Keyence Corporation) Were used to measure the height of the four corners. A value obtained by averaging the measured values at the four corners was taken as the amount of warpage, and the evaluation was made according to the following evaluation criteria.
Landscapes
- Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nonlinear Science (AREA)
- General Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Mathematical Physics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Adhesives Or Adhesive Processes (AREA)
- Polarising Elements (AREA)
- Liquid Crystal (AREA)
- Adhesive Tapes (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020177026001A KR101948462B1 (ko) | 2015-03-31 | 2016-03-18 | 액정 패널 및 화상 표시 장치 |
| CN201680018048.2A CN107430300B (zh) | 2015-03-31 | 2016-03-18 | 液晶面板及图像显示装置 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015072681A JP6068543B2 (ja) | 2015-03-31 | 2015-03-31 | 液晶パネル及び画像表示装置 |
| JP2015-072681 | 2015-03-31 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016158513A1 true WO2016158513A1 (ja) | 2016-10-06 |
Family
ID=57004252
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2016/058794 WO2016158513A1 (ja) | 2015-03-31 | 2016-03-18 | 液晶パネル及び画像表示装置 |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP6068543B2 (ko) |
| KR (1) | KR101948462B1 (ko) |
| CN (1) | CN107430300B (ko) |
| TW (2) | TWI677736B (ko) |
| WO (1) | WO2016158513A1 (ko) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019188971A1 (ja) * | 2018-03-30 | 2019-10-03 | 日東電工株式会社 | 粘着剤層付偏光フィルム、及び画像表示装置 |
| CN110462497A (zh) * | 2017-03-28 | 2019-11-15 | 日东电工株式会社 | 内嵌型液晶面板及液晶显示装置 |
| JP2019215573A (ja) * | 2019-08-28 | 2019-12-19 | 日東電工株式会社 | 粘着剤層付偏光フィルム、及び画像表示装置 |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6970723B2 (ja) * | 2019-10-04 | 2021-11-24 | 日東電工株式会社 | 表示装置及び基材積層体 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014141647A (ja) * | 2012-12-28 | 2014-08-07 | Nitto Denko Corp | 透明導電層用水分散型粘着剤組成物、透明導電層用粘着剤層、粘着剤層付き光学フィルム、及び、液晶表示装置 |
| WO2014208696A1 (ja) * | 2013-06-28 | 2014-12-31 | 日東電工株式会社 | 粘着剤組成物、透明導電層用粘着剤層、積層体、及び画像表示装置 |
| JP2015010195A (ja) * | 2013-06-28 | 2015-01-19 | リンテック株式会社 | 粘着性組成物、粘着剤および粘着シート |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000155213A (ja) * | 1998-11-19 | 2000-06-06 | Nitto Denko Corp | 広視野角偏光板及び液晶表示装置 |
| TW200535517A (en) * | 2003-12-26 | 2005-11-01 | Zeon Corp | Polarizing plate protective film, polarizing plate with reflection preventing function and optical product |
| WO2006019086A1 (ja) * | 2004-08-17 | 2006-02-23 | Zeon Corporation | 偏光板及び液晶表示装置 |
| TWI380900B (zh) * | 2004-09-17 | 2013-01-01 | Sumitomo Chemical Co | 光學疊層體 |
| TW200630226A (en) * | 2004-11-09 | 2006-09-01 | Zeon Corp | Antireflective film, polarizing plate and display |
| JP4515357B2 (ja) * | 2005-01-27 | 2010-07-28 | リンテック株式会社 | 偏光板用粘着剤、粘着剤付き偏光板及びその製造方法 |
| WO2006120887A1 (ja) * | 2005-05-12 | 2006-11-16 | Nippon Kayaku Kabushiki Kaisha | 感光性樹脂組成物、その硬化物及びそれを含有するフィルム |
| JP4697871B2 (ja) * | 2005-10-20 | 2011-06-08 | 日東電工株式会社 | 積層フィルム |
| JP5145081B2 (ja) * | 2008-03-04 | 2013-02-13 | 京セラドキュメントソリューションズ株式会社 | エラー予測装置 |
| JP5517831B2 (ja) * | 2010-08-19 | 2014-06-11 | リンテック株式会社 | 粘着性組成物、粘着剤および粘着シート |
| JP6097474B2 (ja) * | 2010-12-13 | 2017-03-15 | 日東電工株式会社 | 光学フィルム用粘着剤組成物、光学フィルム用粘着剤層、粘着型光学フィルム、および画像表示装置 |
| JP5967654B2 (ja) * | 2012-11-28 | 2016-08-10 | 日本化薬株式会社 | 樹脂組成物及びその硬化物(2) |
| JP6472172B2 (ja) * | 2013-06-28 | 2019-02-20 | 日東電工株式会社 | 光学フィルム用粘着剤組成物、光学フィルム用粘着剤層、粘着剤層付き光学フィルム、液晶表示装置、及び、積層体 |
-
2015
- 2015-03-31 JP JP2015072681A patent/JP6068543B2/ja active Active
-
2016
- 2016-03-18 CN CN201680018048.2A patent/CN107430300B/zh active Active
- 2016-03-18 WO PCT/JP2016/058794 patent/WO2016158513A1/ja active Application Filing
- 2016-03-18 KR KR1020177026001A patent/KR101948462B1/ko active IP Right Grant
- 2016-03-25 TW TW107128065A patent/TWI677736B/zh active
- 2016-03-25 TW TW105109515A patent/TWI645233B/zh active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014141647A (ja) * | 2012-12-28 | 2014-08-07 | Nitto Denko Corp | 透明導電層用水分散型粘着剤組成物、透明導電層用粘着剤層、粘着剤層付き光学フィルム、及び、液晶表示装置 |
| WO2014208696A1 (ja) * | 2013-06-28 | 2014-12-31 | 日東電工株式会社 | 粘着剤組成物、透明導電層用粘着剤層、積層体、及び画像表示装置 |
| JP2015010195A (ja) * | 2013-06-28 | 2015-01-19 | リンテック株式会社 | 粘着性組成物、粘着剤および粘着シート |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110462497A (zh) * | 2017-03-28 | 2019-11-15 | 日东电工株式会社 | 内嵌型液晶面板及液晶显示装置 |
| US11256129B2 (en) | 2017-03-28 | 2022-02-22 | Nitto Denko Corporation | In-cell liquid crystal panel and liquid crystal display device |
| WO2019188971A1 (ja) * | 2018-03-30 | 2019-10-03 | 日東電工株式会社 | 粘着剤層付偏光フィルム、及び画像表示装置 |
| JP2019179211A (ja) * | 2018-03-30 | 2019-10-17 | 日東電工株式会社 | 粘着剤層付偏光フィルム、及び画像表示装置 |
| CN111712741A (zh) * | 2018-03-30 | 2020-09-25 | 日东电工株式会社 | 带粘合剂层的偏振膜、以及图像显示装置 |
| TWI723359B (zh) * | 2018-03-30 | 2021-04-01 | 日商日東電工股份有限公司 | 附黏著劑層之偏光薄膜及影像顯示裝置 |
| US11169312B2 (en) | 2018-03-30 | 2021-11-09 | Nitto Denko Corporation | Pressure-sensitive-adhesive-layer-attached polarizing film, and image display device |
| CN111712741B (zh) * | 2018-03-30 | 2022-03-22 | 日东电工株式会社 | 带粘合剂层的偏振膜、以及图像显示装置 |
| JP2019215573A (ja) * | 2019-08-28 | 2019-12-19 | 日東電工株式会社 | 粘着剤層付偏光フィルム、及び画像表示装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107430300A (zh) | 2017-12-01 |
| CN107430300B (zh) | 2020-10-20 |
| JP6068543B2 (ja) | 2017-01-25 |
| JP2016191865A (ja) | 2016-11-10 |
| KR101948462B1 (ko) | 2019-02-14 |
| KR20170117556A (ko) | 2017-10-23 |
| TW201702700A (zh) | 2017-01-16 |
| TW201841028A (zh) | 2018-11-16 |
| TWI677736B (zh) | 2019-11-21 |
| TWI645233B (zh) | 2018-12-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6175094B2 (ja) | 粘着剤組成物、透明導電層用粘着剤層、粘着剤層付偏光フィルム、及び画像表示装置 | |
| JP6743314B2 (ja) | 粘着剤層、粘着剤層付光学フィルム、光学積層体、および画像表示装置 | |
| JP6182174B2 (ja) | 粘着剤組成物、粘着剤層、粘着剤層付偏光フィルム、及び画像表示装置 | |
| JP2010275524A (ja) | 光学フィルム用粘着剤組成物、光学フィルム用粘着剤層、粘着型光学フィルムおよび画像表示装置 | |
| JP6697625B2 (ja) | 粘着剤層、粘着剤層付光学フィルム、光学積層体、および画像表示装置 | |
| WO2014125684A1 (ja) | アクリル系またはシクロオレフィン系偏光フィルム用粘着剤組成物、粘着剤層、粘着剤層付アクリル系またはシクロオレフィン系偏光フィルムおよび画像形成装置 | |
| JP6725674B2 (ja) | 光学用粘着剤層、光学用粘着剤層の製造方法、粘着剤層付光学フィルム、及び、画像表示装置 | |
| WO2013077270A1 (ja) | 粘着剤組成物、粘着剤層、粘着剤層付偏光フィルムおよび画像形成装置 | |
| WO2018062288A1 (ja) | 光学用粘着剤層、光学用粘着剤層の製造方法、粘着剤層付光学フィルム、及び、画像表示装置 | |
| JP7128945B2 (ja) | 光学用粘着剤層、光学用粘着剤層の製造方法、粘着剤層付光学フィルム、及び、画像表示装置 | |
| WO2016158513A1 (ja) | 液晶パネル及び画像表示装置 | |
| JP6363772B2 (ja) | 粘着剤組成物、粘着剤層、粘着剤層付偏光フィルム、及び画像表示装置 | |
| KR102358985B1 (ko) | 편광 필름용 점착제 조성물, 편광 필름용 점착제층의 제조 방법, 점착제층을 구비한 편광 필름, 및 화상 표시 장치 | |
| TWI741031B (zh) | 附黏著劑層之偏光膜、圖像顯示面板及圖像顯示裝置 | |
| JP2019085436A (ja) | 光学フィルム用粘着剤組成物、光学フィルム用粘着剤層、及び、粘着剤層付光学フィルム | |
| JP7307749B2 (ja) | 光学フィルム用粘着剤組成物、光学フィルム用粘着剤層、及び、粘着剤層付光学フィルム | |
| WO2018062283A1 (ja) | 粘着剤組成物、粘着剤層、粘着剤層付偏光フィルム、液晶パネル、及び、画像表示装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16772395 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20177026001 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 16772395 Country of ref document: EP Kind code of ref document: A1 |