WO2016072446A1 - ウイルスベクター、細胞およびコンストラクト - Google Patents
ウイルスベクター、細胞およびコンストラクト Download PDFInfo
- Publication number
- WO2016072446A1 WO2016072446A1 PCT/JP2015/081145 JP2015081145W WO2016072446A1 WO 2016072446 A1 WO2016072446 A1 WO 2016072446A1 JP 2015081145 W JP2015081145 W JP 2015081145W WO 2016072446 A1 WO2016072446 A1 WO 2016072446A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cells
- gene
- protein
- genome
- cell
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/155—Paramyxoviridae, e.g. parainfluenza virus
- A61K39/165—Mumps or measles virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/48—Reproductive organs
- A61K35/54—Ovaries; Ova; Ovules; Embryos; Foetal cells; Germ cells
- A61K35/545—Embryonic stem cells; Pluripotent stem cells; Induced pluripotent stem cells; Uncharacterised stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/13—B-cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/46—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
- A61K48/0058—Nucleic acids adapted for tissue specific expression, e.g. having tissue specific promoters as part of a contruct
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/38—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells
- A61L27/3804—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells characterised by specific cells or progenitors thereof, e.g. fibroblasts, connective tissue cells, kidney cells
- A61L27/3834—Cells able to produce different cell types, e.g. hematopoietic stem cells, mesenchymal stem cells, marrow stromal cells, embryonic stem cells
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4702—Regulators; Modulating activity
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/10—Cells modified by introduction of foreign genetic material
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K2035/124—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells the cells being hematopoietic, bone marrow derived or blood cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
- C12N2510/02—Cells for production
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/18011—Paramyxoviridae
- C12N2760/18411—Morbillivirus, e.g. Measles virus, canine distemper
- C12N2760/18422—New viral proteins or individual genes, new structural or functional aspects of known viral proteins or genes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/18011—Paramyxoviridae
- C12N2760/18411—Morbillivirus, e.g. Measles virus, canine distemper
- C12N2760/18434—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/18011—Paramyxoviridae
- C12N2760/18411—Morbillivirus, e.g. Measles virus, canine distemper
- C12N2760/18441—Use of virus, viral particle or viral elements as a vector
- C12N2760/18443—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
Definitions
- the present invention relates to viral vectors, cells, and constructs.
- blood cells are most clinically applied as therapeutic cells for regenerative medicine.
- hematopoietic stem cells and hematopoietic progenitor cells have been used for the treatment of malignant tumors centering on the hematopoietic organ for the past about 30 years, and are still used for general therapy.
- blood cells such as lymphocytes, NK cells, NKT cells, and dendritic cells have been clinically studied as immune cell therapy for malignant tumor cells.
- lentiviral vectors or retroviral vectors are used for gene transfer into T cells.
- genomic toxicity due to insertion into genomic DNA is a problem.
- a method using an adenovirus vector and a method using a non-viral vector using mRNA, a plasmid, or the like are known.
- adenoviral vectors and non-viral vectors have very low introduction efficiency, and the cell types that can be used are limited.
- Sendai virus vector is also known as a gene transfer method with low possibility of insertion into genomic DNA.
- Patent Document 1 discloses a Sendai virus vector that has been modified so that the F gene is deleted and secondary infectious virus particles are not released.
- Patent Document 2 discloses a Sendai virus vector carrying a gene related to cell reprogramming.
- Measles virus belonging to the same Paramyxoviridae family as Sendai virus is a virus that has extremely high infectivity in immune cells and epithelial cells.
- Patent Document 3 discloses a measles virus that retains a plurality of segmented genomes and includes at least one segmented genome that contains a foreign gene that can be expressed in a host.
- the Sendai virus vector disclosed in Patent Literature 1 and Patent Literature 2 and the measles virus disclosed in Patent Literature 3 described above are preceded by T Cells need to be stimulated and activated. For this reason, there is a concern that T cell differentiation is induced by activation of T cells, and it is difficult to introduce genes into naive T cells and undifferentiated B cells.
- the present invention has been made in view of the above circumstances, and an object of the present invention is to provide a virus vector, a cell, and a construct that are highly safe and can efficiently introduce a gene into a wide range of cells.
- the viral vector according to the first aspect of the present invention is: A genome derived from a virus belonging to the family Paramyxoviridae, in which both the gene encoding the H protein and the gene encoding the F protein have been modified,
- the genome is Contains foreign genes.
- the genome is Divided into multiple segments Each segmented genome Having a leader arrangement and a trailer arrangement; It is good as well.
- the modification is A deletion of a gene encoding the H protein, or a mutation in which one or several amino acids of the H protein are substituted, deleted or added, and a deletion of the gene encoding the F protein, or one or more of the F protein A mutation in which several amino acids are substituted, deleted or added, It is good as well.
- the gene encoding the M protein in the genome is Having a mutation in which one or several amino acids of the M protein are substituted, deleted or added; It is good as well.
- the virus belonging to the Paramyxoviridae family is Is a measles virus, It is good as well.
- the foreign gene is Including OCT3 / 4, SOX2 and KLF4, It is good as well.
- the cell according to the second aspect of the present invention is:
- the foreign gene is introduced by the virus vector according to the first aspect of the present invention.
- the cell according to the second aspect of the present invention is Blood cells including hematopoietic stem cells, It is good as well.
- the blood cells are Naive T cells, stem cell-like memory T cells or undifferentiated B cells, It is good as well.
- the foreign gene is Including OCT3 / 4, SOX2 and KLF4, Basal state pluripotent stem cells, It is good as well.
- the construct according to the third aspect of the present invention is: From a virus belonging to the family Paramyxoviridae, comprising a nucleic acid serving as a template of a plurality of segmented genomes each having a leader sequence at the 3 ′ end and a trailer sequence at the 5 ′ end, Both the gene encoding the H protein and the gene encoding the F protein in the segmented genome have been modified.
- the gene is highly safe and can be efficiently introduced into a wide range of cells.
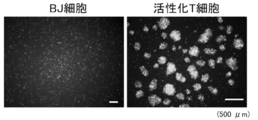
- FIG. 1 It is a figure which shows the structure of the segmented genome of the non-transmissible measles virus vector carrying 6 genes. It is a figure which shows the structure of the measles virus vector plasmid shown in FIG. It is a figure which shows the fluorescence image of the BJ cell which introduce
- the viral vector according to the present embodiment includes a virus-derived genome belonging to the Paramyxoviridae family.
- Viruses belonging to the Paramyxoviridae family are RNA viruses having a ( ⁇ ) strand RNA genome. Viruses include measles virus (Measles virus), Sendai virus (Sendai virus), Newcastle disease virus (Newcastle disease virus), mumps virus (Mumps virus), type 1 parainfluenza virus (Parainfluenza virus 1), type 2 parainfluenza virus (Parainfluenza virus 2), type 3 parainfluenza virus (Parainfluenza virus 3), type 5 parainfluenza virus (Parainfluenza virus 5, Simian virus 5), metapneumovirus (Metapneumo virus), RS virus (Respiratorysyncytial virus), rinderpest virus ( Rinderpest virus), and Distemper virus.
- the virus belonging to the Paramyxoviridae is preferably a measles virus.
- the genome of a virus belonging to the Paramyxoviridae family is a non-segmented single-stranded RNA having a leader sequence (Le) at the 3 'end and a trailer sequence (Tr) at the 5' end.
- Various genes encoding functional proteins constituting the virus are arranged between the leader sequence and the trailer sequence.
- the leader sequence has promoter activity.
- the trailer sequence has promoter activity in the case of antigenome.
- the main genes encoding functional proteins are: a gene encoding N protein (N gene), a gene encoding P protein (P gene), a gene encoding M protein (M gene), and a gene encoding F protein (F gene), a gene encoding H protein (H gene) and a gene encoding L protein (L gene).
- N gene a gene encoding N protein
- P gene a gene encoding P protein
- M gene gene
- F gene gene
- H protein H gene
- L gene gene encoding L protein
- the N protein binds to the viral genome in order from the 5 'end and wraps the genomic RNA.
- the P protein functions as a small subunit of RNA-dependent RNA polymerase and is involved in transcriptional replication of the viral genome.
- accessory proteins such as V protein and C protein may be synthesized by an RNA editing mechanism or the like.
- the L protein functions as the large subunit of RNA-dependent RNA polymerase and is involved in transcriptional replication of the viral genome together with the P protein.
- M protein is a matrix protein that supports the structure of virus particles from the inside.
- the F protein is involved in cell fusion and brings pathogenicity to the virus.
- the H protein is a viral receptor binding protein.
- the H protein binds to SLAM (Signaling lymphocyte activation module) or Nectin4 as a receptor for infection of wild type viruses. Since SLAM and Nectin4 are expressed only in limited cell types, they limit the host range of the virus.
- the genome derived from a virus belonging to the family Paramyxoviridae means a genome that has a gene encoding the functional protein and can be amplified in infected cells.
- both the H gene and the F protein are modified.
- the modification of the H gene is, for example, a defect of the H gene.
- the modification of the H gene may be a mutation in which one or several amino acids of the H protein are substituted, deleted or added.
- the modification of the H gene is performed by, for example, replacing the 390th asparagine with isoleucine (N390I), the 416th asparagine with aspartic acid (N416D), and the 446th threonine serine.
- Substitution T446S
- 481st asparagine substitution to tyrosine N481Y
- 492nd glutamic acid substitution to glycine E492G.
- the structure or function of the H protein can be changed relative to the wild type H protein.
- the virus vector can utilize molecules other than SLAM and Nectin4 as a receptor by modifying the H gene. That is, by modifying the H gene, cell types that can be infected with a viral vector can be increased.
- the modification of the F gene is, for example, a defect in the F gene.
- deleting the F gene the expression of the F protein can be eliminated.
- a virus vector lacking the F protein cannot produce a virus having pathogenicity in other cells except for a special cell line (Vero / SLAM / F cell). For this reason, unless the special cell line is infected, the spread of the viral vector can be lost.
- the modification of the F gene may be a mutation in which one or several amino acids of the F protein are substituted, deleted or added. What is necessary is just to change the structure or function of F protein with respect to wild-type F protein by the said mutation.
- the gene encoding the M protein in the genome may have a mutation in which one or several amino acids of the M protein are substituted, deleted or added.
- mutations include substitution of the M protein at the 64th proline with serine (P64S) and the substitution of the 89th glutamic acid with glycine (E89G). Since the M protein is a matrix protein that supports the structure of the virus particle from the inside, introducing the mutation into the M protein can improve the virus particle forming ability and further reduce the cell fusion ability.
- the genome included in the virus vector according to the present embodiment has a foreign gene.
- the viral vector can express a foreign gene.
- the foreign gene is not limited, but, for example, genes encoding various proteins that cause pathogenicity such as viruses, bacteria and parasites, genes encoding various cytokines, genes encoding various peptide hormones, and cell reprogramming Examples include genes encoding factors.
- a reporter gene such as a gene encoding GFP (Green Fluorescent Protein) or EGFP (Enhanced Green Fluorescent Protein) may be inserted.
- the number of foreign genes is not particularly limited, but is preferably 2-6.
- Specific examples of the foreign gene include reprogramming factors such as OCT3 / 4, SOX2 and KLF4.
- reprogramming factors such as OCT3 / 4, SOX2 and KLF4.
- other reprogramming factors such as L-MYC and PIN1 may be further inserted into the genome as foreign genes.
- the arrangement of the gene encoding the functional protein and the foreign gene in the genome is not particularly limited as long as it is between the leader sequence and the trailer sequence.
- the mutual positional relationship between the genes encoding the functional proteins in the genome can be arbitrarily determined regardless of the position of the wild-type virus in the genome order or the like.
- the genome included in the virus vector according to the present embodiment may be segmented into a plurality of segments.
- a segment means that genomic RNA is fragmented into multiple RNAs. That is, a plurality of genomes is a set of RNAs that function as one genome.
- the number of segmented genomes is not limited, but is preferably at most 6 and particularly preferably 2.
- each segmented genome has a leader sequence and a trailer sequence.
- a leader sequence is placed at one end of each segmented genome and a trailer sequence is placed at the other end.
- one of the genes encoding the functional protein and a foreign gene are arranged between the leader sequence and the trailer sequence of each segmented genome.
- a gene encoding a functional protein and a foreign gene may be inserted into one of a plurality of segmented genomes, or may be inserted into two or more.
- all of the plurality of foreign genes may be inserted into one of the multiple segmented genomes, or to two or more of the multiple segmented genomes.
- a plurality of foreign genes may be inserted as a whole by inserting one or more foreign genes. When a plurality of foreign genes are inserted into one segmented genome, the number is not limited as long as the expression efficiency of the gene encoding the functional protein is not significantly reduced.
- the arrangement of foreign genes in a genome segmented into multiple segments is not particularly limited.
- the F gene is deleted, and a reprogramming factor is inserted as a foreign gene
- the first segmented genome is directed from the 3 'end to the 5' end.
- the leader sequence, OCT3 / 4, N gene, P gene, modified M gene, SOX2, trailer sequence are arranged in this order, and in the second segmented genome, from the 3 ′ end to the 5 ′ end , Leader sequence, KLF4, modified H gene, L gene, trailer sequence.
- L-MYC and PIN1 are further inserted as foreign genes
- L-MYC is preferably inserted between SOX2 and the trailer sequence
- PIN1 is inserted between H gene and L gene.
- Genes encoding the above functional proteins excluding the H gene, F gene and M gene have the wild-type activity in transcription and replication even if they are not completely identical to the base sequence of each gene contained in the genome of the wild-type virus. As long as it is equal to or higher than the activity, a mutation may be introduced.
- a gene encoding a functional protein may be one or several, for example, 1 to 15, preferably 1 to 8, if the activity in transcription and replication is equal to or higher than that of each natural protein. It may be a base sequence encoding a protein consisting of amino acids in which 1 to 5 amino acids are preferably deleted, substituted or added.
- the homology of the amino acid sequence between the mutant protein and the natural protein is preferably 90 to 100%, for example, but if the activity in transcription and replication is maintained, the amino acid homology is For example, it may be 40 to 90%.
- FIG. 1 shows a preferred example of a segmented genome having a gene encoding the above reprogramming factor and a gene encoding EGFP as a foreign gene.
- the H gene is modified so that mutations of N390I, N416D, T446S, N481Y and E492G are added to the H protein (H8), and the F gene is missing.
- the M gene has been modified so that mutations of P64S and E89G are added to the M protein (M6489).
- the P gene is indicated as P / V / C.
- the virus vector has cell infectivity and transmission ability.
- the cell infectivity is the ability to introduce a virus internal genome into a cell by maintaining the ability to adhere to a host cell and membrane fusion ability.
- the propagation power is the ability to replicate the genome introduced into the cell, form an infectious particle or a complex equivalent thereto, and propagate the genome to another cell.
- the viral vector according to the present embodiment can be produced by a known viral vector production method.
- the virus vector can be prepared by reconstructing virus particles using cDNA corresponding to the genome of the virus or using the genome of the virus.
- the rearrangement of virus particles means that the genome of the virus is artificially produced and the original virus or recombinant virus is produced in vitro or in a cell.
- genomic cDNA is prepared by reverse transcription reaction or the like. Subsequently, when the genome is segmented, the cDNA is fragmented into a plurality of cDNAs by a known gene recombination technique and a nucleic acid amplification method, and then an operation such as insertion of a foreign gene is performed.
- the fragmentation method is not particularly limited as long as a plurality of cDNA fragments are prepared as a result based on the base sequence of cDNA prepared from the genome.
- Examples of the preparation method based on the base sequence of cDNA prepared from a genome include a method of obtaining an amplified fragment by a PCR method using a predetermined region in cDNA prepared from a genome as a template.
- the predetermined region can be appropriately set in consideration of the structure of the genome to be expressed, and is not limited.
- the predetermined region is preferably set so that each gene fragment encoding the functional protein of the virus can be amplified individually. When the region is set in this way, a cDNA fragment can be prepared by linking the obtained amplified fragments of each gene in a desired type, number and arrangement order (position).
- a separately prepared DNA fragment containing the foreign gene may be inserted into the segmented cDNA using a known gene recombination technique.
- the base sequence of cDNA corresponding to the first segmented genome containing the N gene shown in FIG. 1 and the base sequence of cDNA corresponding to the second segmented genome containing the H gene are SEQ ID NO: 1 and SEQ ID NO: 1, respectively. It is shown in 2.
- DNA containing the prepared cDNA fragment preferably plasmid DNA, or RNA obtained by previously transcribed cDNA in vitro may be used.
- RNA-dependent RNA polymerase Even if a genome of a virus belonging to the Paramyxoviridae family or its antigenome is introduced into a host cell as naked RNA, it does not serve as a template for an RNA-dependent RNA polymerase. In order to become a template, N protein, P protein and L protein exist in the initial stage of RNA synthesis reaction by the RNA-dependent RNA polymerase, and it is necessary to form a complex (RNP complex) of these proteins and genomic RNA. is there. Therefore, in order to reconstruct a viral vector, it is desirable to express N protein, P protein and L protein together, or to use a host capable of expressing N protein, P protein and L protein. In addition, when the F gene of RNA contained in the viral vector is deleted, it is preferable to use a host that can also express F protein.
- the viral vector can be prepared by, for example, introducing the genome or its cRNA into a host that expresses viral N, P, and L proteins.
- DNA containing cDNA serving as a template for the genome or its cRNA and a transcription unit of the DNA may be introduced into a host that expresses viral N, P, and L proteins.
- DNA containing cDNA as a template for the genome or its cRNA, DNA containing viral N gene, DNA containing viral P gene, DNA containing viral L gene, and these A DNA transcription unit may be introduced.
- the N protein, the P protein, and the L protein expressed by the host need not be completely the same as long as they are equivalent to or higher than the activity of these natural proteins.
- 1 to 15, preferably 1 to 8, more preferably 1 to 5 amino acids may be a protein composed of amino acids deleted, substituted or added, or derived from other viruses Alternatively, it may be a completely different protein having a greatly different amino acid sequence.
- the host is not particularly limited as long as it is a cell capable of expressing the functional protein and a foreign gene, and examples thereof include cultured mammalian or avian cells and chicken eggs.
- the cultured cells include, for example, BHK-T7 / 9 cells, CHO, 293 cells, B95a, monkey cells COS-7, Vero, mouse L cells, rat GH3, human FL cells, LLCMK2, MDCK, MDBK, CV-1, HeLa, HepG2, P19, F9, PClZ, BAF3, Jurkat, human PBMCN, MT-4, Molt-4, NIH3T3, L929, Vero / hSLAM, CHO / hSLAM, A549 / hSLAM, HeLa / hSLAM, 293T BHK, chicken embryo fibroblasts, and the like can be used.
- Insect cells such as Sf9 cells and Sf21 cells can also be used.
- the transcription unit is preferably, for example, a recombinant vaccinia virus that expresses a predetermined DNA-dependent RNA polymerase, DNA containing a predetermined DNA-dependent RNA polymerase gene, or the like.
- the virus vector obtained by the above production method can be selectively and efficiently propagated by co-culture with other cells.
- the cultured cells containing the reconstituted viral vector obtained by the above production method are seeded on Vero / SLAM / F cells that have been cultured in advance and co-cultured.
- Vero / SLAM / F cell giant cells infected and propagated with the viral vector can be obtained.
- two plasmids corresponding to the segmented genome shown in FIG. 1 are optionally combined with other plasmids containing a gene encoding a functional protein. What is necessary is just to introduce
- the measles virus vector may be infected with new Vero / SLAM / F cells and amplified before releasing the measles virus vector. In this case, the supernatant containing the virus can be recovered as a virus solution by centrifugation.
- the H gene is modified in the virus vector according to the present embodiment, it can infect various cell types, that is, the host range can be expanded.
- the F vector has a modified F gene, it is possible to prevent the production of a virus having cell infectivity and to improve the safety by eliminating the spreadability.
- the viral vector since the entire replication process is carried out in the cytoplasm and is not accompanied by DNA synthesis, the viral vector has no concern about genome toxicity and is extremely safe.
- the genome contained in the virus vector according to the present embodiment may be segmented into a plurality of segments. In this way, a plurality of foreign genes or large genes can be loaded.
- each segmented genome may have a leader sequence and a trailer sequence.
- a virus belonging to the Paramyxoviridae family has a known gene expression pattern in which the expression is lower in the downstream (5 ′ side) gene.
- Each segmented genome has a leader sequence and a trailer sequence.
- a polymerase can act on each segmented genome, and the expression level of each gene can be increased.
- a plurality of foreign genes can be expressed simultaneously in the host.
- the genome size is suitable for maintaining high protein expression efficiency. Of course, the same function can be achieved with a single unsegmented genome.
- the gene encoding the M protein on the segmented genome may have a mutation in which one or several amino acids of the M protein are substituted, deleted, or added. . By doing so, the gene transfer efficiency and safety of the virus vector into cells can be further increased.
- the virus may be a measles virus. Since measles virus has a strong directivity to immune cells, the above-described viral vector enables highly efficient gene transfer to B cells, T cells and granulocytes. Furthermore, as shown in the Examples below, the above viral vector is highly efficient in gene expression even in non-activated naive T cells, central memory T cells, effector memory T cells and B cells, and blood cells including hematopoietic stem cells. Introduction is possible. In addition, since measles virus originally uses humans as its host, knowledge about the effects on humans is accumulated, so it can be used appropriately while considering safety.
- OCT3 / 4 OCT3 / 4, SOX2 and KLF4 are given as examples of foreign genes.
- a ground state iPS cell that is very difficult to produce by the conventional method using the virus vector carrying a gene encoding a reprogramming factor. Can be produced.
- RNA contained in the virus vector according to the present embodiment may be a (+) strand RNA, if necessary, in addition to the ( ⁇ ) strand RNA as in the genome originally possessed by the virus.
- a construct suitable for producing a genome contained in the virus vector includes a nucleic acid serving as a template of a plurality of segmented genomes derived from viruses belonging to the Paramyxoviridae family, each having a leader sequence at the 3 ′ end and a trailer sequence at the 5 ′ end. Both the gene encoding the H protein and the gene encoding the F protein in the genome have been modified.
- the nucleic acid may be a cDNA or cRNA prepared based on each segmented genome described above.
- the nucleic acid may be a plurality of cDNA or plasmid DNA corresponding to each segmented genome.
- the above construct is composed of a leader sequence, N gene, P gene, M gene and trailer sequence from the 3 ′ end to the 5 ′ end. And a cDNA corresponding to the genome arranged in the order of leader sequence, H gene, L gene and trailer sequence from the 3 ′ end to the 5 ′ end.
- This construct can be used to easily insert a foreign gene using a genetic recombination technique in conventional genetic engineering.
- a virus vector containing each segmented genome can be efficiently produced.
- the construct can be introduced into prokaryotic or eukaryotic cells via conventional transformation or transfection techniques.
- the expression construct can be introduced into various cells in the form of a plasmid.
- the nucleic acid contained in the construct was a cRNA of a segmented genome derived from the genome of a virus belonging to the family Paramyxoviridae, a segmented genome having a leader sequence at the 3 ′ end and a trailer sequence at the 5 ′ end. May be.
- the construct includes a nucleic acid serving as a template for a genome derived from a virus belonging to the family Paramyxoviridae, having a leader sequence at the 3 ′ end and a trailer sequence at the 5 ′ end, and encodes an H protein in the genome. Both the gene that encodes and the gene that encodes the F protein may be modified.
- the cell according to the present embodiment is a cell into which a foreign gene has been introduced by the virus vector according to the first embodiment.
- Virus vectors can be infected into cells by known methods. For example, a viral vector may be added to a culture solution containing cells and centrifuged at 1,200 ⁇ g for 45 minutes at room temperature.
- methods for infecting cells with a viral vector include methods for infecting cells with various viruses such as electroporation, lipofection, heat shock, PEG, calcium phosphate, and DEAE dextran.
- the cells are not particularly limited, but include human somatic cells, fibroblasts, blood cells, monkey somatic cells, and the like. Suitable cells include B cells, activated or not activated T cells, granulocytes, blood cells including hematopoietic stem cells, and the like. Particularly preferred cells are naive T cells, stem cell-like memory T cells or undifferentiated B cells.
- the titer of the viral vector when a foreign gene is introduced into a cell is not particularly limited, but the MOI (multiplicity of infection) is 1 to 100, preferably 3 to 20, more preferably 5 to 10.
- a cell into which a foreign gene has been introduced with the above-described viral vector containing at least OCT3 / 4, SOX2 and KLF4 as a foreign gene expresses an undifferentiated marker and is induced to iPS cells having the ability to differentiate into three germ layers.
- basal state iPS cells can also be induced.
- the gene according to the present embodiment is introduced by the above-described virus vector, a desired plurality of foreign genes can be expressed simultaneously over a long period of time.
- the cells according to the present embodiment are genes using blood cells whose genes are modified. It is very useful for therapy, especially for genetically modified T cell therapy.
- the cells are introduced into a basal state iPS cell by introducing a foreign gene with the above viral vector containing OCT3 / 4, SOX2 and KLF4. Since basal state iPS cells have higher proliferation efficiency and can be proliferated even when seeded as single cells, large-scale production and easy storage are possible. Moreover, since it is in a ground state, it can be differentiated into a wide range of cell types.
- the use of the cell which concerns on this Embodiment varies according to a foreign gene.
- a foreign gene By making a foreign gene to be introduced into a cell a T cell receptor gene or a chimeric antigen receptor gene, it can be used for T cell therapy for malignant tumors.
- Zn finger nuclease or the like into a T cell as a foreign gene and destroying the CCR5 gene (gene editing), it can be used for treatment of HIV / AIDS.
- Treatment of enzyme deficiency is possible by introducing a gene encoding the enzyme into cells of an enzyme deficiency patient such as adenosine deaminase (ADA).
- ADA adenosine deaminase
- a method for producing iPS cells includes an infection step of infecting the cell with the virus vector according to the second embodiment.
- the cells may be blood cells, immune cells that are not activated (stimulated), preferably na ⁇ ve T cells.
- the method for producing iPS cells includes a method for producing basal state iPS cells.
- Cell culture in the following examples was carried out as follows.
- DMEM Dulbecco's modified easy's medium
- FBS fetal bovine serum
- Vero / SLAM / F cells were cultured in a culture solution containing DMEM at 0.5 mg / mL G418 (manufactured by Nacalai Tesque) and FBS at 7.5%.
- BHK-T7 / 9 cells were cultured in a culture solution containing ⁇ -modified minimum essential medium ( ⁇ -MEM) (Life Technologies) and 600 ⁇ g / mL of Hygrocin B (Nacalai Tesque) and 10% FBS. did.
- Peripheral blood-derived blood cells of a healthy person were cultured in a culture solution containing 10% FBS in RPMI 1640 (manufactured by Nacalai Tesque).
- Umbilical cord blood-derived or healthy person-derived T cells are cultured in RPMI1640 containing 10% FBS, 175 IU / mL human recombinant IL-2 (manufactured by Peprotech), or The cells were cultured in KBM502 culture solution (manufactured by Kojin Bio).
- Umbilical cord blood-derived hematopoietic stem cells were cultured in a culture solution in which human recombinant SCF, human recombinant Flt-3L, and human recombinant TPO (all manufactured by Peprotech) were added to Stemspan culture solution (manufactured by VERITAS).
- All primed iPS cells were cultured on MEF cells in a human ES cell maintenance medium.
- the composition of the human ES cell maintenance culture solution is DMEM / F12 (manufactured by Nacalai Tesque), 20% KSR (Life Technologis), 2-mercaptoethanol (Sigma), 2 mM L-glutamine (manufactured by Nacalai Tesque). 1% non-essential amino acids (manufactured by Nacalai Tesque), 4 ng / mL basic FGF (manufactured by Peprotech).
- the basal state iPS cells were cultured on the MEF cells in a mixed culture solution.
- the composition of the mixed culture solution was as follows: DMEM / F12, 20% KSR, 2-mercaptoethanol, 2 mM L-glutamine, 1% non-essential amino acids, 1 ⁇ M CHIR99021 (manufactured by Militini Biotec), 1 ⁇ M PD03tenyMil ), 1,000 units / mL human recombinant LIF (manufactured by Nacalai Tesque).
- Non-transmissible genetically modified measles virus vector assists virus synthesis with two plasmids (MV-HL-K-Pin1-EGFP and MV-NPM-OSM) containing genes encoding functional proteins that make up measles virus
- plasmids pCITE-IC-N, pCITE-IC-P ⁇ C, pCITEko-9301B-L, pCA7-IC-F
- OCT3 / 4 gene, SOX2 gene, L-MYC gene, KLF4 gene and PIN1 gene used for the production of iPS cells, and EGFP gene as a reporter gene were inserted.
- the wild type H gene and M gene in the measles virus strain MV-IC323-EGFP were inserted so that the mutations of N390I, N416D, N481Y, E492G were inserted into the H protein, and the mutations of P64S, E89K were inserted into the M protein. Modified by gene recombination.
- the MV-NPM-OSM plasmid is composed of the mutated MV-IC323-EGFP EGFP gene in the OCT3 / 4 gene, the F gene in the SOX2 gene, the H gene and the L gene.
- the MV-HL-K-Pin1-EGFP plasmid recombines the N gene, P gene, M gene and F gene of MV-IC323-EGFP with the above mutation into the KLF4 gene, and between the L gene and the H gene. It was prepared by inserting the PIN1 gene into.
- MV-NPM-OSM plasmid and MV-HL-K-Pin1-EGFP plasmid and four plasmids (pCAG-T7-IC-F, pCITE-IC-N, pCITE-IC-P ⁇ C, pCITE-ko-9301B-L)
- the gene was introduced into BHK-T7 / 9 cells. Two days later, the cells were collected from the dish, seeded on Vero / SLAM / F cells, and giant cells were collected two days later to recover the measles virus vector. The recovered measles virus vector was infected with new Vero / SLAM / F cells, and the cells were recovered after amplification.
- the collected cells were resuspended in DMEM and repeatedly frozen and thawed to release measles virus vector (MVV) from the cells into the culture solution. Thereafter, only the supernatant was collected as a virus solution by centrifugation, and dispensed and stored at ⁇ 80 ° C.
- the titer was determined by adding the virus solution to Vero / SLAM cells and analyzing the proportion of GFP positive cells by flow cytometry two days later.
- Example 2 Evaluation of non-transmissible genetically modified measles virus vector
- the MVV prepared in Example 1 was evaluated using activated T cells or BJ cells.
- Activated T cells were prepared by stimulating peripheral blood derived from healthy subjects for 5 days using Dynabeads (registered trademark) Human T-Activator Cd3 / CD28 (manufactured by Life Technologies) in KBM502 culture medium. More specifically, 5 ⁇ 10 4 cells were seeded in a 12-well plate, and an amount of virus solution corresponding to the titer was added. Next, the gene was introduced by centrifugation (room temperature, 1,200 ⁇ g, 45 minutes), then washed once with PBS, and the culture medium was replaced.
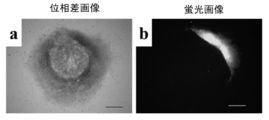
- FIG. 3 shows fluorescence images of BJ cells and activated T cells. Both cells showed fluorescence. Therefore, it was confirmed that MVV introduces genes into BJ cells and activated T cells.
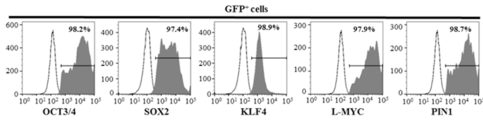
- FIG. 4 shows the result of analyzing the GFP expression of activated T cells. Genes were introduced into activated T cells using MVV, and the expression of the five genes in GFP positive cells on the third day after introduction was analyzed. From this result, it was confirmed that all five genes were expressed simultaneously in addition to the EGFP gene.
- Example 3 Gene transfer by non-transmissible genetically modified measles virus vector and its analysis
- About 10 mL of peripheral blood or umbilical cord blood was collected from a healthy person who obtained consent, and mononuclear cells were separated using a lymphocyte separation solution (manufactured by Nacalai Tesque).
- the culture solution was exchanged after washing with PBS (-) (PBS tablet (manufactured by Takara Bio Inc.) dissolved in pure water and autoclaved). Two days later, the cells were collected, and the gene transfer rate of each blood cell lineage and measles virus receptor expression in each cell lineage were analyzed by flow cytometry method using GFP expression as an index.
- PBS PBS tablet (manufactured by Takara Bio Inc.) dissolved in pure water and autoclaved.
- MVV Sendai virus vector
- PasmEx registered trademark
- -AG obtained from Medical Biology Laboratory
- the antibodies used for the analysis of each blood cell lineage are monocyte system: APC-Cy7-labeled anti-CD14 antibody (manufactured by Biolegend), PE-labeled anti-CD11b antibody (manufactured by BD), neutrophil system: PerCP-Cy5.5.
- B cell system APC-Cy7 labeled anti-CD19 antibody (manufactured by Bio legend)
- T cell system APC-labeled anti-CD3 antibody (manufactured by Bio legend), APC-Cy7 labeled anti-antibody CD4 antibody (manufactured by Bio legend), PE-Cy7-labeled anti-CD8 antibody (manufactured by Bio legend), APC-labeled anti-CD45RA (manufactured by Bio legend), PE-labeled anti-CD197 antibody (manufactured by Bio legend), NK cells: PE -Cy7-labeled anti-CD56 antibody (manufactured by Bio legend).
- the antibodies against the virus receptor used were PE-labeled anti-CD46 antibody (manufactured by eBioscience), PE-labeled anti-CD150 antibody (manufactured by Biolegend), and PE-labeled anti-Nectin4 antibody (manufactured by R & D).
- Monocytes were defined as CD14 + and CD11b + .
- B cells were defined as CD19 + and CD3 ⁇ .
- T cells were defined as CD3 + and CD19 ⁇ .
- Neutrophils were defined as CD15 + .
- NK cells were defined as CD3 ⁇ and CD19 ⁇ and CD56 + .
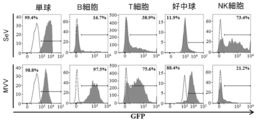
- FIG. 5 shows the GFP positive rate of each blood cell derived from peripheral blood.
- monocytes both vectors showed high gene transfer rates, but MVV in B cells, T cells, and neutrophils showed higher gene transfer efficiency than SeV.
- T cells were fractionated with CD45RA and CCR7 (CD197), and the gene transfer efficiency in each fraction was examined. As shown in FIG. 7, na ⁇ ve and stem cell-like memory T cell fractions (CD45RA high and CD197 high) prior to exposure to antigen, and central memory T cell fraction (CD45RA low) proliferating upon antigen re-exposure. In addition, high-efficiency gene transfer by MVV was confirmed in CD197 high) and effector memory T cell fractions (CD45RA low and CD197 low). On the other hand, from the results of CD8 + cells, the efficiency of gene transfer by MVV was not confirmed in the effector T cell fraction (CD45RA high and CD197 low).
- MVV high-efficiency gene transfer by MVV is possible for naive T cells and stem cell-like memory T cells that have been difficult to transfer by conventional methods including Sendai virus vectors. Therefore, the MVV according to this example strongly suggested the possibility of becoming an innovative therapeutic vector in gene therapy using gene-modified T cells, particularly gene-modified T cell therapy. Moreover, MVV was able to introduce a gene with high efficiency into primary cultured B cells.
- Example 4 Establishment of iPS cells using a non-transmissible genetically modified measles virus vector
- BJ cells were seeded on MEF cells and cultured in a 37 ° C., 5% CO 2 incubator for one day, and the following day, the medium was replaced with a human ES cell maintenance medium. Thereafter, the culture solution was changed every other day and cultured in a 37 ° C., 5% CO 2 incubator.
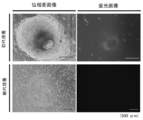
- human ES cell-like colonies were collected (see FIG. 8).
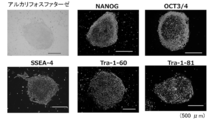
- the above iPS cells established from BJ cells were subjected to immunoantibody staining and RT-PCR to confirm the expression of undifferentiated markers.
- human iPS cells were fixed with 4% paraformaldehyde-phosphate buffer solution (Nacalai Tesque) at 4 ° C. for 30 minutes, and 0.1% Triton X-100 (Nacalai Tesque) After treatment with 5% skimmed milk (manufactured by Nacalai Tesque).
- Anti-NANOG antibody manufactured by R & D
- anti-OCT3 / 4 antibody anti-SSEA-4 antibody
- anti-Tra-1-60 antibody anti-Tra-1-81 antibody (all manufactured by Santa Cruz) were used as primary antibodies. Staining was carried out overnight at room temperature for 30 minutes with a secondary antibody (anti-goat InG (Life Technologies)).
- Alkaline Phosphate Detection Kit Merck Millipore was used for alkaline phosphatase staining.
- RNA is extracted from the above iPS cells established from BJ cells using RNeasy mini kit (manufactured by Qiagen), and complementary strand DNA (cDNA) is obtained from SuperScript III First-Synthesis System for RT-PCR. (Life Technologies). Then, amplification reaction was performed using Takara ExTaq polymerase (manufactured by Takara Bio Inc.), and electrophoresis was performed using 1.5% agarose gel (manufactured by Nacalai Tesque).
- the base sequences of the primers used in the RT-PCR method are SEQ ID NO: 3 and SEQ ID NO: 4 for Endo OCT3 / 4, SEQ ID NO: 5 and SEQ ID NO: 6 for Endo SOX2, and SEQ ID NO: 7 and Endo KLF4.
- SEQ ID NO: 8 SEQ ID NO: 9 and SEQ ID NO: 10 for Endo MYC
- SEQ ID NO: 11 and SEQ ID NO: 12 for NANOG
- SEQ ID NO: 13 and SEQ ID NO: 14 for TERT
- SEQ ID NO: 15 and sequence for DNMT3B SEQ ID NO: 17, SEQ ID NO: 18 for MV-N protein
- SEQ ID NO: 19 and SEQ ID NO: 20 for MV-L protein
- SEQ ID NO: 21 and SEQ ID NO: 22 for ⁇ -ACTIN.
- the above iPS cells established from BJ cells were dissociated from MEF using ES cell dissociation solution (Collagenase IV (Life Technologies), 0.25% trypsin (Life Technologies) and KSR) and human ES. It was resuspended in a cell maintenance medium, seeded in a non-adherent 6-well plate, and cultured in a 37 ° C., 5% CO 2 incubator. On the next day, the floating cells were collected, resuspended in a human ES cell maintenance medium without addition of basic FGF, and cultured in a 37 ° C., 5% CO 2 incubator.
- ES cell dissociation solution Collagenase IV (Life Technologies), 0.25% trypsin (Life Technologies) and KSR
- human ES cell maintenance medium
- the floating cells were collected, resuspended in a human ES cell maintenance medium without addition of basic FGF, and cultured in a 37 ° C., 5% CO 2 incubator.
- the cells were collected, dissociated using a 0.25% trypsin / EDTA mixture (Nacalai Tesque), and coated with a 0.1% gelatin solution (Nacalai Tesque).
- the seeds were seeded and cultured for 7 days at 37 ° C. in a 5% CO 2 incubator.
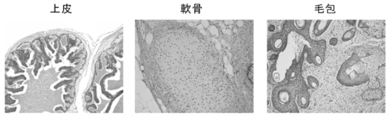
- the three germ layer differentiation of the iPS cells established from BJ cells was confirmed using an immuno-antibody staining method.
- Anti-alpha-fetoprotein antibody manufactured by R & D
- anti-vimentin antibody manufactured by Santa cruz
- anti-nestin antibody manufactured by Santa cruz
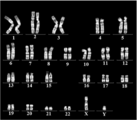
- a teratoma formation test was performed on the iPS cells established from BJ cells. 1 ⁇ 10 6 iPS cells were injected into the testis of immunodeficient mice (obtained from the Central Laboratory for Experimental Animals), and teratomas were removed 9 to 13 weeks later. Thereafter, the teratoma tissue was fixed with 20% formalin (manufactured by Wako Pure Chemical Industries, Ltd.) and stained with hematoxylin-iodine. Moreover, the karyotype analysis of the said iPS cell established from the BJ cell was performed (Chromocenter).
- the iPS cells had 46 chromosomes and had a normal karyotype.
- Example 5 Establishment, differentiation induction and analysis of ground state iPS cells
- Umbilical cord blood-derived CD34-positive cells obtained from RIKEN were thawed and cultured in a 37 ° C., 5% CO 2 incubator using a culture solution in which SCF, TPO, and Flt3L were added to Stemspan.
- gene transfer was performed by centrifugation (room temperature, 1,200 ⁇ g, 45 minutes) using MVV, and cultured in the above culture solution for 2 days.
- the culture medium was replaced with human ES cell maintenance medium. Thereafter, the culture was continued in a 37 ° C., 5% CO 2 incubator while changing the culture medium every other day.
- Colonies that appeared on day 14 after gene transfer were collected, dissociated into single cells with a 0.25% trypsin / EDTA mixed solution, seeded on MEF cells, and placed in a 37 ° C., 5% CO 2 incubator. Incubated in
- basal state iPS cells could be established.
- basal state iPS cells were dissociated to single cells using a 0.25% trypsin / EDTA mixed solution every 4 days and passaged, colony morphology could be maintained for more than 10 passages without using Y-27632. It was. Moreover, the appearance of human ES cell-like colonies could be confirmed by exchanging the culture solution with a human ES cell maintenance culture solution and reducing the MEF cell seeding concentration.
- the present invention is suitable for viral vectors and constructs for gene introduction into blood cells. Therefore, the present invention is expected to be applied to immunotherapy, particularly T cell therapy.
- the present invention is suitable for the production of pluripotent stem cells, in addition to regenerative medicine using tissue cells obtained by differentiation induction, disease analysis using iPS cells established from cells derived from patients with intractable diseases And can be applied to drug discovery research. Furthermore, it can be applied to genome editing technology for blood cells.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Cell Biology (AREA)
- Biochemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Virology (AREA)
- Microbiology (AREA)
- Medicinal Chemistry (AREA)
- Developmental Biology & Embryology (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Immunology (AREA)
- Reproductive Health (AREA)
- Physics & Mathematics (AREA)
- Pharmacology & Pharmacy (AREA)
- Plant Pathology (AREA)
- Hematology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Gynecology & Obstetrics (AREA)
- Toxicology (AREA)
- Communicable Diseases (AREA)
- Pulmonology (AREA)
- Mycology (AREA)
- Urology & Nephrology (AREA)
- Botany (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/523,547 US20170304430A1 (en) | 2014-11-05 | 2015-11-05 | Virus vector, cell, and construct |
| KR1020177015408A KR20200066748A (ko) | 2014-11-05 | 2015-11-05 | 바이러스 벡터, 세포 및 작제물 |
| EP15857426.9A EP3216868A4 (en) | 2014-11-05 | 2015-11-05 | Virus vector, cell, and construct |
| CN201580068057.8A CN107109436A (zh) | 2014-11-05 | 2015-11-05 | 病毒载体、细胞及构建体 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014225642A JP6854461B2 (ja) | 2014-11-05 | 2014-11-05 | ウイルスベクター、iPS細胞の作製方法およびコンストラクト |
| JP2014-225642 | 2014-11-05 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016072446A1 true WO2016072446A1 (ja) | 2016-05-12 |
Family
ID=55909170
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/081145 Ceased WO2016072446A1 (ja) | 2014-11-05 | 2015-11-05 | ウイルスベクター、細胞およびコンストラクト |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20170304430A1 (enExample) |
| EP (1) | EP3216868A4 (enExample) |
| JP (1) | JP6854461B2 (enExample) |
| KR (1) | KR20200066748A (enExample) |
| CN (1) | CN107109436A (enExample) |
| WO (1) | WO2016072446A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023127879A1 (ja) * | 2021-12-28 | 2023-07-06 | Jcrファーマ株式会社 | 安全な遺伝子治療のための抗トランスフェリン受容体抗体と生理活性を有する蛋白質との融合蛋白質 |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3074003B1 (en) | 2013-11-26 | 2023-01-11 | Zoetis Services LLC | Compositions for induction of immune response |
| JP7462863B2 (ja) * | 2019-04-15 | 2024-04-08 | 国立感染症研究所長 | 組換え麻疹ウイルスの増殖方法及び宿主細胞 |
| CN110904232B (zh) * | 2019-12-12 | 2022-05-27 | 上海东方肝胆外科医院 | 评估索拉非尼疗效的分子标记及其检测试剂盒 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007007921A1 (ja) * | 2005-07-13 | 2007-01-18 | Kyushu University, National University Corporation | 分節ゲノム型組換えモノネガウイルスベクター |
| WO2010008054A1 (ja) * | 2008-07-16 | 2010-01-21 | ディナベック株式会社 | 染色体非組み込み型ウイルスベクターを用いてリプログラムされた細胞を製造する方法 |
| WO2012029770A1 (ja) * | 2010-08-30 | 2012-03-08 | ディナベック株式会社 | 多能性幹細胞を誘導するための組成物およびその使用 |
| WO2012063817A1 (ja) * | 2010-11-09 | 2012-05-18 | 独立行政法人産業技術総合研究所 | 末梢血単球由来多能性幹細胞作製方法 |
| WO2015046229A1 (ja) * | 2013-09-24 | 2015-04-02 | ディナベック株式会社 | 多能性幹細胞の誘導効率を改善する方法 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS602058A (ja) | 1983-06-15 | 1985-01-08 | Mitsubishi Electric Corp | 電機子の製造方法 |
| US20030170897A1 (en) * | 2000-06-27 | 2003-09-11 | Enyu Imai | Virus vector for transferring gene into renal cells |
-
2014
- 2014-11-05 JP JP2014225642A patent/JP6854461B2/ja active Active
-
2015
- 2015-11-05 WO PCT/JP2015/081145 patent/WO2016072446A1/ja not_active Ceased
- 2015-11-05 KR KR1020177015408A patent/KR20200066748A/ko not_active Withdrawn
- 2015-11-05 EP EP15857426.9A patent/EP3216868A4/en not_active Withdrawn
- 2015-11-05 CN CN201580068057.8A patent/CN107109436A/zh active Pending
- 2015-11-05 US US15/523,547 patent/US20170304430A1/en not_active Abandoned
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007007921A1 (ja) * | 2005-07-13 | 2007-01-18 | Kyushu University, National University Corporation | 分節ゲノム型組換えモノネガウイルスベクター |
| WO2010008054A1 (ja) * | 2008-07-16 | 2010-01-21 | ディナベック株式会社 | 染色体非組み込み型ウイルスベクターを用いてリプログラムされた細胞を製造する方法 |
| WO2012029770A1 (ja) * | 2010-08-30 | 2012-03-08 | ディナベック株式会社 | 多能性幹細胞を誘導するための組成物およびその使用 |
| WO2012063817A1 (ja) * | 2010-11-09 | 2012-05-18 | 独立行政法人産業技術総合研究所 | 末梢血単球由来多能性幹細胞作製方法 |
| WO2015046229A1 (ja) * | 2013-09-24 | 2015-04-02 | ディナベック株式会社 | 多能性幹細胞の誘導効率を改善する方法 |
Non-Patent Citations (5)
| Title |
|---|
| HIRAMOTO, TAKAFUMI ET AL.: "Newly Developed Measles Viral Vector Can Efficiently Transduce Multiple Genes into naive T Cells", BLOOD, vol. 124, 6 December 2014 (2014-12-06), XP009502736 * |
| See also references of EP3216868A4 * |
| TAHARA, MAINO ET AL.: "Altered interaction of the matrix protein with the cytoplasmic tail of hemagglutinin modulates measles virus growth by affecting virus assembly and cell - cell fusion", J. VIROL., vol. 81, no. 13, 2007, pages 6827 - 6836, XP055440097 * |
| TAHARA, MAINO ET AL.: "Multiple amino acid substitutions in hemagglutinin are necessary for wild-type measles virus to acquire the ability to use receptor CD 46 efficiently", J. VIROL., vol. 81, no. 6, 2007, pages 2564 - 2572, XP055440086 * |
| TAKAFUMI HIRAMOTO ET AL.: "Idenshi Kaihen Hidenpagata Hashika Viral Vector o Mochiita Hito iPS Saibo to Kitei Jotai iPS-yo Saibo no Juritsu", REGENERATIVE MEDICINE SPECIAL EXTRA ISSUE DAI 14 KAI THE JAPANESE SOCIETY FOR REGENERATIVE MEDICINE SOKAI PROGRAM SHOROKU, vol. 14, 1 February 2015 (2015-02-01), pages 259, XP009502750 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023127879A1 (ja) * | 2021-12-28 | 2023-07-06 | Jcrファーマ株式会社 | 安全な遺伝子治療のための抗トランスフェリン受容体抗体と生理活性を有する蛋白質との融合蛋白質 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN107109436A (zh) | 2017-08-29 |
| KR20200066748A (ko) | 2020-06-11 |
| EP3216868A1 (en) | 2017-09-13 |
| JP6854461B2 (ja) | 2021-04-07 |
| JP2016086744A (ja) | 2016-05-23 |
| US20170304430A1 (en) | 2017-10-26 |
| EP3216868A4 (en) | 2018-08-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7072808B2 (ja) | 多能性幹細胞から免疫細胞療法用t細胞を誘導する方法 | |
| JP6865199B2 (ja) | リプログラミングt細胞および造血細胞 | |
| JP7061961B2 (ja) | 多能性幹細胞の免疫細胞への分化を誘導する方法 | |
| JPWO2017179720A1 (ja) | Cd8陽性t細胞を誘導する方法 | |
| CN102144027B (zh) | 生产多能干细胞的方法 | |
| JP5751548B2 (ja) | イヌiPS細胞及びその製造方法 | |
| JPWO2016010155A1 (ja) | 抗原特異的t細胞受容体遺伝子を有する多能性幹細胞の製造方法 | |
| WO2020027094A1 (ja) | iPS細胞を介して再生T細胞集団を製造する方法 | |
| KR20200139799A (ko) | 재프로그래밍 벡터 | |
| JP6854461B2 (ja) | ウイルスベクター、iPS細胞の作製方法およびコンストラクト | |
| JP6275646B2 (ja) | Mait様細胞およびその作製方法 | |
| WO2015099134A1 (ja) | 再構成されたt細胞レセプター遺伝子を有する多能性幹細胞由来のt前駆細胞を用いる免疫細胞療法 | |
| JP5856949B2 (ja) | 人工多能性幹細胞の製造方法 | |
| WO2016010153A1 (ja) | 免疫細胞療法用t細胞の誘導方法 | |
| Schlaeger | Nonintegrating human somatic cell reprogramming methods | |
| Hiramoto et al. | Non-transmissible MV vector with segmented RNA genome establishes different types of iPSCs from hematopoietic cells | |
| JP2019170393A (ja) | iPS細胞の作製方法 | |
| US20220233665A1 (en) | Medicinal composition | |
| Walsh | Modeling Normal and Malignant Hematopoiesis Using Human Pluripotent Stem Cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15857426 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 15523547 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015857426 Country of ref document: EP |