WO2015178007A1 - Sunscreen products in which excessive whiteness due to titanium dioxide and zinc oxide is visually masked upon skin application - Google Patents
Sunscreen products in which excessive whiteness due to titanium dioxide and zinc oxide is visually masked upon skin application Download PDFInfo
- Publication number
- WO2015178007A1 WO2015178007A1 PCT/JP2015/002486 JP2015002486W WO2015178007A1 WO 2015178007 A1 WO2015178007 A1 WO 2015178007A1 JP 2015002486 W JP2015002486 W JP 2015002486W WO 2015178007 A1 WO2015178007 A1 WO 2015178007A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- inclusive
- sunscreen product
- type
- oil
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/11—Encapsulated compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/04—Dispersions; Emulsions
- A61K8/06—Emulsions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/04—Dispersions; Emulsions
- A61K8/06—Emulsions
- A61K8/064—Water-in-oil emulsions, e.g. Water-in-silicone emulsions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/19—Cosmetics or similar toiletry preparations characterised by the composition containing inorganic ingredients
- A61K8/27—Zinc; Compounds thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/19—Cosmetics or similar toiletry preparations characterised by the composition containing inorganic ingredients
- A61K8/29—Titanium; Compounds thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q17/00—Barrier preparations; Preparations brought into direct contact with the skin for affording protection against external influences, e.g. sunlight, X-rays or other harmful rays, corrosive materials, bacteria or insect stings
- A61Q17/04—Topical preparations for affording protection against sunlight or other radiation; Topical sun tanning preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/20—Chemical, physico-chemical or functional or structural properties of the composition as a whole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/56—Compounds, absorbed onto or entrapped into a solid carrier, e.g. encapsulated perfumes, inclusion compounds, sustained release forms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/60—Particulates further characterized by their structure or composition
- A61K2800/61—Surface treated
- A61K2800/62—Coated
- A61K2800/63—More than one coating
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/60—Particulates further characterized by their structure or composition
- A61K2800/65—Characterized by the composition of the particulate/core
- A61K2800/651—The particulate/core comprising inorganic material
Definitions
- the present invention generally relates to sunscreen products (or sunscreen cosmetic compositions) that contain titanium dioxide and zinc oxide to provide protection to skin from ultraviolet light. These products do not require (though they may contain) organic, ultraviolet-absorbing agents for effective ultraviolet protection, and yet exhibit colors attractive to users of the products both in their pre-application state and after application to the skin.
- the present invention generally relates to precursors (or materials) of these sunscreen products.
- the sunscreen product may be generated by stirring or agitating the precursor.
- the present invention further relates generally to (cosmetic) methods of protecting human skin from ultraviolet light by applying the sunscreen product to the skin, or by generating the sunscreen product from the precursor and applying it to the skin.
- UV-absorbing agents are usually organic compounds, such as ethylhexyl methoxycinnamate and octocrylene.
- ultraviolet-scattering agents include inorganic, powder components, such as titanium dioxide and zinc oxide.
- non-chemical sunscreens are intended to exert the effects of protecting the skin from ultraviolet light by containing inorganic, ultraviolet-scattering agents, but not organic, ultraviolet-absorbing agents. This is because these organic, ultraviolet-absorbing agents are apt to irritate or sensitize the skin of a user.
- these non-chemical sunscreen products must contain an increased amount of inorganic, powder components such as titanium dioxide and zinc oxide so that they can compensate for the absence of organic, ultraviolet-absorbing agents.
- a sunscreen product contains these inorganic, powder components in an increased amount, however, the product appears excessively white to the eye. While a product appearing excessively white in its pre-application state may not be problematic, the sunscreen product will not be attractive to a user if such excessive whiteness remains and lingers on even after the product is applied to the skin.
- the issue of excessive whiteness arises whenever an elevated amount of titanium dioxide, zinc oxide, or both is used, regardless of the presence or absence of organic, ultraviolet-absorbing agents in the product. The issue of excessive whiteness is thus not limited to non-chemical sunscreens.
- pigments may be added to a non-chemical sunscreen product to suppress or mask the appearance of excessive whiteness
- pigments that impart natural-looking color to the product post-application i.e., when it is spread out on the skin
- pre-application state i.e., in bulk
- One aspect of the present invention relates to sunscreen products that contain multilayer-type encapsulations containing pigments. These multilayer-type encapsulations remain intact within a sunscreen product before the product is applied to the skin, thereby reducing or hiding the visual effects of a pigment’s color to the eye. When the product is applied to the skin, however, the encapsulations are broken by the force exerted during strokes of application (for example, by the palm of a hand or fingers), and the pigments are released and spread out over the skin to compensate for or visually override the excessive whiteness that may arise from the inorganic, powder components.
- a precursor may be a mixture of two phases separated on a macroscopic scale in a container, in which the upper layer is an oil phase and the lower layer is an aqueous phase. Titanium dioxide, zinc oxide, and multilayer-type encapsulations containing pigments may be in either phase.
- the container may further hold inside a stirring ball or a stirring object, and right before use, the precursor may be stirred and mixed by manually shaking the container to move the stirring ball or object around, thereby producing a water-in-oil-emulsion-type emulsion, which is then applied to the skin.
- Similar stirring and mixing effects may be achieved by simply shaking the container if there is no stirring object inside but sufficient free space is available within the container, or by inserting a stick or an elongated object from the outside and actively agitating the mixture. While the multilayer-type encapsulations containing pigments break apart when manually applied to the skin, the inventor of the present invention has discovered that they remain intact when the precursor is manually stirred and mixed.
- Yet another aspect of the present invention relates to methods of protecting the skin from ultraviolet light by applying a sunscreen product described above, or by preparing a sunscreen product from its precursor as described above and then applying it to the skin.
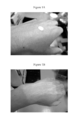
- Figures 1A and 1B are color photographs of a comparative sunscreen product that contains 5 weight% of hydrophobized titanium dioxide and 25 weight% of hydrophobized zinc oxide, but no organic, ultraviolet-absorbing agents, no naked pigments, and no multilayer-type encapsulations containing pigments. (This is Comparative Example 4 later on.)
- Figure 1A shows the comparative sunscreen product before it is spread out on the skin
- Figure 1B shows the comparative sunscreen product after it has been spread out on the skin.
- Figures 2A and 2B are color photographs of a comparative sunscreen product that contains 5 weight% of hydrophobized titanium dioxide, 25 weight% of hydrophobized zinc oxide, 0.048 weight% of Unipure Red LC 381 LL (i.e., naked red pigment), and 0.096 weight% of Unipure Yellow LC 182 LL (i.e., naked yellow pigment), but no organic, ultraviolet-absorbing agents and no multilayer-type encapsulations containing pigments. (This is Comparative Example 3 later on.)
- Figure 2A shows the comparative sunscreen product in bulk, that is, before it is applied to the skin
- Figure 2B shows the comparative sunscreen product after it has been spread out on the skin.
- Figures 3A and 3B are color photographs of a sunscreen product of the present invention, which contains 5 weight% of hydrophobized titanium dioxide, 25 weight% of hydrophobized zinc oxide, 0.2 weight% of MAGICOLOR (Registered Trademark) 103RP (which contains red pigment), and 0.4 weight% of MAGICOLOR (Registered Trademark) 103YP (which contains yellow pigment), but no organic, ultraviolet-absorbing agents and no naked pigments.
- Figure 3A shows the inventive sunscreen product before it is spread out on the skin
- Figure 3B shows the inventive sunscreen product after it has been spread out on the skin.
- the sunscreen products of the present invention may be of water-in-oil-emulsion types, of single-oil-phase types, or of other types. As described below, they contain multilayer-type encapsulations that contain pigments, and titanium dioxide powder and zinc oxide powder whose surface has been treated or processed to be hydrophobic. They may also exhibit low viscosity, such as 10,000 mPa ⁇ s or less at 30oC, and may further exhibit SPF of certain values, such as 40 or more.
- the sunscreen product precursors of the present invention are mixtures of a plurality of phases separated on a macroscopic scale, which are capable of producing the sunscreen products mentioned above, for example, those of water-in-oil-emulsion types, when physically stirred or agitated.
- the methods of protecting the skin from ultraviolet light of the present invention involve applying a sunscreen product mentioned above to the skin, or stirring or agitating a sunscreen product precursor mentioned above to prepare a sunscreen product and applying that product to the skin.
- Figures 1A and 1B are color photographs of a comparative sunscreen product that contains 5 weight% of hydrophobized titanium dioxide and 25 weight% of hydrophobized zinc oxide, but no organic, ultraviolet-absorbing agents, no naked pigments, and no multilayer-type encapsulations containing pigments.
- Figure 1A shows the comparative sunscreen product before it is spread out on the skin
- Figure 1B shows the comparative sunscreen product after it has been spread out on the skin.
- the weight percentages of the hydrophobized titanium dioxide and zinc oxide are chosen so that the comparative sunscreen product will have a desirable level of ultraviolet-protection effects. While a user may not mind the whiteness appearing in Figure 1A before spreading it out on the skin, the excessive whiteness that remains or lingers on after spreading it out on the skin as shown in Figure 1B will not be attractive to the user.
- Figures 2A and 2B are color photographs of a comparative sunscreen product that contains 5 weight% of hydrophobized titanium dioxide, 25 weight% of hydrophobized zinc oxide, 0.048 weight% of Unipure Red LC 381 LL (i.e., naked red pigment), and 0.096 weight% of Unipure Yellow LC 182 LL (i.e., naked yellow pigment), but no organic, ultraviolet-absorbing agents and no multilayer-type encapsulations containing pigments.
- Figure 2A shows the comparative sunscreen product in bulk, that is, before it is applied to the skin
- Figure 2B shows the comparative sunscreen product after it has been spread out on the skin.
- the weight percentages of the naked red and yellow pigments are chosen so that the excessive whiteness that would otherwise appear is masked when it is applied to the skin as shown in Figure 2B. However, when such an adjustment is made, the color of the comparative sunscreen product in bulk, that is, before use, ends up being too thick, and thus unattractive to a user, as shown in Figure 2A.
- Figures 3A and 3B are color photographs of a sunscreen product of the present invention, which contains 5 weight% of hydrophobized titanium dioxide, 25 weight% of hydrophobized zinc oxide, 0.2 weight% of MAGICOLOR (Registered Trademark) 103RP (which contains red pigment), and 0.4 weight% of MAGICOLOR (Registered Trademark) 103YP (which contains yellow pigment), but no organic, ultraviolet-absorbing agents and no naked pigments.
- MAGICOLOR (Registered Trademark) 103RP and 103YP refer to certain commercial products of multilayer-type encapsulations containing pigments.
- Figure 3A shows the inventive sunscreen product before it is spread out on the skin
- Figure 3B shows the inventive sunscreen product after it has been spread out on the skin.
- the sunscreen product of the present invention does not exhibit a thick color in bulk, that is, before use, that may be unattractive to a user, as shown in Figure 3A. And yet, it is able to mask the excessive whiteness that would otherwise appear and to impart a natural-looking color when spread out on the skin as shown in Figure 3B.
- Water-in-Oil-Emulsion-Type Sunscreen Products Conventional water-in-oil-emulsion-type sunscreen products may be used as the bases of the sunscreen products of the present invention, as long as they are compatible with the multilayer-type encapsulations containing pigments that are to be added.
- Water-in-oil emulsions commonly refer to mixtures of water and one or more kinds of oil in which aqueous droplets of less than macroscopic sizes are dispersed in the oil.
- Substances that may form the solvent of the oil phase of a water-in-oil-emulsion-type sunscreen product include decamethyl cyclopentasiloxane, isononyl isononanoate, dimethyl polysiloxane, heptamethyl octyl trisiloxane, trimethylsiloxysilicate, liquid paraffin, squalane, avocado oil, macadamia nut oil, corn oil, olive oil, canola oil, evening primrose oil, castor oil, sunflower seed oil, tea oil, rice bran oil, jojoba oil, cacao butter, palm oil, squalene, beef tallow, japan wax, bees wax, candelilla wax, carnauba wax, spermaceti, lanolin, polyoxyethylene (8 mol) oleyl alcohol ether, glyceryl monooleate, cyclomethicone, diphenyl polysiloxane, isodecane, isodode
- decamethyl cyclopentasiloxane dimethyl polysiloxane, liquid paraffin, and isododecane.
- dimethyl polysiloxane dimethyl polysiloxane
- liquid paraffin liquid paraffin
- isododecane isododecane
- Water may be contained in conventional water-in-oil-emulsion-type sunscreen products in an amount ranging between 0.01 weight% inclusive and 98 weight% inclusive, preferably between 1 weight% inclusive and 60 weight% inclusive, more preferably between 3 weight% inclusive and 40 weight% inclusive, and most preferably between 5 weight% inclusive and 25 weight% inclusive, relative to the total amount of the water-in-oil-emulsion-type sunscreen product.
- the less water the product contains the lower its viscosity becomes.
- the oil phase of conventional water-in-oil-emulsion-type sunscreen products usually contains organic, ultraviolet-absorbing agents and inorganic, ultraviolet-scattering agents, in which the surface of the inorganic, ultraviolet-scattering agents has been treated or processed to be hydrophobic.
- the water-in-oil-emulsion-type sunscreen products of the present invention require the use of hydrophobized, inorganic, ultraviolet-scattering agents, they do not require the use of organic, ultraviolet-absorbing agents.
- conventional water-in-oil-emulsion-type sunscreen products may also contain other powder ingredients, liquid fats and oils, solid fats and oils, waxes, hydrocarbon oils, higher fatty acids, higher alcohols, ester oils, silicone oils, anionic surfactants, cationic surfactants, ampholytic surfactants, hydrophilic nonionic surfactants, lipophilic surfactants, humectants, natural water-soluble polymers, semisynthetic water-soluble polymers, synthetic water-soluble polymers, thickeners, metal ion sequestering agents, lower alcohols, polyhydric alcohols, monosaccharides, oligosaccharides, polysaccharides, amino acids, amino acid derivatives, organic amines, polymer emulsions, pH adjusting agents, vitamins, antioxidants, antioxidation assistants, and other possible ingredients, including skin nutrients and perfumes. Examples of these optional ingredients are described below.
- the water-in-oil-emulsion-type sunscreen products of the present invention may contain any of
- organic, ultraviolet-absorbing agents include para-aminobenzoic acid (“PABA”), PABA monoglycerin ester, N,N-dipropoxy PABA ethyl ester, N,N-diethoxy PABA ethyl ester, N,N-dimethyl PABA ethyl ester, N,N-dimethyl PABA butyl ester, homo mentyl-N-acetyl anthranilate, amyl salicylate, mentyl salicylate, homo mentyl salicylate, octyl salicylate, phenyl salicylate, benzyl salicylate, p-isopropanol phenyl salicylate, octyl cinnamate, ethyl-4-isopropyl cinnamate, methyl-2,5-diisopropyl cinnamate, ethyl-2,4-diis
- the water-in-oil-emulsion-type sunscreen products of the present invention may contain organic, ultraviolet-absorbing agents in no detectable amount, in an amount of no more than 3 weight%, in an amount of no more than 5 weight%, or in an amount of no more than 10 weight%, relative to the total weight of the sunscreen product, as long as the organic, ultraviolet-absorbing agents are compatible with the multilayer-type encapsulations containing pigments that are used.
- powder ingredients include: inorganic powders (for example, talc, kaolin, mica, sericite, muscovite, phlogopite, synthetic mica, lepidolite, biotite, vermiculite, magnesium carbonate, calcium carbonate, aluminum silicate, barium silicate, calcium silicate, magnesium silicate, strontium silicate, tungstic acid metal salt, magnesium, silica, zeolite, barium sulfate, firing calcium sulfate (calcined gypsum), calcium phosphate, fluorine-apatite, hydroxy apatite, ceramic powder, metallic soaps (for example, myristic acid zinc, calcium palmitate, and aluminum stearate), and boron nitride); and organic powders (for example, polyamide resin powder (nylon powder), polyethylene powder, poly-methyl methacrylate powder, polystyrene powder, powders of the copolymer resin of styrene
- inorganic powders for
- liquid fats and oils examples include avocado oil, tsubaki oil, turtle fatty acid, macadamia nut oil, corn oil, mink oil, olive oil, rapeseed oil, egg yolk oil, sesame oil, persic oil, wheat germ oil, sasanqua oil, castor oil, linseed oil, safflower oil, cotton seed oil, perilla oil, soybean oil, peanut oil, tea seed oil, Japanese nutmeg oil, rice bran oil, Chinese gimlet oil, Japanese gimlet oil, jojoba oil, germ oil, and triglycerin.
- Solid fats and oils examples include cacao butter, coconut oil, hydrogenated coconut oil, palm oil, palm kernel oil, Japanese core wax nucleus oil, hydrogenated oil, Japanese core wax, and hydrogenated castor oil.
- waxes examples include beeswax, candelilla wax, cotton wax, carnauba wax, bayberry wax, tree wax, whale wax, montan wax, bran wax, lanolin, kapok wax, lanolin acetate, liquid lanolin, sugar cane wax, lanolin fatty acid isopropyl ester, hexyl laurate, reduced lanolin, jojoba wax, hard lanolin, shellac wax, POE lanolin alcohol ether, POE lanolin alcohol acetate, POE cholesterol ether, lanolin fatty acid polyethylene glycol, and POE hydrogenated lanolin ethyl alcohol ether.
- hydrocarbon oils examples include liquid petrolatum, ozocerite, squalane, pristane, paraffin, ceresin, squalene, petrolatum, and microcrystalline wax.
- Higher fatty acids include lauric acid, myristic acid, palmitic acid, stearic acid, behenic acid, oleic acid, undecylenic acid, tall oil fatty acid, isostearic acid, linolic acid, linoleic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA).
- Higher alcohols include straight-chain alcohols (for example, lauryl alcohol, cetyl alcohol, stearyl alcohol, behenyl alcohol, myristyl alcohol, oleyl alcohol, and cetostearyl alcohol) and branched-chain alcohols (for example, mono stearyl glycerin ether (batyl alcohol), 2-decyltetradecynol, lanolin alcohol, cholesterol, phytosterol, hexyl dodecanol, isostearyl alcohol, and octyl dodecanol).
- straight-chain alcohols for example, lauryl alcohol, cetyl alcohol, stearyl alcohol, behenyl alcohol, myristyl alcohol, oleyl alcohol, and cetostearyl alcohol
- branched-chain alcohols for example, mono stearyl glycerin ether (batyl alcohol), 2-decyltetradecynol, lanolin alcohol, cholesterol, phytosterol, hexyl
- ester oils examples include isopropyl myristate, cetyl octanoate, octyl dodecyl myristate, isopropyl palmitate, butyl stearate, hexyl laurate, myristil myristate, decyl oleate, dimethyl hexyl decyl octanoate, cetyl lactate, myristil lactate, lanolin acetate, isocetyl stearate, isocetyl isostearate, cholesteryl 12-hydroxystearate, di-2-ethylene glycol ethylhexanoate, dipentaerythritol fatty acid ester, n-alkylene glycol monoisostearate, neopentyl glycol dicaprate, diisostearyl malate, glyceryl di-2-heptylundecanoate, trimethylolpropane tri-2-
- silicone oils examples include: chain-type polysiloxanes (for example, dimethylpolysiloxane, methylphenyl polysiloxane, and diphenyl polysiloxane); ring-type polysiloxanes (for example, octamethylcyclotetrasiloxane, decamethyl cyclopentasiloxane, and dodecamethyl cyclohexasiloxane); silicone resins forming a three-dimensional network structure; silicone rubbers; and various modified polysiloxanes (for example, amino-modified polysiloxanes, polyether-modified polysiloxanes, alkyl-modified polysiloxanes, and fluorine-modified polysiloxanes).
- chain-type polysiloxanes for example, dimethylpolysiloxane, methylphenyl polysiloxane, and diphenyl polysiloxane
- ring-type polysiloxanes for example
- anionic surfactants examples include: fatty acid soaps (for example, sodium laurate and sodium palmitate); higher alkyl sulfuric ester salts (for example, sodium lauryl sulfate and potassium lauryl sulfate); alkylether sulfuric ester salts (for example, POE-triethanolamine lauryl sulfate and sodium POE-lauryl sulfate); N-acyl sarcosinic acids (for example, sodium N-lauroyl sarcosinate); higher fatty acid amide sulfonic acid salts (for example, sodium N-myristoyl N-methyl taurate, sodium cocoyl methyl taurate, and sodium laurylmethyl taurate); phosphoric ester salts (for example, sodium POE-oleyl ether phosphate and POE stearyl ether phosphoric acid); sulfosuccinates (for example, sodium di-2-ethylhexylsulfate); sulfos
- cationic surfactants include alkyltrimethylammonium salts (for example, stearyltrimethyl ammonium chloride and lauryltrimethyl ammonium chloride); alkylpyridinium salts (for example, cetylpyridinium chloride); distearyldimethylammonium chloride dialkyldimethylammonium salt; poly(N,N’-dimethyl-3-methylene piperidinium)chloride; alkyl quaternary ammonium salts; alkyl dimethylbenzyl ammonium salts; alkyl isoquinolinium salts; dialkylmorpholine salts; POE alkyl amines; alkyl amine salts; polyamine fatty acid derivatives; amylalcohol fatty acid derivatives; benzalkonium chloride; and benzethonium chloride.
- alkyltrimethylammonium salts for example, stearyltrimethyl ammonium chloride and lauryltrimethyl ammonium chloride
- ampholytic surfactants examples include: imidazoline-type ampholytic surfactants (for example, 2-undecyl-N,N,N-(hydroxyethyl carboxymethyl)-2-imidazoline sodium salt and 2-cocoyl-2-imidazolinium hydroxidel-carboxyethyloxy 2 sodium salt); and betaine-type surfactants (for example, 2-heptadecyl-n-carboxymethyl-n-hydroxyethyl imidazolinium betaine, lauryldimethylaminoacetic acid betaine, alkyl betaine, amide betaine, and sulfobetaine).
- imidazoline-type ampholytic surfactants for example, 2-undecyl-N,N,N-(hydroxyethyl carboxymethyl)-2-imidazoline sodium salt and 2-cocoyl-2-imidazolinium hydroxidel-carboxyethyloxy 2 sodium salt
- betaine-type surfactants for example, 2-
- hydrophilic nonionic surfactants include: polyglycerin fatty acid esters (for example, hexaglyceryl monolaurate (HLB 14.5), hexaglyceryl monomyristate (HLB 11), hexaglyceryl monostearate (HLB 9.0), hexaglyceryl monooleate (HLB 9.0), decaglyceryl monolaurate (HLB 15.5), decaglyceryl monomyristate (HLB 14.0), decaglyceryl monostearate (HLB 12.0), decaglyceryl monoisostearate (HLB 12.0), decaglyceryl monooleate (HLB 12.0), decaglyceryl distearate (HLB 9.5), and decaglyceryl diisostearate (HLB 10.0)); polyoxyethylene glycerin fatty acid esters (for example, polyoxyethylene (“POE”) (5)
- lipophilic surfactants examples include POE (2) stearyl ether (HLB 4.0), self-emulsified propylene glycol monostearate (HLB 4.0), glyceryl myristate (HLB 3.5), glyceryl monostearate (HLB 4.0), self-emulsified glyceryl monostearate (HLB 4.0), glyceryl monoisostearate (HLB 4.0), glyceryl monooleate (HLB 2.5), hexaglyceryl tristearate (HLB 2.5), decaglyceryl pentastearate (HLB 3.5), decaglyceryl pentaisostearate (HLB 3.5), decaglyceryl pentaoleate (HLB 3.5), sorbitan monostearate (HLB 4.7), sorbitan tristearate (HLB 2.1), sorbitan monoisostearate (HLB 5.0), sorbitan sesquiisost
- humectants examples include polyethylene glycol, propylene glycol, glycerin, 1,3-butylene glycol, xylitol, sorbitol, maltitol, chondroitin sulfate, hyaluronic acid, mucoitin sulfuric acid, charonic acid, atelocollagen, cholesteryl-12-hydroxy stearate, sodium lactate, bile salt, dl-pyrrolidone carboxylic acid salt, short-chain soluble collagen, diglycerin (EO) PO adduct, chestnut rose fruit extract, yarrow extract, and sweet clover extract.
- EO diglycerin
- Natural water-soluble polymers examples include: plant-type polymers (for example, gum arabic, gum tragacanth, galactan, guar gum, carob gum, karaya gum, carrageenan, pectin, agar, quince seed (Cyclonia oblonga), algae colloids (brown algae extract), starches (rice, corn, potato, and wheat), and glycyrrhizic acid); microorganism-type polymers (for example, xanthan gum, dextran, succinoglucan, and pullulan); and animal-type polymers (for example, collagen, casein, albumin, and gelatin).
- plant-type polymers for example, gum arabic, gum tragacanth, galactan, guar gum, carob gum, karaya gum, carrageenan, pectin, agar, quince seed (Cyclonia oblonga), algae colloids (brown algae extract), starches (rice, corn, potato, and wheat
- semisynthetic water-soluble polymers include: starch-type polymers (for example, carboxymethyl starch and methylhydroxypropyl starch); cellulosic polymers (for example, methyl cellulose, ethyl cellulose, methylhydroxypropyl cellulose, hydroxyethyl cellulose, cellulose sodium sulfate, hydroxypropyl cellulose, carboxymethyl cellulose, sodium carboxymethyl cellulose, crystal cellulose, and cellulose powder); and alginic acid-type polymers (for example, sodium alginate and propyleneglycol alginate).
- starch-type polymers for example, carboxymethyl starch and methylhydroxypropyl starch
- cellulosic polymers for example, methyl cellulose, ethyl cellulose, methylhydroxypropyl cellulose, hydroxyethyl cellulose, cellulose sodium sulfate, hydroxypropyl cellulose, carboxymethyl cellulose, sodium carboxymethyl cellulose, crystal cellulose, and cellulose powder
- Synthetic water-soluble polymers examples include: vinyl polymers (for example, polyvinyl alcohol, polyvinyl methyl ether, polyvinyl pyrrolidone, and carboxy vinyl polymer); polyoxyethylene-type polymers (for example, a copolymer of polyethylene glycol 20,000, 40,000, or 60,000, and polyoxyethylene polyoxypropylene); acrylic polymers (for example, sodium polyacrylate, polyethylacrylate, and polyacrylamide); polyethyleneimine; and cationic polymers.
- vinyl polymers for example, polyvinyl alcohol, polyvinyl methyl ether, polyvinyl pyrrolidone, and carboxy vinyl polymer
- polyoxyethylene-type polymers for example, a copolymer of polyethylene glycol 20,000, 40,000, or 60,000, and polyoxyethylene polyoxypropylene
- acrylic polymers for example, sodium polyacrylate, polyethylacrylate, and polyacrylamide
- polyethyleneimine examples include cationic polymers.
- thickeners examples include gum arabic, carrageenan, karaya gum, gum tragacanth, carob gum, quince seed (Cyclonia oblonga), casein, dextrin, gelatin, sodium pectate, sodium arginate, methyl cellulose, ethyl cellulose, CMC, hydroxy ethyl cellulose, hydroxypropyl cellulose, PVA, PVM, PVP, sodium polyacrylate, carboxy vinyl polymer, locust bean gum, guar gum, tamarind gum, cellulose dialkyl dimethylammonium sulfate, xanthan gum, aluminum magnesium silicate, bentonite, hectorite, Al-Mg silicate (beagum), laponite, and silicic acid anhydride.
- thickeners include gum arabic, carrageenan, karaya gum, gum tragacanth, carob gum, quince seed (Cyclonia oblonga), casein, dextrin
- Metal ion sequestering agents include 1-hydroxy ethane-1,1-diphosphonic acid, 1-hydroxy ethane-1,1-diphosphonic acid tetrasodium salt, disodium edetate, trisodium edetate, tetrasodium edetate, sodium citrate, sodium polyphosphate, sodium metaphosphate, gluconic acid, phosphoric acid, citric acid, ascorbic acid, succinic acid, and trisodium ethylenediaminehydroxyethyl triacetate.
- Lower alcohols examples include ethanol, propanol, isopropanol, isobutyl alcohol, and t-butyl alcohol.
- polyhydric alcohols examples include: dihydric alcohols (for example, ethylene glycol, propylene glycol, trimethylene glycol, 1,2-butylene glycol, 1,3-butylene glycol, tetramethylene glycol, 2,3-butylene glycol, pentamethylene glycol, 2-butene-1,4-diol, hexylene glycol, and octylene glycol); trihydric alcohols (for example, glycerin and trimethylolpropane); tetrahydric alcohols (for example, pentaerythritol such as 1,2,6-hexanetriol); pentahydric alcohols (for example, xylitol); hexahydric alcohols (for example, sorbitol and mannitol); polyhydric alcohol polymers (for example, diethylene glycol, dipropylene glycol, triethylene glycol, polypropylene glycol, tetraethylene glycol,
- monosaccharides include: trioses (for example, D-glyceryl aldehyde and dihydroxyacetone); tetroses (for example, D-erythrose, D-erythrulose, D-threose, and erythritol); pentoses (for example, L-arabinose, D-xylose, L-lyxose, D-arabinose, D-ribose, D-ribulose, D-xylulose, and L-xylulose); hexoses (for example, D-glucose, D-talose, D-psicose, D-galactose, D-fructose, L-galactose, L-mannose, and D-tagatose); heptoses (for example, aldoheptose and heprose); octoses (for example, octurose); deoxysugar
- Oligosaccharides include sucrose, umbelliferose, lactose, planteose, isolignoses, ⁇ , ⁇ -trehalose, raffinose, lignoses, umbilicine, stachyose, and verbascose.
- polysaccharides examples include cellulose, quince seed, chondroitin sulfate, starch, galactan, dermatan sulfate, glycogen, gum arabic, heparan sulfate, hyaluronic acid, traganth gum, keratan sulfate, chondroitin, xanthan gum, mucoitin sulfuric acid, guar gum, dextran, kerato sulfate, locustbean gum, succinoglucane, and charonic acid.
- amino acids examples include neutral amino acids (for example, threonine and cysteine) and basic amino acids (for example, hydroxylysine).
- amino acid derivatives examples include sodium acyl sarcosinate (for example, sodium N-lauroyl sarcosinate), acyl glutamate, sodium acyl ⁇ -alanine, glutathione, and pyrrolidone carboxylic acid.
- sodium acyl sarcosinate for example, sodium N-lauroyl sarcosinate
- acyl glutamate for example, sodium N-lauroyl sarcosinate
- sodium acyl ⁇ -alanine sodium acyl ⁇ -alanine
- glutathione and pyrrolidone carboxylic acid.
- Organic amines examples include monoethanolamine, diethanolamine, triethanolamine, morpholine, triisopropanolamine, 2-amino-2-methyl-1,3-propanediol, and 2-amino-2-methyl-1-propanol.
- polymer emulsions examples include acrylic resin emulsions, ethyl polyacrylate emulsions, acryl resin liquids, polyacrylic alkyl ester emulsions, polyvinyl acetate resin emulsions, and natural rubber latex.
- pH Adjusting agents examples include buffers such as lactic acid-sodium lactate, citric acid-sodium citrate, and succinic acid-sodium succinate.
- vitamins include vitamin A, B1, B2, B6, C, and E as well as their derivatives, pantothenic acid and its derivatives, and biotin.
- antioxidants include tocopherols, dibutyl hydroxytoluene, butyl hydroxyanisole, and gallic ester.
- antioxidation assistants examples include phosphoric acid, citric acid, ascorbic acid, maleic acid, malonic acid, succinic acid, fumaric acid, cephalin, hexametaphosphate, phytic acid, and ethylene diamine tetraacetic acid.
- compositions include: skin nutrients; perfumes; antiseptics (for example, methylparaben, ethylparaben, butylparaben, and phenoxyethanol); anti-inflammatory agents (for example, glycyrrhizic acid derivatives, glycyrrhetinic acid derivatives, salicylic acid derivatives, hinokitiol, zinc oxide, allantoin, tranexamic acid, thiotaurine, and hypotaurine); whitening agents (for example, creeping saxifrage extract and arbutin); various extracts (for example, Phellodendri Cortex, goldthread, lithospermum root, Paeonia lactiflora, Swertia japonica, Birch, sage, loquat, carrot, aloe, Malva sylvestris, Iris, grape, Coix ma-yuen, sponge gourd, lily, saffron, C
- a single-oil-phase-type sunscreen product of the present invention may be prepared by omitting the aqueous phase from a water-in-oil-emulsion-type sunscreen product of the present invention, that is, by not including a sufficient amount of water to form aqueous droplets within the oil phase.
- Such a single-oil-phase-type sunscreen product will still contain the essential ingredients of hydrophobized titanium dioxide, hydrophobized zinc oxide, and multilayer-type encapsulations containing pigments.
- such a separated composition is simply a precursor of the sunscreen product, as long as the sunscreen product can be restored or generated by stirring or agitating the separated composition for a reasonable time.
- a sunscreen product precursor of the present invention can be manufactured without ever forming a sunscreen product itself, and a sunscreen product precursor of the present invention might not and need not form a sunscreen product until right before it is being applied to the skin.
- the sunscreen product precursor of the present invention may be provided in a container with a stirring ball or a stirring object inside, in the form of a sunscreen product package (or kit).
- the shape of the stirring object is not particularly limited. It may be spherical, or it may be of any other shape.
- the material forming the stirring object is also not particularly limited, as long as it is compatible with the sunscreen product precursor of the present invention and the container.
- the stirring object may be made of stainless steel.
- the container may be shaken so that the movement of the ball or the object stirs or agitates the sunscreen product precursor to generate the corresponding sunscreen product.
- Stirring or agitating with a physical object placed inside a container may be particularly effective when the sunscreen product precursor of the present invention contains a large amount of titanium dioxide powder and zinc oxide powder.
- the container may have sufficient free space inside so that the open airspace acts as stirring objects when shaken.
- the sunscreen product precursor of the present invention may be stirred or agitated by inserting a stick or an elongated object from the outside as necessary. The inventor has discovered that the multilayer-type encapsulations containing pigments that are used in the present invention do not rupture when the container holding a sunscreen product precursor of the present invention is stirred or agitated manually to produce the corresponding sunscreen product.
- a multilayer-type encapsulation containing pigments has a structure with an inner core or layer and an outer layer or layers in which different pigments are located in the inner and outer parts.
- Commercially available products of multilayer-type encapsulations containing pigments include MAGICOLOR (Registered Trademark) currently sold by Biogenics, Inc., Sugarcapsule Magic SP Series currently sold by Daito Kasei Kogyo Co., Ltd., TagraCap TM currently sold by Tagra Biotechnology, and MicroBeads TM currently sold by Salvona Technologies.
- Multilayer-type encapsulations containing pigments may be made, for example, by: preparing a solution of first pigments, a plasticizer, and a polymer; spray-drying this solution to form core particles in which the pigments are enveloped by the polymer; dispersing these core particles in a solution containing second pigments; and spray-drying this dispersion to form encapsulated particles in which the second pigments are coated onto the polymer; such as described in United States Patent Application Publication No. US 2011/0165208 A1 (or equivalently, EP 2 474 299 A2, JP 2011-529104 A, KR 10-0978583 B1, or WO 2011/027960 A2), whose content is herein incorporated by reference in its entirety.
- multilayer-type encapsulations containing pigments are sometimes referred to as “color reveal technology.”
- the encapsulations are stable enough for the pigments to remain inside such that they do not exert the same visual, color effects as when they are exposed outside.
- the encapsulations break up to release the pigments, thereby causing a change in the appearance of color between before and after application.
- the effect of encapsulation is usually to suppress largely, but not completely, a pigment’s color. For example, a bulk of multilayer-type encapsulations containing red pigments appears faintly red to the eye, and a bulk of multilayer-type encapsulations containing yellow pigments appears faintly yellow to the eye.
- the amount of multilayer-type encapsulations containing pigments that may be contained in a sunscreen product or a sunscreen product precursor is not particularly limited as long as the amount is effective in achieving desirable visual effects upon application to the skin.
- it is within a range between 0.1 weight% inclusive and 1.7 weight% inclusive, more preferably between 0.1 weight% inclusive and 1.6 weight% inclusive, still more preferably between 0.2 weight% inclusive and 1.2 weight% inclusive, and most preferably between 0.3 weight% inclusive and 0.9 weight% inclusive, relative to the total weight of the sunscreen product or the sunscreen product precursor.
- it is less than 0.1 weight% the excessive whiteness that appears upon application of the sunscreen product to the skin due to titanium dioxide and zinc oxide may not be sufficiently masked.
- it is more than 1.7 weight%, the appearance of color that results upon skin application may be too thick to be natural-looking and no longer attractive to a user.
- the diameter of the multilayer-type encapsulations containing pigments that are used in a sunscreen product or a sunscreen product precursor of the present invention is preferably less than 300 ⁇ m, more preferably no more than 280 ⁇ m, still more preferably no more than 250 ⁇ m, even more preferably no more than 150 ⁇ m, yet more preferably no more than 100 ⁇ m, and most preferably no more than 60 ⁇ m.
- the diameter refers to number-average diameter. In some cases, if the diameter is 300 ⁇ m or more, a user may start to feel rough or sandy sensations when the product is applied to the skin, or there may be difficulty encountered in dispersing the encapsulations uniformly in the sunscreen product by simple stirring or agitation.
- the color of the pigments contained in the multilayer-type encapsulations that are used in a sunscreen product or a sunscreen product precursor is not particularly limited as long as the appearance of the product is attractive to a user both before and after application to the skin.
- combinations of multilayer-type encapsulations containing red pigments and multilayer-type encapsulations containing yellow pigments can be utilized to mimic a natural-looking color of the skin, such as in a ratio of red to yellow between 1:1.8 inclusive and 1:2.2 inclusive. Combinations in different proportions and of other colors may be utilized as appropriate.
- Titanium Dioxide The titanium dioxide powder (TiO 2 ) used in the present invention has been treated or processed so that its surface becomes hydrophobic (“hydrophobized titanium dioxide”). This facilitates the dispersion of titanium dioxide powder in the oil phase of a sunscreen product or a sunscreen product precursor.
- agents that may be used for hydrophobizing the surface of titanium dioxide include higher alcohols, hydrocarbons, triglycerides, esters, silicone oils, silicone resins, fluorine compounds, and fatty acids, such as alkyl triethoxysilane, alkyl trimethoxysilane, perfluoroalkyl phosphate, (alkyl acrylate/dimethyl silicone) copolymer, dextrin palmitate, triethoxysilylethylpolydimethylsiloxyethylhexyldimethicone, hydrogen dimethicone, dimethicone, polymeric silicone, sodium acryloyl dimethyl taurate/methacrylamide lauric acid copolymer, stearic acid, and myristic acid.
- fatty acids such as alkyl triethoxysilane, alkyl trimethoxysilane, perfluoroalkyl phosphate, (alkyl acrylate/dimethyl silicone) copolymer,
- the amount of hydrophobized titanium dioxide contained in a sunscreen product or a sunscreen product precursor is preferably within a range between 3 weight% inclusive and 13 weight% inclusive, more preferably between 3 weight% inclusive and 11 weight% inclusive, and most preferably between 4 weight% inclusive and 9 weight% inclusive, relative to the total weight of the sunscreen product or the sunscreen product precursor.
- hydrophobized titanium dioxide is contained in an amount that is less than 3 weight%, sufficient ultraviolet-protection effects may not be attained.
- hydrophobized titanium dioxide is contained in an amount that is more than 13 weight%, the amount of the multilayer-type encapsulations containing pigments required to visually mask the excessive whiteness caused by titanium dioxide becomes too much and leads to degradation of the appearance of the sunscreen product upon application to the skin.
- the size of the hydrophobized titanium dioxide powder that is used in a sunscreen product or a sunscreen product precursor of the present invention is not particularly limited as long as the powder exerts sufficient ultraviolet-protection effects, as commonly utilized in the art.
- Zinc Oxide The zinc oxide powder (ZnO) used in the present invention has been treated or processed so that its surface becomes hydrophobic (“hydrophobized zinc oxide”). This facilitates the dispersion of zinc oxide powder in the oil phase of a sunscreen product or a sunscreen product precursor.
- agents that may be used for hydrophobizing the surface of zinc oxide include higher alcohols, hydrocarbons, triglycerides, esters, silicone oils, silicone resins, fluorine compounds, and fatty acids, such as alkyl triethoxysilane, alkyl trimethoxysilane, perfluoroalkyl phosphate, (alkyl acrylate/dimethyl silicone) copolymer, dextrin palmitate, triethoxysilylethylpolydimethylsiloxyethylhexyldimethicone, hydrogen dimethicone, dimethicone, polymeric silicone, sodium acryloyl dimethyl taurate/methacrylamide lauric acid copolymer, stearic acid, and myristic acid.
- fatty acids such as alkyl triethoxysilane, alkyl trimethoxysilane, perfluoroalkyl phosphate, (alkyl acrylate/dimethyl silicone) copolymer,
- the combined amount of hydrophobized titanium dioxide and hydrophobized zinc oxide contained in a sunscreen product or a sunscreen product precursor is preferably within a range between 8 weight% inclusive and 40 weight% inclusive, more preferably between 9 weight% inclusive and 40 weight% inclusive, still more preferably between 15 weight% inclusive and 35 weight% inclusive, even more preferably between 20 weight% inclusive and 30 weight% inclusive, and most preferably between 20 weight% inclusive and 27 weight% inclusive, relative to the total weight of the sunscreen product or the sunscreen product precursor.
- hydrophobized titanium dioxide and hydrophobized zinc oxide are contained in a combined amount that is less than 8 weight%, sufficient ultraviolet-protection effects may not be attained.
- hydrophobized titanium dioxide and hydrophobized zinc oxide are contained in a combined amount that is more than 40 weight%, the amount of the multilayer-type encapsulations containing pigments required to visually mask the excessive whiteness caused by titanium dioxide and zinc oxide becomes too much and leads to degradation of the appearance of the sunscreen product upon application to the skin.
- the size of the hydrophobized zinc oxide powder that is used in a sunscreen product or a sunscreen product precursor of the present invention is not particularly limited as long as the powder exerts sufficient ultraviolet-protection effects, as commonly utilized in the art.
- SPF Sun Protection Factor or Sunburn Protection Factor (“SPF”) is described by the U.S. Food and Drug Administration (“FDA”) as “a measure of how much solar energy (UV radiation) is required to produce sunburn on protected skin (i.e., in the presence of sunscreen) relative to the amount of solar energy required to produce sunburn on unprotected skin” (from the FDA’s webpage titled “About FDA ⁇ Sunburn Protection Factor (SPF)”).
- FDA U.S. Food and Drug Administration
- a higher SPF means better ultraviolet-protection effects provided by a sunscreen product.
- an SPF exhibited by a sunscreen product is not particularly limited as long as it provides a desirable level of ultraviolet-protection effects depending on the intended use.
- a sunscreen product of the present invention has an SPF of 15 or more, more preferably an SPF of 20 or more, still more preferably an SPF of 30 or more, even more preferably an SPF of 40 or more, and most preferably an SPF of 50 or more.
- a sunscreen product that has an SPF of less than 15 may not provide a desirable level of ultraviolet-protection effects.
- Viscosity For the purpose of the present invention, viscosity is measured at 30oC by Type-B viscometer with a rotor revolution rate of 12 per minute.
- the viscosity of a sunscreen product of the present invention is not particularly limited as long as it does not hamper or interfere with its application to a user’s skin and it does not affect negatively the user’s sensation afterwards, and in terms of a precursor, it also does not prevent the multilayer-type encapsulations containing pigments from being uniformly dispersed and the aqueous and oil phases from forming a water-in-oil-type emulsion.
- the viscosity of a sunscreen product of the present invention is 10,000 mPa ⁇ s or less at 30oC, more preferably 7,000 mPa ⁇ s or less at 30oC, still more preferably 5,000 mPa ⁇ s or less at 30oC, and most preferably 3,000 mPa ⁇ s or less at 30oC.
- the viscosity of a sunscreen product exceeds 10,000 mPa ⁇ s at 30oC, a user may find the product rather unwieldy in applying it to the skin or may have a sticky feeling afterwards.
- the multilayer-type encapsulations containing pigments may not be uniformly dispersed when a precursor is stirred or agitated to form a water-in-oil-emulsion-type sunscreen product, and in addition, the aqueous and oil phases may not be sufficiently mixed to form a water-in-oil emulsion.
- a method of reducing skin’s exposure to ultraviolet light is provided by applying a sunscreen product of the present invention to the skin. Further, in the present invention, a method of reducing skin’s exposure to ultraviolet light is provided by preparing a sunscreen product of the present invention by stirring or agitating the corresponding sunscreen product precursor of the present invention, and then applying the sunscreen product to the skin.
- Table 1 lists the trade names and the INCI names of the ingredients used in those examples, which are of water-in-oil-emulsion types. (INCI stands for “International Nomenclature of Cosmetic Ingredients.”) In what follows, unless otherwise specified, the amounts of the ingredients in an example composition are all expressed in weight% relative to the total amount of the composition.
- Table 2 lists the number-average diameters or ranges of diameter of the multilayer-type encapsulations containing pigments used in the examples.
- Table 3 shows the compositions of Comparative Examples 1 and 2, as well as some of the properties observed for each.
- the compositions contain no pigments, and differ in the amounts of hydrophobized titanium dioxide and hydrophobized zinc oxide that were added. The difference in weight was made up for by adjusting the amount of the oil component (Vegelight 1214 LC).
- Comparative Example 3 contains naked pigments (i.e., Unipure Red LC 381 LL and Unipure Yellow LC 182 LL), and Practice Example 2 contains multilayer-type encapsulations containing pigments (i.e., MAGICOLOR R 103RP and MAGICOLOR R 103YP).
- the amount of the naked pigments in Comparative Example 3 was chosen so that it would contain the same amount of raw pigments as in Practice Example 2 based on the fact that the iron oxide content in MAGICOLOR R is 48 weight%. The difference in weight was made up for by adjusting the amount of the oil component (Vegelight 1214 LC).
- Table 5 shows the compositions of Comparative Examples 4 and 5 and Practice Examples 1-5, as well as some of the properties observed for each.
- the compositions differ in the amount of the multilayer-type encapsulations containing pigments that were added, and the differences in weight were made up for by adjusting the amount of the oil component (Vegelight 1214 LC).
- Table 6 shows the compositions of Practice Examples 3, 6, and 7 and Comparative Example 6, as well as some of the properties observed for each.
- the examples differ only in the size of the multilayer-type encapsulations containing pigments that were used as expressed by number-average diameter or range of diameter.
- the amount of the multilayer-type encapsulations containing pigments was increased as necessary so that the excessive whiteness arising from the hydrophobized titanium dioxide and the hydrophobized zinc oxide was sufficiently masked, but this amount was capped at 1.5 weight% since Table 5 above shows that the appearance of color on the skin was natural-looking in Practice Example 5 (which contained 1.5 weight% of the encapsulations) but was not natural-looking in Comparative Example 5 (which contained 1.8 weight% of the encapsulations).
- the differences in weight were made up for by adjusting the amounts of the oil component (Vegelight 1214 LC) and the powder component (Sunsphere L-51S and Penstia TM Powder).
- the present invention has industrial applicability in that it provides, among other things, a sunscreen product useful in protecting the skin from ultraviolet light, a precursor useful in preparing such a sunscreen product, and a method of preparing the sunscreen product from its precursor.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Birds (AREA)
- Epidemiology (AREA)
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Dermatology (AREA)
- Cosmetics (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15795751.5A EP3145473B1 (en) | 2014-05-19 | 2015-05-18 | Sunscreen products in which excessive whiteness due to titanium dioxide and zinc oxide is visually masked upon skin application |
| ES15795751T ES2729881T3 (es) | 2014-05-19 | 2015-05-18 | Productos de protección solar donde el exceso de blancura debido al dióxido de titanio y al óxido de zinc se enmascara visualmente después de aplicarlos sobre la piel |
| JP2016567946A JP6434993B2 (ja) | 2014-05-19 | 2015-05-18 | 二酸化チタン及び酸化亜鉛による過度の白さが皮膚塗布時に視覚的に隠蔽される日焼け止め製品 |
| KR1020167031329A KR20170005804A (ko) | 2014-05-19 | 2015-05-18 | 피부에 도포시 이산화티탄 및 산화아연으로 인한 과잉의 백색도가 시각적으로 마스킹되는 자외선 차단제 |
| CN201580025592.5A CN106488762B (zh) | 2014-05-19 | 2015-05-18 | 皮肤施用时由于二氧化钛和氧化锌造成的过度白度被视觉遮蔽的防晒品 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/280,983 US9186305B1 (en) | 2014-05-19 | 2014-05-19 | Sunscreen products in which excessive whiteness due to titanium dioxide and zinc oxide is visually masked upon skin application |
| US14/280,983 | 2014-05-19 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015178007A1 true WO2015178007A1 (en) | 2015-11-26 |
Family
ID=54434436
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/002486 Ceased WO2015178007A1 (en) | 2014-05-19 | 2015-05-18 | Sunscreen products in which excessive whiteness due to titanium dioxide and zinc oxide is visually masked upon skin application |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US9186305B1 (enExample) |
| EP (1) | EP3145473B1 (enExample) |
| JP (1) | JP6434993B2 (enExample) |
| KR (1) | KR20170005804A (enExample) |
| CN (1) | CN106488762B (enExample) |
| ES (1) | ES2729881T3 (enExample) |
| TW (1) | TWI657828B (enExample) |
| WO (1) | WO2015178007A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021197802A1 (en) | 2020-03-31 | 2021-10-07 | Unilever Ip Holdings B.V. | Topical composition based on porous particles and a crosspolymer comprising adipic acid and neopentylglycol |
| WO2021254723A1 (en) | 2020-06-19 | 2021-12-23 | Unilever Ip Holdings B.V. | A personal care composition based on titanium oxide and a crosspolymer of adipic acid and neopentyl glycol |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017055454A1 (en) | 2015-09-30 | 2017-04-06 | Novaliq Gmbh | Semifluorinated compounds and their compositions |
| JP7412068B2 (ja) * | 2016-12-16 | 2024-01-12 | 株式会社 資生堂 | 水中油型組成物 |
| JP7038133B2 (ja) * | 2017-10-03 | 2022-03-17 | 株式会社日本色材工業研究所 | 油性化粧料 |
| WO2019206956A1 (en) | 2018-04-27 | 2019-10-31 | Novaliq Gmbh | Ophthalmic compositions comprising tafluprost for the treatment of glaucoma |
| AU2019345929B2 (en) | 2018-09-27 | 2022-02-03 | Dermaliq Therapeutics, Inc. | Topical sunscreen formulation |
| CA3112504A1 (en) | 2018-09-27 | 2020-04-02 | Novaliq Gmbh | Lipid barrier repair |
| CA3117776A1 (en) * | 2018-10-24 | 2020-04-30 | Beiersdorf Ag | Sunscreen formulation |
| US20200138681A1 (en) * | 2018-11-06 | 2020-05-07 | L'oreal | Mineral sunscreen compositions |
| WO2020118369A1 (en) * | 2018-12-12 | 2020-06-18 | Advance NanoTek Ltd. | Sunscreen composition |
| CN114173744B (zh) | 2019-07-29 | 2025-05-09 | 株式会社资生堂 | 固体化妆品 |
| CN114173755B (zh) | 2019-07-29 | 2024-02-06 | 株式会社资生堂 | 油性化妆品 |
| JP2021084879A (ja) * | 2019-11-28 | 2021-06-03 | 株式会社 資生堂 | 油中水型乳化化粧料 |
| WO2021118781A1 (en) * | 2019-12-11 | 2021-06-17 | Avon Products, Inc. | Compositions with increased color shade stability based on pigmentary tio2, organic pigments and metal oxide particles |
| EP4230435A4 (en) * | 2020-10-16 | 2024-04-03 | ENEOS Materials Corporation | RUBBER BALE, MANUFACTURING METHOD THEREOF, POLYMER COMPOSITION, CROSS-LINKED OBJECT AND TYRE |
| JP7760236B2 (ja) * | 2020-10-29 | 2025-10-27 | 株式会社 資生堂 | 油中水型乳化日焼け止め化粧料 |
| JP7761992B2 (ja) * | 2020-12-16 | 2025-10-29 | 株式会社コーセー | 油中水型化粧料 |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09507866A (ja) * | 1994-11-03 | 1997-08-12 | エスティー ローダー,インコーポレーテッド | 分散粉体を含有するスプレー可能な組成物およびその使用法 |
| JP2003514008A (ja) * | 1999-11-17 | 2003-04-15 | タグラ バイオテクノロジーズ リミテッド | マイクロカプセル封入方法 |
| JP2007513934A (ja) * | 2003-12-11 | 2007-05-31 | インペリアル・ケミカル・インダストリーズ・ピーエルシー | 金属酸化物分散液 |
| JP2007217393A (ja) * | 2006-02-20 | 2007-08-30 | Shiseido Co Ltd | 低粘度油中水型乳化化粧料 |
| JP2008266283A (ja) * | 2006-09-15 | 2008-11-06 | Nippon Shokubai Co Ltd | 化粧料用紫外線カット剤およびそれを用いた化粧料 |
| JP2011079804A (ja) * | 2009-10-09 | 2011-04-21 | Daito Kasei Kogyo Kk | 顔料内包マイクロカプセル及びこれを配合した化粧料 |
| JP2011236182A (ja) * | 2010-05-13 | 2011-11-24 | Nippon Shikizai Inc | 日焼け止め化粧料 |
| JP2012211114A (ja) * | 2011-03-31 | 2012-11-01 | Kose Corp | 油中水型乳化化粧料 |
| WO2013031374A1 (ja) * | 2011-08-26 | 2013-03-07 | 株式会社資生堂 | 油中水型乳化日焼け止め化粧料 |
| WO2013107350A1 (en) * | 2012-01-17 | 2013-07-25 | L'oreal | Colour changing composition |
| WO2013132914A1 (ja) * | 2012-03-05 | 2013-09-12 | 大塚製薬株式会社 | 日焼け止め用組成物 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3942767B2 (ja) * | 1999-04-06 | 2007-07-11 | 花王株式会社 | 紫外線遮蔽性超微粒子分散体 |
| JP3756043B2 (ja) * | 2000-06-30 | 2006-03-15 | 花王株式会社 | 皮膚化粧料 |
| JP2004002356A (ja) * | 2002-03-29 | 2004-01-08 | Shiseido Co Ltd | 変色性化粧料及びそれを用いた化粧方法 |
| JP4212924B2 (ja) * | 2002-08-08 | 2009-01-21 | 花王株式会社 | 複合ポリマー粒子及びその製法 |
| JP2004277289A (ja) * | 2003-03-12 | 2004-10-07 | Kao Corp | 紫外線防御化粧料 |
| US7153573B2 (en) * | 2002-08-08 | 2006-12-26 | Kao Corporation | Polymer composite particle comprising metal oxide and silicone and/or fluorine and method of producing the same |
| US7368105B2 (en) * | 2004-07-02 | 2008-05-06 | L'oreal | Photostabilization of dibenzoylmethane UV-screening agents with arylalkyl benzoate/bis-resorcinyl triazine compounds and photoprotective compositions comprised thereof |
| JP5240967B2 (ja) * | 2006-02-20 | 2013-07-17 | 株式会社 資生堂 | 油中水型乳化日焼け止め化粧料 |
| US20080038557A1 (en) * | 2006-08-08 | 2008-02-14 | The Procter & Gamble Company | Process for making collapsible water-containing capsules |
| WO2009138978A2 (en) * | 2008-05-12 | 2009-11-19 | Tagra Biotechnologies Ltd | Compositions for topical application comprising microencapsulated colorants |
| JP4881961B2 (ja) * | 2009-01-08 | 2012-02-22 | 株式会社トキワ | 油中水型日焼け止め化粧料 |
| KR100978583B1 (ko) * | 2009-09-02 | 2010-08-27 | (주)바이오제닉스 | 화장품용 색조캡슐 조성물, 이의 제조방법 및 이를 이용한 화장품 제제 |
-
2014
- 2014-05-19 US US14/280,983 patent/US9186305B1/en active Active
-
2015
- 2015-05-18 EP EP15795751.5A patent/EP3145473B1/en active Active
- 2015-05-18 KR KR1020167031329A patent/KR20170005804A/ko not_active Withdrawn
- 2015-05-18 WO PCT/JP2015/002486 patent/WO2015178007A1/en not_active Ceased
- 2015-05-18 JP JP2016567946A patent/JP6434993B2/ja active Active
- 2015-05-18 ES ES15795751T patent/ES2729881T3/es active Active
- 2015-05-18 TW TW104115792A patent/TWI657828B/zh active
- 2015-05-18 CN CN201580025592.5A patent/CN106488762B/zh active Active
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09507866A (ja) * | 1994-11-03 | 1997-08-12 | エスティー ローダー,インコーポレーテッド | 分散粉体を含有するスプレー可能な組成物およびその使用法 |
| JP2003514008A (ja) * | 1999-11-17 | 2003-04-15 | タグラ バイオテクノロジーズ リミテッド | マイクロカプセル封入方法 |
| JP2007513934A (ja) * | 2003-12-11 | 2007-05-31 | インペリアル・ケミカル・インダストリーズ・ピーエルシー | 金属酸化物分散液 |
| JP2007217393A (ja) * | 2006-02-20 | 2007-08-30 | Shiseido Co Ltd | 低粘度油中水型乳化化粧料 |
| JP2008266283A (ja) * | 2006-09-15 | 2008-11-06 | Nippon Shokubai Co Ltd | 化粧料用紫外線カット剤およびそれを用いた化粧料 |
| JP2011079804A (ja) * | 2009-10-09 | 2011-04-21 | Daito Kasei Kogyo Kk | 顔料内包マイクロカプセル及びこれを配合した化粧料 |
| JP2011236182A (ja) * | 2010-05-13 | 2011-11-24 | Nippon Shikizai Inc | 日焼け止め化粧料 |
| JP2012211114A (ja) * | 2011-03-31 | 2012-11-01 | Kose Corp | 油中水型乳化化粧料 |
| WO2013031374A1 (ja) * | 2011-08-26 | 2013-03-07 | 株式会社資生堂 | 油中水型乳化日焼け止め化粧料 |
| WO2013107350A1 (en) * | 2012-01-17 | 2013-07-25 | L'oreal | Colour changing composition |
| WO2013132914A1 (ja) * | 2012-03-05 | 2013-09-12 | 大塚製薬株式会社 | 日焼け止め用組成物 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021197802A1 (en) | 2020-03-31 | 2021-10-07 | Unilever Ip Holdings B.V. | Topical composition based on porous particles and a crosspolymer comprising adipic acid and neopentylglycol |
| WO2021254723A1 (en) | 2020-06-19 | 2021-12-23 | Unilever Ip Holdings B.V. | A personal care composition based on titanium oxide and a crosspolymer of adipic acid and neopentyl glycol |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3145473A1 (en) | 2017-03-29 |
| TWI657828B (zh) | 2019-05-01 |
| US9186305B1 (en) | 2015-11-17 |
| ES2729881T3 (es) | 2019-11-06 |
| CN106488762B (zh) | 2018-04-06 |
| JP6434993B2 (ja) | 2018-12-05 |
| EP3145473A4 (en) | 2018-03-07 |
| CN106488762A (zh) | 2017-03-08 |
| TW201622691A (zh) | 2016-07-01 |
| JP2017515864A (ja) | 2017-06-15 |
| US20150328088A1 (en) | 2015-11-19 |
| KR20170005804A (ko) | 2017-01-16 |
| EP3145473B1 (en) | 2019-05-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9186305B1 (en) | Sunscreen products in which excessive whiteness due to titanium dioxide and zinc oxide is visually masked upon skin application | |
| EP2181697B1 (en) | Oil-in-water emulsion type sunscreen preparation | |
| US8404258B2 (en) | External preparation for the skin | |
| US20110098231A1 (en) | Skin Cosmetic | |
| TW201834631A (zh) | 水中油型組成物 | |
| US7445790B2 (en) | External preparations for skin | |
| CN113015559A (zh) | 化妆品 | |
| WO2017073758A1 (ja) | 組成物 | |
| JP7114164B2 (ja) | 粉体含有水系組成物及び皮膚外用剤 | |
| CN114466647A (zh) | 固体状油包水型乳化化妆品 | |
| JP7132763B2 (ja) | 組成物 | |
| CN112996473A (zh) | 粉末分散组合物及其分散方法 | |
| JP2010047506A (ja) | ゾル−ゲル可逆性組成物、増粘剤及び化粧料。 | |
| EP3892337A1 (en) | Emulsified cosmetic composition | |
| HK1229223B (en) | Sunscreen products in which excessive whiteness due to titanium dioxide and zinc oxide is visually masked upon skin application | |
| HK1229223A1 (en) | Sunscreen products in which excessive whiteness due to titanium dioxide and zinc oxide is visually masked upon skin application | |
| WO2025142560A1 (ja) | 水中油型乳化組成物 | |
| HK1141709B (en) | Oil-in-water emulsion type sunscreen preparation | |
| HK1141713B (en) | Oil-in-water emulsion composition and method for producing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15795751 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20167031329 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2016567946 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015795751 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015795751 Country of ref document: EP |