WO2013140692A1 - ノンハロゲン難燃性樹脂組成物およびそれを用いた電線・ケーブル - Google Patents
ノンハロゲン難燃性樹脂組成物およびそれを用いた電線・ケーブル Download PDFInfo
- Publication number
- WO2013140692A1 WO2013140692A1 PCT/JP2012/083388 JP2012083388W WO2013140692A1 WO 2013140692 A1 WO2013140692 A1 WO 2013140692A1 JP 2012083388 W JP2012083388 W JP 2012083388W WO 2013140692 A1 WO2013140692 A1 WO 2013140692A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- flame retardant
- resin composition
- parts
- mass
- halogen flame
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/04—Homopolymers or copolymers of ethene
- C08L23/08—Copolymers of ethene
- C08L23/0846—Copolymers of ethene with unsaturated hydrocarbons containing atoms other than carbon or hydrogen
- C08L23/0853—Ethene vinyl acetate copolymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/20—Oxides; Hydroxides
- C08K3/22—Oxides; Hydroxides of metals
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3467—Heterocyclic compounds having nitrogen in the ring having more than two nitrogen atoms in the ring
- C08K5/3477—Six-membered rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3467—Heterocyclic compounds having nitrogen in the ring having more than two nitrogen atoms in the ring
- C08K5/3477—Six-membered rings
- C08K5/3492—Triazines
- C08K5/34928—Salts
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/04—Homopolymers or copolymers of ethene
- C08L23/06—Polyethene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/04—Homopolymers or copolymers of ethene
- C08L23/08—Copolymers of ethene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L25/00—Compositions of, homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an aromatic carbocyclic ring; Compositions of derivatives of such polymers
- C08L25/02—Homopolymers or copolymers of hydrocarbons
- C08L25/04—Homopolymers or copolymers of styrene
- C08L25/08—Copolymers of styrene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L31/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid or of a haloformic acid; Compositions of derivatives of such polymers
- C08L31/02—Homopolymers or copolymers of esters of monocarboxylic acids
- C08L31/04—Homopolymers or copolymers of vinyl acetate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L53/00—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers
- C08L53/02—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers of vinyl-aromatic monomers and conjugated dienes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L53/00—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers
- C08L53/02—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers of vinyl-aromatic monomers and conjugated dienes
- C08L53/025—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers of vinyl-aromatic monomers and conjugated dienes modified
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/28—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances natural or synthetic rubbers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/30—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes
- H01B3/44—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/30—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes
- H01B3/44—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins
- H01B3/441—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins from alkenes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/30—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes
- H01B3/44—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins
- H01B3/448—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins from other vinyl compounds
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B7/00—Insulated conductors or cables characterised by their form
- H01B7/02—Disposition of insulation
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B7/00—Insulated conductors or cables characterised by their form
- H01B7/17—Protection against damage caused by external factors, e.g. sheaths or armouring
- H01B7/29—Protection against damage caused by extremes of temperature or by flame
- H01B7/295—Protection against damage caused by extremes of temperature or by flame using material resistant to flame
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/20—Oxides; Hydroxides
- C08K3/22—Oxides; Hydroxides of metals
- C08K2003/2217—Oxides; Hydroxides of metals of magnesium
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/20—Oxides; Hydroxides
- C08K3/22—Oxides; Hydroxides of metals
- C08K2003/2217—Oxides; Hydroxides of metals of magnesium
- C08K2003/2224—Magnesium hydroxide
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K2201/00—Specific properties of additives
- C08K2201/002—Physical properties
- C08K2201/003—Additives being defined by their diameter
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2201/00—Properties
- C08L2201/02—Flame or fire retardant/resistant
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2201/00—Properties
- C08L2201/22—Halogen free composition
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2203/00—Applications
- C08L2203/20—Applications use in electrical or conductive gadgets
- C08L2203/202—Applications use in electrical or conductive gadgets use in electrical wires or wirecoating
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2205/00—Polymer mixtures characterised by other features
- C08L2205/03—Polymer mixtures characterised by other features containing three or more polymers in a blend
- C08L2205/035—Polymer mixtures characterised by other features containing three or more polymers in a blend containing four or more polymers in a blend
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2207/00—Properties characterising the ingredient of the composition
- C08L2207/06—Properties of polyethylene
- C08L2207/062—HDPE
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2312/00—Crosslinking
- C08L2312/06—Crosslinking by radiation
Definitions
- the present invention relates to a non-halogen flame retardant resin composition suitably used as a coating layer for electric wires and the like, and an electric wire / cable using the resin composition.
- a wire harness is an assembly of terminals such as connectors that can be inserted into and removed from a terminal by bundling multiple wires and cables.
- PVC electric wires using polyvinyl chloride (PVC) as an insulating material are used for electric wires for wire harnesses. Since PVC wires are excellent in flexibility, they are easy to handle even when used as wire harnesses, and have sufficient strength, so there is no problem that the insulator breaks or wears during wiring of the wire harness. Excellent workability for attaching pressure contact connectors to be mounted on.
- PVC electric wire contains a halogen element
- a toxic gas of hydrogen chloride is generated when the wire harness is incinerated after use, and dioxin is generated depending on the incineration conditions.
- PVC is not a preferable material as an insulating material.
- halogen-free electric wires using a coating material that does not contain polyvinyl chloride resin or a halogen-based flame retardant have been developed in order to meet the increasing demand for reducing the environmental burden.
- insulated wires and insulated cables used for in-machine wiring of electronic devices are generally required to have various characteristics that conform to UL (Underwriters Laboratories Inc.) standards.
- the UL standard stipulates in detail various properties such as flame retardancy, heat deformability, low temperature properties, initial properties of coating materials and tensile properties after heat aging.
- wires used for pressure welding or crimping it is necessary to route the wire harness inside the electronic device. Since the insulation coating of the electric wire may be damaged or broken during this operation, it may become defective, so that the insulated wire used for the wire harness is required to have high cut-through strength. Abrasion resistance is also required.
- Patent Document 1 discloses a flame retardant resin obtained by heating and kneading a metal hydrate to a thermoplastic resin component in which an elastomer such as ethylene propylene rubber or styrene butadiene rubber is blended with polypropylene.
- a composition and a wiring material using the composition are disclosed.
- Filler acceptability can be increased by blending elastomers, and by cross-linking these elastomers, it has been studied to balance mechanical properties such as flexibility and elongation with extrudability and flame retardancy. ing.
- such materials have poor wear resistance and edge resistance (cut-through characteristics) compared with PVC, and there is a problem that flexibility is lost and the balance of characteristics is lost when trying to improve these characteristics. It was.
- JP-A-2006-36813 Patent Document 2
- JP-A-2007-302907 Patent Document 3
- a resin composition is disclosed. Since these materials are not crosslinked by irradiation with ionizing radiation, the strength of these materials is increased by dynamic crosslinking.
- the dynamic cross-linking material has insufficient stability during extrusion, and depending on the extrusion conditions, tensile properties such as elongation and strength may be insufficient.
- a resin composition mainly composed of polyethylene that is crosslinked by irradiation with ionizing radiation is used, the required strength can be obtained by adjusting the degree of crosslinking.
- a flame retardant such as a metal hydroxide
- the cut-through strength and wear resistance required for electric wires for pressure welding or crimping applications Can not be satisfied.
- the tensile properties defined in the UL standard cannot be satisfied.
- the present invention provides a non-halogen flame retardant resin composition having cut-through strength, abrasion resistance, and flame resistance required for pressure welding wires, and tensile properties satisfying UL standards, and the flame retardant resin composition. It is an object to provide an electric wire / cable using an object as a covering layer.
- the present invention is a non-halogen flame retardant resin composition containing 60 to 100 parts by mass of a metal hydroxide and 5 to 20 parts by mass of a nitrogen-based flame retardant with respect to 100 parts by mass of the resin component, wherein 100 parts by mass of the resin component Non-halogen containing 50 to 80 parts by mass of a polyolefin resin and 20 to 50 parts by mass of a styrene elastomer and 2 to 15 parts by mass of an epoxy group-containing ethylene copolymer as a part of the polyolefin resin. It is a flame retardant resin composition.
- ⁇ A polyolefin resin and a styrene elastomer are used in combination as the resin component.
- Polyolefin resins are widely used as wire coatings and are not only excellent in extrusion processability but also capable of crosslinking by irradiation with ionizing radiation, contributing to both tensile properties and heat resistance.
- the styrene elastomer is flexible, it can not only supplement the filler acceptability, but can further improve and stabilize the tensile properties. By combining both, the tensile properties required by the UL standard can be stably obtained. Further, by containing a metal hydroxide and a nitrogen-based flame retardant, flame retardance that can pass the VW-1 vertical combustion test can be obtained.
- An epoxy group-containing ethylene copolymer is used as part of the polyolefin resin. Since the epoxy group-containing ethylene copolymer is a polyethylene resin, it is crosslinked by irradiation with ionizing radiation. Further, by containing 2 to 15 parts by mass of the epoxy group-containing ethylene copolymer in 100 parts by mass of the resin component, the cut-through strength is remarkably improved as compared with the case where no epoxy group-containing ethylene copolymer is contained. .
- the polyolefin-based resin preferably contains an ethylene-vinyl acetate copolymer having a vinyl acetate content of 20 to 50 parts by mass. Moreover, it is preferable to contain a high density polyethylene.
- the ethylene-vinyl acetate copolymer not only has excellent acceptability for flame retardants such as magnesium hydroxide due to its excellent flexibility, but also contributes to an improvement in elongation. High-density polyethylene also contributes to improved cut-through strength and wear resistance.
- As the polyolefin-based resin it is preferable to use an ethylene-based resin that can be cross-linked by ionizing radiation irradiation, such as the above-described two types of resins. In addition to these two types of polyolefin resins, other resins such as low density polyethylene may be used.
- the styrene elastomer it is preferable to use a block copolymer elastomer of styrene and a rubber component. Since the block copolymer elastomer of styrene and a rubber component is stretched and has high strength, the tensile properties of the flame retardant resin composition can be further improved.
- the nitrogen flame retardant is preferably melamine cyanurate having an average particle size of 5 ⁇ m or less.
- Melamine cyanurate has good thermal stability during mixing, and is particularly excellent in flame retardancy among nitrogen-based flame retardants. Furthermore, the dispersibility at the time of mixing improves because an average particle diameter is 5 micrometers or less.
- the metal hydroxide is preferably magnesium hydroxide having an average particle size of 0.1 ⁇ m or more and 3 ⁇ m or less.
- the average particle size means 50% particle size (D50), measured by a particle size distribution measuring device (Nikkiso Co., Ltd., Nanotrac (registered trademark) particle size distribution measuring device UPA-EX150) applying the laser Doppler method. it can.
- Another aspect of the present invention is an electric wire / cable using the non-halogen flame retardant resin composition as a coating layer.
- a non-halogen insulated electric wire / cable having cut-through strength, abrasion resistance, flame retardancy, and tensile properties satisfying UL standards can be obtained.
- the thickness of the covering layer is preferably 0.4 mm or less.
- the thickness of the coating layer is as thin as 0.4 mm or less, the difference from the electric wire according to the prior art becomes remarkable in characteristics such as cut-through strength, and an excellent effect is exhibited.
- the covering layer is preferably cross-linked by irradiation with ionizing radiation. Since the coating layer is crosslinked, heat resistance and tensile properties are improved.
- non-halogen flame retardant resin composition having cut-through strength, abrasion resistance, and flame retardancy and tensile properties satisfying UL standards, and an electric wire / cable using the same. Can do.
- Polyolefin resins include polyethylene (high density polyethylene, linear low density polyethylene, low density polyethylene, ultra low density polyethylene), ethylene-vinyl acetate copolymer, ethylene-methyl methacrylate copolymer, ethylene-acrylic acid.
- An epoxy group-containing ethylene copolymer is used as part of the polyolefin resin.
- the epoxy group-containing ethylene copolymer is obtained by copolymerizing an epoxy group-containing olefin monomer such as glycidyl methacrylate and an ethylene monomer. Specifically, ethylene-glycidyl methacrylate copolymer, ethylene-propylene-glycidyl methacrylate copolymer, ethylene-butene-1-glycidyl methacrylate copolymer, ethylene-vinyl acetate-glycidyl methacrylate copolymer, And ethylene-acrylic acid-glycidyl methacrylate copolymer.
- the content of the epoxy group-containing ethylene-based copolymer is 2 parts by mass or more and 15 parts by mass or less, more preferably 5 parts by mass or more and 10 parts by mass or less based on the entire resin component.
- the polyolefin-based resin it is preferable to contain an ethylene-vinyl acetate copolymer having a vinyl acetate content of 20 to 50 parts by mass.
- the vinyl acetate content is less than 20 parts by mass, the flame retardancy is lowered and the UL standard cannot be satisfied.
- the vinyl acetate content exceeds 50 parts by mass, flame retardancy is improved, but the cut-through strength and wear resistance are also lowered along with the decrease in tensile strength, and the required characteristics cannot be satisfied.
- the content of the ethylene-vinyl acetate copolymer is preferably 10 parts by mass or more and 30 parts by mass or less of the entire resin component.
- high density polyethylene is contained as part of the polyolefin resin.
- the high density polyethylene is a homopolyethylene or a polyethylene copolymer, and is a polyethylene having a density of 0.942 g / cm 3 or more.
- MFR melt flow rate
- a lower MFR tends to improve the wear resistance.
- the content of the high density polyethylene is preferably 10 parts by mass or more and 30 parts by mass or less of the entire resin component.
- styrene elastomers examples include styrene / ethylene butene / styrene copolymers, styrene / ethylene propylene / styrene copolymers, styrene / ethylene / ethylene propylene / styrene copolymers, and styrene / butylene / styrene copolymers.
- hydrogenated polymers and partially hydrogenated polymers can be exemplified.
- transduced carboxylic acid such as maleic anhydride, can also be blended suitably and used.
- Block copolymers include hydrogenated styrene / butylene / styrene block copolymers, triblock copolymers such as styrene / isobutylene / styrene copolymers, styrene / ethylene copolymers, styrene / ethylene propylene, etc. It is preferable that the triblock component in the styrene elastomer is contained in an amount of 50% by mass or more because the strength and hardness of the coating layer are improved.
- those having a styrene content of 20% by mass or more contained in the styrene elastomer can be suitably used from the viewpoint of tensile properties (strength, elongation) and flame retardancy.
- tensile properties strength, elongation
- flame retardancy When the styrene content is less than 20% by mass, the hardness and extrusion processability are lowered. On the other hand, when the styrene content exceeds 60% by mass, the tensile elongation is lowered, which is not preferable.
- the MFR as an index of molecular weight is in the range of 0.8 to 15 g / 10 min. This is because if the MFR is less than 0.8 g / 10 min, the extrusion processability is lowered, and if it exceeds 15 g / 10 min, the mechanical strength is lowered.
- nitrogen-based flame retardants examples include melamine resin and melamine cyanurate.
- Nitrogen-based flame retardants do not generate toxic gases such as hydrogen halides even when incinerated after use, and can reduce the environmental burden.
- melamine cyanurate When melamine cyanurate is used as a nitrogen-based flame retardant, it is preferable in terms of heat stability at the time of mixing and an effect of improving flame retardancy.
- Melamine cyanurate can also be used after surface treatment with a silane coupling agent or a titanate coupling agent.
- the average particle size of the nitrogen flame retardant is preferably 5 ⁇ m or less. Content of a nitrogen-type flame retardant shall be 5 to 20 mass parts with respect to 100 mass parts of resin components. If the amount is less than 5 parts by mass, the flame resistance of the insulated wire is insufficient, and if it exceeds 20 parts by mass, the elongation and extrusion processability are deteriorated.
- metal hydroxides examples include aluminum hydroxide, magnesium hydroxide, calcium hydroxide and the like. Among these, from the viewpoint of extrusion processability, magnesium hydroxide having an average particle size of 0.1 ⁇ m or more and 3 ⁇ m or less is preferable. Content of a metal hydroxide shall be 60 to 100 mass parts with respect to 100 mass parts of resin components. When the amount is less than 60 parts by mass, the flame resistance of the insulated wire is insufficient, and when the amount exceeds 100 parts by mass, elongation and extrusion processability are deteriorated.
- a crosslinking aid can be further added to the non-halogen flame retardant resin composition.
- a polyfunctional monomer having a plurality of carbon-carbon double bonds in the molecule such as trimethylolpropane trimethacrylate, triallyl cyanurate, triallyl isocyanurate and the like can be preferably used.
- a crosslinking adjuvant is a liquid at normal temperature. This is because when it is a liquid, it can be easily mixed with a polyolefin resin or a styrene elastomer.
- Use of trimethylolpropane trimethacrylate as a crosslinking aid is preferred because compatibility with the resin is improved.
- an antioxidant an antioxidant, a processing stabilizer, a colorant, a heavy metal deactivator, a foaming agent and the like can be appropriately mixed as necessary.
- These materials can be prepared by mixing using a known melt mixer such as a short screw extruder, a pressure kneader, or a Banbury mixer.
- the electric wire (also referred to as an insulated wire) of the present invention has a coating layer made of the above-mentioned flame retardant resin composition, and the coating layer is formed directly on the conductor or via another layer.
- a known extruder such as a melt extruder can be used.
- the coating layer is preferably cross-linked by irradiating with ionizing radiation.
- a cable also referred to as an insulated cable
- has a sheath such as a shield layer on the surface of an electric wire.
- the conductor copper wire, aluminum wire, etc. having excellent conductivity can be used.
- the diameter of the conductor can be appropriately selected according to the intended use, but is preferably 2 mm or less in order to enable wiring in a narrow space. In consideration of ease of handling, the thickness is preferably 0.1 mm or more.
- the conductor may be a single wire or may be a strand of a plurality of strands.
- the thickness of the coating layer can be appropriately selected according to the conductor diameter, but the mechanical strength can be obtained by applying the flame-retardant resin composition of the present invention even when the thickness of the coating layer is 0.4 mm or less. Will be good.
- the wear resistance and the cut-through strength are reduced when the thickness of the coating layer is 0.4 mm or less, but according to the present invention, excellent performance is achieved even when the thickness of the coating layer is 0.4 mm or less. As a result, the difference from the electric wire according to the prior art appears remarkably.
- an electric wire having a coating layer thickness of 0.4 mm or less is preferably used from the viewpoint of fitting property with the connector.
- the coating layer is cross-linked by irradiation with ionizing radiation because the mechanical strength is improved.
- ionizing radiation sources include accelerated electron beams, gamma rays, X-rays, ⁇ rays, ultraviolet rays, and the like. Accelerated electron beams are used from the viewpoint of industrial use, such as ease of use of the radiation source, transmission thickness of ionizing radiation, and speed of crosslinking treatment. Is most preferably used.
- Examples 1 to 14 (Creation of non-halogen flame retardant resin composition pellets) Each component was mixed by the compounding prescription (unit: mass part) shown in Table 1. Using a 300 mm ⁇ open roll mixer, the mixture was melted and mixed at a temperature of 160 ° C. to 180 ° C., and the resulting strip-shaped resin composition was cut with a pelletizer to produce pellets.
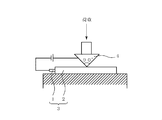
- Cut-through strength was measured using the measuring apparatus shown in FIG.
- the conductor and the sharp edge are insulated by the covering layer 2 and no current flows.
- a current flows between the conductor and the sharp edge.
- a load is applied to the blade 4 and the maximum load that can be withstood without cutting the coating layer 2 is measured.
- the test atmosphere is a temperature of 23 ° C. and a humidity of 50% RH. A load of 35N or more was regarded as an acceptable level.
- Insulated wires were produced in the same manner as in Examples 1 to 14 except that a resin composition having the formulation (unit: parts by mass) shown in Table 2 was used, and a series of evaluations were performed.
- EVA1 ethylene vinyl acetate copolymer having a vinyl acetate content of 25% by mass: Mitsui DuPont Polychemical Co., Ltd.
- EVA2 Ethylene vinyl acetate copolymer having a vinyl acetate content of 33% by mass: Evaflex EV170, manufactured by Mitsui DuPont Polychemical Co., Ltd.
- St-based elastomer 1 SEEPS (polystyrene-poly (ethylene-ethylene / propylene) block-polystyrene) having a styrene content of 30% by mass: Septon (registered trademark) 4033 manufactured by Kuraray Co., Ltd.
- St-based elastomer 2 SEEPS-OH (SEEPS having an OH group at the end): Kuraray Co., Ltd.
- Septon (registered trademark) HG252 St-based elastomer 3 SEBS (polystyrene-poly (ethylene / butylene) block-polystyrene): manufactured by Asahi Kasei Corporation, Tuftec 1041 St elastomer 4: SEBS: Asahi Kasei Corporation, Tuftec H1051 St elastomer 5: SEBS: Kuraray Co., Ltd.
- Septon (registered trademark) 8104 Magnesium hydroxide: Kisuma 5SDK manufactured by Kyowa Chemical Industry Co., Ltd. Melamine cyanurate: MC6000 manufactured by Nissan Chemical Industries, Ltd.
- Antiaging agent Irganox 1010 manufactured by BASF Japan Copper protection: Adeka Stab CDA-1 manufactured by ADEKA Corporation Lubricant: Stearic acid crosslinking aid: Trimethylolpropane trimethacrylate: TD1500S manufactured by DIC Corporation
- the insulated wires of Examples 1 to 14 each have a cut-through strength of 35 N or more and a scrape wear resistance of 650 times or more, and are high strength.
- the original tensile properties (elongation, strength) and the tensile properties after heat aging (elongation, residual ratio of strength) are acceptable levels, and the flame retardancy is also satisfied.
- the non-halogen flame retardant resin compositions used for the insulated wires of Comparative Examples 1 to 6 do not contain an epoxy group-containing ethylene copolymer.
- Original tensile properties elongation, strength
- tensile properties after heat aging elongation, residual ratio of strength
- flame retardancy are acceptable levels, but the cut-through strength is low. From this result, it can be seen that the cut-through strength is improved when an appropriate amount of the epoxy group-containing ethylene copolymer is contained in the resin composition.
- there is a scrape abrasion resistance which is not an acceptable level (Comparative Example 3 and Comparative Example 6).
- the non-halogen flame retardant resin composition used for the insulated wires of Comparative Examples 7 to 11 contains an epoxy group-containing ethylene-based copolymer. Since the comparative example 7 has too much content of a styrene-type elastomer, the cut-through intensity
- a nitrogen-type flame retardant melamine cyanurate
- a resin composition obtained by dynamically crosslinking polypropylene is used for the coating layer.
- the dynamic cross-linking material has insufficient stability during extrusion, and tensile properties such as elongation and strength may be insufficient depending on the extrusion conditions.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Insulated Conductors (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Organic Insulating Materials (AREA)
- Inorganic Insulating Materials (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201280046230.0A CN104220511A (zh) | 2012-03-22 | 2012-12-25 | 无卤阻燃树脂组合物、以及使用该无卤阻燃树脂组合物的电线和电缆 |
| KR1020147007421A KR20140049606A (ko) | 2012-03-22 | 2012-12-25 | 비할로젠 난연성 수지 조성물 및 그것을 이용한 전선·케이블 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012065592A JP2013194214A (ja) | 2012-03-22 | 2012-03-22 | ノンハロゲン難燃性樹脂組成物およびそれを用いた電線・ケーブル |
| JP2012-065592 | 2012-03-22 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013140692A1 true WO2013140692A1 (ja) | 2013-09-26 |
Family
ID=49222178
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/083388 Ceased WO2013140692A1 (ja) | 2012-03-22 | 2012-12-25 | ノンハロゲン難燃性樹脂組成物およびそれを用いた電線・ケーブル |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP2013194214A (enExample) |
| KR (1) | KR20140049606A (enExample) |
| CN (1) | CN104220511A (enExample) |
| WO (1) | WO2013140692A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103524843A (zh) * | 2013-09-30 | 2014-01-22 | 芜湖航天特种电缆厂 | 一种控制信号电缆用护套料及其制备方法 |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6229942B2 (ja) * | 2014-03-05 | 2017-11-15 | 日立金属株式会社 | 鉄道車両用絶縁電線及び鉄道車両用ケーブル |
| CN105330930A (zh) * | 2015-10-30 | 2016-02-17 | 无锡市长安曙光手套厂 | 一种无卤阻燃复合材料及其制备方法 |
| KR102408450B1 (ko) * | 2020-10-22 | 2022-06-14 | 에이치디씨현대이피 주식회사 | 고내열 케이블 피복용 난연성 조성물 및 상기 조성물로 제조된 고분자 복합수지 |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07331086A (ja) * | 1994-06-07 | 1995-12-19 | Sumitomo Bakelite Co Ltd | 熱可塑性樹脂組成物 |
| JPH11349742A (ja) * | 1998-06-04 | 1999-12-21 | Hitachi Cable Ltd | ポリオレフィン樹脂組成物および電線・ケーブル |
| JP2001060414A (ja) * | 1999-01-20 | 2001-03-06 | Furukawa Electric Co Ltd:The | 絶縁樹脂組成物および絶縁電線 |
| JP2001200106A (ja) * | 1999-03-02 | 2001-07-24 | Jsr Corp | 難燃性重合体組成物 |
| JP2001226536A (ja) * | 2000-02-18 | 2001-08-21 | Sumitomo Wiring Syst Ltd | オレフィン系樹脂組成物および被覆電線 |
| WO2003106554A1 (ja) * | 2002-06-14 | 2003-12-24 | 三井化学株式会社 | 熱可塑性樹脂組成物、重合体組成物、該組成物からなる成形体 |
| JP2004256748A (ja) * | 2003-02-27 | 2004-09-16 | Fujikura Ltd | ノンハロゲン難燃性組成物および難燃性電源コード |
| WO2008078406A1 (ja) * | 2006-12-22 | 2008-07-03 | Mitsubishi Chemical Corporation | 難燃性熱可塑性樹脂組成物 |
| JP2009114230A (ja) * | 2007-11-01 | 2009-05-28 | Furukawa Electric Co Ltd:The | 難燃性樹脂組成物およびそれが被覆されている絶縁電線 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5477658A (en) * | 1977-12-02 | 1979-06-21 | Mitsubishi Petrochem Co Ltd | Self-extinguishing resin composition |
| JPS55139443A (en) * | 1979-04-17 | 1980-10-31 | Showa Electric Wire & Cable Co Ltd | Heat-resisting, flame-retardant crosslinked polyethylene composition |
| JPH0275642A (ja) * | 1988-09-12 | 1990-03-15 | Fujikura Ltd | 難燃性組成物及びそれを用いた電線・ケーブル |
| US6576691B2 (en) * | 1999-03-02 | 2003-06-10 | Jsr Corporation | Flame resistant polymer composition |
| US6646205B2 (en) * | 2000-12-12 | 2003-11-11 | Sumitomo Wiring Systems, Ltd. | Electrical wire having a resin composition covering |
| JP2003151376A (ja) * | 2001-11-14 | 2003-05-23 | Fujikura Ltd | 難燃性電源コード |
| JP2005200574A (ja) * | 2004-01-16 | 2005-07-28 | Hitachi Cable Ltd | ノンハロゲン難燃性樹脂組成物及びこれを用いた電線・ケーブル |
| JP2011501346A (ja) * | 2007-10-11 | 2011-01-06 | ディーエスエム アイピー アセッツ ビー.ブイ. | 電子機器用の可撓性の難燃性絶縁電線 |
-
2012
- 2012-03-22 JP JP2012065592A patent/JP2013194214A/ja active Pending
- 2012-12-25 KR KR1020147007421A patent/KR20140049606A/ko not_active Ceased
- 2012-12-25 CN CN201280046230.0A patent/CN104220511A/zh active Pending
- 2012-12-25 WO PCT/JP2012/083388 patent/WO2013140692A1/ja not_active Ceased
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07331086A (ja) * | 1994-06-07 | 1995-12-19 | Sumitomo Bakelite Co Ltd | 熱可塑性樹脂組成物 |
| JPH11349742A (ja) * | 1998-06-04 | 1999-12-21 | Hitachi Cable Ltd | ポリオレフィン樹脂組成物および電線・ケーブル |
| JP2001060414A (ja) * | 1999-01-20 | 2001-03-06 | Furukawa Electric Co Ltd:The | 絶縁樹脂組成物および絶縁電線 |
| JP2001200106A (ja) * | 1999-03-02 | 2001-07-24 | Jsr Corp | 難燃性重合体組成物 |
| JP2001226536A (ja) * | 2000-02-18 | 2001-08-21 | Sumitomo Wiring Syst Ltd | オレフィン系樹脂組成物および被覆電線 |
| WO2003106554A1 (ja) * | 2002-06-14 | 2003-12-24 | 三井化学株式会社 | 熱可塑性樹脂組成物、重合体組成物、該組成物からなる成形体 |
| JP2004256748A (ja) * | 2003-02-27 | 2004-09-16 | Fujikura Ltd | ノンハロゲン難燃性組成物および難燃性電源コード |
| WO2008078406A1 (ja) * | 2006-12-22 | 2008-07-03 | Mitsubishi Chemical Corporation | 難燃性熱可塑性樹脂組成物 |
| JP2009114230A (ja) * | 2007-11-01 | 2009-05-28 | Furukawa Electric Co Ltd:The | 難燃性樹脂組成物およびそれが被覆されている絶縁電線 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103524843A (zh) * | 2013-09-30 | 2014-01-22 | 芜湖航天特种电缆厂 | 一种控制信号电缆用护套料及其制备方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN104220511A (zh) | 2014-12-17 |
| JP2013194214A (ja) | 2013-09-30 |
| KR20140049606A (ko) | 2014-04-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TWI409322B (zh) | 無鹵素難燃性樹脂組成物及使用它之電線/電纜 | |
| KR101601286B1 (ko) | 고난연 전자 기기 전선용 고분자 조성물과 이를 이용한 전선 | |
| JP6229942B2 (ja) | 鉄道車両用絶縁電線及び鉄道車両用ケーブル | |
| JP5825536B2 (ja) | ノンハロゲン難燃性樹脂組成物並びにこれを用いた絶縁電線及びチューブ | |
| JP5549675B2 (ja) | ノンハロゲン難燃性樹脂組成物およびそれを用いた電線・ケーブル | |
| US7586043B2 (en) | Non-halogenous insulated wire and a wiring harness | |
| JP6975718B2 (ja) | 改善された引張特性を有するハロゲンフリー難燃剤組成物 | |
| JP5387944B2 (ja) | ハロゲンフリー難燃絶縁電線 | |
| JP2015000913A (ja) | ノンハロゲン難燃性樹脂組成物、並びにこれを用いた電線及びケーブル | |
| JP5269476B2 (ja) | 電線・ケーブル | |
| JPWO2008078406A1 (ja) | 難燃性熱可塑性樹脂組成物 | |
| JP4255368B2 (ja) | 架橋型難燃性樹脂組成物ならびにこれを用いた絶縁電線およびワイヤーハーネス | |
| US20140141240A1 (en) | Halogen-free resin composition, electric wire and cable | |
| US20140290976A1 (en) | Halogen-free extra-high-voltage cable for railway rolling stock | |
| JP5182580B2 (ja) | ハロゲンフリー難燃絶縁電線 | |
| WO2013140692A1 (ja) | ノンハロゲン難燃性樹脂組成物およびそれを用いた電線・ケーブル | |
| WO2006057120A1 (ja) | ノンハロゲン電線、電線束及び自動車用ワイヤーハーネス | |
| JP6756692B2 (ja) | 絶縁電線 | |
| JP4776208B2 (ja) | 樹脂組成物及びそれを被覆した絶縁電線 | |
| JP2007197619A (ja) | ノンハロゲン難燃性樹脂組成物およびそれを用いた電線・ケーブル | |
| JP2008037927A (ja) | 難燃性樹脂組成物及び絶縁電線 | |
| JP4964412B2 (ja) | ノンハロゲン難燃性組成物及び電線 | |
| JP7064697B2 (ja) | 難燃性電気絶縁組成物及び電線 | |
| JP2003160709A (ja) | ノンハロゲン難燃性樹脂組成物 | |
| JP2007262418A (ja) | 伝送線被覆用樹脂組成物および伝送線 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12872223 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20147007421 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12872223 Country of ref document: EP Kind code of ref document: A1 |