WO2013140692A1 - Non-halogen flame retardant resin composition, and electric wire and cable using same - Google Patents
Non-halogen flame retardant resin composition, and electric wire and cable using same Download PDFInfo
- Publication number
- WO2013140692A1 WO2013140692A1 PCT/JP2012/083388 JP2012083388W WO2013140692A1 WO 2013140692 A1 WO2013140692 A1 WO 2013140692A1 JP 2012083388 W JP2012083388 W JP 2012083388W WO 2013140692 A1 WO2013140692 A1 WO 2013140692A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- parts
- flame retardant
- mass
- resin composition
- halogen flame
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/04—Homopolymers or copolymers of ethene
- C08L23/08—Copolymers of ethene
- C08L23/0846—Copolymers of ethene with unsaturated hydrocarbons containing other atoms than carbon or hydrogen atoms
- C08L23/0853—Vinylacetate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/20—Oxides; Hydroxides
- C08K3/22—Oxides; Hydroxides of metals
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3467—Heterocyclic compounds having nitrogen in the ring having more than two nitrogen atoms in the ring
- C08K5/3477—Six-membered rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/16—Nitrogen-containing compounds
- C08K5/34—Heterocyclic compounds having nitrogen in the ring
- C08K5/3467—Heterocyclic compounds having nitrogen in the ring having more than two nitrogen atoms in the ring
- C08K5/3477—Six-membered rings
- C08K5/3492—Triazines
- C08K5/34928—Salts
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/04—Homopolymers or copolymers of ethene
- C08L23/06—Polyethene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/04—Homopolymers or copolymers of ethene
- C08L23/08—Copolymers of ethene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L25/00—Compositions of, homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an aromatic carbocyclic ring; Compositions of derivatives of such polymers
- C08L25/02—Homopolymers or copolymers of hydrocarbons
- C08L25/04—Homopolymers or copolymers of styrene
- C08L25/08—Copolymers of styrene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L31/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid or of a haloformic acid; Compositions of derivatives of such polymers
- C08L31/02—Homopolymers or copolymers of esters of monocarboxylic acids
- C08L31/04—Homopolymers or copolymers of vinyl acetate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L53/00—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers
- C08L53/02—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers of vinyl-aromatic monomers and conjugated dienes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L53/00—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers
- C08L53/02—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers of vinyl-aromatic monomers and conjugated dienes
- C08L53/025—Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers of vinyl-aromatic monomers and conjugated dienes modified
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/28—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances natural or synthetic rubbers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/30—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes
- H01B3/44—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/30—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes
- H01B3/44—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins
- H01B3/441—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins from alkenes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B3/00—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties
- H01B3/18—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances
- H01B3/30—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes
- H01B3/44—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins
- H01B3/448—Insulators or insulating bodies characterised by the insulating materials; Selection of materials for their insulating or dielectric properties mainly consisting of organic substances plastics; resins; waxes vinyl resins; acrylic resins from other vinyl compounds
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B7/00—Insulated conductors or cables characterised by their form
- H01B7/02—Disposition of insulation
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B7/00—Insulated conductors or cables characterised by their form
- H01B7/17—Protection against damage caused by external factors, e.g. sheaths or armouring
- H01B7/29—Protection against damage caused by extremes of temperature or by flame
- H01B7/295—Protection against damage caused by extremes of temperature or by flame using material resistant to flame
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/20—Oxides; Hydroxides
- C08K3/22—Oxides; Hydroxides of metals
- C08K2003/2217—Oxides; Hydroxides of metals of magnesium
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K3/00—Use of inorganic substances as compounding ingredients
- C08K3/18—Oxygen-containing compounds, e.g. metal carbonyls
- C08K3/20—Oxides; Hydroxides
- C08K3/22—Oxides; Hydroxides of metals
- C08K2003/2217—Oxides; Hydroxides of metals of magnesium
- C08K2003/2224—Magnesium hydroxide
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K2201/00—Specific properties of additives
- C08K2201/002—Physical properties
- C08K2201/003—Additives being defined by their diameter
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2201/00—Properties
- C08L2201/02—Flame or fire retardant/resistant
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2201/00—Properties
- C08L2201/22—Halogen free composition
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2203/00—Applications
- C08L2203/20—Applications use in electrical or conductive gadgets
- C08L2203/202—Applications use in electrical or conductive gadgets use in electrical wires or wirecoating
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2205/00—Polymer mixtures characterised by other features
- C08L2205/03—Polymer mixtures characterised by other features containing three or more polymers in a blend
- C08L2205/035—Polymer mixtures characterised by other features containing three or more polymers in a blend containing four or more polymers in a blend
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2207/00—Properties characterising the ingredient of the composition
- C08L2207/06—Properties of polyethylene
- C08L2207/062—HDPE
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2312/00—Crosslinking
- C08L2312/06—Crosslinking by radiation
Definitions
- the present invention relates to a non-halogen flame retardant resin composition suitably used as a coating layer for electric wires and the like, and an electric wire / cable using the resin composition.
- a wire harness is an assembly of terminals such as connectors that can be inserted into and removed from a terminal by bundling multiple wires and cables.
- PVC electric wires using polyvinyl chloride (PVC) as an insulating material are used for electric wires for wire harnesses. Since PVC wires are excellent in flexibility, they are easy to handle even when used as wire harnesses, and have sufficient strength, so there is no problem that the insulator breaks or wears during wiring of the wire harness. Excellent workability for attaching pressure contact connectors to be mounted on.
- PVC electric wire contains a halogen element
- a toxic gas of hydrogen chloride is generated when the wire harness is incinerated after use, and dioxin is generated depending on the incineration conditions.
- PVC is not a preferable material as an insulating material.
- halogen-free electric wires using a coating material that does not contain polyvinyl chloride resin or a halogen-based flame retardant have been developed in order to meet the increasing demand for reducing the environmental burden.
- insulated wires and insulated cables used for in-machine wiring of electronic devices are generally required to have various characteristics that conform to UL (Underwriters Laboratories Inc.) standards.
- the UL standard stipulates in detail various properties such as flame retardancy, heat deformability, low temperature properties, initial properties of coating materials and tensile properties after heat aging.
- wires used for pressure welding or crimping it is necessary to route the wire harness inside the electronic device. Since the insulation coating of the electric wire may be damaged or broken during this operation, it may become defective, so that the insulated wire used for the wire harness is required to have high cut-through strength. Abrasion resistance is also required.
- Patent Document 1 discloses a flame retardant resin obtained by heating and kneading a metal hydrate to a thermoplastic resin component in which an elastomer such as ethylene propylene rubber or styrene butadiene rubber is blended with polypropylene.
- a composition and a wiring material using the composition are disclosed.
- Filler acceptability can be increased by blending elastomers, and by cross-linking these elastomers, it has been studied to balance mechanical properties such as flexibility and elongation with extrudability and flame retardancy. ing.
- such materials have poor wear resistance and edge resistance (cut-through characteristics) compared with PVC, and there is a problem that flexibility is lost and the balance of characteristics is lost when trying to improve these characteristics. It was.
- JP-A-2006-36813 Patent Document 2
- JP-A-2007-302907 Patent Document 3
- a resin composition is disclosed. Since these materials are not crosslinked by irradiation with ionizing radiation, the strength of these materials is increased by dynamic crosslinking.
- the dynamic cross-linking material has insufficient stability during extrusion, and depending on the extrusion conditions, tensile properties such as elongation and strength may be insufficient.
- a resin composition mainly composed of polyethylene that is crosslinked by irradiation with ionizing radiation is used, the required strength can be obtained by adjusting the degree of crosslinking.
- a flame retardant such as a metal hydroxide
- the cut-through strength and wear resistance required for electric wires for pressure welding or crimping applications Can not be satisfied.
- the tensile properties defined in the UL standard cannot be satisfied.
- the present invention provides a non-halogen flame retardant resin composition having cut-through strength, abrasion resistance, and flame resistance required for pressure welding wires, and tensile properties satisfying UL standards, and the flame retardant resin composition. It is an object to provide an electric wire / cable using an object as a covering layer.
- the present invention is a non-halogen flame retardant resin composition containing 60 to 100 parts by mass of a metal hydroxide and 5 to 20 parts by mass of a nitrogen-based flame retardant with respect to 100 parts by mass of the resin component, wherein 100 parts by mass of the resin component Non-halogen containing 50 to 80 parts by mass of a polyolefin resin and 20 to 50 parts by mass of a styrene elastomer and 2 to 15 parts by mass of an epoxy group-containing ethylene copolymer as a part of the polyolefin resin. It is a flame retardant resin composition.
- ⁇ A polyolefin resin and a styrene elastomer are used in combination as the resin component.
- Polyolefin resins are widely used as wire coatings and are not only excellent in extrusion processability but also capable of crosslinking by irradiation with ionizing radiation, contributing to both tensile properties and heat resistance.
- the styrene elastomer is flexible, it can not only supplement the filler acceptability, but can further improve and stabilize the tensile properties. By combining both, the tensile properties required by the UL standard can be stably obtained. Further, by containing a metal hydroxide and a nitrogen-based flame retardant, flame retardance that can pass the VW-1 vertical combustion test can be obtained.
- An epoxy group-containing ethylene copolymer is used as part of the polyolefin resin. Since the epoxy group-containing ethylene copolymer is a polyethylene resin, it is crosslinked by irradiation with ionizing radiation. Further, by containing 2 to 15 parts by mass of the epoxy group-containing ethylene copolymer in 100 parts by mass of the resin component, the cut-through strength is remarkably improved as compared with the case where no epoxy group-containing ethylene copolymer is contained. .
- the polyolefin-based resin preferably contains an ethylene-vinyl acetate copolymer having a vinyl acetate content of 20 to 50 parts by mass. Moreover, it is preferable to contain a high density polyethylene.
- the ethylene-vinyl acetate copolymer not only has excellent acceptability for flame retardants such as magnesium hydroxide due to its excellent flexibility, but also contributes to an improvement in elongation. High-density polyethylene also contributes to improved cut-through strength and wear resistance.
- As the polyolefin-based resin it is preferable to use an ethylene-based resin that can be cross-linked by ionizing radiation irradiation, such as the above-described two types of resins. In addition to these two types of polyolefin resins, other resins such as low density polyethylene may be used.
- the styrene elastomer it is preferable to use a block copolymer elastomer of styrene and a rubber component. Since the block copolymer elastomer of styrene and a rubber component is stretched and has high strength, the tensile properties of the flame retardant resin composition can be further improved.
- the nitrogen flame retardant is preferably melamine cyanurate having an average particle size of 5 ⁇ m or less.
- Melamine cyanurate has good thermal stability during mixing, and is particularly excellent in flame retardancy among nitrogen-based flame retardants. Furthermore, the dispersibility at the time of mixing improves because an average particle diameter is 5 micrometers or less.
- the metal hydroxide is preferably magnesium hydroxide having an average particle size of 0.1 ⁇ m or more and 3 ⁇ m or less.
- the average particle size means 50% particle size (D50), measured by a particle size distribution measuring device (Nikkiso Co., Ltd., Nanotrac (registered trademark) particle size distribution measuring device UPA-EX150) applying the laser Doppler method. it can.
- Another aspect of the present invention is an electric wire / cable using the non-halogen flame retardant resin composition as a coating layer.
- a non-halogen insulated electric wire / cable having cut-through strength, abrasion resistance, flame retardancy, and tensile properties satisfying UL standards can be obtained.
- the thickness of the covering layer is preferably 0.4 mm or less.
- the thickness of the coating layer is as thin as 0.4 mm or less, the difference from the electric wire according to the prior art becomes remarkable in characteristics such as cut-through strength, and an excellent effect is exhibited.
- the covering layer is preferably cross-linked by irradiation with ionizing radiation. Since the coating layer is crosslinked, heat resistance and tensile properties are improved.
- non-halogen flame retardant resin composition having cut-through strength, abrasion resistance, and flame retardancy and tensile properties satisfying UL standards, and an electric wire / cable using the same. Can do.
- Polyolefin resins include polyethylene (high density polyethylene, linear low density polyethylene, low density polyethylene, ultra low density polyethylene), ethylene-vinyl acetate copolymer, ethylene-methyl methacrylate copolymer, ethylene-acrylic acid.
- An epoxy group-containing ethylene copolymer is used as part of the polyolefin resin.
- the epoxy group-containing ethylene copolymer is obtained by copolymerizing an epoxy group-containing olefin monomer such as glycidyl methacrylate and an ethylene monomer. Specifically, ethylene-glycidyl methacrylate copolymer, ethylene-propylene-glycidyl methacrylate copolymer, ethylene-butene-1-glycidyl methacrylate copolymer, ethylene-vinyl acetate-glycidyl methacrylate copolymer, And ethylene-acrylic acid-glycidyl methacrylate copolymer.
- the content of the epoxy group-containing ethylene-based copolymer is 2 parts by mass or more and 15 parts by mass or less, more preferably 5 parts by mass or more and 10 parts by mass or less based on the entire resin component.
- the polyolefin-based resin it is preferable to contain an ethylene-vinyl acetate copolymer having a vinyl acetate content of 20 to 50 parts by mass.
- the vinyl acetate content is less than 20 parts by mass, the flame retardancy is lowered and the UL standard cannot be satisfied.
- the vinyl acetate content exceeds 50 parts by mass, flame retardancy is improved, but the cut-through strength and wear resistance are also lowered along with the decrease in tensile strength, and the required characteristics cannot be satisfied.
- the content of the ethylene-vinyl acetate copolymer is preferably 10 parts by mass or more and 30 parts by mass or less of the entire resin component.
- high density polyethylene is contained as part of the polyolefin resin.
- the high density polyethylene is a homopolyethylene or a polyethylene copolymer, and is a polyethylene having a density of 0.942 g / cm 3 or more.
- MFR melt flow rate
- a lower MFR tends to improve the wear resistance.
- the content of the high density polyethylene is preferably 10 parts by mass or more and 30 parts by mass or less of the entire resin component.
- styrene elastomers examples include styrene / ethylene butene / styrene copolymers, styrene / ethylene propylene / styrene copolymers, styrene / ethylene / ethylene propylene / styrene copolymers, and styrene / butylene / styrene copolymers.
- hydrogenated polymers and partially hydrogenated polymers can be exemplified.
- transduced carboxylic acid such as maleic anhydride, can also be blended suitably and used.
- Block copolymers include hydrogenated styrene / butylene / styrene block copolymers, triblock copolymers such as styrene / isobutylene / styrene copolymers, styrene / ethylene copolymers, styrene / ethylene propylene, etc. It is preferable that the triblock component in the styrene elastomer is contained in an amount of 50% by mass or more because the strength and hardness of the coating layer are improved.
- those having a styrene content of 20% by mass or more contained in the styrene elastomer can be suitably used from the viewpoint of tensile properties (strength, elongation) and flame retardancy.
- tensile properties strength, elongation
- flame retardancy When the styrene content is less than 20% by mass, the hardness and extrusion processability are lowered. On the other hand, when the styrene content exceeds 60% by mass, the tensile elongation is lowered, which is not preferable.
- the MFR as an index of molecular weight is in the range of 0.8 to 15 g / 10 min. This is because if the MFR is less than 0.8 g / 10 min, the extrusion processability is lowered, and if it exceeds 15 g / 10 min, the mechanical strength is lowered.
- nitrogen-based flame retardants examples include melamine resin and melamine cyanurate.
- Nitrogen-based flame retardants do not generate toxic gases such as hydrogen halides even when incinerated after use, and can reduce the environmental burden.
- melamine cyanurate When melamine cyanurate is used as a nitrogen-based flame retardant, it is preferable in terms of heat stability at the time of mixing and an effect of improving flame retardancy.
- Melamine cyanurate can also be used after surface treatment with a silane coupling agent or a titanate coupling agent.
- the average particle size of the nitrogen flame retardant is preferably 5 ⁇ m or less. Content of a nitrogen-type flame retardant shall be 5 to 20 mass parts with respect to 100 mass parts of resin components. If the amount is less than 5 parts by mass, the flame resistance of the insulated wire is insufficient, and if it exceeds 20 parts by mass, the elongation and extrusion processability are deteriorated.
- metal hydroxides examples include aluminum hydroxide, magnesium hydroxide, calcium hydroxide and the like. Among these, from the viewpoint of extrusion processability, magnesium hydroxide having an average particle size of 0.1 ⁇ m or more and 3 ⁇ m or less is preferable. Content of a metal hydroxide shall be 60 to 100 mass parts with respect to 100 mass parts of resin components. When the amount is less than 60 parts by mass, the flame resistance of the insulated wire is insufficient, and when the amount exceeds 100 parts by mass, elongation and extrusion processability are deteriorated.
- a crosslinking aid can be further added to the non-halogen flame retardant resin composition.
- a polyfunctional monomer having a plurality of carbon-carbon double bonds in the molecule such as trimethylolpropane trimethacrylate, triallyl cyanurate, triallyl isocyanurate and the like can be preferably used.
- a crosslinking adjuvant is a liquid at normal temperature. This is because when it is a liquid, it can be easily mixed with a polyolefin resin or a styrene elastomer.
- Use of trimethylolpropane trimethacrylate as a crosslinking aid is preferred because compatibility with the resin is improved.
- an antioxidant an antioxidant, a processing stabilizer, a colorant, a heavy metal deactivator, a foaming agent and the like can be appropriately mixed as necessary.
- These materials can be prepared by mixing using a known melt mixer such as a short screw extruder, a pressure kneader, or a Banbury mixer.
- the electric wire (also referred to as an insulated wire) of the present invention has a coating layer made of the above-mentioned flame retardant resin composition, and the coating layer is formed directly on the conductor or via another layer.
- a known extruder such as a melt extruder can be used.
- the coating layer is preferably cross-linked by irradiating with ionizing radiation.
- a cable also referred to as an insulated cable
- has a sheath such as a shield layer on the surface of an electric wire.
- the conductor copper wire, aluminum wire, etc. having excellent conductivity can be used.
- the diameter of the conductor can be appropriately selected according to the intended use, but is preferably 2 mm or less in order to enable wiring in a narrow space. In consideration of ease of handling, the thickness is preferably 0.1 mm or more.
- the conductor may be a single wire or may be a strand of a plurality of strands.
- the thickness of the coating layer can be appropriately selected according to the conductor diameter, but the mechanical strength can be obtained by applying the flame-retardant resin composition of the present invention even when the thickness of the coating layer is 0.4 mm or less. Will be good.
- the wear resistance and the cut-through strength are reduced when the thickness of the coating layer is 0.4 mm or less, but according to the present invention, excellent performance is achieved even when the thickness of the coating layer is 0.4 mm or less. As a result, the difference from the electric wire according to the prior art appears remarkably.
- an electric wire having a coating layer thickness of 0.4 mm or less is preferably used from the viewpoint of fitting property with the connector.
- the coating layer is cross-linked by irradiation with ionizing radiation because the mechanical strength is improved.
- ionizing radiation sources include accelerated electron beams, gamma rays, X-rays, ⁇ rays, ultraviolet rays, and the like. Accelerated electron beams are used from the viewpoint of industrial use, such as ease of use of the radiation source, transmission thickness of ionizing radiation, and speed of crosslinking treatment. Is most preferably used.
- Examples 1 to 14 (Creation of non-halogen flame retardant resin composition pellets) Each component was mixed by the compounding prescription (unit: mass part) shown in Table 1. Using a 300 mm ⁇ open roll mixer, the mixture was melted and mixed at a temperature of 160 ° C. to 180 ° C., and the resulting strip-shaped resin composition was cut with a pelletizer to produce pellets.
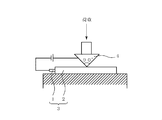
- Cut-through strength was measured using the measuring apparatus shown in FIG.
- the conductor and the sharp edge are insulated by the covering layer 2 and no current flows.
- a current flows between the conductor and the sharp edge.
- a load is applied to the blade 4 and the maximum load that can be withstood without cutting the coating layer 2 is measured.
- the test atmosphere is a temperature of 23 ° C. and a humidity of 50% RH. A load of 35N or more was regarded as an acceptable level.
- Insulated wires were produced in the same manner as in Examples 1 to 14 except that a resin composition having the formulation (unit: parts by mass) shown in Table 2 was used, and a series of evaluations were performed.
- EVA1 ethylene vinyl acetate copolymer having a vinyl acetate content of 25% by mass: Mitsui DuPont Polychemical Co., Ltd.
- EVA2 Ethylene vinyl acetate copolymer having a vinyl acetate content of 33% by mass: Evaflex EV170, manufactured by Mitsui DuPont Polychemical Co., Ltd.
- St-based elastomer 1 SEEPS (polystyrene-poly (ethylene-ethylene / propylene) block-polystyrene) having a styrene content of 30% by mass: Septon (registered trademark) 4033 manufactured by Kuraray Co., Ltd.
- St-based elastomer 2 SEEPS-OH (SEEPS having an OH group at the end): Kuraray Co., Ltd.
- Septon (registered trademark) HG252 St-based elastomer 3 SEBS (polystyrene-poly (ethylene / butylene) block-polystyrene): manufactured by Asahi Kasei Corporation, Tuftec 1041 St elastomer 4: SEBS: Asahi Kasei Corporation, Tuftec H1051 St elastomer 5: SEBS: Kuraray Co., Ltd.
- Septon (registered trademark) 8104 Magnesium hydroxide: Kisuma 5SDK manufactured by Kyowa Chemical Industry Co., Ltd. Melamine cyanurate: MC6000 manufactured by Nissan Chemical Industries, Ltd.
- Antiaging agent Irganox 1010 manufactured by BASF Japan Copper protection: Adeka Stab CDA-1 manufactured by ADEKA Corporation Lubricant: Stearic acid crosslinking aid: Trimethylolpropane trimethacrylate: TD1500S manufactured by DIC Corporation
- the insulated wires of Examples 1 to 14 each have a cut-through strength of 35 N or more and a scrape wear resistance of 650 times or more, and are high strength.
- the original tensile properties (elongation, strength) and the tensile properties after heat aging (elongation, residual ratio of strength) are acceptable levels, and the flame retardancy is also satisfied.
- the non-halogen flame retardant resin compositions used for the insulated wires of Comparative Examples 1 to 6 do not contain an epoxy group-containing ethylene copolymer.
- Original tensile properties elongation, strength
- tensile properties after heat aging elongation, residual ratio of strength
- flame retardancy are acceptable levels, but the cut-through strength is low. From this result, it can be seen that the cut-through strength is improved when an appropriate amount of the epoxy group-containing ethylene copolymer is contained in the resin composition.
- there is a scrape abrasion resistance which is not an acceptable level (Comparative Example 3 and Comparative Example 6).
- the non-halogen flame retardant resin composition used for the insulated wires of Comparative Examples 7 to 11 contains an epoxy group-containing ethylene-based copolymer. Since the comparative example 7 has too much content of a styrene-type elastomer, the cut-through intensity
- a nitrogen-type flame retardant melamine cyanurate
- a resin composition obtained by dynamically crosslinking polypropylene is used for the coating layer.
- the dynamic cross-linking material has insufficient stability during extrusion, and tensile properties such as elongation and strength may be insufficient depending on the extrusion conditions.
Abstract
Provided are: a non-halogen flame retardant resin composition which has cut-through strength, wear resistance and flame retardance required for an electric wire for insulation displacement and has tensile properties that satisfy UL standards; and an electric wire and a cable which use said flame retardant resin composition as a coating layer thereof. This non-halogen flame retardant resin composition comprises 60 to 100 parts by mass of a metal hydroxide and 5 to 20 parts by mass of a nitrogen-based flame retardant with respect to 100 parts by mass of a resin component, wherein the resin component contains in 100 parts by mass thereof, 50 to 80 parts by mass of a polyolefin-based resin and 20 to 50 parts by mass of a styrene-based elastomer as well as 2 to 15 parts by mass of an epoxy group-containing ethylene-based copolymer as part of the polyolefin-based resin.
Description
本発明は、電線などの被覆層として好適に用いられるノンハロゲン難燃性樹脂組成物及びこの樹脂組成物を用いた電線・ケーブルに関する。
The present invention relates to a non-halogen flame retardant resin composition suitably used as a coating layer for electric wires and the like, and an electric wire / cable using the resin composition.
複写機、プリンタなどのOA機器、電子機器の内部配線では、プリント基板間やプリント基板とセンサー、アクチュエータ、モータ等の電子部品間で給電や信号電送を行うワイヤーハーネスが多量に使用されている。
In the internal wiring of OA equipment and electronic equipment such as copiers and printers, a large amount of wire harnesses are used for power supply and signal transmission between printed circuit boards and between electronic parts such as printed circuit boards and sensors, actuators, and motors.
ワイヤーハーネスとは、複数本の電線やケーブルを束ねて端末に挿抜可能なコネクタ等の端子を組み付けしたものである。難燃性、電気絶縁性等の点から、ワイヤーハーネス用の電線には絶縁材料としてポリ塩化ビニル(PVC)を適用したPVC電線が使用されている。PVC電線は柔軟性に優れるのでワイヤーハーネスとした場合も取り回し性が良く、また充分な強度を有しているのでワイヤーハーネスの配線中に絶縁体が破れたり摩耗したりする問題が無く、更に端末に取り付ける圧接コネクタの取り付け作業性にも優れている。
A wire harness is an assembly of terminals such as connectors that can be inserted into and removed from a terminal by bundling multiple wires and cables. From the viewpoints of flame retardancy, electrical insulation, etc., PVC electric wires using polyvinyl chloride (PVC) as an insulating material are used for electric wires for wire harnesses. Since PVC wires are excellent in flexibility, they are easy to handle even when used as wire harnesses, and have sufficient strength, so there is no problem that the insulator breaks or wears during wiring of the wire harness. Excellent workability for attaching pressure contact connectors to be mounted on.
しかし、PVC電線にはハロゲン元素が含まれるため、使用後のワイヤーハーネスの焼却処理を行う場合に塩化水素系の有毒ガスが発生したり、また焼却条件によってはダイオキシンを発生するという問題がある。環境負荷の低減が求められる中、PVCは絶縁材料として好ましい材料とはいえない。
However, since the PVC electric wire contains a halogen element, there is a problem that a toxic gas of hydrogen chloride is generated when the wire harness is incinerated after use, and dioxin is generated depending on the incineration conditions. While the reduction of environmental load is required, PVC is not a preferable material as an insulating material.
近年、環境負荷の低減に対する要求の高まりに応えるために、ポリ塩化ビニル樹脂やハロゲン系難燃剤を含有しない被覆材料を用いたハロゲンフリー電線が開発されている。他方、電子機器の機内配線に使用する絶縁電線や絶縁ケーブルには、一般に、UL(Underwriters Laboratories inc.)規格に適合する諸特性を有することが求められている。UL規格には製品が満たすべき難燃性、加熱変形性、低温特性、被覆材料の初期と熱老化後の引張特性などの諸特性が詳細に規定されている。
In recent years, halogen-free electric wires using a coating material that does not contain polyvinyl chloride resin or a halogen-based flame retardant have been developed in order to meet the increasing demand for reducing the environmental burden. On the other hand, insulated wires and insulated cables used for in-machine wiring of electronic devices are generally required to have various characteristics that conform to UL (Underwriters Laboratories Inc.) standards. The UL standard stipulates in detail various properties such as flame retardancy, heat deformability, low temperature properties, initial properties of coating materials and tensile properties after heat aging.
圧接あるいは圧着用途の電線では、電子機器内でワイヤーハーネスを引き回す必要がある。この作業中に電線の絶縁被覆に傷や破れが生じて不良となる可能性があるため、ワイヤーハーネスに使用される絶縁電線には高いカットスルー強度が求められる。また耐摩耗性も求められる。
For wires used for pressure welding or crimping, it is necessary to route the wire harness inside the electronic device. Since the insulation coating of the electric wire may be damaged or broken during this operation, it may become defective, so that the insulated wire used for the wire harness is required to have high cut-through strength. Abrasion resistance is also required.
特開2002-105255号公報(特許文献1)にはポリプロピレンにエチレンプロピレンゴムやスチレンブタジエンゴム等のエラストマーを配合した熱可塑性樹脂成分に対して、金属水和物を加熱・混練した難燃性樹脂組成物、及びそれを用いた配線材が開示されている。エラストマーを配合することでフィラー受容性を高めることができ、またこれらのエラストマーを架橋することで、柔軟性、伸び等の機械的物性と押出加工性及び難燃性のバランスを取ることが検討されている。しかし、このような材料はPVCと比べると耐摩耗性や耐エッジ性(カットスルー特性)が悪く、これらの特性を向上させようとすると柔軟性が低下して特性のバランスを失うという問題があった。
Japanese Patent Application Laid-Open No. 2002-105255 (Patent Document 1) discloses a flame retardant resin obtained by heating and kneading a metal hydrate to a thermoplastic resin component in which an elastomer such as ethylene propylene rubber or styrene butadiene rubber is blended with polypropylene. A composition and a wiring material using the composition are disclosed. Filler acceptability can be increased by blending elastomers, and by cross-linking these elastomers, it has been studied to balance mechanical properties such as flexibility and elongation with extrudability and flame retardancy. ing. However, such materials have poor wear resistance and edge resistance (cut-through characteristics) compared with PVC, and there is a problem that flexibility is lost and the balance of characteristics is lost when trying to improve these characteristics. It was.
特開2006-36813号公報(特許文献2)及び特開2007-302907号公報(特許文献3)には、ポリプロピレンを主体とした樹脂混合物を有機過酸化物の存在下に動的架橋して得られる樹脂組成物が開示されている。これらの材料は、ポリプロピレンは電離放射線照射では架橋しないことから動的架橋により強度を高めている。しかし動的架橋材料は押出加工時の安定性が不十分であり、押出条件によっては伸び、強度といった引張特性が不十分となることがある。
JP-A-2006-36813 (Patent Document 2) and JP-A-2007-302907 (Patent Document 3) are obtained by dynamically crosslinking a resin mixture mainly composed of polypropylene in the presence of an organic peroxide. A resin composition is disclosed. Since these materials are not crosslinked by irradiation with ionizing radiation, the strength of these materials is increased by dynamic crosslinking. However, the dynamic cross-linking material has insufficient stability during extrusion, and depending on the extrusion conditions, tensile properties such as elongation and strength may be insufficient.
電離放射線照射で架橋するポリエチレンを主体とする樹脂組成物を用いれば、架橋度を調整することで必要な強度を得ることができる。しかしノンハロゲン系で難燃特性を出すためには金属水酸化物等の難燃剤を樹脂組成物中に多量に添加する必要があり、圧接あるいは圧着用途の電線に求められるカットスルー強度や耐摩耗性を満足することができない。また伸びが低下することからUL規格に定める引張特性を満足できない。
If a resin composition mainly composed of polyethylene that is crosslinked by irradiation with ionizing radiation is used, the required strength can be obtained by adjusting the degree of crosslinking. However, in order to produce flame retardant properties in a non-halogen system, it is necessary to add a large amount of a flame retardant such as a metal hydroxide to the resin composition, and the cut-through strength and wear resistance required for electric wires for pressure welding or crimping applications. Can not be satisfied. Further, since the elongation is lowered, the tensile properties defined in the UL standard cannot be satisfied.
そこで本発明は、圧接用電線に求められるカットスルー強度、耐摩耗性、及び難燃性を有すると共に、UL規格を満足する引張特性を有するノンハロゲン難燃性樹脂組成物及びこの難燃性樹脂組成物を被覆層として用いた電線・ケーブルを提供することを課題とする。
Accordingly, the present invention provides a non-halogen flame retardant resin composition having cut-through strength, abrasion resistance, and flame resistance required for pressure welding wires, and tensile properties satisfying UL standards, and the flame retardant resin composition. It is an object to provide an electric wire / cable using an object as a covering layer.
本発明は、樹脂成分100質量部に対して金属水酸化物60~100質量部及び窒素系難燃剤5~20質量部を含有するノンハロゲン難燃性樹脂組成物であって、前記樹脂成分100質量部中にポリオレフィン系樹脂50~80質量部及びスチレン系エラストマー20~50質量部を含有するとともに、前記ポリオレフィン系樹脂の一部としてエポキシ基含有エチレン系共重合体を2~15質量部含有するノンハロゲン難燃性樹脂組成物である。
The present invention is a non-halogen flame retardant resin composition containing 60 to 100 parts by mass of a metal hydroxide and 5 to 20 parts by mass of a nitrogen-based flame retardant with respect to 100 parts by mass of the resin component, wherein 100 parts by mass of the resin component Non-halogen containing 50 to 80 parts by mass of a polyolefin resin and 20 to 50 parts by mass of a styrene elastomer and 2 to 15 parts by mass of an epoxy group-containing ethylene copolymer as a part of the polyolefin resin. It is a flame retardant resin composition.
樹脂成分としてポリオレフィン系樹脂とスチレン系エラストマーとを併用する。ポリオレフィン系樹脂は電線被覆として広く用いられており押出加工性に優れているだけでなく、電離放射線照射による架橋が可能であり、引張特性と耐熱性の両立に寄与している。また、スチレン系エラストマーは柔軟であることからフィラー受容性を補うだけでなく、引張特性の更なる向上、安定化を図ることが可能となる。両者を組み合わせることで、UL規格で求められる引張特性を安定して得ることができる。また金属水酸化物及び窒素系難燃剤を含有することで、VW-1垂直燃焼試験に合格できる難燃性を得ることができる。
¡A polyolefin resin and a styrene elastomer are used in combination as the resin component. Polyolefin resins are widely used as wire coatings and are not only excellent in extrusion processability but also capable of crosslinking by irradiation with ionizing radiation, contributing to both tensile properties and heat resistance. In addition, since the styrene elastomer is flexible, it can not only supplement the filler acceptability, but can further improve and stabilize the tensile properties. By combining both, the tensile properties required by the UL standard can be stably obtained. Further, by containing a metal hydroxide and a nitrogen-based flame retardant, flame retardance that can pass the VW-1 vertical combustion test can be obtained.
ポリオレフィン系樹脂の一部としてエポキシ基含有エチレン系共重合体を使用する。エポキシ基含有エチレン系共重合体はポリエチレン系樹脂であるため電離放射線照射により架橋する。またエポキシ基含有エチレン系共重合体を樹脂成分100質量部中に2~15質量部含有することで、エポキシ基含有エチレン系共重合体を含有しない場合に比べてカットスルー強度が格段に向上する。
An epoxy group-containing ethylene copolymer is used as part of the polyolefin resin. Since the epoxy group-containing ethylene copolymer is a polyethylene resin, it is crosslinked by irradiation with ionizing radiation. Further, by containing 2 to 15 parts by mass of the epoxy group-containing ethylene copolymer in 100 parts by mass of the resin component, the cut-through strength is remarkably improved as compared with the case where no epoxy group-containing ethylene copolymer is contained. .
ポリオレフィン系樹脂として、酢酸ビニル含有量が20質量部以上50質量部以下のエチレン-酢酸ビニル共重合体を含有すると好ましい。また、高密度ポリエチレンを含有すると好ましい。エチレン-酢酸ビニル共重合体は優れた柔軟性により水酸化マグネシウムなどの難燃剤受容性に優れるだけでなく、伸びの向上にも寄与する。また高密度ポリエチレンはカットスルー強度及び耐摩耗性の向上に寄与する。ポリオレフィン系樹脂としては、上記の2種の樹脂のように電離放射線照射によって架橋可能なエチレン系樹脂を使用することが好ましい。なおポリオレフィン系樹脂としてこの2種以外に低密度ポリエチレン等の他の樹脂を使用しても良い。
The polyolefin-based resin preferably contains an ethylene-vinyl acetate copolymer having a vinyl acetate content of 20 to 50 parts by mass. Moreover, it is preferable to contain a high density polyethylene. The ethylene-vinyl acetate copolymer not only has excellent acceptability for flame retardants such as magnesium hydroxide due to its excellent flexibility, but also contributes to an improvement in elongation. High-density polyethylene also contributes to improved cut-through strength and wear resistance. As the polyolefin-based resin, it is preferable to use an ethylene-based resin that can be cross-linked by ionizing radiation irradiation, such as the above-described two types of resins. In addition to these two types of polyolefin resins, other resins such as low density polyethylene may be used.
スチレン系エラストマーとしては、スチレンとゴム成分のブロック共重合エラストマーを使用することが好ましい。スチレンとゴム成分のブロック共重合エラストマーは伸び、強度が高いため難燃性樹脂組成物の引張特性をさらに向上することができる。
As the styrene elastomer, it is preferable to use a block copolymer elastomer of styrene and a rubber component. Since the block copolymer elastomer of styrene and a rubber component is stretched and has high strength, the tensile properties of the flame retardant resin composition can be further improved.
窒素系難燃剤は、平均粒子径が5μm以下のメラミンシアヌレートが好ましい。メラミンシアヌレートは混合時の熱安定性が良く、また窒素系難燃剤の中でも特に難燃性に優れている。さらに平均粒子径が5μm以下であることにより混合時の分散性が向上する。また金属水酸化物は、平均粒子径が0.1μm以上3μm以下の水酸化マグネシウムが好ましい。なお平均粒子径とは50%粒子径(D50)を指し、レーザードップラー法を応用した粒度分布測定装置(日機装(株)製、ナノトラック(登録商標)粒度分布測定装置UPA-EX150)等により測定できる。
The nitrogen flame retardant is preferably melamine cyanurate having an average particle size of 5 μm or less. Melamine cyanurate has good thermal stability during mixing, and is particularly excellent in flame retardancy among nitrogen-based flame retardants. Furthermore, the dispersibility at the time of mixing improves because an average particle diameter is 5 micrometers or less. The metal hydroxide is preferably magnesium hydroxide having an average particle size of 0.1 μm or more and 3 μm or less. The average particle size means 50% particle size (D50), measured by a particle size distribution measuring device (Nikkiso Co., Ltd., Nanotrac (registered trademark) particle size distribution measuring device UPA-EX150) applying the laser Doppler method. it can.
本発明の別の態様は、上記のノンハロゲン難燃性樹脂組成物を被覆層として用いた電線・ケーブルである。本発明により、カットスルー強度、耐摩耗性、難燃性、及びUL規格を満足する引張特性を有するノンハロゲン絶縁電線・ケーブルが得られる。
Another aspect of the present invention is an electric wire / cable using the non-halogen flame retardant resin composition as a coating layer. According to the present invention, a non-halogen insulated electric wire / cable having cut-through strength, abrasion resistance, flame retardancy, and tensile properties satisfying UL standards can be obtained.
前記電線・ケーブルにおいて、前記被覆層の厚みは0.4mm以下であることが好ましい。被覆層の厚みが0.4mm以下と薄い場合には、カットスルー強度等の特性において従来技術による電線との差が顕著となり、優れた効果を発揮する。
In the electric wire / cable, the thickness of the covering layer is preferably 0.4 mm or less. When the thickness of the coating layer is as thin as 0.4 mm or less, the difference from the electric wire according to the prior art becomes remarkable in characteristics such as cut-through strength, and an excellent effect is exhibited.
前記電線・ケーブルにおいて、前記被覆層は電離放射線の照射により架橋されていることが好ましい。被覆層が架橋されていることで、耐熱性や引張特性が向上する。
In the electric wire / cable, the covering layer is preferably cross-linked by irradiation with ionizing radiation. Since the coating layer is crosslinked, heat resistance and tensile properties are improved.
本発明によれば、カットスルー強度、耐摩耗性、及び難燃性を有すると共に、UL規格を満足する引張特性を有するノンハロゲン難燃性樹脂組成物及びこれを用いた電線・ケーブルを提供することができる。
According to the present invention, there is provided a non-halogen flame retardant resin composition having cut-through strength, abrasion resistance, and flame retardancy and tensile properties satisfying UL standards, and an electric wire / cable using the same. Can do.
まずノンハロゲン難燃性樹脂組成物に使用する各種材料について説明する。ポリオレフィン系樹脂としては、ポリエチレン(高密度ポリエチレン、直鎖状低密度ポリエチレン、低密度ポリエチレン、超低密度ポリエチレン)、エチレン-酢酸ビニル共重合体、エチレン-メタクリル酸メチル共重合体、エチレン-アクリル酸メチル共重合体、エチレン-アクリル酸エチル共重合体、エチレン-メタクリル酸エチル共重合体、エチレン-アクリル酸ブチル共重合体、エチレン-プロピレンゴム、エチレンアクリルゴム、エチレン-グリシジルメタクリレート共重合体、エチレン-メタクリル酸共重合体、ポリプロピレン(ホモポリマー、ブロックポリマー、ランダムポリマー)、ポリプロピレン系熱可塑性エラストマー、アイオノマー樹脂等を使用できる。
First, various materials used for the non-halogen flame retardant resin composition will be described. Polyolefin resins include polyethylene (high density polyethylene, linear low density polyethylene, low density polyethylene, ultra low density polyethylene), ethylene-vinyl acetate copolymer, ethylene-methyl methacrylate copolymer, ethylene-acrylic acid. Methyl copolymer, ethylene-ethyl acrylate copolymer, ethylene-ethyl methacrylate copolymer, ethylene-butyl acrylate copolymer, ethylene-propylene rubber, ethylene acrylic rubber, ethylene-glycidyl methacrylate copolymer, ethylene -Methacrylic acid copolymer, polypropylene (homopolymer, block polymer, random polymer), polypropylene-based thermoplastic elastomer, ionomer resin, etc. can be used.
ポリオレフィン系樹脂の一部として、エポキシ基含有エチレン系共重合体を使用する。エポキシ基含有エチレン系共重合体は、メタクリル酸グリシジル等のエポキシ基含有オレフィン系モノマーとエチレン系モノマーとを共重合させたものである。具体的には、エチレン-メタクリル酸グリシジル共重合体、エチレン-プロピレン-メタクリル酸グリシジル共重合体、エチレン-ブテン-1-メタクリル酸グリシジル共重合体、エチレン-酢酸ビニル-メタクリル酸グリシジル共重合体、エチレン-アクリル酸-メタクリル酸グリシジル共重合体等が挙げられる。エポキシ基含有エチレン系共重合体の含有量は、樹脂成分全体の2質量部以上15質量部以下、さらに好ましくは5質量部以上10質量部以下である。
An epoxy group-containing ethylene copolymer is used as part of the polyolefin resin. The epoxy group-containing ethylene copolymer is obtained by copolymerizing an epoxy group-containing olefin monomer such as glycidyl methacrylate and an ethylene monomer. Specifically, ethylene-glycidyl methacrylate copolymer, ethylene-propylene-glycidyl methacrylate copolymer, ethylene-butene-1-glycidyl methacrylate copolymer, ethylene-vinyl acetate-glycidyl methacrylate copolymer, And ethylene-acrylic acid-glycidyl methacrylate copolymer. The content of the epoxy group-containing ethylene-based copolymer is 2 parts by mass or more and 15 parts by mass or less, more preferably 5 parts by mass or more and 10 parts by mass or less based on the entire resin component.
ポリオレフィン系樹脂の一部として、酢酸ビニル含有量が20質量部以上50質量部以下のエチレン-酢酸ビニル共重合体を含有すると好ましい。酢酸ビニル含有量が20質量部を下回る場合には難燃性が低下しUL規格を満足できない。また、酢酸ビニル含有量が50質量部を上回ると難燃性は向上するが、引張強さの低下と共に、カットスルー強度や耐摩耗性も低下し要求特性を満足できない。エチレン-酢酸ビニル共重合体の含有量は、樹脂成分全体の10質量部以上30質量部以下とすると好ましい。
As part of the polyolefin-based resin, it is preferable to contain an ethylene-vinyl acetate copolymer having a vinyl acetate content of 20 to 50 parts by mass. When the vinyl acetate content is less than 20 parts by mass, the flame retardancy is lowered and the UL standard cannot be satisfied. In addition, when the vinyl acetate content exceeds 50 parts by mass, flame retardancy is improved, but the cut-through strength and wear resistance are also lowered along with the decrease in tensile strength, and the required characteristics cannot be satisfied. The content of the ethylene-vinyl acetate copolymer is preferably 10 parts by mass or more and 30 parts by mass or less of the entire resin component.
ポリオレフィン系樹脂の一部として、高密度ポリエチレンを含有すると好ましい。高密度ポリエチレンはホモポリエチレン又はポリエチレンコポリマーであり、密度0.942g/cm3以上のポリエチレンである。また分子量の指標となるメルトフローレート(以下「MFR」と略記;JIS K 7210に従って、230℃×2.16kgfで測定、単位g/10min)が0.80以下のものを使用することが好ましい。MFRが低いほど耐摩耗性が向上する傾向にある。高密度ポリエチレンの含有量は、樹脂成分全体の10質量部以上30質量部以下とすると好ましい。
It is preferable that high density polyethylene is contained as part of the polyolefin resin. The high density polyethylene is a homopolyethylene or a polyethylene copolymer, and is a polyethylene having a density of 0.942 g / cm 3 or more. In addition, it is preferable to use one having a melt flow rate (hereinafter abbreviated as “MFR”, measured at 230 ° C. × 2.16 kgf, unit g / 10 min in accordance with JIS K 7210, unit g / 10 min) serving as an index of molecular weight of 0.80 or less. A lower MFR tends to improve the wear resistance. The content of the high density polyethylene is preferably 10 parts by mass or more and 30 parts by mass or less of the entire resin component.
スチレン系エラストマーとしては、スチレン・エチレンブテン・スチレン共重合体、スチレン・エチレンプロピレン・スチレン共重合体、スチレン・エチレン・エチレンプロピレン・スチレン共重合体、スチレン・ブチレン・スチレン共重合体等が挙げられ、これらの水素添加ポリマーや部分水素添加ポリマーを例示できる。また無水マレイン酸等のカルボン酸を導入したものを適宜ブレンドして使用することもできる。
Examples of styrene elastomers include styrene / ethylene butene / styrene copolymers, styrene / ethylene propylene / styrene copolymers, styrene / ethylene / ethylene propylene / styrene copolymers, and styrene / butylene / styrene copolymers. These hydrogenated polymers and partially hydrogenated polymers can be exemplified. Moreover, what introduce | transduced carboxylic acid, such as maleic anhydride, can also be blended suitably and used.
この中でも、スチレンとゴム成分のブロック共重合エラストマーを使用すると、押出加工性が向上することに加え、引張伸びが向上し、また耐衝撃性が向上するなどの点で好ましい。またブロック共重合体として、水素化スチレン・ブチレン・スチレンブロック共重合体やスチレン・イソブチレン・スチレン系共重合体等のトリブロック型共重合体、及びスチレン・エチレン共重合体、スチレン・エチレンプロピレン等のジブロック型共重合体を使用することができ、スチレン系エラストマー中トリブロック成分が50質量%以上含まれていると被覆層の強度及び硬度が向上するため好ましい。
Among these, the use of a block copolymer elastomer of styrene and a rubber component is preferable from the viewpoints of improving extrudability, improving tensile elongation, and improving impact resistance. Block copolymers include hydrogenated styrene / butylene / styrene block copolymers, triblock copolymers such as styrene / isobutylene / styrene copolymers, styrene / ethylene copolymers, styrene / ethylene propylene, etc. It is preferable that the triblock component in the styrene elastomer is contained in an amount of 50% by mass or more because the strength and hardness of the coating layer are improved.
またスチレン系エラストマー中に含まれるスチレン含有量が20質量%以上のものが引張特性(強度、伸び)、難燃性の点から好適に使用できる。スチレン含有量が20質量%より少ないと硬度や押出加工性が低下する。またスチレン含有量が60質量%を超えると引張伸びが低下するため好ましくない。更に、分子量の指標となるMFRが0.8~15g/10minの範囲であることが好ましい。MFRが0.8g/10minより小さいと押出加工性が低下し、また15g/10minを超えると機械強度が低下するからである。
Also, those having a styrene content of 20% by mass or more contained in the styrene elastomer can be suitably used from the viewpoint of tensile properties (strength, elongation) and flame retardancy. When the styrene content is less than 20% by mass, the hardness and extrusion processability are lowered. On the other hand, when the styrene content exceeds 60% by mass, the tensile elongation is lowered, which is not preferable. Further, it is preferable that the MFR as an index of molecular weight is in the range of 0.8 to 15 g / 10 min. This is because if the MFR is less than 0.8 g / 10 min, the extrusion processability is lowered, and if it exceeds 15 g / 10 min, the mechanical strength is lowered.
窒素系難燃剤としては、メラミン樹脂、メラミンシアヌレート等を例示できる。窒素系難燃剤は使用後に焼却処理してもハロゲン化水素等の有毒ガスが発生せず、環境負荷の低減を図ることができる。窒素系難燃剤としてメラミンシアヌレートを使用すると混合時の熱安定性や難燃性向上効果の面で好ましい。メラミンシアヌレートは、シランカップリング剤やチタネート系カップリング剤で表面処理して使用することも可能である。また窒素系難燃剤の平均粒子系は5μm以下が好ましい。窒素系難燃剤の含有量は、樹脂成分100質量部に対して5質量部以上20質量部以下とする。5質量部を下回ると絶縁電線の難燃性が不充分であり、20質量部を超えると伸びや押出加工性が低下する。
Examples of nitrogen-based flame retardants include melamine resin and melamine cyanurate. Nitrogen-based flame retardants do not generate toxic gases such as hydrogen halides even when incinerated after use, and can reduce the environmental burden. When melamine cyanurate is used as a nitrogen-based flame retardant, it is preferable in terms of heat stability at the time of mixing and an effect of improving flame retardancy. Melamine cyanurate can also be used after surface treatment with a silane coupling agent or a titanate coupling agent. The average particle size of the nitrogen flame retardant is preferably 5 μm or less. Content of a nitrogen-type flame retardant shall be 5 to 20 mass parts with respect to 100 mass parts of resin components. If the amount is less than 5 parts by mass, the flame resistance of the insulated wire is insufficient, and if it exceeds 20 parts by mass, the elongation and extrusion processability are deteriorated.
金属水酸化物としては、水酸化アルミニウム、水酸化マグネシウム、水酸化カルシウム等を例示できる。この中でも押出加工性の観点から、平均粒子径が0.1μm以上3μm以下の水酸化マグネシウムが好ましい。金属水酸化物の含有量は樹脂成分100質量部に対して60質量部以上100質量部以下とする。60質量部を下回ると絶縁電線の難燃性が不十分であり、100質量部を超えると伸びや押出加工性が低下する。
Examples of metal hydroxides include aluminum hydroxide, magnesium hydroxide, calcium hydroxide and the like. Among these, from the viewpoint of extrusion processability, magnesium hydroxide having an average particle size of 0.1 μm or more and 3 μm or less is preferable. Content of a metal hydroxide shall be 60 to 100 mass parts with respect to 100 mass parts of resin components. When the amount is less than 60 parts by mass, the flame resistance of the insulated wire is insufficient, and when the amount exceeds 100 parts by mass, elongation and extrusion processability are deteriorated.
ノンハロゲン難燃性樹脂組成物には、さらに架橋助剤を添加することができる。架橋助剤としてはトリメチロールプロパントリメタクリレート、トリアリルシアヌレート、トリアリルイソシアヌレート等の分子内に複数の炭素-炭素二重結合を持つ多官能性モノマーが好ましく使用できる。また架橋助剤は常温で液体であることが好ましい。液体であるとポリオレフィン系樹脂やスチレン系エラストマーとの混合がしやすいからである。架橋助剤としてトリメチロールプロパントリメタクリレートを使用すると、樹脂への相溶性が向上して好ましい。
A crosslinking aid can be further added to the non-halogen flame retardant resin composition. As the crosslinking aid, a polyfunctional monomer having a plurality of carbon-carbon double bonds in the molecule such as trimethylolpropane trimethacrylate, triallyl cyanurate, triallyl isocyanurate and the like can be preferably used. Moreover, it is preferable that a crosslinking adjuvant is a liquid at normal temperature. This is because when it is a liquid, it can be easily mixed with a polyolefin resin or a styrene elastomer. Use of trimethylolpropane trimethacrylate as a crosslinking aid is preferred because compatibility with the resin is improved.
本発明のノンハロゲン難燃性樹脂組成物には、さらに必要に応じて酸化防止剤、老化防止剤、加工安定剤、着色剤、重金属不活性化材、発泡剤等を適宜混合することができ、これらの材料を短軸押出型混合機、加圧ニーダー、バンバリーミキサー等の既知の溶融混合機を用いて混合して作成することができる。
In the non-halogen flame retardant resin composition of the present invention, an antioxidant, an antioxidant, a processing stabilizer, a colorant, a heavy metal deactivator, a foaming agent and the like can be appropriately mixed as necessary. These materials can be prepared by mixing using a known melt mixer such as a short screw extruder, a pressure kneader, or a Banbury mixer.
本発明の電線(絶縁電線とも言う)は、上記の難燃性樹脂組成物からなる被覆層を有するものであり、導体上に被覆層が直接又は他の層を介して形成される。被覆層の形成には溶融押出機など既知の押出成形機を用いることができる。また被覆層に電離放射線を照射して架橋することが好ましい。またケーブル(絶縁ケーブルとも言う)は、電線の表面にシールド層等の外被を有するものである。
The electric wire (also referred to as an insulated wire) of the present invention has a coating layer made of the above-mentioned flame retardant resin composition, and the coating layer is formed directly on the conductor or via another layer. For forming the coating layer, a known extruder such as a melt extruder can be used. The coating layer is preferably cross-linked by irradiating with ionizing radiation. A cable (also referred to as an insulated cable) has a sheath such as a shield layer on the surface of an electric wire.
導体としては、導電性に優れる銅線、アルミ線などが使用できる。導体の径は使用用途に応じて適宜選択できるが、狭いスペースへの配線を可能とするためには2mm以下とすることが好ましい。また取り扱いの容易さを考慮すると0.1mm以上とすることが好ましい。導体は単線であっても良いし、複数の素線を撚り線したものでも良い。
As the conductor, copper wire, aluminum wire, etc. having excellent conductivity can be used. The diameter of the conductor can be appropriately selected according to the intended use, but is preferably 2 mm or less in order to enable wiring in a narrow space. In consideration of ease of handling, the thickness is preferably 0.1 mm or more. The conductor may be a single wire or may be a strand of a plurality of strands.
被覆層の厚みは、導体径に応じて適宜選択することができるが、被覆層の厚みが0.4mm以下のサイズにおいても、本発明の難燃性樹脂組成物を適用することで機械的強度は良好なものとなる。従来技術によるハロゲンフリー電線では、被覆層の厚みが0.4mm以下の場合に耐摩耗性やカットスルー強度が低下するが、本発明によると被覆層の厚みが0.4mm以下でも優れた性能が得られ、従来技術による電線との差が顕著に現れる。また圧接用電線においては、コネクタとの嵌合性の点から被覆層厚みが0.4mm以下の電線が好ましく使用される。
The thickness of the coating layer can be appropriately selected according to the conductor diameter, but the mechanical strength can be obtained by applying the flame-retardant resin composition of the present invention even when the thickness of the coating layer is 0.4 mm or less. Will be good. In the halogen-free electric wire according to the prior art, the wear resistance and the cut-through strength are reduced when the thickness of the coating layer is 0.4 mm or less, but according to the present invention, excellent performance is achieved even when the thickness of the coating layer is 0.4 mm or less. As a result, the difference from the electric wire according to the prior art appears remarkably. Moreover, in the pressure welding electric wire, an electric wire having a coating layer thickness of 0.4 mm or less is preferably used from the viewpoint of fitting property with the connector.
被覆層が電離放射線の照射により架橋されていると、機械的強度が向上して好ましい。電離放射線源としては、加速電子線やガンマ線、X線、α線、紫外線等が例示でき、線源利用の簡便さや電離放射線の透過厚み、架橋処理の速度など工業的利用の観点から加速電子線が最も好ましく利用できる。
It is preferable that the coating layer is cross-linked by irradiation with ionizing radiation because the mechanical strength is improved. Examples of ionizing radiation sources include accelerated electron beams, gamma rays, X-rays, α rays, ultraviolet rays, and the like. Accelerated electron beams are used from the viewpoint of industrial use, such as ease of use of the radiation source, transmission thickness of ionizing radiation, and speed of crosslinking treatment. Is most preferably used.
次に、本発明を実施例に基づいてさらに詳細に説明する。実施例は本発明の範囲を限定するものではない。
Next, the present invention will be described in more detail based on examples. The examples are not intended to limit the scope of the invention.
[実施例1~14]
(ノンハロゲン難燃性樹脂組成物ペレットの作成)
表1に示す配合処方(単位:質量部)で各成分を混合した。300mmφのオープンロール混合機を用い、160℃~180℃の温度にて溶融混合し、得られた帯状の樹脂組成物をペレタイザーで切断することによりペレットを作製した。 [Examples 1 to 14]
(Creation of non-halogen flame retardant resin composition pellets)
Each component was mixed by the compounding prescription (unit: mass part) shown in Table 1. Using a 300 mmφ open roll mixer, the mixture was melted and mixed at a temperature of 160 ° C. to 180 ° C., and the resulting strip-shaped resin composition was cut with a pelletizer to produce pellets.
(ノンハロゲン難燃性樹脂組成物ペレットの作成)
表1に示す配合処方(単位:質量部)で各成分を混合した。300mmφのオープンロール混合機を用い、160℃~180℃の温度にて溶融混合し、得られた帯状の樹脂組成物をペレタイザーで切断することによりペレットを作製した。 [Examples 1 to 14]
(Creation of non-halogen flame retardant resin composition pellets)
Each component was mixed by the compounding prescription (unit: mass part) shown in Table 1. Using a 300 mmφ open roll mixer, the mixture was melted and mixed at a temperature of 160 ° C. to 180 ° C., and the resulting strip-shaped resin composition was cut with a pelletizer to produce pellets.
(絶縁電線の作製)
単軸押出機(30mmφ、L/D=24)を用いて、導体(錫メッキ軟銅線を7本撚りしたもの。導体径0.48mm)上に肉厚が0.25mmになるように押出被覆した後、加速電圧2MeVの電子線を60kGy照射して絶縁電線を作成した。なお引張特性(オリジナル及び熱老化後)は作成した絶縁電線から導体を取り除いて被覆層のみとしたものを使用して評価した。 (Production of insulated wires)
Using a single-screw extruder (30 mmφ, L / D = 24), extrusion coating is performed so that the thickness is 0.25 mm on a conductor (7 tin-plated annealed copper wires, conductor diameter 0.48 mm). After that, an insulated wire was created by irradiating an electron beam with an acceleration voltage of 2 MeV by 60 kGy. The tensile properties (original and after heat aging) were evaluated using a conductor layer removed from the prepared insulated wire to make only the coating layer.
単軸押出機(30mmφ、L/D=24)を用いて、導体(錫メッキ軟銅線を7本撚りしたもの。導体径0.48mm)上に肉厚が0.25mmになるように押出被覆した後、加速電圧2MeVの電子線を60kGy照射して絶縁電線を作成した。なお引張特性(オリジナル及び熱老化後)は作成した絶縁電線から導体を取り除いて被覆層のみとしたものを使用して評価した。 (Production of insulated wires)
Using a single-screw extruder (30 mmφ, L / D = 24), extrusion coating is performed so that the thickness is 0.25 mm on a conductor (7 tin-plated annealed copper wires, conductor diameter 0.48 mm). After that, an insulated wire was created by irradiating an electron beam with an acceleration voltage of 2 MeV by 60 kGy. The tensile properties (original and after heat aging) were evaluated using a conductor layer removed from the prepared insulated wire to make only the coating layer.
(被覆層の評価:引張特性)
作製した電線から導体を抜き取り、被覆層の引張試験を行った。試験条件は引張速度=500mm/分、標線間距離=25mm、温度=23℃とし、引張強さ、及び引張伸び(破断伸び)を各3点の試料で測定し、それらの平均値を求めた。引張強さが10.3MPa以上かつ引張伸び150%以上のものを「合格」と判定した。 (Evaluation of coating layer: tensile properties)
A conductor was extracted from the produced electric wire, and a tensile test of the coating layer was performed. The test conditions were: tensile speed = 500 mm / min, distance between marked lines = 25 mm, temperature = 23 ° C., tensile strength and tensile elongation (breaking elongation) were measured with three samples each, and the average value was obtained. It was. A sample having a tensile strength of 10.3 MPa or more and a tensile elongation of 150% or more was judged as “pass”.
作製した電線から導体を抜き取り、被覆層の引張試験を行った。試験条件は引張速度=500mm/分、標線間距離=25mm、温度=23℃とし、引張強さ、及び引張伸び(破断伸び)を各3点の試料で測定し、それらの平均値を求めた。引張強さが10.3MPa以上かつ引張伸び150%以上のものを「合格」と判定した。 (Evaluation of coating layer: tensile properties)
A conductor was extracted from the produced electric wire, and a tensile test of the coating layer was performed. The test conditions were: tensile speed = 500 mm / min, distance between marked lines = 25 mm, temperature = 23 ° C., tensile strength and tensile elongation (breaking elongation) were measured with three samples each, and the average value was obtained. It was. A sample having a tensile strength of 10.3 MPa or more and a tensile elongation of 150% or more was judged as “pass”.
(被覆層の評価:セカントモジュラス)
上記引張試験と同様のサンプルを用いて、引張速度=50mm/分、標線間距離=25mm、温度=23℃で引張試験を行った後、応力-伸び曲線から伸びが2%となる点の弾性率を計算した。 (Evaluation of coating layer: secant modulus)
Using a sample similar to the above tensile test, after performing a tensile test at a tensile rate of 50 mm / min, a distance between marked lines of 25 mm, and a temperature of 23 ° C., the elongation at which the elongation becomes 2% from the stress-elongation curve The elastic modulus was calculated.
上記引張試験と同様のサンプルを用いて、引張速度=50mm/分、標線間距離=25mm、温度=23℃で引張試験を行った後、応力-伸び曲線から伸びが2%となる点の弾性率を計算した。 (Evaluation of coating layer: secant modulus)
Using a sample similar to the above tensile test, after performing a tensile test at a tensile rate of 50 mm / min, a distance between marked lines of 25 mm, and a temperature of 23 ° C., the elongation at which the elongation becomes 2% from the stress-elongation curve The elastic modulus was calculated.
(被覆層の評価:耐熱老化性)
絶縁電線を136℃に設定したギアオーブン内で168時間(7日間)放置した後、引張特性評価と同様に引張試験を行い、加熱処理前の引張強度、引張伸びとの比較を行った。加熱処理前の引張強度に対し残率70%以上、引張伸びに対し残率45%以上を合格レベルとした。 (Evaluation of coating layer: heat aging resistance)
After leaving the insulated wire in a gear oven set at 136 ° C. for 168 hours (7 days), a tensile test was performed in the same manner as in the tensile property evaluation, and the tensile strength and tensile elongation before heat treatment were compared. A residual rate of 70% or more with respect to the tensile strength before the heat treatment and a residual rate of 45% or more with respect to the tensile elongation were regarded as acceptable levels.
絶縁電線を136℃に設定したギアオーブン内で168時間(7日間)放置した後、引張特性評価と同様に引張試験を行い、加熱処理前の引張強度、引張伸びとの比較を行った。加熱処理前の引張強度に対し残率70%以上、引張伸びに対し残率45%以上を合格レベルとした。 (Evaluation of coating layer: heat aging resistance)
After leaving the insulated wire in a gear oven set at 136 ° C. for 168 hours (7 days), a tensile test was performed in the same manner as in the tensile property evaluation, and the tensile strength and tensile elongation before heat treatment were compared. A residual rate of 70% or more with respect to the tensile strength before the heat treatment and a residual rate of 45% or more with respect to the tensile elongation were regarded as acceptable levels.
(絶縁電線の評価:カットスルー強度)
図1に示す測定装置を用いてカットスルー強度を測定した。導体1及び被覆層2からなる絶縁電線3の上に90°シャープエッジ(先端R=0.125mm、先端角度90°)を有する刃4を当て、導体とシャープエッジとの間に流れる電流値を測定する。初期状態では導体とシャープエッジとは被覆層2によって絶縁されており電流は流れないが、被覆層2が刃4によって切断されると導体とシャープエッジとの間に電流が流れる。刃4に荷重を加え、被覆層2が切断されないで耐える最大荷重を測定する。なお試験雰囲気は温度23℃、湿度50%RHとする。荷重35N以上を合格レベルとした。 (Evaluation of insulated wires: cut-through strength)
Cut-through strength was measured using the measuring apparatus shown in FIG. A blade 4 having a 90 ° sharp edge (tip R = 0.125 mm, tip angle 90 °) is applied on the insulated wire 3 composed of the conductor 1 and the covering layer 2, and the current value flowing between the conductor and the sharp edge is determined. taking measurement. In the initial state, the conductor and the sharp edge are insulated by the covering layer 2 and no current flows. However, when the covering layer 2 is cut by the blade 4, a current flows between the conductor and the sharp edge. A load is applied to the blade 4 and the maximum load that can be withstood without cutting the coating layer 2 is measured. The test atmosphere is a temperature of 23 ° C. and a humidity of 50% RH. A load of 35N or more was regarded as an acceptable level.
図1に示す測定装置を用いてカットスルー強度を測定した。導体1及び被覆層2からなる絶縁電線3の上に90°シャープエッジ(先端R=0.125mm、先端角度90°)を有する刃4を当て、導体とシャープエッジとの間に流れる電流値を測定する。初期状態では導体とシャープエッジとは被覆層2によって絶縁されており電流は流れないが、被覆層2が刃4によって切断されると導体とシャープエッジとの間に電流が流れる。刃4に荷重を加え、被覆層2が切断されないで耐える最大荷重を測定する。なお試験雰囲気は温度23℃、湿度50%RHとする。荷重35N以上を合格レベルとした。 (Evaluation of insulated wires: cut-through strength)
Cut-through strength was measured using the measuring apparatus shown in FIG. A blade 4 having a 90 ° sharp edge (tip R = 0.125 mm, tip angle 90 °) is applied on the insulated wire 3 composed of the conductor 1 and the covering layer 2, and the current value flowing between the conductor and the sharp edge is determined. taking measurement. In the initial state, the conductor and the sharp edge are insulated by the covering layer 2 and no current flows. However, when the covering layer 2 is cut by the blade 4, a current flows between the conductor and the sharp edge. A load is applied to the blade 4 and the maximum load that can be withstood without cutting the coating layer 2 is measured. The test atmosphere is a temperature of 23 ° C. and a humidity of 50% RH. A load of 35N or more was regarded as an acceptable level.
(絶縁電線の評価:スクレープ摩耗性)
絶縁電線を水平に固定して直径1.6mmのピアノ線を接触させ、ピアノ線の上から408gの荷重をかけて50~60Hzの速度で往復させる。被覆層が破壊して導体とピアノ線との間に電流が流れるまでの回数を測定する。650回以上を合格レベルとした。 (Evaluation of insulated wires: scrape wearability)
An insulated electric wire is fixed horizontally, a piano wire having a diameter of 1.6 mm is brought into contact, and a load of 408 g is applied from above the piano wire to reciprocate at a speed of 50-60 Hz. The number of times until the coating layer breaks and a current flows between the conductor and the piano wire is measured. 650 times or more was regarded as an acceptable level.
絶縁電線を水平に固定して直径1.6mmのピアノ線を接触させ、ピアノ線の上から408gの荷重をかけて50~60Hzの速度で往復させる。被覆層が破壊して導体とピアノ線との間に電流が流れるまでの回数を測定する。650回以上を合格レベルとした。 (Evaluation of insulated wires: scrape wearability)
An insulated electric wire is fixed horizontally, a piano wire having a diameter of 1.6 mm is brought into contact, and a load of 408 g is applied from above the piano wire to reciprocate at a speed of 50-60 Hz. The number of times until the coating layer breaks and a current flows between the conductor and the piano wire is measured. 650 times or more was regarded as an acceptable level.
(絶縁電線の評価:垂直燃焼試験)
UL規格1581、1080項に記載のVW-1垂直燃焼試験を5点の試料で行った。各試料に15秒着火を5回繰り返した場合に、60秒以内に消化し、下部に敷いた脱脂綿が燃焼落下物によって類焼せず、かつ試料の上部に取り付けたクラフト紙が燃えたり焦げたりしないものを合格とした。5点の試料を評価し、合格となった試料の点数を数えた。 (Evaluation of insulated wires: vertical combustion test)
The VW-1 vertical combustion test described in UL standard 1581, 1080 was performed on five samples. When each sample is ignited 15 seconds 5 times, it digests within 60 seconds, the absorbent cotton laid underneath is not burnt by burning fallen objects, and the kraft paper attached to the top of the sample does not burn or burn Things were accepted. Five samples were evaluated and the number of samples that passed was counted.
UL規格1581、1080項に記載のVW-1垂直燃焼試験を5点の試料で行った。各試料に15秒着火を5回繰り返した場合に、60秒以内に消化し、下部に敷いた脱脂綿が燃焼落下物によって類焼せず、かつ試料の上部に取り付けたクラフト紙が燃えたり焦げたりしないものを合格とした。5点の試料を評価し、合格となった試料の点数を数えた。 (Evaluation of insulated wires: vertical combustion test)
The VW-1 vertical combustion test described in UL standard 1581, 1080 was performed on five samples. When each sample is ignited 15 seconds 5 times, it digests within 60 seconds, the absorbent cotton laid underneath is not burnt by burning fallen objects, and the kraft paper attached to the top of the sample does not burn or burn Things were accepted. Five samples were evaluated and the number of samples that passed was counted.
(絶縁電線の評価:水平燃焼試験)
UL規格1581、1100項に記載の水平燃焼試験を5点の試料で行い、合格となった試料の点数を数えた。 (Evaluation of insulated wires: horizontal combustion test)
The horizontal combustion test described in UL standards 1581 and 1100 was performed on five samples, and the number of samples that passed was counted.
UL規格1581、1100項に記載の水平燃焼試験を5点の試料で行い、合格となった試料の点数を数えた。 (Evaluation of insulated wires: horizontal combustion test)
The horizontal combustion test described in UL standards 1581 and 1100 was performed on five samples, and the number of samples that passed was counted.
[比較例1~11]
表2に示す配合処方(単位:質量部)を持つ樹脂組成物を用いたこと以外は実施例1~14と同様に絶縁電線を作製し、一連の評価を行った。 [Comparative Examples 1 to 11]
Insulated wires were produced in the same manner as in Examples 1 to 14 except that a resin composition having the formulation (unit: parts by mass) shown in Table 2 was used, and a series of evaluations were performed.
表2に示す配合処方(単位:質量部)を持つ樹脂組成物を用いたこと以外は実施例1~14と同様に絶縁電線を作製し、一連の評価を行った。 [Comparative Examples 1 to 11]
Insulated wires were produced in the same manner as in Examples 1 to 14 except that a resin composition having the formulation (unit: parts by mass) shown in Table 2 was used, and a series of evaluations were performed.
[比較例12]
単軸押出機(30mmφ、L/D=24)を用いて導体(錫メッキ軟銅線を7本撚りしたもの。導体径0.48mm)上に肉厚が0.25mmになるように押出被覆し、動的架橋ポリプロピレン(リケンテクノス(株)製INA-9968)を被覆層とする絶縁電線を作製し、実施例1~14と同様に一連の評価を行った。以上の結果を表1及び表2に示す。 [Comparative Example 12]
Using a single-screw extruder (30 mmφ, L / D = 24), extrusion coating was performed on a conductor (7 tin-plated annealed copper wires, conductor diameter 0.48 mm) to a thickness of 0.25 mm. Then, an insulated wire having a dynamically cross-linked polypropylene (INA-9968 manufactured by Riken Technos Co., Ltd.) as a coating layer was prepared, and a series of evaluations were performed in the same manner as in Examples 1-14. The above results are shown in Tables 1 and 2.
単軸押出機(30mmφ、L/D=24)を用いて導体(錫メッキ軟銅線を7本撚りしたもの。導体径0.48mm)上に肉厚が0.25mmになるように押出被覆し、動的架橋ポリプロピレン(リケンテクノス(株)製INA-9968)を被覆層とする絶縁電線を作製し、実施例1~14と同様に一連の評価を行った。以上の結果を表1及び表2に示す。 [Comparative Example 12]
Using a single-screw extruder (30 mmφ, L / D = 24), extrusion coating was performed on a conductor (7 tin-plated annealed copper wires, conductor diameter 0.48 mm) to a thickness of 0.25 mm. Then, an insulated wire having a dynamically cross-linked polypropylene (INA-9968 manufactured by Riken Technos Co., Ltd.) as a coating layer was prepared, and a series of evaluations were performed in the same manner as in Examples 1-14. The above results are shown in Tables 1 and 2.
(脚注)
EVA1:酢酸ビニル含有量25質量%のエチレン酢酸ビニル共重合体:三井・デュポンポリケミカル(株)製、エバフレックスEV360
EVA2:酢酸ビニル含有量33質量%のエチレン酢酸ビニル共重合体:三井・デュポンポリケミカル(株)製、エバフレックスEV170
HDPE1:MFR=0.8、密度0.951g/cm3、硬度62Dの高密度ポリエチレン:プライムポリマー(株)製、ハイゼックス5305E
HDPE2:MFR=0.55、密度0.959g/cm3、硬度70Dの高密度ポリエチレン:日本ポリエチレン(株)製、ノバテックHY530
HDPE3:MFR=0.25、密度0.961g/cm3、硬度65Dの高密度ポリエチレン:プライムポリマー(株)製、ハイゼックス520MB
HDPE4:MFR=0.3、密度0.964g/cm3、硬度71Dの高密度ポリエチレン:日本ポリエチレン(株)製、ノバテックHB530
E-GMA:住友化学(株)製、ボンドファーストE(グリシジルメタクリレート含有量12質量%のエポキシ基含有エチレン系共重合体)
St系エラストマー1:スチレン含有量30質量%のSEEPS(ポリスチレン-ポリ(エチレン-エチレン/プロピレン)ブロック-ポリスチレン):クラレ(株)製セプトン(登録商標)4033
St系エラストマー2:SEEPS-OH(末端にOH基を持つSEEPS):クラレ(株)製セプトン(登録商標)HG252
St系エラストマー3:SEBS(ポリスチレン-ポリ(エチレン/ブチレン)ブロック-ポリスチレン):旭化成(株)製、タフテック1041
St系エラストマー4:SEBS:旭化成(株)製、タフテックH1051
St系エラストマー5:SEBS:クラレ(株)製セプトン(登録商標)8104
水酸化マグネシウム:(株)協和化学工業製 キスマ5SDK
メラミンシアヌレート:日産化学工業(株)製 MC6000
老防剤:BASFジャパン(株)製Irganox1010
銅防 :(株)ADEKA 製アデカスタブCDA-1
滑剤 :ステアリン酸
架橋助剤:トリメチロールプロパントリメタクリレート:DIC(株)製TD1500S (footnote)
EVA1: ethylene vinyl acetate copolymer having a vinyl acetate content of 25% by mass: Mitsui DuPont Polychemical Co., Ltd., EVAFLEX EV360
EVA2: Ethylene vinyl acetate copolymer having a vinyl acetate content of 33% by mass: Evaflex EV170, manufactured by Mitsui DuPont Polychemical Co., Ltd.
HDPE1: MFR = 0.8, density 0.951 g / cm 3 , hardness 62D high density polyethylene: made by Prime Polymer Co., Ltd., Hi-Zex 5305E
HDPE2: MFR = 0.55, density 0.959 g / cm 3 , high density polyethylene with hardness 70D: manufactured by Nippon Polyethylene Co., Ltd., Novatec HY530
HDPE3: MFR = 0.25, density 0.961 g / cm 3 , high density polyethylene with hardness 65D: made by Prime Polymer Co., Ltd., Hi-Zex 520MB
HDPE4: MFR = 0.3, density 0.964 g / cm 3 , hardness 71D high density polyethylene: manufactured by Nippon Polyethylene Co., Ltd., Novatec HB530
E-GMA: Bond First E (epoxy group-containing ethylene copolymer having a glycidyl methacrylate content of 12% by mass) manufactured by Sumitomo Chemical Co., Ltd.
St-based elastomer 1: SEEPS (polystyrene-poly (ethylene-ethylene / propylene) block-polystyrene) having a styrene content of 30% by mass: Septon (registered trademark) 4033 manufactured by Kuraray Co., Ltd.
St-based elastomer 2: SEEPS-OH (SEEPS having an OH group at the end): Kuraray Co., Ltd. Septon (registered trademark) HG252
St-based elastomer 3: SEBS (polystyrene-poly (ethylene / butylene) block-polystyrene): manufactured by Asahi Kasei Corporation, Tuftec 1041
St elastomer 4: SEBS: Asahi Kasei Corporation, Tuftec H1051
St elastomer 5: SEBS: Kuraray Co., Ltd. Septon (registered trademark) 8104
Magnesium hydroxide: Kisuma 5SDK manufactured by Kyowa Chemical Industry Co., Ltd.
Melamine cyanurate: MC6000 manufactured by Nissan Chemical Industries, Ltd.
Antiaging agent: Irganox 1010 manufactured by BASF Japan
Copper protection: Adeka Stab CDA-1 manufactured by ADEKA Corporation
Lubricant: Stearic acid crosslinking aid: Trimethylolpropane trimethacrylate: TD1500S manufactured by DIC Corporation
EVA1:酢酸ビニル含有量25質量%のエチレン酢酸ビニル共重合体:三井・デュポンポリケミカル(株)製、エバフレックスEV360
EVA2:酢酸ビニル含有量33質量%のエチレン酢酸ビニル共重合体:三井・デュポンポリケミカル(株)製、エバフレックスEV170
HDPE1:MFR=0.8、密度0.951g/cm3、硬度62Dの高密度ポリエチレン:プライムポリマー(株)製、ハイゼックス5305E
HDPE2:MFR=0.55、密度0.959g/cm3、硬度70Dの高密度ポリエチレン:日本ポリエチレン(株)製、ノバテックHY530
HDPE3:MFR=0.25、密度0.961g/cm3、硬度65Dの高密度ポリエチレン:プライムポリマー(株)製、ハイゼックス520MB
HDPE4:MFR=0.3、密度0.964g/cm3、硬度71Dの高密度ポリエチレン:日本ポリエチレン(株)製、ノバテックHB530
E-GMA:住友化学(株)製、ボンドファーストE(グリシジルメタクリレート含有量12質量%のエポキシ基含有エチレン系共重合体)
St系エラストマー1:スチレン含有量30質量%のSEEPS(ポリスチレン-ポリ(エチレン-エチレン/プロピレン)ブロック-ポリスチレン):クラレ(株)製セプトン(登録商標)4033
St系エラストマー2:SEEPS-OH(末端にOH基を持つSEEPS):クラレ(株)製セプトン(登録商標)HG252
St系エラストマー3:SEBS(ポリスチレン-ポリ(エチレン/ブチレン)ブロック-ポリスチレン):旭化成(株)製、タフテック1041
St系エラストマー4:SEBS:旭化成(株)製、タフテックH1051
St系エラストマー5:SEBS:クラレ(株)製セプトン(登録商標)8104
水酸化マグネシウム:(株)協和化学工業製 キスマ5SDK
メラミンシアヌレート:日産化学工業(株)製 MC6000
老防剤:BASFジャパン(株)製Irganox1010
銅防 :(株)ADEKA 製アデカスタブCDA-1
滑剤 :ステアリン酸
架橋助剤:トリメチロールプロパントリメタクリレート:DIC(株)製TD1500S (footnote)
EVA1: ethylene vinyl acetate copolymer having a vinyl acetate content of 25% by mass: Mitsui DuPont Polychemical Co., Ltd., EVAFLEX EV360
EVA2: Ethylene vinyl acetate copolymer having a vinyl acetate content of 33% by mass: Evaflex EV170, manufactured by Mitsui DuPont Polychemical Co., Ltd.
HDPE1: MFR = 0.8, density 0.951 g / cm 3 , hardness 62D high density polyethylene: made by Prime Polymer Co., Ltd., Hi-Zex 5305E
HDPE2: MFR = 0.55, density 0.959 g / cm 3 , high density polyethylene with hardness 70D: manufactured by Nippon Polyethylene Co., Ltd., Novatec HY530
HDPE3: MFR = 0.25, density 0.961 g / cm 3 , high density polyethylene with hardness 65D: made by Prime Polymer Co., Ltd., Hi-Zex 520MB
HDPE4: MFR = 0.3, density 0.964 g / cm 3 , hardness 71D high density polyethylene: manufactured by Nippon Polyethylene Co., Ltd., Novatec HB530
E-GMA: Bond First E (epoxy group-containing ethylene copolymer having a glycidyl methacrylate content of 12% by mass) manufactured by Sumitomo Chemical Co., Ltd.
St-based elastomer 1: SEEPS (polystyrene-poly (ethylene-ethylene / propylene) block-polystyrene) having a styrene content of 30% by mass: Septon (registered trademark) 4033 manufactured by Kuraray Co., Ltd.
St-based elastomer 2: SEEPS-OH (SEEPS having an OH group at the end): Kuraray Co., Ltd. Septon (registered trademark) HG252
St-based elastomer 3: SEBS (polystyrene-poly (ethylene / butylene) block-polystyrene): manufactured by Asahi Kasei Corporation, Tuftec 1041
St elastomer 4: SEBS: Asahi Kasei Corporation, Tuftec H1051
St elastomer 5: SEBS: Kuraray Co., Ltd. Septon (registered trademark) 8104
Magnesium hydroxide: Kisuma 5SDK manufactured by Kyowa Chemical Industry Co., Ltd.
Melamine cyanurate: MC6000 manufactured by Nissan Chemical Industries, Ltd.
Antiaging agent: Irganox 1010 manufactured by BASF Japan
Copper protection: Adeka Stab CDA-1 manufactured by ADEKA Corporation
Lubricant: Stearic acid crosslinking aid: Trimethylolpropane trimethacrylate: TD1500S manufactured by DIC Corporation
実施例1~14の絶縁電線はいずれもカットスルー強度が35N以上、スクレープ摩耗性の回数650回以上であり、高強度である。オリジナルの引張特性(伸び、強度)及び熱老化後の引張特性(伸び、強度の残率)は合格レベルであり、難燃性も満足している。
The insulated wires of Examples 1 to 14 each have a cut-through strength of 35 N or more and a scrape wear resistance of 650 times or more, and are high strength. The original tensile properties (elongation, strength) and the tensile properties after heat aging (elongation, residual ratio of strength) are acceptable levels, and the flame retardancy is also satisfied.
比較例1~6の絶縁電線に使用したノンハロゲン難燃性樹脂組成物にはエポキシ基含有エチレン系共重合体が含まれていない。オリジナルの引張特性(伸び、強度)、熱老化後の引張特性(伸び、強度の残率)、及び難燃性は合格レベルであるが、カットスルー強度が低い。この結果より、エポキシ基含有エチレン系共重合体が樹脂組成物中に適量入っていることでカットスルー強度が向上することがわかる。またスクレープ摩耗性が合格レベルでないものもある(比較例3、比較例6)。
The non-halogen flame retardant resin compositions used for the insulated wires of Comparative Examples 1 to 6 do not contain an epoxy group-containing ethylene copolymer. Original tensile properties (elongation, strength), tensile properties after heat aging (elongation, residual ratio of strength), and flame retardancy are acceptable levels, but the cut-through strength is low. From this result, it can be seen that the cut-through strength is improved when an appropriate amount of the epoxy group-containing ethylene copolymer is contained in the resin composition. Moreover, there is a scrape abrasion resistance which is not an acceptable level (Comparative Example 3 and Comparative Example 6).
比較例7~11の絶縁電線に使用したノンハロゲン難燃性樹脂組成物にはエポキシ基含有エチレン系共重合体が含まれている。比較例7はスチレン系エラストマーの含有率が多すぎるためカットスルー強度が低下している。比較例8~10は金属水酸化物(水酸化マグネシウム)の含有量が多すぎるため伸びが目標特性に達していない。比較例11は窒素系難燃剤(メラミンシアヌレート)の含有量が多すぎるため伸び及び熱老化後の伸び残率が目標特性に達していない。比較例12の絶縁電線は、ポリプロピレンを動的架橋した樹脂組成物を被覆層に用いている。今回の評価では目標特性をほぼ満足しているが、動的架橋材料は押出加工時の安定性が不十分であり、押出条件によっては伸び、強度といった引張特性が不十分となることがある。
The non-halogen flame retardant resin composition used for the insulated wires of Comparative Examples 7 to 11 contains an epoxy group-containing ethylene-based copolymer. Since the comparative example 7 has too much content of a styrene-type elastomer, the cut-through intensity | strength is falling. In Comparative Examples 8 to 10, since the content of metal hydroxide (magnesium hydroxide) is too large, the elongation does not reach the target characteristics. Since the comparative example 11 has too much content of a nitrogen-type flame retardant (melamine cyanurate), the elongation and the residual elongation rate after heat aging do not reach the target characteristics. In the insulated wire of Comparative Example 12, a resin composition obtained by dynamically crosslinking polypropylene is used for the coating layer. Although the target properties are almost satisfied in this evaluation, the dynamic cross-linking material has insufficient stability during extrusion, and tensile properties such as elongation and strength may be insufficient depending on the extrusion conditions.
1 導体
2 被覆層
3 絶縁電線
4 刃 1 conductor 2 coating layer 3 insulated wire 4 blade
2 被覆層
3 絶縁電線
4 刃 1 conductor 2 coating layer 3 insulated wire 4 blade
Claims (9)
- 樹脂成分100質量部に対して金属水酸化物60~100質量部及び窒素系難燃剤5~20質量部を含有するノンハロゲン難燃性樹脂組成物であって、前記樹脂成分100質量部中にポリオレフィン系樹脂50~80質量部及びスチレン系エラストマー20~50質量部を含有するとともに、前記ポリオレフィン系樹脂の一部としてエポキシ基含有エチレン系共重合体を2~15質量部含有する、ノンハロゲン難燃性樹脂組成物。 A non-halogen flame retardant resin composition containing 60 to 100 parts by mass of a metal hydroxide and 5 to 20 parts by mass of a nitrogen-based flame retardant with respect to 100 parts by mass of a resin component, the polyolefin being contained in 100 parts by mass of the resin component Non-halogen flame retardant containing 50 to 80 parts by weight of a resin and 20 to 50 parts by weight of a styrene elastomer and 2 to 15 parts by weight of an epoxy group-containing ethylene copolymer as a part of the polyolefin resin Resin composition.
- 前記ポリオレフィン系樹脂として酢酸ビニル含有量が20質量部以上50質量部以下のエチレン-酢酸ビニル共重合体を含有する、請求項1に記載のノンハロゲン難燃性樹脂組成物。 The non-halogen flame-retardant resin composition according to claim 1, wherein the polyolefin-based resin contains an ethylene-vinyl acetate copolymer having a vinyl acetate content of 20 to 50 parts by mass.
- 前記ポリオレフィン系樹脂として高密度ポリエチレンを含有する、請求項1又は2に記載のノンハロゲン難燃性樹脂組成物。 The non-halogen flame retardant resin composition according to claim 1 or 2, comprising high-density polyethylene as the polyolefin resin.
- 前記スチレン系エラストマーが、スチレンとゴム成分のブロック共重合エラストマーである、請求項1~3のいずれか1項に記載のノンハロゲン難燃性樹脂組成物。 4. The non-halogen flame retardant resin composition according to claim 1, wherein the styrene elastomer is a block copolymer elastomer of styrene and a rubber component.
- 前記窒素系難燃剤は、平均粒子径が5μm以下のメラミンシアヌレートである、請求項1~4のいずれか1項に記載のノンハロゲン難燃性樹脂組成物。 The non-halogen flame retardant resin composition according to any one of claims 1 to 4, wherein the nitrogen-based flame retardant is melamine cyanurate having an average particle size of 5 µm or less. *
- 前記金属水酸化物は、平均粒子径が0.1μm以上3μm以下の水酸化マグネシウムである、請求項1~5のいずれか1項に記載のノンハロゲン難燃性樹脂組成物。 The non-halogen flame retardant resin composition according to any one of claims 1 to 5, wherein the metal hydroxide is magnesium hydroxide having an average particle size of 0.1 µm to 3 µm.
- 請求項1~6のいずれか1項に記載のノンハロゲン難燃性樹脂組成物を被覆層として用いた電線・ケーブル。 An electric wire / cable using the non-halogen flame retardant resin composition according to any one of claims 1 to 6 as a coating layer.
- 前記被覆層の厚みが0.4mm以下である、請求項7に記載の電線・ケーブル。 The electric wire / cable according to claim 7, wherein the coating layer has a thickness of 0.4 mm or less.
- 前記被覆層が電離放射線の照射により架橋されている、請求項7又は8記載の電線・ケーブル。 The electric wire / cable according to claim 7 or 8, wherein the coating layer is crosslinked by irradiation with ionizing radiation.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020147007421A KR20140049606A (en) | 2012-03-22 | 2012-12-25 | Non-halogen flame retardant resin composition and electric wire and cable using same |
| CN201280046230.0A CN104220511A (en) | 2012-03-22 | 2012-12-25 | Non-halogen flame retardant resin composition, and electric wire and cable using same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-065592 | 2012-03-22 | ||
| JP2012065592A JP2013194214A (en) | 2012-03-22 | 2012-03-22 | Non-halogen flame-retardant resin composition, and wire and cable produced by using the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013140692A1 true WO2013140692A1 (en) | 2013-09-26 |
Family
ID=49222178
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/083388 WO2013140692A1 (en) | 2012-03-22 | 2012-12-25 | Non-halogen flame retardant resin composition, and electric wire and cable using same |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP2013194214A (en) |
| KR (1) | KR20140049606A (en) |
| CN (1) | CN104220511A (en) |
| WO (1) | WO2013140692A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103524843A (en) * | 2013-09-30 | 2014-01-22 | 芜湖航天特种电缆厂 | Sheath material for signal controlling cable and preparation method thereof |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6229942B2 (en) * | 2014-03-05 | 2017-11-15 | 日立金属株式会社 | Insulated wires for railway vehicles and cables for railway vehicles |
| CN105330930A (en) * | 2015-10-30 | 2016-02-17 | 无锡市长安曙光手套厂 | Halogen-free flame-retardant composite material and preparation method thereof |
| KR102408450B1 (en) * | 2020-10-22 | 2022-06-14 | 에이치디씨현대이피 주식회사 | Flame retardant composition for high heat resistant cable and composites prepared thereby |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07331086A (en) * | 1994-06-07 | 1995-12-19 | Sumitomo Bakelite Co Ltd | Thermoplastic resin composition |
| JPH11349742A (en) * | 1998-06-04 | 1999-12-21 | Hitachi Cable Ltd | Polyolefin resin composition and electric wire/cable |
| JP2001060414A (en) * | 1999-01-20 | 2001-03-06 | Furukawa Electric Co Ltd:The | Insulated resin composition and insulated wire |
| JP2001200106A (en) * | 1999-03-02 | 2001-07-24 | Jsr Corp | Flame-retarded polymer composition |
| JP2001226536A (en) * | 2000-02-18 | 2001-08-21 | Sumitomo Wiring Syst Ltd | Olefinic resin composition and covered electric wire |
| WO2003106554A1 (en) * | 2002-06-14 | 2003-12-24 | 三井化学株式会社 | Thermoplastic resin composition, polymer composition, and molded object obtained from the composition |
| JP2004256748A (en) * | 2003-02-27 | 2004-09-16 | Fujikura Ltd | Non-halogen flame-retardant composition and flame-retardant power cord |
| WO2008078406A1 (en) * | 2006-12-22 | 2008-07-03 | Mitsubishi Chemical Corporation | Flame-retardant thermoplastic resin composition |
| JP2009114230A (en) * | 2007-11-01 | 2009-05-28 | Furukawa Electric Co Ltd:The | Flame retardant resin composition and insulated electric wire coated with the same |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5477658A (en) * | 1977-12-02 | 1979-06-21 | Mitsubishi Petrochem Co Ltd | Self-extinguishing resin composition |
| JPS55139443A (en) * | 1979-04-17 | 1980-10-31 | Showa Electric Wire & Cable Co Ltd | Heat-resisting, flame-retardant crosslinked polyethylene composition |
| JPH0275642A (en) * | 1988-09-12 | 1990-03-15 | Fujikura Ltd | Flame-retarding composition and wire and cable coated therewith |
| US6576691B2 (en) * | 1999-03-02 | 2003-06-10 | Jsr Corporation | Flame resistant polymer composition |
| US6646205B2 (en) * | 2000-12-12 | 2003-11-11 | Sumitomo Wiring Systems, Ltd. | Electrical wire having a resin composition covering |
| JP2003151376A (en) * | 2001-11-14 | 2003-05-23 | Fujikura Ltd | Flame retardant power supply cord |
| JP2005200574A (en) * | 2004-01-16 | 2005-07-28 | Hitachi Cable Ltd | Non-halogen flame-retardant resin composition and electric wire/cable using the same |
| US8076581B2 (en) * | 2007-10-11 | 2011-12-13 | Dsm Ip Assets B.V. | Flexible flame retardant insulated wires for use in electronic equipment |
-
2012
- 2012-03-22 JP JP2012065592A patent/JP2013194214A/en active Pending
- 2012-12-25 KR KR1020147007421A patent/KR20140049606A/en not_active Application Discontinuation
- 2012-12-25 WO PCT/JP2012/083388 patent/WO2013140692A1/en active Application Filing
- 2012-12-25 CN CN201280046230.0A patent/CN104220511A/en active Pending
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07331086A (en) * | 1994-06-07 | 1995-12-19 | Sumitomo Bakelite Co Ltd | Thermoplastic resin composition |
| JPH11349742A (en) * | 1998-06-04 | 1999-12-21 | Hitachi Cable Ltd | Polyolefin resin composition and electric wire/cable |
| JP2001060414A (en) * | 1999-01-20 | 2001-03-06 | Furukawa Electric Co Ltd:The | Insulated resin composition and insulated wire |
| JP2001200106A (en) * | 1999-03-02 | 2001-07-24 | Jsr Corp | Flame-retarded polymer composition |
| JP2001226536A (en) * | 2000-02-18 | 2001-08-21 | Sumitomo Wiring Syst Ltd | Olefinic resin composition and covered electric wire |
| WO2003106554A1 (en) * | 2002-06-14 | 2003-12-24 | 三井化学株式会社 | Thermoplastic resin composition, polymer composition, and molded object obtained from the composition |
| JP2004256748A (en) * | 2003-02-27 | 2004-09-16 | Fujikura Ltd | Non-halogen flame-retardant composition and flame-retardant power cord |
| WO2008078406A1 (en) * | 2006-12-22 | 2008-07-03 | Mitsubishi Chemical Corporation | Flame-retardant thermoplastic resin composition |
| JP2009114230A (en) * | 2007-11-01 | 2009-05-28 | Furukawa Electric Co Ltd:The | Flame retardant resin composition and insulated electric wire coated with the same |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103524843A (en) * | 2013-09-30 | 2014-01-22 | 芜湖航天特种电缆厂 | Sheath material for signal controlling cable and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20140049606A (en) | 2014-04-25 |
| CN104220511A (en) | 2014-12-17 |
| JP2013194214A (en) | 2013-09-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TWI409322B (en) | Non-halogen flame retardent resin composition and electric wire/cable using the same | |
| US7586043B2 (en) | Non-halogenous insulated wire and a wiring harness | |
| KR101601286B1 (en) | Highly flame-resistant polymer composition for electrical wire insulation and electrical wire produced therewith | |
| JP5825536B2 (en) | Non-halogen flame retardant resin composition and insulated wire and tube using the same | |
| JP5549675B2 (en) | Non-halogen flame retardant resin composition and electric wire and cable using the same | |
| JP6229942B2 (en) | Insulated wires for railway vehicles and cables for railway vehicles | |
| JP5387944B2 (en) | Halogen-free flame retardant insulated wire | |
| JPWO2008078406A1 (en) | Flame retardant thermoplastic resin composition | |
| US20140141240A1 (en) | Halogen-free resin composition, electric wire and cable | |
| JP2015000913A (en) | Non-halogen flame-retardant resin composition, and wires and cables prepared using the same | |
| JP4255368B2 (en) | Cross-linked flame retardant resin composition, insulated wire and wire harness using the same | |
| JP6975718B2 (en) | Halogen-free flame retardant composition with improved tensile properties | |
| WO2006057120A1 (en) | Nonhalogen electric wire, electric wire bundle, and automobile wire harness | |
| JP5269476B2 (en) | Electric wire / cable | |
| JP5182580B2 (en) | Halogen-free flame retardant insulated wire | |
| WO2013140692A1 (en) | Non-halogen flame retardant resin composition, and electric wire and cable using same | |
| JP2005314516A (en) | Non-halogen flame-retardant resin composition | |
| JP2007197619A (en) | Non-halogen flame-retardant resin composition and electric wire/cable using the same | |
| JP2008037927A (en) | Flame-retardant resin composition and insulated wire | |
| JP4776208B2 (en) | Resin composition and insulated wire coated therewith | |
| JP2004256621A (en) | Nonhalogen flame-retardant resin composition | |
| JP2004339317A (en) | Non-halogen flame retardant resin composition | |
| JP4964412B2 (en) | Non-halogen flame retardant composition and electric wire | |
| JP2003160709A (en) | Nonhalogen-based flame-retardant resin composition | |
| JP2004269570A (en) | Flame-retardant resin composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12872223 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20147007421 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12872223 Country of ref document: EP Kind code of ref document: A1 |