WO2013038643A1 - 化成処理めっき鋼板およびその製造方法 - Google Patents
化成処理めっき鋼板およびその製造方法 Download PDFInfo
- Publication number
- WO2013038643A1 WO2013038643A1 PCT/JP2012/005722 JP2012005722W WO2013038643A1 WO 2013038643 A1 WO2013038643 A1 WO 2013038643A1 JP 2012005722 W JP2012005722 W JP 2012005722W WO 2013038643 A1 WO2013038643 A1 WO 2013038643A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- chemical conversion
- plated steel
- conversion treatment
- film
- steel sheet
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/05—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions
- C23C22/06—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6
- C23C22/34—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing fluorides or complex fluorides
- C23C22/36—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing fluorides or complex fluorides containing also phosphates
- C23C22/361—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals using aqueous solutions using aqueous acidic solutions with pH less than 6 containing fluorides or complex fluorides containing also phosphates containing titanium, zirconium or hafnium compounds
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C28/00—Coating for obtaining at least two superposed coatings either by methods not provided for in a single one of groups C23C2/00 - C23C26/00 or by combinations of methods provided for in subclasses C23C and C25C or C25D
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C2222/00—Aspects relating to chemical surface treatment of metallic material by reaction of the surface with a reactive medium
- C23C2222/20—Use of solutions containing silanes
Definitions
- the present invention relates to a chemically treated Zn-based plated steel sheet having excellent weather resistance, water resistance, blackening resistance, film adhesion and workability, and a method for producing the same.
- the plated steel sheet is sometimes formed with a chemical conversion treatment film containing an organic resin on its surface in order to prevent galling during forming (for example, see Patent Documents 1 and 2).
- Patent Documents 1 and 2 describe that a chemical conversion treatment film containing an organic resin such as a urethane resin is formed on the surface of a zinc-based plated steel sheet.
- a chemical conversion treatment film containing an organic resin such as a urethane resin is formed on the surface of a zinc-based plated steel sheet.
- a fluororesin having excellent weather resistance may be used as an organic resin constituting the chemical conversion film.
- an organic solvent type fluororesin composition is used in many cases.
- organic solvent-based fluororesin compositions have problems such as fire hazard and harmfulness and air pollution.
- Patent Document 4 a water-based fluororesin composition capable of forming a film even at a low temperature by introducing a curable site (organic functional group) has been proposed (for example, Patent Document 4). reference).
- a film cured by reacting with an organic functional group the weather resistance deteriorates preferentially from the cured portion, so that the film becomes porous and water resistance decreases.
- the base treatment with an epoxy resin or a urethane resin is performed for improving the adhesion, the organic resin is preferentially deteriorated in weather resistance, and the film adhesion is rapidly lowered. .
- galling resistance, corrosion resistance, discoloration resistance, and the like can be improved by forming a chemical conversion film containing an organic resin on the surface of a plated steel sheet.
- the conventional chemical conversion treatment plated steel sheet formed with the chemical conversion treatment film containing the organic resin may have insufficient weather resistance when used as an exterior building material.
- many organic resins such as urethane resins are deteriorated by ultraviolet rays
- the chemical conversion film covering the surface of the plated steel sheet is lost over time. There is a risk that. If the chemical conversion treatment film is lost in this manner, discoloration, rust, etc. may occur and the appearance may be impaired, which is not preferable as an exterior building material.
- a conventional chemical conversion-treated steel sheet on which a chemical conversion treatment film containing an organic resin is formed may have insufficient weather resistance.
- the use of water-based fluororesin as the organic resin can improve the weather resistance (ultraviolet light resistance) of the chemically treated steel sheet, but on the other hand, the film forming property, water resistance and film adhesion are reduced. Therefore, it is impossible to achieve both weather resistance, water resistance, blackening resistance and film adhesion.
- An object of the present invention is a chemical conversion treatment Zn-plated steel sheet having a chemical conversion treatment film containing an organic resin, which is excellent in all of weather resistance, water resistance, blackening resistance, film adhesion and workability. It is to provide a steel plate.
- the present inventor uses a high molecular weight fluorine-containing resin into which a hydrophilic functional group is introduced as an organic resin, and crosslinks these fluorine-containing resins with a group 4A metal compound, thereby enabling the weather resistance and water resistance of the chemical conversion coating.
- the present invention has been completed through further investigations, which have been found to improve the property, blackening resistance and film adhesion.
- the first of the present invention relates to the following chemical conversion treatment Zn-plated steel sheet.
- An Al-containing Zn-based alloy plated steel sheet having a Zn-based alloy plated layer containing 0.05 to 60% by mass of Al, and a film thickness of 0.5 to 0.5 formed on the surface of the Al-containing Zn-based alloy plated steel sheet A chemical conversion treatment Zn-plated steel sheet having a chemical treatment film of 10 ⁇ m; the chemical conversion treatment film having a hydrophilic functional group of 0.05 to 5 selected from the group consisting of a carboxyl group, a sulfonic acid group and salts thereof
- a fluorine-containing resin containing 5% by mass and 7 to 20% by mass of F atoms, 0.1 to 5% by mass of a 4A group metal compound in terms of metal with respect to the fluorine-containing resin A chemical conversion-treated Zn-based plated steel sheet, comprising 10 ⁇ m resin particles; and an area occupancy ratio of the resin particles on the surface of the chemical conversion coating is
- the chemical conversion coating further contains a polyethylene resin; the polyethylene-fluororesin particles protrude from the surface of the chemical conversion coating in part of the surface of the chemical conversion coating;
- the chemical conversion film further contains a phosphate; the amount of the phosphate with respect to the fluorine-containing resin is in the range of 0.05 to 3% by mass in terms of P.
- the chemical conversion treatment film further contains a silane coupling agent; the amount of the silane coupling agent with respect to the fluorine-containing resin is in the range of 0.5 to 5% by mass.
- An underlayer chemical conversion coating formed between the Al-containing Zn-based alloy-plated steel sheet and the chemical conversion coating and containing a valve metal oxide or hydroxide and a valve metal fluoride.
- 2nd of this invention is related with the manufacturing method of the following chemical conversion treatment Zn-plated steel plate.
- a fluorine-containing resin containing 0.05 to 5% by mass of a group and 7 to 20% by mass of F atoms and having a number average molecular weight in the range of 1,000 to 2,000,000, a group 4A metal oxyacid salt, fluoride, Any one of hydroxide, organic acid salt, carbonate or peroxide salt, and resin particles having an average particle size of 0.1 to 10 ⁇ m; an oxyacid salt of the group 4A metal with respect to the fluorine-containing resin; Fluoride, hydroxide, existence The amount of the acid salt, carbonate or peroxide is in the range of 0.1 to 5% by mass in terms of metal; the amount of the resin particles relative to the solid content in the chemical conversion solution is 0.5 to The manufacturing method of the chemical conversion treatment Zn-plated steel plate which exists in the range of 20 mass%.
- the chemical conversion treatment liquid further contains a phosphate; the amount of the phosphate with respect to the fluorine-containing resin is in the range of 0.05 to 3% by mass in terms of P, [9] to The manufacturing method of the chemical conversion treatment Zn-plated steel plate as described in any one of [12].
- the chemical conversion treatment liquid further contains a silane coupling agent; the amount of the silane coupling agent with respect to the fluorine-containing resin is in the range of 0.5 to 5% by mass. 13] The manufacturing method of the chemical conversion treatment Zn-plated steel plate as described in any one of [13].
- the method further includes the step of applying a base chemical conversion treatment solution to the surface of the Al-containing Zn-based alloy plated steel sheet and drying to form a base chemical conversion treatment film;
- the present invention it is possible to provide a chemically treated Zn-based plated steel sheet that is excellent in all of weather resistance, water resistance, blackening resistance, film adhesion, and workability. Since the chemical conversion treatment Zn-plated steel sheet of the present invention is excellent in weather resistance, water resistance, corrosion resistance, discoloration resistance and workability, it is useful as a plated steel sheet for exterior building materials, for example.
- 1A to 1D are SEM images of a chemical conversion coating containing polyethylene resin particles or polyethylene-fluorine resin particles heated at a predetermined temperature.
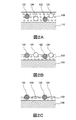
- 2A to 2C are schematic cross-sectional views showing the formation process of the chemical conversion film.
- 3A to 3C are SEM images showing the formation process of the chemical conversion film.
- 2 is a fluorescent X-ray spectrum of polyethylene-fluorine resin particles in a chemical conversion coating. It is a graph which shows the relationship between the quantity of the 4A group metal in a fluororesin membrane
- Chemical conversion treatment Zn-plated steel sheet The chemical conversion treatment Zn-plated steel sheet of the present invention has an Al-containing Zn-based alloy-plated steel sheet (chemical conversion-treated original plate) and a chemical conversion-treated film formed on the surface of the Al-containing Zn-based alloy-plated steel sheet. .
- the chemical conversion treatment Zn-based plated steel sheet of the present invention comprises a high molecular weight fluorine-containing resin, a group 4A metal compound and a resin particle (lubricant) in which a chemical conversion treatment film has introduced a hydrophilic functional group (such as a carboxyl group or a sulfonic acid group). Inclusion is a feature.
- Al-containing Zn-based alloy plated steel plate having excellent corrosion resistance and design properties is used.
- Al-containing Zn-based alloy-plated steel sheet means a steel sheet having a Zn-based alloy plated layer containing 0.05 to 60% by mass of Al.
- Al-containing Zn alloy-plated steel sheets examples include hot-dip Al-Zn-plated steel sheets (hot Zn-0.1% Al plating, hot Zn-55% Al plating, hot Zn-6% Al-3% Mg plating, hot Zn-11% Al-3% Mg-0.2% Si, hot-dip Zn-5% Al-0.75% Mg), alloyed Zn-plated steel sheet (alloyed after hot-dip 0.1% Al-Zn plating) Alloyed molten Al—Zn plating) and the like.
- hot-dip Al-Zn-plated steel sheets hot Zn-0.1% Al plating, hot Zn-55% Al plating, hot Zn-6% Al-3% Mg plating, hot Zn-11% Al-3% Mg-0.2% Si, hot-dip Zn-5% Al-0.75% Mg
- alloyed Zn-plated steel sheet alloyed Zn-plated steel sheet (alloyed after hot-dip 0.1% Al-Zn plating) Alloyed molten Al—Zn plating) and the like
- the base steel of the Al-containing Zn-based alloy plated steel sheet low carbon steel, medium carbon steel, high carbon steel, alloy steel, or the like is used.
- steel sheets for deep drawing such as low carbon Ti-added steel and low carbon Nb-added steel are preferred as the base steel.
- an Al-containing Zn-based alloy-plated steel sheet used as a chemical conversion treatment original plate has a base chemical conversion treatment film containing a valve metal oxide or hydroxide and a valve metal fluoride. is there.
- the base chemical conversion film is formed on the surface of the Al-containing Zn-based alloy plated steel sheet
- the chemical conversion film is formed on the surface of the Al-containing Zn-based alloy plated steel sheet via the base chemical conversion film.
- substrate chemical conversion treatment film containing the oxide or hydroxide of valve metal acts as a resistor with respect to the movement of an electron. Therefore, the reduction reaction of dissolved oxygen contained in the moisture in the atmosphere is suppressed, and the oxidation reaction of the paired Al-containing Zn-based alloy plated steel sheet is also suppressed. As a result, elution (corrosion) of metal components from the Al-containing Zn-based alloy-plated steel sheet serving as the substrate is suppressed.
- the tetravalent compound of IV group A element, such as Ti, Zr, and Hf is a stable compound, and forms the outstanding highly insulating film.
- a film defect is inevitably generated in a chemical conversion treatment film at the time of chemical conversion treatment or molding. Since the base material is exposed at the film defect portion, the corrosion inhibiting action cannot be expected even if chemical conversion treatment is performed.
- the above-mentioned base chemical conversion treatment film also contains a fluoride of valve metal, it has a self-repairing action. That is, the fluoride of the valve metal dissolves in moisture in the atmosphere and then re-deposits as a hardly soluble oxide or hydroxide on the surface of the base steel exposed from the film defect portion. As a result, since the film defect portion is filled, a self-repairing action is exhibited.
- the chemical conversion-treated Zn-based alloy-plated steel sheet of the present invention is manufactured by subjecting an Al-containing Zn-based alloy-plated steel sheet to chemical conversion treatment, but the Zn-based alloy-plated steel sheet before chemical conversion treatment has insufficient corrosion resistance. Therefore, corrosion may occur during storage or transportation of the Zn-based alloy-plated steel sheet before chemical conversion treatment or during forming. Therefore, by forming a base chemical conversion coating on the Zn-based alloy plated steel sheet before the chemical conversion treatment, it is possible to reliably prevent the occurrence of corrosion in the Zn-based alloy plated steel sheet before the chemical conversion treatment.
- the Al-containing Zn-based alloy-plated steel sheet on which the base chemical conversion treatment film is formed is stored, transported or formed (including welding), a part of the base chemical conversion treatment film may be peeled off, missing, or missing. Thereby, the surface of the Al-containing Zn-based alloy-plated steel sheet is exposed, and the chemical conversion treatment film containing the fluorine-containing resin and the 4A group metal compound is in direct contact with the exposed surface.
- the base chemical conversion treatment film exhibits an effect of improving the adhesion between the Al-containing Zn-based alloy-plated steel sheet and the chemical conversion treatment film. For this reason, the chemical conversion treatment film formed at the place where the base chemical conversion treatment film is peeled is generally considered to have a reduced film adhesion.
- Al is eluted from the plating layer in direct contact with the chemical conversion coating.
- the corrosion resistance and film adhesion of the chemical conversion film are improved by Al eluted in the chemical conversion film.
- the adhesiveness of a chemical conversion treatment film and an Al containing Zn type alloy plating steel plate is high, and the corrosion resistance of a chemical conversion treatment film increases. The mechanism by which the presence of Al in the chemical conversion film improves the corrosion resistance and film adhesion of the chemical conversion film will be described later.
- the base chemical conversion treatment film can be formed by drying a coating film of the base chemical conversion treatment liquid formed on the surface of the Al-containing Zn-based alloy-plated steel sheet as the base material.
- the base chemical conversion treatment solution contains valve metal salt, fluoride ions, and water as a solvent. By drying the coating film of the base chemical conversion treatment solution, the valve metal salt becomes an oxide or hydroxide or a fluoride of valve metal contained in the base chemical conversion coating film.
- valve metals examples include Ti, Zr, Hf, V, Nb, Ta, Mo, and W.
- the valve metal salt added to the base chemical conversion treatment liquid may be a valve metal halide or oxyacid salt. If the added valve metal salt is fluoride, it also acts as a fluoride ion source.
- titanium salts examples include K n TiF 6 (K: alkali metal or alkaline earth metal, n: 1 or 2), K 2 [TiO (COO) 2 ], (NH 4 ) 2 TiF 6 , TiCl 4 , TiOSO 4 , Ti (SO 4 ) 2 , Ti (OH) 4 and the like are included.
- the fluoride ion source contained in the base chemical conversion treatment liquid may be a valve metal salt containing a fluorine atom or a soluble fluoride (for example, (NH 4 ) F).
- an organic acid having a chelating action is added to the base chemical conversion treatment solution in order to stabilize the valve metal salt.
- the organic acid can chelate metal ions and stabilize the chemical conversion solution. Therefore, the addition amount of the organic acid is set so that the molar ratio of organic acid / metal ion is 0.02 or more.
- the organic acid include tartaric acid, tannic acid, citric acid, succinic acid, malonic acid, lactic acid, acetic acid, ascorbic acid and the like.
- oxycarboxylic acids such as tartaric acid and polyhydric phenols such as tannic acid stabilize the base chemical conversion solution and also complement the fluoride's self-healing action, improving adhesion. It is valid.
- Various metal orthophosphates or polyphosphates may be added to the base chemical conversion treatment solution. This is because the base chemical conversion treatment film to be formed contains a soluble or hardly soluble metal phosphate or composite phosphate.
- Soluble metal phosphate or composite phosphate elutes from the base chemical conversion coating to the film defects and reacts with the plating components (Zn, Al, etc.) of the Al-containing Zn-based alloy-plated steel sheet as the substrate. Insoluble phosphate is precipitated. In this way, the self-healing action of titanium fluoride is complemented. Further, since the atmosphere is slightly acidified when the soluble phosphate is dissociated, the hydrolysis of titanium fluoride, and hence the generation of hardly soluble titanium oxide or hydroxide, is promoted.
- the metal of the soluble phosphate or the composite phosphate can be an alkali metal, an alkaline earth metal, Mn, or the like.
- the soluble phosphate or the composite phosphate may be added to the base chemical conversion treatment liquid in the form of various metal phosphates, or may be formed by combining various metal salts with phosphoric acid, polyphosphoric acid or phosphate. It may be added to the treatment liquid.
- poorly soluble metal phosphates or composite phosphates are dispersed in the base chemical conversion treatment film to eliminate film defects and improve the film strength.
- the metal of the poorly soluble phosphate or the composite phosphate may be Al, Ti, Zr, Hf, Zn or the like.
- the hardly soluble phosphate or the composite phosphate may be added to the chemical conversion treatment liquid in the form of various metal phosphates, or a chemical conversion treatment by combining various metal salts with phosphoric acid, polyphosphoric acid or phosphate. It may be added to the liquid.
- organic waxes such as fluorine, polyethylene, and styrene
- inorganic lubricants such as silica, molybdenum disulfide, and talc can be added.
- the lubricity of the base chemical conversion coating can be improved. It is considered that the low melting point organic wax bleeds on the film surface and develops lubricity when the coating film of the base chemical conversion treatment liquid is dried.
- high-melting organic waxes and inorganic lubricants are present in a dispersed state in the film, but it is considered that the outermost surface layer of the film is exposed to the surface of the film in an island-like distribution to exhibit lubricity.
- the concentrations of O and F contained in the base chemical conversion film are measured.
- the concentration ratio F / O (atomic ratio) of these elements is preferably 1/100 or more. It is for suppressing the corrosion of the obtained chemical conversion treatment steel plate.
- the element concentration ratio F / O (atomic ratio) is 1/100 or more, the occurrence of corrosion starting from a film defect portion is significantly reduced. This is presumably because a sufficient amount of titanium fluoride is contained in the base chemical conversion treatment film and exhibits a self-repairing action.
- the chemical conversion treatment film is formed on the surface of the above-mentioned Al-containing Zn-based alloy plated steel sheet (chemical conversion treatment original plate).
- the surface of the chemical conversion treatment original plate may be subjected to a base chemical conversion treatment such as forming a coating as a base, but the base chemical conversion treatment may not be performed.
- a chemical conversion treatment film is directly formed on the surface of the chemical conversion treatment original plate. This chemical conversion treatment film improves the weather resistance, blackening resistance, workability (lubricity), and the like of the Al-containing Zn-based alloy plated steel sheet.
- the object of the present invention is to improve all of the weather resistance, water resistance, blackening resistance, film adhesion and workability of the chemically treated Zn-based plated steel sheet.
- a fluorine-containing resin may be used as the organic resin. Fluorine-containing resins are roughly classified into solvent-based fluorine-containing resins and water-based fluorine-containing resins. When a chemical conversion film is formed using a solvent-based fluorine-containing resin, recovery of the volatilized solvent becomes a problem, but when a water-based fluorine-containing resin is used, such a problem does not occur.
- the present inventor uses a water-based fluorine-containing resin that is easy to handle to form a chemically treated Zn-based plated steel sheet that is excellent in all of weather resistance, water resistance, blackening resistance, film adhesion, and workability. Tried.
- the present inventor examined not only suppressing the decrease in water resistance of the chemical conversion coating but also improving the water resistance. As a result of studying from various viewpoints, it was found that the water resistance of the chemical conversion coating can be remarkably improved by increasing the molecular weight of the aqueous fluorine-containing resin and crosslinking the aqueous fluorine resin with a group 4A metal compound. It was.
- the present inventor has studied to improve the workability (lubricity) of the chemical conversion treatment Zn-plated steel sheet. As a result of studying various means, the present inventor disperses resin particles having an average particle diameter of 0.1 to 10 ⁇ m in the chemical conversion film, thereby improving weather resistance, water resistance, blackening resistance, and film adhesion. In addition to the properties, it has been found that a chemical conversion film excellent in workability (lubricity) can be formed.
- 1) weather resistance is improved by blending a fluorine-containing resin (preferably a fluorine-containing olefin resin).
- a fluorine-containing resin preferably a fluorine-containing olefin resin
- 2) the use of a fluorine-containing resin into which a hydrophilic functional group has been introduced reduces the use of an emulsifier during emulsion production
- 3) the molecular weight of the fluorine-containing resin is increased
- 4) the fluorine-containing resin is 4A.
- By cross-linking with a group metal compound weather resistance (ultraviolet light resistance) and water resistance are improved.
- Workability lubricity is improved by dispersing resin particles having an average particle diameter of 0.1 to 10 ⁇ m.
- the chemical conversion film contains a fluorine-containing resin, more specifically, a fluorine-containing olefin resin as a main component.
- the amount of the fluorine-containing resin contained as a main component in the chemical conversion film is preferably in the range of 70 to 99% by mass.
- the weather resistance (ultraviolet resistance) of the chemical conversion coating can be improved.
- the fluorine-containing resin is preferably a water-based fluorine-containing resin that is easier to handle than the organic solvent-based fluorine resin.
- “Aqueous fluorine-containing resin” refers to a fluorine-containing resin having a hydrophilic functional group. Preferred examples of the hydrophilic functional group include a carboxyl group, a sulfonic acid group, and salts thereof. Examples of the salt of the carboxyl group or sulfonic acid group include ammonium salt, amine salt, alkali metal salt and the like.
- a preferred aqueous fluorine-containing resin (preferably a fluorine-containing olefin resin) has a hydrophilic functional group of 0.05 to 5% by mass.
- a fluorine-containing resin having 0.05 to 5% by mass of a hydrophilic functional group can be made into an aqueous emulsion with little use of an emulsifier.
- the chemical conversion film containing almost no emulsifier can be a chemical conversion film excellent in water resistance.
- the content of the hydrophilic functional group in the aqueous fluorine-containing resin may be obtained by dividing the total molar mass of the hydrophilic functional group contained in the aqueous fluorine-containing resin by the number average molecular weight of the aqueous fluorine-containing resin. Since the molar mass of the carboxyl group is 45 and the molar mass of the sulfonic acid group is 81, the number of each of the carboxyl group and sulfonic acid group contained in the aqueous fluorine-containing resin is determined, and each is multiplied by the molar mass. The total molar mass of the hydrophilic functional group contained in the aqueous fluorine-containing resin is obtained. The number average molecular weight of the water-based fluorine-containing resin is measured by GPC.
- the carboxyl group in the water-based fluorine-containing resin forms a hydrogen bond with the plating layer surface and contributes to improving the adhesion between the chemical conversion film and the plating layer surface.
- H + is difficult to dissociate, The cross-linking reaction is unlikely to occur.
- the sulfonic acid group in the water-based fluorine-containing resin tends to dissociate H +, but if it remains unreacted in the film without cross-linking reaction with the group 4A metal compound, the water molecule adsorbing action is strong, so There is a risk that the water resistance will be significantly reduced.
- the water-based fluorine-containing resin includes both a carboxyl group and a sulfonic acid group in order to make use of each feature.
- the ratio of carboxyl group to sulfonic acid group is preferably in the range of 5 to 60 in terms of the molar ratio of carboxyl group / sulfonic acid group.
- the number average molecular weight of the water-based fluorine-containing resin (preferably fluorine-containing olefin resin) contained in the chemical conversion film is preferably 1000 or more, more preferably 10,000 or more, and particularly preferably 200,000 or more.
- the molecular weight of the water-based fluorine-containing resin contained in the chemical conversion coating is too small, the water permeability and water resistance of the chemical conversion coating cannot be sufficiently improved. In such a case, moisture or corrosive gas easily penetrates the chemical conversion coating and reaches the plated steel sheet, so that the plated steel sheet may be easily corroded.
- an aqueous fluorine-containing resin with a low molecular weight is used, radicals generated by the action of light energy or the like are likely to act on the end of the polymer chain, so the aqueous fluorine-containing resin is easily hydrolyzed by a synergistic action such as water. There is a risk of being.
- the molecular weight of the aqueous fluorine-containing resin contained in the chemical conversion coating may be increased to some extent, or a crosslinked structure may be formed between the aqueous fluorine-containing resins.
- the intermolecular force increases and the cohesive strength of the chemical conversion coating increases, so that the water resistance is improved.
- hydrolysis is less likely to occur.
- the number average molecular weight of the water-based fluorine-containing resin contained in the chemical conversion coating is preferably 2 million or less.
- the number average molecular weight exceeds 2 million, there is a possibility that a problem may occur in the stability of the treatment liquid such as gelation.
- the content of F atoms in the aqueous fluorine-containing resin contained in the chemical conversion coating is preferably within the range of 7 to 20% by mass.
- content of F atom is less than 7 mass%, the weather resistance of a chemical conversion treatment film cannot fully be improved.
- the content of F atoms is more than 20% by mass, it is difficult to form a paint and the adhesion and drying properties may be reduced.
- the content of F atoms in the aqueous fluorine-containing resin can be measured by using a fluorescent X-ray analyzer.
- water-based fluorine-containing resin examples include a copolymer of a fluoroolefin and a hydrophilic functional group-containing monomer.
- the hydrophilic functional group-containing monomer is a carboxyl group-containing monomer or a sulfonic acid group-containing monomer.
- fluoroolefins examples include tetrafluoroethylene, trifluoroethylene, chlorotrifluoroethylene, hexafluoropropylene, vinyl fluoride, vinylidene fluoride, pentafluoropropylene, 2,2,3,3-tetrafluoropropylene, 3, 3,3-trifluoropropylene, bromotrifluoroethylene, 1-chloro-1,2-difluoroethylene, 1,1-dichloro-2,2-difluoroethylene and the like are included. These fluoroolefins may be used alone or in combination of two or more.
- perfluoroolefins such as tetrafluoroethylene and hexafluoropropylene, and vinylidene fluoride are preferable.
- Fluoroolefins containing chlorine such as chlorotrifluoroethylene are not preferred because corrosion due to chlorine ions may occur.
- carboxyl group-containing monomer examples include unsaturated carboxylic acids represented by the following formula (1) and unsaturated carboxylic acids such as esters or acid anhydrides thereof.
- R 1 , R 2 and R 3 are the same or different, and each is a hydrogen atom, an alkyl group, a carboxyl group or an ester group.
- N is in the range of 0-20.
- Examples of the unsaturated carboxylic acid represented by the above formula (1) include acrylic acid, methacrylic acid, vinyl acetic acid, crotonic acid, cinnamic acid, itaconic acid, itaconic acid monoester, maleic acid, maleic acid monoester, fumaric acid , Fumaric acid monoester, 5-hexenoic acid, 5-heptenoic acid, 6-heptenoic acid, 7-octenoic acid, 8-nonenoic acid, 9-decenoic acid, 10-undecylene acid, 11-dodecylene acid, 17-octadecylenic acid Oleic acid and the like.
- carboxyl group-containing monomer includes a carboxyl group-containing vinyl ether monomer represented by the following formula (2). (Wherein R 4 and R 5 are the same or different, and each is a saturated or unsaturated linear or cyclic alkyl group. N is 0 or 1. m is 0 or 1.)
- Examples of the carboxyl group-containing vinyl ether monomer represented by the above formula (2) include 3- (2-allyloxyethoxycarbonyl) propionic acid, 3- (2-allyloxybutoxycarbonyl) propionic acid, 3- (2-vinylidene). Roxyethoxycarbonyl) propionic acid, 3- (2-vinyloxybutoxycarbonyl) propionic acid and the like.
- sulfonic acid group-containing monomers examples include vinyl sulfonic acid, allyl sulfonic acid, methallyl sulfonic acid, styrene sulfonic acid, 2-acrylamido-2-methylpropane sulfonic acid, 2-methacryloyloxyethane sulfonic acid, and 3-methacryloyloxy.
- Propanesulfonic acid 4-methacryloyloxybutanesulfonic acid, 3-methacryloyloxy-2-hydroxypropanesulfonic acid, 3-acryloyloxypropanesulfonic acid, allyloxybenzenesulfonic acid, methallyloxybenzenesulfonic acid, isoprenesulfonic acid, 3-allyloxy-2-hydroxypropane sulfonic acid and the like are included.
- the copolymer of the fluoroolefin and the hydrophilic functional group-containing monomer may be copolymerized with another copolymerizable monomer as necessary.
- examples of other copolymerizable monomers include carboxylic acid vinyl esters, alkyl vinyl ethers, non-fluorinated olefins, and the like.
- Carboxylic acid vinyl esters can improve compatibility and gloss, and increase the glass transition temperature.
- vinyl carboxylates include vinyl acetate, vinyl propionate, vinyl butyrate, vinyl isobutyrate, vinyl pivalate, vinyl caproate, vinyl versatate, vinyl laurate, vinyl stearate, vinyl cyclohexylcarboxylate, benzoate Vinyl acid, para-t-butyl vinyl benzoate and the like are included.
- Alkyl vinyl ethers can improve gloss and flexibility.
- alkyl vinyl ethers include methyl vinyl ether, ethyl vinyl ether, butyl vinyl ether and the like.
- Non-fluorinated olefins can improve flexibility.
- Examples of non-fluorinated olefins include ethylene, propylene, n-butene, isobutene and the like.
- a fluoroolefin copolymer having a hydrophilic functional group can be obtained by copolymerizing the above monomers by a known polymerization method. At this time, by adjusting the amount of the fluoroolefin in the raw material monomer composition so that the fluoroolefin copolymer has a hydrophilic functional group of 0.05 to 5% by mass, the fluoroolefin copolymer can be used with almost no emulsifier. An aqueous emulsion of the polymer can be produced. The chemical conversion film formed using an emulsion of a fluoroolefin copolymer containing almost no emulsifier (1% by mass or less) contains almost no emulsifier.
- a chemical conversion treatment film containing almost no emulsifier can be easily formed.
- the chemical conversion film thus formed exhibits excellent water resistance with little deterioration in water resistance due to the residual emulsifier.
- the chemical conversion film contains a group 4A metal compound.
- the 4A group metal compound easily reacts with a functional group such as a carboxyl group or a sulfonic acid group in the aqueous fluorine-containing resin, and accelerates the curing or crosslinking reaction of the aqueous fluorine-containing resin. Therefore, the water resistance of the chemical conversion film can be improved even by low-temperature drying.
- a melamine resin or an isocyanate resin is used for crosslinking of the fluorine-containing resin, there is a problem that the weather resistance is easily deteriorated.
- weather resistance deterioration is immediately caused by oxidation and hydrolysis of ester bonds and form ether bonds.
- weathering deterioration also proceeds when the crosslinked structure is cut by an acidic substance such as sulfate ion or nitrate ion contained in acid rain.
- the urethane bond formed at the cross-linked portion is weaker than the F bond, so that the cross-linked structure is preferentially cut, and the weather resistance deteriorates.
- the group 4A metal compound also improves film adhesion, water resistance and blackening resistance. That is, the strong Al oxide present on the surface of the Al-containing Zn-based alloy-plated steel sheet decreases the adhesion of the chemical conversion coating, but this Al oxide can be obtained by including a group 4A metal compound in the chemical conversion coating. It is possible to suppress a decrease in film adhesion due to.
- the group 4A metal compound also serves as a supply source of group 4A metal ions that react with Al ions eluted by the etching reaction. The reaction product is concentrated at the interface between the plating layer and the chemical conversion film to improve the initial corrosion resistance and blackening resistance. Examples of Group 4A metals include Ti, Zr, Hf, and the like.
- the content of the group 4A metal compound in the chemical conversion film is preferably in the range of 0.1 to 5% by mass in terms of metal with respect to the fluorine-containing resin.
- the content is less than 0.1% by mass in terms of metal, the adverse effect due to the concentration of Al oxide cannot be sufficiently suppressed, and the aqueous fluorine-containing resin fat cannot be sufficiently crosslinked. As a result, the water resistance of the chemical conversion coating cannot be sufficiently improved.
- the content is more than 5% by mass in terms of metal, the chemical conversion film becomes porous, and the workability and weather resistance may be reduced.
- the metal equivalent amount of the group 4A metal compound in the chemical conversion coating can be measured by using a fluorescent X-ray analyzer.
- Al eluted from the plating layer is present in the chemical conversion film.
- This Al contributes to improvement of corrosion resistance. It is presumed that the corrosion resistance is improved by the presence of Al due to the following mechanism. That is, 1) Since the chemical conversion treatment solution is weakly alkaline, when the chemical conversion treatment solution is applied, Al oxide and metal Al contained in the plating layer are selectively eluted into the chemical conversion treatment solution (Zn is almost eluted). do not do). 2) In the pH range of the chemical conversion treatment liquid, Al is Al (OH) 4 - is dissolved in the chemical conversion treatment liquid state. 3) When forming the chemical conversion film by drying the chemical conversion liquid, Al in the chemical conversion liquid is taken into the chemical conversion film by dehydration condensation or the like. 4) As a result, the insulating properties and density of the chemical conversion coating are improved, and the corrosion resistance is improved.
- the chemical conversion film contains resin particles.
- the resin particles are dispersed in the chemical conversion treatment film, and at least a part thereof is exposed (protruded) on the surface of the chemical conversion treatment film (see FIG. 3C).
- the resin particles protruding on the surface of the chemical conversion treatment film function like a “roller” during the molding process, thereby improving the lubricity of the chemical conversion treatment film surface. As a result, the workability of the chemical conversion treated Zn-plated steel sheet can be improved.
- the average particle diameter of the resin particles is preferably within the range of 0.1 to 10 ⁇ m.
- the average particle diameter of the resin particles is less than 0.1 ⁇ m, most of the resin particles are buried in the chemical conversion coating, so that the lubricity of the chemical conversion coating surface cannot be improved efficiently.
- the average particle diameter of the resin particles exceeds 10 ⁇ m, the resin particles may be easily dropped during the molding process. If the resin particles fall off in this way, defects will occur in the chemical conversion film, and the corrosion resistance will be reduced.
- the average particle diameter of the resin particles means a particle diameter (median diameter) at an integrated value of 50% in a particle diameter distribution measured by a laser diffraction scattering method.
- the content of the resin particles in the chemical conversion coating is adjusted so that the area occupancy ratio of the resin particles on the surface of the chemical conversion coating is 0.1 area% or more. If the area occupancy of the resin particles is less than 0.1 area%, the lubricity of the chemical conversion coating cannot be sufficiently improved.
- the area occupancy ratio of the resin particles on the surface of the chemical conversion coating can be determined by observing the surface of the chemical conversion coating with a scanning electron microscope (SEM).
- the type of resin particles is not particularly limited, but polyethylene-fluorine resin particles are preferable from the viewpoint of heat resistance and dispersibility.
- Polyethylene resin particles are excellent in dispersibility in the chemical conversion liquid because of low specific gravity (eg, 0.95), but poor in heat resistance because of low melting point (eg, 123 ° C.).
- the shape of the particles may not be maintained when the chemical conversion solution is heated and dried to form a chemical conversion coating. Specifically, when the chemical conversion treatment liquid is heated and dried at about 140 to 180 ° C., the polyethylene resin particles are melted. When the resin particles are thus melted, the resin particles cannot function as “rollers” and cannot contribute to improvement of lubricity.
- the fluororesin particles have a high melting point (eg, 330 ° C.), they are excellent in heat resistance, but because of their large specific gravity (eg, 2.2), they are inferior in dispersibility in the chemical conversion treatment liquid. Thus, when resin particles with low dispersibility in the chemical conversion treatment liquid are used, it becomes difficult to uniformly disperse the resin particles in the chemical conversion treatment film.
- the polyethylene-fluororesin particles are obtained by bonding (adsorbing) fluororesin fine particles to the surface of the polyethylene resin particles by bringing the fluororesin fine particles into contact with the polyethylene resin particles softened by heating.
- the heat resistance is such that the shape of the particles can be maintained even when heated at about 140 to 180 ° C., and the dispersibility in the chemical conversion liquid (specific gravity: For example, 1.1) can be made compatible.
- a weather resistance can also be improved by couple
- FIG. 1 is an SEM image (plan view) of a chemical conversion coating containing polyethylene resin particles or polyethylene-fluororesin particles heated to 50 ° C. or 150 ° C.
- FIG. 1A is an SEM image of a chemical conversion coating formed by applying a chemical conversion treatment liquid containing polyethylene resin particles to the surface of a plated steel sheet and drying at 50 ° C.
- FIG. 1B is an SEM image of a chemical conversion film formed by applying a chemical conversion treatment liquid containing polyethylene resin particles to the surface of a plated steel sheet and drying at 150 ° C.
- FIG. 1 is an SEM image (plan view) of a chemical conversion coating containing polyethylene resin particles or polyethylene-fluororesin particles heated to 50 ° C. or 150 ° C.
- FIG. 1A is an SEM image of a chemical conversion coating formed by applying a chemical conversion treatment liquid containing polyethylene resin particles to the surface of a plated steel sheet and drying at 50 ° C.
- FIG. 1B is an SEM
- FIG. 1C is an SEM image of a chemical conversion film formed by applying a chemical conversion liquid containing polyethylene-fluorine resin particles to the surface of a plated steel sheet and drying at 50 ° C.
- FIG. 1D is an SEM image of a chemical conversion coating formed by applying a chemical conversion treatment liquid containing polyethylene-fluorine resin particles to the surface of a plated steel sheet and drying at 150 ° C.
- the average particle diameter of the polyethylene resin particles constituting the polyethylene-fluorine resin particles is not particularly limited as long as the average particle diameter of the polyethylene-fluorine resin particles is within the range of 0.1 to 10 ⁇ m.
- Examples of commercially available polyethylene resin particles that can be used include HYTEC E-9016, HYTEC E-1000 (all are Toho Chemical Co., Ltd.), CJ-172B, CJ-137 (all are Koyo Chemical Co., Ltd.), and Permarin KUE. -4, Permarin KUE-5 (both are Sanyo Chemical Industries).
- the average particle diameter of the fluororesin fine particles constituting the polyethylene-fluororesin particles may be appropriately set according to the average particle diameter of the polyethylene resin particles.
- the average particle diameter of the fluororesin fine particles is preferably 0.3 ⁇ m or less.
- the ratio of the fluororesin in the polyethylene-fluororesin particles is preferably in the range of 5 to 40% by mass.
- the ratio of the fluororesin in the polyethylene-fluororesin particles can be measured by using a fluorescent X-ray analyzer.
- the chemical conversion film preferably further contains a polyethylene resin.
- the polyethylene resin covers all or a part of the surface of the chemical conversion film where the polyethylene-fluororesin particles do not protrude (see FIG. 2C).
- the polyethylene resin that coats the surface of the chemical conversion coating is a polyethylene resin that bleeds on the surface of the chemical conversion coating in the manufacturing process.
- the polyethylene resin particles, together with the polyethylene-fluorine resin particles improve the lubricity of the surface of the chemical conversion treatment film, thereby further improving the workability of the chemical conversion treatment Zn-plated steel sheet.
- the content of the polyethylene resin in the chemical conversion coating is preferably in the range of 0.1 to 16% by mass with respect to the chemical conversion coating.
- the content of the polyethylene resin is less than 0.1% by mass, the effect of the polyethylene resin cannot be exhibited sufficiently.
- the content of the polyethylene resin is more than 16% by mass, the weather resistance may be lowered.
- the chemical conversion treatment film preferably further contains a phosphate. Phosphate reacts with the plating layer surface of the Al-containing Zn-based alloy plated steel sheet to improve the adhesion of the chemical conversion coating to the Al-containing Zn-based alloy plated steel sheet.
- the type of phosphate is not particularly limited as long as it is a compound having a phosphate anion and is water-soluble.
- phosphates include sodium phosphate, ammonium phosphate, magnesium phosphate, potassium phosphate, manganese phosphate, zinc phosphate, orthophosphoric acid, metaphosphoric acid, pyrophosphoric acid (diphosphoric acid), triphosphoric acid, Tetraphosphate etc. are included. These phosphates may be used alone or in combination of two or more.
- the content of phosphate in the chemical conversion coating is preferably in the range of 0.05 to 3% by mass in terms of P with respect to the fluorine-containing resin.
- P conversion amount is less than 0.05% by mass, the reaction with the plating layer surface is insufficient, and the adhesion of the chemical conversion film cannot be sufficiently improved.
- P conversion amount exceeds 3 mass%, reaction with 4A group metal compound will advance excessively, and the crosslinking effect by 4A group metal compound will be impaired.
- the amount of phosphate converted to P in the chemical conversion coating can be measured by using a fluorescent X-ray analyzer.
- Silane coupling agent It is preferable that a chemical conversion treatment film contains a silane coupling agent further. By mix
- Silane coupling agents include silanes containing one or more functional groups such as amino, epoxy, mercapto, acryloxy, methacryloxy, alkoxy, vinyl, styryl, isocyanate, and chloropropyl groups. A compound is used.
- the content of the silane coupling agent in the chemical conversion film is preferably in the range of 0.5 to 5% by mass with respect to the fluorine-containing resin.
- content of a silane coupling agent is less than 0.5 mass%, the adhesiveness of a chemical conversion treatment film cannot fully be improved.
- content of the silane coupling agent exceeds 5% by mass, the film adhesion is saturated and no further improvement is observed.
- the content of the silane coupling agent in the chemical conversion film can be measured by using a fluorescent X-ray analyzer.
- the film thickness of the chemical conversion coating is preferably in the range of 0.5 to 10 ⁇ m.
- the film thickness is less than 0.5 ⁇ m, sufficient corrosion resistance and discoloration resistance cannot be imparted.
- the film thickness exceeds 10 ⁇ m, it cannot be expected to improve the performance with the increase in film thickness.
- the manufacturing method of the chemical conversion treatment Zn-plated steel plate Although the manufacturing method of the chemical conversion treatment Zn-plated steel plate of this invention is not specifically limited, For example, it can manufacture by the following method.
- the manufacturing method of the chemical conversion treatment Zn-plated steel sheet of the present invention includes 1) a first step of preparing an Al-containing Zn-based alloy plated steel sheet (chemical conversion treatment original plate), and 2) a second step of preparing a chemical conversion treatment liquid. And 3) a third step of forming a chemical conversion film on the surface of the Al-containing Zn-based alloy plated steel sheet.
- a chemical conversion treatment film on the surface of an Al content Zn system alloy plating steel plate via a foundation chemical conversion treatment film before the 3rd step of forming a chemical conversion treatment film, an Al content Zn system alloy plating steel plate
- the method further includes the step of forming a base chemical conversion coating on the surface of the substrate.
- the aforementioned Al-containing Zn-based alloy plated steel sheet is prepared as a chemical conversion treatment original sheet.
- a chemical conversion liquid containing a fluorine-containing resin having a hydrophilic functional group preferably a fluorine-containing olefin resin
- a group 4A metal compound preferably a group 4A metal compound, and resin particles
- a group 4A metal compound and resin particles are added to the aqueous emulsion of the fluorine-containing resin having a hydrophilic functional group (preferably fluorine-containing olefin resin).
- a group 4A metal compound to be added to the chemical conversion treatment liquid a group 4A metal oxyacid salt, fluoride, hydroxide, organic acid salt, carbonate, peroxide salt, or the like is used.
- oxyacid salts include hydrates, ammonium salts, alkali metal salts, alkaline earth metal salts, and the like. You may add a polyethylene resin particle, a phosphate, a silane coupling agent, etc. to a chemical conversion liquid as needed.
- the number average molecular weight of the fluorine-containing resin contained in the aqueous emulsion is preferably 1000 or more, more preferably 10,000 or more, and particularly preferably 200,000 or more. As described above, this is to impart water resistance to the chemical conversion coating. On the other hand, from the viewpoint of the stability of the treatment liquid, the number average molecular weight of the fluorine-containing resin is preferably 2 million or less.
- the fluorine-containing resin preferably has a hydrophilic functional group of 0.05 to 5% by mass from the viewpoint of preparing an aqueous emulsion without using an emulsifier.
- the content of the emulsifier in the aqueous emulsion of the fluorine-containing resin is preferably 1% by mass or less.
- the emulsifier exceeds 1% by mass, the emulsifier may remain in the chemical conversion film depending on the drying temperature when the chemical conversion film is formed in the third step. If the emulsifier remains in the chemical conversion coating as described above, the water resistance of the chemical conversion coating is remarkably lowered, which is not preferable.
- an aqueous emulsion can be prepared even if the amount of the emulsifier is 1% by mass or less as long as it is a fluorine-containing resin having a hydrophilic functional group.
- the emulsifier that may be contained in the aqueous emulsion of the fluorine-containing resin is preferably a fluorine-based emulsifier such as an ammonium salt of perfluorooctanoic acid or an ammonium salt of perfluorononanoic acid from the viewpoint of weather resistance and water resistance.
- a known fluorosurfactant can also be used as an emulsifier.
- the content of the fluorine-containing resin in the chemical conversion treatment liquid is preferably in the range of 10 to 70 parts by mass with respect to 100 parts by mass of water.
- the content of the fluorine-containing resin is less than 10 parts by mass, the amount of water evaporation increases in the drying process, and the film formability and denseness of the chemical conversion film may be reduced.
- content of fluorine-containing resin is more than 70 mass parts, there exists a possibility that the storage stability of a chemical conversion liquid may fall.
- the content of group 4A metal oxyacid salt, fluoride, hydroxide, organic acid salt, carbonate or peroxide in the chemical conversion solution is 0. A range of 1 to 5 parts by mass is preferable.
- the content of these salts is less than 0.1 part by mass, the crosslinking reaction and the reaction with the plating layer surface are insufficient, and the water resistance and film adhesion of the chemical conversion film cannot be sufficiently improved.
- the content of these salts exceeds 5 parts by mass, the cross-linking reaction proceeds and the storage stability of the chemical conversion solution may be reduced.
- the content of the resin particles (for example, polyethylene-fluorine resin particles) in the chemical conversion liquid is in the range of 0.5 to 20 parts by mass with respect to 100 parts by mass of the solid content (fluorine-containing resin, 4A group metal compound, etc.)
- the inside is preferable.
- the resin particle content is less than 0.5% by mass, the lubricity of the chemical conversion coating cannot be sufficiently improved.
- the content of the resin particles is more than 20% by mass, the weather resistance of the chemical conversion film may be deteriorated.
- the content of the polyethylene resin particles in the chemical conversion treatment liquid is 0.1 to 16 masses with respect to 100 mass parts of the solid content. Within the range of parts is preferred. As described above, when the content of the polyethylene resin particles is less than 0.1% by mass, the effect of the polyethylene resin cannot be exhibited sufficiently. On the other hand, when the addition amount exceeds 16% by mass, the weather resistance of the chemical conversion coating may be lowered.
- the average particle diameter of the polyethylene resin particles is preferably in the range of 0.1 to 10 ⁇ m.
- the average particle diameter is less than 0.1 ⁇ m, most of the polyethylene resin particles are buried in the chemical conversion film, and the polyethylene resin cannot be bleed on the surface of the chemical conversion film. On the other hand, when the average particle size is more than 10 ⁇ m, the polyethylene resin particles may fall off while the chemical conversion solution is being dried.
- the phosphate content in the chemical conversion liquid is preferably in the range of 0.05 to 3 parts by mass in terms of P with respect to 100 parts by mass of the fluorine-containing resin. .
- the phosphate content is less than 0.05 parts by mass, the adhesion of the chemical conversion film cannot be sufficiently improved.
- the content of the phosphate is more than 3 parts by mass, the reaction with the 4A group metal compound may proceed excessively and the crosslinking effect by the 4A group metal compound may be impaired.
- the content of the silane coupling agent in the chemical conversion solution is preferably in the range of 0.5 to 5 parts by mass with respect to 100 parts by mass of the fluorine-containing resin.
- content of a silane coupling agent is less than 0.5 mass part, the adhesiveness of a chemical conversion treatment film cannot fully be improved.
- content of the silane coupling agent exceeds 5 parts by mass, the film adhesion is saturated and no further improvement is observed.
- the stability of the chemical conversion liquid may be reduced.
- Etching agents, inorganic compounds, inorganic lubricants, color pigments, dyes, and the like may be added to the chemical conversion treatment liquid as necessary as other components.
- Fluoride etc. are used as an etching agent.
- An etching agent improves the adhesiveness of a chemical conversion treatment film more by activating the plating layer surface.
- Inorganic compounds (oxides, phosphates, etc.) such as Mg, Ca, Sr, V, W, Mn, B, Si, and Sn improve the water resistance by densifying the chemical conversion film.
- Inorganic lubricants such as molybdenum disulfide and talc further improve the lubricity of the chemical conversion coating and, further, the workability of the chemical conversion Zn-plated steel sheet.
- prescribed color tone can be provided to a chemical conversion treatment film by mix
- a chemical conversion treatment film is formed on the surface of the Al-containing Zn-based alloy plated steel sheet prepared in the first step.
- the chemical conversion solution prepared in the second step may be applied to the surface of the Al-containing Zn-based alloy plated steel sheet prepared in the first step and dried.
- the method for applying the chemical conversion liquid is not particularly limited, and may be appropriately selected from known methods.
- Examples of such a coating method include a roll coating method, a curtain flow method, a spin coating method, a spray method, and a dip pulling method.
- the chemical conversion solution may be dried at room temperature, but considering continuous operation, it is preferable to keep the temperature at 50 ° C. or higher to shorten the drying time. However, when the temperature is kept above 300 ° C., the organic component may be thermally decomposed to deteriorate the performance of the chemical conversion coating. In the production method of the present invention, since the content of the emulsifier contained in the chemical conversion treatment liquid is small, even if the drying temperature is about 50 ° C., the emulsifier is hardly contained, and a chemical conversion treatment film having excellent water resistance can be formed. .
- FIG. 2 is a schematic cross-sectional view showing the formation process of the chemical conversion coating.
- FIG. 2 shows a state in which a chemical conversion treatment liquid to which polyethylene-fluorine resin particles and polyethylene resin particles are added is applied.
- a chemical conversion treatment liquid is applied to the surface of the Al-containing Zn-based alloy plated steel sheet 110 to form a coating film 120 of the chemical conversion treatment liquid (see FIG. 2A).
- Polyethylene-fluorine resin particles 122 and polyethylene resin particles 124 are dispersed in the coating film 120 of the chemical conversion treatment liquid.
- FIG. 3A is an SEM image (plan view) of the chemical conversion coating after the chemical conversion coating is dried at 50 ° C.
- FIG. Although it is not possible to distinguish between polyethylene-fluorine resin particles and polyethylene resin particles, it can be seen that these particles protrude from the surface of the chemical conversion coating (see “PE-F or PE” in the figure).
- FIG. 3B is an SEM image (plan view) of the chemical conversion coating after the coating of the chemical conversion solution is dried at 150 ° C. It can be seen that only the polyethylene-fluororesin particles protrude from the surface of the chemical conversion coating (see “PE-F” in the figure).
- FIG. 3B is an SEM image (plan view) of the chemical conversion coating after the coating of the chemical conversion solution is dried at 150 ° C. It can be seen that only the polyethylene-fluororesin particles protrude from the surface of the chemical conversion coating (see “PE-F” in the figure).
- 3C is an SEM image (cross-sectional view) of polyethylene-fluororesin particles protruding from the surface of the chemical conversion coating. It can be confirmed by fluorescent X-ray analysis that the particles protruding from the surface of the chemical conversion coating are polyethylene-fluororesin particles.
- FIG. 4 is a fluorescent X-ray spectrum of polyethylene-fluororesin particles protruding from the surface of the chemical conversion coating.
- the chemical conversion-treated Zn-based plated steel sheet of the present invention which is excellent in all of weather resistance, water resistance, blackening resistance, film adhesion and workability can be produced.
- the base chemical conversion treatment liquid is applied to the surface of the Al-containing Zn-based alloy plated steel sheet prepared in the first step to form a coating film.
- the base chemical conversion treatment liquid can be applied by, for example, a roll coating method, a spin coating method, a spray method, or the like.
- the coating amount of the base chemical conversion treatment liquid is preferably adjusted so that the valve metal adhesion amount is 1 mg / m 2 or more. It is for providing sufficient corrosion resistance to the chemical conversion treatment steel plate obtained.
- substrate chemical conversion liquid so that the thickness of the foundation
- An undercoat chemical conversion coating can be formed by drying the coating film formed on the surface of the Al-containing Zn-based alloy-plated steel sheet without washing with water. Although it can dry at normal temperature, when continuous operation is considered, it is preferable to hold at 50 degreeC or more and to shorten drying time. However, when the drying temperature exceeds 200 ° C., the organic component contained in the chemical conversion film is thermally decomposed, and the properties imparted with the organic component may be impaired.
- Example 1 Preparation of chemical conversion treated Zn-based plated steel sheet The following three types of molten Al-containing Zn-based alloy plated steel sheets were prepared using SPCC having a thickness of 0.8 mm as a base material. In this example, these three types of molten Al-containing Zn-based alloy-plated steel sheets were used as chemical conversion treatment original sheets.
- a chemical conversion treatment liquid having the composition shown in Table 1 was applied to the surface of each Al-containing Zn-based alloy-plated steel sheet, and dried by heating at a final plate temperature of 140 ° C. to form a chemical conversion treatment film having a thickness of 2.0 ⁇ m.
- the chemical conversion treatment liquids of treatment liquid Nos. 1 to 12 shown in Table 1 are aqueous emulsions containing a predetermined amount of a fluorine-containing resin containing a carboxyl group and a sulfonic acid group and an emulsifier (nonvolatile content: 25% by mass; see Table 2) 4A group metal compound, polyethylene-fluorine resin particles (resin particles) and the like.
- the chemical conversion treatment liquid of treatment liquid No. 13 is a 4A group metal compound, polyethylene-fluorine resin particles (resin particles), etc. added to an aqueous emulsion containing a urethane resin and an emulsifier (nonvolatile content: 25% by mass; see Table 2). Prepared.
- An aqueous emulsion containing a fluorine-containing resin was obtained by adding a predetermined amount of a fluoroolefin, a carboxyl group-containing monomer, a sulfonic acid group-containing monomer and an emulsifier to an aqueous solvent and copolymerizing them.
- PR135 Sudika essence Urethane Co., Ltd.
- A-1891 Momentive Performance Materials Japan GK
- the amounts of Group 4A metal, phosphate, and silane coupling agent relative to the organic resin in the chemical conversion coating of each chemical conversion treatment Zn-plated steel sheet were measured using a fluorescent X-ray analyzer. The contents of phosphate and silane coupling agent were calculated from the measured values of P and Si. Further, the area occupancy ratio of the polyethylene-fluororesin particles on the surface of the chemical conversion coating of each chemical conversion treatment Zn-plated steel sheet was measured using a scanning electron microscope. Table 3 shows the amounts of Group 4A metal, phosphate, and silane coupling agent relative to the organic resin in the chemical conversion coating formed, and the area occupancy ratio of the polyethylene-fluororesin particles on the surface of the chemical conversion coating for each chemical conversion coating. Shown in
- the weather resistance was evaluated by the coating film remaining rate of the chemical conversion coating after the accelerated weather resistance test.
- the chemical conversion treatment Zn-plated steel sheets of Comparative Example 6 Comparative Example 12 and Comparative Example 18 in which a chemical conversion treatment film containing a urethane resin was formed, the chemical conversion treatment film disappeared after 500 cycles (equivalent to outdoor exposure for 5 years).
- the conversion-treated Zn-based plated steel sheets of Comparative Example 4 Comparative Example 10 and Comparative Example 16 in which the chemical conversion treatment film containing excessively large polyethylene-fluororesin particles was formed, the polyethylene-fluororesin particles dropped off from the chemical conversion treatment film. As a result, the weather resistance was poor.
- Blackening resistance was evaluated by the difference in brightness ( ⁇ L * value) before and after the accelerated weather resistance test.
- the chemical conversion treatment Zn-plated steel sheets of Comparative Example 6 Comparative Example 12 and Comparative Example 18 in which a chemical conversion treatment film containing a urethane resin was formed, as the number of cycles increased, the blackening of the plating layer progressed and the brightness decreased. I have.
- the chemical conversion-treated Zn-based plated steel sheets of Examples 1 to 21 in which a chemical conversion film containing a fluorine-containing resin having a predetermined amount of a hydrophilic functional group and a group 4A metal compound was formed 1000 cycles (equivalent to 10 years of outdoor exposure) Even after repeating, the brightness was hardly lowered.
- Corrosion resistance was evaluated based on the white rust generation area ratio after the salt spray test.
- Comparative Example 6 Comparative Example 12 and Comparative Example 18 in which a chemical conversion treatment film containing a urethane resin was formed, although the corrosion resistance was good before the accelerated weather resistance test, the corrosion resistance was accompanied by the disappearance of the film. Has fallen significantly.
- the chemical conversion of Comparative Example 1, Comparative Example 2, Comparative Example 7, Comparative Example 8, Comparative Example 13, and Comparative Example 14 in which a chemical conversion treatment film containing a fluorine-containing resin having an excessive amount or a small amount of a hydrophilic functional group was formed.
- Lubricity was evaluated by the pulling force required for pulling out a test piece to which a load was applied.
- the chemical conversion-treated Zn-plated steel sheets of Comparative Example 1, Comparative Example 7 and Comparative Example 13 were inferior in lubricity because the amount of polyethylene-fluororesin particles in the chemical conversion coating was small.
- the chemically treated Zn-based plated steel sheets of Comparative Example 5, Comparative Example 11 and Comparative Example 17 that did not contain polyethylene-fluorine resin particles also had poor lubricity.
- the chemical conversion-treated Zn-based plated steel sheets of Comparative Example 6, Comparative Example 12 and Comparative Example 18 have poor lubricity because the average particle diameter of the polyethylene-fluorine resin particles is small and buried in the chemical conversion film. It was.
- the chemical conversion treatment Zn-plated steel sheets of Examples 1 to 21 in which the chemical conversion treatment film containing a predetermined amount of polyethylene-fluororesin particles having an average particle diameter within a predetermined range was excellent in lubricity.
- the chemically treated Zn-based plated steel sheet of the present invention is excellent in weather resistance, blackening resistance and workability (lubricity).
- a predetermined amount of group 4A was added to an aqueous emulsion of a fluorine-containing resin having a hydrophilic functional group prepared by adding a hydrophilic functional group-containing monomer to 1% by mass and adding an emulsifier to 1% by mass.
- the chemical conversion treatment solution prepared by adding the metal compound was applied to the surface of the plated steel plate with a bar coater and dried by heating at a final plate temperature of 140 ° C. to form a fluororesin film having a thickness of 30 ⁇ m. This fluororesin film was peeled off from the plated steel sheet and cut into a predetermined size to obtain a test piece. About each test piece (free fluororesin film
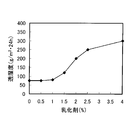
- FIG. 5 is a graph showing the relationship between the amount of group 4A metal in the fluororesin film and the water vapor transmission rate. From this graph, it is understood that the moisture permeability of the fluororesin film can be remarkably lowered by setting the amount of the group 4A metal in the fluororesin film to 0.1 mass% or more.
- FIG. 6 is a graph showing the relationship between the concentration of the emulsifier in the aqueous emulsion of the fluorine-containing resin and the moisture permeability of the fluorine resin film. From this graph, it is understood that the moisture permeability of the fluororesin film can be remarkably reduced by setting the concentration of the emulsifier in the emulsion to 1% by mass or less.
- the fluororesin film having a large amount of the group 4A metal compound and a small amount of the remaining emulsifier has excellent water resistance.
- the chemical conversion-treated Zn-based plated steel sheet of the present invention is useful in various applications such as exterior building materials because it is excellent in weather resistance, water resistance, blackening resistance, film adhesion and workability.
- the chemical conversion-treated Zn-based plated steel sheet of the present invention includes: 1) steel pipe, shape steel, support, beam, conveying member for a greenhouse or agricultural house, 2) sound insulation wall, sound insulation wall, sound absorption wall, snow insulation wall, guardrail, It can be suitably used for applications such as railings, protective fences, columns, 3) railway vehicle members, overhead wire members, electrical equipment members, safety environment members, structural members, and solar mounts.
- the Al-containing Zn-based alloy-plated steel sheet is sufficiently in close contact with the coating film under high temperature and high humidity and has excellent corrosion resistance. Therefore, the chemical conversion treatment Zn-plated steel sheet of the present invention is particularly suitable as an exterior material used in a high temperature and high humidity environment.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Treatment Of Metals (AREA)
- Other Surface Treatments For Metallic Materials (AREA)
- Laminated Bodies (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011-199465 | 2011-09-13 | ||
| JP2011199465A JP5674605B2 (ja) | 2011-09-13 | 2011-09-13 | 化成処理めっき鋼板およびその製造方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013038643A1 true WO2013038643A1 (ja) | 2013-03-21 |
Family
ID=47882894
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/005722 Ceased WO2013038643A1 (ja) | 2011-09-13 | 2012-09-10 | 化成処理めっき鋼板およびその製造方法 |
Country Status (3)
| Country | Link |
|---|---|
| JP (1) | JP5674605B2 (enExample) |
| TW (1) | TWI531677B (enExample) |
| WO (1) | WO2013038643A1 (enExample) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5808626B2 (ja) * | 2011-09-14 | 2015-11-10 | 日新製鋼株式会社 | 化成処理めっき鋼板およびその製造方法 |
| CN110494591B (zh) | 2017-03-30 | 2022-02-25 | 杰富意钢铁株式会社 | 镀锌系钢板及其制造方法 |
| JP6962215B2 (ja) * | 2018-01-24 | 2021-11-05 | 日本製鉄株式会社 | めっき鋼板の端面防錆処理液、めっき鋼板の端面の化成処理方法、化成処理鋼板および成形加工品 |
| JP6962216B2 (ja) * | 2018-01-24 | 2021-11-05 | 日本製鉄株式会社 | 溶接鋼管用防錆処理液、溶接鋼管の化成処理方法、溶接鋼管および溶接鋼管の成形加工品 |
| KR102428620B1 (ko) * | 2018-05-25 | 2022-08-03 | 닛폰세이테츠 가부시키가이샤 | 표면 처리 강판 |
| JP2020037659A (ja) * | 2018-09-05 | 2020-03-12 | Dicグラフィックス株式会社 | 水性コーティング剤、及びこれを塗布してなる印刷物 |
| BR112022008338A2 (pt) | 2019-11-21 | 2022-07-26 | Nippon Steel Corp | Chapa de aço elétrico não orientado, e, método para produzir uma chapa de aço elétrico não orientado |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0328380A (ja) * | 1989-06-26 | 1991-02-06 | Kawasaki Steel Corp | 潤滑性及び耐食性に優れた表面処理鋼板 |
| JP2001072819A (ja) * | 1999-09-06 | 2001-03-21 | Daikin Ind Ltd | 架橋性含フッ素樹脂水性分散液組成物 |
| JP2003105555A (ja) * | 2001-07-23 | 2003-04-09 | Nkk Corp | 耐白錆性に優れた表面処理鋼板及びその製造方法 |
| JP2003293169A (ja) * | 2002-04-04 | 2003-10-15 | Furukawa Electric Co Ltd:The | フッ素系樹脂被覆Al材 |
| JP2004501252A (ja) * | 2000-06-22 | 2004-01-15 | ポーハング アイアン アンド スティール シーオー.,エルティディ. | 水性ウレタン樹脂組成物の作製方法 |
| JP2006175817A (ja) * | 2004-12-24 | 2006-07-06 | Nippon Steel Corp | 反射性と成形加工性に優れる塗装金属板及びその製造方法 |
| JP2007098864A (ja) * | 2005-10-06 | 2007-04-19 | Kobe Steel Ltd | プレコート金属板およびその製造方法 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH06248227A (ja) * | 1993-02-24 | 1994-09-06 | Asahi Glass Co Ltd | フッ素樹脂塗料組成物 |
| JP2004250560A (ja) * | 2003-02-19 | 2004-09-09 | Nippon Yushi Basf Coatings Kk | 水系塗料組成物 |
| JP2005138294A (ja) * | 2003-11-04 | 2005-06-02 | Nisshin Steel Co Ltd | 潤滑性,加工性に優れた塗装金属板 |
| JP2005162993A (ja) * | 2003-12-05 | 2005-06-23 | Daikin Ind Ltd | 水性含フッ素硬化性組成物 |
| TWI500500B (zh) * | 2010-06-18 | 2015-09-21 | Nisshin Steel Co Ltd | 化學轉化鍍鋼板及其製造方法 |
-
2011
- 2011-09-13 JP JP2011199465A patent/JP5674605B2/ja active Active
-
2012
- 2012-09-10 WO PCT/JP2012/005722 patent/WO2013038643A1/ja not_active Ceased
- 2012-09-12 TW TW101133355A patent/TWI531677B/zh not_active IP Right Cessation
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0328380A (ja) * | 1989-06-26 | 1991-02-06 | Kawasaki Steel Corp | 潤滑性及び耐食性に優れた表面処理鋼板 |
| JP2001072819A (ja) * | 1999-09-06 | 2001-03-21 | Daikin Ind Ltd | 架橋性含フッ素樹脂水性分散液組成物 |
| JP2004501252A (ja) * | 2000-06-22 | 2004-01-15 | ポーハング アイアン アンド スティール シーオー.,エルティディ. | 水性ウレタン樹脂組成物の作製方法 |
| JP2003105555A (ja) * | 2001-07-23 | 2003-04-09 | Nkk Corp | 耐白錆性に優れた表面処理鋼板及びその製造方法 |
| JP2003293169A (ja) * | 2002-04-04 | 2003-10-15 | Furukawa Electric Co Ltd:The | フッ素系樹脂被覆Al材 |
| JP2006175817A (ja) * | 2004-12-24 | 2006-07-06 | Nippon Steel Corp | 反射性と成形加工性に優れる塗装金属板及びその製造方法 |
| JP2007098864A (ja) * | 2005-10-06 | 2007-04-19 | Kobe Steel Ltd | プレコート金属板およびその製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| TWI531677B (zh) | 2016-05-01 |
| TW201323657A (zh) | 2013-06-16 |
| JP2013060624A (ja) | 2013-04-04 |
| JP5674605B2 (ja) | 2015-02-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5328980B2 (ja) | 化成処理めっき鋼板およびその製造方法 | |
| JP5328981B2 (ja) | 化成処理めっき鋼板およびその製造方法 | |
| JP5674605B2 (ja) | 化成処理めっき鋼板およびその製造方法 | |
| JP5595305B2 (ja) | 溶接めっき鋼管 | |
| JP5837245B1 (ja) | 化成処理鋼板およびその製造方法ならびに化成処理液 | |
| JP5469556B2 (ja) | 化成処理めっき鋼板およびその製造方法 | |
| JP5575009B2 (ja) | めっき鋼板の成形加工品およびその製造方法ならびに化成処理液 | |
| JP6271062B1 (ja) | 水系処理液、化成処理方法および化成処理鋼板 | |
| JP5808626B2 (ja) | 化成処理めっき鋼板およびその製造方法 | |
| JP5469577B2 (ja) | 化成処理めっき鋼板およびその製造方法 | |
| JP5631231B2 (ja) | 化成処理Zn系めっき鋼板およびその製造方法 | |
| CN111630206A (zh) | 焊接钢管用防锈处理液、焊接钢管的化学转化处理方法、焊接钢管及焊接钢管的成型产品 | |
| JP5674606B2 (ja) | 化成処理めっき鋼板およびその製造方法 | |
| WO2015145514A1 (ja) | 化成処理鋼板およびその製造方法ならびに化成処理液 | |
| JP5631239B2 (ja) | 化成処理Al系めっき鋼板およびその製造方法 | |
| JP2019127617A (ja) | めっき鋼板の端面防錆処理液、めっき鋼板の端面の化成処理方法、化成処理鋼板および成形加工品 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12831504 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12831504 Country of ref document: EP Kind code of ref document: A1 |