WO2013035722A1 - 非水電解液電池 - Google Patents
非水電解液電池 Download PDFInfo
- Publication number
- WO2013035722A1 WO2013035722A1 PCT/JP2012/072562 JP2012072562W WO2013035722A1 WO 2013035722 A1 WO2013035722 A1 WO 2013035722A1 JP 2012072562 W JP2012072562 W JP 2012072562W WO 2013035722 A1 WO2013035722 A1 WO 2013035722A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- positive electrode
- weight
- flame retardant
- phosphazene compound
- active material
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/42—Methods or arrangements for servicing or maintenance of secondary cells or secondary half-cells
- H01M10/4235—Safety or regulating additives or arrangements in electrodes, separators or electrolyte
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/139—Processes of manufacture
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to a non-aqueous electrolyte battery.
- a non-aqueous electrolyte battery such as a lithium ion secondary battery has a high energy density, and a volatile organic solvent is used as the non-aqueous electrolyte. For this reason, when the nonaqueous electrolyte battery is placed in a high temperature environment, or when abnormal heat is generated, such as when overcharge or overdischarge occurs, the battery may burst or expand due to the increase in the internal pressure of the battery due to vaporization of the nonaqueous electrolyte. In addition, there is a problem that the battery ignites and emits smoke due to combustion of the non-aqueous electrolyte and the positive electrode active material.
- Patent Document 1 Japanese Patent Application Laid-Open No. 2000-173619
- a flame retardant layer is formed by applying a flame retardant to the surface of a negative electrode plate containing a carbon material, thereby making the battery flame retardant.
- Technology is disclosed.
- the flame retardant layer formed on the surface of the electrode plate By forming the flame retardant layer on the surface of the electrode plate, the ignition and rupture of the battery due to the rise in the internal temperature of the nonaqueous electrolyte battery can be suppressed to some extent.
- the flame retardant layer formed on the surface of the electrode plate due to the presence of the flame retardant layer formed on the surface of the electrode plate, there is a problem that the ionic conductivity between the electrode and the non-aqueous electrolyte is hindered and the battery characteristics are deteriorated.
- An object of the present invention is to provide a nonaqueous electrolyte battery that does not significantly reduce battery performance even when a flame retardant layer is formed on the surface of an electrode plate.
- the non-aqueous electrolyte battery to be improved by the present invention has a positive electrode active material layer formed on at least one of the front and back surfaces of a positive electrode current collector, and is bound to the surface of the positive electrode active material layer by a porous binder. And a positive electrode plate on which a flame retardant layer including the solid flame retardant is formed.
- the positive electrode current collector is made of a highly conductive metal material such as aluminum.
- the positive electrode active material layer is formed by binding a positive electrode active material to a positive electrode current collector with a binder.
- the binder of this positive electrode active material layer may be the same component as the porous binder described later or a different component.
- a cyclic phosphazene compound having a melting point of 90 ° C. or higher is used as the flame retardant. Since the phosphazene compound having a melting point of 90 ° C. or higher maintains a solid state when the battery is normal (internal temperature is less than 90 ° C.), the phosphazene compound hardly affects the battery characteristics.
- This flame retardant is a radical or active species generated in the vicinity of the positive electrode active material layer when part of or all of the flame retardant becomes liquid when the battery is abnormally heated (internal temperature is over 90 ° C.). Demonstrate the ability to trap. Since the flame retardant layer includes such a flame retardant, a chain reaction of radicals or active species that are the basis of combustion can be suppressed on the surface of the positive electrode active material layer.
- the cyclic phosphazene compound used as the flame retardant is represented by the general formula (NPR 2 ) 3 or (NPR 2 ) 4 , wherein R in the general formula is a halogen element or a monovalent substituent,
- R in the general formula is a halogen element or a monovalent substituent
- a cyclic phosphazene compound in which the substituent is an alkoxy group, an aryloxy group, an alkyl group, an aryl group, an amino group, an alkylthio group or an arylthio group is preferable. Since the cyclic phosphazene compound having such a chemical structure has a melting point of 90 ° C.
- the higher the flame retardant content the higher the flame retardancy of the battery. Therefore, the inventors of the present application have considered that it is preferable to set the content of the flame retardant to 10% by weight or more with respect to the weight of the positive electrode active material when the priority is given to enhancing the flame retardancy of the battery.
- the content of the flame retardant increases, the flame retardancy improves, but the presence of the flame retardant itself becomes a factor that inhibits ion permeability, and the battery characteristics deteriorate.
- the content of the cyclic phosphazene compound is set to 3.
- the content of polyvinylidene fluoride is 5 to 7.5% by weight and the content of polyvinylidene fluoride is 15 to 25% by weight with respect to the weight of the flame retardant, the necessary flame retardancy is ensured and the battery performance is greatly improved.
- the inventors have found that there is no decrease. The present invention is based on this research result.
- the ion permeability in the positive electrode plate is hardly inhibited (without significantly reducing the battery performance) and non-aqueous.
- the electrolyte battery can be made flame retardant to the extent that there is no practical problem. Moreover, since the content of the flame retardant is reduced, the cost for increasing the flame retardancy of the non-aqueous electrolyte battery can be reduced. In addition, when content of a cyclic phosphazene compound is less than 3.5 weight%, since there is little content of a flame retardant, sufficient flame retardance cannot be exhibited.
- polyvinylidene fluoride having a weight average molecular weight of about 200,000 to 350,000 as the polyvinylidene fluoride.
- the weight average molecular weight indicates the molecular weight averaged by the total weight of the polymer.
- the weight average molecular weight of the polyvinylidene fluoride may be 350,000.
- the non-aqueous electrolyte battery of the present invention can be applied to a lithium ion secondary battery.
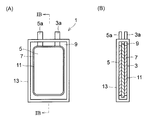
- (A) is the schematic which showed the inside of the lithium ion secondary battery used as a nonaqueous electrolyte battery of this invention in the state seen through
- (B) is the IB-IB sectional view taken on the line of (A).
- FIG. 1A is a schematic view showing the inside of a lithium ion secondary battery as an embodiment of the nonaqueous electrolyte battery of the present invention as seen through
- FIG. IB is a cross-sectional view of IB-IB.
- the lithium ion secondary battery (laminated battery) 1 includes a positive electrode plate 3 having a positive electrode lead terminal 3 a, a negative electrode plate 5 having a negative electrode lead terminal 5 a, and a separator disposed between the positive electrode plate 3 and the negative electrode plate 5. 7 and a non-aqueous electrolyte 9 in which a lithium salt is dissolved in an organic solvent.
- the positive electrode plate 3, the negative electrode plate 5, and the separator 7 are laminated to constitute an electrode plate group 11 made of a laminate.
- the electrode plate group 11 is housed in the case 13 with the positive electrode lead terminal 3a and the negative electrode lead terminal 5a being connectable to the outside.
- the inside of the case 13 is evacuated with the nonaqueous electrolyte 9 filled.
- such a lithium ion secondary battery 1 was produced as follows.
- a lithium cobalt composite oxide (LiCoO 2 ) is prepared as a positive electrode active material for the positive electrode plate.
- This lithium cobalt composite oxide, acetylene black as a conductive agent, and polyvinylidene fluoride as a binder are mixed at a mass ratio of 90: 5: 5 and dispersed in a solvent of N-methylpyrrolidone.
- a slurry was prepared. This slurry was applied to a positive electrode current collector made of aluminum foil and dried, and then subjected to press working to produce a positive electrode sheet.
- a polyvinylidene fluoride having a weight average molecular weight of 280,000, a solvent comprising N-methylpyrrolidone, a cyclic phosphazene compound having a melting point of 112 ° C. [Phoslite (registered by Bridgestone Corporation) (Trademark)] was applied to form a coating layer, and the coating layer was dried under drying conditions determined so that the cured binder was porous. Specific drying conditions were set such that the drying temperature was 100 to 120 ° C. and the drying time was 120 seconds.
- the chemical structure of the cyclic phosphazene compound used is represented by the general formula (NPR 2 ) 3 and R is represented by a phenoxy group.
- the coating layer after drying formed on the surface of the positive electrode sheet is not particularly shown, it is a porous layer in which a large number of continuous pores are formed.
- the positive electrode sheet on which such a coating layer was formed was cut into 10 cm ⁇ 20 cm, and a current collecting tab made of aluminum foil was welded to prepare a positive electrode plate 3. Thereby, a positive electrode active material layer is formed on the surface of the positive electrode plate 3, and a flame retardant layer is further formed on the surface of the positive electrode active material layer.
- Artificial graphite is prepared as a negative electrode active material. This artificial graphite and a polyvinylidene fluoride as a binder were mixed at a mass ratio of 90:10 and dispersed in a solvent of N-methylpyrrolidone to prepare a slurry. The slurry was applied to a negative electrode current collector made of copper foil and dried, and then subjected to press working to produce a negative electrode sheet. The negative electrode sheet was cut to 10 cm ⁇ 20 cm, and a nickel foil current collecting tab was welded to the cut sheet to prepare the negative electrode plate 5.
- Non-aqueous electrolyte A mixed solvent consisting of 50% by volume of ethylene carbonate and 50% by volume of dimethyl carbonate was prepared. In this mixed solvent, lithium hexafluorophosphate (LiPF 6 ) was dissolved to a concentration of 1 mol / L to prepare an electrolyte solution (non-aqueous electrolyte 9).
- LiPF 6 lithium hexafluorophosphate
- the prepared laminate 11 is inserted into an exterior material (which will later become a case 13) made of a heat-sealing film (aluminum laminate film), and the prepared non-aqueous electrolyte 9 is placed in the exterior material. Injected. Then, the inside of the exterior material was evacuated, and the opening of the exterior material was quickly heat-sealed to produce a non-aqueous electrolyte battery (lithium ion secondary battery) having the structure of the flat laminate battery 1.
- an exterior material which will later become a case 13
- a heat-sealing film aluminum laminate film
- the non-aqueous electrolyte battery (laminated battery) 1 produced as described above was evaluated for flame retardancy (battery safety).
- the flame retardancy was evaluated by a nail penetration test. In the nail penetration test, first, a charge / discharge cycle with a current density of 0.1 mA / cm 2 was repeated twice in a voltage range of 4.2 to 3.0 V in an environment of 25 ° C., and the battery was further reduced to 4.2 V. Was charged.
- the battery characteristics of the produced nonaqueous electrolyte battery were evaluated.
- the battery characteristics were evaluated by a high rate discharge test.
- the high rate discharge test first, the battery was charged to 4.2 V by repeating the charge / discharge cycle under the same conditions as in the nail penetration test. After charging, constant current discharge with a current of 24 A and a final voltage of 3.0 V was performed. The discharge capacity thus obtained was defined as a high rate discharge capacity.
- the relationship between the content of the flame retardant (cyclic phosphazene compound) and the flame retardancy of the battery was confirmed.
- Experimental Examples 1 to 7 see Table 1 in which the content of the cyclic phosphazene compound (phoslite) was changed, the internal temperature of the battery, ignition / smoke, and explosion / expansion were confirmed.
- content of a cyclic phosphazene compound is shown as weight% of the cyclic phosphazene compound with respect to 100 weight% of positive electrode active materials which comprise a positive electrode active material layer.
- the content of the binder contained in the flame retardant layer was set to 20% by weight with respect to 100% by weight of the flame retardant.
- the evaluation results of flame retardancy are as shown in Table 1.
- the internal temperature of the battery exceeded 200 ° C. in both the example not containing the cyclic phosphazene compound (Experimental Example 1) and the example containing 1.0% by weight of the cyclic phosphazene compound (Experimental Example 2). (It reached the ignition temperature range of the battery), and the smoke and expansion of the battery were confirmed. Further, in the example (Experimental Example 3) containing 2.5% by weight of the phosphazene compound, although the internal temperature of the battery did not reach the ignition temperature range of the battery, the expansion of the battery was confirmed.
- the battery temperature was less than 150 ° C. (not reaching the ignition temperature range of the battery). Ignition / smoke and battery rupture / expansion were not confirmed. From these results, the non-aqueous electrolyte battery containing 3.5 to 10.0% by weight of the cyclic phosphazene compound can suppress thermal runaway at the time of internal short circuit, and the safety of the non-aqueous electrolyte battery is increased. I understood. That is, it was found that when the content of the cyclic phosphazene compound is less than 3.5% by weight, the effect of suppressing thermal runaway of the battery is insufficient.
- the content of the cyclic phosphazene compound is preferably at least 3.5% by weight with respect to 100% by weight of the positive electrode active material. Note that the flame retardant property was not obtained when the cyclic phosphazene compound was 1.0 to 2.5% by weight, because the content of the electric flame retardant is the minimum content necessary to make the battery flame retardant. This is considered to be due to not satisfying the above.
- the relationship between the content of the flame retardant (cyclic phosphazene compound) and the battery characteristics was confirmed. Specifically, a high rate discharge capacity was confirmed for Experimental Examples 8 to 14 (see Table 2) in which the content of the cyclic phosphazene compound was changed. Also in this case, the content of the cyclic phosphazene compound is shown as the weight% of the cyclic phosphazene compound with respect to 100% by weight of the positive electrode active material constituting the positive electrode active material layer, and the content of the binder contained in the flame retardant layer is flame retardant. It was set to 20% by weight with respect to 100% by weight of the agent. The evaluation results of the battery characteristics are as shown in Table 2.
- the upper limit of the content of the cyclic phosphazene compound may not be determined if only the viewpoint of flame retardancy of the battery is taken into consideration.
- the battery characteristics deteriorate when the content of the cyclic phosphazene compound is 10 to 15% by weight. Therefore, considering the battery performance relative to the flame retardant content, the upper limit of the cyclic phosphazene compound content is preferably 7.5% by weight.
- the battery characteristics are maintained when the content of the cyclic phosphazene compound is 3.5 to 7.5% by weight because the cyclic phosphazene compound having a content of 3.5 to 7.5% by weight does not inhibit the ion permeability.
- the relationship between the content of the binder (polyvinylidene fluoride) and the battery characteristics was confirmed. Specifically, high rate discharge capacity was confirmed for Experimental Examples 15 to 23 (see Table 3) in which the content of polyvinylidene fluoride was changed.
- content of polyvinylidene fluoride is shown as weight% of polyvinylidene fluoride with respect to 100 weight% of flame retardants (cyclic phosphazene compound).
- the content of the flame retardant contained in the flame retardant layer was set to 3.5% by weight with respect to 100% by weight of the positive electrode active material contained in the positive electrode active material layer.
- the evaluation results of the battery characteristics are as shown in Table 3.
- Battery characteristics are maintained at a polyvinylidene fluoride content of 15 to 25% by weight because a large number of pores are formed inside the flame retardant layer, and a porous layer having ion permeability is formed. This is probably due to this. Moreover, it is thought that the battery characteristics are lowered when the content of polyvinylidene fluoride is 10% by weight because the ion permeability is lowered due to a decrease in the pore volume in the flame retardant layer. In addition, when the content of polyvinylidene fluoride is 30 to 60% by weight, the battery characteristics may be decreased because the ion permeability is decreased due to the thickening of the flame retardant layer itself.
- the electrode plate group 11 is composed of the laminate itself.
- the present invention is naturally applied to a cylindrical lithium ion secondary battery in which the laminate is wound to form a wound electrode plate group. Can be applied.
- the content of the cyclic phosphazene compound as a flame retardant contained in the flame retardant layer is 3.5 to 7.5% by weight with respect to the weight of the positive electrode active material layer, and the flame retardant Even if the flame retardant layer is formed by setting the content of polyvinylidene fluoride as a porous binder to bind the agent to 15 to 25% by weight with respect to the weight of the flame retardant, the battery It is possible to provide a non-aqueous electrolyte battery that does not significantly reduce the performance.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Secondary Cells (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Cell Electrode Carriers And Collectors (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011-193290 | 2011-09-05 | ||
| JP2011193290A JP5896374B2 (ja) | 2011-09-05 | 2011-09-05 | 非水電解液電池 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013035722A1 true WO2013035722A1 (ja) | 2013-03-14 |
Family
ID=47832167
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/072562 Ceased WO2013035722A1 (ja) | 2011-09-05 | 2012-09-05 | 非水電解液電池 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP5896374B2 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2013035722A1 (cg-RX-API-DMAC7.html) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019046733A (ja) * | 2017-09-06 | 2019-03-22 | トヨタ自動車株式会社 | 非水電解質二次電池 |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6501129B2 (ja) * | 2017-02-22 | 2019-04-17 | マツダ株式会社 | リチウムイオン電池の電極構造 |
| KR102148512B1 (ko) * | 2017-09-01 | 2020-08-27 | 주식회사 엘지화학 | 양극 활물질의 제조방법 및 이를 이용한 양극 활물질 및 리튬 이차전지 |
| US12113190B2 (en) * | 2020-11-23 | 2024-10-08 | Nano And Advanced Materials Institute Limited | Thermal responsive electrode structure for lithium-ion batteries |
| JP2024101616A (ja) | 2023-01-18 | 2024-07-30 | 三星エスディアイ株式会社 | 非水電解質二次電池用正極及び非水電解質二次電池 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006107753A (ja) * | 2004-09-30 | 2006-04-20 | Dainippon Printing Co Ltd | 正極活物質層用塗工組成物、非水電解液二次電池用正極板、及び非水電解液二次電池 |
| JP2006127839A (ja) * | 2004-10-27 | 2006-05-18 | Bridgestone Corp | 電池用セパレータ及びそれを備えた非水電解質電池 |
| WO2010101180A1 (ja) * | 2009-03-03 | 2010-09-10 | 株式会社Nttファシリティーズ | 非水電解液電池 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5623199B2 (ja) * | 2010-09-06 | 2014-11-12 | 株式会社Nttファシリティーズ | 非水電解液電池 |
-
2011
- 2011-09-05 JP JP2011193290A patent/JP5896374B2/ja not_active Expired - Fee Related
-
2012
- 2012-09-05 WO PCT/JP2012/072562 patent/WO2013035722A1/ja not_active Ceased
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006107753A (ja) * | 2004-09-30 | 2006-04-20 | Dainippon Printing Co Ltd | 正極活物質層用塗工組成物、非水電解液二次電池用正極板、及び非水電解液二次電池 |
| JP2006127839A (ja) * | 2004-10-27 | 2006-05-18 | Bridgestone Corp | 電池用セパレータ及びそれを備えた非水電解質電池 |
| WO2010101180A1 (ja) * | 2009-03-03 | 2010-09-10 | 株式会社Nttファシリティーズ | 非水電解液電池 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019046733A (ja) * | 2017-09-06 | 2019-03-22 | トヨタ自動車株式会社 | 非水電解質二次電池 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5896374B2 (ja) | 2016-03-30 |
| JP2013054969A (ja) | 2013-03-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7237053B2 (ja) | リチウムイオン電気化学的電池、その成分、その製造法およびその使用 | |
| JP5656521B2 (ja) | 非水電解液電池 | |
| JP7231188B2 (ja) | リチウムイオン電池の製造方法 | |
| JP5728721B2 (ja) | 耐熱性リチウムイオン二次電池 | |
| JP5055350B2 (ja) | 非水電解質二次電池及び非水電解質二次電池用の電極 | |
| JP5526488B2 (ja) | 電気化学デバイス | |
| US8801810B1 (en) | Conducting formation cycles | |
| US20060019151A1 (en) | Non-aqueous electrolyte battery | |
| JP5740118B2 (ja) | 非水電解液電池 | |
| JP7000856B2 (ja) | リチウムイオン二次電池 | |
| JP2012059404A5 (cg-RX-API-DMAC7.html) | ||
| US20150303482A1 (en) | Reduction of Gassing in Lithium Titanate Cells | |
| JP2015115168A (ja) | リチウムイオン二次電池用電極及びそれを用いたリチウムイオン二次電池 | |
| KR20150070971A (ko) | 리튬 이온 2차 전지 | |
| JP2012059405A5 (cg-RX-API-DMAC7.html) | ||
| WO2013035720A1 (ja) | 非水電解液電池用セパレータ及び非水電解液電池 | |
| JP2014049294A (ja) | リチウムイオン二次電池用非水電解液及びリチウムイオン二次電池 | |
| JP2015125948A (ja) | リチウムイオン二次電池 | |
| JP5896374B2 (ja) | 非水電解液電池 | |
| JP2013054969A5 (cg-RX-API-DMAC7.html) | ||
| JP5432746B2 (ja) | リチウムイオン二次電池 | |
| WO2012101693A1 (ja) | リチウムイオン電池用負極集電体及びリチウムイオン電池 | |
| JP5614432B2 (ja) | リチウムイオン二次電池用非水電解液及びリチウムイオン二次電池 | |
| JP2023034053A (ja) | パウチ型非水電解質二次電池 | |
| JP2015125950A (ja) | リチウムイオン二次電池 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12830602 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 12830602 Country of ref document: EP Kind code of ref document: A1 |