WO2012169285A1 - 微細結晶子高機能金属合金部材とその製造方法 - Google Patents
微細結晶子高機能金属合金部材とその製造方法 Download PDFInfo
- Publication number
- WO2012169285A1 WO2012169285A1 PCT/JP2012/060518 JP2012060518W WO2012169285A1 WO 2012169285 A1 WO2012169285 A1 WO 2012169285A1 JP 2012060518 W JP2012060518 W JP 2012060518W WO 2012169285 A1 WO2012169285 A1 WO 2012169285A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- metal alloy
- gadolinium
- crystallite
- ppm
- alloy
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22D—CASTING OF METALS; CASTING OF OTHER SUBSTANCES BY THE SAME PROCESSES OR DEVICES
- B22D25/00—Special casting characterised by the nature of the product
- B22D25/06—Special casting characterised by the nature of the product by its physical properties
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D5/00—Heat treatments of cast-iron
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D6/00—Heat treatment of ferrous alloys
- C21D6/001—Heat treatment of ferrous alloys containing Ni
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
- C22C1/02—Making non-ferrous alloys by melting
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
- C22C1/02—Making non-ferrous alloys by melting
- C22C1/026—Alloys based on aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
- C22C1/06—Making non-ferrous alloys with the use of special agents for refining or deoxidising
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C14/00—Alloys based on titanium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C21/00—Alloys based on aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C21/00—Alloys based on aluminium
- C22C21/06—Alloys based on aluminium with magnesium as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C23/00—Alloys based on magnesium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C23/00—Alloys based on magnesium
- C22C23/02—Alloys based on magnesium with aluminium as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C30/00—Alloys containing less than 50% by weight of each constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C30/00—Alloys containing less than 50% by weight of each constituent
- C22C30/02—Alloys containing less than 50% by weight of each constituent containing copper
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/02—Ferrous alloys, e.g. steel alloys containing silicon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/06—Ferrous alloys, e.g. steel alloys containing aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/08—Ferrous alloys, e.g. steel alloys containing nickel
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C5/00—Alloys based on noble metals
- C22C5/02—Alloys based on gold
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C5/00—Alloys based on noble metals
- C22C5/04—Alloys based on a platinum group metal
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C5/00—Alloys based on noble metals
- C22C5/06—Alloys based on silver
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C5/00—Alloys based on noble metals
- C22C5/06—Alloys based on silver
- C22C5/08—Alloys based on silver with copper as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C9/00—Alloys based on copper
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C9/00—Alloys based on copper
- C22C9/04—Alloys based on copper with zinc as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/04—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/04—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon
- C22F1/047—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon of alloys with magnesium as the next major constituent
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/06—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of magnesium or alloys based thereon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/08—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of copper or alloys based thereon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/14—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of noble metals or alloys based thereon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/16—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of other metals or alloys based thereon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/16—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of other metals or alloys based thereon
- C22F1/18—High-melting or refractory metals or alloys based thereon
- C22F1/183—High-melting or refractory metals or alloys based thereon of titanium or alloys based thereon
-
- A—HUMAN NECESSITIES
- A44—HABERDASHERY; JEWELLERY
- A44C—PERSONAL ADORNMENTS, e.g. JEWELLERY; COINS
- A44C27/00—Making jewellery or other personal adornments
- A44C27/001—Materials for manufacturing jewellery
- A44C27/002—Metallic materials
- A44C27/003—Metallic alloys
Definitions
- the present invention relates to a high-performance elastic limit metal alloy member suitable for electronic members, automobile / aviation members, physics and chemistry members, medical members, jewelry members, musical instrument members, tableware members, structural members, and the like, and a manufacturing method.
- the present invention improves the various characteristics without losing the excellent characteristics of the metal / noble metal alloy, and is a new modified alloy that can adjust these characteristics without harmful effects, and is a fine crystallite high-performance metal / noble metal alloy It is characterized by providing.
- the fine crystallites there are strength, Young's modulus, elongation, heat resistance, corrosion resistance, spring properties, control durability, etc., providing a fine crystallite high-performance metal alloy that is easy to work and has good workability It is characterized by doing. .
- the present invention improves the functional characteristics while maintaining or improving the hardness, tensile strength, Young's modulus, elongation, corrosion resistance, discoloration, high temperature characteristics, workability in order to take advantage of the excellent characteristics of the alloy material itself, Providing fine crystallite high-functionality metal alloy members and their manufacturing methods that improve durability and workability, are easy to work, and do not waste.
- Conventional metal materials have mechanical properties when used in applications in various fields. However, physical properties, chemical properties, etc. are not necessarily sufficient. There is also a problem that workability is poor.
- An object of the present invention is to obtain the required new fine crystallite high-functional alloy member by maintaining the characteristics of the metal material and improving, improving, and improving these defective characteristics.
- the crystallite is refined (10 ⁇ 9 m to 10 ⁇ 3 m), the size and shape thereof are controlled, and the performance, quality, function, processing, processing, and the like are high, such as physical, electrical, mechanical, and chemical properties. It was found that a new fine crystallite high-performance metal alloy member excellent in work and the like was obtained, and a manufacturing method thereof was established.
- the fine crystallite metal alloy member of the present invention is controlled by controlling the size and shape of the newly developed fine crystallite, so that the hardness, tensile strength, elongation, Young's modulus, proof stress, softening property, conductivity of conventional metal alloys Because it can maintain and improve various characteristics such as thermal conductivity, workability, workability, etc., and control these characteristics, it is possible to eliminate waste in function, performance, quality, processing, work, etc. Features.
- the fine crystallite metal alloy of the present invention can be processed by 90% or more without annealing. Features such as no cracking even if the rolling direction is changed. In the case of commercialization, since it is easy to process, hardly deforms, and has durability, it is suitable for miniaturization.
- the fine crystallite high-performance metal / noble metal alloy of the present invention has high properties such as hardness, tensile strength, Young's modulus, yield strength, elastic limit, elongation, and spring property. It is easy to process and workability is good. Since it has high purity, fine crystallites, and small volume occupancy of the additive element, an electronic material having high electrical conductivity and high thermal conductivity can be obtained. Since various properties can be improved without lowering the Young's modulus, the product development range is large. When you create a musical instrument, you can get creative tone / sound. Since the spring property is improved, it is flexible and a firm wire and plate can be obtained. Since the heat resistance can be increased, its application is wide. Materials with high physical properties, mechanical properties, electrical properties and chemical properties can be obtained.
- the fine crystallite high-performance alloy of the present invention has a crystallite of nanometer size (10 ⁇ 9 m to 10 ⁇ 6 m) and micrometer size (10 ⁇ 6 m to 10 ⁇ 3 m). The same high-performance characteristics can be obtained with a plating film.
- the present invention has arisen from the market demands as described above, and its purpose is to maintain, improve and adjust the mechanical / physical / chemical properties, and to achieve excellent target function, performance, quality and workability.
- Another object of the present invention is to provide a fine crystallite high-performance metal alloy member that is toxic-free and a method for producing the same.
- a new method for refining the most important crystallites has been found and established to improve and maintain various properties such as mechanical properties, electrical properties, physical properties, and chemical properties of metal alloys.
- a fine crystallite metal alloy member excellent in corrosion resistance and discoloration and its production method and its properties at normal temperature as well as a fine crystallite metal alloy member excellent in various properties at high temperatures and its production method The purpose is to provide.
- PCT / JP96 / 00510, PCT / JP97 / 2014, PCT / JP00 / 04411, PCT / 03/01993, and PCT / JP2007 / 073133 have been proposed to solve the shortcomings of noble metal alloys.
- the present invention extends to a wider range. We found a new phenomenon in fine crystallite high-performance precious metal alloys and fine crystallite high-performance metal alloys, and established their practical application.
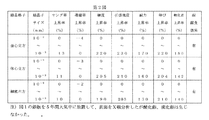
- FIG. 1 shows the addition of Gd to a metal alloy containing a high-purity metal alloy of Au, Ag, Pt, Pd, Al, Mg, Cu, Fe and Ti, and after melting, cast into a 30 mm wide ⁇ 10 mm thick plate. Further, the average crystallite size of a plate rolled into a 0.2 mm thick plate after solution treatment and aging is shown. It has been found that the crystallite size can be adjusted by a casting method, a processing method, and a heat treatment method. Since the crystallite size can be reduced to a nanometer as shown in FIG. 1, the size can be adjusted in the range of 10 ⁇ 9 nm to 10 ⁇ 3 nm, and the tissue shape can also be adjusted.
- FIG. 2 shows the crystal lattice of the fine crystallite high-functional alloy of the present invention and its electrical conductivity, Young's modulus, hardness, tensile strength, yield strength, elongation, softening temperature increase ratio and corrosion resistance. It shows that hardness, tensile strength, proof stress, elongation, and softening point can be greatly improved and adjusted without lowering Young's modulus and electrical conductivity. It was found that the corrosion resistance was improved and the effect of preventing metal corrosion was great.
- the fine crystallite high-performance metal alloy member according to the first embodiment of the present invention is composed of a metal alloy in which gadolinium Gd is contained in a range of 5 ppm or more and less than 30000 ppm in a gold Au alloy including a high-purity gold Au alloy. .
- the first fine crystallite high-function gold Au alloy member according to the first embodiment of the present invention is obtained by adding 500 ppm of gadolinium Gd to an alloy having a gold Au content of 99.95 wt. After casting, solution treatment, and aging, a 0.3 mm thick plate was rolled.
- gadolinium Gd was added to a gold alloy consisting of gold Au 90% -Ag 10% weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate. Further, 500 g of gadolinium Gd was added to a gold alloy composed of 50% gold Au-50% weight by weight, and a 30 mm wide ⁇ 10 mm thick plate was cast. After heat treatment, it was rolled into a 0.3 mm thick plate.
- gadolinium Gd 500 g was added to a gold alloy consisting of gold Au 10% -Ag 90% weight, a 30 mm wide ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate.
- the finished 0.3 mm thick plate was subjected to X-ray analysis, and the average crystallite diameters derived using Scherrer's formula were 27 nm, 15 nm, 19 nm, and 23 nm, respectively.
- Hardness, tensile strength, and 0.2% proof stress increased 2-3 times with almost no decrease in conductivity and Young's modulus. It was found that the elongation can be increased by 2 to 3 times. The softening temperature could be increased by 2.3 times. Rolling can also be performed without heat treatment, and cross processing can be performed. Processability has improved and various properties have been improved, and it has become possible to balance these properties. The effect was confirmed with the plate, wire, thin film, and powder. Next, a method for producing an alloy member having the above characteristics will be described.
- an alloy material having the above composition is cast, and if necessary, a solution treatment is performed by heating to a predetermined temperature and then rapidly cooling, and if necessary, an aging treatment is then performed at a predetermined temperature. Apply.
- an alloy material having the above composition is cast, and if necessary, the material is subjected to a solution treatment that is heated to a predetermined temperature and then rapidly cooled, and the material is processed into a predetermined shape. If necessary, an aging treatment is applied to the material before or after processing.
- the gold alloy material at this time is cast, and the solution treatment temperature can be 500 ° C. to 2700 ° C., and the aging treatment temperature can be 100 ° C.
- Particularly preferable production conditions for obtaining a high-performance gold alloy are a solution treatment temperature of 600 to 1000 ° C. and an aging treatment temperature of 150 to 550 ° C. Although it processed with the said processing method, in any case, the gadolinium Gd addition effect is seen notably. Similar results were obtained after solution treatment and aging treatment.
- a gold Au alloy containing a high-purity gold Au alloy whose crystal lattice is a face-centered cubic lattice was made to contain gadolinium Gd in a range of 5 ppm or more and less than 30000 ppm, and a trial evaluation was performed, but the same gadolinium Gd addition effect was shown.
- the fine crystallite high-performance metal alloy member according to the second embodiment of the present invention is composed of a metal alloy containing gadolinium Gd in a range of 5 ppm or more and less than 30000 ppm in a silver Ag alloy containing a high-purity silver Ag alloy. .
- the fine crystallite high-performance silver Ag alloy member according to the second embodiment of the present invention is obtained by casting 500 mm of gadolinium Gd to an alloy having a silver Ag content of 99.95% by weight and casting a 30 mm wide ⁇ 10 mm thick plate. After solution treatment and aging, a 0.3 mm thick plate was rolled.
- gadolinium Gd was added to a silver Ag alloy composed of Ag 90% -Pd 10% weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate. Further, 500 g of gadolinium Gd was added to a silver Ag alloy composed of 50% Ag-Pd 50% by weight, a 30 mm wide ⁇ 10 mm thick plate was cast, and after heat treatment, rolled to a 0.3 mm thick plate.
- gadolinium Gd 500 g was added to a silver Ag alloy composed of Ag 10% -Pd 90% by weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate.
- the finished 0.3 mm thick plate was subjected to X-ray analysis, and the average crystallite diameters derived using Scherrer's formula were 8 nm, 19 nm, 23 nm, and 25 nm.
- Hardness, tensile strength, and 0.2% proof stress increased 2-3 times with almost no decrease in conductivity and Young's modulus. It was found that the elongation can also be extended by 2 to 3 times. The softening temperature could also be increased by 2.2 times. Rolling could also be done without heat treatment, and cross processing could be done. It was found that processability was also improved. It was also possible to balance these characteristics. Various characteristics have been improved, and it has become possible to balance these characteristics. The effect was confirmed with the plate, wire, thin film, and powder. Next, a method for producing an alloy member having the above characteristics will be described.
- an alloy material having the above composition is cast, and if necessary, a solution treatment is performed by heating to a predetermined temperature and then rapidly cooling, and if necessary, an aging treatment is then performed at a predetermined temperature. Apply.
- an alloy material having the above composition is cast, and if necessary, the material is subjected to a solution treatment that is heated to a predetermined temperature and then rapidly cooled, and the material is processed into a predetermined shape. If necessary, an aging treatment is applied to the material before or after processing.
- the silver Ag alloy can have a solution treatment temperature of 450 to 2200 ° C. and an aging treatment temperature of 100 to 600 ° C.
- Particularly preferred conditions are a solution treatment temperature of 500 to 1550 ° C. and an aging treatment temperature of 150 to 500 ° C.
- the processing efficiency at the time of processing is arbitrary, but the preferable range is the same as that of the first embodiment. It was found that by adding gadolinium Gd, the tensile strength, hardness, proof stress, elongation, heat resistance can be increased while slightly increasing without lowering the Young's modulus, and sulfidation and oxidation can be delayed with little decrease in electrical conductivity. . Although it processed with the said processing method, in any case, the gadolinium Gd addition effect was seen notably. Similar results were obtained after solution treatment and aging treatment.
- a silver Ag alloy containing a high-purity silver Ag alloy having a face-centered cubic lattice and containing gadolinium Gd in a range of 5 ppm to less than 30000 ppm was also experimentally evaluated.
- the same gadolinium Gd addition effect was shown.
- the above-mentioned silver Ag alloy composition was also experimentally evaluated, it was the same gadolinium Gd addition effect.
- the properties of hardness, tensile strength, Young's modulus and heat resistance were improved and spring properties were exhibited. Easy to process and improved workability. Little decrease in conductivity was observed.
- the fine crystallite high-performance metal alloy member according to the third embodiment of the present invention is composed of a metal alloy in which gadolinium Gd is contained in a range of 5 ppm or more and less than 30000 ppm in a platinum alloy including a high-purity platinum alloy. .
- the fine crystallite high-function platinium Pt alloy according to the third embodiment of the present invention is obtained by adding 500 ppm of gadolinium Gd to an alloy having a platinum content of 99.95% by weight and casting a 30 mm wide ⁇ 10 mm thick plate, After solution treatment and aging, a 0.3 mm thick plate was rolled. Next, 500 g of gadolinium Gd was added to a platinum alloy of Pt 90% -Pd 10% by weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate.
- gadolinium Gd was added to a platinum platinum Pt alloy composed of Pt 50% -Cu 50% weight, a 30 mm wide ⁇ 10 mm thick plate was cast, and after heat treatment, rolled to a 0.3 mm thick plate.
- 500 g of gadolinium Gd was added to a platinum alloy composed of Pt 10% -Cu 90% by weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate.
- the finished 0.3 mm thick plate was subjected to X-ray analysis, and the average crystallite diameters derived using Scherrer's formula were 11 nm, 19 nm, 17 nm, and 22 nm.
- the crystallite size is nanometer (10 -9 m ⁇ 10 -6 m) and a micrometer (10 -6 m ⁇ 10 -3 m).
- Hardness, tensile strength, and 0.2% proof stress increased 2-3 times with almost no decrease in conductivity and Young's modulus. It was found that the elongation can also be extended by 2 to 3 times.
- the softening temperature could be increased more than twice. Rolling could also be done without heat treatment, and cross processing could be done. It was found that processability was also improved.
- an aging treatment is applied to the material before or after processing. It is composed of a noble metal alloy in which gadolinium Gd is contained in a range of 5 ppm or more and less than 30000 ppm in a platinum alloy including a high-purity platinum PT alloy.
- the solution treatment temperature can be 600 to 2800 ° C.
- the aging treatment temperature can be 150 to 1400 ° C.
- Particularly preferred conditions are a solution treatment temperature of 500 to 1600 ° C. and an aging treatment temperature of 150 to 1000 ° C.
- the processing efficiency at the time of processing is arbitrary, but the preferable range is the same as that of the first embodiment.
- gadolinium Gd addition effect showed notably. Similar results were obtained after solution treatment and aging treatment. Trial evaluation was also conducted to determine whether or not gadolinium Gd was contained in a platinum Pt alloy including a high-purity platinum Pt alloy having a face-centered cubic lattice in the range of 5 ppm or more and less than 30000 ppm, but the same effect of adding gadolinium Gd was shown. Although the above-mentioned blended platinum Pt composition was also experimentally evaluated, it was the same gadolinium Gd addition effect.
- gadolinium Gd After the addition of gadolinium Gd, the properties of hardness, tensile strength, elongation, proof stress, heat resistance were improved and spring properties were exhibited. Easy to process and improved workability. Little decrease in Young's modulus and conductivity was observed.
- gadolinium Gd is contained in a range of 50 ppm or more and less than 15000 ppm in a metal alloy composed of at least one element selected from the group consisting of the same, various characteristics and balance characteristics are also significantly increased. .
- the fine crystallite high-performance metal alloy member according to the fourth embodiment of the present invention is composed of a metal alloy in which gadolinium Gd is contained in a range of 5 ppm or more and less than 30000 ppm in a palladium Pd alloy including a high-purity gold-palladium Pd alloy.
- the fine crystallite high-performance palladium Pd alloy member according to the fourth embodiment of the present invention is obtained by casting 500 mm of gadolinium Gd to an alloy having a palladium Pd content of 99.95% by weight and casting a 30 mm wide ⁇ 10 mm thick plate. After solution treatment and aging, a 0.3 mm thick plate was rolled.
- the finished 0.3 mm thick plate was subjected to X-ray analysis, and the average crystallite diameter derived using Scherrer's equation was 15 nm.
- the crystallite size is nanometer (10 -9 m ⁇ 10 -6 m) and a micrometer (10 -6 m ⁇ 10 -3 m).
- Hardness, tensile strength, and 0.2% proof stress increased 2-3 times with almost no decrease in conductivity and Young's modulus. It was found that the elongation can also be extended by 2 to 3 times.
- the softening temperature could be increased by 2.3 times. Rolling could also be done without heat treatment, and cross processing could be done. It was found that processability was also improved. It was also possible to balance these characteristics. Various characteristics have been improved and these characteristics can be adjusted.
- an alloy member having the above characteristics In the case of a cast alloy, an alloy material having the above composition is cast, and if necessary, a solution treatment is performed by heating to a predetermined temperature and then rapidly cooling, and if necessary, an aging treatment is then performed at a predetermined temperature. Apply.
- an alloy material having the above composition is cast, and if necessary, the material is subjected to a solution treatment that is heated to a predetermined temperature and then rapidly cooled, and the material is processed into a predetermined shape. If necessary, an aging treatment is applied to the material before or after processing.
- the palladium Pd alloy can have a solution treatment temperature of 500 to 2700 ° C. and an aging treatment temperature of 150 to 1300 ° C. Particularly preferred conditions are a solution treatment temperature of 550 to 1500 ° C. and an aging treatment temperature of 150 to 900 ° C.
- the processing efficiency at the time of processing is arbitrary, but the preferable range is the same as that of the first embodiment. Although it processed with the said processing method, in any case, the gadolinium Gd addition effect showed notably.
- the fine crystallite high-performance metal alloy member according to the fifth embodiment of the present invention is composed of a metal alloy in which gadolinium Gd is contained in a range of 5 ppm to less than 30000 ppm in an aluminum Al alloy containing a high-purity aluminum Al alloy. .
- the fine crystallite high-performance aluminum Al alloy member according to the fifth embodiment of the present invention is obtained by casting a 30 mm wide ⁇ 10 mm thick plate by adding 500 ppm of gadolinium Gd to an alloy having an aluminum Al content of 99.95 wt%. After solution treatment and aging, a 0.3 mm thick plate was rolled. Next, 500 g of gadolinium Gd was added to an aluminum Al alloy composed of aluminum Al 90% -magnesium Mg 10% by weight, a 30 mm wide ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate. .
- gadolinium Gd was added to an aluminum Al alloy composed of aluminum Al 50% -magnesium Mg 50% by weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after heat treatment, rolled to a 0.3 mm thick plate.
- 500 g of gadolinium Gd was added to an aluminum Al alloy composed of aluminum Al 10% -magnesium Mg 90% by weight, a 30 mm wide ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate. .
- the finished 0.3 mm thick plate was subjected to X-ray analysis, and the average crystallite diameters derived using Scherrer's formula were 6 nm, 13 nm, 27 nm, and 19 nm.
- the crystallite size is nanometer (10 -9 m ⁇ 10 -6 m) and a micrometer (10 -6 m ⁇ 10 -3 m).
- the finished 0.3 mm thick plate was subjected to X-ray analysis, and the average crystallite diameters derived using Scherrer's formula were 8 nm, 13 nm, 27 nm, and 19 nm.
- the crystallite size is nanometer (10 -9 m ⁇ 10 -6 m) and a micrometer (10 -6 m ⁇ 10 -3 m).
- the aluminum Al alloy member is made of an aluminum Al alloy containing gadolinium Gd in a range of 5 ppm or more and less than 30000 ppm.
- the aluminum Al alloy can have a solution treatment temperature of 300 to 2000 ° C. and an aging treatment temperature of 50 to 450 ° C.
- Particularly preferred conditions are a solution treatment temperature of 500 to 1600 ° C. and an aging treatment temperature of 50 to 400 ° C.
- the processing efficiency at the time of processing is arbitrary, but the preferable range is the same as that of the first embodiment. It has been found that by adding gadolinium Gd, a highly functional and durable member that is easy to process can be obtained. Although it processed with the said processing method, in any case, the gadolinium Gd addition effect is seen notably. Similar results were obtained after solution treatment and aging treatment.
- the fine crystallite high-performance metal alloy member according to the sixth embodiment of the present invention is composed of a metal alloy in which gadolinium Gd is contained in a range of 5 ppm to less than 30000 ppm in a magnesium Mg alloy including a high-purity magnesium Mg alloy. .
- the fine crystallite high-performance magnesium Mg alloy member according to the sixth embodiment of the present invention is obtained by adding 500 ppm of gadolinium Gd to a 99.95 wt% alloy, casting a 30 mm wide ⁇ 10 mm thick plate, After aging and aging, it was rolled into a 0.3 mm thick plate. The finished 0.3 mm thick plate was subjected to X-ray analysis, and the average crystallite diameter derived using Scherrer's equation was 12 nm. The crystallite size is nanometer (10 -9 m ⁇ 10 -6 m) and a micrometer (10 -6 m ⁇ 10 -3 m).
- the fine crystallite high-performance magnesium alloy of the present invention is constituted by containing magnesium Mg ingot and magnesium Mg alloy in a range of gadolinium Gd 5 ppm or more and less than 30000 ppm.
- Hardness, tensile strength, and 0.2% proof stress increased 2-3 times with almost no decrease in conductivity and Young's modulus. It was found that the elongation can also be extended by 2 to 3 times. The softening temperature could be increased more than twice. Rolling could also be done without heat treatment, and cross processing could be done. It was found that processability was also improved. It was also possible to balance these characteristics. The effect was confirmed with the plate, wire, thin film, and powder. Next, a method for producing an alloy member having the above characteristics will be described.
- an alloy material having the above composition is cast, and if necessary, a solution treatment is performed by heating to a predetermined temperature and then rapidly cooling, and if necessary, an aging treatment is then performed at a predetermined temperature. Apply.
- an alloy material having the above composition is cast, and if necessary, the material is subjected to a solution treatment that is heated to a predetermined temperature and then rapidly cooled, and the material is processed into a predetermined shape. If necessary, an aging treatment is applied to the material before or after processing.
- the magnesium Mg alloy can have a solution treatment temperature of 250 to 1050 ° C. and an aging treatment temperature of 110 to 500 ° C.
- Particularly preferred conditions are a solution treatment temperature of 500 to 1000 ° C. and an aging treatment temperature of 50 to 450 ° C.
- the processing efficiency at the time of processing is arbitrary, but the preferable range is the same as that of the first embodiment. It has been found that by adding gadolinium Gd, a highly functional, durable and easily processable member can be obtained. Although it processed with the said processing method, in any case, the gadolinium Gd addition effect was notably shown. Similar results were obtained after solution treatment and aging treatment. A magnesium grit alloy containing a high-purity magnesium Mg alloy having a face-centered cubic lattice containing gadolinium Gd in a range of 5 ppm or more and less than 30000 ppm was also experimentally evaluated.
- the fine crystallite high-performance metal alloy member according to the seventh embodiment of the present invention is composed of a metal alloy in which gadolinium Gd is contained in a range of 5 ppm to less than 30000 ppm in a copper Cu alloy including a high-purity copper Cu alloy. .
- the fine crystallite high-functionality copper Cu alloy member according to the seventh embodiment of the present invention is obtained by adding 500 ppm of gadolinium Gd to a 99.95 wt% alloy, casting a 30 mm wide ⁇ 10 mm thick plate, After aging and aging, it was rolled into a 0.3 mm thick plate. Next, 500 g of gadolinium Gd was added to a copper Cu alloy consisting of copper Cu 90% -zinc Zn 10% by weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate. .
- gadolinium Gd was added to a copper Cu alloy composed of copper Cu 65% -zinc Zn% weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after heat treatment, rolled to a 0.3 mm thick plate.
- 500 g of gadolinium Gd was added to a copper Cu alloy consisting of copper Cu 10% -silver Ag 90% weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate. .
- the finished 0.3 mm thick plate was subjected to X-ray analysis, and the average crystallite diameters derived using Scherrer's formula were 17 nm, 7 nm, 21 nm, and 13 nm.
- the crystallite size is nanometer (10 -9 m ⁇ 10 -6 m) and a micrometer (10 -6 m ⁇ 10 -3 m).
- Hardness, tensile strength, and 0.2% proof stress increased 2-3 times with almost no decrease in conductivity and Young's modulus. It was found that the elongation can also be extended by 2 to 3 times.
- the softening temperature could be increased by 2.3 times. Rolling could also be done without heat treatment, and cross processing could be done. It was found that processability was also improved.
- the copper Cu alloy can have a solution treatment temperature of 600 to 2500 ° C. and an aging treatment temperature of 150 to 850 ° C. Particularly preferred conditions are a solution treatment temperature of 600 to 1600 ° C. and an aging treatment temperature of 150 to 780 ° C. It has been found that by adding gadolinium Gd, a highly functional, durable and easily processable member can be obtained. Although it processed by the said processing method, in any case, the gadolinium Gd addition effect was remarkable. Similar results were obtained even after solution treatment and aging treatment.
- gadolinium Gd was contained in a range of 5 ppm or more and less than 30000 ppm in a copper Cu alloy containing a high-purity copper Cu alloy having a face-centered cubic lattice.
- the copper Cu alloy composition was also prototyped and evaluated, but showed the same gadolinium Gd addition effect. After the addition of gadolinium Gd, the properties of hardness, tensile strength, proof stress, elongation and heat resistance were improved, and it was easy to process and workability was improved. Little decrease in Young's modulus and conductivity was observed.
- the fine crystallite high-performance metal alloy member according to the eighth embodiment of the present invention is composed of a metal alloy containing gadolinium Gd in a range of 5 ppm or more and less than 30000 ppm in an iron Fe alloy including a high-purity iron Fe alloy. .
- the fine crystallite high-functional iron Fe alloy member according to the eighth embodiment of the present invention is obtained by adding 500 ppm of gadolinium Gd to a 99.95 wt% alloy, casting a 30 mm wide ⁇ 10 mm thick plate, After aging and aging, it was rolled into a 0.3 mm thick plate.
- gadolinium Gd was added to an iron Fe alloy composed of iron Fe 99% -silicon Si 1% weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after solution treatment and aging, it was rolled into a 0.3 mm thick plate.
- 500 g of gadolinium Gd was added to an iron Fe alloy composed of iron Fe% -nickel Ni 17% -aluminum Al 8% weight, a 30 mm width ⁇ 10 mm thick plate was cast, and after heat treatment, rolled to a 0.3 mm thick plate.
- the finished 0.3 mm thick plate was subjected to X-ray analysis, and the average crystallite diameters derived using Scherrer's formula were 7 nm, 27 nm, and 18 nm, respectively.
- the crystallite size is nanometer (10 -9 m ⁇ 10 -6 m) and a micrometer (10 -6 m ⁇ 10 -3 m).
- Hardness, tensile strength, and 0.2% proof stress increased 2-3 times with almost no decrease in conductivity and Young's modulus. It was found that the elongation can also be extended by 2 to 3 times.
- the softening temperature could be increased more than twice. Rolling could also be done without heat treatment, and cross processing could be done. It was found that processability was also improved. It was also possible to balance these characteristics.
- an alloy member having the above characteristics is cast, and if necessary, a solution treatment is performed by heating to a predetermined temperature and then rapidly cooling, and if necessary, an aging treatment is then performed at a predetermined temperature. Apply.
- an alloy material having the above composition is cast, and if necessary, the material is subjected to a solution treatment that is heated to a predetermined temperature and then rapidly cooled, and the material is processed into a predetermined shape.
- an aging treatment is applied to the material before or after processing.
- the solution treatment was performed at 820 ° C. for 1 hour, and the aging treatment was performed at 480 ° C. for 3 hours.
- the iron Fe alloy can have a solution treatment temperature of 600 to 2800 ° C. and an aging treatment temperature of 150 to 700 ° C. Particularly preferred conditions are a solution treatment temperature of 600 to 2000 ° C. and an aging treatment temperature of 150 to 700 ° C.
- the processing efficiency at the time of processing is arbitrary, but the preferable range is the same as that of the first embodiment. It has been found that by adding gadolinium Gd, functional characteristics are improved, and a durable and easy-to-process member can be obtained.
- the gadolinium Gd addition effect is seen notably. Similar results were obtained after solution treatment and aging treatment. Trial evaluation was also conducted on the gadolinium Gd content in a range of 5 ppm to less than 30000 ppm in an iron Fe alloy including a high-purity iron Fe alloy having a body-centered cubic lattice, and the same gadolinium Gd addition effect was shown.
- the copper iron Fe alloy composition was also prototyped and evaluated, but showed the same effect of adding gadolinium Gd. After addition of gadolinium Gd, properties such as hardness, tensile strength, proof stress, elongation, heat resistance and the like were improved, and it was easy to work and workability was also improved.
- the fine crystallite high-function alloy member according to the ninth embodiment of the present invention is made of a metal alloy in which gadolinium Gd is contained in a range of 5 ppm or more and less than 30000 ppm in a titanium Ti alloy containing a high-purity titanium Ti alloy.
- the fine crystallite high-performance titanium Ti alloy member according to the ninth embodiment of the present invention is obtained by adding 500 ppm of gadolinium Gd to a 99.95 wt% alloy, casting a 30 mm wide ⁇ 10 mm thick plate, After aging and aging, it was rolled into a 0.3 mm thick plate.
- gadolinium Gd is added to an iron-Fe alloy composed of titanium Ti 99.8% -palladium Pd 0.2% by weight, a 30 mm width ⁇ 10 mm thick plate is cast, solutionized, after aging, a 0.3 mm thick plate It was rolled into.
- X-ray analysis of the above finished 0.3 mm thick plate and the average crystallite diameter derived using Scherrer's formula is They were 7 nm and 27 nm, respectively.
- the crystallite size is nanometer (10 -9 m ⁇ 10 -6 m) and a micrometer (10 -6 m ⁇ 10 -3 m). Hardness, tensile strength, and 0.2% proof stress increased 2-3 times with almost no decrease in conductivity and Young's modulus.
- an alloy material having the above composition is cast, and if necessary, the material is subjected to a solution treatment that is heated to a predetermined temperature and then rapidly cooled, and the material is processed into a predetermined shape. If necessary, an aging treatment is applied to the material before or after processing.
- the titanium Ti alloy can have a solution treatment temperature of 600 to 2700 ° C. and an aging treatment temperature of 150 to 500 ° C. Particularly preferred conditions are a solution treatment temperature of 500 to 1550 ° C. and an aging treatment temperature of 300 to 800 ° C.
- the processing efficiency at the time of processing is arbitrary, but the preferable range is the same as that of the first embodiment. Similar results were obtained after solution treatment and aging treatment.
- gadolinium Gd was contained in a range of 5 ppm or more and less than 30000 ppm in a titanium Ti alloy containing a high-purity titanium Ti alloy having a close-packed hexagonal crystal lattice.
- the trial manufacture evaluation was carried out also with the said copper titanium Ti alloy composition, the same gadolinium Gd addition effect was shown.
- properties such as hardness, tensile strength, proof stress, elongation, heat resistance and the like were improved, and it was easy to work and workability was also improved. Little decrease in conductivity and Young's modulus was observed.
- the titanium titanium alloy composition was also prototyped and evaluated, but showed the same effect of adding gadolinium Gd.
- a metal alloy composed of at least one element selected from the group consisting of the same group showed similar remarkable high performance characteristics.
- the alloy applied to the embodiment is not particularly limited.
- Components other than the above-described function improving additive may be any components as long as they can be used for ordinary metal alloys, and are not particularly limited. That is, the said function improvement additive is effective also with respect to the existing general metal alloy.
- the manufacturing of the alloy member according to these embodiments is the same as the actual form of the metal alloy.
- an alloy material having the above composition is cast, and the material is subjected to a solution treatment in which the material is heated to a predetermined temperature and then rapidly cooled. Thereafter, an aging treatment is performed at a predetermined temperature as necessary.
- a processed alloy an alloy material having the above composition is cast, and the material is subjected to a solution treatment for rapid cooling after heating at a predetermined temperature, and the material is processed into a predetermined shape before or after the processing. Aging treatment is applied to the previous term material.
- Gadolinium Gd is the most effective and highly functional element considering the volume occupancy, and the improvement in heat resistance is also remarkable. In particular, extremely high Young's modulus and elastic limit can be obtained by adding Gd.
- Gd has a large effect of improving hardness, Young's modulus, and tensile strength
- the improvement of functional characteristics is remarkable.
- the addition amount is small and the occupied volume is small, the characteristics unique to the alloy can be utilized.
- the effect as a functional property improving additive is exhibited by Gd alone, but it is also possible to obtain excellent properties by a synergistic effect by adding in combination with at least one element selected from the group consisting of the above elements other than Gd. it can.
- the fine crystallite resistant high-performance metal alloy member of the present invention has enhanced functional properties, so it has strength, high yield strength, Young's modulus, electrical conductivity, thermal conductivity, softening point, etc., strong tensile strength and no brittleness. .
- the fine crystallite high-performance metal alloy member of the present invention has improved functionality, high properties such as hardness, tensile strength, Young's modulus, proof stress, heat resistance, electrical conductivity, thermal conductivity, etc. Easy to work and good workability. Different from conventional alloy members. Further, it is a great feature that these characteristics can be adjusted according to the user's preference. Therefore, the most important feature is that an individual functional metal alloy adjusted according to the user's preference is obtained by increasing the important functional characteristics of the above-described high-performance metal alloy.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Conductive Materials (AREA)
- Adornments (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP12796739.6A EP2719780A4 (en) | 2011-06-06 | 2012-04-10 | FINE CRYSTALLINE HIGH PERFORMANCE METAL ALLOYING ELEMENT AND METHOD FOR THE PRODUCTION THEREOF |
| CN201280037682.2A CN103748243A (zh) | 2011-06-06 | 2012-04-10 | 细微晶高功能金属合金元件及其制造方法 |
| US14/124,212 US20140212324A1 (en) | 2011-06-06 | 2012-04-10 | Fine crystallite high-function metal alloy member and method for manufacturing same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011138909A JP2012251235A (ja) | 2011-06-06 | 2011-06-06 | 微細結晶子高機能金属合金部材とその製造方法 |
| JP2011-138909 | 2011-06-06 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012169285A1 true WO2012169285A1 (ja) | 2012-12-13 |
Family
ID=47295856
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/060518 Ceased WO2012169285A1 (ja) | 2011-06-06 | 2012-04-10 | 微細結晶子高機能金属合金部材とその製造方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20140212324A1 (enExample) |
| EP (1) | EP2719780A4 (enExample) |
| JP (1) | JP2012251235A (enExample) |
| CN (1) | CN103748243A (enExample) |
| WO (1) | WO2012169285A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106893882A (zh) * | 2015-12-18 | 2017-06-27 | 北京有色金属研究总院 | 一种铜钆中间合金的制备方法 |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103258687B (zh) * | 2013-04-24 | 2015-04-22 | 浙江理工大学 | 一种突跳式温控器 |
| KR101677146B1 (ko) * | 2015-03-17 | 2016-11-17 | 한국생산기술연구원 | 듀플렉스 스테인리스강 및 그 제조방법 |
| US11674193B2 (en) | 2017-05-25 | 2023-06-13 | Sumitomo Electric Industries, Ltd. | Canted coil spring and connector |
| RU2744837C2 (ru) | 2017-10-19 | 2021-03-16 | Зе Боинг Компани | Сплав на основе титана и способ получения комплектующей детали из сплава на основе титана с помощью аддитивного технологического процесса |
| CN110144482B (zh) * | 2019-06-24 | 2020-03-24 | 昆明理工大学 | 一种稀土增强钯合金及其制备方法 |
| CN111763844B (zh) * | 2020-05-20 | 2021-12-17 | 上杭县紫金佳博电子新材料科技有限公司 | 一种键合金带及其制备方法 |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08157983A (ja) * | 1994-11-30 | 1996-06-18 | Kuwayama Kikinzoku:Kk | Au高純度の硬質Au合金製装飾部材 |
| WO1996031632A1 (en) * | 1995-04-07 | 1996-10-10 | Kazuo Ogasa | High-purity hard gold alloy and process for production thereof |
| JPH093612A (ja) * | 1995-04-07 | 1997-01-07 | Nishiki Tokushu Kinzoku Kenkyusho:Kk | 高純度金合金の硬質化方法 |
| WO1997047778A1 (en) * | 1996-06-12 | 1997-12-18 | Kazuo Ogasa | High purity hard gold alloy and method of manufacturing same |
| JP2001049364A (ja) * | 2000-07-03 | 2001-02-20 | Kazuo Ogasa | 硬質貴金属合金部材とその製造方法 |
| WO2003074745A1 (fr) * | 2002-03-01 | 2003-09-12 | Kazuo Ogasa | Element d'alliage de metal dur et procede de fabrication de celui-ci |
| WO2008072485A1 (ja) * | 2006-11-24 | 2008-06-19 | Kazuo Ogasa | 高性能弾性金属合金部材とその製造方法 |
| JP2009030146A (ja) * | 2007-07-26 | 2009-02-12 | Kazuo Ogasa | 高性能弾性金属合金部材とその製造方法 |
-
2011
- 2011-06-06 JP JP2011138909A patent/JP2012251235A/ja active Pending
-
2012
- 2012-04-10 WO PCT/JP2012/060518 patent/WO2012169285A1/ja not_active Ceased
- 2012-04-10 CN CN201280037682.2A patent/CN103748243A/zh active Pending
- 2012-04-10 EP EP12796739.6A patent/EP2719780A4/en not_active Withdrawn
- 2012-04-10 US US14/124,212 patent/US20140212324A1/en not_active Abandoned
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08157983A (ja) * | 1994-11-30 | 1996-06-18 | Kuwayama Kikinzoku:Kk | Au高純度の硬質Au合金製装飾部材 |
| WO1996031632A1 (en) * | 1995-04-07 | 1996-10-10 | Kazuo Ogasa | High-purity hard gold alloy and process for production thereof |
| JPH093612A (ja) * | 1995-04-07 | 1997-01-07 | Nishiki Tokushu Kinzoku Kenkyusho:Kk | 高純度金合金の硬質化方法 |
| WO1997047778A1 (en) * | 1996-06-12 | 1997-12-18 | Kazuo Ogasa | High purity hard gold alloy and method of manufacturing same |
| JP2001049364A (ja) * | 2000-07-03 | 2001-02-20 | Kazuo Ogasa | 硬質貴金属合金部材とその製造方法 |
| WO2002002834A1 (fr) * | 2000-07-03 | 2002-01-10 | Kazuo Ogasa | Alliage dur de metal noble et son procede d'obtention |
| WO2003074745A1 (fr) * | 2002-03-01 | 2003-09-12 | Kazuo Ogasa | Element d'alliage de metal dur et procede de fabrication de celui-ci |
| WO2008072485A1 (ja) * | 2006-11-24 | 2008-06-19 | Kazuo Ogasa | 高性能弾性金属合金部材とその製造方法 |
| JP2009030146A (ja) * | 2007-07-26 | 2009-02-12 | Kazuo Ogasa | 高性能弾性金属合金部材とその製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2719780A4 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106893882A (zh) * | 2015-12-18 | 2017-06-27 | 北京有色金属研究总院 | 一种铜钆中间合金的制备方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN103748243A (zh) | 2014-04-23 |
| JP2012251235A (ja) | 2012-12-20 |
| EP2719780A1 (en) | 2014-04-16 |
| EP2719780A4 (en) | 2014-12-31 |
| US20140212324A1 (en) | 2014-07-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TWI521074B (zh) | 電子/電氣機器用銅合金、銅合金薄板及導電構件 | |
| WO2012169285A1 (ja) | 微細結晶子高機能金属合金部材とその製造方法 | |
| JP4230218B2 (ja) | 硬質貴金属合金部材およびその製造方法 | |
| TWI327601B (en) | Copper alloy containing cobalt, nickel and silicon | |
| TWI704240B (zh) | 電阻材料用銅合金材料及其製造方法、以及電阻器 | |
| JP5319700B2 (ja) | 電子材料用Cu−Ni−Si−Co系銅合金及びその製造方法 | |
| TW526272B (en) | High strength copper alloy excellent in bendability and method for producing the same and terminal and connector using the same | |
| TW201211282A (en) | Copper alloy sheet and manufacturing method for same | |
| JP2005532477A5 (enExample) | ||
| JPWO2002002834A1 (ja) | 硬質貴金属合金部材およびその製造方法 | |
| WO2011152124A1 (ja) | 電子材料用Cu-Co-Si系銅合金及びその製造方法 | |
| JP3800279B2 (ja) | プレス打抜き性が優れた銅合金板 | |
| JPWO2008072485A1 (ja) | 高性能弾性金属合金部材とその製造方法 | |
| JP2019507252A5 (enExample) | ||
| JP4620173B1 (ja) | Cu−Co−Si合金材 | |
| TW200426232A (en) | Cu-Ni-Si alloy and production method thereof | |
| JP5610789B2 (ja) | 銅合金板材および銅合金板材の製造方法 | |
| JP5297855B2 (ja) | 銅合金板材およびその製造方法 | |
| JPH07166279A (ja) | 耐食性、打抜き加工性及び切削性が優れた銅基合金及びその製造方法 | |
| CN103031466B (zh) | 一种锡黄铜合金及其制造方法 | |
| JP4417115B2 (ja) | 硬質金属合金部材とその製造方法 | |
| KR101741681B1 (ko) | 내변색성 및 경도가 우수한 Ag-Cu계 합금 조성물 및 이의 제조방법 | |
| TW201718888A (zh) | 電子.電氣機器用銅合金、電子.電氣機器用銅合金薄板、電子.電氣機器用導電構件及端子 | |
| JP2009030146A (ja) | 高性能弾性金属合金部材とその製造方法 | |
| TW200829707A (en) | Copper alloy material for electric and electronic instruments and method of producing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12796739 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14124212 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012796739 Country of ref document: EP |