WO2012133945A1 - 胚性幹細胞から心筋シートを製造する方法 - Google Patents
胚性幹細胞から心筋シートを製造する方法 Download PDFInfo
- Publication number
- WO2012133945A1 WO2012133945A1 PCT/JP2012/059427 JP2012059427W WO2012133945A1 WO 2012133945 A1 WO2012133945 A1 WO 2012133945A1 JP 2012059427 W JP2012059427 W JP 2012059427W WO 2012133945 A1 WO2012133945 A1 WO 2012133945A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cells
- sheet
- cell
- cardiomyocytes
- embryonic stem
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/34—Muscles; Smooth muscle cells; Heart; Cardiac stem cells; Myoblasts; Myocytes; Cardiomyocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/44—Vessels; Vascular smooth muscle cells; Endothelial cells; Endothelial progenitor cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/70—Web, sheet or filament bases ; Films; Fibres of the matrix type containing drug
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/38—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells
- A61L27/3804—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells characterised by specific cells or progenitors thereof, e.g. fibroblasts, connective tissue cells, kidney cells
- A61L27/3834—Cells able to produce different cell types, e.g. hematopoietic stem cells, mesenchymal stem cells, marrow stromal cells, embryonic stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/38—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells
- A61L27/3886—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells comprising two or more cell types
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/54—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0657—Cardiomyocytes; Heart cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0697—Artificial constructs associating cells of different lineages, e.g. tissue equivalents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/412—Tissue-regenerating or healing or proliferative agents
- A61L2300/414—Growth factors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/20—Materials or treatment for tissue regeneration for reconstruction of the heart, e.g. heart valves
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/01—Modulators of cAMP or cGMP, e.g. non-hydrolysable analogs, phosphodiesterase inhibitors, cholera toxin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/04—Immunosuppressors, e.g. cyclosporin, tacrolimus
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/165—Vascular endothelial growth factor [VEGF]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/13—Coculture with; Conditioned medium produced by connective tissue cells; generic mesenchyme cells, e.g. so-called "embryonic fibroblasts"
- C12N2502/1329—Cardiomyocytes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/13—Coculture with; Conditioned medium produced by connective tissue cells; generic mesenchyme cells, e.g. so-called "embryonic fibroblasts"
- C12N2502/1347—Smooth muscle cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/28—Vascular endothelial cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2506/00—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells
- C12N2506/02—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from embryonic cells
Definitions
- the present invention relates to a method for producing a myocardial sheet using embryonic stem cell-derived cardiomyocytes, endothelial cells and mural cells.
- the present invention also relates to a therapeutic agent for heart disease comprising a myocardial sheet obtained by the above method.
- Non-patent Document 1 Patent Document 2
- the expected therapeutic effect cannot be obtained because the amount of cells in the sheet is insufficient, it is considered that the sheets need to be laminated and administered (Non-patent Document 2).
- An object of the present invention relates to a method for producing a myocardial sheet using embryonic stem cell-derived cardiomyocytes, endothelial cells and mural cells, and a therapeutic agent for heart disease comprising the myocardial sheet obtained thereby. Accordingly, an object of the present invention is to provide a myocardial sheet prepared by mixing cells obtained by inducing differentiation from embryonic stem cells into cardiomyocytes, endothelial cells and mural cells.
- the inventors of the present invention manufactured embryonic stem cells from Flk / KDR-positive cells on a culture dish coated with a temperature-responsive polymer, and cardiomyocytes prepared from the Flk / KDR-positive cells.
- the present inventors succeeded in treating a myocardial infarction model with a myocardial sheet produced using cardiomyocytes derived from embryonic stem cells, endothelial cells and mural cells, and completed the present invention. It came. That is, the present invention includes the following features.
- a mixed cell comprising cardiomyocytes, endothelial cells and mural cells.
- the cell according to any one of [11] to [15], wherein the cardiomyocyte, endothelial cell and wall cell are cells produced from embryonic stem cells.
- FIG. 1 shows the results of FACS of CM and EC component before co-culture on a temperature sensitive dish and the result of FACS after sheet formation.
- APC allophycocyanin, PE; phycoerythrin, SSC; side scatter, GFP; green fluorescent protein, FITC; fluorescein isothiocyanate.
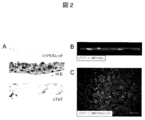
- FIG. 2 shows (A) Sirius red-stained image (upper): the formation of a layered extracellular matrix at the cell culture dish adhesion.
- HE-stained image (middle): 3-4 layers of cells constitute a sheet.
- FIG. 3 shows (A) a sheet image adhered on a MED64 probe. (B) The extracellular potential at each electrode is shown. (C) The distribution of potential measured in (B) is shown. The electrode having the highest peak negative potential (lower part of the figure) was regarded as a zero point, and color classification was performed by the time difference (seconds) from there to the peak negative potential of each electrode. The conduction from the bottom to the top is recognized.
- FIG. 4 shows the measurement results of each cytokine by ELISA on the culture supernatant.
- VEGF was shown to be extremely high compared to serum-free medium.
- SF serum free, TNFa
- tumor necrosis factor- ⁇ IGF-1
- insulin-like growth factor-1 VEGF
- basal endoral cell growth factor bFGF
- bass FGF An epidermal growth factor, HGF
- hepatocyte growth factor a hepatocyte growth factor.
- FIG. 5 shows a schematic diagram of the cell sheet lamination method.
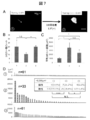
- FIG. 6 shows a graph comparing the amount of VEGF produced by sheets produced with or without cardiomyocytes (CM).
- CM cardiomyocytes
- FIG. 7 shows (A) a representative undifferentiated colony (arrow) in Group 1 (left figure: immediately after collection of the myocardial sheet) and an expanded colony of Group 2 (right figure: continuously cultured for 3 days with LIF added). . (B) The number of undifferentiated colonies in each Group is shown. (C) The average colony area in each Group is shown. (D) Show all colonies of each Group in order of size. Those of 5,000 ⁇ m 2 or more are displayed in red.
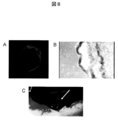
- FIG. 8 shows (A) macroscopic findings of the laminated sheet.
- B The optical microscope image of the sheet
- C Macroscopic findings after transplanting the sheet. The arrow points to the laminated sheet.
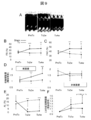
- FIG. 9 shows the results of cardiac ultrasonography.
- A The time-dependent change of the M mode figure in a myocardial sheet transplantation group is shown.
- FS left ventricular diameter shortening rate
- FAC left ventricular cross-sectional change rate
- D systolic wall thickness increase rate
- (E) shows the time course of the non-shrinkage range (AL) (infarct site range index).
- (F) shows the rate of change of diastolic left ventricular area relative to pre-treatment (PreTx) (an index of left ventricular expansion).
- PreTx before treatment, Tx2w; 2 weeks after treatment, Tx4w; 4 weeks after treatment, LV; left ventricle.
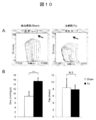
- FIG. 10 shows the results of the left ventricular volume pressure curve measurement test
- A The volume pressure curve shift accompanying the decrease in the preload in the sham group (left figure) and the treatment group (right figure).
- End systolic left ventricular elastic modulus (Ees) is indicated by the slope of a straight line indicated by an arrow.
- B Ees (left figure) and time constant (Tau) (right figure) of the Sham group (white) and the treatment group (black) are shown, respectively.
- Ees indicates left ventricular contractility (high value indicates high contractility), and Tau indicates left ventricular expandability (low value indicates high expandability).
- FIG. 11 shows (A) FISH (mouse-derived cells: yellow) and cTnT immunostained image (red) on day 1 (left), day 1 (middle), and day 4 (right) after transplantation. Show. The engraftment site on the 28th day after transplantation (Tx-d28) is indicated by an arrow. (B) Connexin 43 (green) immunostained image 7 days after transplantation. The figure surrounded by the white line at the lower left shows an image of a normal myocardium.
- FIG. 12 shows FISH of graft-derived engrafted cardiomyocytes on day 1 after transplantation (Tx-d1), day 3 (Tx-d3), week 1 (Tx-d7), and week 4 (Tx-d28). (Mouse-derived cells: yellow) and cTnT immunostained images (red) are shown.
- FIG. 13 shows Sirius red stained images of (A) Sham group (left) and treatment group (Tx: right). In the treatment group, thinning of the wall is suppressed and a decrease in infarct area is observed.
- FIG. 14 shows (A) FISH (mouse-derived cells: 1 day after transplantation (Tx-d1: left figure), 3 days (Tx-d3: middle figure), and 7 days (Tx-d7 right figure)). (Yellow), cTnT immunostained image (red), von Willebrand factor (vWF) immunostained image (green). (B) Each enlarged image of the fluorescence microscope on the third day after transplantation is shown.
- FIG. 15 shows the measurement result of neovascular density at 4 weeks after transplantation.
- A Masson trichrome stained image of the treatment group 4 weeks after transplantation.
- FIG. 1 the infarct central part (Central-MI) and the infarct peripheral part (Peri-MI) are shown.
- B Stained images of cTnT (red) and vWF (green) in the periphery and center of the infarct in the treatment group (Tx) and the Sham group, respectively. A marked formation of a vWF-positive site with a luminal structure is observed in the treatment group in both the peripheral part and the central part.
- C Capillary density of the Central-MI and Peri-MI sites in each group shown in FIG. In the figure, **: p ⁇ 0.01, ***: p ⁇ 0.001 (unpaired t-test).
- the present invention includes (a) a step of producing Flk / KDR positive cells, cardiomyocytes, endothelial cells and mural cells from embryonic stem cells, respectively, and (b) the Flk / KDR positive cells,
- a method for producing a myocardial sheet from embryonic stem cells comprising a step of forming a myocardial sheet by mixing with endothelial cells and mural cells, and a method for producing a heart disease such as ischemic heart disease comprising a myocardial sheet obtained by this method It relates to a therapeutic agent.
- ES cells are stem cells established from the inner cell mass of early embryos (for example, blastocysts) of mammals such as humans and mice and having the pluripotency and the ability to proliferate by self-replication.
- ES cells are embryonic stem cells derived from the inner cell mass of the blastocyst, which is the embryo after the morula, at the 8-cell stage of a fertilized egg, and have the ability to differentiate into any cell that constitutes an adult, so-called differentiation differentiation. And ability to proliferate by self-replication.
- ES cells were discovered in 1981 in mice (MJ Evans and MH Kaufman (1981), Nature 292: 154-156), and then ES cell lines were also established in primates such as humans and monkeys.
- ES cells can be established by removing an inner cell mass from a blastocyst of a fertilized egg of a subject animal and culturing the inner cell mass on a fibroblast feeder.
- LIF leukemia inhibitory factor
- bFGF basic fibroblast growth factor
- DMEM / F-12 culture supplemented with 0.1 mM 2-mercaptoethanol, 0.1 mM non-essential amino acid, 2 mM L-glutamic acid, 20% KSR and 4 ng / ml ⁇ -FGF as a culture solution for ES cell production
- the solution can be used to maintain human ES cells in a humidified atmosphere at 37 ° C., 2% CO 2 /98% air (O. Fumitaka et al. (2008), Nat. Biotechnol., 26: 215- 224).
- ES cells need to be passaged every 3 to 4 days, and at this time, passage is performed by, for example, 0.25% trypsin and 0.1 mg / ml in PBS containing 1 mM CaCl 2 and 20% KSR. Can be performed using ml collagenase IV.
- ES cells can be selected by the Real-Time PCR method using the expression of gene markers such as alkaline phosphatase, Oct-3 / 4, Nanog as an index.
- gene markers such as OCT-3 / 4, NANOG, and ECAD can be used as an index (E. Kroon et al. (2008), Nat. Biotechnol., 26: 443). -452).
- Human ES cell lines are obtained from, for example, WA01 (H1) and WA09 (H9) from the WiCell Research Institute, and KhES-1, KhES-2 and KhES-3 from the Institute of Regenerative Medicine, Kyoto University (Kyoto, Japan). Is possible.
- ⁇ Differentiation medium Examples of differentiation media used for the production of Flk / KDR positive cells (also called Flk + cells), cardiomyocytes, endothelial cells and mural cells for producing myocardial sheets are described below.
- the medium can be prepared using a medium used for culturing animal cells as a basal medium.
- IMDM medium for example, IMDM medium, Medium 199 medium, Eagle's Minimum Essential Medium (EMEM) medium, ⁇ MEM medium, Dolbecco's modified Eagle's Medium (DMEM) medium, Ham's F16 medium, Ham's F16 medium, Fischer's medium, mixed media thereof, and the like are included.
- the medium may contain serum or may be serum-free.
- the medium can also contain, for example, albumin, transferrin, Knockout Serum Replacement (KSR) (serum substitute for FBS during ES cell culture), fatty acid, insulin, collagen precursor, trace element, 2-mercaptoethanol, One or more serum replacements such as 3′-thiolglycerol may be included, as well as lipids, amino acids, non-essential amino acids, vitamins, growth factors, cytokines, antibiotics, antioxidants, pyruvate, buffers, One or more substances such as inorganic salts may also be included.
- KSR Knockout Serum Replacement
- a Flk / KDR positive cell is a cell that expresses at least Flk / KDR, where Flk / KDR means Flk1 or KDR and is a receptor for vascular endothelial growth factor (VEGF). It is.
- Flk1 is exemplified by NCBI accession number NM_010612
- KDR is exemplified by NCBI accession number NM_002253.
- Flk / KDR positive cells can be prepared by inducing differentiation of embryonic stem cells by any method (Yamashita J, et al, Nature.
- cardiomyocytes mean cells expressing at least cardiac troponin (cTnT) or ⁇ MHC.
- cTnT is exemplified by NCBI accession number NM_000364 in the case of humans and NM_001130174 in the case of mice.
- ⁇ MHC is exemplified by NCBI accession number NM_002471 in the case of humans, and NM_001164171 in the case of mice.
- Embryonic stem cells can be induced to differentiate into cardiomyocytes by forming a cell mass (embryoid body) by suspension culture.
- a known method can be used as a method for inducing differentiation of cardiomyocytes from embryonic stem cells, and is not particularly specified. For example, differentiation in the presence of a substance that suppresses BMP signaling is performed.
- a method of induction (WO2005 / 033298), a method of inducing differentiation by sequentially adding Activin A and BMP (WO2007 / 002136), a method of inducing differentiation in the presence of a substance that promotes activation of the canonical Wnt signal pathway ( WO2007 / 126077) and FIk / KDR positive cells can be isolated from embryonic stem cells and cardiomyocytes can be induced to differentiate from embryonic stem cells using the method of inducing differentiation in the presence of cyclosporin A (WO2009 / 118928) .
- embryonic stem cells are adherently cultured on a culture device to produce Flk / KDR positive cells, and then differentiation is induced in the presence of cyclosporin A.
- the content of cyclosporin A in the medium is, for example, 0.1 to 30 ⁇ g / mL, and preferably 1 to 3 ⁇ g / mL, but can be any amount that allows differentiation of cardiomyocytes.
- the culture equipment is coated with a cell support material such as collagen, gelatin, poly-L-lysine, poly-D-lysine, laminin, fibronectin, Matrigel TM (Becton Dickinson). ) And the like.
- cardiomyocytes may be isolated and purified, or may be mixed with other cell types. Preferably, it is an isolated and purified cell.
- the method of isolation and purification is not particularly limited, but for example, a method of selecting using a cardiomyocyte marker such as N-cadherin as an index (Honda M, et al, Biochem Biophys Res Commun.
- endothelial cells mean cells expressing at least one of PE-CAM, VE-cadherin, and von Willebrand factor (vWF).
- the mural cell means a cell that expresses at least Smooth muscle actin (SMA).
- the PE-CAM is exemplified by NCBI accession number NM_000442 in the case of humans, and NM_001032378 in the case of mice.
- VE-cadherin is exemplified by NCBI accession number NM_001795 for humans and NM_009868 for mice.
- NCBI accession number NM_000552 is exemplified for humans
- NM_011708 is exemplified for mice.
- SMA is exemplified by NCBI accession number NM_001141945 for humans, and NM_007392 for mice.
- Embryonic stem cells can be induced to differentiate into endothelial cells or mural cells by forming a cell mass (embryoid body) by suspension culture.
- a known method can be used as a method for inducing differentiation of endothelial cells or mural cells from embryonic stem cells, and is not particularly specified.
- FLk / KDR positive cells from embryonic stem cells And cardiomyocytes can be induced to differentiate from embryonic stem cells using a method of inducing differentiation in the presence of VEGF and cAMP (Yamashita J, et al. Nature. 408: 92-6, 2000).
- the content of VEGF and cAMP in the medium is not limited as long as it can induce differentiation of endothelial cells or mural cells.
- VEGF for example, it is 25 ng / mL to 150 ng / mL, preferably 50 to 100 ng / mL.
- cAMP is used to include its derivatives (eg 8-bromo-cAMP), but its content is not limited to eg 0.1 mmol / L to 2 mmol / l, preferably 0.5 to 1 mmol / l L.
- the culture days are not particularly limited, but are 1 to 10 days, preferably 3 days after culturing Flk / KDR positive cells in the presence of VEGF and cAMP.
- the endothelial cells or mural cells may be isolated from each other and purified, or the endothelial cells or mural cells may be present in a mixture with other cell types.
- a mixed cell containing cardiomyocytes, endothelial cells and mural cells obtained using the above method can be produced.
- the content of cardiomyocytes is, for example, 10%, 20% or more, 30% or more, 40% or more, or 50% or more, 90% or less, 80% or less, 70% or less, 60% or less, or 50 % Or less.
- the preferred content is 40% or more, and the more preferred content is in the range of 40% or more and 50% or less.
- the content of endothelial cells is, for example, 1%, 2% or more, 3% or more, 4% or more, 5% or more, or 10% or more, 50% or less, 40% or less, 30% or less, 20 % Or less or 10% or less.
- a preferable content rate is 3% or more, and a more preferable content rate is the range of 3% or more and 10% or less.
- the content of mural cells is, for example, 10%, 20% or more, 30% or more, 40% or more, 50% or more, or 60% or more, 90% or less, 80% or less, 70% or less, or 60 % Or less.
- a preferable content rate is 20% or more, 30% or more, 40% or more, or 50% or more, and a more preferable content rate is a range of 50% or more and 60% or less.
- the mixed cell may be isolated as a cell culture, and is preferably a mixed cell in the form of a sheet.
- the myocardial sheet is a sheet-like cell aggregate composed of various cells forming the heart or blood vessels and connected to each other by intercellular bonding.
- examples of the various cells forming the heart or blood vessels include the above-described cardiomyocytes, endothelial cells and wall cells.
- a preferred myocardial sheet in the present invention has an electrical connection and orientation between cells, and secretes VEGF out of the cell.
- the myocardial sheet is produced by mixing and culturing cells containing at least cardiomyocytes, endothelial cells and mural cells prepared by the above-described method. At this time, the number of cells to be cultured is, for example, 1 ⁇ 10 4 to 1 ⁇ 10 6 .
- cells other than cardiomyocytes, endothelial cells, and mural cells may be included.
- the above-described embryonic stem cell-derived Flk / KDR positive cells are cultured by mixing cardiomyocytes, endothelial cells and mural cells into cells cultured for 1 to 7 days, preferably 3 days.
- VEGF may be added to the culture medium for further culturing, and the culture days at this time may be 1 to 10 days, preferably 4 days.
- the culture was coated with a temperature-responsive polymer obtained by polymerizing a (meth) acrylamide compound, an N- (or N, N-di) alkyl-substituted (meth) acrylamide derivative (Japanese Patent Laid-Open No.
- a culture device may be used, and a culture device to which poly-N-isopropylacrylamide is fixed is preferable.
- this culture equipment can also be purchased from Cellseed Co. as UpCell.
- the size of the myocardial sheet depends on the culture equipment, but preferably has a sufficient area to cover the site to be transplanted.
- the prepared myocardial sheet may be used by being laminated, and is preferably a myocardial sheet having three layers. In the lamination, the myocardial sheets are stacked in the culture solution (preferably, the myocardial sheets are shifted and stacked), and then joined by removing the culture solution.
- the myocardial sheet manufactured by the above method has, for example, the following characteristics.
- the cell composition ratio comprising at least cardiomyocytes, endothelial cells and wall cells and constituting the myocardial sheet is not limited to the following, but for example, cardiomyocytes 35-50%, endothelial cells 0.5-7%, It is 45 to 63% of wall cells.
- -Endothelial cells are scattered between cardiomyocytes.
- the myocardial sheet provided in the present invention can be used as a therapeutic agent for animal (preferably human) heart disease.
- a method for treating a heart disease is achieved by arranging a myocardial sheet so as to cover a desired portion.
- the arrangement so as to cover a desired portion can be performed using a technique well known in the art.
- the desired part if the desired part is large, it may be placed so as to surround the tissue.
- the myocardial sheet in order to obtain a desired effect, can be arranged several times in the same portion. When arranging several times, it is desirable to allow sufficient time for the myocardial sheet to engraft in the tissue and perform angiogenesis.
- Examples of the heart disease in the present invention include heart failure, ischemic heart disease, myocardial infarction, cardiomyopathy, myocarditis, hypertrophic cardiomyopathy, dilated phase hypertrophic cardiomyopathy, deficiency due to dilated cardiomyopathy, and the like. .
- Example 1 Preparation of myocardial sheet Yamashita JK, et al. FASEB J.H. 19: 1534-6, 2005.
- Flk + cells were generated by previously reported methods (Yamashita J, et al. Nature. 408: 92-6, 2000 or Yamashita JK, et al. FASEB J. 19: 1534-6, 2005).
- EMG7 or 20D17 was cultured for 4 days on gelatin-coated dishes using differentiation medium ( ⁇ MEM supplemented with 10% fetal bovine serum and 5 ⁇ 10 5 mol / L 2-mercaptoethanol), followed by Flk by FACS. It was prepared by purifying positive cells. Mixed cells of endothelial cells and mural cells were produced from the Flk + cells obtained by the above-described method using the method reported so far (Yamashita J, et al. Nature. 408: 92-6, 2000 or Yurgi- Kobayashi T, et al. Arterioscler Thromb Vasc Biol.26: 1977-84, 2006).
- Cardiomyocytes were prepared from the Flk + cells obtained by the method described above using the methods reported so far (WO 2009/118928 or Yan P, et al. Biochem Biophys Res Commun. 379: 115-20, 2009). Briefly, it was obtained by culturing on mitomycin C-treated OP9 cells for 4 days using a differentiation medium supplemented with 1-3 ⁇ g / mL Cyclosporin-A, and then separating the GFP positive fraction.
- the myocardial sheet was produced by the following method using the above-described plural types of cells. From 2.5 ⁇ 10 4 to 4.0 ⁇ 10 4 Flk + cells were seeded on a temperature-sensitive culture dish (UpCell, Cellu Seed) and cultured using a differentiation medium. On day 3 of culture, 5.0 ⁇ 10 5 mixed cells of the above endothelial cells and mural cells and 5.0 ⁇ 10 5 of the above cardiomyocytes were seeded in the same dish and differentiation medium supplemented with VEGF And cultured at 37 ° C. By returning to room temperature 4 days after the addition of cardiomyocytes (7 days after the start of culture), the cells were detached from the culture dish into a sheet to obtain a myocardial sheet.
- a temperature-sensitive culture dish UpCell, Cellu Seed
- FIG. 1 shows the results of quantification of the ratio of cardiomyocytes (CM), endothelial cells (EC), and mural cells (MC) based on the positive rates of GFP (FITC) and PE.
- CM cardiomyocytes
- EC endothelial cells
- MC mural cells
- Example 3 Histological evaluation of myocardial sheet
- a myocardial sheet prepared from EMG7 was fixed using 4% PFA (paraformaldehyde), blocked with 1% skim milk, and then antibody (primary Antibody: mouse anti-cTnT, rat anti-VE-Cadherin, secondary antibody: anti-mouse Alexa Flour 546, anti-rat Alexa Flour 488) are used to label CM and EC, and DAPI (4 ′, 6-diamino) After nuclei staining with 2-phenylindole), the images were observed with a multiphoton laser microscope (LSM510, Carl-Zeiss) or a fluorescence microscope (BZ-9000, Keyence) (FIGS.
- LSM510 multiphoton laser microscope
- Carl-Zeiss Carl-Zeiss
- BZ-9000 fluorescence microscope
- EMG7 Electrophysiological evaluation of myocardial sheet
- EMG7 was prepared on an electrode of a culture dish with an electrode (MED64 system, Alpha Med Science) coated with 0.1% gelatin. The myocardial sheet was left still. Subsequently, the medium was aspirated and incubated at 37 ° C. for 30 minutes to fix the electrode and the sheet, and the electric potential conduction on the sheet was recorded by measuring the electric potential of each electrode. The result is shown in FIG. By measuring the extracellular potential, it was confirmed that the pulsation was electrically continuously transmitted in one direction (FIG. 3C).
- Example 5 Cytokine production ability of myocardial sheet The amount of cytokine in the culture supernatant during formation (Condition 1) and after completion (Condition 2) of a myocardial sheet prepared from EMG7 was measured using ELISA (enzyme-linked immunosorbent assay). TNF ⁇ , IGF-1, VEGF, IL-6, bFGF, IFN ⁇ , EGF, Leptin and HGF were measured (HGF: mouse HGF EIA kit, IIM, otherwise: mouse angiogenesis ELISA strip, Signosis). The result is shown in FIG.
- VEGF vascular endothelial growth factor
- ⁇ Condition 2 Spread the myocardial sheet prepared by the above method on a gelatin-coated dish and leave it to stand, suck the medium, fix the culture dish and the sheet, add the medium, and add at 37 ° C for 30 minutes. Incubate. Another myocardial sheet was spread and allowed to stand on the sheet fixed to the dish, and the medium was sucked and laminated. Repeat for 3 layers in the same way. The second and third layers were laminated by shifting the position little by little from the original sheet. Subsequently, the medium was allowed to flow along the bottom of the culture dish using a pipetteman, and the laminated cell sheets were peeled from the culture dish (FIG. 5).
- VEGF production ability of myocardial sheet RNA was extracted from the myocardial sheet prepared as described above using RNeasy mini (QIAGEN), and the expression level of vegf164 was measured by quantitative RT-PCR (Step One Plus, Appliedbiosystems, vegf164 forward primer: 5′-CCAGCACATAGGAGAGATGAGCTT-3 ′ (SEQ ID NO: 1) and reverse primer: 5′-CAAGGCTCACAGTGATTTTCTGG-3 ′ (SEQ ID NO: tAC 3 '(SEQ ID NO: 3) and reverse primer: 5'-ATGGAGCCACCGATCCCAC -3 '(SEQ ID NO: 4)).
- FIG. 6A a cell sheet prepared using neonatal mouse cardiac fibroblasts (CF) was used as a control.
- CF neonatal mouse cardiac fibroblasts
- FIG. 6A the culture supernatant is aspirated before collecting (before lowering to room temperature) the sheet added with cardiomyocytes (CM (+)) and the sheet not added (CM ( ⁇ )).
- CM (+) cardiomyocytes
- CM ( ⁇ ) the sheet not added
- the supernatant after culturing for 3 hours with serum-free medium was collected, and the amount of VEGF was measured (Quantikine mouse VEGF, R & D). The result is shown in FIG.
- CM (+) sheet secretes VEGF much more than the CM ( ⁇ ) sheet.
- Example 7 Evaluation of contamination of undifferentiated cells into myocardial sheet The myocardial sheet was collected and allowed to stand on a gelatin-coated dish, and the medium was sucked and incubated at 37 ° C for 30 minutes to fix the dish and the sheet.
- Group 1 Fluorescent immunostaining is performed immediately after collection of the myocardial sheet.
- Group 2 After recovery of myocardial sheet, continuous culture for 3 days in a medium for ES cells (Yamashita J, et al. Nature. 408: 92-6, 2000) supplemented with LIF (leukemia inhibitory factor), followed by fluorescence immunization Perform staining.
- -Group 3 Fluorescent immunostaining is performed after continuous culturing for 3 days in a medium for ES cells without addition of LIF.
- Example 8 Myocardial sheet transplantation for disease model rats 10-13 weeks old, 250-330 g athymic immunodeficient rats (F344 / N Jcl-rnu / rnu) (CLEA, Japan) An infarct (MI) model was created. The rat was subjected to respiratory management with a rat ventilator and anesthetized by isoflurane aspiration. Subsequently, the heart was exposed by pericardiotomy through left intercostal thoracotomy under artificial respiration with a small amount of oxygen, and the anterior descending branch was ligated with 6-0 polypropylene thread at the periphery of the first septal branch.
- MI infarct
- the wound was closed with 4-0 polypropylene yarn.
- the presence or absence of MI was confirmed by cardiac ultrasonography (Vivid 7, GE Yokogawa Medical). Those with a left ventricular diameter shortening rate (FS) exceeding 40% were excluded as inappropriate models.
- the myocardial sheet (FIG. 8) prepared from EMG7 was transplanted 7 days after the introduction of MI. For transplantation, three myocardial sheets were laminated and used by the method described above (FIG. 5).
- an MI model rat was introduced with anesthesia with diethyl ether, and then respiratory management was performed with a rat ventilator, and anesthesia was maintained with isoflurane.
- Thoracotomy was performed with left intercostal thoracotomy and adhesion with the lungs and chest wall was carefully peeled to expose the myocardial infarction, and a laminated myocardial sheet was transplanted into the infarct. After standing for 15 minutes, the wound was closed with 4-0 polypropylene yarn.
- the Sham operation group was exposed in the same manner to myocardial infarction, and similarly closed after 15 minutes. All 12 cases observed until 4 weeks after transplantation survived.
- Example 10 Histological evaluation by transplantation of myocardial sheet Graft cell engraftment over time, localization of engraftment cells, and evaluation of changes in graft-derived cardiomyocyte morphology (maturation) The following method was carried out at 1 day, 4 weeks. After induction of anesthesia with diethyl ether, respiratory management was performed with a rat ventilator and anesthesia was maintained with isoflurane. The chest was opened at the midline, and the superior vena cava, left superior vena cava and inferior vena cava were secured and blocked. A 23G needle was inserted into the apex of the heart, and the left ventricular blood was drained by physiological saline injection.
- the right atrium was opened to prevent overexpansion and edema of the heart.
- 4% PFA was similarly injected for 45 minutes to fix the tissue (perfusion fixation method).
- the heart was removed, infiltrated with 4% PFA, and after 4 ° C over night, it was infiltrated with 15% sucrose solution (4 ° C, 2 changes, total 24 hours). Freeze embedding was performed using a solution obtained by adding dry ice to isopentane and an OCT compound.
- a 6 ⁇ m section was prepared at the center of the infarcted region, blocked with a blocking agent (Protein Block Serum-Free, DAKO), and then antibody (primary antibody: mouse anti-cTnT, rabbit anti-GFP and secondary anti-GFP).
- Primary antibody mouse anti-cTnT, rabbit anti-GFP and secondary anti-GFP.
- Antibody GFP-positive cardiomyocytes were labeled using anti-mouse Alexa Floor 546 or anti-rat Alexa Floor 488) and observed using a fluorescence microscope (BZ-9000, Keyence).
- nexin 43 is labeled on the 1-week post-transplant model (primary antibody: rabbit anti-connexin 43, secondary antibody: anti-rabbit Alexa Floor 488), and the presence or absence of the expression of gap junction at the graft site is fluorescent.
- mice cells in rat heart tissue were detected by the following method using a FISH (fluorescence in situ hybridization) probe that recognizes a species-specific repetitive sequence.
- FISH fluorescence in situ hybridization
- Rat genomic DNA FISH probe (Cy5 label) and mouse genomic DNA FISH probe (digoxigenin label) manufactured by Chromosome Science Lab were used as the above probes.
- the tissue sections were used after pretreatment by the following method. After washing with PBS, it was fixed with 4% PFA / PBS for 15 minutes, washed with PBS, then dehydrated and dried with an alcohol series.
- the hybridized chromosome specimen was subjected to stringency wash with 50% formamide / 2 ⁇ SSC at 37 ° C., and then rat and mouse nuclear signals were detected by anti-Dig-Cy3 using the signal of mouse genomic DNA FISH probe.
- a solution prepared by diluting mouse anti-cTnT with Can Get Signal Solution 1 (TOYOBO) to a specified concentration (1: 200) was added dropwise to the FISH-finished section and reacted at 37 ° C. for 1 hour.
- the plate was washed with PBST for 5 minutes ⁇ 3 times, and 1: 500 diluted secondary antibodies (anti-rabbit-Alexa 488 and anti-mouse-Alexa 594) were added with Can Get Signal Solution 2 and reacted for 30 minutes to perform immunostaining. It was. After the reaction, the plate was washed with PBST for 5 minutes ⁇ 3 times, and nuclear staining was also performed with DAPI. After staining, the genomic DNA probe signal and the fluorescently labeled antibody were microscopically observed using a Leica CW-4000 system. As a result, the number of engrafted graft cells derived from mice decreased with time, and only a small number were observed on the 28th day.
- the site engrafted after the 7th day was mainly the periphery of the infarct where more recipient myocardium remained.
- the cardiomyocyte engraftment site it was confirmed that a gap junction was being formed on the seventh day after transplantation (FIG. 11).
- cTnT cardiac troponin T
- cardiomyocyte sarcomere supermolecular aggregate
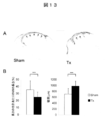
- Example 11 Evaluation of left ventricular remodeling after infarction by myocardial sheet transplantation
- 5 sections were prepared for each individual at 50 ⁇ m intervals from the center of the infarction at a thickness of 6 ⁇ m, stained with Sirius red, and fluorescent. Microscopic observation was performed. The intraventricular cavity length and infarct site length were measured, and the ratio of infarct site was calculated. The infarct site area was divided by the infarct site length to calculate the infarct site average wall thickness.
- the treatment group and the Sham group were each performed (5 individuals each) and compared.
- the number of neovascularization (capillary density) in the treatment group and the Sham group at the 4th week was measured by comparing the infarct site into the central part (Central-MI) and the peripheral part (Peri-MI) (for each three individuals). , Random 5 views each).
- Masson trichrome staining was performed for one example of the treatment group in order to illustrate the central portion and the peripheral portion.
- the distribution of neovascularization the presence or absence of neovascularization at the graft-derived cardiomyocyte engraftment portion at the stage of 1st, 3rd, 1st week was observed.
- vWF positive cells were scattered inside the graft site, and on the third day, accumulation of vWF positive cells was observed so as to surround the myocardial cell mass of the graft site from the inside. Did not show an obvious lumen structure. Since mouse signals are not shown, accumulation from the recipient (rat) side is considered. On day 7 after transplantation, the accumulation shown on day 3 was no longer observed. Furthermore, when the state on the third day was observed at a high magnification, a partially vWF-positive luminal structure was observed in the graft myocardial cell mass, and erythrocytes were observed inside. It showed that it was growing.
- FISH and vWF immunostaining were performed simultaneously at the same site, indicating that mouse-derived cells were taken up in part of the new blood vessels. It was considered that not only recipient cells but also graft-derived cells contributed to neovascularization (FIG. 14). As described above, the graft-derived cell engraftment itself is considerably reduced at 4 weeks after transplantation, but the capillary density showed that angiogenesis was significantly promoted in the treatment group. It was also shown that the angiogenesis occurred more frequently around the infarct in the treatment group (FIG. 15).
- the myocardial sheet of the present invention can be transplanted to a diseased part of a patient's heart disease to proliferate and engraft normal cardiomyocytes and promote angiogenesis with blood flow. Therefore, the present invention is applied to regenerative medicine for the treatment of heart diseases such as heart failure, ischemic heart disease, myocardial infarction, cardiomyopathy, myocarditis, hypertrophic cardiomyopathy, dilated phase hypertrophic cardiomyopathy, dilated cardiomyopathy and the like.
- the myocardial sheet can be used.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Cell Biology (AREA)
- General Health & Medical Sciences (AREA)
- Zoology (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Biotechnology (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Developmental Biology & Embryology (AREA)
- Pharmacology & Pharmacy (AREA)
- Genetics & Genomics (AREA)
- Wood Science & Technology (AREA)
- Transplantation (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Dermatology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Cardiology (AREA)
- Vascular Medicine (AREA)
- Immunology (AREA)
- Virology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Botany (AREA)
- Rheumatology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Heart & Thoracic Surgery (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/009,018 US9623052B2 (en) | 2011-03-30 | 2012-03-30 | Method for producing myocardial sheet from embryonic stem cell |
| EP12763936.7A EP2692859B1 (en) | 2011-03-30 | 2012-03-30 | Method for producing cardiomyocyte sheet from embryonic stem cells |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011-076235 | 2011-03-30 | ||
| JP2011076235A JP5840855B2 (ja) | 2011-03-30 | 2011-03-30 | 胚性幹細胞から心筋シートを製造する方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012133945A1 true WO2012133945A1 (ja) | 2012-10-04 |
Family
ID=46931617
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/059427 Ceased WO2012133945A1 (ja) | 2011-03-30 | 2012-03-30 | 胚性幹細胞から心筋シートを製造する方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US9623052B2 (enExample) |
| EP (1) | EP2692859B1 (enExample) |
| JP (1) | JP5840855B2 (enExample) |
| WO (1) | WO2012133945A1 (enExample) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013137491A1 (ja) * | 2012-03-15 | 2013-09-19 | 国立大学法人京都大学 | 人工多能性幹細胞から心筋および血管系混合細胞群を製造する方法 |
| WO2014192909A1 (ja) | 2013-05-31 | 2014-12-04 | iHeart Japan株式会社 | ハイドロゲルを組み込んだ積層化細胞シート |
| WO2017038562A1 (ja) * | 2015-08-31 | 2017-03-09 | 学校法人東京女子医科大学 | 多能性幹細胞を減少させる方法、多能性幹細胞を減少させた細胞集団の製造方法 |
| WO2018101466A1 (ja) | 2016-12-02 | 2018-06-07 | タカラバイオ株式会社 | 内皮細胞の製造方法 |
| WO2019189554A1 (ja) | 2018-03-30 | 2019-10-03 | 国立大学法人京都大学 | 心筋細胞成熟促進剤 |
| WO2019189553A1 (ja) | 2018-03-30 | 2019-10-03 | 国立大学法人京都大学 | 複素環化合物 |
| US11124770B2 (en) * | 2014-11-12 | 2021-09-21 | Terumo Kabushiki Kaisha | Myocardial cell sheet |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9598668B2 (en) | 2008-10-14 | 2017-03-21 | Cellseed Inc. | Temperature-responsive cell culture substrate and method for producing the same |
| JP6248617B2 (ja) * | 2013-12-25 | 2017-12-20 | 大日本印刷株式会社 | 細胞シートの製造方法 |
| EP3138906B1 (en) * | 2014-05-01 | 2019-08-21 | iHeart Japan Corporation | Cd82-positive cardiac progenitor cells |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5843780A (en) | 1995-01-20 | 1998-12-01 | Wisconsin Alumni Research Foundation | Primate embryonic stem cells |
| WO2002008387A1 (en) | 2000-07-21 | 2002-01-31 | Cellseed Inc. | Heart muscle-like cell sheet, three-dimensional construct, heart muscle-like tissue and process for producing the same |
| WO2005033298A1 (ja) | 2003-10-03 | 2005-04-14 | Keiichi Fukuda | 幹細胞から心筋細胞を分化誘導する方法 |
| WO2006022377A1 (ja) | 2004-08-27 | 2006-03-02 | Daiichi Asubio Pharma Co., Ltd. | 細胞内ミトコンドリアを指標とした心筋細胞の選択方法 |
| WO2007002136A2 (en) | 2005-06-22 | 2007-01-04 | Geron Corporation | Differentiation of primate pluripotent stem cells to cardiomyocyte-lineage cells |
| WO2007088874A1 (ja) | 2006-01-31 | 2007-08-09 | Asubio Pharma Co., Ltd. | 幹細胞及び胎児由来の心筋細胞及び予定心筋細胞の精製方法 |
| JP2007528755A (ja) | 2003-08-01 | 2007-10-18 | 株式会社カルディオ | 三次元組織構造体 |
| WO2007126077A1 (ja) | 2006-04-28 | 2007-11-08 | Asubio Pharma Co., Ltd. | 多能性幹細胞から心筋細胞を分化誘導する方法 |
| WO2009118928A1 (en) | 2008-03-26 | 2009-10-01 | Kyoto University | Efficient production and use of highly cardiogenic progenitors and cardiomyocytes from embryonic and induced pluripotent stem cells |
| JP2010255001A (ja) | 2010-06-28 | 2010-11-11 | Cellseed Inc | アクリルアミド誘導体および該誘導体を含む重合体 |
| JP2011076235A (ja) | 2009-09-29 | 2011-04-14 | Brother Industries Ltd | サーバに接続されるプリンタ及びサーバ |
-
2011
- 2011-03-30 JP JP2011076235A patent/JP5840855B2/ja not_active Expired - Fee Related
-
2012
- 2012-03-30 US US14/009,018 patent/US9623052B2/en not_active Expired - Fee Related
- 2012-03-30 EP EP12763936.7A patent/EP2692859B1/en not_active Not-in-force
- 2012-03-30 WO PCT/JP2012/059427 patent/WO2012133945A1/ja not_active Ceased
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5843780A (en) | 1995-01-20 | 1998-12-01 | Wisconsin Alumni Research Foundation | Primate embryonic stem cells |
| WO2002008387A1 (en) | 2000-07-21 | 2002-01-31 | Cellseed Inc. | Heart muscle-like cell sheet, three-dimensional construct, heart muscle-like tissue and process for producing the same |
| JP2007528755A (ja) | 2003-08-01 | 2007-10-18 | 株式会社カルディオ | 三次元組織構造体 |
| WO2005033298A1 (ja) | 2003-10-03 | 2005-04-14 | Keiichi Fukuda | 幹細胞から心筋細胞を分化誘導する方法 |
| WO2006022377A1 (ja) | 2004-08-27 | 2006-03-02 | Daiichi Asubio Pharma Co., Ltd. | 細胞内ミトコンドリアを指標とした心筋細胞の選択方法 |
| WO2007002136A2 (en) | 2005-06-22 | 2007-01-04 | Geron Corporation | Differentiation of primate pluripotent stem cells to cardiomyocyte-lineage cells |
| WO2007088874A1 (ja) | 2006-01-31 | 2007-08-09 | Asubio Pharma Co., Ltd. | 幹細胞及び胎児由来の心筋細胞及び予定心筋細胞の精製方法 |
| WO2007126077A1 (ja) | 2006-04-28 | 2007-11-08 | Asubio Pharma Co., Ltd. | 多能性幹細胞から心筋細胞を分化誘導する方法 |
| WO2009118928A1 (en) | 2008-03-26 | 2009-10-01 | Kyoto University | Efficient production and use of highly cardiogenic progenitors and cardiomyocytes from embryonic and induced pluripotent stem cells |
| JP2011076235A (ja) | 2009-09-29 | 2011-04-14 | Brother Industries Ltd | サーバに接続されるプリンタ及びサーバ |
| JP2010255001A (ja) | 2010-06-28 | 2010-11-11 | Cellseed Inc | アクリルアミド誘導体および該誘導体を含む重合体 |
Non-Patent Citations (33)
| Title |
|---|
| 0. FUMITAKA ET AL., NAT. BIOTECHNOL., vol. 26, 2008, pages 215 - 224 |
| BEL, A ET AL., CIRCULATION, vol. 122, 2010, pages 118 - 23 |
| E. KROON ET AL., NAT. BIOTECHNOL., vol. 26, 2008, pages 443 - 452 |
| H. KAWASAKI ET AL., PROC. NATL. ACAD. SCI. U.S.A., vol. 99, 2002, pages 1580 - 1585 |
| H. SUEMORI ET AL., BIOCHEM. BIOPHYS. RES. COMMUN., vol. 345, 2006, pages 926 - 932 |
| H. SUEMORI ET AL., DEV. DYN., vol. 222, 2001, pages 273 - 279 |
| HONDA M ET AL., BIOCHEM BIOPHYS RES COMMUN., vol. 29, no. 351, 2006, pages 877 - 82 |
| J. A. THOMSON ET AL., BIOL. REPROD., vol. 55, 1996, pages 254 - 259 |
| J. A. THOMSON ET AL., PROC. NATL. ACAD. SCI. U.S.A., vol. 92, 1995, pages 7844 - 7848 |
| J. A. THOMSON; V. S. MARSHALL, CURR. TOP. DEV. BIOL., vol. 38, 1998, pages 133 - 165 |
| J.A: THOMSON ET AL., SCIENCE, vol. 282, 1998, pages 1145 - 1147 |
| KLIMANSKAYA I ET AL., NATURE, vol. 444, 2006, pages 481 - 485 |
| KOBAYASHI H. ET AL.: "Fibroblast sheets co- cultured with endothelial progenitor cells improve cardiac function of infarcted hearts.", JOURNAL OF ARTIFICIAL ORGANS, vol. 11, 2008, pages 141 - 147, XP019635651 * |
| M. UENO ET AL., PROC. NATL. ACAD. SCI. U.S.A., vol. 103, 2006, pages 9554 - 9559 |
| M.J. EVANS; M.H. KAUFMAN, NATURE, vol. 292, 1981, pages 154 - 156 |
| MASUMOTO H. ET AL.: "Embryonic Stem (ES) Cell- engineered Tissue Sheets with Defined Cardiac Cell Populations Ameliorate Function after Myocardial Infarction.", CIRCULATION JOURNAL 1346-9843, vol. 75, no. SUP.1, 1 March 2011 (2011-03-01), pages 610, XP008171083 * |
| See also references of EP2692859A4 * |
| SEKINE H. ET AL.: "Endothelial Cell Coculture Within Tissue-Engineered Cardiomyocyte Sheets Enhances Neovascularization and Improves Cardiac Function of Ischemic Hearts.", CIRCULATION, vol. 118, no. SUP.1, 2008, pages S145 - S152, XP002680531 * |
| SHIMIZU, T ET AL., BIOMATERIALS, vol. 24, 2003, pages 2309 - 2316 |
| SHIMIZU, T ET AL., FASEB J., vol. 20, 2006, pages 708 - 10 |
| SHUICHI SEKINE ET AL.: "Kyoketsusei Shinfuzen ni Taisuru Naihi-Shinkin Kyobaiyo Saibo Sheet no Ishoku", DAI 19 KAI JAPANESE ASSOCIATION OF CARDIOVASCULAR PHARMACOLOGY KOEN YOSHISHU, vol. 19, 2009, pages 34, XP008170821 * |
| SHUICHI SEKINE ET AL.: "Naihi-Shinkin Kyobaiyo Saibo Sheet Ishoku ni yoru Saisei Shinkin Soshikinai Kekkanmo Shinsei no Sokushin to Shinkino Kaizen Koka", JAPAN RESEARCH PROMOTION SOCIETY FOR CARDIOVASCULAR DISEASE, HEISEI 18 NENDO KENKYU GYOSEKISHU, 2006, pages 5 - 8, XP008170823 * |
| TATSUYA SHIMIZU ET AL.: "Shinkin-Naihi Kyobaiyo Saibo Sheet o Mochiita Kekkanmo no Seigyo", KOSEI RODO KAGAKU KENKYUHI HOJOKIN HITO GENOME SAISEI IRYO-TO KENKYU JIGYO, KAN'YOKEI KANSAIBO O MOCHIITA SHINKIN KEKKAN SAISEI RYOHO NO KISO OYOBI RINSHO KENKYU, HEISEI 17 NENDO SOKATSU BUNTAN KENKYU HOKOKUSHO, 2006, pages 17 - 18, XP008170818 * |
| THOMSON JA ET AL., PROC NATL. ACAD. SCI. U.S.A., vol. 92, 1995, pages 7844 - 7848 |
| THOMSON JA ET AL., SCIENCE, vol. 282, 1998, pages 1145 - 1147 |
| YAMASHITA J ET AL., NATURE, vol. 408, 2000, pages 92 - 6 |
| YAMASHITA J. ET AL.: "Flkl-positive cells derived from embryonic stem cells serve as vascular progenitors.", NATURE, vol. 408, 2000, pages 92 - 96, XP001146921 * |
| YAMASHITA JK ET AL., FASEB J., vol. 19, 2005, pages 1534 - 6 |
| YAN P ET AL., BIOCHEM BIOPHYS RES COMMUN., vol. 379, 2009, pages 115 - 20 |
| YAN P. ET AL.: "Cyclosporin-A potently induces highly cardiogenic progenitors from embryonic stem cells.", BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS, vol. 379, 2009, pages 115 - 120, XP025770136 * |
| YANG L. ET AL.: "Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell- derived population.", NATURE, vol. 453, 2008, pages 524 - 528, XP002561095 * |
| YURUGI-KOBAYASHI T ET AL., ARTERIOSCLER THROMB VASE BIOL., vol. 26, 2006, pages 1977 - 84 |
| YURUGI-KOBAYASHI T. ET AL.: "Adrenomedullin/ Cyclic AMP Pathway Induces Notch Activation and Differentiation of Arterial Endothelial Cells From Vascular Progenitors.", ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY, vol. 26, 2006, pages 1977 - 1984, XP055118884 * |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013137491A1 (ja) * | 2012-03-15 | 2013-09-19 | 国立大学法人京都大学 | 人工多能性幹細胞から心筋および血管系混合細胞群を製造する方法 |
| WO2014192909A1 (ja) | 2013-05-31 | 2014-12-04 | iHeart Japan株式会社 | ハイドロゲルを組み込んだ積層化細胞シート |
| CN105247041A (zh) * | 2013-05-31 | 2016-01-13 | 爱心细胞有限公司 | 组入有水凝胶的层叠细胞膜片 |
| EP3006559A4 (en) * | 2013-05-31 | 2017-01-04 | iHeart Japan Corporation | Layered cell sheet incorporating hydrogel |
| CN105247041B (zh) * | 2013-05-31 | 2018-04-20 | 爱心细胞有限公司 | 组入有水凝胶的层叠细胞膜片 |
| US10159766B2 (en) | 2013-05-31 | 2018-12-25 | Iheart Japan Corporation | Layered cell sheet incorporating hydrogel |
| US11124770B2 (en) * | 2014-11-12 | 2021-09-21 | Terumo Kabushiki Kaisha | Myocardial cell sheet |
| US10711247B2 (en) | 2015-08-31 | 2020-07-14 | Tokyo Women's Medical University | Method for reducing pluripotent stem cells, method for producing cell population having reduced pluripotent stem cells |
| WO2017038562A1 (ja) * | 2015-08-31 | 2017-03-09 | 学校法人東京女子医科大学 | 多能性幹細胞を減少させる方法、多能性幹細胞を減少させた細胞集団の製造方法 |
| JPWO2017038562A1 (ja) * | 2015-08-31 | 2018-06-21 | 学校法人東京女子医科大学 | 多能性幹細胞を減少させる方法、多能性幹細胞を減少させた細胞集団の製造方法 |
| WO2018101466A1 (ja) | 2016-12-02 | 2018-06-07 | タカラバイオ株式会社 | 内皮細胞の製造方法 |
| KR20190079686A (ko) | 2016-12-02 | 2019-07-05 | 다카라 바이오 가부시키가이샤 | 내피세포의 제조 방법 |
| US11225643B2 (en) | 2016-12-02 | 2022-01-18 | Takara Bio Inc. | Method for producing endothelial cells |
| WO2019189553A1 (ja) | 2018-03-30 | 2019-10-03 | 国立大学法人京都大学 | 複素環化合物 |
| WO2019189554A1 (ja) | 2018-03-30 | 2019-10-03 | 国立大学法人京都大学 | 心筋細胞成熟促進剤 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5840855B2 (ja) | 2016-01-06 |
| US20140056859A1 (en) | 2014-02-27 |
| US9623052B2 (en) | 2017-04-18 |
| EP2692859A1 (en) | 2014-02-05 |
| JP2012210156A (ja) | 2012-11-01 |
| EP2692859A4 (en) | 2015-01-07 |
| EP2692859B1 (en) | 2016-12-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5840855B2 (ja) | 胚性幹細胞から心筋シートを製造する方法 | |
| JP5671670B2 (ja) | 間葉系幹細胞を含む細胞シート | |
| Du et al. | Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering | |
| Wanjare et al. | Perivascular cells in blood vessel regeneration | |
| JP5920741B2 (ja) | 人工多能性幹細胞から心筋および血管系混合細胞群を製造する方法 | |
| JP6832049B2 (ja) | 心臓神経堤細胞、及びその使用方法 | |
| Mendes et al. | Perivascular-like cells contribute to the stability of the vascular network of osteogenic tissue formed from cell sheet-based constructs | |
| Yeh et al. | Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model | |
| JP2020000245A (ja) | シート状細胞培養物の製造方法 | |
| US20040009589A1 (en) | Endothelial cells derived from human embryonic stem cells | |
| Zakharova et al. | Endothelial and smooth muscle cells derived from human cardiac explants demonstrate angiogenic potential and suitable for design of cell-containing vascular grafts | |
| WO2012118099A1 (ja) | サイトカイン産生細胞シートとその利用方法 | |
| US11666604B2 (en) | Multilayered cell sheet of cardiac stem cells and method of preparing the same | |
| US9428735B2 (en) | Smooth muscle-like cells (SMLCs) dervided from human pluripotent stem cells | |
| Meijer et al. | 3D Human iPSC Blood Vessel Organoids as a Source of Flow‐Adaptive Vascular Cells for Creating a Human‐Relevant 3D‐Scaffold Based Macrovessel Model | |
| He et al. | Generation of alveolar epithelium using reconstituted basement membrane and hiPSC‐derived organoids | |
| JP6670040B2 (ja) | シート状細胞培養物の製造方法 | |
| KR20110024660A (ko) | 인간 배아줄기세포 또는 인간 유도만능줄기세포의 혈관내피세포로의 혈청 및 이종 비함유 분화방법 | |
| WO2005100549A1 (ja) | 臓器特異的幹細胞の増殖方法及び増殖装置 | |
| WO2021065984A1 (ja) | 心筋細胞のシート化方法 | |
| US20240368552A1 (en) | Pre-epicardial cells and uses thereof | |
| US20200095557A1 (en) | Cell spheroids containing capillary structures and methods of using same | |
| Colunga | Human pluripotent stem cell-derived mesothelium functions inregenerative medicine as a multipotent vascular progenitor | |
| Macklin | Defining the Regenerative Potential of Vascular Stem Cell Derivatives | |
| Glaser | Defining the Microenvironment for Directing Embryonic Stem Cells into Endothelial Cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12763936 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14009018 Country of ref document: US |
|
| REEP | Request for entry into the european phase |
Ref document number: 2012763936 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012763936 Country of ref document: EP |