WO2011158933A1 - 腎線維症処置剤 - Google Patents
腎線維症処置剤 Download PDFInfo
- Publication number
- WO2011158933A1 WO2011158933A1 PCT/JP2011/063910 JP2011063910W WO2011158933A1 WO 2011158933 A1 WO2011158933 A1 WO 2011158933A1 JP 2011063910 W JP2011063910 W JP 2011063910W WO 2011158933 A1 WO2011158933 A1 WO 2011158933A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- kidney
- extracellular matrix
- carrier
- producing cells
- retinoid
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/045—Hydroxy compounds, e.g. alcohols; Salts thereof, e.g. alcoholates
- A61K31/07—Retinol compounds, e.g. vitamin A
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/10—Alcohols; Phenols; Salts thereof, e.g. glycerol; Polyethylene glycols [PEG]; Poloxamers; PEG/POE alkyl ethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/542—Carboxylic acids, e.g. a fatty acid or an amino acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6905—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion
- A61K47/6911—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion the form being a liposome
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6905—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion
- A61K47/6919—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion the form being a ribbon or a tubule cochleate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6921—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Liposomes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/111—General methods applicable to biologically active non-coding nucleic acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering N.A.
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2320/00—Applications; Uses
- C12N2320/30—Special therapeutic applications
- C12N2320/32—Special delivery means, e.g. tissue-specific
Definitions
- the present invention relates to a substance delivery carrier that targets extracellular matrix-producing cells in the kidney, a composition for treating renal fibrosis and a method for treating renal fibrosis using the same.
- the kidney is an important organ for maintaining the homeostasis of the internal environment by excretion of waste products and regulation of body fluid, electrolyte, and acid-base balance.
- the kidney controls the concentration of various compounds in the blood, such as hydrogen, sodium, potassium and silicon, and excretes waste products in the form of urine. Any decrease in renal function can interfere with the body's ability to sufficiently remove metabolites from the blood and can disrupt the body's electrolyte balance. Reduction or failure of kidney function can be fatal in its most severe form.
- the kidney has functions such as cellular immunity, endocrine function, and metabolism.
- the kidney as an endocrine organ produces 1,25 dihydroxyvitamin D3, which is an active vitamin D3, and erythropoietin that regulates red blood cell production, renin and erythropoietin, which are blood pressure regulators, kinin, kallikrein, and prostaglandins.
- renin and erythropoietin which are blood pressure regulators, kinin, kallikrein, and prostaglandins.
- Chronic renal failure is a state in which these renal functions have gradually declined irreversibly, making it impossible to maintain homeostasis.
- Chronic renal failure is known to be caused by diabetic nephropathy, chronic glomerulonephritis, malignant nephrosclerosis, polycystic kidney disease, and the like. All kidney diseases are accompanied by renal fibrosis and eventually lead to end stage renal failure. In particular, since chronic decrease in renal function is deeply related to the progression of renal fibrosis, inhibition of fibrosis progression is thought to lead to suppression of progression of chronic renal failure.
- renal fibrosis is caused by an inflammatory reaction resulting from endothelial cell damage, and finally fibrosis caused by an excessively produced extracellular matrix.
- glomerular endothelial cells are damaged, cytokines such as chemokines and growth factors are secreted, and monocytes and macrophages migrate to promote an inflammatory reaction.
- cytokines such as chemokines and growth factors
- monocytes and macrophages migrate to promote an inflammatory reaction.
- activation, proliferation, and transformation of mesangial cells occur, and an excessive amount of extracellular matrix is produced from extracellular matrix-producing cells such as mesangial cells, and fibrosis occurs, leading to glomerulosclerosis.
- diabetic nephropathy is the biggest cause of chronic renal failure and is one of the serious complications of diabetes.
- the characteristic of diabetic nephropathy is the growth and expansion of glomerular mesangium, which is mainly due to increased accumulation of ECM proteins such as type I and type IV collagen, fibronectin, and laminin.
- uremic disease occurs if treatment such as dialysis is not performed.
- treatment methods for chronic renal failure dietary therapy such as a low protein diet and salt restriction, and administration of an antihypertensive agent to prevent the glomerulus from being loaded are first performed.
- angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists are used to suppress the progression of renal failure.
- these are thought to have a renoprotective effect on their own.
- the glomerular blood pressure is lowered too much, the glomerular blood flow will be reduced and prerenal renal failure will occur, which requires careful administration.
- angiotensin converting enzyme inhibitors examples include captolyl, enalapril, delapril, imidapril, quinapril, temocapril, perindopril erbumine, and lisinopril.
- Angiotensin II receptor antagonists include losartan, valsartan, candesartan cilexetil, olmisartan, olmisartan, Medoxomil and irbesartan.
- cremedin is used as an adsorbed charcoal that adsorbs harmful substances in the intestines in order to improve the symptoms of uremia and delay the introduction of dialysis.
- calcium polystyrene sulfonate is used as an ion exchange resin that adsorbs potassium in the intestine and excretes it together with feces.
- an angiotensin converting enzyme inhibitor an AT1 angiotensin II receptor antagonist, an aldosterone antagonist, a drug that acts on the renin-angiotensin-aldosterone system such as a renin inhibitor, an endothelin receptor antagonist, an ⁇ blocker, a ⁇ Blocking agents, immunosuppressive agents, extracellular matrix metabolism inhibitors, complement system inhibitors, chemokine inhibitors, phosphodiesterase 5 inhibitors and other drugs that act on the nitric oxide system, NF ⁇ B inhibitors, Rho inhibitors, p38 MAPK inhibitors , PI3K ⁇ inhibitor, vascular endothelial growth factor (VEGF) inhibitor, kallikrein, relaxin, interleukin 1 receptor antagonist, bone morphogenetic factor 7 (BMP-7), anti-tumor necrosis factor ⁇ antibody (Anti-TNF ⁇ antibody
- angiotensin converting enzyme inhibitor Patent Document 1
- angiotensin II receptor antagonist Patent Document 2
- TGF- ⁇ transforming growth factor ⁇
- Patent Document 3 plasminogen activator inhibitor 1 (PAI-1) production inhibitor
- Patent Document 4 prostaglandin receptor 4 selective agonist
- Patent Document 5 type I collagen synthesis inhibitor
- Patent Document 6 Chondroitin sulfate proteoglycan sulfate transferase inhibitor
- Patent Document 8 vitamin K epoxide reductase inhibitor
- glycation end product formation inhibitor Patent Document 9
- A2A adenosine receptor 2A agonist Patent Document 10
- Endothelin receptor antagonist Patent Document 11
- VEGF inhibitor Patent Document 12
- An object of the present invention is to provide a carrier capable of specifically delivering a substance such as a drug to extracellular matrix-producing cells in the kidney, a therapeutic agent for renal fibrosis and a method for treating renal fibrosis using the carrier.
- the present inventors have effectively treated renal fibrosis by administering a composition comprising an extracellular matrix production inhibitor supported on a carrier containing a retinoid as a targeting agent.
- the present invention has been completed.
- Carriers containing vitamin A deliver drugs to stellate cells that store vitamin A (patent document 13), and compositions in which siRNA for HSP47 is supported on the same carrier are liver fibrosis (patent document 13), lung Although it has been known to improve fibrosis (Patent Document 14) and myelofibrosis (Patent Document 15), the relationship with renal fibrosis, renal interstitial tissue, and mesangium has never been known. It was.
- the present invention relates to the following.
- a carrier for substance delivery to an extracellular matrix-producing cell in the kidney comprising a retinoid as a targeting agent for the extracellular matrix-producing cell in the kidney.
- the carrier according to (1) above wherein the retinoid contains retinol.
- the carrier according to (1) or (2) above which has a liposome form and has a molar ratio of retinoid to lipid contained in the liposome of 8: 1 to 1: 4.
- a pharmaceutical composition for treating renal fibrosis comprising the carrier according to any one of (1) to (3) above and a drug that controls the activity or proliferation of extracellular matrix-producing cells in the kidney.
- Drugs that control the activity or proliferation of extracellular matrix-producing cells in the kidney are PAI-1 activity or production inhibitors, cell activity inhibitors, growth inhibitors, apoptosis inducers, and extracellular matrix constituent molecules Or selected from the group consisting of RNAi molecules, ribozymes, antisense nucleic acids, DNA / RNA chimeric polynucleotides targeting at least one molecule involved in the production or secretion of the extracellular matrix constituent molecules, and vectors expressing them.
- a method for producing a carrier for delivering a substance to an extracellular matrix-producing cell in the kidney comprising a step of blending a retinoid as a targeting agent to the extracellular matrix-producing cell in the kidney.
- a method for producing a pharmaceutical composition comprising a step of incorporating a retinoid as a targeting agent to extracellular matrix-producing cells in the kidney and a drug that controls the activity or proliferation of extracellular matrix-producing cells in the kidney as an active ingredient.
- retinoids are targeted to extracellular matrix-producing cells in the kidney such as fibroblasts and myofibroblasts. It is considered that an anti-renal fibrosis effect is exerted by delivering an active ingredient such as a drug that controls the activity or proliferation of extracellular matrix-producing cells in the kidney to the same cell. Therefore, since the active ingredient can be efficiently delivered to the site of action and further to the target cell by the carrier of the present invention, it is possible to cure or suppress the progression of renal fibrosis, particularly diabetic nephritis, which has been difficult to treat until now.
- the carrier of the present invention can be combined with an arbitrary drug (for example, an existing therapeutic drug for renal fibrosis) to increase its action efficiency, it has a wide range of pharmaceutical applications and can produce an effective therapeutic agent. There is also an advantage that it can be performed easily.
- FIG. 1 shows representative microscopic images of glomeruli by Sirius Red staining of kidney cortex sections of mice in each group. Images were taken at a magnification of 800 times using an oil immersion lens.
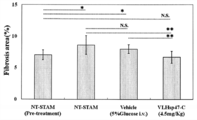
- FIG. 2 is a graph showing the ratio of the fibrosis region of the renal cortex quantified by Sirius red staining. Twenty visual fields of the renal cortex area were randomly photographed for each mouse, and the ratio of fibrosis area (Fibrosis area (%)) was calculated ( * P ⁇ 0.05, ** P ⁇ 0.01).
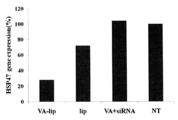
- FIG. 3 is a graph showing gene knockdown by siRNA-containing VA-bound liposomes in mouse kidney extracellular matrix-producing cells.
- HSP47 gene expression was corrected by the GAPDH expression level of the internal control gene, and No treatment (untreated) was taken as 100% (HSP47 gene expression (%) ) was graphed.
- VA-lip indicates VA-liposome-siRNA Hsp47C
- lip indicates liposome-siRNA Hsp47C
- VA + siRNA indicates VA + siRNA Hsp47C
- NT indicates No treatment.
- the extracellular matrix-producing cell in the kidney is not particularly limited as long as it is a cell having an extracellular matrix-producing ability present in the kidney.
- mesangial cells, tubulointerstitial cells, peripheral cells present in the kidney It includes skin cells, fibroblasts, fibrosite and myofibroblasts which are fibroblast precursor cells.
- Matrix-producing cells present in the kidney include not only those derived from cells present in the kidney, but also those derived from fibrocytes in the circulating blood and those converted from endothelial cells by endothelial mesenchymal transdifferentiation. obtain.
- Myofibroblasts are characterized by the expression of ⁇ SMA ( ⁇ smooth muscle actin).
- the myofibroblast in the present invention is identified by immunostaining using an anti- ⁇ SMA antibody that is detectably labeled.
- Fibroblasts express vimentin characteristic of mesenchymal cells, but do not express ⁇ SMA, and therefore can be identified by double staining of vimentin and ⁇ SMA.
- Extracellular matrix-producing cells in the kidney also, after the renal tissue was treated with collagenase and protease, which a density gradient centrifugation (e.g., final concentration 8% Nycodenz (R) in) may be obtained by separating by .
- a density gradient centrifugation e.g., final concentration 8% Nycodenz (R) in
- the retinoid in the present invention functions as a targeting agent (targeting agent) to extracellular matrix-producing cells in the kidney and promotes specific substance delivery to such cells.
- targeting agent targeting agent
- RBP retinol binding protein
- a retinoid is a member of a group of compounds with a skeleton in which four isoprenoid units are linked in a head-to-tail manner (G. P. Moss, “Biochemical Nomenclature and Related Documents,” 2nd Ed. Portland Press, pp. 247-251 (1992)), vitamin A is a general descriptor of retinoids that qualitatively shows the biological activity of retinol.
- the retinoid that can be used in the present invention is not particularly limited.
- retinol including all-trans retinol
- retinal retinoic acid (including tretinoin)
- ester of retinol and fatty acid aliphatic alcohol and retinoic acid
- retinoid derivatives such as esters, etretinate, isotretinoin, adapalene, acitretin, tazarotene, retinol palmitate, and vitamin A analogs such as fenretinide (4-HPR) and bexarotene.
- retinol, retinal, retinoic acid, esters of retinol with fatty acids (eg retinyl acetate, retinyl palmitate, retinyl stearate, and retinyl laurate) and esters of fatty alcohols with retinoic acid (Eg, ethyl retinoate) is preferred in terms of the efficiency of delivery of specific substances to extracellular matrix-producing cells in the kidney. All isomers, including cis-trans of retinoids, are within the scope of the present invention.
- the retinoid may also be substituted with one or more substituents.
- the retinoid in the present invention includes not only an isolated state but also a retinoid in a state of being dissolved or mixed in a medium capable of dissolving or holding the same.
- the carrier of the present invention may be composed of these retinoids themselves, or may be composed by binding or including a retinoid in another carrier component. Therefore, the carrier of the present invention may contain carrier components other than retinoids. Such components are not particularly limited, and any of those known in the pharmaceutical and pharmaceutical fields can be used, but those that can include or bind to retinoids are preferred.
- Such components include lipids, phospholipids such as glycerophospholipids, sphingolipids such as sphingomyelin, sterols such as cholesterol, vegetable oils such as soybean oil and poppy oil, lecithins such as mineral oil and egg yolk lecithin, polymers, etc. However, it is not limited to these.
- liposomes for example, natural phospholipids such as lecithin, semisynthetic phospholipids such as dimyristoylphosphatidylcholine (DMPC), dipalmitoylphosphatidylcholine (DPPC), distearoylphosphatidylcholine (DSPC), and dioleylphosphatidylethanol Amine (DOPE), dilauroyl phosphatidylcholine (DLPC), cholesterol and the like are preferable.
- natural phospholipids such as lecithin
- semisynthetic phospholipids such as dimyristoylphosphatidylcholine (DMPC), dipalmitoylphosphatidylcholine (DPPC), distearoylphosphatidylcholine (DSPC), and dioleylphosphatidylethanol Amine (DOPE), dilauroyl phosphatidylcholine (DLPC), cholesterol and the like are preferable.
- DMPC dimyristoylphosphatidy
- Particularly preferred components include components that can avoid capture by the reticuloendothelial system, such as N- ( ⁇ -trimethylammonioacetyl) -didodecyl-D-glutamate chloride (TMAG), N, N ′, N ′′, N ′ ′′-tetramethyl-N, N ′, N ′′, N ′ ′′-tetrapalmitylspermine (TMTPS), 2,3-dioleyloxy-N- [2 (sperminecarboxamido) ethyl] -N , N-dimethyl-1-propanaminium trifluoroacetate (DOSPA), N- [1- (2,3-dioleyloxy) propyl] -N, N, N-trimethylammonium chloride (DOTMA), dioctadecyldimethyl Ammonium chloride (DODAC), didodecyl ammonium bromide (DDAB), 1,2-dioleyloxy-3-tri
- the carrier in the present invention may have a specific three-dimensional structure.
- a specific three-dimensional structure examples include, but are not limited to, a linear or branched linear structure, a film-like structure, and a spherical structure.
- the carrier may have any three-dimensional form such as, but not limited to, micelles, liposomes, emulsions, microspheres, nanospheres.
- Binding or inclusion of the retinoid to the carrier of the present invention is also possible by binding or including the retinoid to other components of the carrier by chemical and / or physical methods.
- the retinoid can be bound or included in the carrier of the present invention by mixing the retinoid and other carrier components at the time of producing the carrier.
- the amount of retinoid in the carrier of the present invention can be, for example, 0.01 to 1000 nmol / ⁇ l, preferably 0.1 to 100 nmol / ⁇ l.
- the binding or inclusion of the retinoid to the carrier may be performed before loading the drug or the like on the carrier, may be performed by mixing the carrier, the retinoid and the drug simultaneously, or the like.
- the present invention also includes a step of binding a retinoid to any existing drug-binding carrier or drug-encapsulating carrier, for example, a liposome preparation such as DaunoXome (R) , Doxil, Caelyx (R) , Myocet (R) ,
- a liposome preparation such as DaunoXome (R) , Doxil, Caelyx (R) , Myocet (R)
- the present invention also relates to a method for producing a preparation specific for extracellular matrix-producing cells in the kidney.
- the form of the carrier of the present invention may be any form as long as it can transport a desired substance or substance to the extracellular matrix-producing cells in the target kidney.
- polymer micelles, liposomes, emulsions are not limited thereto. , Microspheres, nanospheres, and the like.
- the form of liposomes is preferred, and among these, cationic liposomes containing cationic lipids are particularly preferred.
- the molar ratio of the retinoid to the other liposome-constituting lipid is preferably 8: 1 to 1: 4, considering the efficiency of binding or inclusion of the retinoid to the carrier.
- the ratio is preferably 4: 1 to 1: 2.
- the retinoid contained therein may contain a transported material inside, or may be attached to the outside of the transported material, It may be mixed with a transported item.
- functioning as a targeting agent means that a carrier containing a retinoid reaches and / or takes up extracellular matrix-producing cells in the target kidney, more rapidly and / or in a larger amount than a carrier containing no retinoid. This can be easily confirmed, for example, by adding a carrier with a label or containing a label to a culture of target cells and analyzing the site of the label after a predetermined time. .
- the above requirement can be satisfied if the retinoid is at least partially exposed to the outside of the preparation containing the carrier before reaching the target cell at the latest.
- the “formulation” is a concept including the composition of the present invention described later and further having a form. Whether or not the retinoid is exposed to the outside of the preparation is evaluated by contacting the preparation with a substance that specifically binds to the retinoid, for example, retinol binding protein (RBP) and investigating the binding to the preparation. be able to.
- a substance that specifically binds to the retinoid for example, retinol binding protein (RBP)
- At least partially exposing the retinoid to the outside of the preparation before reaching the target cell at the latest can be achieved, for example, by adjusting the mixing ratio of the retinoid and the carrier component other than the retinoid. it can.
- the carrier has a form of a lipid structure such as a liposome
- a lipid structure first comprising a carrier component other than retinoid can be diluted in an aqueous solution and then contacted and mixed with the retinoid.
- the retinoid may be in a state dissolved in a solvent, for example, an organic solvent such as DMSO.
- the lipid structure means a structure having an arbitrary three-dimensional structure, for example, a linear shape, a film shape, a spherical shape, and the like, which includes lipid as a constituent component, and is not limited to liposomes, micelles. , Lipid microspheres, lipid nanoglobules, lipid emulsions and the like.
- the same targeting agent that targeted liposomes can be applied to other drug carriers, e.g. Zhao and Lee, Adv Drug Deliv Rev. 2004; 56 (8): 1193-204, Temming et al., Drug Resist Updat. 2005; 8 (6): 381-402.
- Lipid structures for example, salts, sugars such as sucrose, glucose, maltose, polyhydric alcohols such as glycerin, propylene glycol, etc., preferably osmotic pressure is adjusted using an osmotic pressure regulator such as sucrose or glucose By doing so, it can be stabilized. Moreover, you may adjust pH by adding pH adjusters, such as a moderate salt and a buffer solution. Therefore, the production and storage of lipid structures can be performed in a medium containing these substances. In this case, the concentration of the osmotic pressure adjusting agent is preferably adjusted to be isotonic with blood.
- the concentration in the medium is not limited and may be 3 to 15% by weight, preferably 5 to 12% by weight, more preferably 8 to 10% by weight, particularly 9% by weight.
- the concentration in the medium is not limited and may be 1 to 10% by weight, preferably 3 to 8% by weight, more preferably 4 to 6% by weight, especially 5% by weight.
- the present invention also relates to a method for producing a carrier for substance delivery to extracellular matrix-producing cells in the kidney, comprising a step of blending a retinoid as a targeting agent for extracellular matrix-producing cells in the kidney.
- the retinoid compounding method is not particularly limited as long as the retinoid can function as a targeting agent to the extracellular matrix-producing cells in the kidney in the compounded carrier.
- various methods described in the present specification can be used.
- the retinoid formulation involves combining or including the retinoid with other components of the carrier by chemical and / or physical methods, or combining the retinoid and other carrier components during carrier production. It can be performed by mixing.
- the amount of retinoid compounded is as already described for the carrier of the present invention.
- the substance or object to be delivered by the carrier is not particularly limited, but is preferably sized so that it can be physically moved from the administration site to the lesion site where the target cells are present. Therefore, the carrier of the present invention carries not only substances such as atoms, molecules, compounds, proteins, and nucleic acids but also objects such as vectors, virus particles, cells, drug release systems composed of one or more elements, and micromachines. be able to.
- the substance or object preferably has a property that has some influence on the target cell, such as one that labels the target cell or one that controls (eg, enhances or suppresses) the activity or proliferation of the target cell. including.
- the substance delivered by the carrier is a “drug that controls the activity or proliferation of extracellular matrix-producing cells in the kidney”.
- the activity of extracellular matrix-producing cells in the kidney refers to various activities such as secretion, uptake, migration, etc. exhibited by the extracellular matrix-producing cells in the kidney.
- it typically, In particular, it means an activity involved in the onset, progression and / or recurrence of renal fibrosis.
- Examples of such activities include, but are not limited to, production and secretion of physiologically active substances such as PAI-1, extracellular matrix components such as collagen, proteoglycan, tenascin, fibronectin, thrombospondin, osteopontin, osteonectin, and elastin. And suppression of the degradation activity of these extracellular matrix components.
- physiologically active substances such as PAI-1
- extracellular matrix components such as collagen, proteoglycan, tenascin, fibronectin, thrombospondin, osteopontin, osteonectin, and elastin. And suppression of the degradation activity of these extracellular matrix components.
- a drug that regulates the activity or proliferation of extracellular matrix-producing cells in the kidney refers to the physical, chemical and / or cellular properties involved in the onset, progression and / or recurrence of renal fibrosis.
- any drug that directly or indirectly suppresses a physiological action or the like may be used without limitation, and a drug that inhibits the activity or production of the physiologically active substance, an antibody that neutralizes the physiologically active substance, and Including antibody fragments, RNAi molecules that suppress the expression of the physiologically active substance (eg, siRNA, shRNA, ddRNA, miRNA, piRNA, rasiRNA, etc.), ribozymes, antisense nucleic acids (RNA, DNA, PNA, or a complex thereof) ) Or a dominant negative effect such as a dominant negative mutant Substances, vectors expressing them, drugs that inhibit the production / secretion of the extracellular matrix components, such as RNAi molecules that suppress the expression of extracellular matrix components (for example, siRNA, shRNA, ddRNA, miRNA, substances such as piRNA, rasiRNA, etc.), ribozymes, antisense nucleic acids (including RNA, DNA, PNA, or a complex thereof), substances having a dominant negative effect such as
- drugs that inhibit the production and secretion of extracellular matrix components include, but are not limited to, collagen-specific molecular chaperones essential for intracellular transport and molecular maturation that are common in the synthesis of various types of collagen. HSP47 etc. are mentioned.
- the “drug that controls the activity or proliferation of extracellular matrix-producing cells in the kidney” in the present invention refers to extracellular matrix production in the kidney that is directly or indirectly related to suppression of the onset, progression, and / or recurrence of renal fibrosis. Any of the physical, chemical and / or physiological actions of cells such as MMP (including MMP1, MMP2, etc.), plasminogen activator (PA) etc. It may be a drug. Examples of such drugs include, but are not limited to, activators and expression enhancers for these substances.
- the carrier of the present invention can deliver one or more of the above drugs.
- siRNA small interfering RNA
- the siRNA includes, in addition to siRNA in a narrow sense, miRNA (micro RNA), shRNA (short hairpin RNA), piRNA (Piwi-interacting RNA), rasiRNA (repeat associated siRNA) Also included are double stranded RNA such as and variants thereof.
- siRNA and siRNA expressing a functional small molecule RNA in a broad sense for example, according to the teachings of general text (Experimental Medicine Separate Volume Revised RNAi Experiment Protocol 2004 Yodosha, Q & A for RNAi Experiments 2006 Yodosha) Can be used.
- siRNA designs can be performed by those skilled in the art by referring to the messenger RNA sequence of a target gene and the sequence of a known siRNA to obtain a general text (Experimental Medicine Separate Volume Revised RNAi Experimental Protocol 2004 Yodosha, The RNAi experiment can be appropriately performed according to the teaching of Q & A 2006 (Yodosha).
- the delivery of the carrier of the present invention is also not limited to drugs other than those described above that suppress the onset, progression and / or recurrence of renal fibrosis, such as, but not limited to, angiotensin converting enzyme inhibitor, AT1 angiotensin II receptor antagonist, aldosterone antagonist, endothelin receptor antagonist, ⁇ blocker, ⁇ blocker, immunosuppressant, extracellular matrix metabolism inhibitor, complement system inhibitor, chemokine inhibitor, TGF- ⁇ inhibitor, Drugs that act on the nitric oxide system such as phosphodiesterase 5 inhibitor, NF ⁇ B inhibitor, Rho inhibitor, p38 MAPK inhibitor, PI3K ⁇ inhibitor, kallikrein, interleukin 1 receptor antagonist, BMP-7, antitumor necrosis factor ⁇ antibody, antiplatelet-derived growth factor D antibody, plasminogen activator inhibitor-1 production inhibitor, Rostaglandin receptor 4 selective agonist, type I collagen synthesis inhibitor, chondroitin sulfate proteoglycan
- the expression enhancer includes, for example, a gene therapy agent such as a vector containing a nucleic acid encoding a protein to be enhanced in expression, and the like.
- these drugs can also be used in combination with the composition of the present invention described below.
- the “combination” includes administration of the composition of the present invention and the drug substantially simultaneously, and administration at time intervals within the same treatment period. In the latter case, the composition of the present invention may be administered before or after the drug.
- drugs that control the activity or proliferation of extracellular matrix-producing cells in the kidney include inhibitors of HSP47, such as siRNA for each of these.
- the substance or object delivered by the carrier of the present invention may or may not be labeled. By labeling, it becomes possible to monitor the success or failure of delivery to target cells and the increase / decrease of target cells, which is useful not only at the test / research level but also at the clinical level.
- the label may be any substance known to those skilled in the art, for example, any radioisotope, magnetic substance, substance that binds to the labeling substance (such as an antibody), fluorescent substance, fluorophore, chemiluminescent substance, enzyme, and the like. You can choose.
- the label may also be attached to the carrier component or may be carried on the carrier as an independent delivery.
- the extracellular matrix-producing cells in the kidney are suitable for use as target cells. Includes, for example, the ability to deliver substances to the same cell more rapidly, efficiently and / or in larger quantities than other cells, eg, normal cells.
- the carrier of the present invention is an extracellular matrix-producing cell in the kidney that is 1.1 times or more, 1.2 times or more, 1.3 times or more, 1.5 times or more, 2 times or more compared to other cells.
- the substance can be delivered at a rate and / or efficiency that is three times or more.
- the present invention also includes the carrier and a drug that controls the activity or proliferation of extracellular matrix-producing cells in the kidney, or for controlling the activity or proliferation of extracellular matrix-producing cells in the kidney, or renal fibrosis As well as the use of said carriers for the production of these compositions.
- the renal fibrosis in the present invention is any interstitial nephritis, such as streptococcal nephritis, staphylococcal nephritis, pneumococcal nephritis, chickenpox, hepatitis B, hepatitis C, viral nephritis associated with HIV, malaria, etc.
- the carrier may contain the delivery product inside or be attached to the outside of the delivery product. It may also be mixed with a delivery product. Thus, depending on the route of administration, mode of drug release, etc., the composition may be coated with a suitable material, such as an enteric coating or a time-disintegrating material, and a suitable drug release system. It may be incorporated. Furthermore, the composition of the present invention may take the form of a complex of a retinoid-bound liposome and an active ingredient, that is, a lipoplex. When the carrier is composed only of a retinoid, the composition of the present invention may take the form of a complex of a drug and a retinoid that controls the activity or proliferation of extracellular matrix-producing cells in the kidney.
- compositions of the invention can be used as medicaments (ie, pharmaceutical compositions), and include various routes including both oral and parenteral, such as, without limitation, oral, intravenous, intramuscular, subcutaneous, Local, intrapulmonary, intratracheal, intratracheal, intrabronchial, intranasal, rectal, intraarterial, intraportal, intraventricular, intramedullary, intralymphatic, intralymphatic, intracerebral, intrathecal, intraventricular , Transmucosal, transdermal, intranasal, intraperitoneal, and intrauterine routes, and may be formulated into dosage forms suitable for each route of administration.
- oral and parenteral such as, without limitation, oral, intravenous, intramuscular, subcutaneous, Local, intrapulmonary, intratracheal, intratracheal, intrabronchial, intranasal, rectal, intraarterial, intraportal, intraventricular, intramedullary, intralymphatic, intralymphatic, intracerebral, intrat
- dosage forms suitable for oral administration include, but are not limited to, powders, granules, tablets, capsules, solutions, suspensions, emulsions, gels, syrups, etc.
- Suitable dosage forms include injections such as solution injections, suspension injections, emulsion injections, and injections prepared at the time of use.
- Formulations for parenteral administration can be in the form of aqueous or non-aqueous isotonic sterile solutions or suspensions.
- the present invention also includes a step of blending a retinoid as a targeting agent to extracellular matrix-producing cells in the kidney and a drug that controls the activity or proliferation of extracellular matrix-producing cells in the kidney as an active ingredient, respectively.
- the present invention relates to a method for producing a pharmaceutical composition for treatment.
- the method for blending retinoid is not particularly limited as long as the retinoid can function as a targeting agent for extracellular matrix-producing cells in the kidney in the blended composition.
- various methods described in the present specification can be used.
- blending method of an active ingredient will not be specifically limited if an active ingredient can have a predetermined effect, Arbitrary well-known methods can be used.
- the active ingredient may be blended simultaneously with the retinoid, or before or after the retinoid is blended.
- the active ingredient may be blended by mixing a carrier in which the retinoid is already blended as a targeting agent and the active ingredient, etc. It may be carried out by mixing the carrier component other than the retinoid and the active ingredient at the same time, or after mixing the active ingredient with the carrier component other than the retinoid and mixing it with the retinoid. Also good.
- the amount of retinoid blended is as already described for the carrier of the present invention.
- the compounding amount of the active ingredient, when administered as a composition is preferably an amount that can suppress the onset or recurrence of renal fibrosis, improve the disease state, reduce the symptoms, or delay or stop the progression. May be an amount that can prevent or cure the onset and recurrence of renal fibrosis. In addition, an amount that does not cause adverse effects exceeding the benefits of administration is preferred. Such an amount is known or can be appropriately determined by an in vitro test using cultured cells, or a test in a model animal such as a mouse, rat, dog or pig. Such a test method is well known to those skilled in the art. Are known.
- Examples of renal fibrosis model animals include those described in JP-A-2009-178143.
- the compounding amount of the active ingredient may vary depending on the dosage form of the composition. For example, when a composition of a plurality of units is used for one administration, the amount of the active ingredient blended in one unit of the composition may be a plurality of the amount of the active ingredient necessary for one administration. it can. Those skilled in the art can appropriately adjust the blending amount.
- the carrier or composition of the present invention may be supplied in any form, but from the viewpoint of storage stability, it is preferably in a form ready for use, for example, at or near the medical site and / or It can be provided in a form that can be prepared by a pharmacist, nurse, or other paramedical.
- the carrier or composition of the present invention is provided as one or more containers comprising at least one of the essential components thereof, and is used before use, for example, within 24 hours, preferably 3 Prepared within an hour and more preferably immediately before use.
- reagents, solvents, dispensing devices and the like that are usually available at the place of preparation can be appropriately used.
- the present invention also provides a kit for preparing a carrier or composition comprising one or more containers containing retinoids and / or deliverables and / or carrier constituents other than retinoids, alone or in combination, It also relates to the necessary components of the carrier or composition provided in the form of such a kit.
- the kit of the present invention may contain instructions relating to the preparation method and administration method of the carrier and composition of the present invention, such as instructions and electronic recording media such as CD and DVD.
- the kit of the present invention may contain all of the components for completing the carrier or composition of the present invention, but may not necessarily contain all of the components. Therefore, the kit of the present invention may not contain reagents and solvents that are usually available at medical sites, experimental facilities, etc., such as sterile water, physiological saline, and glucose solution.
- the present invention further includes administering an effective amount of the composition to a subject in need thereof, for controlling the activity or proliferation of extracellular matrix-producing cells in the kidney, or treating renal fibrosis Related to the method.
- the effective amount is, for example, the amount that suppresses the onset and recurrence of renal fibrosis, improves the disease state, reduces the symptoms, or delays or stops the progression of the latter.
- the amount that prevents or cures the onset and recurrence of fibrosis is preferred.
- Such an amount can be appropriately determined by an in vitro test using cultured cells or the like, or a test in a model animal such as a mouse, rat, dog or pig, and such a test method is well known to those skilled in the art. .
- the doses of retinoid contained in the carrier and the drug used in the method of the present invention are known to those skilled in the art or can be appropriately determined by the above-described tests and the like.
- Examples of renal fibrosis model animals include those described in JP-A-2009-178143.
- the specific dose of the composition to be administered in the methods of the present invention can vary depending on various conditions related to the subject in need of treatment, such as severity of symptoms, general health of the subject, age, weight, subject sex, diet, administration It can be determined in consideration of the route, the timing and frequency of administration, the drugs used in combination, the responsiveness to treatment, compliance with treatment, and the like.
- Administration routes include various routes including both oral and parenteral, such as oral, intravenous, intramuscular, subcutaneous, topical, intrapulmonary, intratracheal, intratracheal, intrabronchial, nasal, rectal, Includes routes such as intraarterial, intraportal, intraventricular, intramedullary, intralymphatic, intralymphatic, intracerebral, intrathecal, intraventricular, transmucosal, transdermal, intranasal, intraperitoneal, and intrauterine. It is.
- the frequency of administration varies depending on the properties of the composition used and the conditions of the subject as described above.
- the term “subject” means any living individual, preferably an animal, more preferably a mammal, more preferably a human individual.
- the subject may be healthy or afflicted with some disease, but when treatment of renal fibrosis is contemplated, typically diabetic nephritis or renal fibrosis Means a subject suffering from or at risk of suffering from.
- a typical example is a subject suffering from diabetic nephritis, particularly diabetic nephritis due to type II diabetes, without limitation.
- treatment is also intended to encompass all types of medically acceptable prophylactic and / or therapeutic interventions intended to cure, temporarily ameliorate or prevent disease.
- treatment includes medically acceptable interventions for various purposes, including delaying or stopping the progression of renal fibrosis, regression or disappearance of lesions, preventing or preventing relapse of renal fibrosis, etc. Is included.
- the present invention also relates to a method for delivering a drug to extracellular matrix-producing cells in the kidney using the carrier.
- This method is not limited, and includes, for example, a step of carrying a delivery product on the carrier, and administering or adding the carrier carrying the delivery product to an organism or medium containing extracellular matrix-producing cells in the kidney, such as a culture medium. Including the step of. These steps can be appropriately achieved according to any known method, the method described in the present specification, and the like.
- the delivery method can also be combined with other delivery methods, such as other delivery methods that target the kidney.
- the said method includes the aspect made in vitro, and the aspect which targets the extracellular matrix production cell in the kidney in a body.
- siRNA-containing VA-bound liposomes siRNA having the following sequence was used. Sequence name: Hsp47-C 5'-GGACAGGCCUGUACAACUA-dTdT-3 '(sense, SEQ ID NO: 1) 5'-UAGUUGUACAGGCCUGUCC-dTdT-3 '(antisense, SEQ ID NO: 2)
- lipid-constituting lipid As a pre-mix solution, 10 mM vitamin A (retinol, Sigma; hereinafter also referred to as VA; dissolved in dimethyl sulfoxide), 1 mM Lipotrust® SR (Hokkaido System Science Co., Ltd., hereinafter also referred to as liposome or liposome-constituting lipid. Nuclease-free water) And 10 ⁇ g / ⁇ l of siRNA (Hsp47-C dissolved in nuclease-free water) were prepared.
- VA dissolved in dimethyl sulfoxide was added at a ratio of 1: 1 (mol / mol) to Lipotrust® SR in the nuclease-free water prepared above, stirred for 15 seconds with vortex, and then allowed to stand at room temperature for 5 minutes in a light-shielded state. A complex was formed. SiRNA was added to this complex and mixed to obtain an administration solution VA Liposome-siRNA Hsp47C.
- This administration solution contains 75 nmol of VA, 75 nmol of liposome-constituting lipid, and siRNA 112.5 ⁇ g per 100 ⁇ l, which includes 3.00 ⁇ mol / kg body weight of VA, 3.00 ⁇ mol / kg body weight of liposome-constituting lipid, siRNA 4.5 mg. Corresponds to / kg body weight siRNA. VA was exposed on the liposome surface.
- Example 2 Examination of therapeutic effect in renal fibrosis model mouse (1) Production of renal fibrosis model animal A renal fibrosis model mouse was produced by requesting the Stellick Regenerative Medicine Institute. Specifically, N-acetyl- ⁇ -D-glucosaminidase inhibitor was given to male C57BL6J / JcL mice (Nippon Claire) 2 days old after birth, and feed CE-2 (manufactured by Claire Japan) until 4 weeks of age. After weaning at 4 weeks of age, we fed High Fat Diet32 (Claire Japan), which has a higher crude fat content than normal food, and sterilized water, and raised it to 12 weeks of age. Produced.
- N-acetyl- ⁇ -D-glucosaminidase inhibitor was given to male C57BL6J / JcL mice (Nippon Claire) 2 days old after birth, and feed CE-2 (manufactured by Claire Japan) until 4 weeks of age. After weaning at 4 weeks of age, we fed High Fat Diet32 (
- mice This model mouse is known to develop diabetic nephritis (see JP-A-2009-178143), and renal fibrosis due to diabetic nephritis can be observed.
- the model mice were divided into the following 4 groups of 10 mice at the age of 12 weeks and 3 days.
- No treatment-STAM mouse group hereinafter NT-STAM group
- 3) 5% glucose administration group hereinafter referred to as Vehicle group
- VA Liposome-siRNA Hsp47C administration group hereinafter referred to as VL-Hsp47C group
- mice were euthanized before the start of treatment after grouping, and the disease state was confirmed.
- the following corresponding administration solutions were administered by tail vein injection 10 times every other day from the age of 12 weeks and 5 days.
- Group 3 As a solvent control, an administration solution (5% glucose or vehicle) in which nuclease-free water 0.75 ml / kg body weight and 5% glucose (Otsuka Pharmaceutical Co., Ltd.) 3.250 ml / kg body weight was mixed was used. .
- VA Liposome-siRNA Hsp47C or VL An administration solution (VA Liposome-siRNA Hsp47C or VL) in which VA is 75 nmol, liposome-constituting lipid is 75 nmol, siRNA is 112.5 ⁇ g per 100 ⁇ l of the administration solution, and final adjustment is performed with 5% glucose. -Hsp47C) was used. Each administration solution was administered with 4 ml / kg body weight from the tail vein when the body weight on the administration start date was set as the reference body weight and the weight change rate on the administration day was within 20% of the reference body weight. When it exceeded 20%, the dose was reset after that with the body weight as a new reference body weight.
- FIG. 1 shows microscopic images of representative glomeruli in the renal cortex region in each administration group.
- Sirius red staining is collagen-specific fiber staining, and the fibrosis site is stained red to pink.
- Microscopic results showed no difference in glomerular basement membrane thickening in all groups, but fibrosis around the mesangial region (stroma) and tubular cells was more fibrotic in the VL-Hsp47C administration group than in the other three groups. The degree of conversion was only mild, and improvement in renal fibrosis was observed (the increased portion of the mesangial region is indicated by an arrow).
- 20 fields of the renal cortex area were randomly photographed for each mouse, and the ratio of the fibrosis area was calculated. From the results shown in FIG.
- Example 3 Gene knockdown in renal extracellular matrix-producing cells by siRNA-containing VA-bound liposomes (1) Separation and recovery of cells Extracellular matrix-producing cells in the kidney showing properties similar to hepatic stellate cells were as follows. Separated and recovered. First, the following five types of solutions were prepared in advance. All solutions were stored at 4 ° C. -EGTA solution: HBES (Invitrogen 14170) 500ml, HEPES 1.19g and EGTA 0.1g was added and mixed.
- HBES Invitrogen 14170
- Collagenase solution To 500 ml of HBSS (Invitrogen 24020), 1.19 g of HEPES, 0.235 g of CaCl 2 2H 2 O and 0.1 g of Collagenase (Yakult YK-102) were added and mixed. -0.02% Collagenase + 0.1% Protease solution: 40 mg of Protease (Sigma P6911-1G) was added to 40 ml of 0.02% Collagenase and mixed. Hanks solution: 0.05 g of MgSO 4 was added to and mixed with 500 ml of HBSS (Invitrogen 24020). -10% Nycodenz (R) solution (Axis-Shield Prod. No 1114542-1): 50 g Nycodenz (R) was added to 500 ml of distilled water and mixed and dissolved.
- R Nycodenz
- mice (Male C57BL6 / J, 20-30 g, 6-8 weeks old) were anesthetized, the abdomen was shaved, the abdomen was opened along the midline, and the kidneys were removed. The removed kidney was washed with 30 ml of EGTA solution about 3 times to remove blood. The kidney was placed in 5 ml of EGTA solution and minced with scissors. The EGTA solution was added to the chopped kidney to make a total volume of 30 ml and centrifuged at 1300 rpm for 5 minutes.
- siRNA-containing VA-binding liposome As siRNA, one having the following sequence was used. Sequence name: Hsp47-C 5'-GGACAGGCCUGUACAACUA-dTdT-3 '(sense, SEQ ID NO: 1) 5'-UAGUUGUACAGGCCUGUCC-dTdT-3 '(antisense, SEQ ID NO: 2)
- vitamin A retinol, Sigma® R7632, abbreviated as VA, dissolved in dimethyl sulfoxide
- lipotrust® SR Hokkaido System Science Co., Ltd., N301, hereinafter also referred to as liposome or liposome-constituting lipid.
- Nuclease Dissolved in free water and 10 mg / ml siRNA (Hsp47-C dissolved in nuclease-free water).
- VA dissolved in dimethyl sulfoxide was added at a ratio of 1: 1 (mol: mol) to Lipotrust® SR in the nuclease-free water prepared above, and then stirred for 15 seconds with vortex and kept at room temperature for 5 minutes in a light-shielded state. Allowed to form a complex.
- 10 mg / ml siRNA was added and mixed to obtain VA Liposome-siRNA Hsp47C.
- an equivalent amount of dimethyl sulfoxide was added instead of VA. Preparation was made so that the final concentration of lipotrust® SR / VA® was 7.7 ⁇ M and siRNA was 869 nM at the time of transfection into cells.
- Transfection experiment As an experimental cell, extracellular matrix-producing cells recovered from the mouse kidney by the above-described recovery method were seeded in a 6-well plate at a cell count of 0.2 ⁇ 10 5 cells / well. The cells were cultured at 37 ° C. for 10 days in 10% FBS DMEM. After 2 days of culture, all 10% FBS DMEM in each well was removed before transfection, and 900 ⁇ l of fresh 10% FBS DMEM was added, followed by incubation at 37 ° C. for about 15 minutes.
- siRNA against the HSP47 gene used in Example 2 suppresses the expression of HSP47 gene in the extracellular matrix-producing cells of the kidney, and siRNA contained in the therapeutic agent of the present invention is This suggests that the progression of renal fibrosis was suppressed by specifically taking up the extracellular matrix-producing cells of the cells and suppressing the expression of the target gene in the cells.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Dispersion Chemistry (AREA)
- Biomedical Technology (AREA)
- Organic Chemistry (AREA)
- Biochemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Zoology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Plant Pathology (AREA)
- Microbiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Urology & Nephrology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
Abstract
Description
ビタミンAを含む担体が、ビタミンAを貯蔵する星細胞に薬物を送達すること(特許文献13)や、同担体にHSP47に対するsiRNAを担持させた組成物が肝線維症(特許文献13)、肺線維症(特許文献14)および骨髄線維症(特許文献15)を改善させることは知られていたが、腎線維症、腎の間質組織、メサンギウムとの関係についてはこれまで全く知られていなかった。
(1)レチノイドを腎臓における細胞外マトリックス産生細胞への標的化剤として含む、腎臓における細胞外マトリックス産生細胞への物質送達用担体。
(2)レチノイドがレチノールを含む、上記(1)の担体。
(3)リポソームの形態を有し、レチノイドと、リポソームに含まれる脂質とのモル比が8:1~1:4である、上記(1)または(2)の担体。
(4)上記(1)~(3)のいずれかの担体と、腎臓における細胞外マトリックス産生細胞の活性または増殖を制御する薬物とを含む、腎線維症処置用医薬組成物。
(5)腎臓における細胞外マトリックス産生細胞の活性または増殖を制御する薬物が、PAI-1の活性または産生阻害剤、細胞活性抑制剤、増殖阻害剤、アポトーシス誘導剤、および、細胞外マトリックス構成分子または該細胞外マトリックス構成分子の産生もしくは分泌に関与する分子の少なくとも1つを標的とするRNAi分子、リボザイム、アンチセンス核酸、DNA/RNAキメラポリヌクレオチドおよびこれらを発現するベクターからなる群から選択される、上記(4)の医薬組成物。
(7)薬物と担体とを、医療の現場またはその近傍で混合してなる、上記(4)~(6)のいずれかの医薬組成物。
(8)腎における細胞外マトリックス産生細胞の活性または増殖を制御する薬物、レチノイド、ならびに、必要に応じてレチノイド以外の担体構成物質を、単独でまたは組み合わせて含む1つまたはそれ以上の容器を含む、上記(4)~(7)のいずれかの医薬組成物の調製キット。
(9)レチノイドを腎臓における細胞外マトリックス産生細胞への標的化剤として配合する工程を含む、腎臓における細胞外マトリックス産生細胞への物質送達用担体の製造方法。
(10)レチノイドを腎臓における細胞外マトリックス産生細胞への標的化剤として、腎臓における細胞外マトリックス産生細胞の活性または増殖を制御する薬物を有効成分としてそれぞれ配合する工程を含む、腎線維症処置用医薬組成物の製造方法。
したがって、本発明の担体により、有効成分を作用部位、さらには標的細胞に効率的に送達できるため、腎線維症、特にこれまで治療が困難であった糖尿病性腎炎などの治癒、進行の抑制または発症の予防が可能となり、ヒト医療および獣医療への貢献は極めて大きい。
また、本発明の担体は、任意の薬剤(例えば、既存の腎線維症治療薬)と組み合わせてその作用効率を高めることができるため、製剤的な応用範囲が広く、効果的な処置剤の製造を簡便に行うことができるという利点もある。
レチノイドのシス-トランスを含む異性体全ては、本発明の範囲内に入る。レチノイドはまた、1または2以上の置換基で置換されることもある。本発明におけるレチノイドは、単離された状態のものはもちろんのこと、これを溶解または保持することができる媒体に溶解または混合した状態のレチノイドをも含む。
本発明における担体は、特定の3次元構造を有してもよい。かかる構造としては、限定されずに、直鎖状または分枝状の線状構造、フィルム状構造、球状構造などが挙げられる。したがって、担体は、限定されずに、ミセル、リポソーム、エマルジョン、微小球、ナノ小球などの任意の3次元形態を有してもよい。
また、本発明における「腎における細胞外マトリックス産生細胞の活性または増殖を制御する薬物」は、腎線維症の発症、進行および/または再発の抑制に直接または間接に関係する腎における細胞外マトリックス産生細胞の物理的、化学的および/または生理的な作用等、例えば、MMP(MMP1、MMP2などを含む)、プラスミノーゲンアクチベーター(PA)などの産生・分泌を直接または間接に促進する何れの薬物であってもよい。かかる薬物としては、限定することなく、例えば、これらの物質の活性化剤や発現増強剤などが挙げられる。本発明の担体は、上記薬物の1種または2種以上を送達することができる。
これらのsiRNA設計は、当業者であれば、標的とする遺伝子のメッセンジャーRNA配列および既知のsiRNAの配列を参照することにより、一般的なテキスト(実験医学別冊 改訂RNAi実験プロトコール 2004年 羊土社、RNAi実験なるほどQ&A 2006年 羊土社)の教示に従って適宜行なうことができる。
本発明における腎線維症は、任意の間質性腎炎、例えば、レンサ球菌腎炎、ブドウ球菌腎炎、肺炎球菌腎炎、水痘、B型肝炎、C型肝炎、HIVなどに伴うウイルス性腎炎、マラリアなどの寄生虫感染による腎炎、真菌性腎炎、マイコプラズマ腎炎などに伴う感染性間質性腎炎、全身性エリテマトーデス(ループス腎炎)、全身性強皮症(膠原病腎)、シェーグレン症候群などの膠原病に伴う間質性腎炎、紫斑病性腎炎、多発性動脈炎、急速進行性糸球体腎炎などの血管の免疫疾患に伴う腎炎、放射線被曝に伴う間質性腎炎、金製剤、NSAIDs、ペニシラミン、ブレオマイシンなどの抗癌剤、抗生物質、パラコートなどによる薬剤性間質性腎炎、昆虫の刺し傷、花粉、ウルシ科の植物などによるアレルギー性腎炎、アミロイドーシス腎炎、糖尿病性腎症、慢性糸球体腎炎、悪性腎硬化症、多発性嚢胞腎症などに伴う腎炎、尿細管間質性腎炎、妊娠中毒症や癌に伴う腎炎、膜性増殖性糸球体腎炎、IgA腎症、混合型クリオグロブリン血症腎炎、グッドパスチャー症候群腎炎、ヴェーゲナー肉芽腫症腎炎、急性間質性腎炎などの特発性間質性腎炎などに起因し得、したがって、これらの間質性腎炎が慢性化したものを含む。本発明における腎線維症は、好ましくは糖尿病性腎炎、薬剤性間質性腎炎および特発性間質性腎炎が慢性化したものを含む。
例えば、経口投与に適した剤形としては、限定することなく、散剤、顆粒剤、錠剤、カプセル剤、液剤、懸濁剤、乳剤、ゲル剤、シロップ剤などが挙げられ、また非経口投与に適した剤形としては、溶液性注射剤、懸濁性注射剤、乳濁性注射剤、用時調製型注射剤などの注射剤が挙げられる。非経口投与用製剤は、水性または非水性の等張性無菌溶液または懸濁液の形態であることができる。
投与経路としては、経口および非経口の両方を包含する種々の経路、例えば、経口、静脈内、筋肉内、皮下、局所、肺内、気道内、気管内、気管支内、経鼻、直腸内、動脈内、門脈内、心室内、骨髄内、リンパ節内、リンパ管内、脳内、髄液腔内、脳室内、経粘膜、経皮、鼻内、腹腔内および子宮内等の経路が含まれる。
投与頻度は、用いる組成物の性状や、上記のような対象の条件によって異なるが、例えば、1日多数回(すなわち1日2、3、4回または5回以上)、1日1回、数日毎(すなわち2、3、4、5、6、7日毎など)、1週間に数回(例えば、1週間に2、3、4回など)、1週間毎、数週間毎(すなわち2、3、4週間毎など)であってもよい。
また、用語「処置」は、疾患の治癒、一時的寛解または予防などを目的とする医学的に許容される全ての種類の予防的および/または治療的介入を包含するものとする。例えば、「処置」の用語は、腎線維症の進行の遅延または停止、病変の退縮または消失、腎線維症発症の予防または再発の防止などを含む、種々の目的の医学的に許容される介入を包含する。
例1 siRNA含有VA結合リポソームの作製
siRNAとして、以下の配列を有するものを用いた。
配列名:Hsp47-C
5'-GGACAGGCCUGUACAACUA-dTdT-3'(センス、配列番号1)
5'-UAGUUGUACAGGCCUGUCC-dTdT-3'(アンチセンス、配列番号2)
(1)腎線維症モデル動物の作製
腎線維症モデルマウスを株式会社ステリック再生医科学研究所に依頼して作製した。具体的には、出生後2日齢のC57BL6J/JcLマウス雄(日本クレア社)にN-アセチル-β-D-グルコサミニダーゼ阻害剤を与え、4週齢まで飼料CE-2(日本クレア社製)および滅菌水を与えて飼育し、満4週齢で離乳後、粗脂肪含量が通常食よりも高いHigh Fat Diet32(日本クレア社)および滅菌水を与え、12週齢まで飼育し、STAMマウスを作製した。本モデルマウスは、糖尿病性腎炎を発症することが知られており(特開2009-178143参照)、糖尿病性腎炎による腎線維症を観察することができる。
上記モデルマウスを、12週3日齢時に10匹ずつ以下の4群に振り分けた。
(第1群)No treatment-STAM マウス、処置前対照群(以下、 NT-STAM (Pre) 群)
(第2群)No treatment-STAM マウス群(以下、 NT-STAM群)
(第3群)5%グルコース投与群(以下、Vehicle群)
(第4群)VA Liposome-siRNA Hsp47C投与群(以下、VL-Hsp47C群)
NT-STAM (Pre)群については、群分け後の治療開始前にマウスを安楽死させ、病態確認を行った。NT-STAM群を除く上記2群に対し、対応する以下の投与液を、12週5日齢時から1日おきに合計10回の尾静脈注射による投与を行った。
(第3群)溶媒対照として、ヌクレアーゼフリー水 0.75ml/kg体重と5%グルコース(大塚製薬株式会社)3.250ml/kg体重とを混合した投与液(5%グルコースまたはVehicle)を用いた。
(第4群)投与液100μlあたり、VAを75nmol、リポソーム構成脂質を75nmol、siRNAを112.5μgとなるよう混合し、5%グルコースで最終調整を行った投与液(VA Liposome-siRNA Hsp47CまたはVL-Hsp47C)を用いた。
各投与液は、投与開始日の体重を基準体重とし、投与日の体重変化率が基準体重の20%以内の場合、4ml/kg体重を尾静脈より投与した。20%を超えた場合は、以降その体重を新たな基準体重として投与量を再設定した。
最終投与終了後2日目(15週4日齢時)にジエチルエーテル麻酔下で心臓より採血し、マウスを安楽死させてから腎臓を摘出した。
摘出した腎臓は、4%パラホルムアルデヒド-リン酸緩衝液にて固定後、パラフィン包埋して薄切標本を作製した。腎線維症に対する治療効果を検討するため、シリウス・レッド染色(コラーゲンを特異的に赤染する線維染色)を行い、オールインワン蛍光顕微鏡BZ-9000(株式会社キーエンス)を用いて、80倍にて撮影した。解析は、腎皮質領域から無作為に20視野を撮影して、BZ-9000付属の解析ソフトを用いて定量化した。
以上の結果から、VL-Hsp47C投与群において線維化の改善傾向が認められた。siRNAが基本的に細胞質内で作用することを考慮すれば、この結果は、レチノイドが腎における細胞外マトリックス産生細胞への標的化剤として機能し、同細胞に効率的に薬物を送達することにより、腎線維症の進行を抑制できることを示すものである。
(1)細胞の分離・回収
肝星細胞に類似した性状を示す腎における細胞外マトリックス産生細胞を、以下のようにして分離・回収した。
まず、事前に以下の5種の溶液の調製を行った。すべての溶液は4℃にて保存した。
・EGTA液:HBSS (Invitrogen 14170)500mlに対し、HEPES1.19gと EGTA0.1gを加え混合した。
・0.02% Collagenase液:HBSS (Invitrogen 24020)500mlに対し、 HEPES1.19g、CaCl2 2H2O 0.235gとCollagenase(Yakult YK-102) 0.1gを加え混合した。
・0.02% Collagenase+0.1% Protease 液:0.02% Collagenase 40mlに対し、Protease(Sigma P6911-1G)40mgを加え混合した。
・Hanks液:HBSS (Invitrogen 24020)500mlに対し、MgSO40.05gを加え混合した。
・10% Nycodenz(R)液(Axis-Shield Prod. No 1114542-1):蒸留水500mlに対し、50g Nycodenz(R)を加え混合溶解した。
1.siRNA含有VA結合liposomeの作製
siRNAとして、以下の配列を有するものを用いた。
配列名:Hsp47-C
5'-GGACAGGCCUGUACAACUA-dTdT-3'(センス、配列番号1)
5'-UAGUUGUACAGGCCUGUCC-dTdT-3'(アンチセンス、配列番号2)
事前に実験用の細胞として、上述の回収方法にてマウス腎臓より回収した細胞外マトリックス産生細胞を、細胞数0.2×105個/ウェルにて6ウェルプレートに播種し、2日間、37℃、10% FBS DMEMにて培養した。
培養2日後、トランスフェクション前に各ウェルの10% FBS DMEMをすべて取り除き、900μlのフレッシュな10% FBS DMEMを加え、15分程度37℃で培養した。
以上の結果は、例2で用いたHSP47遺伝子に対するsiRNAが、腎の細胞外マトリックス産生細胞においてHSP47遺伝子の発現を抑制することを示すものであり、本発明の治療剤に含まれるsiRNAが、腎の細胞外マトリックス産生細胞特異的に取り込まれ、同細胞内で標的遺伝子の発現を抑制することによって、腎線維症の進行の抑制をもたらしたことを示唆するものである。
Claims (10)
- レチノイドを腎臓における細胞外マトリックス産生細胞への標的化剤として含む、腎臓における細胞外マトリックス産生細胞への物質送達用担体。
- レチノイドがレチノールを含む、請求項1に記載の担体。
- リポソームの形態を有し、レチノイドと、リポソームに含まれる脂質とのモル比が8:1~1:4である、請求項1または2に記載の担体。
- 請求項1~3のいずれか一項に記載の担体と、腎臓における細胞外マトリックス産生細胞の活性または増殖を制御する薬物とを含む、腎線維症処置用医薬組成物。
- 腎臓における細胞外マトリックス産生細胞の活性または増殖を制御する薬物が、PAI-1の活性または産生阻害剤、細胞活性抑制剤、増殖阻害剤、アポトーシス誘導剤、および、細胞外マトリックス構成分子または該細胞外マトリックス構成分子の産生もしくは分泌に関与する分子の少なくとも1つを標的とするRNAi分子、リボザイム、アンチセンス核酸、DNA/RNAキメラポリヌクレオチドおよびこれらを発現するベクターからなる群から選択される、請求項4に記載の医薬組成物。
- 細胞外マトリックス産生細胞の活性または増殖を制御する薬物が、HSP47の阻害剤である、請求項4に記載の医薬組成物。
- 薬物と担体とを、医療の現場またはその近傍で混合してなる、請求項4~6のいずれか一項に記載の医薬組成物。
- 腎における細胞外マトリックス産生細胞の活性または増殖を制御する薬物、レチノイド、ならびに、必要に応じてレチノイド以外の担体構成物質を、単独でまたは組み合わせて含む1つまたはそれ以上の容器を含む、請求項4~7のいずれか一項に記載の医薬組成物の調製キット。
- レチノイドを腎臓における細胞外マトリックス産生細胞への標的化剤として配合する工程を含む、腎臓における細胞外マトリックス産生細胞への物質送達用担体の製造方法。
- レチノイドを腎臓における細胞外マトリックス産生細胞への標的化剤として、腎臓における細胞外マトリックス産生細胞の活性または増殖を制御する薬物を有効成分としてそれぞれ配合する工程を含む、腎線維症処置用医薬組成物の製造方法。
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CA2802414A CA2802414C (en) | 2010-06-17 | 2011-06-17 | Agent for treating renal fibrosis |
| CN2011800279298A CN102933233A (zh) | 2010-06-17 | 2011-06-17 | 肾纤维化处理剂 |
| AU2011266057A AU2011266057B2 (en) | 2010-06-17 | 2011-06-17 | Agent for treating renal fibrosis |
| KR1020137001156A KR101967868B1 (ko) | 2010-06-17 | 2011-06-17 | 신장 섬유증 치료 물질 |
| ES11795835T ES2712086T3 (es) | 2010-06-17 | 2011-06-17 | Agente para tratar la fibrosis renal |
| US13/704,437 US20130136789A1 (en) | 2010-06-17 | 2011-06-17 | Agent for treating renal fibrosis |
| EP11795835.5A EP2583691B1 (en) | 2010-06-17 | 2011-06-17 | Agent for treating renal fibrosis |
| RU2013101969A RU2635460C2 (ru) | 2010-06-17 | 2011-06-17 | Средство для лечения фиброза почек |
| IN342CHN2013 IN2013CN00342A (ja) | 2010-06-17 | 2011-06-17 | |
| US14/668,618 US20150259683A1 (en) | 2004-12-22 | 2015-03-25 | Agent for treating renal fibrosis |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010138070 | 2010-06-17 | ||
| JP2010-138070 | 2010-06-17 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/867,346 Continuation-In-Part US20130216611A1 (en) | 2004-12-22 | 2013-04-22 | Therapeutic agent for fibroid lung |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/704,437 A-371-Of-International US20130136789A1 (en) | 2010-06-17 | 2011-06-17 | Agent for treating renal fibrosis |
| US14/668,618 Continuation US20150259683A1 (en) | 2004-12-22 | 2015-03-25 | Agent for treating renal fibrosis |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011158933A1 true WO2011158933A1 (ja) | 2011-12-22 |
Family
ID=45348325
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/063910 WO2011158933A1 (ja) | 2004-12-22 | 2011-06-17 | 腎線維症処置剤 |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US20130136789A1 (ja) |
| EP (1) | EP2583691B1 (ja) |
| JP (2) | JP2012020995A (ja) |
| KR (1) | KR101967868B1 (ja) |
| CN (1) | CN102933233A (ja) |
| AU (1) | AU2011266057B2 (ja) |
| CA (1) | CA2802414C (ja) |
| ES (1) | ES2712086T3 (ja) |
| IN (1) | IN2013CN00342A (ja) |
| RU (2) | RU2711531C2 (ja) |
| TW (1) | TWI549691B (ja) |
| WO (1) | WO2011158933A1 (ja) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9976142B2 (en) | 2014-04-02 | 2018-05-22 | Nitto Denko Corporation | Targeting molecule and a use thereof |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009221164A (ja) | 2008-03-17 | 2009-10-01 | Nitto Denko Corp | 肺線維症処置剤 |
| US9572886B2 (en) | 2005-12-22 | 2017-02-21 | Nitto Denko Corporation | Agent for treating myelofibrosis |
| JP5950428B2 (ja) | 2010-08-05 | 2016-07-13 | 日東電工株式会社 | 線維化組織から正常組織を再生するための組成物 |
| JP6340162B2 (ja) | 2012-12-20 | 2018-06-06 | 日東電工株式会社 | アポトーシス誘導剤 |
| EP2945701B1 (en) | 2013-01-18 | 2023-04-05 | Cornell University | Methods of treating diseases associated with high fat diet and vitamin a deficiency using retinoic acid receptor agonists |
| CN106133024B (zh) | 2014-04-07 | 2019-07-05 | 日东电工株式会社 | 用于疏水性药物递送的新颖的基于聚合物的助水溶物 |
| CN105597085A (zh) * | 2015-12-17 | 2016-05-25 | 哈尔滨博翱生物医药技术开发有限公司 | 成纤维细胞生长因子-21在治疗肾纤维化中的应用 |
| JP6833456B2 (ja) * | 2016-11-02 | 2021-02-24 | 日東電工株式会社 | 皮膚線維症処置剤 |
| KR102352489B1 (ko) | 2019-12-09 | 2022-01-18 | 전남대학교산학협력단 | 소수성 개질된 글리콜 키토산 나노입자 및 이를 이용한 신장 표적화 약물 전달체 |
Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5238924A (en) | 1984-05-03 | 1993-08-24 | Merck & Co., Inc. | Treatment of renal diseases with ace inhibitors |
| JPH072667A (ja) | 1993-04-22 | 1995-01-06 | Takeda Chem Ind Ltd | 腎疾患の予防または治療剤 |
| JP2001233792A (ja) | 2000-01-31 | 2001-08-28 | Pfizer Prod Inc | 急性及び慢性腎不全の処置のためのプロスタグランジン(pge2)受容体4(ep4)選択的アゴニストの使用 |
| JP2004043459A (ja) | 2002-05-24 | 2004-02-12 | Sumitomo Pharmaceut Co Ltd | TGF−β阻害活性を有する低分子化合物からなる医薬 |
| JP2004534760A (ja) | 2001-05-24 | 2004-11-18 | ハダジット メディカル リサーチ サービシズ アンド ディベラップメント リミテッド | 腎線維症の治療 |
| WO2006068232A1 (ja) | 2004-12-22 | 2006-06-29 | Sapporo Medical University | 線維化抑制のための薬物担体および薬物担体キット |
| JP2006519817A (ja) | 2003-03-06 | 2006-08-31 | シュペーデル・ファルマ・アーゲー | 新しい医薬 |
| JP2007099641A (ja) | 2005-09-30 | 2007-04-19 | Tsumura & Co | インドールキノキサリン類化合物、その製造方法およびそれを用いた医薬組成物 |
| JP2007536241A (ja) | 2004-05-03 | 2007-12-13 | ユニバーシティ オブ バージニア パテント ファウンデーション | 糖尿病性腎症の処置のためのa2aアデノシンレセプターアゴニスト |
| JP2009007258A (ja) | 2007-06-26 | 2009-01-15 | Kowa Pharmaceutical Co Ltd | Pai−1産生抑制作用を有する3−アニリノ−2−シクロアルケノン誘導体 |
| JP2009029750A (ja) | 2007-07-27 | 2009-02-12 | Kowa Co | アリールプロパン誘導体を有効成分とする糖化最終産物形成阻害剤 |
| WO2009036368A2 (en) * | 2007-09-14 | 2009-03-19 | Nitto Denko Corporation | Drug carriers |
| JP2009178143A (ja) | 2008-02-01 | 2009-08-13 | Stelic Institute Of Regenerative Medicine | 脂肪性肝炎−肝癌モデル動物 |
| JP2009221164A (ja) | 2008-03-17 | 2009-10-01 | Nitto Denko Corp | 肺線維症処置剤 |
| JP2009292725A (ja) | 2006-09-01 | 2009-12-17 | Stelic Institute Of Regenerative Medicine | 腎疾患改善剤 |
| JP2010059124A (ja) | 2008-09-05 | 2010-03-18 | Nitto Denko Corp | 骨髄線維症処置剤 |
| JP2010077101A (ja) | 2008-09-29 | 2010-04-08 | Toray Ind Inc | 腎線維症の治療剤又は予防剤 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6183774B1 (en) * | 1996-01-31 | 2001-02-06 | Collaborative Laboratories, Inc. | Stabilizing vitamin A derivatives by encapsulation in lipid vesicles formed with alkylammonium fatty acid salts |
| US6334999B1 (en) * | 1999-08-27 | 2002-01-01 | Research Development Foundation | Liposomal aerosols for delivery of chemotherapeutic retinoids to the lungs |
| BR0006556A (pt) * | 2000-12-28 | 2002-09-17 | Apsen Farmaceutica S A | Composição farmacêutica para uso tópico à base de corticosteróide para tratamento de fimose |
| KR100613342B1 (ko) | 2004-12-16 | 2006-08-21 | 동부일렉트로닉스 주식회사 | 반도체 소자 및 그 제조방법 |
| TWI407971B (zh) * | 2007-03-30 | 2013-09-11 | Nitto Denko Corp | Cancer cells and tumor-related fibroblasts |
| CA2732412C (en) * | 2008-07-30 | 2014-12-09 | Lei Yu | Retinoid-targeted drug carriers |
| CA2736368A1 (en) * | 2008-09-12 | 2010-03-18 | Nitto Denko Corporation | Imaging agents of fibrotic diseases |
| PL2509991T3 (pl) * | 2009-12-09 | 2016-04-29 | Nitto Denko Corp | Modulacja ekspresji hsp47 |
| DK2998289T3 (da) * | 2011-06-08 | 2019-09-16 | Nitto Denko Corp | Forbindelser til at målrette lægemiddellevering og fremme sirna-aktivitet |
-
2011
- 2011-06-17 CA CA2802414A patent/CA2802414C/en active Active
- 2011-06-17 ES ES11795835T patent/ES2712086T3/es active Active
- 2011-06-17 WO PCT/JP2011/063910 patent/WO2011158933A1/ja active Application Filing
- 2011-06-17 RU RU2017136720A patent/RU2711531C2/ru active
- 2011-06-17 RU RU2013101969A patent/RU2635460C2/ru active
- 2011-06-17 CN CN2011800279298A patent/CN102933233A/zh active Pending
- 2011-06-17 US US13/704,437 patent/US20130136789A1/en not_active Abandoned
- 2011-06-17 TW TW100121244A patent/TWI549691B/zh active
- 2011-06-17 IN IN342CHN2013 patent/IN2013CN00342A/en unknown
- 2011-06-17 KR KR1020137001156A patent/KR101967868B1/ko active IP Right Grant
- 2011-06-17 JP JP2011134708A patent/JP2012020995A/ja active Pending
- 2011-06-17 EP EP11795835.5A patent/EP2583691B1/en active Active
- 2011-06-17 AU AU2011266057A patent/AU2011266057B2/en active Active
-
2015
- 2015-03-25 US US14/668,618 patent/US20150259683A1/en not_active Abandoned
- 2015-05-18 JP JP2015100873A patent/JP5873589B2/ja active Active
Patent Citations (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5238924A (en) | 1984-05-03 | 1993-08-24 | Merck & Co., Inc. | Treatment of renal diseases with ace inhibitors |
| JPH072667A (ja) | 1993-04-22 | 1995-01-06 | Takeda Chem Ind Ltd | 腎疾患の予防または治療剤 |
| JP2001233792A (ja) | 2000-01-31 | 2001-08-28 | Pfizer Prod Inc | 急性及び慢性腎不全の処置のためのプロスタグランジン(pge2)受容体4(ep4)選択的アゴニストの使用 |
| JP2004534760A (ja) | 2001-05-24 | 2004-11-18 | ハダジット メディカル リサーチ サービシズ アンド ディベラップメント リミテッド | 腎線維症の治療 |
| JP2004043459A (ja) | 2002-05-24 | 2004-02-12 | Sumitomo Pharmaceut Co Ltd | TGF−β阻害活性を有する低分子化合物からなる医薬 |
| JP2006519817A (ja) | 2003-03-06 | 2006-08-31 | シュペーデル・ファルマ・アーゲー | 新しい医薬 |
| JP2007536241A (ja) | 2004-05-03 | 2007-12-13 | ユニバーシティ オブ バージニア パテント ファウンデーション | 糖尿病性腎症の処置のためのa2aアデノシンレセプターアゴニスト |
| WO2006068232A1 (ja) | 2004-12-22 | 2006-06-29 | Sapporo Medical University | 線維化抑制のための薬物担体および薬物担体キット |
| JP2007099641A (ja) | 2005-09-30 | 2007-04-19 | Tsumura & Co | インドールキノキサリン類化合物、その製造方法およびそれを用いた医薬組成物 |
| JP2009292725A (ja) | 2006-09-01 | 2009-12-17 | Stelic Institute Of Regenerative Medicine | 腎疾患改善剤 |
| JP2009007258A (ja) | 2007-06-26 | 2009-01-15 | Kowa Pharmaceutical Co Ltd | Pai−1産生抑制作用を有する3−アニリノ−2−シクロアルケノン誘導体 |
| JP2009029750A (ja) | 2007-07-27 | 2009-02-12 | Kowa Co | アリールプロパン誘導体を有効成分とする糖化最終産物形成阻害剤 |
| WO2009036368A2 (en) * | 2007-09-14 | 2009-03-19 | Nitto Denko Corporation | Drug carriers |
| JP2009178143A (ja) | 2008-02-01 | 2009-08-13 | Stelic Institute Of Regenerative Medicine | 脂肪性肝炎−肝癌モデル動物 |
| JP2009221164A (ja) | 2008-03-17 | 2009-10-01 | Nitto Denko Corp | 肺線維症処置剤 |
| JP2010059124A (ja) | 2008-09-05 | 2010-03-18 | Nitto Denko Corp | 骨髄線維症処置剤 |
| JP2010077101A (ja) | 2008-09-29 | 2010-04-08 | Toray Ind Inc | 腎線維症の治療剤又は予防剤 |
Non-Patent Citations (8)
| Title |
|---|
| "Hyojun Yakuzaigaku", 2003, NANKODO |
| "Revised RNAi Experimental Protocol", 2004, YODOSHA, article "Experimental Medicine" |
| "RNAi Experimental Frequently Asked Questions", 2006, YODOSHA |
| G. P. MOSS: "Biochemical Nomenclature and Related Documents I, 2nd Ed.", 1992, PORTLAND PRESS, pages: 247 - 251 |
| KIDA YUJIRO ET AL.: "Characterterization of vitamin A-storing cells in mouse fibrous kidneys using Cygb/STAP as a marker of activated stellate cells", ARCH HISTOL CYTOL, vol. 70, no. 2, 2007, pages 95 - 106, XP055073691 * |
| NEPHROLOGY, DIALYSIS, TRANSPLANTATION, vol. 22, no. 12, 2007, pages 3391 - 407 |
| TEMMING ET AL., DRUG RESIST UPDAT., vol. 8, no. 6, 2005, pages 381 - 402 |
| ZHAO; LEE, ADV DRUG DELIV REV., vol. 56, no. 8, 2004, pages 1193 - 204 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9976142B2 (en) | 2014-04-02 | 2018-05-22 | Nitto Denko Corporation | Targeting molecule and a use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2015155459A (ja) | 2015-08-27 |
| TWI549691B (zh) | 2016-09-21 |
| TW201204389A (en) | 2012-02-01 |
| RU2017136720A (ru) | 2019-02-08 |
| CN102933233A (zh) | 2013-02-13 |
| IN2013CN00342A (ja) | 2015-07-03 |
| JP5873589B2 (ja) | 2016-03-01 |
| RU2635460C2 (ru) | 2017-11-13 |
| CA2802414C (en) | 2018-01-09 |
| ES2712086T3 (es) | 2019-05-09 |
| US20150259683A1 (en) | 2015-09-17 |
| RU2013101969A (ru) | 2014-07-27 |
| AU2011266057B2 (en) | 2014-12-04 |
| US20130136789A1 (en) | 2013-05-30 |
| EP2583691B1 (en) | 2019-01-16 |
| EP2583691A1 (en) | 2013-04-24 |
| CA2802414A1 (en) | 2011-12-22 |
| KR101967868B1 (ko) | 2019-08-19 |
| RU2711531C2 (ru) | 2020-01-17 |
| RU2017136720A3 (ja) | 2019-02-08 |
| EP2583691A4 (en) | 2016-04-27 |
| AU2011266057A1 (en) | 2013-01-10 |
| KR20130121813A (ko) | 2013-11-06 |
| JP2012020995A (ja) | 2012-02-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5873589B2 (ja) | 腎線維症処置剤 | |
| US10098953B2 (en) | Therapeutic agent for fibroid lung | |
| EP2135600B1 (en) | Targeting agent for cancer cell or cancer-associated fibroblast | |
| US8574623B2 (en) | Therapeutic agent for pulmonary fibrosis | |
| JP2015205934A (ja) | 核酸および/または他の構成要素を含有している合成ナノ構造体 | |
| JP5873419B2 (ja) | 腸管線維症処置剤 | |
| JP6833456B2 (ja) | 皮膚線維症処置剤 | |
| JP5517306B2 (ja) | 肺線維症処置剤 | |
| JP5976874B2 (ja) | 肺線維症処置剤 | |
| JP5727557B2 (ja) | 肺線維症処置剤 | |
| JPWO2010110318A1 (ja) | 核酸を含有する動脈硬化性疾患治療剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180027929.8 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11795835 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2802414 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2011266057 Country of ref document: AU Date of ref document: 20110617 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011795835 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 20137001156 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2013101969 Country of ref document: RU Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13704437 Country of ref document: US |