WO2010101121A1 - プロピレンの製造方法 - Google Patents
プロピレンの製造方法 Download PDFInfo
- Publication number
- WO2010101121A1 WO2010101121A1 PCT/JP2010/053265 JP2010053265W WO2010101121A1 WO 2010101121 A1 WO2010101121 A1 WO 2010101121A1 JP 2010053265 W JP2010053265 W JP 2010053265W WO 2010101121 A1 WO2010101121 A1 WO 2010101121A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- zeolite
- catalyst
- reaction
- hours
- water
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/90—Regeneration or reactivation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/40—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the pentasil type, e.g. types ZSM-5, ZSM-8 or ZSM-11, as exemplified by patent documents US3702886, GB1334243 and US3709979, respectively
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/40—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the pentasil type, e.g. types ZSM-5, ZSM-8 or ZSM-11, as exemplified by patent documents US3702886, GB1334243 and US3709979, respectively
- B01J29/42—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the pentasil type, e.g. types ZSM-5, ZSM-8 or ZSM-11, as exemplified by patent documents US3702886, GB1334243 and US3709979, respectively containing iron group metals, noble metals or copper
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/40—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the pentasil type, e.g. types ZSM-5, ZSM-8 or ZSM-11, as exemplified by patent documents US3702886, GB1334243 and US3709979, respectively
- B01J29/42—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the pentasil type, e.g. types ZSM-5, ZSM-8 or ZSM-11, as exemplified by patent documents US3702886, GB1334243 and US3709979, respectively containing iron group metals, noble metals or copper
- B01J29/44—Noble metals
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/0009—Use of binding agents; Moulding; Pressing; Powdering; Granulating; Addition of materials ameliorating the mechanical properties of the product catalyst
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/0009—Use of binding agents; Moulding; Pressing; Powdering; Granulating; Addition of materials ameliorating the mechanical properties of the product catalyst
- B01J37/0027—Powdering
- B01J37/0045—Drying a slurry, e.g. spray drying
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J38/00—Regeneration or reactivation of catalysts, in general
- B01J38/04—Gas or vapour treating; Treating by using liquids vaporisable upon contacting spent catalyst
- B01J38/12—Treating with free oxygen-containing gas
- B01J38/14—Treating with free oxygen-containing gas with control of oxygen content in oxidation gas
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C6/00—Preparation of hydrocarbons from hydrocarbons containing a different number of carbon atoms by redistribution reactions
- C07C6/02—Metathesis reactions at an unsaturated carbon-to-carbon bond
- C07C6/04—Metathesis reactions at an unsaturated carbon-to-carbon bond at a carbon-to-carbon double bond
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2229/00—Aspects of molecular sieve catalysts not covered by B01J29/00
- B01J2229/30—After treatment, characterised by the means used
- B01J2229/42—Addition of matrix or binder particles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2529/00—Catalysts comprising molecular sieves
- C07C2529/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites, pillared clays
- C07C2529/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- C07C2529/70—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of types characterised by their specific structure not provided for in groups C07C2529/08 - C07C2529/65
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/52—Improvements relating to the production of bulk chemicals using catalysts, e.g. selective catalysts
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/584—Recycling of catalysts
Definitions
- the present invention relates to a method for producing propylene from a hydrocarbon raw material containing ethylene using a zeolite-containing catalyst.
- zeolite-containing catalyst examples include a catalyst containing Ag in an intermediate pore size zeolite substantially free of protons, and its silica / alumina (SiO 2 / Al 2 O 3 ). Catalysts having a molar ratio in the range of 200 to 5000 are known.
- olefins encompasses a broad concept, raw material “olefins” that have been put to practical use in the production of propylene are limited to those having 4 or more carbon atoms.
- Patent Document 1 states that “a hydrocarbon raw material containing one or more C 4 or more olefin components is brought into contact with a crystalline silicate catalyst to have substantially the same olefin weight content as that of the raw material. While producing a effluent having a second composition of one or more olefinic components of C 3 or higher ”(Claim 1), wherein“ preferably said ethylene is said hydrocarbon. It should be comprised between 0.1 and 50% by weight of the raw material "(paragraph 0028). Patent Document 2 describes a method for producing propylene from ethylene and methanol and / or dimethyl ether.
- ethanol that is, ethylene produced through a dehydration reaction
- H-ZSM5 zeolite into which metal ions have been introduced
- phosphate zeolite such as SAPO-34
- Patent Documents 1 and 2 described above describe that propylene can be produced from a raw material containing ethylene. Nevertheless, the reason why the catalyst has not been put into practical use is considered to be because the conventionally proposed catalyst could not achieve sufficient activity for converting stable ethylene while maintaining high selectivity. .
- Patent Document 3 discloses a method for producing propylene from ethanol as a raw material. That is, it is described that phosphate-based zeolite exhibits high selectivity in a method for producing propylene from a mixed raw material of ethylene and water produced by a dehydration reaction of ethanol.
- the conversion rate of ethylene is as low as 40% or less, and the conversion rate is remarkably lowered in only a few hours.
- a catalyst whose activity decreases in a short time cannot be said to withstand industrial use.
- a method of using ethylene obtained by steam cracking of ethane as a raw material without separation and purification, or a method of using biomass-derived ethanol as a raw material ethylene source is advantageous. That is, it can be said that it is a preferable embodiment that the ethylene source is subjected to the reaction while containing water from the viewpoint of easy availability of raw materials.
- the zeolite-containing catalyst comes into contact with water vapor at a high temperature. Deterioration due to dealumination is likely to proceed, and this degradation is so-called permanent degradation that does not recover even when regenerated by removing coke fuel, so that such a catalyst cannot withstand repeated use.
- the problem to be solved by the present invention is to produce propylene from ethylene in a high yield in a method for producing propylene from a hydrocarbon raw material containing ethylene exceeding 50% by mass in the presence of water. And providing a method for stably producing in the long term while repeating the reaction / regeneration.
- the present inventors conducted a catalytic conversion reaction of ethylene-containing hydrocarbons using a zeolite-containing catalyst containing MFI-type zeolite having a specific composition and physical properties.
- it is difficult to cause permanent deterioration of the catalyst even in the presence of water the deterioration of the catalyst due to the deposited coke can be regenerated by removing the coke combustion, and the permanent deterioration of the catalyst due to water vapor generated during the removal of the coke combustion is also suppressed.
- the inventors have found that propylene can be stably produced with high yield and long-term activity, and the present invention has been completed.
- a method for producing propylene comprising a step of catalytic conversion of a hydrocarbon raw material containing ethylene in excess of 50% by mass with a zeolite-containing catalyst while supplying water, wherein the zeolite contained in the zeolite-containing catalyst is (1 )
- MFI type zeolite MFI type zeolite
- the zeolite crystallization index determined from the X-ray diffraction spectrum is 3.3 or more
- the silica / alumina (SiO 2 / Al 2 O 3 ) molar ratio is 20 to 300.
- [2] The method for producing propylene according to the above [1], wherein 10% by mass or more of water is supplied to the hydrocarbon raw material.
- [3] The method for producing propylene according to the above [1] or [2], further comprising a step of heat-treating the zeolite-containing catalyst at a temperature of 550 ° C. or higher.
- [4] The method for producing propylene according to any one of the above [1] to [3], further comprising a step of heat-treating the zeolite-containing catalyst at a temperature of 300 ° C. or higher in the presence of water vapor.
- the crystallization index measurement result (X-ray diffraction spectrum) of the zeolite used in Example 1 is shown.
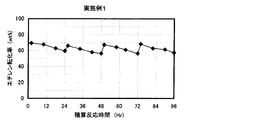
- the result of the reaction / regeneration repetition test in Example 1 is shown.
- the crystallization index measurement result (X-ray diffraction spectrum) of the zeolite used in Example 3 is shown.
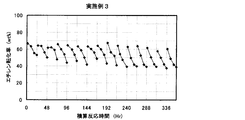
- the result of the reaction / regeneration repetition test in Example 3 is shown.
- the crystallization index measurement result (X-ray diffraction spectrum) of the zeolite used in Example 4 is shown.
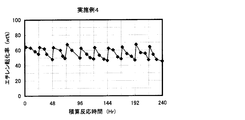
- the result of the reaction / regeneration repetition test in Example 4 is shown.
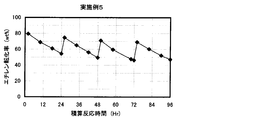
- the result of the reaction / regeneration repetition test in Example 5 is shown.
- exponent measurement result (X-ray diffraction spectrum) of the zeolite used for the comparative example 1 is shown.
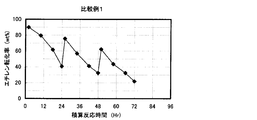
- the result of the reaction / regeneration repetition test in Comparative Example 1 is shown.
- the crystallization index measurement result (X-ray diffraction spectrum) of the zeolite used in Example 6 is shown.
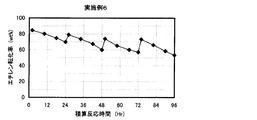
- the result of the reaction / regeneration repetition test in Example 6 is shown.
- exponent measurement result (X-ray diffraction spectrum) of the zeolite used for the comparative example 2 is shown.
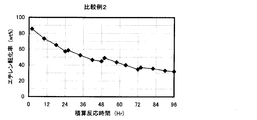
- the result of the reaction / regeneration repetition test in Comparative Example 2 is shown.
- the present embodiment a mode for carrying out the present invention (hereinafter abbreviated as “the present embodiment”) will be described in detail.
- this invention is not limited to the following embodiment, It can deform
- the method for producing propylene of the present embodiment is as follows: A method for producing propylene comprising a step of catalytic conversion of a hydrocarbon raw material containing ethylene exceeding 50% by mass with a zeolite-containing catalyst while supplying water, A method for producing propylene wherein the zeolite contained in the zeolite-containing catalyst satisfies the following (1) to (3): (1) MFI type zeolite, (2) The zeolite crystallization index determined from the X-ray diffraction spectrum is 3.3 or more, (3) The silica / alumina (SiO 2 / Al 2 O 3 ) molar ratio is 20 to 300.
- the zeolite contained in the zeolite-containing catalyst of the present embodiment is (1) MFI type zeolite. That is, the zeolite is classified into the MFI structure by the skeletal structure type according to the IUPAC recommendation, and specifically, the zeolite is ZSM-5 type.
- the zeolite contained in the zeolite-containing catalyst is an MFI-type zeolite, it exhibits high activity and selectivity in the catalytic conversion reaction of olefin, and also shows high resistance to coking deterioration.
- the MFI type zeolite is a medium pore size zeolite.
- the zeolite crystallization index obtained from the (2) X-ray diffraction spectrum of the zeolite contained in the zeolite-containing catalyst of the present embodiment is 3.3 or more.
- the crystallization index is preferably 3.5 or more, more preferably 4.0 or more.
- the peak intensity of 2 ⁇ 23 to 24 degrees is an index indicating the crystallinity of MFI-type zeolite (see JP-A-10-52646).
- the use of an internal standard sample is also widely known, and rutile titania is known to have little change in diffraction line intensity due to pulverization or grinding (Advances in X-ray analysis 5, Ikuo Kukiya) Published by Toshihiro Nakamura, published by Science and Technology Corporation (1973), pages 133-144).
- a continuous extraction / continuous regeneration method using a fluidized bed method or a fixed bed swing reactor method can be adopted industrially, and a high yield can be stably maintained over a long period of time.
- Patent Document 3 described above also describes an example in which H-ZSM5 zeolite having a silica / alumina molar ratio of 23.8 is used as a catalyst, but details of the zeolite are not described. Therefore, the crystallization index of zeolite is unknown, there is no example of repeated use of the catalyst, and the resistance to permanent deterioration is also unknown. The present inventor tried to confirm the crystallization index of this zeolite, but it was not usually available and the production method was not described, so the crystallization index could not be confirmed. Patent Document 3 describes a method for producing propylene through a dehydration reaction of ethanol.
- a zeolite having a crystallization index of 3.3 or higher is a substance that is difficult to produce unless it is intentionally synthesized. Based on the above facts, it can be predicted that the crystallization index of H-ZSM5 zeolite described in the same document is less than 3.3.
- the (3) silica / alumina (SiO 2 / Al 2 O 3 ) molar ratio of the zeolite contained in the zeolite-containing catalyst of the present embodiment is in the range of 20 to 300. From the viewpoint of stably producing the catalyst, the silica / alumina molar ratio of the zeolite is 20 or more. On the other hand, when the silica / alumina molar ratio exceeds 300, the ethylene conversion activity is low, and when a high conversion is obtained with the catalyst, the propylene selectivity is low. In particular, as described later, when the pretreatment includes a step of heat treatment at a temperature of 300 ° C. or higher in the presence of water vapor, the activity tends to further decrease.
- the silica / alumina molar ratio of zeolite can be measured by a known method.
- the zeolite can be completely dissolved in an alkaline aqueous solution, and the resulting solution can be analyzed and analyzed by plasma emission spectroscopy.
- metalloaluminosilicates in which some of the aluminum atoms constituting the zeolite skeleton are substituted with elements such as Ga, Fe, B, and Cr, and all the aluminum atoms constituting the zeolite skeleton are substituted with the above elements.
- Metallosilicates can also be used. In that case, the silica / alumina molar ratio is calculated after converting the content of the substituted element in the metalloaluminosilicate or metallosilicate to the number of moles of alumina.
- the acid amount (hereinafter referred to as TPD acid amount) determined from the high temperature desorption amount in the ammonia temperature programmed desorption (TPD) spectrum of the zeolite-containing catalyst is 50 ⁇ mol / g- It is preferable to be more than zeolite. It can be said that the TPD acid amount of 50 ⁇ mol / g-zeolite or higher is relatively high as the acid amount of the zeolite-containing catalyst.
- TPD acid amount determined from the high temperature desorption amount in the ammonia temperature programmed desorption (TPD) spectrum of the zeolite-containing catalyst is 50 ⁇ mol / g- It is preferable to be more than zeolite. It can be said that the TPD acid amount of 50 ⁇ mol / g-zeolite or higher is relatively high as the acid amount of the zeolite-containing catalyst.
- TPD acid amount the acid amount determined from the high temperature desorption amount in the ammonia temperature programmed desorption (TPD) spectrum of the zeolite
- the problem of activity deterioration due to coke formation is solved by the coexistence of water, and the problem of permanent deterioration caused by the coexistence of water is solved by increasing the crystallization index of zeolite, so that the propylene yield can be increased over a long period of time. Can be maintained. Even if coking deterioration is suppressed by the coexistence of water, if the crystallization index of the zeolite in the catalyst is 3.3 or less, the permanent deterioration proceeds at the same rate as the coking deterioration rate, and the activity activation rate after removing the coke is increased. Since the decrease is significant, high activity cannot be maintained.
- the amount of TPD acid in the present embodiment is measured by the following method.
- the sample catalyst is placed in the measurement cell of the thermal desorption spectrometer, the inside of the measurement cell is replaced with helium gas, the temperature is stabilized at 100 ° C., and the inside of the cell is once vacuum-treated, followed by ammonia gas. To a pressure of 100 Torr. This state is maintained for 30 minutes, and ammonia is adsorbed on the catalyst. Thereafter, the inside of the cell is again evacuated to remove ammonia that is not adsorbed on the catalyst, the carrier gas is switched to helium, and the inside of the cell is returned to atmospheric pressure.
- the measurement cell was connected to a quadrupole mass spectrometer, the pressure in the cell was set to 200 Torr, and the temperature in the cell was increased to 600 ° C. at a temperature increase rate of 8.33 ° C./min. The desorbed ammonia is detected. The pressure in the cell during desorption is set to be kept at about 200 Torr.
- the temperature-programmed desorption spectrum obtained was divided by waveform separation based on Gaussian distribution, and the ammonia desorption amount was obtained from the sum of the areas of the waveform having a peak top at a desorption temperature of 240 ° C or higher, and this was included in the catalyst It is expressed by the value divided by the weight of zeolite (unit: ⁇ mol / g-zeolite).
- 240 ° C.” is an index used only for determining the position of the peak top, and does not mean that the area of the portion of 240 ° C. or higher is obtained.
- the “waveform area” is the total area including portions other than 240 ° C.
- the method for synthesizing the MFI zeolite having the specific physical properties and composition described above is not particularly limited, but can be produced by optimizing various conditions of conventionally known hydrothermal synthesis methods for MFI zeolite.
- structure-directing agent SDA
- hydrothermal synthesis method by adding hydrothermally synthesized MFI zeolite as a seed crystal or as a seed slurry in a crystallization stage.
- organic SDA inorganic cations and anions are known to be involved in the structure, and zeolite synthesis depends on the combined action of each component.
- a method of synthesizing in the presence of an ammonium salt an aluminum source needs to be added as appropriate so as to obtain a desired silica-alumina molar ratio of the zeolite used in the present embodiment.
- zeolite can also be used as long as it has the above-mentioned specific physical properties and composition.
- examples of such commercially available products include MFI-27 from Zude Chemy AG, and Zeolis International. ZD03030 (MFI-42) of the company.
- the zeolite-containing catalyst of the present embodiment can be produced by molding, for example, as follows using the zeolite having the specific physical properties and composition described above.
- the molding method is not particularly limited, and may be a general method. Specifically, a method of compression molding a catalyst component, a method of extrusion molding, and a spray dry molding method optimal for a fluidized bed reaction method can be mentioned.
- a binder can be used for molding. It does not restrict
- the mass ratio of zeolite / binder is preferably in the range of 10/90 to 90/10, more preferably in the range of 20/80 to 80/20.
- the zeolite-containing catalyst of the present embodiment may contain at least one metal element selected from the group consisting of elements belonging to Group IB of the periodic table. This means that the zeolite in the catalyst contains or is supported on the catalyst in the corresponding cationic state. It is one of the preferred embodiments that the zeolite-containing catalyst contains at least one metal selected from the group consisting of metals belonging to Group IB of the Periodic Table, that is, copper, silver, and gold. More preferred group IB metals include copper and silver, and more preferably silver.
- the term "periodic table" in the present embodiment CRC Handbook of Chemistry and Physics, 75 th edition David R. Lide et al, CRC Press Inc. issued (1994- 1995), 1-15 pp Means the listed periodic table.
- ion exchange site of the zeolite contained in the zeolite-containing catalyst is exchanged with a group IB metal cation and / or proton.
- ion exchange sites other than those exchanged with group IB metal cations and / or protons may be exchanged with alkali metal cations, alkaline earth metal cations, and other metal cations.
- Examples of the method of causing the zeolite-containing catalyst to contain at least one metal element selected from the group consisting of metal elements belonging to Group IB of the Periodic Table include a method of causing zeolite to contain a Group IB metal element.
- a method of treating a zeolite or zeolite-containing catalyst not containing a group IB metal by an ion exchange method more specifically, a liquid phase ion exchange treatment method or an impregnated supported catalyst at a high temperature to treat solid phase ions.
- a method of exchanging processing is mentioned.
- group IB metal salt examples include silver nitrate, silver acetate, silver sulfate, copper chloride, copper sulfate, copper nitrate, and gold chloride. Silver nitrate and copper nitrate are preferable, and silver nitrate is more preferably used.
- the content of the group IB metal in the zeolite is preferably 0.1 to 5% by mass, more preferably 0.2 to 3% by mass.
- the content of the group IB metal in the zeolite can be determined by X-ray fluorescence analysis or the like.
- a pretreatment step may be performed on the zeolite-containing catalyst.
- a pretreatment step (A) a heat treatment step at a temperature of 550 ° C. or higher, (B) a step of heat treatment at a temperature of 300 ° C. or higher in the presence of water vapor; Is mentioned.
- the treatment is preferably performed at a temperature of 550 ° C. or higher and 1000 ° C. or lower (although the atmosphere is not particularly limited) under an inert gas flow condition such as air or nitrogen.
- a mixed gas of an inert gas such as air or nitrogen and steam (water vapor) is circulated at a temperature of 300 ° C. or higher and 900 ° C. or lower (although the atmosphere is not particularly limited). It is preferable to perform the treatment under conditions of a pressure of 0.01 atmosphere or more. In the present specification, the step (B) may be simply referred to as “steam treatment”.
- the reaction system since water coexists in the reaction system, the reaction system is in the same state as or similar to the case where the steam treatment is performed in a heated state. For this reason, prior to the reaction, (A) heat treatment at a temperature of 550 ° C. or higher and / or (B) steam treatment, that is, so-called pretreatment, is performed for the reaction and regeneration for burning and removing the deposited coke is performed. By repeating the reaction / regeneration cycle, the performance of the pretreated catalyst is gradually approached. In other words, these pretreatments can be said to be methods for obtaining the effect of improving the selectivity of the catalyst of the present embodiment over time by shortening the time. At this time, as described above, the zeolite having a crystallization index of 3.3 or higher in the present embodiment has a small decrease in activity in these pretreatments (and also in the repetition of reaction / regeneration).
- the hydrocarbon raw material contains ethylene in a range exceeding 50% by mass.
- the ethylene content in the hydrocarbon raw material is preferably 55% by mass or more, and more preferably 60% by mass or more.
- the ethylene content is 50% by mass or less as in the prior art, it may be necessary to mix a diluent gas with the hydrocarbon raw material, which is not preferable because productivity tends to decrease.
- hydrocarbon raw material containing ethylene for example, one obtained by thermal decomposition and / or oxidative dehydrogenation reaction of ethane or ethanol dehydration reaction can be used.
- ethanol may be derived from biomass.
- the hydrocarbon raw material can contain alkanes and other olefins.
- alkanes include methane, ethane, propane, butane, pentane, hexane, heptane, octane, and nonane.
- olefins include propylene, butene, pentene, hexene, heptene, octene, and nonene.
- cycloalkanes such as cyclopentane, methylcyclopentane and cyclohexane; cycloolefins such as cyclopentene, methylcyclopentene and cyclohexene; and / or dienes such as cyclohexadiene, butadiene, pentadiene and cyclopentadiene and acetylene
- acetylenes such as methylacetylene.
- oxygen-containing compounds such as t-butyl alcohol, methyl-t-butyl ether, diethyl ether, methyl ethyl ether, dimethyl ether, ethanol and methanol.
- the hydrocarbon raw material containing ethylene may contain hydrogen, nitrogen, carbon dioxide, carbon monoxide and the like.
- hydrocarbons such as unreacted ethane and acetylene and water, hydrogen, carbon dioxide, monoxide are included in the reaction product produced by the so-called steam cracking method of ethane, in which ethane is thermally decomposed in the presence of water vapor. Although carbon etc. are contained, this reaction product can be used as a raw material as it is.
- Biomass ethanol is not particularly limited as long as it is ethanol obtained from plant resources.
- Specific examples of biomass ethanol include ethanol obtained by fermentation of sugarcane and corn, and ethanol obtained from wood resources such as waste wood, thinned wood, rice straw, and crops.
- Propylene can be separated from the reaction product (propylene-containing gas) produced by contacting the hydrocarbon raw material with the zeolite-containing catalyst by a method such as distillation separation, and at least a part of the remaining can be recycled to the reactor.
- the residue obtained by removing propylene from the reaction product contains a low boiling component containing ethylene and / or a high boiling component containing butene.
- the ethylene content in the mixed raw material of the recycled component and the feedstock has a concentration exceeding 50% by mass.
- the zeolite-containing catalyst containing a zeolite having specific physical properties and composition used in the present embodiment hardly causes permanent deterioration even when water is present in the reaction system. Therefore, in recent years, the reaction product produced by the so-called steam cracking method of ethane, in which ethane, which has been attracting attention as a raw material for a new propylene production reaction, is thermally decomposed in the presence of water vapor, can be used as it is, or biomass ethanol ( This is extremely advantageous for industrial use in that ethylene and water can be used as they are by dehydration.
- the method for producing propylene of the present embodiment includes a step of catalytically converting a hydrocarbon raw material containing ethylene exceeding 50 mass% with a zeolite-containing catalyst containing the specific zeolite while supplying water.
- the “catalytic conversion” is a catalytic reaction that proceeds at an interface of a heterogeneous phase, that is, a reaction in which a reaction material in a gas phase and / or a liquid phase is brought into contact with a solid catalyst. It shows a reaction in which a substance conversion occurs.
- a heterogeneous phase that is, a reaction in which a reaction material in a gas phase and / or a liquid phase is brought into contact with a solid catalyst. It shows a reaction in which a substance conversion occurs.
- catalytic conversion of ethylene when a raw material containing ethylene is supplied to a reactor containing a zeolite-containing catalyst, the raw material comes into contact with the catalyst, and at least a part of ethylene contained in the raw material undergoes a conversion reaction to produce propylene.
- the propylene production method of the present embodiment is carried out while supplying water to the reactor together with the hydrocarbon raw material containing ethylene.
- the coexistence of water is known to be effective in improving the reaction selectivity and extending the life of the catalyst by suppressing coke formation, and also has the effect of reducing the ethylene partial pressure in the reaction system by diluting the hydrocarbon raw material. is there.
- the reduction of the olefin partial pressure is advantageous for improving the yield of propylene due to the reaction equilibrium, but if the water pressure in the reaction system is high, there is a risk of promoting permanent deterioration due to structural destruction of the zeolite.
- the amount of water supplied to the reactor is preferably 10% by mass or more, more preferably, based on the hydrocarbon raw material, from the viewpoint of reaction results, coke suppression, productivity, and permanent deterioration suppression. It is 20% by mass or more, more preferably 30 to 80% by mass.
- the reaction product obtained by the ethane steam cracking method contains water as a raw material, and when ethanol is used as a raw material, ethylene and water are produced by a dehydration reaction. It is not necessary to supply.
- the conversion rate of ethylene is preferably in the range of 45 to 85%, more preferably in the range of 50 to 80%.
- the produced olefin has thermal equilibrium, and from the viewpoint of obtaining a high propylene yield, a high reaction temperature exceeding 500 ° C. is suitable.
- the temperature is preferably in the range of 520 ° C to 600 ° C.
- catalyst deterioration is accelerated.
- the reaction pressure is preferably in the range of 0.1 to 30 atmospheres, more preferably in the range of 0.5 to 10 atmospheres.
- the feed rate of the hydrocarbon raw material is a space velocity (WHSV) based on the zeolite mass of the zeolite-containing catalyst, preferably 0.1 to 20 Hr ⁇ 1 , more preferably 0.5 to 10 Hr ⁇ 1 .
- WHSV space velocity
- the reactor for reacting the hydrocarbon raw material containing ethylene with the zeolite-containing catalyst is not particularly limited, and any reactor such as a fixed bed type, a fluidized bed type, and a moving bed type can be used.
- a zeolite-containing catalyst When a zeolite-containing catalyst is used in a propylene production reaction, a carbonaceous compound (coke) is gradually formed on the catalyst, and the catalytic activity may be lowered.
- a fixed bed reactor When a fixed bed reactor is used for propylene production reaction, temporarily stop the supply of hydrocarbon raw material, and burn the coke accumulated in the zeolite-containing catalyst using a gas containing oxygen.
- the zeolite-containing catalyst When moving bed and fluidized bed reactors are used, a part of the zeolite-containing catalyst is continuously or intermittently removed from the reactor, and the coke deposited with oxygen-containing gas is burned.
- the zeolite-containing catalyst can be regenerated.
- the regenerated zeolite-containing catalyst can be returned to the reactor.
- the regeneration of the zeolite-containing catalyst is usually carried out under conditions of 400 to 700 ° C. in air or in a mixed gas composed of air and an inert gas.
- the present embodiment will be described more specifically with reference to examples.
- the present embodiment is not limited to only these examples.
- the measuring method performed in the Example and the comparative example is as follows.
- zeolite silica / alumina (SiO 2 / Al 2 O 3 ) molar ratio 0.2 g of zeolite was added to 50 g of a 5N sodium hydroxide (NaOH) aqueous solution. This was transferred to a stainless steel micro cylinder with a Teflon (registered trademark) inner tube, and the micro cylinder was sealed. The zeolite was completely dissolved by holding the micro bomb in an oil bath for 15 to 70 hours. The resulting zeolite solution is diluted with ion-exchanged water, and the silicon and aluminum concentrations in the diluted solution are measured with the following plasma emission spectrometer (ICP device).
- ICP device plasma emission spectrometer
- silica / alumina molar ratio of the zeolite is calculated.
- Apparatus JOBIN YVON (JY138 ULTRACE) manufactured by Rigaku Denki Co., Ltd. Measurement conditions Silicon measurement wavelength: 251.60 nm Aluminum measurement wavelength: 396.152 nm Plasma power: 1.0 kW Nebulizer gas: 0.28 L / min Sheath gas: 0.3 to 0.8 L / min Coolant gas: 13L / min
- the ammonia desorption amount was calculated from the total area of waveforms having a peak top at a desorption temperature of 240 ° C. or higher, based on a separately obtained calibration curve, and converted per zeolite weight (unit: ⁇ mol / g-zeolite).
- Example 1 [Hydrothermal synthesis of raw material zeolite]
- Special No. 3 sodium silicate (Fuji Chemical Co., Ltd., SiO 2 25 mass% Na 2 O 8 mass%) 92 kg, water 95 kg, aluminum sulfate 16-hydrate 7.3 kg, and sulfuric acid (purity 97%) 3.0 kg
- FIG. 1 shows an X-ray diffraction spectrum of a sample obtained by adding 1 g of rutile type titania to 5 g of the obtained H-type ZSM-5 zeolite and collecting it with an electric mortar for 30 minutes. From the measurement result of X-ray diffraction, the crystallization index of H-type ZSM-5 zeolite was determined to be 3.73. The silica / alumina molar ratio was 40.
- Example 2 Using the zeolite obtained in Example 1, catalyst preparation, molding and pretreatment were carried out in the same manner as in Example 1. As a result of measuring the amount of TPD acid of the obtained steam-treated zeolite-containing catalyst, it was 89 ⁇ mol / g-zeolite. 50 g of the obtained steam treatment catalyst was filled in a stainless steel reaction tube having an inner diameter of 21.2 mm ⁇ , and the test was repeated three times with the reaction temperature of the first and third reactions being 520 ° C. and the reaction temperature of the second reaction being 580 ° C. A reaction evaluation experiment was conducted in the same manner as in Example 1 except that. The test results are shown in Table 2.
- Example 3 NH 4 type MFI type zeolite ZD03030 (silica / alumina molar ratio 42) manufactured by Zeolis International was kneaded with silica sol and extruded. The content of zeolite was 50% by mass. The obtained extrusion molded catalyst was dried at 120 ° C. for 6 hours and then calcined at 700 ° C. for 2 hours to obtain a columnar zeolite-containing molded catalyst having a diameter of 2 mm and a length of 3 to 5 mm. The obtained shaped catalyst was stirred and ion exchanged in a 0.5N nitric acid aqueous solution, washed with water, and dried at 120 ° C. for 5 hours. On the other hand, the crystallization index of this zeolite was 4.45. An X-ray diffraction spectrum is shown in FIG.
- H-MFI type zeolite MFI-27 (silica alumina molar ratio 27) manufactured by Sud Chemie AG was kneaded with silica sol and extruded. The content of zeolite was 50% by mass.
- the obtained extrusion molded catalyst was dried at 120 ° C. for 6 hours and then calcined at 700 ° C. for 2 hours to obtain a columnar zeolite-containing molded catalyst having a diameter of 2 mm and a length of 3 to 5 mm.
- the obtained shaped catalyst was stirred and ion exchanged in a 0.5N nitric acid aqueous solution, washed with water, and dried at 120 ° C. for 5 hours.
- the crystallization index of this zeolite was 3.37.
- An X-ray diffraction spectrum is shown in FIG.
- Example 5 Silver exchange was performed on the zeolite-containing shaped catalyst obtained in Example 1. 50 g of the zeolite-containing shaped catalyst was added to 450 g of a 0.1N silver nitrate aqueous solution and stirred at room temperature for 2 hours. Thereafter, filtration and washing were performed, and the zeolite-containing molded catalyst was dried at 120 ° C. for 5 hours to obtain a silver-supporting zeolite-containing molded catalyst. The amount of silver supported was 0.95% by mass as measured by fluorescent X-ray analysis.
- Example 6 [Hydrothermal synthesis of raw material zeolite] To a solution in which 130 g of ethyl silicate was dissolved in 278 g of ethanol, 291 g of a 10% by mass tetrapropylammonium hydroxide aqueous solution in which 1.5 g of aluminum sulfate hexahydrate was dissolved was added. This mixed solution was mixed and stirred at 5000 rpm for 10 minutes with a homogenizer to obtain a uniform transparent solution. 350 g of this solution was charged into a 1 liter autoclave and hydrothermally synthesized at 125 ° C. for 110 hours while stirring at 500 rpm for crystallization.
- FIG. 10 shows an X-ray diffraction spectrum of a sample obtained by adding 1 g of rutile type titania to 5 g of the obtained H-type ZSM-5 zeolite and performing the analysis for 30 minutes with an electric mortar. From the measurement result of X-ray diffraction, the crystallization index of H-type ZSM-5 zeolite was determined to be 4.37. The silica / alumina molar ratio was 290.
- NH 4 type MFI type zeolite CBV2802 (silica / alumina molar ratio 280) manufactured by Zeolis International was kneaded with silica sol and extruded. The content of zeolite was 50% by mass.
- the obtained extrusion molded catalyst was dried at 120 ° C. for 6 hours and then calcined at 700 ° C. for 2 hours to obtain a columnar zeolite-containing molded catalyst having a diameter of 2 mm and a length of 3 to 5 mm.
- the obtained shaped catalyst was stirred and ion exchanged in a 0.5N nitric acid aqueous solution, washed with water, and dried at 120 ° C. for 5 hours.
- water of 10% by mass or more is supplied to a hydrocarbon raw material from a hydrocarbon raw material containing ethylene exceeding 50% by mass efficiently and stably over the long term.
- it is possible to produce propylene.
- it is an industrially useful method also from the viewpoint of the diversity of propylene production raw materials.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Crystallography & Structural Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Catalysts (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/254,373 US8450551B2 (en) | 2009-03-02 | 2010-03-01 | Method for producing propylene |
| JP2011502746A JP5607024B2 (ja) | 2009-03-02 | 2010-03-01 | プロピレンの製造方法 |
| BRPI1009564-0A BRPI1009564B1 (pt) | 2009-03-02 | 2010-03-01 | Method for producing propylene. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009-048369 | 2009-03-02 | ||
| JP2009048369 | 2009-03-02 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010101121A1 true WO2010101121A1 (ja) | 2010-09-10 |
Family
ID=42709678
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2010/053265 Ceased WO2010101121A1 (ja) | 2009-03-02 | 2010-03-01 | プロピレンの製造方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8450551B2 (cg-RX-API-DMAC7.html) |
| JP (1) | JP5607024B2 (cg-RX-API-DMAC7.html) |
| BR (1) | BRPI1009564B1 (cg-RX-API-DMAC7.html) |
| TW (1) | TWI399358B (cg-RX-API-DMAC7.html) |
| WO (1) | WO2010101121A1 (cg-RX-API-DMAC7.html) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011135785A1 (en) * | 2010-04-30 | 2011-11-03 | Toyota Jidosha Kabushiki Kaisha | Propylene production method |
| WO2013099220A1 (en) * | 2011-12-28 | 2013-07-04 | Showa Denko K.K. | Method of producing catalyst and method of producing unsaturated hydrocarbon using said catalyst |
| JP2014047176A (ja) * | 2012-08-31 | 2014-03-17 | Mitsubishi Chemicals Corp | プロピレンの製造方法 |
| JPWO2016017794A1 (ja) * | 2014-08-01 | 2017-04-27 | 千代田化工建設株式会社 | 複合体触媒、複合体触媒の製造方法、低級オレフィンの製造方法および複合体触媒の再生方法 |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11492264B2 (en) * | 2018-03-23 | 2022-11-08 | Ngk Insulators, Ltd. | Seed crystals, method of producing seed crystals, method of producing seed crystals attachment support, and method of producing zeolite membrane complex |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH03293031A (ja) * | 1990-04-10 | 1991-12-24 | Tosoh Corp | 高耐熱水性ゼオライト及びその製造方法 |
| JPH05147925A (ja) * | 1991-11-27 | 1993-06-15 | Tosoh Corp | ゼオライト及びその製造方法 |
| JPH07291620A (ja) * | 1994-04-22 | 1995-11-07 | Mizusawa Ind Chem Ltd | 高耐熱水性高シリカゼオライト及びその製造方法 |
| JPH1052646A (ja) * | 1996-04-18 | 1998-02-24 | Sanyo Sekiyu Kagaku Kk | 高シリカゼオライト系触媒 |
| JP2005232122A (ja) * | 2004-02-23 | 2005-09-02 | Mitsubishi Chemicals Corp | プロピレンの製造方法 |
| JP2005281254A (ja) * | 2004-03-30 | 2005-10-13 | Mitsubishi Chemicals Corp | プロピレンの製造方法 |
| JP2007191444A (ja) * | 2006-01-20 | 2007-08-02 | Tokyo Institute Of Technology | プロピレンの製造方法、触媒の再生方法、固体酸触媒 |
| WO2007114195A1 (ja) * | 2006-03-30 | 2007-10-11 | Mitsubishi Chemical Corporation | プロピレンの製造方法 |
| WO2009031445A1 (ja) * | 2007-09-06 | 2009-03-12 | Asahi Kasei Chemicals Corporation | プロピレンの製造方法 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4150062A (en) * | 1976-12-20 | 1979-04-17 | Mobil Oil Corporation | Light olefin processing |
| JPS58145616A (ja) * | 1982-02-22 | 1983-08-30 | Teijin Yuka Kk | 新規結晶性アルミノシリケ−トゼオライト及びその製造法 |
| US4845063A (en) * | 1982-10-15 | 1989-07-04 | Mobil Oil Corporation | Zeolite catalyst of improved hydrothermal stability |
| US4527001A (en) * | 1983-11-15 | 1985-07-02 | Union Carbide Corporation | Small olefin interconversions |
| US4605807A (en) * | 1984-04-27 | 1986-08-12 | Atlantic Richfield Company | Process for catalytic conversion of ethylene to higher hydrocarbons |
| JPS6168319A (ja) * | 1984-09-10 | 1986-04-08 | Asahi Chem Ind Co Ltd | 結晶性アルミノシリケ−トの合成法 |
| US4849194A (en) * | 1987-05-26 | 1989-07-18 | Mobil Oil Corporation | Measurement and control of zeolite synthesis |
| EP1061116A1 (en) | 1999-06-16 | 2000-12-20 | Fina Research S.A. | Production of olefins |
| RU2240312C1 (ru) | 2001-02-14 | 2004-11-20 | Асахи Касеи Кабусики Кайся | Способ получения эпсилон-капролактама |
| WO2005056504A1 (ja) * | 2003-12-12 | 2005-06-23 | Mitsubishi Chemical Corporation | プロピレンの製造方法 |
| JP4774813B2 (ja) | 2005-06-03 | 2011-09-14 | 三菱化学株式会社 | プロピレンの製造方法 |
| BRPI0619761A2 (pt) * | 2005-12-01 | 2011-10-18 | Basf Catalysts Llc | métodos de produzir um captador de hidrocarboneto de zeólito hidrotermicamente estável e de tratar gás de escapamento de um motor de combustão interna, e, material captador de hidrocarboneto |
-
2010
- 2010-03-01 BR BRPI1009564-0A patent/BRPI1009564B1/pt active IP Right Grant
- 2010-03-01 JP JP2011502746A patent/JP5607024B2/ja active Active
- 2010-03-01 US US13/254,373 patent/US8450551B2/en active Active
- 2010-03-01 WO PCT/JP2010/053265 patent/WO2010101121A1/ja not_active Ceased
- 2010-03-02 TW TW099106014A patent/TWI399358B/zh active
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH03293031A (ja) * | 1990-04-10 | 1991-12-24 | Tosoh Corp | 高耐熱水性ゼオライト及びその製造方法 |
| JPH05147925A (ja) * | 1991-11-27 | 1993-06-15 | Tosoh Corp | ゼオライト及びその製造方法 |
| JPH07291620A (ja) * | 1994-04-22 | 1995-11-07 | Mizusawa Ind Chem Ltd | 高耐熱水性高シリカゼオライト及びその製造方法 |
| JPH1052646A (ja) * | 1996-04-18 | 1998-02-24 | Sanyo Sekiyu Kagaku Kk | 高シリカゼオライト系触媒 |
| JP2005232122A (ja) * | 2004-02-23 | 2005-09-02 | Mitsubishi Chemicals Corp | プロピレンの製造方法 |
| JP2005281254A (ja) * | 2004-03-30 | 2005-10-13 | Mitsubishi Chemicals Corp | プロピレンの製造方法 |
| JP2007191444A (ja) * | 2006-01-20 | 2007-08-02 | Tokyo Institute Of Technology | プロピレンの製造方法、触媒の再生方法、固体酸触媒 |
| WO2007114195A1 (ja) * | 2006-03-30 | 2007-10-11 | Mitsubishi Chemical Corporation | プロピレンの製造方法 |
| WO2009031445A1 (ja) * | 2007-09-06 | 2009-03-12 | Asahi Kasei Chemicals Corporation | プロピレンの製造方法 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011135785A1 (en) * | 2010-04-30 | 2011-11-03 | Toyota Jidosha Kabushiki Kaisha | Propylene production method |
| WO2013099220A1 (en) * | 2011-12-28 | 2013-07-04 | Showa Denko K.K. | Method of producing catalyst and method of producing unsaturated hydrocarbon using said catalyst |
| JP2014047176A (ja) * | 2012-08-31 | 2014-03-17 | Mitsubishi Chemicals Corp | プロピレンの製造方法 |
| JPWO2016017794A1 (ja) * | 2014-08-01 | 2017-04-27 | 千代田化工建設株式会社 | 複合体触媒、複合体触媒の製造方法、低級オレフィンの製造方法および複合体触媒の再生方法 |
| US10556229B2 (en) | 2014-08-01 | 2020-02-11 | Chiyoda Corporation | Composite catalyst, method for producing composite catalyst, method for producing lower olefin and method for regenerating composite catalyst |
Also Published As
| Publication number | Publication date |
|---|---|
| BRPI1009564A2 (cg-RX-API-DMAC7.html) | 2016-03-22 |
| US20120004490A1 (en) | 2012-01-05 |
| BRPI1009564B1 (pt) | 2017-12-26 |
| US8450551B2 (en) | 2013-05-28 |
| JP5607024B2 (ja) | 2014-10-15 |
| TWI399358B (zh) | 2013-06-21 |
| JPWO2010101121A1 (ja) | 2012-09-10 |
| TW201036939A (en) | 2010-10-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5116043B2 (ja) | プロピレンの製造方法 | |
| JP3707607B2 (ja) | エチレンおよびプロピレンの製造方法 | |
| KR101227221B1 (ko) | 에탄올로부터의 올레핀의 제조 방법 | |
| KR20130025412A (ko) | 피독된 산성 촉매에서의 알코올의 탈수화 | |
| JPWO2000010948A1 (ja) | エチレンおよびプロピレンの製造方法 | |
| JP5607024B2 (ja) | プロピレンの製造方法 | |
| US10773250B2 (en) | Modified crystalline aluminosilicate for dehydration of alcohols | |
| KR20080081288A (ko) | 프로필렌 및 방향족 탄화수소의 제조 방법 및 상기 제조장치 | |
| US20170252731A1 (en) | Composite catalyst, method for producing composite catalyst, method for producing lower olefin and method for regenerating composite catalyst | |
| JP2025157508A (ja) | エタノールの変換方法、及びその他炭化水素の製造方法 | |
| US10472300B2 (en) | Process for preparing olefins by dehydrating alcohols with less side effects comprising addition of sulfur containing compounds | |
| JP4921788B2 (ja) | エチレン及びプロピレンを製造する方法 | |
| JPWO2007032447A1 (ja) | エチレン及びプロピレンを製造する方法 | |
| TWI900821B (zh) | 乙醇之轉換方法、及其他烴之製造方法 | |
| US8168842B2 (en) | Process for the alkylation of a cycloalkene | |
| TWI886774B (zh) | 乙醇之轉換方法、含沸石之觸媒、含沸石之觸媒之製造方法、及烴等之製造方法 | |
| EP4596525A1 (en) | Ethanol conversion method, hydrocarbon production method, propylene production method, aromatic compound production method, and ethanol conversion device | |
| JP2023177331A (ja) | アルコールの変換方法、及び炭化水素の製造方法 | |
| JP2024121026A (ja) | エタノール及びエチレンの変換方法、プロピレンの製造方法、芳香族化合物の製造方法、並びに、エタノール及びエチレンの変換装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10748716 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011502746 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13254373 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10748716 Country of ref document: EP Kind code of ref document: A1 |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: PI1009564 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: PI1009564 Country of ref document: BR Kind code of ref document: A2 Effective date: 20110902 |