WO1999002516A1 - Compuestos derivados de tiofeno y benzotiofeno y utilizacion y composicion correspondientes - Google Patents
Compuestos derivados de tiofeno y benzotiofeno y utilizacion y composicion correspondientes Download PDFInfo
- Publication number
- WO1999002516A1 WO1999002516A1 PCT/ES1998/000191 ES9800191W WO9902516A1 WO 1999002516 A1 WO1999002516 A1 WO 1999002516A1 ES 9800191 W ES9800191 W ES 9800191W WO 9902516 A1 WO9902516 A1 WO 9902516A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- piperazin
- propan
- methoxyphenyl
- alkyl
- thiophene
- Prior art date
Links
- 0 *=*C1OC1c1ccc[s]1 Chemical compound *=*C1OC1c1ccc[s]1 0.000 description 2
- QKQJRUOBJMLOJX-UHFFFAOYSA-N CC(CCC(CC1)N)(CC1NCCNC)N Chemical compound CC(CCC(CC1)N)(CC1NCCNC)N QKQJRUOBJMLOJX-UHFFFAOYSA-N 0.000 description 1
- PWUNNUBHWLOPSS-UHFFFAOYSA-N CN(CC1)CCN1[AlH2] Chemical compound CN(CC1)CCN1[AlH2] PWUNNUBHWLOPSS-UHFFFAOYSA-N 0.000 description 1
- QUIMJTKRVOBTQN-UHFFFAOYSA-N Cc1ccc(CO)c(C)c1 Chemical compound Cc1ccc(CO)c(C)c1 QUIMJTKRVOBTQN-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D409/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms
- C07D409/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings
- C07D409/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having sulfur atoms as the only ring hetero atoms containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/14—Radicals substituted by singly bound hetero atoms other than halogen
- C07D333/20—Radicals substituted by singly bound hetero atoms other than halogen by nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/22—Radicals substituted by doubly bound hetero atoms, or by two hetero atoms other than halogen singly bound to the same carbon atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/28—Halogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/30—Hetero atoms other than halogen
- C07D333/32—Oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/26—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D333/42—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms with nitro or nitroso radicals directly attached to ring carbon atoms
- C07D333/44—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms with nitro or nitroso radicals directly attached to ring carbon atoms attached in position 5
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D333/52—Benzo[b]thiophenes; Hydrogenated benzo[b]thiophenes
- C07D333/54—Benzo[b]thiophenes; Hydrogenated benzo[b]thiophenes with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to carbon atoms of the hetero ring
- C07D333/58—Radicals substituted by nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/50—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom condensed with carbocyclic rings or ring systems
- C07D333/52—Benzo[b]thiophenes; Hydrogenated benzo[b]thiophenes
- C07D333/62—Benzo[b]thiophenes; Hydrogenated benzo[b]thiophenes with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to carbon atoms of the hetero ring
- C07D333/64—Oxygen atoms
Definitions
- the present invention relates to compounds derived from thiophene and benzothiophene, their salts, optical isomers and polymorphs, of general formula (I):
- the last class of antidepressants introduced in the market is that of selective serotonin reuptake inhibitors, among which the following stand out: fluoxetine (Lilly ES 433720), paroxetine (Ferrosan, ES 422734) and sertraline (Pfizer, ES 496443).
- This class of compounds has a high degree of structural diversity compared to other types of serotonin reuptake inhibitors, such as tricyclic antidepressants.
- These compounds despite their structural variety, have a high selectivity for the serotonin receptor. In fact, its binding to a and ⁇ adrenergic, dopaminergic, histamine, and muscarinic receptors is little or significant. It is postulated that this could be due to a great structural similarity with the pharmacophore that is responsible for its specificity and relative affinity towards the corresponding serotonin receptor.
- WAY-100635 (Fornal CA et al., Brit.]. Pharmacol: 1 12, (2), 92P, 1994; Fletcher A . et al., Brit. ⁇ . Pharmacol. 1 12, (2) 91 P, 1994)
- the object of this invention is the synthesis of compounds that exhibit this dual activity, that is, serotonin reuptake inhibitors with antagonistic activity towards the 5-HT, A receptor.
- the present invention relates to the synthesis and pharmacological activity of new compounds derived from thiophene and benzothiophene of general formula I.
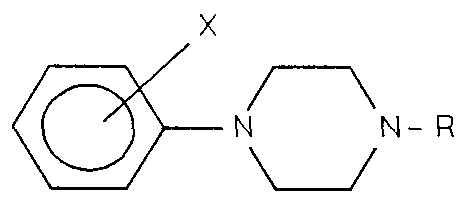
- X is generally: -S-, or -S (O) - but it can be, among others: -C (O) -; -CH (OR) -; -C (N-OR) -; -CH (NH 2 ) -;
- A can be an alkylene group and T is generally a 1,2-benzoisoxazole or 1,2-benzothiazole ring, but it can be any other aromatic ring.
- none of the products claimed in the present invention are described and, on the other hand, the products of said patent are claimed as antipsychotics but not as antidepressants.
- R can be: -CH 2 -CH 2 -C (0) -Ar and Ar can be among others a thiophene ring.
- R is 2-F or 4-F or 4-CI (possibilities not included in the present invention).
- R is 2-F or 4-F or 4-CI (possibilities not included in the present invention).
- GB 1096341 its therapeutic action as antidepressants is not claimed.
- R is H, methyl or halogen.
- GB 1294720 products are described with some analogy with the desritos in the present invention but in which the union between the group Z (referred to the present invention) and the piperazine ring is made through an alkenyl chain of at least 3 carbon atoms DESCRIPTION OF THE INVENTION
- the object of the present invention are the new thiophene and benzothiophene derivatives of general formula (I) as well as the corresponding compositions and their use for obtaining compositions with pharmacological activity.
- Z is: -CO-, -CH (OR 6 ) -, -C (NOR 7 ) -;
- R is: H, C, -C 6 alkyl, halogen, or -0-R ] 2 ;
- R 4 and R 5 the same or different, mean: H, C alkyl, -C 6 , halogen, haloalkyl, -OR 12 , nitro, -NR 13 R 14 , -COR 12 , -C0 2 R 12 , -S0 2 NR 13 R 14 , -S0 2 R 12 , -SR 12 , cyano, -CONR 13 R 14 or R 4 and R 5 together form a benzene ring fused to the phenyl ring; with the following exceptions: [a] one of them R 4 or R 5 cannot be: H, C alkyl, -C 6 , or halogen when the other (R 5 or R 4 ) is H, and R ,, R 2 and R 3 means hydrogen, Z is -CO- and is attached to position 2 of the thiophene ring; and [b] R 4 or R 5 cannot both be H or one of them halogen or C alkyl, -C 6 , when the
- R 6 is: H, C alkyl, -C 6 , -C0 2 R 12 , -C (0) NR 13 R 14 naphthyl or optional phenyl Nally substituted by one or more substituents chosen from: H, haloalkyl C, -C 6 , C-alkyl, -C 6 , halogen, C-alkoxy, -C 6 , methylenedioxy, nitro and cyano;
- R 7 is: H or C alkyl, -C 6 ;
- R 8 , Re ,, R I 0 and R are independent of each other and means H, C alkyl, -C 6 , halogen, -OR 12 , nitro, cyano, -NR 13 R 14 , -COR 12 , -C0 2 R 12 , -S0 2 NR 13 R 14 , - S0 2 R 12 , -SR 12 , -CONR 13 R 14 ;
- R, 2 is H, C, -C 6 alkyl, or phenyl
- R 13 and R I 4 are independent of each other and mean H, C, -C 6 alkyl, or phenyl or R 13 and R I 4 together with the N to which they are attached form a ring of 5 or 6 links in which optionally there may be an N, O or S. 5
- the invention also comprises the salts, solvates and salts of the physiologically acceptable solvates of the compounds of formula (I) and which include the acid addition salts formed with inorganic or organic acids, for example, hydrochlorides, hydrobromides, sulfates, nitrates, phosphates, formates, mesylates, citrates, benzoates, smokers, maleates, lactates and succinates among others.
- a salt of a compound of formula (I) is formed with a dicarboxylic acid, such as succinic acid
- the salt may contain between one and two moles of the compound of formula (I) per mole of acid. 5
- Preferred salts are hydrochlorides.
- Preferred solvates are hydrates.
- R is H or lower alkyl.
- R 4 is H or halogen
- R 5 is H, hydroxy or lower alkoxy
- R 6 is H or naphthyl
- R 7 is H
- R 8 , R 9 , R 10 and R are independent of each other and mean H, low molecular weight alkyl, halogen, -OR 17 , nitro, NR 13 R 14
- ⁇ 12 ⁇ - 13 / ⁇ 14 are independent of each other and mean H or alkyl
- the compounds object of the present invention are useful in the treatment of alterations related to the reuptake of serotonin and other alterations related to the post or presynaptic transmission of serotonin and in particular in the treatment of depression.
- the treatments may be preventive or curative and are performed by administration by any conventional route of administration of a compound of formula (1) or a physiologically acceptable salt or solvate thereof.
- the invention also provides a pharmaceutical composition acceptable for use in medicine, comprising: (a) a pharmaceutically effective amount of a compound of formula (I) and / or a salt or solvate thereof and (b ) a pharmaceutically acceptable excipient for oral, sublingual, parenteral, retard or intranasal administration
- the invention also relates to the use of a thiophene or benzothiophene derivative of formula (I) for the preparation of a medicament for therapeutic application as an antidepressant.
- compositions for oral administration may be solid, such as tablets or capsules prepared by conventional means with pharmaceutically acceptable excipients, or liquids such as aqueous or oily solutions, syrups, elixirs, emulsions or suspensions prepared by conventional means with pharmaceutically acceptable additives.
- R ,, R 2 , R 3 , R 4 and R 5 have the aforementioned meanings.
- a third procedure for obtaining the ketones of formula (la) consists in the transformation, by the methods described in the literature, of a substituent in a compound of formula (la) into a different substituent, thus obtaining a different compound that responds structurally to the same type of formula (la).
- An example of such transformations is the reduction of an aromatic N0 2 group, by the methods described in the literature, to an amino group.
- a preferred method of reduction consists in the use of sodium borohydride as a reducing agent in a medium of methyl or ethyl alcohol and at a temperature between -20 ° C and the reflux of the corresponding alcohol, preferably the reduction is carried out at 0 ° C.
- R, R 5 have the meanings indicated above.
- aryl ether derivatives of formula (le) where Z is -CH (OR 6 ) - R 6 being an optionally substituted naphthyl or phenyl aromatic ring are obtained by the following methods:
- reaction is preferably carried out in a solvent such as dimethylformamide, dimethylacetamide or any other suitable high boiling solvent:
- the derivative (le) can also be obtained by transforming the alcohols of the general formula (la) into derivatives with a good leaving group, such as a mesyl group, and reaction of these intermediates with the corresponding Phenolic derivative preferably in the presence of a base such as sodium or potassium hydroxide in an alcoholic medium
- R, R 5 have the meaning described above and where R 15 means H, halogen, C alkyl, -C 6 , haloalkyl, alkoxy, alkylenedioxy, nitro, cyano or a fused phenyl ring between any 2 positions .
- R, R 5 and R 13 are defined as indicated above.
- IR ⁇ cm 1 3380 (m, NH 2 ); 1245 (mf, Ar-O-).
- a mixture of 3 g of 1-naphthiamine (20.95 x 10 "3 moles) is refluxed for 24 hours together with 3.68 g of bis (2- cIoroethyl) amine hydrochloride (20.95 x 10 " 3 moles) and 2.90 g of Na 2 C0 3 (27.36 x 10 3 moles) 47 ml of chlorobenzene.
- the pH of the medium is controlled in order to keep it basic; For this, Na 2 C0 3 is added when necessary.
- the reaction is diluted with H 2 0 and the two phases are separated.
- the aqueous phase is extracted with AcOEt twice with 100 ml.
- the aqueous phase is extracted with AcOEt, washed with H 2 0, dried with Na 2 S0 4 and the solvent is removed to dryness. It is purified by hydrochloride formation and recrystallization of it in isopropanol. Yield: 29%.
- Example 17 l - (3-Methylbenzorbltiophene-2-yl) -3-r4- (2- methoxyphenePpiperazin-1 -illpropan-1-one) hydrochloride (VN-7012)
- Example 18 l - (3-Methylbenzorb1thiophene-2-yl) -3-r4- (2- hydroxyphenyl) piperazin-1 -illpropan-1-one hydrochloride. (VN-701 H)
- Example 25 l- (benzorb1thiophene-3-yl) -3-r4- (5-fluoro-2- methoxyphenePpiperazin-1 -illpropan-1 -ol (VN-222F) hydrochloride
- Example 27 Hbenzorb1tiofen-3-yl) -3-r4- (4-chloro phenyl) piperazin-l-illpropan-l-ol (VN-2225) Mp: 148-150 ° C.
- Example 28 3-f 4- (2-chlorophenyl) piperazin-1 -iH-1 - (thiophene-3-yl) propan-1 - oKVN-2120)
- Example 33 H2,5-dimethylthiophene-3-yl) -3-r4- (2-hydroxyphenyl) piperazin-1-i-propan-1-ol (VN-252H) Mp .: 117 ° C.
- Example 35 1 - (3,5-dimethylbenzorb1thiophene-2-yl) -3-F4- (2-methoxyphenyppi-perazin-1 -ill propan-1 -oI. (VN-7122)
- Example 36 l- (3-methylbenzofbltiophene-2-yl) -3-r4- (2-methoxyphenyl) piperazin-1 -illpropan-1 -ol. (VN-7022)
- Example 38 1- (3-methylthiophene-2-yl) -3-r4- (2-methoxyphenyl) piperazin-1- illpropan-l-ol (VN-2722)
- Example 40 Z-Oxime of 1 - (2,5-dimethylthiophene-3-yl) -3- [4- (2-methoxy-feniQpiperazin-1 -illpropan-1-one (VN-2582A)
- Example 41 E-oxime of l- (2,5-dimethylthiophene-3-yl) -3-r4- (2-methoxyphenyl) piperazin-1-inpropan-l-one (VN-2582B)
- Example 44 E, Z-Oxime of 3-r4- (2-methoxyphenylPpiperazin-1-yl " I-1 - (thiophene-3-yl) propan-1-one (VN-) dihydrochloride dihydrochloride
- Example 48 Oxime of 0-ethyl-3-r4- (2-methoxyphenyl) piperazin-1 -ill- 1 -
- Example 54 l - (2-methoxyphenyl) -4-r3-thiophene-3-yl-3- (3-trifluoromethylphene-xp-propylpiperazine (VN-2152)
- Example 55 Enantiomeric resolution of the product Hbenzorbltio-fen-3-iP-3-r4- (2-methoxyphenyl) piperazin-1 -ill-propan-1 -ol (VN-2222)
- HPLC HPLC Waters 600E; Waters 994 photodiode detector; Millennium workstation; Supelcosil LC-CN column 25 x 0.46 cm; FM: (hexane-
- the next step is hydrolysis under non-racemizing conditions.
- Each of the diastereomers is dissolved in methanol (40 mL), added
- VN-2222 1 H-NMR (CDCI 3 , 200 MHz) r (ppm): 2.09 (C, 2H, CHOH-CH 2 ), 2.6-2.9 (m, 6H, (CH 2 ) 3 N), 3, 1 -3.3 (m, 4H, (CH 2 ) 2 N-Ar), 3.86 (s, 3H, OCH 3 ), 5.35 (t, 1 H, CHOH), 7.01-7.31 (m, 4H, benzene), 7.4 (m, 2H, 5 H 5 + H 6 ), 7.44 (d, 1 H, H 2 ), 7.78-7.789 ( m, 2H, H 4 + H 7 )
- each enantiomer is derivatized with (R) - (+) - ⁇ -methoxy- ⁇ - (trifluoromethyl) phenylacetic acid chloride.
- Two vials are prepared with 5 mg (0.013 mmol) of each enantiomer, chlorine form (2 mL), triethylamine (6 ⁇ L, 0.039 mmol) and 4-dimethylaminopyridine (2 mg, 0.016 mmol). Two solutions are obtained which are added on each of the flasks containing the (R) - (+) - ⁇ -methoxy- ⁇ (trifluoromethyl) phenylacetic acid chloride obtained from the corresponding acid by the methods described in the literature.
- the frontal cortex of the rat is dissected and homogenized in 5 mM Tris-HCl pH 7.7 at 4 ° C.
- the resulting homogenate is centrifuged at 25,000 rpm for 15 min. and the pellet obtained is resuspended in Tris-HCl and incubated at 37 ° C for 10 min.
- the resulting resuspension is re-centrifuged and resuspended in Tris-HCl containing 4mM CaCI 2 .
- the incubation mixture contains the membrane suspension, 3H- or DPAT (1 nM) and the cold displacer. To separate the fraction fixed to the receptors, rapid filtration is performed.
- the membrane fraction of the frontal cortex of the rat is obtained as described for the determination of binding to 5-HT 1A receptors.
- the membrane suspension is incubated for 60 min at 22 ° C with 3 H-paroxetine using fluoxetine as a displacer. At the end of the incubation, the membrane fraction is separated by rapid filtration.
- the technique used is that described by Marcusson et al. (]. Neurochemistry 44,
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pain & Pain Management (AREA)
- Psychiatry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
- Plural Heterocyclic Compounds (AREA)
- Medicines Containing Plant Substances (AREA)
- Saccharide Compounds (AREA)
- Cosmetics (AREA)
Abstract
Description
Claims
Priority Applications (17)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IL13356698A IL133566A (en) | 1997-07-08 | 1998-07-01 | Compounds derived from benzothiophene, and related utilisation and composition |
| PL98337930A PL337930A1 (en) | 1997-07-08 | 1998-07-01 | Derivatives of benzothiophene as well as their application and compositions |
| SK27-2000A SK272000A3 (en) | 1997-07-08 | 1998-07-01 | Compounds derived from thiophene and benzothiophene, and related utilisation and composition |
| NZ502128A NZ502128A (en) | 1997-07-08 | 1998-07-01 | Benzothiophene compounds and their use in treating neurological disorders |
| BR9810557-4A BR9810557A (pt) | 1997-07-08 | 1998-07-01 | Compostos derivados de tiofeno e benzotiofeno e utilização e composição correspondentes |
| EA200000101A EA002687B1 (ru) | 1997-07-08 | 1998-07-01 | Производные бензотиофена, их применение и композиции |
| DE69822478T DE69822478D1 (de) | 1997-07-08 | 1998-07-01 | Thiophen- und benzothiophenderivate, deren verwendung und sie enthaltende zubereitung |
| AU81113/98A AU735637B2 (en) | 1997-07-08 | 1998-07-01 | Benzothiophene derivative and corresponding use and composition |
| JP50819399A JP2002511883A (ja) | 1997-07-08 | 1998-07-01 | ベンゾチオフェン誘導体ならびに対応する使用および組成物 |
| KR1020007000190A KR20010021632A (ko) | 1997-07-08 | 1998-07-01 | 티오펜 및 벤조티오펜으로부터 유도된 화합물, 그 관련용도 및 조성물 |
| CA002295715A CA2295715A1 (en) | 1997-07-08 | 1998-07-01 | Compounds derived from thophene and benzothiophene, and related utilisation and composition |
| EP98930807A EP1008594B1 (en) | 1997-07-08 | 1998-07-01 | Compounds derived from thophene and benzothiophene, and related utilisation and composition |
| AT98930807T ATE261956T1 (de) | 1997-07-08 | 1998-07-01 | Thiophen- und benzothiophenderivate, deren verwendung und sie enthaltende zubereitung |
| HU0002861A HUP0002861A3 (en) | 1997-07-08 | 1998-07-01 | Benzothiophene derivatives, and compositions containing the same |
| IS5328A IS5328A (is) | 1997-07-08 | 1999-12-29 | Bensóþíófenafleiður og samsvarandi notkun þeirra og sametning |
| NO20000038A NO20000038L (no) | 1997-07-08 | 2000-01-05 | Benzodiazepinderivater og tilsvarende anvendelse og sammensetning |
| US09/480,120 US6262056B1 (en) | 1997-07-08 | 2000-01-10 | Benzothiophene derivatives and corresponding use and composition |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES009701517A ES2128266B1 (es) | 1997-07-08 | 1997-07-08 | Compuestos derivados de tiofeno y benzotiofeno y utilizacion y composicion correspondientes. |
| ESP9701517 | 1997-07-08 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/480,120 Continuation-In-Part US6262056B1 (en) | 1997-07-08 | 2000-01-10 | Benzothiophene derivatives and corresponding use and composition |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1999002516A1 true WO1999002516A1 (es) | 1999-01-21 |
Family
ID=8299986
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/ES1998/000191 WO1999002516A1 (es) | 1997-07-08 | 1998-07-01 | Compuestos derivados de tiofeno y benzotiofeno y utilizacion y composicion correspondientes |
Country Status (21)
| Country | Link |
|---|---|
| US (1) | US6262056B1 (es) |
| EP (1) | EP1008594B1 (es) |
| JP (1) | JP2002511883A (es) |
| KR (1) | KR20010021632A (es) |
| CN (1) | CN1125821C (es) |
| AT (1) | ATE261956T1 (es) |
| AU (1) | AU735637B2 (es) |
| BR (1) | BR9810557A (es) |
| CA (1) | CA2295715A1 (es) |
| DE (1) | DE69822478D1 (es) |
| EA (1) | EA002687B1 (es) |
| ES (1) | ES2128266B1 (es) |
| HU (1) | HUP0002861A3 (es) |
| IL (1) | IL133566A (es) |
| IS (1) | IS5328A (es) |
| NZ (1) | NZ502128A (es) |
| OA (1) | OA11274A (es) |
| PL (1) | PL337930A1 (es) |
| SK (1) | SK272000A3 (es) |
| TR (1) | TR200000058T2 (es) |
| WO (1) | WO1999002516A1 (es) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001049678A1 (en) * | 1999-12-30 | 2001-07-12 | H. Lundbeck A/S | Substituted phenyl-piperazine derivatives, their preparation and use |
| WO2001007431A3 (en) * | 1999-07-21 | 2001-08-16 | Lilly Co Eli | Benzothiophene derivatives |
| WO2002044170A2 (en) * | 2000-11-29 | 2002-06-06 | Laboratorios Vita, S.A. | Benzothiophene derivative compounds, process of preparation and use thereof |
| US7094786B2 (en) | 2003-01-13 | 2006-08-22 | Dynogen Pharmaceuticals, Inc. | Method of treating nausea, vomiting, retching or any combination thereof |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10043659A1 (de) * | 2000-09-05 | 2002-03-14 | Merck Patent Gmbh | Arylpiperazinderivate |
| WO2004082686A2 (en) * | 2003-03-13 | 2004-09-30 | Dynogen Pharmaceuticals, Inc. | Use of compounds with combined 5-ht1a and ssri activities to treat sexual dysfunction |
| TWI320783B (en) | 2005-04-14 | 2010-02-21 | Otsuka Pharma Co Ltd | Heterocyclic compound |
| WO2008140198A1 (en) | 2007-05-14 | 2008-11-20 | Sk Holdings Co., Ltd. | Novel carbamoyloxy arylalkanoyl arylpiperazine compound, pharmaceutical compositions comprising the compound and method for treating pain, anxiety and depression by administering the compound |
| CN101613347B (zh) * | 2008-06-23 | 2012-07-04 | 中国人民解放军军事医学科学院毒物药物研究所 | 胺类化合物及其医药用途 |
| CN101619056B (zh) | 2008-07-02 | 2013-07-17 | 石药集团中奇制药技术(石家庄)有限公司 | 苯并噻吩烷醇哌嗪衍生物及其作为抗抑郁症药物的应用 |
| EP2539706B1 (en) * | 2010-02-24 | 2015-03-04 | Research Triangle Institute | Arylpiperazine opioid receptor antagonists |
| CN104725359B (zh) | 2013-12-20 | 2017-05-03 | 广东东阳光药业有限公司 | 取代的哌嗪化合物及其使用方法和用途 |
| WO2021152113A1 (en) | 2020-01-31 | 2021-08-05 | Bayer Aktiengesellschaft | Substituted 2,3-benzodiazepines derivatives |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2979507A (en) * | 1959-03-26 | 1961-04-11 | Paul Adriaan J Janssen | 1-(2-thenoyl) alkyl-4-arylpiperazines |
| US3002976A (en) * | 1959-10-12 | 1961-10-03 | Paul A J Janssen | 1-(2-thienyl)-omega-(4-arylpiperazine)alkanols |
| GB1096341A (en) * | 1965-03-09 | 1967-12-29 | Mauvernay Roland Yves | Novel derivatives of the phenyl-piperazine series and process for their preparation |
| US4515793A (en) * | 1983-07-27 | 1985-05-07 | Edna Mcconnell Clark Foundation | Phenylpiperazines which are useful in the treatment of schistosomiasis |
| EP0574313A1 (fr) * | 1992-06-12 | 1993-12-15 | Adir Et Compagnie | Pipérazines 1,4-disubstituées pour le traitement des maladies du système nerveux central et des maladies neuro endocriniennes |
| EP0596120A1 (en) * | 1991-08-09 | 1994-05-11 | Yoshitomi Pharmaceutical Industries, Ltd. | Thiophene compound |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5632898A (en) * | 1996-08-13 | 1997-05-27 | Isis Pharmaceuticals, Inc. | Method for removing unreacted electrophiles from a reaction mixture |
-
1997
- 1997-07-08 ES ES009701517A patent/ES2128266B1/es not_active Expired - Fee Related
-
1998
- 1998-07-01 NZ NZ502128A patent/NZ502128A/en unknown
- 1998-07-01 SK SK27-2000A patent/SK272000A3/sk unknown
- 1998-07-01 JP JP50819399A patent/JP2002511883A/ja active Pending
- 1998-07-01 DE DE69822478T patent/DE69822478D1/de not_active Expired - Lifetime
- 1998-07-01 AU AU81113/98A patent/AU735637B2/en not_active Ceased
- 1998-07-01 WO PCT/ES1998/000191 patent/WO1999002516A1/es not_active Application Discontinuation
- 1998-07-01 HU HU0002861A patent/HUP0002861A3/hu unknown
- 1998-07-01 CA CA002295715A patent/CA2295715A1/en not_active Abandoned
- 1998-07-01 PL PL98337930A patent/PL337930A1/xx unknown
- 1998-07-01 IL IL13356698A patent/IL133566A/xx not_active IP Right Cessation
- 1998-07-01 CN CN98807490A patent/CN1125821C/zh not_active Expired - Fee Related
- 1998-07-01 TR TR2000/00058T patent/TR200000058T2/xx unknown
- 1998-07-01 EA EA200000101A patent/EA002687B1/ru not_active IP Right Cessation
- 1998-07-01 BR BR9810557-4A patent/BR9810557A/pt not_active IP Right Cessation
- 1998-07-01 KR KR1020007000190A patent/KR20010021632A/ko not_active Application Discontinuation
- 1998-07-01 EP EP98930807A patent/EP1008594B1/en not_active Expired - Lifetime
- 1998-07-01 AT AT98930807T patent/ATE261956T1/de not_active IP Right Cessation
-
1999
- 1999-12-29 IS IS5328A patent/IS5328A/is unknown
-
2000
- 2000-01-06 OA OA1200000002A patent/OA11274A/en unknown
- 2000-01-10 US US09/480,120 patent/US6262056B1/en not_active Expired - Fee Related
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2979507A (en) * | 1959-03-26 | 1961-04-11 | Paul Adriaan J Janssen | 1-(2-thenoyl) alkyl-4-arylpiperazines |
| US3002976A (en) * | 1959-10-12 | 1961-10-03 | Paul A J Janssen | 1-(2-thienyl)-omega-(4-arylpiperazine)alkanols |
| GB1096341A (en) * | 1965-03-09 | 1967-12-29 | Mauvernay Roland Yves | Novel derivatives of the phenyl-piperazine series and process for their preparation |
| US4515793A (en) * | 1983-07-27 | 1985-05-07 | Edna Mcconnell Clark Foundation | Phenylpiperazines which are useful in the treatment of schistosomiasis |
| EP0596120A1 (en) * | 1991-08-09 | 1994-05-11 | Yoshitomi Pharmaceutical Industries, Ltd. | Thiophene compound |
| EP0574313A1 (fr) * | 1992-06-12 | 1993-12-15 | Adir Et Compagnie | Pipérazines 1,4-disubstituées pour le traitement des maladies du système nerveux central et des maladies neuro endocriniennes |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001007431A3 (en) * | 1999-07-21 | 2001-08-16 | Lilly Co Eli | Benzothiophene derivatives |
| WO2001049678A1 (en) * | 1999-12-30 | 2001-07-12 | H. Lundbeck A/S | Substituted phenyl-piperazine derivatives, their preparation and use |
| JP2003519224A (ja) * | 1999-12-30 | 2003-06-17 | ハー・ルンドベック・アクチエゼルスカベット | 置換されたフェニル−ピペラジン誘導体、その製造方法及びその使用方法 |
| US6699864B2 (en) | 1999-12-30 | 2004-03-02 | H. Lundbeck A/S | Substituted phenyl-piperazine derivatives, their preparation and use |
| WO2002044170A2 (en) * | 2000-11-29 | 2002-06-06 | Laboratorios Vita, S.A. | Benzothiophene derivative compounds, process of preparation and use thereof |
| WO2002044170A3 (en) * | 2000-11-29 | 2002-09-06 | Vita Invest Sa | Benzothiophene derivative compounds, process of preparation and use thereof |
| ES2188344A1 (es) * | 2000-11-29 | 2003-06-16 | Vita Invest Sa | Compuestos derivados de benzotiofeno, su procedimiento de obtencion y utilizacion de los mismos. |
| US7094786B2 (en) | 2003-01-13 | 2006-08-22 | Dynogen Pharmaceuticals, Inc. | Method of treating nausea, vomiting, retching or any combination thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| EA002687B1 (ru) | 2002-08-29 |
| IS5328A (is) | 1999-12-29 |
| OA11274A (en) | 2003-07-29 |
| SK272000A3 (en) | 2000-07-11 |
| EP1008594B1 (en) | 2004-03-17 |
| ES2128266A1 (es) | 1999-05-01 |
| PL337930A1 (en) | 2000-09-11 |
| AU8111398A (en) | 1999-02-08 |
| US6262056B1 (en) | 2001-07-17 |
| DE69822478D1 (de) | 2004-04-22 |
| BR9810557A (pt) | 2000-08-08 |
| CN1265106A (zh) | 2000-08-30 |
| JP2002511883A (ja) | 2002-04-16 |
| EA200000101A1 (ru) | 2000-08-28 |
| CN1125821C (zh) | 2003-10-29 |
| TR200000058T2 (tr) | 2000-09-21 |
| ATE261956T1 (de) | 2004-04-15 |
| AU735637B2 (en) | 2001-07-12 |
| CA2295715A1 (en) | 1999-01-21 |
| IL133566A (en) | 2003-10-31 |
| HUP0002861A3 (en) | 2001-09-28 |
| EP1008594A1 (en) | 2000-06-14 |
| ES2128266B1 (es) | 2000-01-16 |
| KR20010021632A (ko) | 2001-03-15 |
| IL133566A0 (en) | 2001-04-30 |
| NZ502128A (en) | 2001-09-28 |
| HUP0002861A2 (hu) | 2001-08-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA1167036A (en) | Synthesis of acylated benzothiophenes | |
| EP1337528B1 (en) | Benzothiophene derivative compounds, process of preparation and use thereof | |
| WO1999002516A1 (es) | Compuestos derivados de tiofeno y benzotiofeno y utilizacion y composicion correspondientes | |
| AU2005209441B2 (en) | Derivatives of N-` (1,5-diphenyl-1H-pyrazol-3-yl) sulphonamide with CB1 receptor affinity | |
| US5290951A (en) | Intermediates useful in preparing propenone oxime ethers | |
| PT834508E (pt) | Novos derivados substituidos de bifenilo ou de fenilpiridina processo para a sua preparacao e as composicoes farmaceuticas que os contem | |
| PT1578740E (pt) | Derivados de 1, 2, 4-triaminobenzeno úteis para tratamento de distúrbios do sistema nervoso central | |
| US4857543A (en) | Thiophene derivative and process for preparing the same | |
| WO2008081476A2 (en) | Process for preparing duloxetine hydrochloride | |
| US6403814B1 (en) | Method for synthesizing diaryl-substituted heterocyclic compounds, including tetrahydrofurans | |
| US4946862A (en) | Thiophene derivative and process for preparing the same | |
| AU597319B2 (en) | Heteroaromatic acetylenes useful as antihypertensive agents | |
| MXPA00000351A (es) | Compuestos derivados de benzotiofeno y utilizacion y composicion correspondientes | |
| DK160043B (da) | Analogifremgangsmaade til fremstilling af n-alk-2-en-4-yn-yl-benzothienylalkylaminer eller syreadditionssalte deraf | |
| NZ230628A (en) | Ring-substituted alkylene amine derivatives, preparatory intermediates and pharmaceutical compositions | |
| CZ479499A3 (cs) | Deriváty benzothiofenu, použití a přípravky | |
| NO301327B1 (no) | Tienocyklopentanonoksimetere, og farmasöytiske blandinger som inneholder dem | |
| US20020128491A1 (en) | Method for synthesizing diaryl-substituted heterocyclic compounds, including tetrahydrofurans | |
| JPS63501796A (ja) | ジヒドロベンゾチオフエンおよびチオクロマンアミノアルコ−ル類 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 133566 Country of ref document: IL Ref document number: 98807490.7 Country of ref document: CN |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AL AM AT AU AZ BA BB BG BR BY CA CH CN CU CZ DE DK EE ES FI GB GE GH GM GW HU ID IL IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT UA UG US UZ VN YU ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 81113/98 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PV1999-4794 Country of ref document: CZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 502128 Country of ref document: NZ |
|
| ENP | Entry into the national phase |
Ref document number: 2295715 Country of ref document: CA Ref document number: 2295715 Country of ref document: CA Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2000/00058 Country of ref document: TR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 272000 Country of ref document: SK Ref document number: PA/a/2000/000351 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020007000190 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 09480120 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1998930807 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 200000101 Country of ref document: EA |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWP | Wipo information: published in national office |
Ref document number: PV1999-4794 Country of ref document: CZ |
|
| WWP | Wipo information: published in national office |
Ref document number: 1998930807 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020007000190 Country of ref document: KR |
|
| WWG | Wipo information: grant in national office |
Ref document number: 81113/98 Country of ref document: AU |
|
| WWG | Wipo information: grant in national office |
Ref document number: 1998930807 Country of ref document: EP |
|
| WWR | Wipo information: refused in national office |
Ref document number: PV1999-4794 Country of ref document: CZ |
|
| WWR | Wipo information: refused in national office |
Ref document number: 1020007000190 Country of ref document: KR |