RU2604395C2 - Эхогенное устройство и система для блокады нерва - Google Patents
Эхогенное устройство и система для блокады нерва Download PDFInfo
- Publication number
- RU2604395C2 RU2604395C2 RU2013119467/14A RU2013119467A RU2604395C2 RU 2604395 C2 RU2604395 C2 RU 2604395C2 RU 2013119467/14 A RU2013119467/14 A RU 2013119467/14A RU 2013119467 A RU2013119467 A RU 2013119467A RU 2604395 C2 RU2604395 C2 RU 2604395C2
- Authority
- RU
- Russia
- Prior art keywords
- echogenic
- catheter
- needle
- working end
- tubular element
- Prior art date
Links
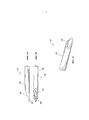
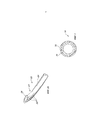
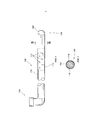
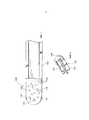
- 210000005036 nerve Anatomy 0.000 title claims abstract description 37
- 239000000463 material Substances 0.000 claims abstract description 76
- 238000000034 method Methods 0.000 claims abstract description 35
- 238000000576 coating method Methods 0.000 claims description 28
- 239000011248 coating agent Substances 0.000 claims description 22
- 239000012528 membrane Substances 0.000 claims description 10
- 238000012377 drug delivery Methods 0.000 claims description 6
- 239000000599 controlled substance Substances 0.000 claims description 5
- 239000003814 drug Substances 0.000 abstract description 4
- 239000000126 substance Substances 0.000 abstract description 4
- 229940079593 drug Drugs 0.000 abstract description 3
- 238000003384 imaging method Methods 0.000 description 25
- 230000005641 tunneling Effects 0.000 description 22
- 229910052751 metal Inorganic materials 0.000 description 19
- 239000002184 metal Substances 0.000 description 19
- 239000011521 glass Substances 0.000 description 18
- ORQBXQOJMQIAOY-UHFFFAOYSA-N nobelium Chemical compound [No] ORQBXQOJMQIAOY-UHFFFAOYSA-N 0.000 description 15
- 239000011324 bead Substances 0.000 description 13
- 229920000642 polymer Polymers 0.000 description 13
- 239000012530 fluid Substances 0.000 description 12
- 239000007787 solid Substances 0.000 description 11
- 239000011159 matrix material Substances 0.000 description 8
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 8
- 239000004926 polymethyl methacrylate Substances 0.000 description 8
- 210000004872 soft tissue Anatomy 0.000 description 8
- 229910000684 Cobalt-chrome Inorganic materials 0.000 description 7
- 239000010952 cobalt-chrome Substances 0.000 description 7
- 239000002245 particle Substances 0.000 description 7
- WAIPAZQMEIHHTJ-UHFFFAOYSA-N [Cr].[Co] Chemical class [Cr].[Co] WAIPAZQMEIHHTJ-UHFFFAOYSA-N 0.000 description 6
- 239000000654 additive Substances 0.000 description 6
- 230000000903 blocking effect Effects 0.000 description 6
- 239000012634 fragment Substances 0.000 description 6
- 239000007789 gas Substances 0.000 description 6
- 239000000017 hydrogel Substances 0.000 description 6
- 239000010453 quartz Substances 0.000 description 6
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 5
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 4
- NRTOMJZYCJJWKI-UHFFFAOYSA-N Titanium nitride Chemical compound [Ti]#N NRTOMJZYCJJWKI-UHFFFAOYSA-N 0.000 description 4
- 230000000996 additive effect Effects 0.000 description 4
- UQZIWOQVLUASCR-UHFFFAOYSA-N alumane;titanium Chemical compound [AlH3].[Ti] UQZIWOQVLUASCR-UHFFFAOYSA-N 0.000 description 4
- 239000004020 conductor Substances 0.000 description 4
- PMHQVHHXPFUNSP-UHFFFAOYSA-M copper(1+);methylsulfanylmethane;bromide Chemical compound Br[Cu].CSC PMHQVHHXPFUNSP-UHFFFAOYSA-M 0.000 description 4
- JMANVNJQNLATNU-UHFFFAOYSA-N oxalonitrile Chemical compound N#CC#N JMANVNJQNLATNU-UHFFFAOYSA-N 0.000 description 4
- 239000010936 titanium Substances 0.000 description 4
- 229910052719 titanium Inorganic materials 0.000 description 4
- MTPVUVINMAGMJL-UHFFFAOYSA-N trimethyl(1,1,2,2,2-pentafluoroethyl)silane Chemical compound C[Si](C)(C)C(F)(F)C(F)(F)F MTPVUVINMAGMJL-UHFFFAOYSA-N 0.000 description 4
- 238000002604 ultrasonography Methods 0.000 description 4
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 3
- 230000003444 anaesthetic effect Effects 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- TZCXTZWJZNENPQ-UHFFFAOYSA-L barium sulfate Chemical compound [Ba+2].[O-]S([O-])(=O)=O TZCXTZWJZNENPQ-UHFFFAOYSA-L 0.000 description 3
- 239000005321 cobalt glass Substances 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 238000001125 extrusion Methods 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 229920002635 polyurethane Polymers 0.000 description 3
- 239000004814 polyurethane Substances 0.000 description 3
- 229910052709 silver Inorganic materials 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- 230000004936 stimulating effect Effects 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 238000012285 ultrasound imaging Methods 0.000 description 3
- CDMDQYCEEKCBGR-UHFFFAOYSA-N 1,4-diisocyanatocyclohexane Chemical compound O=C=NC1CCC(N=C=O)CC1 CDMDQYCEEKCBGR-UHFFFAOYSA-N 0.000 description 2
- 229910000531 Co alloy Inorganic materials 0.000 description 2
- 210000003484 anatomy Anatomy 0.000 description 2
- QXJJQWWVWRCVQT-UHFFFAOYSA-K calcium;sodium;phosphate Chemical compound [Na+].[Ca+2].[O-]P([O-])([O-])=O QXJJQWWVWRCVQT-UHFFFAOYSA-K 0.000 description 2
- IUWCPXJTIPQGTE-UHFFFAOYSA-N chromium cobalt Chemical compound [Cr].[Co].[Co].[Co] IUWCPXJTIPQGTE-UHFFFAOYSA-N 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 239000003292 glue Substances 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 239000012798 spherical particle Substances 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 229920001169 thermoplastic Polymers 0.000 description 2
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 239000010937 tungsten Substances 0.000 description 2
- 238000012800 visualization Methods 0.000 description 2
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 208000003098 Ganglion Cysts Diseases 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 208000004550 Postoperative Pain Diseases 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 208000005400 Synovial Cyst Diseases 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 150000001553 barium compounds Chemical class 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 150000001621 bismuth Chemical class 0.000 description 1
- 239000003153 chemical reaction reagent Chemical class 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 125000005442 diisocyanate group Chemical class 0.000 description 1
- 229940003304 dilt Drugs 0.000 description 1
- 150000002009 diols Chemical class 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 239000002923 metal particle Substances 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 230000001095 motoneuron effect Effects 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000010355 oscillation Effects 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 229920005594 polymer fiber Polymers 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- -1 polytetrafluoroethylene Polymers 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 239000004416 thermosoftening plastic Substances 0.000 description 1
- 230000037303 wrinkles Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/34—Trocars; Puncturing needles
- A61B17/3401—Puncturing needles for the peridural or subarachnoid space or the plexus, e.g. for anaesthesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B8/00—Diagnosis using ultrasonic, sonic or infrasonic waves
- A61B8/48—Diagnostic techniques
- A61B8/481—Diagnostic techniques involving the use of contrast agents, e.g. microbubbles introduced into the bloodstream
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M19/00—Local anaesthesia; Hypothermia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0108—Steering means as part of the catheter or advancing means; Markers for positioning using radio-opaque or ultrasound markers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/178—Syringes
- A61M5/31—Details
- A61M5/32—Needles; Details of needles pertaining to their connection with syringe or hub; Accessories for bringing the needle into, or holding the needle on, the body; Devices for protection of needles
- A61M5/3286—Needle tip design, e.g. for improved penetration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/178—Syringes
- A61M5/31—Details
- A61M5/32—Needles; Details of needles pertaining to their connection with syringe or hub; Accessories for bringing the needle into, or holding the needle on, the body; Devices for protection of needles
- A61M5/329—Needles; Details of needles pertaining to their connection with syringe or hub; Accessories for bringing the needle into, or holding the needle on, the body; Devices for protection of needles characterised by features of the needle shaft
- A61M5/3291—Shafts with additional lateral openings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/34—Trocars; Puncturing needles
- A61B17/3403—Needle locating or guiding means
- A61B2017/3413—Needle locating or guiding means guided by ultrasound
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/39—Markers, e.g. radio-opaque or breast lesions markers
- A61B2090/3925—Markers, e.g. radio-opaque or breast lesions markers ultrasonic
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/39—Markers, e.g. radio-opaque or breast lesions markers
- A61B2090/3925—Markers, e.g. radio-opaque or breast lesions markers ultrasonic
- A61B2090/3929—Active markers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B8/00—Diagnosis using ultrasonic, sonic or infrasonic waves
- A61B8/08—Clinical applications
- A61B8/0833—Clinical applications involving detecting or locating foreign bodies or organic structures
- A61B8/0841—Clinical applications involving detecting or locating foreign bodies or organic structures for locating instruments
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Hematology (AREA)
- Surgery (AREA)
- Molecular Biology (AREA)
- Pathology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biophysics (AREA)
- Medical Informatics (AREA)
- Pulmonology (AREA)
- Physics & Mathematics (AREA)
- Radiology & Medical Imaging (AREA)
- Vascular Medicine (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US39404010P | 2010-10-18 | 2010-10-18 | |
| US61/394,040 | 2010-10-18 | ||
| US13/272,643 US9254146B2 (en) | 2010-10-18 | 2011-10-13 | Echogenic nerve block apparatus and system |
| US13/272,643 | 2011-10-13 | ||
| PCT/IB2011/054599 WO2012052907A2 (en) | 2010-10-18 | 2011-10-17 | Echogenic nerve block apparatus and system |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2013119467A RU2013119467A (ru) | 2014-11-27 |
| RU2604395C2 true RU2604395C2 (ru) | 2016-12-10 |
Family
ID=45934739
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2013119467/14A RU2604395C2 (ru) | 2010-10-18 | 2011-10-17 | Эхогенное устройство и система для блокады нерва |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US9254146B2 (enExample) |
| EP (2) | EP2629671B1 (enExample) |
| JP (1) | JP6009449B2 (enExample) |
| KR (1) | KR101857968B1 (enExample) |
| CN (2) | CN105686866B (enExample) |
| AU (2) | AU2011319493B2 (enExample) |
| BR (1) | BR112013008840A2 (enExample) |
| CA (2) | CA3078068A1 (enExample) |
| ES (1) | ES2523389T3 (enExample) |
| MX (1) | MX339109B (enExample) |
| RU (1) | RU2604395C2 (enExample) |
| WO (1) | WO2012052907A2 (enExample) |
Families Citing this family (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8882673B2 (en) * | 2010-02-12 | 2014-11-11 | I-Flow Corporation | Continuous transversus abdominis plane block |
| US9254146B2 (en) * | 2010-10-18 | 2016-02-09 | Avent, Inc. | Echogenic nerve block apparatus and system |
| US20170049993A1 (en) * | 2012-08-14 | 2017-02-23 | Cosman Medical Inc. | Echogenic probe |
| US9795508B2 (en) * | 2012-11-06 | 2017-10-24 | Alcon Research, Ltd. | Ocular infusion system |
| EP2945683A4 (en) * | 2013-01-18 | 2017-03-15 | SRI International | Anchoring nerve block catheter |
| DK2959509T3 (en) | 2013-02-14 | 2018-08-13 | Nanopareil Llc | Electrospun hybrid nanofiber felt, method of making it and method of purifying biomolecules |
| US9622719B2 (en) | 2013-02-26 | 2017-04-18 | Allen Maizes | Color ultrasound needle |
| US11224724B2 (en) | 2013-03-12 | 2022-01-18 | Teleflex Medical Incorporated | Catheter insertion device |
| US10357635B2 (en) | 2013-03-12 | 2019-07-23 | Teleflex Medical Incorporated | Catheter insertion device |
| US9717886B2 (en) | 2013-03-12 | 2017-08-01 | Teleflex Medical Incorporated | Safety clip for a needle |
| WO2014160278A1 (en) * | 2013-03-14 | 2014-10-02 | Allergan, Inc. | Polymer system for securing implants in syringe needles |
| CA3008509C (en) * | 2013-09-23 | 2020-11-10 | Becton, Dickinson And Company Ltd. | Piercing member for container access device |
| USD767127S1 (en) * | 2013-10-14 | 2016-09-20 | Tsk Laboratory Europe B.V. | Needle with dome shaped tip |
| US9629981B2 (en) | 2013-12-13 | 2017-04-25 | Dolcera Information Technology Services Private Limited | Drainage catheter |
| US20150374348A1 (en) * | 2014-06-26 | 2015-12-31 | Boston Scientific Scimed, Inc. | Use Of Vibration For EUS-FNA Tissue Acquisition |
| US12186129B2 (en) * | 2015-03-31 | 2025-01-07 | Boston Scientific Scimed, Inc. | Devices and methods for ultrasound imaging |
| MX391267B (es) * | 2015-06-18 | 2025-03-21 | Avent Inc | Miembro de espiral ecogénico para un ensamble de catéter. |
| CA2989772C (en) | 2015-06-18 | 2021-06-01 | Avent, Inc. | Echogenic catheter member |
| CA2992974A1 (en) | 2015-07-21 | 2017-01-26 | Avent, Inc. | Ultrasonic catheter assembly |
| USD842984S1 (en) | 2015-12-09 | 2019-03-12 | Dentsply Ih Ab | Catheter |
| USD833606S1 (en) * | 2016-02-29 | 2018-11-13 | Csp Technologies, Inc. | Cannula sensor carrier |
| WO2017222523A1 (en) * | 2016-06-23 | 2017-12-28 | Avent, Inc. | Echogenic coil member for a catheter assembly |
| JP7153650B2 (ja) * | 2016-12-19 | 2022-10-14 | ニュー ワールド メディカル インコーポレイテッド | 眼球治療装置、及び関連する使用方法 |
| US10328251B2 (en) | 2017-04-03 | 2019-06-25 | Becton, Dickinson And Company | Systems and methods to prevent catheter occlusion |
| CA3172718C (en) | 2017-04-13 | 2025-10-07 | Teleflex Medical Incorporated | CATHETER INSERTION DEVICE |
| US11179178B2 (en) | 2017-08-31 | 2021-11-23 | Cook Medical Technologies Llc | Vaginal positioner for uterine tamponade device and methods of using the same |
| USD1023297S1 (en) * | 2018-06-04 | 2024-04-16 | Airlift Concrete Experts, LLC | Subterranean injection rod tip |
| EP3808251A4 (en) | 2018-06-18 | 2022-04-06 | Gibello Alejandro Fernández | High-visibility protected ultrasound needle for carrying out ultrasound-guided percutaneous neuromodulation or electrolysis techniques |
| US12251527B2 (en) * | 2018-09-20 | 2025-03-18 | Becton, Dickinson And Company | Catheter having a closed tip and slit for a peripheral intravenous catheter assembly |
| EP3852861A1 (en) * | 2018-09-21 | 2021-07-28 | Cook Medical Technologies LLC | Introducer for uterine tamponade assembly with echogenic element |
| EP3941369A1 (en) * | 2019-03-19 | 2022-01-26 | Atropos Limited | Curette and use thereof |
| CN109939323B (zh) * | 2019-04-30 | 2021-07-30 | 浙江超乐医疗科技有限公司 | 一种用于区域阻滞的麻醉装置 |
| CN211884905U (zh) | 2019-08-22 | 2020-11-10 | 贝克顿·迪金森公司 | 球囊扩张导管及其球囊 |
| CN211856471U (zh) | 2019-08-22 | 2020-11-03 | 贝克顿·迪金森公司 | 回声医疗器械回声反射性量化测试系统 |
| CN112401971B (zh) | 2019-08-23 | 2025-09-09 | 贝克顿·迪金森公司 | 为经皮肾镜取石术外科手术设计的套件 |
| KR102744705B1 (ko) * | 2019-10-30 | 2024-12-23 | 보스톤 싸이엔티픽 싸이메드 인코포레이티드 | 내시경 카테터 장치 |
| USD959650S1 (en) * | 2020-03-31 | 2022-08-02 | Naslund Medical AB | Injection instrument |
| TWI872280B (zh) * | 2020-09-25 | 2025-02-11 | 財團法人工業技術研究院 | 醫材針具 |
| JP7029005B1 (ja) | 2021-02-02 | 2022-03-02 | 厚夫 森 | カテーテル |
| CN113171161B (zh) * | 2021-04-01 | 2022-04-01 | 深圳市第二人民医院 | 一种显影效果佳的超声阻滞针 |
| GB2608648B (en) * | 2021-07-09 | 2024-06-26 | Intelligent Ultrasound Ltd | Apparatus and method for positioning a tube |
| DE102021122999A1 (de) * | 2021-09-06 | 2023-03-09 | Dionex Softron Gmbh | Nadel für die Verwendung in analytischen Anwendungen |
| KR102329489B1 (ko) * | 2021-09-07 | 2021-11-19 | 이영석 | 의료용 니들 및 이를 제조하는 방법 |
| DE102021131770A1 (de) | 2021-12-02 | 2023-06-07 | Heraeus Deutschland GmbH & Co. KG | Markierung aus porösem Material |
| WO2024123286A1 (en) * | 2022-12-06 | 2024-06-13 | Istanbul Medipol Universitesi Teknoloji Transfer Ofisi Anonim Sirketi | J-tip catheter for nerve, fasial or interfasial plan, epidural and spinal block analgesia or anesthesia |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6770070B1 (en) * | 2000-03-17 | 2004-08-03 | Rita Medical Systems, Inc. | Lung treatment apparatus and method |
| WO2009158012A2 (en) * | 2008-06-27 | 2009-12-30 | Gore Enterprise Holdings, Inc. | Improved catheter |
| WO2010085374A1 (en) * | 2009-01-20 | 2010-07-29 | Abbott Cardiovascular Systems Inc. | Improving the visualization of a catheter viewed under ultrasound imaging |
Family Cites Families (40)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4401124A (en) | 1981-08-13 | 1983-08-30 | Technicare Corporation | Reflection enhancement of a biopsy needle |
| US5289831A (en) | 1989-03-09 | 1994-03-01 | Vance Products Incorporated | Surface-treated stent, catheter, cannula, and the like |
| US5201314A (en) * | 1989-03-09 | 1993-04-13 | Vance Products Incorporated | Echogenic devices, material and method |
| US5081997A (en) * | 1989-03-09 | 1992-01-21 | Vance Products Incorporated | Echogenic devices, material and method |
| US5289381A (en) | 1989-12-04 | 1994-02-22 | Maschinenfabrik Rieter Ag | Method and apparatus for continuously determining the fineness of fibers in slivers |
| JP3328326B2 (ja) | 1991-07-12 | 2002-09-24 | クリティコン・インコーポレイテッド | キンク抵抗性を有し、かつ放射線不透過性で柔軟なポリウレタン管状材料から製造したカテーテル |
| US6461296B1 (en) * | 1998-06-26 | 2002-10-08 | 2000 Injectx, Inc. | Method and apparatus for delivery of genes, enzymes and biological agents to tissue cells |
| US5327891A (en) | 1992-07-30 | 1994-07-12 | Rammler David H | Catheter track and catheter for diagnosis and treatment |
| US5490521A (en) * | 1993-08-31 | 1996-02-13 | Medtronic, Inc. | Ultrasound biopsy needle |
| US5462521A (en) * | 1993-12-21 | 1995-10-31 | Angeion Corporation | Fluid cooled and perfused tip for a catheter |
| US7229413B2 (en) | 1996-11-06 | 2007-06-12 | Angiotech Biocoatings Corp. | Echogenic coatings with overcoat |
| DE19746840B4 (de) * | 1997-10-23 | 2007-02-08 | Robert Bosch Gmbh | Verfahren zum dichten Verschließen einer Bohrung in einem Werkstück aus duktilem Werkstoff |
| US5921933A (en) * | 1998-08-17 | 1999-07-13 | Medtronic, Inc. | Medical devices with echogenic coatings |
| DE19916494A1 (de) | 1999-04-13 | 2000-10-19 | Mannesmann Vdo Ag | Verfahren zur Herstellung eines Kunststoffbauteils und Kraftstoffbehälter für ein Kraftfahrzeug |
| US6350253B1 (en) * | 1999-07-19 | 2002-02-26 | I-Flow Corporation | Catheter for uniform delivery of medication |
| US7004923B2 (en) | 1999-07-19 | 2006-02-28 | I-Flow Corporation | Catheter for uniform delivery of medication |
| US7547302B2 (en) | 1999-07-19 | 2009-06-16 | I-Flow Corporation | Anti-microbial catheter |
| ATE327779T1 (de) | 1999-12-30 | 2006-06-15 | Acrymed | Methode und zusammensetzungen für verbesserte abgabevorrichtungen |
| US6506156B1 (en) | 2000-01-19 | 2003-01-14 | Vascular Control Systems, Inc | Echogenic coating |
| US6592612B1 (en) * | 2000-05-04 | 2003-07-15 | Cardeon Corporation | Method and apparatus for providing heat exchange within a catheter body |
| GB0011568D0 (en) | 2000-05-15 | 2000-06-28 | Nycomed Amersham Plc | Grooved medical devices |
| JP3735019B2 (ja) * | 2000-08-09 | 2006-01-11 | 英雄 中嶋 | 超音波診断可能な医療用器具または部材 |
| US20080287939A1 (en) * | 2002-07-10 | 2008-11-20 | Appling William M | Endovascular thermal treatment device with flexible guide tip and method |
| US8333734B2 (en) * | 2003-07-03 | 2012-12-18 | Walter A. Zohmann | Fenestrated peripheral nerve block needle and method for using the same |
| US7044923B2 (en) * | 2003-11-12 | 2006-05-16 | Santos Perry M | Apparatus and method for measuring a nerve diameter |
| US20060141186A1 (en) | 2004-12-28 | 2006-06-29 | Janssen Robert A | Gloves with hydrogel coating for damp hand donning and method of making same |
| WO2009114556A2 (en) * | 2005-10-04 | 2009-09-17 | Ilh, Llc | Catheters with lubricious linings and methods for making and using them |
| US7867169B2 (en) * | 2005-12-02 | 2011-01-11 | Abbott Cardiovascular Systems Inc. | Echogenic needle catheter configured to produce an improved ultrasound image |
| US8382674B2 (en) * | 2005-12-02 | 2013-02-26 | Abbott Cardiovascular Systems Inc. | Visualization of a catheter viewed under ultrasound imaging |
| EP1960039A2 (en) | 2005-12-12 | 2008-08-27 | Cook Critical Care Incorporated | Hyperechoic stimulating block needle |
| US20080058702A1 (en) * | 2005-12-12 | 2008-03-06 | Cook Critical Care Incorporated | Continuous nerve block assembly |
| WO2007089724A2 (en) | 2006-01-31 | 2007-08-09 | Angiotech Biocoatings Corp. | Lubricious coatings |
| US8672889B2 (en) | 2006-05-05 | 2014-03-18 | Kimberly-Clark Worldwide, Inc. | Soft tissue tunneling device |
| US9289232B2 (en) | 2006-05-05 | 2016-03-22 | Avent, Inc. | Soft tissue tunneling device |
| CN101631584A (zh) * | 2007-02-09 | 2010-01-20 | 史蒂文·J·费里 | 用于在活体脉管系统中进行腔内通过的系统 |
| US9498282B2 (en) | 2007-02-09 | 2016-11-22 | Boston Scientific Scimed, Inc. | Medical probe with echogenic and insulative properties |
| US20090030307A1 (en) | 2007-06-04 | 2009-01-29 | Assaf Govari | Intracorporeal location system with movement compensation |
| US8992538B2 (en) | 2008-09-30 | 2015-03-31 | DePuy Synthes Products, Inc. | Customized patient-specific acetabular orthopaedic surgical instrument and method of use and fabrication |
| CA2726418A1 (en) | 2010-01-12 | 2011-07-12 | Custom Medical Applications, Inc. | Ultrasound guided echogenic catheter and related methods |
| US9254146B2 (en) * | 2010-10-18 | 2016-02-09 | Avent, Inc. | Echogenic nerve block apparatus and system |
-
2011
- 2011-10-13 US US13/272,643 patent/US9254146B2/en active Active
- 2011-10-17 EP EP11778979.2A patent/EP2629671B1/en not_active Not-in-force
- 2011-10-17 WO PCT/IB2011/054599 patent/WO2012052907A2/en not_active Ceased
- 2011-10-17 JP JP2013533323A patent/JP6009449B2/ja active Active
- 2011-10-17 CN CN201610132916.1A patent/CN105686866B/zh not_active Expired - Fee Related
- 2011-10-17 BR BR112013008840A patent/BR112013008840A2/pt not_active IP Right Cessation
- 2011-10-17 CA CA3078068A patent/CA3078068A1/en not_active Abandoned
- 2011-10-17 RU RU2013119467/14A patent/RU2604395C2/ru not_active IP Right Cessation
- 2011-10-17 CN CN201180050075.5A patent/CN103167835B/zh not_active Expired - Fee Related
- 2011-10-17 MX MX2013004375A patent/MX339109B/es active IP Right Grant
- 2011-10-17 CA CA2811736A patent/CA2811736C/en active Active
- 2011-10-17 KR KR1020137009827A patent/KR101857968B1/ko not_active Expired - Fee Related
- 2011-10-17 ES ES11778979.2T patent/ES2523389T3/es active Active
- 2011-10-17 EP EP14151998.3A patent/EP2724672B1/en not_active Not-in-force
- 2011-10-17 AU AU2011319493A patent/AU2011319493B2/en not_active Ceased
-
2016
- 2016-02-04 US US15/015,188 patent/US10080549B2/en active Active
- 2016-04-27 AU AU2016202667A patent/AU2016202667B2/en not_active Ceased
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6770070B1 (en) * | 2000-03-17 | 2004-08-03 | Rita Medical Systems, Inc. | Lung treatment apparatus and method |
| WO2009158012A2 (en) * | 2008-06-27 | 2009-12-30 | Gore Enterprise Holdings, Inc. | Improved catheter |
| WO2010085374A1 (en) * | 2009-01-20 | 2010-07-29 | Abbott Cardiovascular Systems Inc. | Improving the visualization of a catheter viewed under ultrasound imaging |
Also Published As
| Publication number | Publication date |
|---|---|
| CN105686866B (zh) | 2019-06-28 |
| EP2724672B1 (en) | 2018-06-20 |
| EP2724672A1 (en) | 2014-04-30 |
| MX339109B (es) | 2016-05-12 |
| CN103167835B (zh) | 2016-02-10 |
| CN103167835A (zh) | 2013-06-19 |
| CA3078068A1 (en) | 2012-04-26 |
| AU2011319493A1 (en) | 2013-04-11 |
| US20160151049A1 (en) | 2016-06-02 |
| WO2012052907A2 (en) | 2012-04-26 |
| US20120095404A1 (en) | 2012-04-19 |
| BR112013008840A2 (pt) | 2016-06-21 |
| KR20130142117A (ko) | 2013-12-27 |
| MX2013004375A (es) | 2013-06-28 |
| AU2016202667B2 (en) | 2017-02-16 |
| US10080549B2 (en) | 2018-09-25 |
| US9254146B2 (en) | 2016-02-09 |
| WO2012052907A3 (en) | 2012-07-26 |
| AU2016202667A1 (en) | 2016-05-19 |
| CA2811736C (en) | 2020-05-19 |
| JP6009449B2 (ja) | 2016-10-19 |
| CA2811736A1 (en) | 2012-04-26 |
| KR101857968B1 (ko) | 2018-06-28 |
| CN105686866A (zh) | 2016-06-22 |
| RU2013119467A (ru) | 2014-11-27 |
| JP2013543410A (ja) | 2013-12-05 |
| AU2011319493B2 (en) | 2016-03-03 |
| ES2523389T3 (es) | 2014-11-25 |
| EP2629671A2 (en) | 2013-08-28 |
| EP2629671B1 (en) | 2014-08-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2604395C2 (ru) | Эхогенное устройство и система для блокады нерва | |
| EP2343098A1 (en) | Ultrasound guided echogenic catheter and related methods | |
| EP3310262B1 (en) | Echogenic catheter member | |
| TR201810573T4 (tr) | Bir iğnenin ultrason dalgaları için görünür kılınması için düzenekler. | |
| EP3565484A1 (en) | Methods for implanting and reversing stimuli-responsive implants | |
| JP2013538097A (ja) | 頭蓋内出血の治療のための方法および装置関連出願本出願は、2010年8月27日付の米国仮特許出願第61/377,639号の優先権の利益を主張し、この米国特許仮出願の全内容が、ここでの言及によって本明細書に援用される。 | |
| US7985184B2 (en) | Ultrasound-assisted drug-delivery method and system based on time reversal acoustics | |
| US20140039315A1 (en) | Medical devices with visibility-enhancing features | |
| CN115066217A (zh) | 用于标记组织的标记体 | |
| EP3165254A1 (en) | Anchoring control system for medical devices | |
| AU2016410572B2 (en) | Echogenic coil member for a catheter assembly | |
| US20110270090A1 (en) | Needle having ultrasound opaque elements | |
| JP2004344274A (ja) | 超音波カテーテル | |
| US20150011873A1 (en) | Catheters, catheters for use in ultrasound guided procedures, and related methods | |
| HK1160041A (en) | Ultrasound guided echogenic catheter and related methods |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| HZ9A | Changing address for correspondence with an applicant | ||
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20181018 |