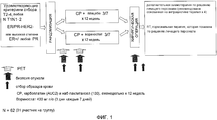
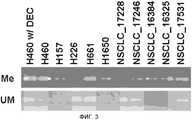
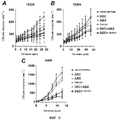
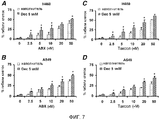
RU2577278C2 - Способы комбинированной терапии для лечения пролиферативных заболеваний - Google Patents
Способы комбинированной терапии для лечения пролиферативных заболеваний Download PDFInfo
- Publication number
- RU2577278C2 RU2577278C2 RU2012156903/15A RU2012156903A RU2577278C2 RU 2577278 C2 RU2577278 C2 RU 2577278C2 RU 2012156903/15 A RU2012156903/15 A RU 2012156903/15A RU 2012156903 A RU2012156903 A RU 2012156903A RU 2577278 C2 RU2577278 C2 RU 2577278C2
- Authority
- RU
- Russia
- Prior art keywords
- individual
- composition
- cancer
- effective amount
- administered
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
- A61K31/165—Amides, e.g. hydroxamic acids having aromatic rings, e.g. colchicine, atenolol, progabide
- A61K31/167—Amides, e.g. hydroxamic acids having aromatic rings, e.g. colchicine, atenolol, progabide having the nitrogen of a carboxamide group directly attached to the aromatic ring, e.g. lidocaine, paracetamol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/28—Compounds containing heavy metals
- A61K31/282—Platinum compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/337—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having four-membered rings, e.g. taxol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/42—Proteins; Polypeptides; Degradation products thereof; Derivatives thereof, e.g. albumin, gelatin or zein
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/146—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
- A61K9/5107—Excipients; Inactive ingredients
- A61K9/513—Organic macromolecular compounds; Dendrimers
- A61K9/5169—Proteins, e.g. albumin, gelatin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Molecular Biology (AREA)
- Dermatology (AREA)
- Pain & Pain Management (AREA)
- Nanotechnology (AREA)
- Optics & Photonics (AREA)
- Biomedical Technology (AREA)
- Physics & Mathematics (AREA)
- Nutrition Science (AREA)
- Reproductive Health (AREA)
- Endocrinology (AREA)
- Pulmonology (AREA)
- Physiology (AREA)
- Urology & Nephrology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Inorganic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US35233310P | 2010-06-07 | 2010-06-07 | |
| US61/352,333 | 2010-06-07 | ||
| US201161446909P | 2011-02-25 | 2011-02-25 | |
| US61/446,909 | 2011-02-25 | ||
| PCT/US2011/037450 WO2011156119A1 (en) | 2010-06-07 | 2011-05-20 | Combination therapy methods for treating proliferative diseases |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2016103126A Division RU2016103126A (ru) | 2010-06-07 | 2011-05-20 | Способы комбинированной терапии для лечения пролиферативных заболеваний |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2012156903A RU2012156903A (ru) | 2014-07-20 |
| RU2577278C2 true RU2577278C2 (ru) | 2016-03-10 |
Family
ID=45098373
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2016103126A RU2016103126A (ru) | 2010-06-07 | 2011-05-20 | Способы комбинированной терапии для лечения пролиферативных заболеваний |
| RU2012156903/15A RU2577278C2 (ru) | 2010-06-07 | 2011-05-20 | Способы комбинированной терапии для лечения пролиферативных заболеваний |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2016103126A RU2016103126A (ru) | 2010-06-07 | 2011-05-20 | Способы комбинированной терапии для лечения пролиферативных заболеваний |
Country Status (20)
| Country | Link |
|---|---|
| US (4) | US20140155344A1 (OSRAM) |
| EP (2) | EP3056201A1 (OSRAM) |
| JP (3) | JP6031437B2 (OSRAM) |
| KR (1) | KR101850566B1 (OSRAM) |
| CN (2) | CN103037846B (OSRAM) |
| AU (2) | AU2011264590B2 (OSRAM) |
| BR (1) | BR112012031163A2 (OSRAM) |
| CA (1) | CA2801891C (OSRAM) |
| CO (1) | CO6670568A2 (OSRAM) |
| CR (1) | CR20120638A (OSRAM) |
| IL (2) | IL223190A0 (OSRAM) |
| MX (1) | MX2012014231A (OSRAM) |
| MY (1) | MY166014A (OSRAM) |
| NI (1) | NI201200181A (OSRAM) |
| NZ (1) | NZ707377A (OSRAM) |
| PH (1) | PH12012502399A1 (OSRAM) |
| RU (2) | RU2016103126A (OSRAM) |
| SG (2) | SG185798A1 (OSRAM) |
| WO (1) | WO2011156119A1 (OSRAM) |
| ZA (1) | ZA201208820B (OSRAM) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11911374B2 (en) | 2019-05-29 | 2024-02-27 | Nelum Corporation | Methods and uses for treating cancer |
Families Citing this family (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8853260B2 (en) | 1997-06-27 | 2014-10-07 | Abraxis Bioscience, Llc | Formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof |
| SI1585548T1 (sl) | 2002-12-09 | 2018-11-30 | Abraxis Bioscience, Llc | Sestave in metode odmerjanja farmakoloških sredstev |
| US8735394B2 (en) | 2005-02-18 | 2014-05-27 | Abraxis Bioscience, Llc | Combinations and modes of administration of therapeutic agents and combination therapy |
| CN103285395A (zh) | 2005-02-18 | 2013-09-11 | 阿布拉科斯生物科学有限公司 | 治疗剂的组合和给予方式以及联合治疗 |
| LT3311805T (lt) | 2005-08-31 | 2020-04-27 | Abraxis Bioscience, Llc | Kompozicijos, apimančios silpnai vandenyje tirpius farmacinius agentus ir priešmikrobinius agentus |
| SI2117520T1 (sl) | 2006-12-14 | 2019-01-31 | Abraxis Bioscience, Llc | Zdravljenje raka dojk glede na status hormonskih receptorjev z nano delci, ki zajemajo taksan |
| AU2008260447B2 (en) * | 2007-06-01 | 2013-10-10 | Abraxis Bioscience, Llc | Methods and compositions for treating recurrent cancer |
| ES2764100T3 (es) | 2009-04-15 | 2020-06-02 | Abraxis Bioscience Llc | Composiciones de nanopartículas exentas de priones y métodos |
| EP2552438B1 (en) | 2010-03-26 | 2016-05-11 | Abraxis BioScience, LLC | Methods of treatment of hepatocellular carcinoma |
| BR112012024442A2 (pt) | 2010-03-29 | 2017-03-21 | Abraxis Bioscience Llc | métodos de tratamento de câncer |
| CA2794147A1 (en) | 2010-03-29 | 2011-10-06 | Abraxis Bioscience, Llc | Use of a composition comprising nanoparticles comprising a taxane and an albumin to improve uptake of chemotherapeutics by tumors and for treating a cancer that is highly fibrotic and/or has a dense stroma |
| RU2576609C2 (ru) | 2010-06-04 | 2016-03-10 | АБРАКСИС БАЙОСАЙЕНС, ЭлЭлСи | Способы лечение рака поджелудочной железы |
| EP3056201A1 (en) * | 2010-06-07 | 2016-08-17 | Abraxis BioScience, LLC | Combination therapy methods for treating proliferative diseases |
| CA2834040C (en) | 2011-04-28 | 2020-01-14 | Abraxis Bioscience, Llc | Intravascular delivery of nanoparticle compositions and uses thereof |
| CN103501812A (zh) | 2011-04-29 | 2014-01-08 | 西莱克塔生物科技公司 | 用于过敏症治疗的致耐受性合成纳米载体 |
| HRP20200793T1 (hr) | 2011-05-09 | 2020-10-16 | Mayo Foundation For Medical Education And Research | Liječenje raka |
| EP2773322A1 (en) | 2011-11-01 | 2014-09-10 | Celgene Corporation | Methods for treating cancers using oral formulations of cytidine analogs |
| HUE045661T2 (hu) | 2011-12-14 | 2020-01-28 | Abraxis Bioscience Llc | Polimer segédanyagok alkalmazása részecskék liofilizálásához vagy fagyasztásához |
| WO2014055415A1 (en) | 2012-10-01 | 2014-04-10 | Mayo Foundation For Medical Education And Research | Cancer treatments |
| US9149455B2 (en) | 2012-11-09 | 2015-10-06 | Abraxis Bioscience, Llc | Methods of treating melanoma |
| US9511046B2 (en) | 2013-01-11 | 2016-12-06 | Abraxis Bioscience, Llc | Methods of treating pancreatic cancer |
| EP2968254B1 (en) | 2013-03-12 | 2020-04-22 | Abraxis BioScience, LLC | Methods of treating lung cancer |
| EP2968191B1 (en) | 2013-03-14 | 2021-06-16 | Abraxis BioScience, LLC | Methods of treating bladder cancer |
| AU2014262164B2 (en) * | 2013-05-03 | 2020-02-27 | Selecta Biosciences, Inc. | Dosing combinations for reducing undesired humoral immune responses |
| CN118370823A (zh) * | 2013-05-03 | 2024-07-23 | 西莱克塔生物科技公司 | 用于降低的或增强的药效学作用的致耐受性合成纳米载体和治疗性大分子 |
| EP3154586B1 (en) | 2014-06-13 | 2020-05-27 | Mayo Foundation for Medical Education and Research | Treating lymphomas |
| MX2016016617A (es) | 2014-06-16 | 2017-03-23 | Mayo Foundation | Tratamiento de mielomas. |
| WO2015195634A1 (en) * | 2014-06-17 | 2015-12-23 | Celgne Corporation | Methods for treating epstein-barr virus (ebv) associated cancers using oral formulations of 5-azacytidine |
| WO2016037162A1 (en) | 2014-09-07 | 2016-03-10 | Selecta Biosciences, Inc. | Methods and compositions for attenuating anti-viral transfer vector immune responses |
| US9446148B2 (en) | 2014-10-06 | 2016-09-20 | Mayo Foundation For Medical Education And Research | Carrier-antibody compositions and methods of making and using the same |
| AU2015355137A1 (en) * | 2014-12-02 | 2017-06-08 | Celgene Corporation | Combination therapies |
| US10527604B1 (en) | 2015-03-05 | 2020-01-07 | Abraxis Bioscience, Llc | Methods of assessing suitability of use of pharmaceutical compositions of albumin and paclitaxel |
| US10705070B1 (en) | 2015-03-05 | 2020-07-07 | Abraxis Bioscience, Llc | Methods of assessing suitability of use of pharmaceutical compositions of albumin and poorly water soluble drug |
| LT3313401T (lt) | 2015-06-29 | 2022-01-10 | Abraxis Bioscience, Llc | Nanodalelės, apimančios sirolimą ir albuminą, skirtos naudoti epitelioidinių ląstelių navikų gydymui |
| TW201707725A (zh) | 2015-08-18 | 2017-03-01 | 美國馬友醫藥教育研究基金會 | 載體-抗體組合物及其製造及使用方法 |
| TW201713360A (en) | 2015-10-06 | 2017-04-16 | Mayo Foundation | Methods of treating cancer using compositions of antibodies and carrier proteins |
| EP3399861A4 (en) | 2016-01-07 | 2019-08-07 | Mayo Foundation for Medical Education and Research | METHOD FOR THE TREATMENT OF CANCER WITH INTERFERON |
| CN105606824B (zh) * | 2016-01-28 | 2018-04-06 | 山东省药物研究院 | 晚期乳腺癌患者外周血循环肿瘤细胞Her‑2基因的检测方法 |
| US11351254B2 (en) | 2016-02-12 | 2022-06-07 | Mayo Foundation For Medical Education And Research | Hematologic cancer treatments |
| CA3018340A1 (en) | 2016-03-21 | 2017-09-28 | Mayo Foundation For Medical Education And Research | Methods for improving the therapeutic index for a chemotherapeutic drug |
| CA3018341A1 (en) | 2016-03-21 | 2017-09-28 | Mayo Foundation For Medical Education And Research | Methods for reducing toxicity of a chemotherapeutic drug |
| US10618969B2 (en) | 2016-04-06 | 2020-04-14 | Mayo Foundation For Medical Education And Research | Carrier-binding agent compositions and methods of making and using the same |
| MX2019002473A (es) | 2016-09-01 | 2019-09-18 | Mayo Found Medical Education & Res | Métodos y composiciones para el direccionamiento de cánceres de células t. |
| KR20220151022A (ko) | 2016-09-01 | 2022-11-11 | 메이오 파운데이션 포 메디칼 에쥬케이션 앤드 리써치 | 암 치료용 담체-pd-l1 결합제 조성물 |
| CA3035653A1 (en) | 2016-09-06 | 2018-03-15 | Mayo Foundation For Medical Education And Research | Paclitaxel-albumin-binding agent compositions and methods for using and making the same |
| CA3035655A1 (en) | 2016-09-06 | 2018-03-15 | Mayo Foundation For Medical Education And Research | Methods of treating pd-l1 expressing cancer |
| US11590098B2 (en) | 2016-09-06 | 2023-02-28 | Mayo Foundation For Medical Education And Research | Methods of treating triple-negative breast cancer using compositions of antibodies and carrier proteins |
| AU2017340913A1 (en) * | 2016-10-07 | 2019-04-18 | Abraxis Bioscience, Llc | Methods of treating biliary tract cancer |
| HUE072005T2 (hu) | 2017-03-11 | 2025-10-28 | Cartesian Therapeutics Inc | Gyulladásgátlókkal és immunszupresszánst tartalmazó szintetikus nanohordozókkal történõ kombinált kezeléssel összefüggõ eljárások és készítmények |
| CA3076919A1 (en) | 2017-10-03 | 2019-04-11 | Crititech, Inc. | Local delivery of antineoplastic particles in combination with systemic delivery of immunotherapeutic agents for the treatment of cancer |
| BR112020018910A2 (pt) | 2018-03-20 | 2020-12-29 | Abraxis Bioscience, Llc | Métodos de tratamento de transtorno do sistema nervoso central através da administração de nanopartículas de um unibidor de mtor e de uma albumina |
| CN108498529A (zh) * | 2018-06-20 | 2018-09-07 | 福建师范大学 | 用于肿瘤预防治疗的DNA甲基转移酶抑制剂与cGAMP药物组合物 |
| US20220008383A1 (en) * | 2018-11-13 | 2022-01-13 | Amy Yee | Compositions and methods of enhancing immunotherapies |
| JP2022553426A (ja) | 2019-10-28 | 2022-12-22 | アブラクシス バイオサイエンス, エルエルシー | アルブミンおよびラパマイシンの医薬組成物 |
| US20230330123A1 (en) * | 2020-08-11 | 2023-10-19 | Auxilla Pharmaceuticals And Research Llp | A non-aqueous suspension of anticancer agent |
| TR202018770A2 (tr) * | 2020-11-23 | 2021-02-22 | Ankara Ueniversitesi | Platine Dirençli Over Kanseri Tedavisine Yönelik Yeni Bir İlaç Taşıyıcı Sistem |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006089290A1 (en) * | 2005-02-18 | 2006-08-24 | Abraxis Bioscience Inc.. | Combinations and modes of administration of therapeutic agents and combination therapy |
Family Cites Families (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6537579B1 (en) | 1993-02-22 | 2003-03-25 | American Bioscience, Inc. | Compositions and methods for administration of pharmacologically active compounds |
| US5916596A (en) | 1993-02-22 | 1999-06-29 | Vivorx Pharmaceuticals, Inc. | Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof |
| US6096331A (en) | 1993-02-22 | 2000-08-01 | Vivorx Pharmaceuticals, Inc. | Methods and compositions useful for administration of chemotherapeutic agents |
| US6749868B1 (en) | 1993-02-22 | 2004-06-15 | American Bioscience, Inc. | Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof |
| DK1023050T3 (da) * | 1997-06-27 | 2013-10-14 | Abraxis Bioscience Llc | Nye formuleringer af farmakologiske midler, fremgangsmåder til fremstillingen deraf og fremgangsmåder til anvendelsen deraf |
| US6613753B2 (en) * | 2001-02-21 | 2003-09-02 | Supergen, Inc. | Restore cancer-suppressing functions to neoplastic cells through DNA hypomethylation |
| SI1585548T1 (sl) | 2002-12-09 | 2018-11-30 | Abraxis Bioscience, Llc | Sestave in metode odmerjanja farmakoloških sredstev |
| US20050187148A1 (en) * | 2004-02-25 | 2005-08-25 | Yoshinori Naoe | Antitumor agent |
| US7951780B2 (en) * | 2004-02-25 | 2011-05-31 | Astellas Pharma Inc. | Antitumor agent |
| AU2006249235B2 (en) * | 2004-05-14 | 2010-11-11 | Abraxis Bioscience, Llc | Sparc and methods of use thereof |
| US20060069060A1 (en) * | 2004-09-27 | 2006-03-30 | Sanjeev Redkar | Salts of decitabine |
| NZ589276A (en) * | 2005-02-03 | 2012-06-29 | Topotarget Uk Ltd | Combination therapies using hdac inhibitors and erlotinib (tarceva) |
| US20070166388A1 (en) * | 2005-02-18 | 2007-07-19 | Desai Neil P | Combinations and modes of administration of therapeutic agents and combination therapy |
| US8735394B2 (en) * | 2005-02-18 | 2014-05-27 | Abraxis Bioscience, Llc | Combinations and modes of administration of therapeutic agents and combination therapy |
| CN101291658B (zh) | 2005-08-31 | 2014-04-16 | 阿布拉科斯生物科学有限公司 | 用于制备稳定性增加的水难溶性药物的组合物和方法 |
| EP1942884A4 (en) * | 2005-11-04 | 2010-01-06 | Merck & Co Inc | METHOD FOR THE TREATMENT OF CANCER WITH SAHA, CARBOPLATIN AND PACLITAXEL AND OTHER COMBINATION THERAPIES |
| WO2008028193A2 (en) * | 2006-09-01 | 2008-03-06 | Pharmion Corporation | Colon-targeted oral formulations of cytidine analogs |
| ES2569477T3 (es) * | 2006-12-04 | 2016-05-11 | Novartis Ag | Combinación de un inhibidor de HDAC y un antimetabolito |
| CA2686736A1 (en) | 2007-05-03 | 2008-11-13 | Abraxis Bioscience, Llc | Nanoparticle compositions comprising rapamycin for treating pulmonary hypertension |
| WO2009145841A1 (en) * | 2008-04-03 | 2009-12-03 | Cognate 3, Llc | Compositions and methods for immunotherapy |
| KR20150136137A (ko) | 2008-04-10 | 2015-12-04 | 아브락시스 바이오사이언스, 엘엘씨 | 소수성 탁산 유도체의 조성물 및 그의 용도 |
| AU2009234127B2 (en) | 2008-04-10 | 2015-04-30 | Abraxis Bioscience, Llc | Compositions of hydrophobic taxane derivatives and uses thereof |
| SI2695609T1 (sl) * | 2008-05-15 | 2020-03-31 | Celgene Corporation | Oral formulacije anologov citidina in postopki za njihovo uporabo |
| US20100055047A1 (en) * | 2008-08-26 | 2010-03-04 | Yiyu Zou | Methods for treating bronchial premalignancy and lung cancer |
| US20120128732A1 (en) * | 2008-12-11 | 2012-05-24 | Vuong Trieu | Combinations and modes of administration of therapeutic agents and combination therapy |
| DK2470173T3 (en) * | 2009-08-25 | 2016-06-06 | Abraxis Bioscience Llc | Combination therapy of nanoparticle composition of the taxane and the hedgehog inhibitors |
| CA2794147A1 (en) * | 2010-03-29 | 2011-10-06 | Abraxis Bioscience, Llc | Use of a composition comprising nanoparticles comprising a taxane and an albumin to improve uptake of chemotherapeutics by tumors and for treating a cancer that is highly fibrotic and/or has a dense stroma |
| BR112012024442A2 (pt) * | 2010-03-29 | 2017-03-21 | Abraxis Bioscience Llc | métodos de tratamento de câncer |
| MX343671B (es) * | 2010-06-02 | 2016-11-16 | Abraxis Bioscience Llc * | Metodos de tratamiento de cancer de vejiga. |
| RU2576609C2 (ru) * | 2010-06-04 | 2016-03-10 | АБРАКСИС БАЙОСАЙЕНС, ЭлЭлСи | Способы лечение рака поджелудочной железы |
| EP3056201A1 (en) * | 2010-06-07 | 2016-08-17 | Abraxis BioScience, LLC | Combination therapy methods for treating proliferative diseases |
| US9149455B2 (en) * | 2012-11-09 | 2015-10-06 | Abraxis Bioscience, Llc | Methods of treating melanoma |
| US9511046B2 (en) * | 2013-01-11 | 2016-12-06 | Abraxis Bioscience, Llc | Methods of treating pancreatic cancer |
-
2011
- 2011-05-20 EP EP16153625.5A patent/EP3056201A1/en not_active Withdrawn
- 2011-05-20 MY MYPI2012005255A patent/MY166014A/en unknown
- 2011-05-20 CN CN201180038243.9A patent/CN103037846B/zh active Active
- 2011-05-20 PH PH1/2012/502399A patent/PH12012502399A1/en unknown
- 2011-05-20 CA CA2801891A patent/CA2801891C/en active Active
- 2011-05-20 SG SG2012088001A patent/SG185798A1/en unknown
- 2011-05-20 RU RU2016103126A patent/RU2016103126A/ru not_active Application Discontinuation
- 2011-05-20 MX MX2012014231A patent/MX2012014231A/es unknown
- 2011-05-20 JP JP2013514194A patent/JP6031437B2/ja active Active
- 2011-05-20 US US13/701,001 patent/US20140155344A1/en not_active Abandoned
- 2011-05-20 EP EP11792867.1A patent/EP2575774A4/en not_active Withdrawn
- 2011-05-20 WO PCT/US2011/037450 patent/WO2011156119A1/en not_active Ceased
- 2011-05-20 SG SG10201504590WA patent/SG10201504590WA/en unknown
- 2011-05-20 AU AU2011264590A patent/AU2011264590B2/en active Active
- 2011-05-20 RU RU2012156903/15A patent/RU2577278C2/ru active
- 2011-05-20 NZ NZ707377A patent/NZ707377A/en unknown
- 2011-05-20 CN CN201610059985.4A patent/CN105832703A/zh active Pending
- 2011-05-20 KR KR1020137000228A patent/KR101850566B1/ko active Active
- 2011-05-20 BR BR112012031163A patent/BR112012031163A2/pt not_active Application Discontinuation
-
2012
- 2012-11-22 IL IL223190A patent/IL223190A0/en unknown
- 2012-11-22 ZA ZA2012/08820A patent/ZA201208820B/en unknown
- 2012-12-06 NI NI201200181A patent/NI201200181A/es unknown
- 2012-12-14 CR CR20120638A patent/CR20120638A/es unknown
-
2013
- 2013-01-04 CO CO13001846A patent/CO6670568A2/es unknown
- 2013-03-01 US US13/782,992 patent/US20140170228A1/en not_active Abandoned
-
2016
- 2016-07-04 JP JP2016132221A patent/JP2016175947A/ja not_active Withdrawn
- 2016-11-28 US US15/361,851 patent/US20170181998A1/en not_active Abandoned
-
2017
- 2017-03-15 AU AU2017201757A patent/AU2017201757A1/en not_active Abandoned
-
2018
- 2018-02-16 JP JP2018025928A patent/JP2018087227A/ja active Pending
- 2018-08-10 US US16/101,027 patent/US20190167629A1/en not_active Abandoned
-
2019
- 2019-10-03 IL IL26980819A patent/IL269808A/en unknown
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006089290A1 (en) * | 2005-02-18 | 2006-08-24 | Abraxis Bioscience Inc.. | Combinations and modes of administration of therapeutic agents and combination therapy |
Non-Patent Citations (1)
| Title |
|---|
| OWONIKOKO TK et al.,Vorinostat increases carboplatin and paclitaxel activity in non-small-cell lung cancer cells, Int J Cancer. 2010 Feb 1;126(3):743-55. doi: 10.1002/ijc.24759. RAMALINGAM SS et al., Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies,Clin Cancer Res. 2007 Jun 15;13(12):3605-10. Epub 2007 May 17. * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11911374B2 (en) | 2019-05-29 | 2024-02-27 | Nelum Corporation | Methods and uses for treating cancer |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2577278C2 (ru) | Способы комбинированной терапии для лечения пролиферативных заболеваний | |
| TWI495467B (zh) | 肝細胞癌之治療方法 | |
| JP6257324B2 (ja) | 膵臓がんの処置方法 | |
| RU2561055C2 (ru) | Комбинированная терапия композициями наночастиц таксана и ингибиторами хэджхог | |
| WO2013085902A1 (en) | Combination therapy methods for treating an inflammatory breast cancer | |
| HK1227760A1 (en) | Combination therapy methods for treating proliferative diseases | |
| HK1227760A (en) | Combination therapy methods for treating proliferative diseases |