RU2299191C2 - Способ многостадийной конверсии загрузки, содержащей олефины с четырьмя, пятью или более атомами углерода, с целью получения пропилена (варианты) - Google Patents
Способ многостадийной конверсии загрузки, содержащей олефины с четырьмя, пятью или более атомами углерода, с целью получения пропилена (варианты) Download PDFInfo
- Publication number
- RU2299191C2 RU2299191C2 RU2004130481/04A RU2004130481A RU2299191C2 RU 2299191 C2 RU2299191 C2 RU 2299191C2 RU 2004130481/04 A RU2004130481/04 A RU 2004130481/04A RU 2004130481 A RU2004130481 A RU 2004130481A RU 2299191 C2 RU2299191 C2 RU 2299191C2
- Authority
- RU
- Russia
- Prior art keywords
- oligomerization
- olefins
- cracking
- reactor
- isobutene
- Prior art date
Links
- 150000001336 alkenes Chemical class 0.000 title claims abstract description 144
- 238000000034 method Methods 0.000 title claims abstract description 123
- 125000004805 propylene group Chemical group [H]C([H])([H])C([H])([*:1])C([H])([H])[*:2] 0.000 title claims abstract description 74
- QQONPFPTGQHPMA-UHFFFAOYSA-N propylene Natural products CC=C QQONPFPTGQHPMA-UHFFFAOYSA-N 0.000 title claims abstract description 71
- 125000004432 carbon atom Chemical group C* 0.000 title claims abstract description 36
- 238000006243 chemical reaction Methods 0.000 title claims abstract description 32
- 230000008569 process Effects 0.000 title abstract description 25
- 238000006384 oligomerization reaction Methods 0.000 claims abstract description 190
- 238000005336 cracking Methods 0.000 claims abstract description 99
- 238000004523 catalytic cracking Methods 0.000 claims abstract description 53
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims abstract description 30
- 239000005977 Ethylene Substances 0.000 claims abstract description 30
- 230000003197 catalytic effect Effects 0.000 claims abstract description 8
- 238000004821 distillation Methods 0.000 claims description 124
- 239000003054 catalyst Substances 0.000 claims description 112
- VQTUBCCKSQIDNK-UHFFFAOYSA-N Isobutene Chemical compound CC(C)=C VQTUBCCKSQIDNK-UHFFFAOYSA-N 0.000 claims description 101
- 239000010457 zeolite Substances 0.000 claims description 74
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 claims description 66
- 229910021536 Zeolite Inorganic materials 0.000 claims description 51
- 238000005984 hydrogenation reaction Methods 0.000 claims description 33
- 229930195733 hydrocarbon Natural products 0.000 claims description 24
- 150000002430 hydrocarbons Chemical class 0.000 claims description 23
- 238000005194 fractionation Methods 0.000 claims description 19
- 238000011068 loading method Methods 0.000 claims description 15
- 150000001993 dienes Chemical class 0.000 claims description 13
- 239000004215 Carbon black (E152) Substances 0.000 claims description 8
- 125000000383 tetramethylene group Chemical group [H]C([H])([*:1])C([H])([H])C([H])([H])C([H])([H])[*:2] 0.000 claims description 8
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 claims description 7
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 6
- 229910052782 aluminium Inorganic materials 0.000 claims description 6
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 6
- 229910052799 carbon Inorganic materials 0.000 claims description 5
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 3
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 claims description 3
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 claims description 3
- 229910052796 boron Inorganic materials 0.000 claims description 3
- 229910052733 gallium Inorganic materials 0.000 claims description 3
- 229910052742 iron Inorganic materials 0.000 claims description 3
- 229910052698 phosphorus Inorganic materials 0.000 claims description 3
- 239000011574 phosphorus Substances 0.000 claims description 3
- 229910052710 silicon Inorganic materials 0.000 claims description 3
- 239000010703 silicon Substances 0.000 claims description 3
- 239000011973 solid acid Substances 0.000 claims description 3
- 125000004429 atom Chemical group 0.000 claims 2
- 230000000694 effects Effects 0.000 abstract description 3
- 239000000126 substance Substances 0.000 abstract description 2
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 33
- 238000004230 steam cracking Methods 0.000 description 24
- 230000008929 regeneration Effects 0.000 description 22
- 238000011069 regeneration method Methods 0.000 description 22
- 150000001875 compounds Chemical class 0.000 description 21
- 239000000203 mixture Substances 0.000 description 19
- 239000003502 gasoline Substances 0.000 description 18
- 239000007789 gas Substances 0.000 description 15
- 230000015572 biosynthetic process Effects 0.000 description 14
- 238000004519 manufacturing process Methods 0.000 description 14
- 239000001257 hydrogen Substances 0.000 description 13
- 229910052739 hydrogen Inorganic materials 0.000 description 13
- 238000009434 installation Methods 0.000 description 12
- VXNZUUAINFGPBY-UHFFFAOYSA-N 1-Butene Chemical compound CCC=C VXNZUUAINFGPBY-UHFFFAOYSA-N 0.000 description 11
- 229910000323 aluminium silicate Inorganic materials 0.000 description 11
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 9
- 238000001816 cooling Methods 0.000 description 9
- 238000000926 separation method Methods 0.000 description 9
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 8
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 8
- 238000009835 boiling Methods 0.000 description 8
- 238000004064 recycling Methods 0.000 description 8
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 7
- 230000008901 benefit Effects 0.000 description 7
- IAQRGUVFOMOMEM-UHFFFAOYSA-N butene Natural products CC=CC IAQRGUVFOMOMEM-UHFFFAOYSA-N 0.000 description 7
- 238000005516 engineering process Methods 0.000 description 7
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 7
- 229910052760 oxygen Inorganic materials 0.000 description 7
- 239000001301 oxygen Substances 0.000 description 7
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 6
- -1 acetylene hydrocarbons Chemical class 0.000 description 6
- 235000013844 butane Nutrition 0.000 description 6
- 239000003350 kerosene Substances 0.000 description 6
- 150000005673 monoalkenes Chemical class 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 239000002283 diesel fuel Substances 0.000 description 5
- 238000000605 extraction Methods 0.000 description 5
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 5
- 239000003921 oil Substances 0.000 description 5
- 239000012188 paraffin wax Substances 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- KAKZBPTYRLMSJV-UHFFFAOYSA-N Butadiene Chemical compound C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical class CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 4
- 239000000571 coke Substances 0.000 description 4
- 150000002431 hydrogen Chemical class 0.000 description 4
- NNPPMTNAJDCUHE-UHFFFAOYSA-N isobutane Chemical compound CC(C)C NNPPMTNAJDCUHE-UHFFFAOYSA-N 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- 125000004817 pentamethylene group Chemical class [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 description 4
- 238000010926 purge Methods 0.000 description 4
- 239000000377 silicon dioxide Substances 0.000 description 4
- 238000012546 transfer Methods 0.000 description 4
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 238000004939 coking Methods 0.000 description 3
- 238000002485 combustion reaction Methods 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 238000000354 decomposition reaction Methods 0.000 description 3
- 239000000539 dimer Substances 0.000 description 3
- 229910052738 indium Inorganic materials 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 229910052763 palladium Inorganic materials 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 239000011347 resin Substances 0.000 description 3
- 229920005989 resin Polymers 0.000 description 3
- 235000012239 silicon dioxide Nutrition 0.000 description 3
- 238000004227 thermal cracking Methods 0.000 description 3
- 238000006276 transfer reaction Methods 0.000 description 3
- 229910001868 water Inorganic materials 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 239000002841 Lewis acid Substances 0.000 description 2
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 206010041067 Small cell lung cancer Diseases 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 2
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 2
- 150000001491 aromatic compounds Chemical class 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- WTEOIRVLGSZEPR-UHFFFAOYSA-N boron trifluoride Chemical compound FB(F)F WTEOIRVLGSZEPR-UHFFFAOYSA-N 0.000 description 2
- 239000001273 butane Substances 0.000 description 2
- NEHMKBQYUWJMIP-UHFFFAOYSA-N chloromethane Chemical compound ClC NEHMKBQYUWJMIP-UHFFFAOYSA-N 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 230000032050 esterification Effects 0.000 description 2
- 238000005886 esterification reaction Methods 0.000 description 2
- 239000003546 flue gas Substances 0.000 description 2
- 239000000446 fuel Substances 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 125000004836 hexamethylene group Chemical class [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 239000001282 iso-butane Substances 0.000 description 2
- 150000007517 lewis acids Chemical class 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- TVMXDCGIABBOFY-UHFFFAOYSA-N octane Chemical compound CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 2
- 230000003606 oligomerizing effect Effects 0.000 description 2
- YWAKXRMUMFPDSH-UHFFFAOYSA-N pentene Chemical compound CCCC=C YWAKXRMUMFPDSH-UHFFFAOYSA-N 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- IAQRGUVFOMOMEM-ONEGZZNKSA-N trans-but-2-ene Chemical compound C\C=C\C IAQRGUVFOMOMEM-ONEGZZNKSA-N 0.000 description 2
- 239000013638 trimer Substances 0.000 description 2
- PMJHHCWVYXUKFD-SNAWJCMRSA-N (E)-1,3-pentadiene Chemical compound C\C=C\C=C PMJHHCWVYXUKFD-SNAWJCMRSA-N 0.000 description 1
- LAAVYEUJEMRIGF-UHFFFAOYSA-N 2,4,4-trimethylpent-2-ene Chemical compound CC(C)=CC(C)(C)C LAAVYEUJEMRIGF-UHFFFAOYSA-N 0.000 description 1
- BKOOMYPCSUNDGP-UHFFFAOYSA-N 2-methylbut-2-ene Chemical group CC=C(C)C BKOOMYPCSUNDGP-UHFFFAOYSA-N 0.000 description 1
- YHQXBTXEYZIYOV-UHFFFAOYSA-N 3-methylbut-1-ene Chemical compound CC(C)C=C YHQXBTXEYZIYOV-UHFFFAOYSA-N 0.000 description 1
- 229910015900 BF3 Inorganic materials 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 229910021627 Tin(IV) chloride Inorganic materials 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 150000000475 acetylene derivatives Chemical class 0.000 description 1
- 238000007259 addition reaction Methods 0.000 description 1
- 150000004996 alkyl benzenes Chemical class 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006392 deoxygenation reaction Methods 0.000 description 1
- 238000006477 desulfuration reaction Methods 0.000 description 1
- 230000023556 desulfurization Effects 0.000 description 1
- 238000006471 dimerization reaction Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000006266 etherification reaction Methods 0.000 description 1
- 238000000895 extractive distillation Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 238000004231 fluid catalytic cracking Methods 0.000 description 1
- 239000000295 fuel oil Substances 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000000622 liquid--liquid extraction Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229940050176 methyl chloride Drugs 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 238000005121 nitriding Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000002941 palladium compounds Chemical class 0.000 description 1
- 238000002161 passivation Methods 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 238000000066 reactive distillation Methods 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 230000001172 regenerating effect Effects 0.000 description 1
- 238000003303 reheating Methods 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- NBRKLOOSMBRFMH-UHFFFAOYSA-N tert-butyl chloride Chemical compound CC(C)(C)Cl NBRKLOOSMBRFMH-UHFFFAOYSA-N 0.000 description 1
- HPGGPRDJHPYFRM-UHFFFAOYSA-J tin(iv) chloride Chemical compound Cl[Sn](Cl)(Cl)Cl HPGGPRDJHPYFRM-UHFFFAOYSA-J 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 150000003738 xylenes Chemical class 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C11/00—Aliphatic unsaturated hydrocarbons
- C07C11/02—Alkenes
- C07C11/06—Propene
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2/00—Preparation of hydrocarbons from hydrocarbons containing a smaller number of carbon atoms
- C07C2/02—Preparation of hydrocarbons from hydrocarbons containing a smaller number of carbon atoms by addition between unsaturated hydrocarbons
- C07C2/04—Preparation of hydrocarbons from hydrocarbons containing a smaller number of carbon atoms by addition between unsaturated hydrocarbons by oligomerisation of well-defined unsaturated hydrocarbons without ring formation
- C07C2/06—Preparation of hydrocarbons from hydrocarbons containing a smaller number of carbon atoms by addition between unsaturated hydrocarbons by oligomerisation of well-defined unsaturated hydrocarbons without ring formation of alkenes, i.e. acyclic hydrocarbons having only one carbon-to-carbon double bond
- C07C2/08—Catalytic processes
- C07C2/12—Catalytic processes with crystalline alumino-silicates or with catalysts comprising molecular sieves
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Crystallography & Structural Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Catalysts (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0203211 | 2002-03-15 | ||
| FR0203211A FR2837199B1 (fr) | 2002-03-15 | 2002-03-15 | Procede de conversion en plusieurs etapes d'une charge comprenant des olefines a quatre, cinq atomes de carbone ou plus, en vue de produire du propylene |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2004130481A RU2004130481A (ru) | 2005-07-20 |
| RU2299191C2 true RU2299191C2 (ru) | 2007-05-20 |
Family
ID=27772142
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2004130481/04A RU2299191C2 (ru) | 2002-03-15 | 2003-03-06 | Способ многостадийной конверсии загрузки, содержащей олефины с четырьмя, пятью или более атомами углерода, с целью получения пропилена (варианты) |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US7262332B2 (enExample) |
| EP (1) | EP1487768B1 (enExample) |
| JP (1) | JP4665397B2 (enExample) |
| CN (1) | CN100475755C (enExample) |
| AU (1) | AU2003227818A1 (enExample) |
| DE (1) | DE60316415T2 (enExample) |
| FR (1) | FR2837199B1 (enExample) |
| RU (1) | RU2299191C2 (enExample) |
| WO (1) | WO2003078364A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2501778C2 (ru) * | 2008-08-29 | 2013-12-20 | Ифп Энержи Нувелль | Способ конверсии тяжелого сырья в бензин и пропилен с регулируемым выходом |
| RU2599749C2 (ru) * | 2012-08-10 | 2016-10-10 | Асахи Касеи Кемикалз Корпорейшн | Способ превращения олефина или спирта и способ получения пропилена или ароматического соединения |
| RU2611498C2 (ru) * | 2011-11-24 | 2017-02-27 | Ифп Энержи Нувелль | Способ получения среднего дистиллята из обычной тяжелой фракции, включающий этап селективного гидрирования фракции нсо из fcc |
| RU2701018C2 (ru) * | 2014-09-30 | 2019-09-24 | Дау Глоубл Текнолоджиз Ллк | Способ увеличения выхода этилена и пропилена на установке получения пропилена |
Families Citing this family (53)
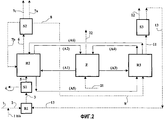
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2877590B1 (fr) * | 2004-11-09 | 2007-01-12 | Inst Francais Du Petrole | Dispositif reactionnel a plusieurs zones en lit mobile avec appoint dans chaque zone de catalyseur regenere ou frais |
| FR2879620B1 (fr) * | 2004-12-21 | 2007-02-23 | Inst Francais Du Petrole | Procede de conversion directe d'une charge comprenant des olefines a quatre et/ou cinq atomes de carbone, pour la production de propylene avec une co-production d'essence |
| CN100413826C (zh) * | 2005-08-15 | 2008-08-27 | 中国石油化工股份有限公司 | 生产丙烯的方法 |
| CN100413823C (zh) * | 2005-09-07 | 2008-08-27 | 中国石油化工股份有限公司 | 催化裂解生产丙烯的方法 |
| FR2892126B1 (fr) * | 2005-10-19 | 2010-04-30 | Inst Francais Du Petrole | Procede de conversion directe d'une charge comprenant des olefines a quatre, et/ou cinq atomes de carbone, pour la production de propylene avec une co-production d'essence desulfuree |
| US8299312B2 (en) * | 2005-10-28 | 2012-10-30 | Neste Oil Oyj | Process for dimerizing olefins |
| US7459593B1 (en) * | 2005-11-18 | 2008-12-02 | Uop Llc | Metathesis unit pretreatment process with formation of octene |
| KR101368416B1 (ko) * | 2006-02-03 | 2014-03-05 | 리액션 35, 엘엘씨 | 천연 가스를 액체 탄화수소로 변환시키는 연속 공정 |
| FR2905122B1 (fr) * | 2006-08-24 | 2009-07-24 | Inst Francais Du Petrole | Procede de production de propylene en presence d'un catalyseur macroporeux se presentant sous forme de billes spheriques |
| JP5355910B2 (ja) * | 2008-03-13 | 2013-11-27 | 旭化成ケミカルズ株式会社 | シリカ成形体 |
| JP5478253B2 (ja) * | 2007-09-18 | 2014-04-23 | 旭化成ケミカルズ株式会社 | プロピレンの製造方法 |
| TW200918486A (en) * | 2007-09-18 | 2009-05-01 | Asahi Kasei Chemicals Corp | Process for production of propylene |
| CN101492334B (zh) * | 2008-01-23 | 2012-11-14 | 中国石油化工股份有限公司 | 提高混合碳四化工利用价值的方法 |
| CN101492335B (zh) * | 2008-01-23 | 2013-07-31 | 中国石油化工股份有限公司 | 综合利用混合碳四的组合方法 |
| RU2460713C1 (ru) * | 2008-11-17 | 2012-09-10 | Юоп Ллк | Способ предварительной обработки в установке метатезиса с образованием октена |
| BR112012024022A2 (pt) * | 2010-03-31 | 2016-08-30 | Uop Llc | processo e aparelho para converter olefinas em compostos maiores |
| US8471084B2 (en) | 2010-03-31 | 2013-06-25 | Uop Llc | Process for increasing weight of olefins |
| US8128879B2 (en) | 2010-03-31 | 2012-03-06 | Uop Llc | Apparatus for increasing weight of olefins |
| CN102884027B (zh) * | 2010-05-10 | 2016-08-17 | 催化蒸馏技术公司 | 从异丁醇制备喷气燃料和其他重质燃料 |
| FR2968010B1 (fr) | 2010-11-25 | 2014-03-14 | Ifp Energies Now | Procede de conversion d'une charge lourde en distillat moyen |
| US8993824B2 (en) | 2011-09-28 | 2015-03-31 | Uop Llc | Fluid catalytic cracking process |
| BR112014019272B1 (pt) * | 2012-03-02 | 2023-11-14 | Petroleo Brasileiro S. A. - Petrobras | Aditivos para maximização de olefinas leves em unidades de craqueamento catalítico fluido e processo |
| CN103420761B (zh) * | 2012-05-16 | 2015-02-11 | 中国石油化工股份有限公司 | 戊烯歧化制丙烯的方法 |
| CN103420758B (zh) * | 2012-05-16 | 2015-06-10 | 中国石油化工股份有限公司 | 戊烯和乙烯制丙烯的方法 |
| US9695096B2 (en) | 2012-07-12 | 2017-07-04 | Lummus Technology Inc. | More energy efficient C5 hydrogenation process |
| CN103539619B (zh) * | 2012-07-12 | 2015-05-13 | 中国石油化工股份有限公司 | 混合碳四芳构化制备芳烃的方法 |
| ES2443539B1 (es) | 2012-07-19 | 2014-12-04 | Consejo Superior De Investigaciones Científicas (Csic) | Proceso de oligomerización de alquenos utilizando la zeolita ITQ-39 |
| US9522373B2 (en) | 2012-11-12 | 2016-12-20 | Uop Llc | Apparatus for oligomerizing light olefins |
| US9644159B2 (en) | 2012-11-12 | 2017-05-09 | Uop Llc | Composition of oligomerate |
| US9567267B2 (en) | 2012-11-12 | 2017-02-14 | Uop Llc | Process for oligomerizing light olefins including pentenes |
| US10508064B2 (en) | 2012-11-12 | 2019-12-17 | Uop Llc | Process for oligomerizing gasoline without further upgrading |
| US9434891B2 (en) | 2012-11-12 | 2016-09-06 | Uop Llc | Apparatus for recovering oligomerate |
| US9522375B2 (en) | 2012-11-12 | 2016-12-20 | Uop Llc | Apparatus for fluid catalytic cracking oligomerate |
| US9914673B2 (en) | 2012-11-12 | 2018-03-13 | Uop Llc | Process for oligomerizing light olefins |
| US9441173B2 (en) | 2012-11-12 | 2016-09-13 | Uop Llc | Process for making diesel by oligomerization |
| US9834492B2 (en) | 2012-11-12 | 2017-12-05 | Uop Llc | Process for fluid catalytic cracking oligomerate |
| US9663415B2 (en) | 2012-11-12 | 2017-05-30 | Uop Llc | Process for making diesel by oligomerization of gasoline |
| WO2014074833A1 (en) | 2012-11-12 | 2014-05-15 | Uop Llc | Process for making gasoline by oligomerization |
| US10011778B2 (en) | 2013-12-17 | 2018-07-03 | Uop Llc | Process and apparatus for improving propylene yield from a fluid catalytic cracking process |
| US9670425B2 (en) | 2013-12-17 | 2017-06-06 | Uop Llc | Process for oligomerizing and cracking to make propylene and aromatics |
| US9732285B2 (en) | 2013-12-17 | 2017-08-15 | Uop Llc | Process for oligomerization of gasoline to make diesel |
| US10556229B2 (en) | 2014-08-01 | 2020-02-11 | Chiyoda Corporation | Composite catalyst, method for producing composite catalyst, method for producing lower olefin and method for regenerating composite catalyst |
| FR3049954A1 (fr) | 2016-04-08 | 2017-10-13 | Ifp Energies Now | Utilisation de zeolithe nu-86 pour le procede de craquage catalytique de naphtha |
| CN111116287A (zh) * | 2018-10-30 | 2020-05-08 | 中国石油化工股份有限公司 | 制备丙烯和乙烯的方法 |
| CN111116288B (zh) * | 2018-10-30 | 2023-09-29 | 中国石油化工股份有限公司 | 生产丙烯和乙烯的方法 |
| CN109369319B (zh) * | 2018-12-07 | 2021-11-12 | 宁波旭合瑞石化工程有限公司 | 一种以c4-c8烯烃为原料最大化生产丙烯的方法 |
| CN111718753B (zh) * | 2019-03-22 | 2021-10-08 | 中国石油化工股份有限公司 | 一种多产丙烯的催化转化方法和系统 |
| CN111718230B (zh) * | 2019-03-22 | 2023-04-11 | 中国石油化工股份有限公司 | 一种生产丙烯的方法和系统 |
| CN111718752B (zh) * | 2019-03-22 | 2021-11-16 | 中国石油化工股份有限公司 | 一种多产丙烯的催化裂化方法和系统 |
| CN111718750B (zh) * | 2019-03-22 | 2021-12-17 | 中国石油化工股份有限公司 | 一种制取丙烯的方法和系统 |
| EP3990420A4 (en) * | 2019-06-27 | 2023-07-12 | GEVO, Inc. | OLIGOMERIZATION OF BIOLOGICALLY DERIVED OLEFINS THROUGH A CHABAZITE-TYPE ZEOLITE CATALYST |
| CN111825514B (zh) * | 2020-08-12 | 2021-06-01 | 浙江科茂环境科技有限公司 | 一种乙烯或丙烯最大化的生产方法 |
| CN115784830B (zh) * | 2021-09-10 | 2024-08-23 | 北京石油化工工程有限公司 | 一种提高低碳烯烃收率的耦合工艺方法和耦合工艺系统 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SU1385517A1 (ru) * | 1986-04-16 | 1999-09-27 | Институт катализа СО АН СССР | Способ получения пропилена |
| RU2184610C2 (ru) * | 1996-11-19 | 2002-07-10 | Энститю Франсэ Дю Петроль | Дезалюминированный цеолит nu-86, способ его получения (варианты), катализатор на его основе и его использование при конверсии углеводородов |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0109059B1 (en) * | 1982-11-10 | 1987-07-15 | MONTEDIPE S.p.A. | Process for converting olefins having 4 to 12 carbon atoms into propylene |
| FR2663946B1 (fr) * | 1990-05-09 | 1994-04-29 | Inst Francais Du Petrole | Procede de craquage catalytique en presence d'un catalyseur renfermant une zeolite zsm a ouverture de pore intermediaire. |
| GB9225183D0 (en) * | 1992-12-02 | 1993-01-20 | British Petroleum Co Plc | Process for the production of branched olefins |
| EP0921181A1 (en) * | 1997-12-05 | 1999-06-09 | Fina Research S.A. | Production of propylene |
| EP0920911A1 (en) * | 1997-12-05 | 1999-06-09 | Fina Research S.A. | Production of catalysts for olefin conversion |
| US6049017A (en) * | 1998-04-13 | 2000-04-11 | Uop Llc | Enhanced light olefin production |
| FR2837213B1 (fr) * | 2002-03-15 | 2004-08-20 | Inst Francais Du Petrole | Procede de production conjointe de propylene et d'essence a partir d'une charge relativement lourde |
-
2002
- 2002-03-15 FR FR0203211A patent/FR2837199B1/fr not_active Expired - Fee Related
-
2003
- 2003-03-06 WO PCT/FR2003/000728 patent/WO2003078364A1/fr not_active Ceased
- 2003-03-06 AU AU2003227818A patent/AU2003227818A1/en not_active Abandoned
- 2003-03-06 CN CNB03806104XA patent/CN100475755C/zh not_active Expired - Lifetime
- 2003-03-06 EP EP03725271A patent/EP1487768B1/fr not_active Expired - Lifetime
- 2003-03-06 US US10/507,853 patent/US7262332B2/en not_active Expired - Lifetime
- 2003-03-06 RU RU2004130481/04A patent/RU2299191C2/ru active
- 2003-03-06 DE DE60316415T patent/DE60316415T2/de not_active Expired - Lifetime
- 2003-03-06 JP JP2003576373A patent/JP4665397B2/ja not_active Expired - Lifetime
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SU1385517A1 (ru) * | 1986-04-16 | 1999-09-27 | Институт катализа СО АН СССР | Способ получения пропилена |
| RU2184610C2 (ru) * | 1996-11-19 | 2002-07-10 | Энститю Франсэ Дю Петроль | Дезалюминированный цеолит nu-86, способ его получения (варианты), катализатор на его основе и его использование при конверсии углеводородов |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2501778C2 (ru) * | 2008-08-29 | 2013-12-20 | Ифп Энержи Нувелль | Способ конверсии тяжелого сырья в бензин и пропилен с регулируемым выходом |
| RU2611498C2 (ru) * | 2011-11-24 | 2017-02-27 | Ифп Энержи Нувелль | Способ получения среднего дистиллята из обычной тяжелой фракции, включающий этап селективного гидрирования фракции нсо из fcc |
| RU2599749C2 (ru) * | 2012-08-10 | 2016-10-10 | Асахи Касеи Кемикалз Корпорейшн | Способ превращения олефина или спирта и способ получения пропилена или ароматического соединения |
| RU2701018C2 (ru) * | 2014-09-30 | 2019-09-24 | Дау Глоубл Текнолоджиз Ллк | Способ увеличения выхода этилена и пропилена на установке получения пропилена |
Also Published As
| Publication number | Publication date |
|---|---|
| FR2837199B1 (fr) | 2005-09-16 |
| WO2003078364A1 (fr) | 2003-09-25 |
| US20050222475A1 (en) | 2005-10-06 |
| JP2005520874A (ja) | 2005-07-14 |
| US7262332B2 (en) | 2007-08-28 |
| DE60316415D1 (de) | 2007-10-31 |
| CN100475755C (zh) | 2009-04-08 |
| EP1487768B1 (fr) | 2007-09-19 |
| RU2004130481A (ru) | 2005-07-20 |
| FR2837199A1 (fr) | 2003-09-19 |
| AU2003227818A1 (en) | 2003-09-29 |
| DE60316415T2 (de) | 2008-01-17 |
| CN1642887A (zh) | 2005-07-20 |
| EP1487768A1 (fr) | 2004-12-22 |
| JP4665397B2 (ja) | 2011-04-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2299191C2 (ru) | Способ многостадийной конверсии загрузки, содержащей олефины с четырьмя, пятью или более атомами углерода, с целью получения пропилена (варианты) | |
| RU2294916C2 (ru) | Способ конверсии углеводородной загрузки | |
| RU2501778C2 (ru) | Способ конверсии тяжелого сырья в бензин и пропилен с регулируемым выходом | |
| RU2674024C2 (ru) | Способ олигомеризации легких олефинов, включая пентены | |
| RU2638933C2 (ru) | Способ получения дизельного топлива с помощью олигомеризации бензина | |
| US9193922B2 (en) | Process of direct conversion of a charge comprising olefins with four and/or five carbon atoms, for the production of propylene with co-production of gasoline | |
| RU2639160C2 (ru) | Способ олигомеризации бензина без дополнительного облагораживания | |
| JP2001040369A (ja) | オレフィンの製造 | |
| RU2563655C2 (ru) | Способ конверсии тяжелой фракции в средний дистиллят | |
| US20160264492A1 (en) | Integrated process for converting light paraffins to gasoline and distillate | |
| EP2649161A2 (en) | A method for production of middle distillate components from gasoline components by oligomerization of olefins | |
| EP2228355A1 (fr) | Procédé de conversion directe d'une charge comprenant des oléfines à quatre et/ou cinq atomes de carbone pour la production de propylène | |
| US9914884B2 (en) | Process and apparatus for recovering oligomerate | |
| JP4958800B2 (ja) | 芳香族のアルキル化を伴うオレフィンの重合によるガソリンの製造 | |
| US9387413B2 (en) | Process and apparatus for recovering oligomerate | |
| RU2135547C1 (ru) | Способ олигомеризации низших олефинов | |
| WO2015094680A1 (en) | Process for oligomerization of gasoline to make diesel | |
| WO2015094678A1 (en) | Process for oligomerization of gasoline | |
| KR101474889B1 (ko) | 올레핀의 중량을 증가시키는 공정 및 장치 | |
| WO2014074970A1 (en) | Process for oligomerizing light olefins |