RU2225837C2 - Способ получения порошкообразных комплексных керамических материалов на основе тугоплавких металлов - Google Patents
Способ получения порошкообразных комплексных керамических материалов на основе тугоплавких металлов Download PDFInfo
- Publication number
- RU2225837C2 RU2225837C2 RU2000125557/15A RU2000125557A RU2225837C2 RU 2225837 C2 RU2225837 C2 RU 2225837C2 RU 2000125557/15 A RU2000125557/15 A RU 2000125557/15A RU 2000125557 A RU2000125557 A RU 2000125557A RU 2225837 C2 RU2225837 C2 RU 2225837C2
- Authority
- RU
- Russia
- Prior art keywords
- calcium
- reaction
- mixed
- preceding paragraphs
- metal
- Prior art date
Links
- 238000000034 method Methods 0.000 title claims description 38
- 229910010293 ceramic material Inorganic materials 0.000 title claims description 24
- 229910000753 refractory alloy Inorganic materials 0.000 title 1
- 238000006243 chemical reaction Methods 0.000 claims abstract description 68
- 239000011575 calcium Substances 0.000 claims abstract description 60
- 239000000843 powder Substances 0.000 claims abstract description 43
- 229910052791 calcium Inorganic materials 0.000 claims abstract description 34
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims abstract description 32
- 229910052751 metal Inorganic materials 0.000 claims abstract description 26
- 239000000203 mixture Substances 0.000 claims abstract description 26
- 239000002184 metal Substances 0.000 claims abstract description 25
- 239000003870 refractory metal Substances 0.000 claims abstract description 22
- 239000002245 particle Substances 0.000 claims abstract description 18
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 claims abstract description 16
- 150000001875 compounds Chemical class 0.000 claims abstract description 13
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims abstract description 11
- 150000001247 metal acetylides Chemical class 0.000 claims abstract description 10
- 150000004767 nitrides Chemical class 0.000 claims abstract description 10
- CJNBYAVZURUTKZ-UHFFFAOYSA-N hafnium(IV) oxide Inorganic materials O=[Hf]=O CJNBYAVZURUTKZ-UHFFFAOYSA-N 0.000 claims abstract description 9
- 229910044991 metal oxide Inorganic materials 0.000 claims abstract description 9
- 150000004706 metal oxides Chemical class 0.000 claims abstract description 9
- 150000003839 salts Chemical class 0.000 claims abstract description 8
- 238000001816 cooling Methods 0.000 claims abstract description 7
- 238000004519 manufacturing process Methods 0.000 claims abstract description 6
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 claims abstract description 5
- ODINCKMPIJJUCX-UHFFFAOYSA-N Calcium oxide Chemical compound [Ca]=O ODINCKMPIJJUCX-UHFFFAOYSA-N 0.000 claims abstract description 4
- 229910052755 nonmetal Inorganic materials 0.000 claims abstract 5
- WHJFNYXPKGDKBB-UHFFFAOYSA-N hafnium;methane Chemical compound C.[Hf] WHJFNYXPKGDKBB-UHFFFAOYSA-N 0.000 claims description 14
- 229910052799 carbon Inorganic materials 0.000 claims description 12
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 11
- 239000010936 titanium Substances 0.000 claims description 7
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 claims description 4
- 230000015572 biosynthetic process Effects 0.000 claims description 4
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 3
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 claims description 3
- 239000007787 solid Substances 0.000 claims description 3
- 229910052719 titanium Inorganic materials 0.000 claims description 3
- BRPQOXSCLDDYGP-UHFFFAOYSA-N calcium oxide Chemical compound [O-2].[Ca+2] BRPQOXSCLDDYGP-UHFFFAOYSA-N 0.000 claims description 2
- 239000000292 calcium oxide Substances 0.000 claims description 2
- 239000000376 reactant Substances 0.000 claims 2
- 150000001669 calcium Chemical class 0.000 claims 1
- MTPVUVINMAGMJL-UHFFFAOYSA-N trimethyl(1,1,2,2,2-pentafluoroethyl)silane Chemical compound C[Si](C)(C)C(F)(F)C(F)(F)F MTPVUVINMAGMJL-UHFFFAOYSA-N 0.000 abstract description 23
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 abstract description 12
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 abstract description 10
- 239000000126 substance Substances 0.000 abstract description 9
- 229910052786 argon Inorganic materials 0.000 abstract description 6
- 239000000919 ceramic Substances 0.000 abstract description 6
- -1 NaCl Chemical class 0.000 abstract description 5
- 239000011780 sodium chloride Substances 0.000 abstract description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 abstract description 4
- 238000000576 coating method Methods 0.000 abstract description 4
- 239000001301 oxygen Substances 0.000 abstract description 4
- 229910052760 oxygen Inorganic materials 0.000 abstract description 4
- 230000000694 effects Effects 0.000 abstract description 3
- 238000002360 preparation method Methods 0.000 abstract description 3
- 238000005520 cutting process Methods 0.000 abstract description 2
- 229910014813 CaC2 Inorganic materials 0.000 abstract 1
- 239000006229 carbon black Substances 0.000 abstract 1
- 238000005498 polishing Methods 0.000 abstract 1
- 239000011541 reaction mixture Substances 0.000 abstract 1
- 239000011819 refractory material Substances 0.000 abstract 1
- 230000001105 regulatory effect Effects 0.000 abstract 1
- MBEGFNBBAVRKLK-UHFFFAOYSA-N sodium;iminomethylideneazanide Chemical compound [Na+].[NH-]C#N MBEGFNBBAVRKLK-UHFFFAOYSA-N 0.000 abstract 1
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 abstract 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 22
- 239000003153 chemical reaction reagent Substances 0.000 description 15
- 239000011777 magnesium Substances 0.000 description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 12
- 229910052757 nitrogen Inorganic materials 0.000 description 11
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 10
- 239000007789 gas Substances 0.000 description 10
- 229910052749 magnesium Inorganic materials 0.000 description 10
- 230000007935 neutral effect Effects 0.000 description 10
- 150000002739 metals Chemical class 0.000 description 9
- 238000002156 mixing Methods 0.000 description 9
- XCNGEWCFFFJZJT-UHFFFAOYSA-N calcium;azanidylidenecalcium Chemical compound [Ca+2].[Ca]=[N-].[Ca]=[N-] XCNGEWCFFFJZJT-UHFFFAOYSA-N 0.000 description 8
- 238000010438 heat treatment Methods 0.000 description 8
- 239000005997 Calcium carbide Substances 0.000 description 7
- 239000000047 product Substances 0.000 description 7
- CLZWAWBPWVRRGI-UHFFFAOYSA-N tert-butyl 2-[2-[2-[2-[bis[2-[(2-methylpropan-2-yl)oxy]-2-oxoethyl]amino]-5-bromophenoxy]ethoxy]-4-methyl-n-[2-[(2-methylpropan-2-yl)oxy]-2-oxoethyl]anilino]acetate Chemical compound CC1=CC=C(N(CC(=O)OC(C)(C)C)CC(=O)OC(C)(C)C)C(OCCOC=2C(=CC=C(Br)C=2)N(CC(=O)OC(C)(C)C)CC(=O)OC(C)(C)C)=C1 CLZWAWBPWVRRGI-UHFFFAOYSA-N 0.000 description 7
- 230000008901 benefit Effects 0.000 description 6
- 239000002360 explosive Substances 0.000 description 5
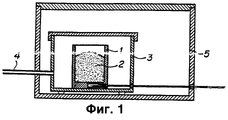
- 238000009434 installation Methods 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 238000009835 boiling Methods 0.000 description 4
- 239000003245 coal Substances 0.000 description 4
- 229910052735 hafnium Inorganic materials 0.000 description 4
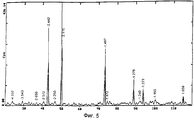
- 238000001228 spectrum Methods 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 229910010413 TiO 2 Inorganic materials 0.000 description 3
- NRTOMJZYCJJWKI-UHFFFAOYSA-N Titanium nitride Chemical compound [Ti]#N NRTOMJZYCJJWKI-UHFFFAOYSA-N 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 235000008733 Citrus aurantifolia Nutrition 0.000 description 2
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 2
- 239000005977 Ethylene Substances 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 229910000831 Steel Inorganic materials 0.000 description 2
- 235000011941 Tilia x europaea Nutrition 0.000 description 2
- 238000002441 X-ray diffraction Methods 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 150000001540 azides Chemical class 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 238000002425 crystallisation Methods 0.000 description 2
- 230000008025 crystallization Effects 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- VBJZVLUMGGDVMO-UHFFFAOYSA-N hafnium atom Chemical compound [Hf] VBJZVLUMGGDVMO-UHFFFAOYSA-N 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 239000004571 lime Substances 0.000 description 2
- 239000000395 magnesium oxide Substances 0.000 description 2
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 2
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 239000012798 spherical particle Substances 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 239000010959 steel Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- ZVWKZXLXHLZXLS-UHFFFAOYSA-N zirconium nitride Chemical compound [Zr]#N ZVWKZXLXHLZXLS-UHFFFAOYSA-N 0.000 description 2
- 229910000975 Carbon steel Inorganic materials 0.000 description 1
- 206010016275 Fear Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- RWDBMHZWXLUGIB-UHFFFAOYSA-N [C].[Mg] Chemical compound [C].[Mg] RWDBMHZWXLUGIB-UHFFFAOYSA-N 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 239000003570 air Substances 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 239000012080 ambient air Substances 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 239000012300 argon atmosphere Substances 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 239000010962 carbon steel Substances 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 238000005229 chemical vapour deposition Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 230000005518 electrochemistry Effects 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000004880 explosion Methods 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- IXCSERBJSXMMFS-UHFFFAOYSA-N hcl hcl Chemical compound Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- IXQWNVPHFNLUGD-UHFFFAOYSA-N iron titanium Chemical compound [Ti].[Fe] IXQWNVPHFNLUGD-UHFFFAOYSA-N 0.000 description 1
- YDZQQRWRVYGNER-UHFFFAOYSA-N iron;titanium;trihydrate Chemical compound O.O.O.[Ti].[Fe] YDZQQRWRVYGNER-UHFFFAOYSA-N 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000010907 mechanical stirring Methods 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000002923 metal particle Substances 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 238000005240 physical vapour deposition Methods 0.000 description 1
- 239000012255 powdered metal Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 239000004071 soot Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 229910052845 zircon Inorganic materials 0.000 description 1
- GFQYVLUOOAAOGM-UHFFFAOYSA-N zirconium(iv) silicate Chemical compound [Zr+4].[O-][Si]([O-])([O-])[O-] GFQYVLUOOAAOGM-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01F—COMPOUNDS OF THE METALS BERYLLIUM, MAGNESIUM, ALUMINIUM, CALCIUM, STRONTIUM, BARIUM, RADIUM, THORIUM, OR OF THE RARE-EARTH METALS
- C01F11/00—Compounds of calcium, strontium, or barium
- C01F11/02—Oxides or hydroxides
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B21/00—Nitrogen; Compounds thereof
- C01B21/06—Binary compounds of nitrogen with metals, with silicon, or with boron, or with carbon, i.e. nitrides; Compounds of nitrogen with more than one metal, silicon or boron
- C01B21/076—Binary compounds of nitrogen with metals, with silicon, or with boron, or with carbon, i.e. nitrides; Compounds of nitrogen with more than one metal, silicon or boron with titanium or zirconium or hafnium
- C01B21/0765—Preparation by carboreductive nitridation
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/90—Carbides
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/90—Carbides
- C01B32/907—Oxycarbides; Sulfocarbides; Mixture of carbides
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/90—Carbides
- C01B32/914—Carbides of single elements
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/90—Carbides
- C01B32/914—Carbides of single elements
- C01B32/921—Titanium carbide
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/70—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data
- C01P2002/72—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data by d-values or two theta-values, e.g. as X-ray diagram
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/70—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data
- C01P2002/77—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data by unit-cell parameters, atom positions or structure diagrams
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/01—Particle morphology depicted by an image
- C01P2004/03—Particle morphology depicted by an image obtained by SEM
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/50—Agglomerated particles
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/60—Particles characterised by their size
- C01P2004/62—Submicrometer sized, i.e. from 0.1-1 micrometer
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/80—Compositional purity
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Carbon And Carbon Compounds (AREA)
- Compositions Of Oxide Ceramics (AREA)
- Inorganic Compounds Of Heavy Metals (AREA)
- Ceramic Products (AREA)
- Oxygen, Ozone, And Oxides In General (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR9803393 | 1998-03-16 | ||
| FR98/03393 | 2000-12-16 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2000125557A RU2000125557A (ru) | 2002-09-27 |
| RU2225837C2 true RU2225837C2 (ru) | 2004-03-20 |
Family
ID=9524243
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2000125557/15A RU2225837C2 (ru) | 1998-03-16 | 1999-03-16 | Способ получения порошкообразных комплексных керамических материалов на основе тугоплавких металлов |
Country Status (10)
| Country | Link |
|---|---|
| EP (1) | EP1064224B1 (enExample) |
| JP (1) | JP4541544B2 (enExample) |
| CN (1) | CN1204043C (enExample) |
| AT (1) | ATE267768T1 (enExample) |
| AU (1) | AU2635299A (enExample) |
| CA (1) | CA2322707C (enExample) |
| DE (1) | DE69917613T2 (enExample) |
| ES (1) | ES2218996T3 (enExample) |
| RU (1) | RU2225837C2 (enExample) |
| WO (1) | WO1999047454A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2729277C1 (ru) * | 2019-12-24 | 2020-08-05 | Федеральное государственное автономное образовательное учреждение высшего образования "Национальный исследовательский технологический университет "МИСиС" | Способ получения сверхвысокотемпературного керамического материала на основе карбонитрида гафния |
| RU2844323C1 (ru) * | 2024-10-07 | 2025-07-28 | Татьяна Олеговна Гаврилова | Реактор для получения тугоплавких неорганических соединений и композиционных материалов на их основе |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2877008B1 (fr) * | 2004-10-26 | 2007-02-09 | Ccn Soc Par Actions Simplifiee | Materiau composite compose d'une matrice organique au moins chargee par des particules non oxydes, quasi-spheriques et de granulometrie comprise entre 0,01mu et 4mu |
| KR100959931B1 (ko) * | 2008-03-07 | 2010-05-26 | 한국과학기술연구원 | 질화티타늄 분말의 제조 방법 |
| JP4997542B2 (ja) * | 2008-05-20 | 2012-08-08 | 独立行政法人物質・材料研究機構 | TiN基結晶体ナノ粒子及びその製造方法、並びにTiN基結晶体ナノ粒子の集合体及びその製造方法 |
| JP4997541B2 (ja) * | 2008-05-20 | 2012-08-08 | 独立行政法人物質・材料研究機構 | TiN基結晶体ナノ粒子及びその製造方法 |
| KR101691410B1 (ko) * | 2014-08-13 | 2017-01-02 | 주식회사 나노테크 | 탄질화티타늄 분말의 제조 방법 |
| JP6850505B2 (ja) * | 2017-10-19 | 2021-03-31 | 国立研究開発法人物質・材料研究機構 | ジルコニウムの窒化物を製造する方法 |
| RU2767111C1 (ru) * | 2020-08-20 | 2022-03-16 | федеральное государственное бюджетное образовательное учреждение высшего образования "Алтайский государственный технический университет им. И.И. Ползунова" (АлтГТУ) | Способ получения композиционного материала преимущественно рассекателя для барботационной установки |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4217948A (en) * | 1977-08-29 | 1980-08-19 | Borovinskaya Inna P | Method for production of two-layer pipe casting |
| US4459363A (en) * | 1983-02-28 | 1984-07-10 | The United States Of America As Represented By The United States Department Of Energy | Synthesis of refractory materials |
| RU2028281C1 (ru) * | 1991-06-17 | 1995-02-09 | Институт проблем горения | Шихта для изготовления формованных керамических изделий |
| RU2076469C1 (ru) * | 1993-10-12 | 1997-03-27 | Николай Валентинович Щербаков | Многоразрядный полупроводниковый индикатор |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2461018A (en) * | 1945-03-02 | 1949-02-08 | Metal Hydrides Inc | Production of titanium nitride |
| JPS58213617A (ja) * | 1982-06-07 | 1983-12-12 | Mitsubishi Metal Corp | 炭窒化チタン粉末の製造法 |
| US4595545A (en) * | 1982-12-30 | 1986-06-17 | Eltech Systems Corporation | Refractory metal borides and composites containing them |
| JPS61106405A (ja) * | 1984-10-29 | 1986-05-24 | Sumitomo Electric Ind Ltd | 炭窒化チタン粉末の製造法 |
| JPS61232211A (ja) * | 1985-04-08 | 1986-10-16 | Kogyo Kaihatsu Kenkyusho | 炭化チタンの製造方法 |
| FR2596745B1 (fr) * | 1986-04-03 | 1991-06-07 | Atochem | Poudres pour ceramiques en carbures et nitrures metalliques par reduction carbothermique et leur procede de fabrication |
| JPS63277506A (ja) * | 1987-05-08 | 1988-11-15 | Masahiro Yoshimura | 窒化チタン、もしくは炭化チタン、もしくはそれら両者の固溶体の合成方法 |
| WO1989005280A1 (en) * | 1987-11-30 | 1989-06-15 | Martin Marietta Corporation | Process for forming fine ceramic powders and products thereof |
| JPH01172205A (ja) * | 1987-12-26 | 1989-07-07 | Ibiden Co Ltd | 金属炭化物製造用原料組成物 |
| US5380688A (en) * | 1993-08-09 | 1995-01-10 | The Dow Chemical Company | Method for making submicrometer carbides, submicrometer solid solution carbides, and the material resulting therefrom |
-
1999
- 1999-03-16 AU AU26352/99A patent/AU2635299A/en not_active Abandoned
- 1999-03-16 CN CNB998039403A patent/CN1204043C/zh not_active Expired - Fee Related
- 1999-03-16 EP EP99906402A patent/EP1064224B1/fr not_active Expired - Lifetime
- 1999-03-16 RU RU2000125557/15A patent/RU2225837C2/ru not_active IP Right Cessation
- 1999-03-16 AT AT99906402T patent/ATE267768T1/de active
- 1999-03-16 WO PCT/IB1999/000441 patent/WO1999047454A1/fr not_active Ceased
- 1999-03-16 ES ES99906402T patent/ES2218996T3/es not_active Expired - Lifetime
- 1999-03-16 JP JP2000536653A patent/JP4541544B2/ja not_active Expired - Fee Related
- 1999-03-16 DE DE69917613T patent/DE69917613T2/de not_active Expired - Lifetime
- 1999-03-16 CA CA002322707A patent/CA2322707C/fr not_active Expired - Fee Related
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4217948A (en) * | 1977-08-29 | 1980-08-19 | Borovinskaya Inna P | Method for production of two-layer pipe casting |
| US4459363A (en) * | 1983-02-28 | 1984-07-10 | The United States Of America As Represented By The United States Department Of Energy | Synthesis of refractory materials |
| RU2028281C1 (ru) * | 1991-06-17 | 1995-02-09 | Институт проблем горения | Шихта для изготовления формованных керамических изделий |
| RU2076469C1 (ru) * | 1993-10-12 | 1997-03-27 | Николай Валентинович Щербаков | Многоразрядный полупроводниковый индикатор |
Non-Patent Citations (1)
| Title |
|---|
| КОИНДЗУМИ М. Химия синтеза сжиганием. - М.: Мир, 1998, с. 87. * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2729277C1 (ru) * | 2019-12-24 | 2020-08-05 | Федеральное государственное автономное образовательное учреждение высшего образования "Национальный исследовательский технологический университет "МИСиС" | Способ получения сверхвысокотемпературного керамического материала на основе карбонитрида гафния |
| WO2021133226A1 (en) * | 2019-12-24 | 2021-07-01 | National University of Science and Technology “MISIS” | Method for fabrication of ultra-high-temperature ceramic material based on hafnium carbide and carbonitride |
| RU2844323C1 (ru) * | 2024-10-07 | 2025-07-28 | Татьяна Олеговна Гаврилова | Реактор для получения тугоплавких неорганических соединений и композиционных материалов на их основе |
Also Published As
| Publication number | Publication date |
|---|---|
| DE69917613T2 (de) | 2005-08-25 |
| EP1064224B1 (fr) | 2004-05-26 |
| ATE267768T1 (de) | 2004-06-15 |
| JP2002506787A (ja) | 2002-03-05 |
| CN1204043C (zh) | 2005-06-01 |
| CA2322707A1 (fr) | 1999-09-23 |
| ES2218996T3 (es) | 2004-11-16 |
| AU2635299A (en) | 1999-10-11 |
| CN1292767A (zh) | 2001-04-25 |
| CA2322707C (fr) | 2008-12-16 |
| JP4541544B2 (ja) | 2010-09-08 |
| EP1064224A1 (fr) | 2001-01-03 |
| DE69917613D1 (de) | 2004-07-01 |
| WO1999047454A1 (fr) | 1999-09-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7964172B2 (en) | Method of manufacturing high-surface-area silicon | |
| Gillan et al. | Synthesis of refractory ceramics via rapid metathesis reactions between solid-state precursors | |
| Lefort et al. | Mechanism of AlN formation through the carbothermal reduction of Al2O3 in a flowing N2 atmosphere | |
| JP2874925B2 (ja) | 均一な、微細なホウ素含有セラミツク粉末を製造する装置および方法 | |
| JP3529100B2 (ja) | ミクロン以下の大きさをもつ遷移金属の炭窒化物の製造法 | |
| Liu et al. | Molten salt dynamic sealing synthesis of MAX phases (Ti3AlC2, Ti3SiC2 et al.) powder in air | |
| TW201829299A (zh) | 高純度氮化矽粉末之製造方法 | |
| Garcia et al. | New combustion synthesis technique for the production of (InxGa1− x) 2O3 powders: Hydrazine/metal nitrate method | |
| RU2225837C2 (ru) | Способ получения порошкообразных комплексных керамических материалов на основе тугоплавких металлов | |
| Omran et al. | Fast synthesis of MgAl2O4‒W and MgAl2O4‒W‒W2B composite powders by self-propagating high-temperature synthesis reactions | |
| Omran et al. | On the self-propagating high-temperature synthesis of tungsten boride containing composite powders from WO3–B2O3–Mg system | |
| Bichurov | Halides in SHS azide technology of nitrides obtaining | |
| Maeda et al. | Synthesis of ultrafine NbB2 powder by rapid carbothermal reduction in a vertical tubular reactor | |
| US11713251B2 (en) | Method for preparing powdered composite carbide of tungsten and titanium | |
| RU2087262C1 (ru) | Способ получения тонкодисперсного монокристаллического порошка диборида металла | |
| RU2200128C2 (ru) | Способ получения карбида вольфрама и карбид вольфрама, полученный этим способом | |
| JP2002187711A (ja) | 炭化チタンまたは2ホウ化チタンの合成方法 | |
| Weimer et al. | Carbothermal nitridation synthesis of α-Si3N4 powder from pyrolysed rice hulls | |
| JP2005047739A (ja) | 金属熱還元法によるTiC系ナノ複合粉末及びその合成方法 | |
| Patsera et al. | Combustion synthesis and consolidation of Ti (C, N)–Si3N4–SiC heterophase ceramic with YAG sintering additives | |
| Roy et al. | Self-propagating high-temperature synthesis of titanium borides | |
| US6451279B1 (en) | Process for making carbides through mechanochemical processing | |
| Ali et al. | Solid state metathesis routes to metal nitrides; use of strontium and barium nitrides as reagents and dilution effects | |
| Manukyan et al. | W and two-dimensional WO3/W nanocrystals produced by controlled self-sustaining reduction of sodium tungstate | |
| Silyakov et al. | Chemical, phase, and structural transformations in the combustion of mixtures with tungsten oxide with aluminum |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20120317 |