RU2177950C2 - Комплексы нуклеиновых кислот - лигандов сосудистого эндотелиального фактора роста (vegf) - Google Patents
Комплексы нуклеиновых кислот - лигандов сосудистого эндотелиального фактора роста (vegf) Download PDFInfo
- Publication number
- RU2177950C2 RU2177950C2 RU99110377/04A RU99110377A RU2177950C2 RU 2177950 C2 RU2177950 C2 RU 2177950C2 RU 99110377/04 A RU99110377/04 A RU 99110377/04A RU 99110377 A RU99110377 A RU 99110377A RU 2177950 C2 RU2177950 C2 RU 2177950C2
- Authority
- RU
- Russia
- Prior art keywords
- vegf
- complex
- ligand
- nucleic acid
- lipid
- Prior art date
Links
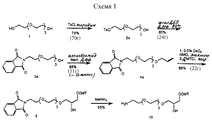
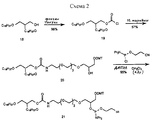
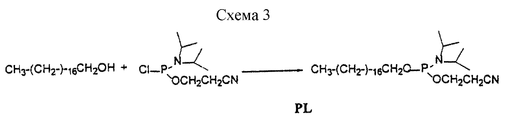
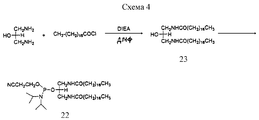
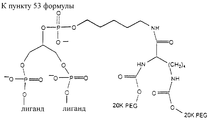
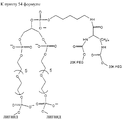
- 0 COP(OCCCCCNC(C(*)NC(O*)=O)=O)(OC(COP(O)(O*)=O)COP(O)(ON*)=O)=O Chemical compound COP(OCCCCCNC(C(*)NC(O*)=O)=O)(OC(COP(O)(O*)=O)COP(O)(ON*)=O)=O 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/115—Aptamers, i.e. nucleic acids binding a target molecule specifically and with high affinity without hybridising therewith ; Nucleic acids binding to non-nucleic acids, e.g. aptamers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/543—Lipids, e.g. triglycerides; Polyamines, e.g. spermine or spermidine
- A61K47/544—Phospholipids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0048—Eye, e.g. artificial tears
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Synthetic bilayered vehicles, e.g. liposomes or liposomes with cholesterol as the only non-phosphatidyl surfactant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H21/00—Compounds containing two or more mononucleotide units having separate phosphate or polyphosphate groups linked by saccharide radicals of nucleoside groups, e.g. nucleic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/88—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation using microencapsulation, e.g. using amphiphile liposome vesicle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/321—2'-O-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/322—2'-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/35—Nature of the modification
- C12N2310/351—Conjugate
- C12N2310/3515—Lipophilic moiety, e.g. cholesterol
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biochemistry (AREA)
- Epidemiology (AREA)
- Zoology (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Immunology (AREA)
- Ophthalmology & Optometry (AREA)
- Physics & Mathematics (AREA)
- Dermatology (AREA)
- Microbiology (AREA)
- Rheumatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Plant Pathology (AREA)
- Dispersion Chemistry (AREA)
- Gastroenterology & Hepatology (AREA)
- Pain & Pain Management (AREA)
- Physical Education & Sports Medicine (AREA)
- Toxicology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US08/739,109 | 1996-10-25 | ||
| US08/739,109 US5859228A (en) | 1995-05-04 | 1996-10-25 | Vascular endothelial growth factor (VEGF) nucleic acid ligand complexes |
| US08/870,930 US6168778B1 (en) | 1990-06-11 | 1997-06-06 | Vascular endothelial growth factor (VEGF) Nucleic Acid Ligand Complexes |
| US08/870,930 | 1997-06-06 | ||
| US08/897,351 | 1997-07-21 | ||
| US08/897,351 US6051698A (en) | 1997-06-06 | 1997-07-21 | Vascular endothelial growth factor (VEGF) nucleic acid ligand complexes |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU99110377A RU99110377A (ru) | 2001-02-27 |
| RU2177950C2 true RU2177950C2 (ru) | 2002-01-10 |
Family
ID=27419245
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU99110377/04A RU2177950C2 (ru) | 1996-10-25 | 1997-10-17 | Комплексы нуклеиновых кислот - лигандов сосудистого эндотелиального фактора роста (vegf) |
Country Status (18)
| Country | Link |
|---|---|
| EP (2) | EP0957929B1 (enExample) |
| JP (2) | JP3626503B2 (enExample) |
| KR (1) | KR100514929B1 (enExample) |
| AT (1) | ATE318143T1 (enExample) |
| AU (1) | AU733674B2 (enExample) |
| CA (1) | CA2269072C (enExample) |
| DE (3) | DE122006000029I1 (enExample) |
| DK (1) | DK0957929T3 (enExample) |
| ES (1) | ES2259188T3 (enExample) |
| FR (1) | FR06C0021I2 (enExample) |
| GE (1) | GEP20094799B (enExample) |
| IL (2) | IL129497A0 (enExample) |
| LU (1) | LU91252I2 (enExample) |
| NL (1) | NL300234I2 (enExample) |
| NZ (1) | NZ334859A (enExample) |
| PT (1) | PT957929E (enExample) |
| RU (1) | RU2177950C2 (enExample) |
| WO (1) | WO1998018480A1 (enExample) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2234097C1 (ru) * | 2003-03-05 | 2004-08-10 | Московский областной научно-исследовательский клинический институт | Способ диагностики саркомы капоши |
| RU2318004C2 (ru) * | 2002-07-24 | 2008-02-27 | Ф.Хоффманн-Ля Рош Аг | Добавки в виде полиалкиленгликолевой кислоты |
| RU2318019C2 (ru) * | 2002-03-02 | 2008-02-27 | Дзе Скриппс Рисерч Инститьют | Днк-вакцина против пролиферирующих эндотелиальных клеток и способы ее применения |
| RU2376373C2 (ru) * | 2003-06-30 | 2009-12-20 | Ридженерон Фармасьютикалз, Инк. | Изолированная молекула нуклеиновой кислоты, кодирующая слитый полипептид, способный связывать фактор роста эндотелиальных клеток сосудов (vegf), слитый полипептид, реплицируемый экспрессионный вектор, способ получения слитого полипептида, ловушка vegf, фармацевтическая композиция, способ лечения и набор для лечения vegf-опосредованного заболевания или состояния |
| EA013878B1 (ru) * | 2005-12-06 | 2010-08-30 | Домантис Лимитед | Лиганды, имеющие специфичность связывания в отношении рецептора эпидермального фактора роста (egfr) и/или сосудистого эндотелиального фактора роста (vegf), и способы их применения |
| RU2569182C2 (ru) * | 2008-12-04 | 2015-11-20 | КьюРНА,Инк.,US | Лечение заболеваний, связанных с фактором роста эндотелия сосудов (vegf), посредством ингибирования природного антисмыслового транскрипта к vegf |
Families Citing this family (61)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6395888B1 (en) | 1996-02-01 | 2002-05-28 | Gilead Sciences, Inc. | High affinity nucleic acid ligands of complement system proteins |
| US6232071B1 (en) * | 1990-06-11 | 2001-05-15 | Gilead Sciences, Inc. | Tenascin-C nucleic acid ligands |
| US6346611B1 (en) * | 1990-06-11 | 2002-02-12 | Gilead Sciences, Inc. | High affinity TGfβ nucleic acid ligands and inhibitors |
| AU782620B2 (en) * | 1991-06-10 | 2005-08-11 | Gilead Sciences, Inc. | High affinity vascular endothelial growth factor (VEGF) receptor nucleic acid ligands and inhibitors |
| US6762290B1 (en) | 1999-07-29 | 2004-07-13 | Gilead Sciences, Inc. | High affinity vascular endothelial growth factor (VEGF) receptor nucleic acid ligands and inhibitors |
| KR20070086708A (ko) | 1999-07-14 | 2007-08-27 | 알자 코포레이션 | 중성 지질중합체 및 그를 함유하는 리포솜 조성물 |
| EP2351855A1 (en) * | 2000-09-26 | 2011-08-03 | Duke University | RNA aptamers and methods for identifying the same |
| DK1438321T5 (da) * | 2001-10-26 | 2011-02-21 | Noxxon Pharma Ag | Modificeret L-nukleinsyre |
| KR20050044372A (ko) * | 2001-11-09 | 2005-05-12 | 아이테크 파마슈티컬즈, 인크. | 눈의 혈관신생성 질환을 치료하는 방법 |
| US7767803B2 (en) | 2002-06-18 | 2010-08-03 | Archemix Corp. | Stabilized aptamers to PSMA and their use as prostate cancer therapeutics |
| ATE516366T1 (de) | 2002-07-25 | 2011-07-15 | Archemix Corp | Regulierte aptamer-therapeutika |
| US9303262B2 (en) | 2002-09-17 | 2016-04-05 | Archemix Llc | Methods for identifying aptamer regulators |
| US8853376B2 (en) | 2002-11-21 | 2014-10-07 | Archemix Llc | Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics |
| US8039443B2 (en) | 2002-11-21 | 2011-10-18 | Archemix Corporation | Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics |
| US10100316B2 (en) | 2002-11-21 | 2018-10-16 | Archemix Llc | Aptamers comprising CPG motifs |
| EP1570085A4 (en) * | 2002-12-03 | 2007-07-25 | Archemix Corp | PROCESS FOR IN VITRO SELECTION OF 2'-SUBSTITUTED NUCLEIC ACIDS |
| FR2852606A1 (fr) * | 2003-03-18 | 2004-09-24 | Inst Nat Sante Rech Med | Moyens pour inhiber simultanement l'expression de plusieurs genes impliques dans une pathologie |
| AU2004232848A1 (en) | 2003-04-21 | 2004-11-04 | Archemix Corp. | Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics |
| CL2004001996A1 (es) * | 2003-08-08 | 2005-05-06 | Eyetech Pharmaceuticals Inc | Aptameros anti-vegf (factor de crecimiento endotelial vascular) con bloqueo nucleotidico 5'-5' o 3'-3' invertido, composicion que lo contiene, util para trastornos de neovascularizacion. |
| EP3736335A1 (en) | 2004-02-12 | 2020-11-11 | Archemix LLC | Aptamer therapeutics useful in the treatment of complement-related disorders |
| US7803931B2 (en) | 2004-02-12 | 2010-09-28 | Archemix Corp. | Aptamer therapeutics useful in the treatment of complement-related disorders |
| CA2562948A1 (en) * | 2004-04-13 | 2005-11-24 | (Osi) Eyetech, Inc. | Enhanced biologically active conjugates |
| US7579450B2 (en) | 2004-04-26 | 2009-08-25 | Archemix Corp. | Nucleic acid ligands specific to immunoglobulin E and their use as atopic disease therapeutics |
| US7566701B2 (en) | 2004-09-07 | 2009-07-28 | Archemix Corp. | Aptamers to von Willebrand Factor and their use as thrombotic disease therapeutics |
| KR20070101226A (ko) | 2004-09-07 | 2007-10-16 | 아케믹스 코포레이션 | 앱타머의 약화학 |
| EP3034089A1 (en) | 2004-11-02 | 2016-06-22 | Archemix LLC | Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics |
| KR20080025181A (ko) | 2005-06-30 | 2008-03-19 | 아케믹스 코포레이션 | 완전 2'-변형된 핵산 전사체를 생성하기 위한 물질 및 방법 |
| US8101385B2 (en) | 2005-06-30 | 2012-01-24 | Archemix Corp. | Materials and methods for the generation of transcripts comprising modified nucleotides |
| JP2009510182A (ja) * | 2005-07-28 | 2009-03-12 | (オーエスアイ)アイテツク・インコーポレーテツド | シクリトールリンカーポリマー抱合体 |
| CN101304748A (zh) | 2005-08-22 | 2008-11-12 | 加利福尼亚大学董事会 | Tlr激动剂 |
| KR100877824B1 (ko) | 2005-11-11 | 2009-01-12 | 한국생명공학연구원 | E2epf ucp-vhl 상호작용 및 그 용도 |
| JP2009519033A (ja) * | 2005-12-16 | 2009-05-14 | ディアト | 核酸を細胞に送達するための細胞貫通ペプチド結合体 |
| EP1970078A4 (en) * | 2006-01-11 | 2010-11-17 | Kyowa Hakko Kirin Co Ltd | COMPOSITION INHIBITING THE EXPRESSION OF A TARGET GENE OF THE OCULAR GLOBE AND REMEDY FOR OCULAR GLOBE DISEASE |
| SG172686A1 (en) | 2006-03-08 | 2011-07-28 | Archemix Corp | Complement binding aptamers and anti-c5 agents useful in the treatment of ocular disorders |
| PL2125007T3 (pl) | 2007-02-07 | 2014-07-31 | Univ California | Koniugaty syntetycznych agonistów TLR i ich zastosowania |
| US20110229498A1 (en) | 2008-05-08 | 2011-09-22 | The Johns Hopkins University | Compositions and methods for modulating an immune response |
| AU2010226434A1 (en) | 2009-03-20 | 2011-10-13 | Egen, Inc. | Polyamine derivatives |
| US20120295811A1 (en) | 2009-11-23 | 2012-11-22 | Jean-Jacques Toulme | Aptamers directed against the matrix protein-1 of type a influenza viruses and uses thereof |
| TWI510246B (zh) | 2010-04-30 | 2015-12-01 | Molecular Partners Ag | 抑制vegf-a受體交互作用的經修飾結合性蛋白質 |
| CA2811601A1 (en) * | 2010-09-24 | 2012-03-29 | Mallinckrodt Llc | Aptamer conjugates for targeting of therapeutic and/or diagnostic nanocarriers |
| US20150337308A1 (en) | 2012-04-11 | 2015-11-26 | Chu De Bordeaux | Matrix metalloproteinase 9 (mmp-9) aptamer and uses thereof |
| US10942184B2 (en) | 2012-10-23 | 2021-03-09 | Caris Science, Inc. | Aptamers and uses thereof |
| EP4170031A1 (en) | 2012-10-23 | 2023-04-26 | Caris Science, Inc. | Aptamers and uses thereof |
| WO2014100434A1 (en) | 2012-12-19 | 2014-06-26 | Caris Science, Inc. | Compositions and methods for aptamer screening |
| EP2977454B1 (en) | 2013-03-22 | 2018-05-02 | The University of Tokyo | Aptamer to il-17 and use thereof |
| SG10201912985RA (en) | 2013-07-12 | 2020-02-27 | Ophthotech Corp | Methods for treating or preventing ophthalmological conditions |
| BR112016004153A2 (pt) | 2013-08-28 | 2019-09-17 | Caris Life Sciences Switzerland Holdings Gmbh | "método para caracterizar uma doença ou distúrbio, kit, composição, método para gerar uma biblioteca de entrada, oligonucleotídeo, pluralidade de oligonucleotídeos e método para identificar um aptâmero |
| AU2015360496B2 (en) | 2014-12-11 | 2021-09-30 | Bayer Healthcare Llc | Treatment of age related macular degeneration with a small active choroidal neovascularization lesion |
| AU2015373071B2 (en) | 2014-12-29 | 2022-03-31 | Toray Industries, Inc. | Composition containing nucleic acid molecule stably |
| US20180066262A1 (en) | 2015-03-09 | 2018-03-08 | Caris Science, Inc. | Oligonucleotide probes and uses thereof |
| EP3314027A4 (en) | 2015-06-29 | 2019-07-03 | Caris Science, Inc. | THERAPEUTIC OLIGONUCLEOTIDES |
| EP3328873B1 (en) | 2015-07-28 | 2025-09-17 | Caris Science, Inc. | Targeted oligonucleotides |
| WO2017046140A1 (en) | 2015-09-18 | 2017-03-23 | Bayer Pharma Aktiengesellschaft | Treatment regimens for dr and rvo in dependence of the extent of retinal ischemia |
| US10731166B2 (en) | 2016-03-18 | 2020-08-04 | Caris Science, Inc. | Oligonucleotide probes and uses thereof |
| US11697851B2 (en) | 2016-05-24 | 2023-07-11 | The Regents Of The University Of California | Early ovarian cancer detection diagnostic test based on mRNA isoforms |
| CA3025486A1 (en) | 2016-05-25 | 2017-11-30 | Caris Science, Inc. | Oligonucleotide probes and uses thereof |
| EP3332812A1 (en) | 2016-12-07 | 2018-06-13 | Rheinische Friedrich-Wilhelms-Universität Bonn | Nucleic acid-based assembly and use of the assembly in cancer therapy |
| TW201904610A (zh) | 2017-06-14 | 2019-02-01 | 德商拜耳製藥公司 | 用於治療新生血管型青光眼之非抗體vegf拮抗劑 |
| EP3650045B1 (en) | 2017-07-04 | 2024-11-13 | Daiichi Sankyo Company, Limited | Drug for retinal degenerative disease associated with photoreceptor degeneration |
| WO2019210097A1 (en) * | 2018-04-25 | 2019-10-31 | Vitrisa Therapeutics, Inc. | Aptamers with stability, potency or half-life for enhanced safety and efficacy |
| GB202405551D0 (en) | 2024-04-19 | 2024-06-05 | Vvb Bio Pte Ltd | Combination treatment |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5459015A (en) * | 1990-06-11 | 1995-10-17 | Nexstar Pharmaceuticals, Inc. | High-affinity RNA ligands of basic fibroblast growth factor |
| WO1996027604A1 (en) * | 1995-03-06 | 1996-09-12 | Nexstar Pharmaceuticals, Inc. | High-affinity oligonucleotide ligands to secretory phospholipase a2(spla2) |
| WO1996034876A1 (en) * | 1995-05-04 | 1996-11-07 | Nexstar Pharmaceuticals, Inc. | Nucleic acid ligand complexes |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK0462145T3 (da) * | 1989-03-07 | 1994-08-08 | Genentech Inc | Covalente konjugater mellem lipid og oligonucleotid |
| US6168778B1 (en) * | 1990-06-11 | 2001-01-02 | Nexstar Pharmaceuticals, Inc. | Vascular endothelial growth factor (VEGF) Nucleic Acid Ligand Complexes |
| US5849479A (en) * | 1990-06-11 | 1998-12-15 | Nexstar Pharmaceuticals, Inc. | High-affinity oligonucleotide ligands to vascular endothelial growth factor (VEGF) |
| US5811533A (en) * | 1990-06-11 | 1998-09-22 | Nexstar Pharmaceuticals, Inc. | High-affinity oligonucleotide ligands to vascular endothelial growth factor (VEGF) |
-
1997
- 1997-10-17 DK DK97912811T patent/DK0957929T3/da active
- 1997-10-17 NZ NZ334859A patent/NZ334859A/xx not_active IP Right Cessation
- 1997-10-17 ES ES97912811T patent/ES2259188T3/es not_active Expired - Lifetime
- 1997-10-17 IL IL12949797A patent/IL129497A0/xx unknown
- 1997-10-17 AU AU49904/97A patent/AU733674B2/en not_active Expired
- 1997-10-17 PT PT97912811T patent/PT957929E/pt unknown
- 1997-10-17 JP JP52055398A patent/JP3626503B2/ja not_active Expired - Lifetime
- 1997-10-17 EP EP97912811A patent/EP0957929B1/en not_active Expired - Lifetime
- 1997-10-17 KR KR10-1999-7003488A patent/KR100514929B1/ko not_active Expired - Lifetime
- 1997-10-17 WO PCT/US1997/018944 patent/WO1998018480A1/en not_active Ceased
- 1997-10-17 DE DE1997635292 patent/DE122006000029I1/de active Pending
- 1997-10-17 DE DE200612000029 patent/DE122006000029I2/de active Active
- 1997-10-17 RU RU99110377/04A patent/RU2177950C2/ru active
- 1997-10-17 AT AT97912811T patent/ATE318143T1/de active
- 1997-10-17 GE GEAP199711120A patent/GEP20094799B/en unknown
- 1997-10-17 CA CA002269072A patent/CA2269072C/en not_active Expired - Lifetime
- 1997-10-17 EP EP06003279A patent/EP1685842A3/en not_active Withdrawn
- 1997-10-17 DE DE69735292T patent/DE69735292T2/de not_active Expired - Lifetime
-
1999
- 1999-04-19 IL IL129497A patent/IL129497A/en not_active IP Right Cessation
-
2004
- 2004-10-15 JP JP2004301813A patent/JP4160038B2/ja not_active Expired - Lifetime
-
2006
- 2006-06-14 LU LU91252C patent/LU91252I2/fr unknown
- 2006-07-03 FR FR06C0021C patent/FR06C0021I2/fr active Active
- 2006-07-20 NL NL300234C patent/NL300234I2/nl unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5459015A (en) * | 1990-06-11 | 1995-10-17 | Nexstar Pharmaceuticals, Inc. | High-affinity RNA ligands of basic fibroblast growth factor |
| WO1996027604A1 (en) * | 1995-03-06 | 1996-09-12 | Nexstar Pharmaceuticals, Inc. | High-affinity oligonucleotide ligands to secretory phospholipase a2(spla2) |
| WO1996034876A1 (en) * | 1995-05-04 | 1996-11-07 | Nexstar Pharmaceuticals, Inc. | Nucleic acid ligand complexes |
Non-Patent Citations (1)
| Title |
|---|
| КОРШАК В.В. и др. Полимеры в процессах иммобилизации и модификации природных соединений. - М.: Наука, 1984, с.199. * |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2318019C2 (ru) * | 2002-03-02 | 2008-02-27 | Дзе Скриппс Рисерч Инститьют | Днк-вакцина против пролиферирующих эндотелиальных клеток и способы ее применения |
| RU2318004C2 (ru) * | 2002-07-24 | 2008-02-27 | Ф.Хоффманн-Ля Рош Аг | Добавки в виде полиалкиленгликолевой кислоты |
| RU2234097C1 (ru) * | 2003-03-05 | 2004-08-10 | Московский областной научно-исследовательский клинический институт | Способ диагностики саркомы капоши |
| RU2376373C2 (ru) * | 2003-06-30 | 2009-12-20 | Ридженерон Фармасьютикалз, Инк. | Изолированная молекула нуклеиновой кислоты, кодирующая слитый полипептид, способный связывать фактор роста эндотелиальных клеток сосудов (vegf), слитый полипептид, реплицируемый экспрессионный вектор, способ получения слитого полипептида, ловушка vegf, фармацевтическая композиция, способ лечения и набор для лечения vegf-опосредованного заболевания или состояния |
| EA013878B1 (ru) * | 2005-12-06 | 2010-08-30 | Домантис Лимитед | Лиганды, имеющие специфичность связывания в отношении рецептора эпидермального фактора роста (egfr) и/или сосудистого эндотелиального фактора роста (vegf), и способы их применения |
| RU2569182C2 (ru) * | 2008-12-04 | 2015-11-20 | КьюРНА,Инк.,US | Лечение заболеваний, связанных с фактором роста эндотелия сосудов (vegf), посредством ингибирования природного антисмыслового транскрипта к vegf |
Also Published As
| Publication number | Publication date |
|---|---|
| NL300234I2 (nl) | 2006-10-02 |
| DE122006000029I1 (de) | 2006-10-19 |
| KR100514929B1 (ko) | 2005-09-15 |
| DE122006000029I2 (de) | 2007-11-08 |
| EP0957929A4 (en) | 2003-06-25 |
| FR06C0021I2 (fr) | 2007-08-03 |
| NL300234I1 (nl) | 2006-09-01 |
| EP0957929B1 (en) | 2006-02-22 |
| ATE318143T1 (de) | 2006-03-15 |
| IL129497A (en) | 2009-06-15 |
| JP4160038B2 (ja) | 2008-10-01 |
| WO1998018480A1 (en) | 1998-05-07 |
| FR06C0021I1 (enExample) | 2006-10-13 |
| DE69735292D1 (de) | 2006-04-27 |
| EP1685842A3 (en) | 2006-11-29 |
| LU91252I2 (fr) | 2006-08-14 |
| AU733674B2 (en) | 2001-05-24 |
| DE69735292T2 (de) | 2006-10-19 |
| KR20000052697A (ko) | 2000-08-25 |
| PT957929E (pt) | 2006-06-30 |
| GEP20094799B (en) | 2009-10-26 |
| EP1685842A2 (en) | 2006-08-02 |
| ES2259188T3 (es) | 2006-09-16 |
| JP2001505191A (ja) | 2001-04-17 |
| JP2005046160A (ja) | 2005-02-24 |
| CA2269072C (en) | 2006-02-14 |
| CA2269072A1 (en) | 1998-05-07 |
| NZ334859A (en) | 2001-02-23 |
| DK0957929T3 (da) | 2006-07-03 |
| AU4990497A (en) | 1998-05-22 |
| EP0957929A1 (en) | 1999-11-24 |
| IL129497A0 (en) | 2000-02-29 |
| JP3626503B2 (ja) | 2005-03-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2177950C2 (ru) | Комплексы нуклеиновых кислот - лигандов сосудистого эндотелиального фактора роста (vegf) | |
| US6051698A (en) | Vascular endothelial growth factor (VEGF) nucleic acid ligand complexes | |
| US6962784B2 (en) | Vascular endothelial growth factor (VEGF) nucleic acid ligand complexes | |
| US6168778B1 (en) | Vascular endothelial growth factor (VEGF) Nucleic Acid Ligand Complexes | |
| US5859228A (en) | Vascular endothelial growth factor (VEGF) nucleic acid ligand complexes | |
| US6147204A (en) | Nucleic acid ligand complexes | |
| AU749273B2 (en) | Platelet derived growth factor (PDGF) nucleic acid ligand complexes | |
| US6465188B1 (en) | Nucleic acid ligand complexes | |
| US6011020A (en) | Nucleic acid ligand complexes | |
| CA2219119C (en) | Nucleic acid ligand complexes | |
| HK1096580A (en) | Vascular endothelial growth factor (vegf) nucleic acid ligand complexes | |
| JP5637528B2 (ja) | 核酸リガンド複合体 | |
| CA2535449A1 (en) | Vascular endothelial growth factor (vegf) nucleic acid ligand complexes |