KR20190119607A - 높은 에너지 밀도 및 연장된 사이클링 수명을 가진 리튬-황 배터리를 가능하게 하는 재생성 폴리설파이드-소거 층 및 그 제조 방법 - Google Patents
높은 에너지 밀도 및 연장된 사이클링 수명을 가진 리튬-황 배터리를 가능하게 하는 재생성 폴리설파이드-소거 층 및 그 제조 방법 Download PDFInfo
- Publication number
- KR20190119607A KR20190119607A KR1020197026289A KR20197026289A KR20190119607A KR 20190119607 A KR20190119607 A KR 20190119607A KR 1020197026289 A KR1020197026289 A KR 1020197026289A KR 20197026289 A KR20197026289 A KR 20197026289A KR 20190119607 A KR20190119607 A KR 20190119607A
- Authority
- KR
- South Korea
- Prior art keywords
- cnts
- rsl
- sulfur
- polysulfide
- lithium
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- JDZCKJOXGCMJGS-UHFFFAOYSA-N [Li].[S] Chemical compound [Li].[S] JDZCKJOXGCMJGS-UHFFFAOYSA-N 0.000 title claims abstract description 25
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 11
- 230000001351 cycling effect Effects 0.000 title abstract description 48
- 239000002041 carbon nanotube Substances 0.000 claims abstract description 227
- 150000004706 metal oxides Chemical class 0.000 claims abstract description 52
- 229910044991 metal oxide Inorganic materials 0.000 claims abstract description 50
- 238000000034 method Methods 0.000 claims abstract description 44
- 239000002070 nanowire Substances 0.000 claims abstract description 36
- 239000002159 nanocrystal Substances 0.000 claims abstract description 20
- 230000001172 regenerating effect Effects 0.000 claims abstract description 17
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 253
- 229910021393 carbon nanotube Inorganic materials 0.000 claims description 217
- 229920001021 polysulfide Polymers 0.000 claims description 110
- 239000005077 polysulfide Substances 0.000 claims description 109
- 150000008117 polysulfides Polymers 0.000 claims description 109
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 88
- 229910052717 sulfur Inorganic materials 0.000 claims description 84
- 239000011593 sulfur Substances 0.000 claims description 84
- 239000004743 Polypropylene Substances 0.000 claims description 31
- 229920001155 polypropylene Polymers 0.000 claims description 31
- 239000002131 composite material Substances 0.000 claims description 28
- 229910052744 lithium Inorganic materials 0.000 claims description 26
- 238000011068 loading method Methods 0.000 claims description 24
- 239000000203 mixture Substances 0.000 claims description 24
- -1 polypropylene Polymers 0.000 claims description 19
- 229910052799 carbon Inorganic materials 0.000 claims description 18
- 239000000725 suspension Substances 0.000 claims description 17
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 17
- 239000008367 deionised water Substances 0.000 claims description 16
- 229910021641 deionized water Inorganic materials 0.000 claims description 16
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 14
- 239000012528 membrane Substances 0.000 claims description 14
- 230000000903 blocking effect Effects 0.000 claims description 12
- 239000006185 dispersion Substances 0.000 claims description 12
- 230000000694 effects Effects 0.000 claims description 12
- 239000002033 PVDF binder Substances 0.000 claims description 9
- 229920002981 polyvinylidene fluoride Polymers 0.000 claims description 9
- 239000006229 carbon black Substances 0.000 claims description 8
- 238000005429 filling process Methods 0.000 claims description 8
- 238000000527 sonication Methods 0.000 claims description 8
- 238000006479 redox reaction Methods 0.000 claims description 6
- 239000002002 slurry Substances 0.000 claims description 6
- 238000001035 drying Methods 0.000 claims description 5
- 238000001914 filtration Methods 0.000 claims description 5
- 238000001027 hydrothermal synthesis Methods 0.000 claims description 5
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical class C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 claims description 5
- 239000002134 carbon nanofiber Substances 0.000 claims description 4
- 238000007599 discharging Methods 0.000 claims description 4
- 239000007788 liquid Substances 0.000 claims description 4
- 238000003756 stirring Methods 0.000 claims description 4
- 229910052782 aluminium Inorganic materials 0.000 claims description 3
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 3
- UNTBPXHCXVWYOI-UHFFFAOYSA-O azanium;oxido(dioxo)vanadium Chemical compound [NH4+].[O-][V](=O)=O UNTBPXHCXVWYOI-UHFFFAOYSA-O 0.000 claims description 3
- 239000011888 foil Substances 0.000 claims description 3
- 238000010438 heat treatment Methods 0.000 claims description 3
- 230000008595 infiltration Effects 0.000 claims description 3
- 238000001764 infiltration Methods 0.000 claims description 3
- 239000002245 particle Substances 0.000 claims description 3
- 238000007582 slurry-cast process Methods 0.000 claims description 3
- 150000003463 sulfur Chemical class 0.000 claims description 3
- 230000002194 synthesizing effect Effects 0.000 claims description 3
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 claims description 2
- 238000004080 punching Methods 0.000 claims description 2
- 238000005266 casting Methods 0.000 claims 1
- 239000010410 layer Substances 0.000 description 56
- 229910018091 Li 2 S Inorganic materials 0.000 description 33
- 230000027455 binding Effects 0.000 description 24
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 20
- 239000000243 solution Substances 0.000 description 20
- 238000001179 sorption measurement Methods 0.000 description 17
- 238000001878 scanning electron micrograph Methods 0.000 description 13
- 230000002000 scavenging effect Effects 0.000 description 13
- 230000008569 process Effects 0.000 description 12
- 230000006870 function Effects 0.000 description 11
- 230000008929 regeneration Effects 0.000 description 11
- 238000011069 regeneration method Methods 0.000 description 11
- 238000006243 chemical reaction Methods 0.000 description 10
- 230000000875 corresponding effect Effects 0.000 description 10
- 241000894007 species Species 0.000 description 10
- 238000001228 spectrum Methods 0.000 description 10
- 230000015572 biosynthetic process Effects 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical class O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 7
- QPLDLSVMHZLSFG-UHFFFAOYSA-N Copper oxide Chemical compound [Cu]=O QPLDLSVMHZLSFG-UHFFFAOYSA-N 0.000 description 7
- 229910010413 TiO 2 Inorganic materials 0.000 description 7
- 238000002441 X-ray diffraction Methods 0.000 description 7
- 238000004833 X-ray photoelectron spectroscopy Methods 0.000 description 7
- 238000009792 diffusion process Methods 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- 238000002411 thermogravimetry Methods 0.000 description 7
- 229910052720 vanadium Inorganic materials 0.000 description 7
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 6
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 6
- 230000007797 corrosion Effects 0.000 description 6
- 238000005260 corrosion Methods 0.000 description 6
- 238000002484 cyclic voltammetry Methods 0.000 description 6
- 230000007423 decrease Effects 0.000 description 6
- 239000003792 electrolyte Substances 0.000 description 6
- 230000014759 maintenance of location Effects 0.000 description 6
- 230000002441 reversible effect Effects 0.000 description 6
- 238000012546 transfer Methods 0.000 description 6
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 6
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 5
- 238000010494 dissociation reaction Methods 0.000 description 5
- 230000005593 dissociations Effects 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- 229910020203 CeO Inorganic materials 0.000 description 4
- 229910002090 carbon oxide Inorganic materials 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 238000002149 energy-dispersive X-ray emission spectroscopy Methods 0.000 description 4
- 238000002173 high-resolution transmission electron microscopy Methods 0.000 description 4
- 238000010348 incorporation Methods 0.000 description 4
- 238000013507 mapping Methods 0.000 description 4
- 230000010287 polarization Effects 0.000 description 4
- 239000002516 radical scavenger Substances 0.000 description 4
- 238000004626 scanning electron microscopy Methods 0.000 description 4
- 125000004434 sulfur atom Chemical group 0.000 description 4
- 229920000557 Nafion® Polymers 0.000 description 3
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 3
- 238000003917 TEM image Methods 0.000 description 3
- 239000011149 active material Substances 0.000 description 3
- 239000002156 adsorbate Substances 0.000 description 3
- 239000003463 adsorbent Substances 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 238000000157 electrochemical-induced impedance spectroscopy Methods 0.000 description 3
- 238000004146 energy storage Methods 0.000 description 3
- 230000007613 environmental effect Effects 0.000 description 3
- 229910021389 graphene Inorganic materials 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 239000011244 liquid electrolyte Substances 0.000 description 3
- 229910001416 lithium ion Inorganic materials 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 230000027756 respiratory electron transport chain Effects 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 229920000049 Carbon (fiber) Polymers 0.000 description 2
- 229910004298 SiO 2 Inorganic materials 0.000 description 2
- 150000001450 anions Chemical class 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000004917 carbon fiber Substances 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 238000005352 clarification Methods 0.000 description 2
- 230000002596 correlated effect Effects 0.000 description 2
- 230000005684 electric field Effects 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 239000011229 interlayer Substances 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 150000003568 thioethers Chemical class 0.000 description 2
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 101100317222 Borrelia hermsii vsp3 gene Proteins 0.000 description 1
- 229910020599 Co 3 O 4 Inorganic materials 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229910018068 Li 2 O Inorganic materials 0.000 description 1
- 229910013553 LiNO Inorganic materials 0.000 description 1
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 229910006404 SnO 2 Inorganic materials 0.000 description 1
- 238000000026 X-ray photoelectron spectrum Methods 0.000 description 1
- GJEAMHAFPYZYDE-UHFFFAOYSA-N [C].[S] Chemical compound [C].[S] GJEAMHAFPYZYDE-UHFFFAOYSA-N 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 239000000908 ammonium hydroxide Substances 0.000 description 1
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 1
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 1
- 235000011130 ammonium sulphate Nutrition 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000006664 bond formation reaction Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- IVMYJDGYRUAWML-UHFFFAOYSA-N cobalt(II) oxide Inorganic materials [Co]=O IVMYJDGYRUAWML-UHFFFAOYSA-N 0.000 description 1
- 239000006258 conductive agent Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- XTVVROIMIGLXTD-UHFFFAOYSA-N copper(II) nitrate Chemical compound [Cu+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O XTVVROIMIGLXTD-UHFFFAOYSA-N 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 238000005315 distribution function Methods 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 238000012983 electrochemical energy storage Methods 0.000 description 1
- 238000002848 electrochemical method Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000000445 field-emission scanning electron microscopy Methods 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 238000000024 high-resolution transmission electron micrograph Methods 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 229910003473 lithium bis(trifluoromethanesulfonyl)imide Inorganic materials 0.000 description 1
- QSZMZKBZAYQGRS-UHFFFAOYSA-N lithium;bis(trifluoromethylsulfonyl)azanide Chemical compound [Li+].FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F QSZMZKBZAYQGRS-UHFFFAOYSA-N 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- GNRSAWUEBMWBQH-UHFFFAOYSA-N nickel(II) oxide Inorganic materials [Ni]=O GNRSAWUEBMWBQH-UHFFFAOYSA-N 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 230000003071 parasitic effect Effects 0.000 description 1
- 238000002161 passivation Methods 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 238000004375 physisorption Methods 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- RAVDHKVWJUPFPT-UHFFFAOYSA-N silver;oxido(dioxo)vanadium Chemical compound [Ag+].[O-][V](=O)=O RAVDHKVWJUPFPT-UHFFFAOYSA-N 0.000 description 1
- XMVONEAAOPAGAO-UHFFFAOYSA-N sodium tungstate Chemical compound [Na+].[Na+].[O-][W]([O-])(=O)=O XMVONEAAOPAGAO-UHFFFAOYSA-N 0.000 description 1
- 238000003980 solgel method Methods 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- 238000004627 transmission electron microscopy Methods 0.000 description 1
- 238000003828 vacuum filtration Methods 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/581—Chalcogenides or intercalation compounds thereof
- H01M4/5815—Sulfides
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/42—Methods or arrangements for servicing or maintenance of secondary cells or secondary half-cells
- H01M10/4235—Safety or regulating additives or arrangements in electrodes, separators or electrolyte
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/42—Methods or arrangements for servicing or maintenance of secondary cells or secondary half-cells
- H01M10/4242—Regeneration of electrolyte or reactants
-
- H01M2/1653—
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/362—Composites
- H01M4/364—Composites as mixtures
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/362—Composites
- H01M4/366—Composites as layered products
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/38—Selection of substances as active materials, active masses, active liquids of elements or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/38—Selection of substances as active materials, active masses, active liquids of elements or alloys
- H01M4/381—Alkaline or alkaline earth metals elements
- H01M4/382—Lithium
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/621—Binders
- H01M4/622—Binders being polymers
- H01M4/623—Binders being polymers fluorinated polymers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/624—Electric conductive fillers
- H01M4/625—Carbon or graphite
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/628—Inhibitors, e.g. gassing inhibitors, corrosion inhibitors
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/661—Metal or alloys, e.g. alloy coatings
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/663—Selection of materials containing carbon or carbonaceous materials as conductive part, e.g. graphite, carbon fibres
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/665—Composites
- H01M4/667—Composites in the form of layers, e.g. coatings
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/70—Carriers or collectors characterised by shape or form
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/70—Carriers or collectors characterised by shape or form
- H01M4/75—Wires, rods or strips
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/70—Carriers or collectors characterised by shape or form
- H01M4/76—Containers for holding the active material, e.g. tubes, capsules
- H01M4/762—Porous or perforated metallic containers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M50/00—Constructional details or processes of manufacture of the non-active parts of electrochemical cells other than fuel cells, e.g. hybrid cells
- H01M50/40—Separators; Membranes; Diaphragms; Spacing elements inside cells
- H01M50/409—Separators, membranes or diaphragms characterised by the material
- H01M50/411—Organic material
- H01M50/414—Synthetic resins, e.g. thermoplastics or thermosetting resins
- H01M50/417—Polyolefins
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0088—Composites
- H01M2300/0094—Composites in the form of layered products, e.g. coatings
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Composite Materials (AREA)
- Inorganic Chemistry (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Secondary Cells (AREA)
- Cell Separators (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762456936P | 2017-02-09 | 2017-02-09 | |
| US62/456,936 | 2017-02-09 | ||
| PCT/US2018/017155 WO2018148233A1 (en) | 2017-02-09 | 2018-02-07 | Regenerative polysulfide-scavenging layers enabling lithium-sulfur batteries with high energy density and prolonged cycling life and methods of making same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190119607A true KR20190119607A (ko) | 2019-10-22 |
Family
ID=63107829
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197026289A Ceased KR20190119607A (ko) | 2017-02-09 | 2018-02-07 | 높은 에너지 밀도 및 연장된 사이클링 수명을 가진 리튬-황 배터리를 가능하게 하는 재생성 폴리설파이드-소거 층 및 그 제조 방법 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US10985368B2 (enExample) |
| EP (1) | EP3580808A4 (enExample) |
| JP (1) | JP6980235B2 (enExample) |
| KR (1) | KR20190119607A (enExample) |
| CN (1) | CN111066194A (enExample) |
| AU (1) | AU2018218262A1 (enExample) |
| WO (1) | WO2018148233A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20220065578A (ko) * | 2020-11-13 | 2022-05-20 | 한국전기연구원 | 플렉시블 리튬-황 전지 집전체용 금속나노와이어-탄소나노튜브 복합체 |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI681587B (zh) * | 2018-01-16 | 2020-01-01 | 中央研究院 | 製造快速充電和長壽鋰-硫電池的方法 |
| KR102680031B1 (ko) * | 2018-12-07 | 2024-06-28 | 주식회사 엘지에너지솔루션 | 분리막 및 이를 포함하는 리튬 이차전지 |
| CN111081945A (zh) * | 2019-12-20 | 2020-04-28 | 河南师范大学 | 一种锂硫电池用多功能Nb2O5/中空碳纤维复合隔膜涂层材料的制备方法 |
| CN111082063B (zh) * | 2019-12-26 | 2023-03-28 | 内蒙古民族大学 | 一种柔性导电碳/金属复合纳米纤维膜及其制备方法和应用、锂硫电池 |
| CN111540868A (zh) * | 2020-01-21 | 2020-08-14 | 武汉船用电力推进装置研究所(中国船舶重工集团公司第七一二研究所) | 一种二维二氧化锰修饰聚丙烯隔膜的制备方法和应用 |
| JP7743984B2 (ja) * | 2020-03-13 | 2025-09-25 | 学校法人早稲田大学 | 二次電池用正極、二次電池用正極の製造方法、二次電池 |
| KR102819965B1 (ko) * | 2020-09-04 | 2025-06-13 | 주식회사 엘지에너지솔루션 | 분리막 및 이를 포함하는 이차 전지 |
| CN111916640B (zh) * | 2020-09-11 | 2023-04-28 | 合肥工业大学 | 一种锂硫电池WS2/CNTs改性隔膜及其制备方法 |
| CN114420928B (zh) * | 2020-10-28 | 2024-04-19 | 山东海科创新研究院有限公司 | 一种具有高性能的锂离子电池用硅碳负极材料及其制备方法、锂离子电池 |
| CN112332025A (zh) * | 2020-11-10 | 2021-02-05 | 南京工业大学 | 一种锂硫电池用隔膜及其制备方法 |
| CN114583165A (zh) * | 2020-12-01 | 2022-06-03 | 河南大学 | 一种金属/金属氧化物锂硫电池正极骨架结构 |
| US20250015437A1 (en) * | 2021-07-07 | 2025-01-09 | Board Of Regents, The University Of Texas System | Separators for use in energy storage devices |
| CN113937418B (zh) * | 2021-10-11 | 2023-11-17 | 中科南京绿色制造产业创新研究院 | 一种锂硫电池隔膜及其制备方法和锂硫电池 |
| CN114530671B (zh) * | 2022-01-25 | 2024-05-28 | 广东工业大学 | 一种基于高比表面积介孔氧化铝的功能性隔膜及其制备方法和应用 |
| CN114497888B (zh) * | 2022-01-25 | 2024-04-05 | 郑州大学 | 一种锂硫电池隔膜修饰材料的制备方法及其应用 |
| CN114605734B (zh) * | 2022-02-17 | 2023-08-01 | 温州大学 | 有机小分子嫁接碳纳米管修饰的功能薄膜复合材料及其制备方法和应用 |
| CN114890478A (zh) * | 2022-05-13 | 2022-08-12 | 武汉科技大学 | 一种超晶格材料及其制备方法以及在锂硫电池隔膜改性中的应用 |
| CN115832617B (zh) * | 2022-11-30 | 2025-09-02 | 惠州锂威新能源科技有限公司 | 一种插层复合薄膜及其制备方法和锂硫电池 |
| CN116159595B (zh) * | 2022-12-15 | 2024-08-06 | 温州大学 | 一种改性血蓝蛋白催化剂复合材料及其应用 |
| US20240204355A1 (en) * | 2022-12-16 | 2024-06-20 | Lyten, Inc. | Protective layer on anode-facing surface of separator for mitigating polysufide shuttling in lithium-based batteries |
| CN116936777B (zh) * | 2023-09-15 | 2023-12-29 | 山东海化集团有限公司 | 一种锂硫电池正、负极材料及其制备方法和应用 |
| CN119401056B (zh) * | 2024-11-01 | 2025-09-30 | 南通大学 | 具有球链状金属氧化物结构的锂硫电池隔膜及其制备方法 |
| CN120601077B (zh) * | 2025-08-07 | 2025-09-26 | 苏州工学院 | 一种橄榄形结构In2Ox修饰的锂硫电池隔膜及其制备方法和应用 |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8236446B2 (en) | 2008-03-26 | 2012-08-07 | Ada Technologies, Inc. | High performance batteries with carbon nanomaterials and ionic liquids |
| WO2010019648A2 (en) | 2008-08-15 | 2010-02-18 | The Regents Of The University Of California | Hierarchical nanowire composites for electrochemical energy storage |
| WO2012109648A1 (en) | 2011-02-11 | 2012-08-16 | The Penn State Research Foundation | Carbon-metal oxide-sulfur cathodes for high-performance lithium-sulfur batteries |
| CN102263257B (zh) | 2011-06-28 | 2013-08-07 | 中国科学院金属研究所 | 高能量柔性电极材料及其制备方法和在二次电池中的应用 |
| CN104620416A (zh) * | 2012-08-17 | 2015-05-13 | 得克萨斯州大学系统董事会 | 用于锂-硫电池的多孔碳中间层 |
| US10164231B2 (en) * | 2013-02-05 | 2018-12-25 | Hrl Laboratories, Llc | Separators for lithium-sulfur batteries |
| CN104143630A (zh) * | 2013-05-09 | 2014-11-12 | 中国科学院大连化学物理研究所 | 石墨烯-纳米金属氧化物复合材料在锂硫电池中应用 |
| US20150056493A1 (en) | 2013-08-21 | 2015-02-26 | GM Global Technology Operations LLC | Coated porous separators and coated electrodes for lithium batteries |
| US9373829B2 (en) * | 2013-10-11 | 2016-06-21 | GM Global Technology Operations LLC | Porous interlayer for a lithium-sulfur battery |
| CN104716382B (zh) * | 2013-12-15 | 2017-08-25 | 中国科学院大连化学物理研究所 | 一种锂‑硫电池结构 |
| CN104183820B (zh) * | 2014-03-03 | 2016-08-17 | 河南师范大学 | 一种锂硫电池正极用膜材料 |
| KR101684645B1 (ko) * | 2015-01-27 | 2016-12-07 | 한국과학기술연구원 | 바나듐 산화물 제로겔/카본 나노복합체의 제조방법, 이를 포함하는 리튬-황 이차전지 양극 및 이의 제조방법 |
| US9564656B1 (en) | 2015-09-14 | 2017-02-07 | Nanotek Instruments, Inc. | Process for producing alkali metal or alkali-ion batteries having high volumetric and gravimetric energy densities |
| CN105552282B (zh) * | 2015-11-13 | 2019-04-23 | 北京理工大学 | 基于功能性碳纤维布作为正极阻挡层的锂硫电池 |
| CN105489818A (zh) * | 2015-12-29 | 2016-04-13 | 长沙矿冶研究院有限责任公司 | 锂硫电池用改性隔膜的制备方法、改性隔膜及具有该改性隔膜的锂硫电池 |
-
2018
- 2018-02-06 US US15/889,603 patent/US10985368B2/en active Active
- 2018-02-07 EP EP18751931.9A patent/EP3580808A4/en not_active Withdrawn
- 2018-02-07 JP JP2019542702A patent/JP6980235B2/ja not_active Expired - Fee Related
- 2018-02-07 WO PCT/US2018/017155 patent/WO2018148233A1/en not_active Ceased
- 2018-02-07 AU AU2018218262A patent/AU2018218262A1/en not_active Abandoned
- 2018-02-07 KR KR1020197026289A patent/KR20190119607A/ko not_active Ceased
- 2018-02-07 CN CN201880011264.3A patent/CN111066194A/zh active Pending
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20220065578A (ko) * | 2020-11-13 | 2022-05-20 | 한국전기연구원 | 플렉시블 리튬-황 전지 집전체용 금속나노와이어-탄소나노튜브 복합체 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2018218262A1 (en) | 2019-08-29 |
| JP2020507896A (ja) | 2020-03-12 |
| WO2018148233A1 (en) | 2018-08-16 |
| US20180241039A1 (en) | 2018-08-23 |
| US10985368B2 (en) | 2021-04-20 |
| EP3580808A4 (en) | 2020-11-25 |
| JP6980235B2 (ja) | 2021-12-15 |
| EP3580808A1 (en) | 2019-12-18 |
| CN111066194A (zh) | 2020-04-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10985368B2 (en) | Regenerative polysulfide-scavenging layers enabling lithium-sulfur batteries with high energy density and prolonged cycling life and methods of making same | |
| Eftekhari et al. | Cathode materials for lithium–sulfur batteries: a practical perspective | |
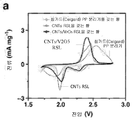
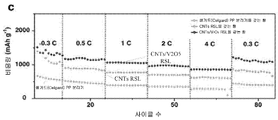
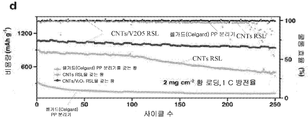
| Liu et al. | Regenerative polysulfide-scavenging layers enabling lithium–sulfur batteries with high energy density and prolonged cycling life | |
| Pang et al. | Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries | |
| Liu et al. | Chemical routes toward long-lasting lithium/sulfur cells | |
| JP6156939B2 (ja) | リチウムイオン二次電池 | |
| Ma et al. | High electrical conductivity of 3D mesporous carbon nanocage as an efficient polysulfide buffer layer for high sulfur utilization in lithium-sulfur batteries | |
| Zhang et al. | Conductive bridging effect of TiN nanoparticles on the electrochemical performance of TiN@ CNT-S composite cathode | |
| Wang et al. | Fast lithium-ion storage of Nb 2 O 5 nanocrystals in situ grown on carbon nanotubes for high-performance asymmetric supercapacitors | |
| KR20170003604A (ko) | 리튬-황 배터리용 이작용성 세퍼레이터 | |
| CN103201885A (zh) | 用于可充电电池的多组分电极 | |
| Liu et al. | Efficient separators with fast Li-ion transfer and high polysulfide entrapment for superior lithium-sulfur batteries | |
| JP2015531968A (ja) | リチウム硫黄バッテリのための多孔性炭素内層 | |
| Liao et al. | Novel flower-like hierarchical carbon sphere with multi-scale pores coated on PP separator for high-performance lithium-sulfur batteries | |
| Kong et al. | Polydopamine-derived porous nanofibers as host of ZnFe 2 O 4 nanoneedles: towards high-performance anodes for lithium-ion batteries | |
| Lin et al. | NiCo2O4/CNF separator modifiers for trapping and catalyzing polysulfides for high-performance lithium–sulfur batteries with high sulfur loadings and lean electrolytes | |
| Xian et al. | A trifunctional separator based on a blockage-adsorption-catalysis synergistic effect for Li-S batteries | |
| US20170092954A1 (en) | Multi-layer carbon-sulfur cathodes | |
| Iqbal et al. | Efficient sulfur host based on NiCo2O4 hollow microtubes for advanced Li-S batteries | |
| Lu et al. | Polypyrrole-modified carbon nanotubes@ manganese dioxide@ sulfur composite for lithium–sulfur batteries | |
| JP2023505050A (ja) | リチウム二次電池用正極、この製造方法及び前記正極を含むリチウム二次電池 | |
| G. Buonomenna et al. | Lithium-sulfur batteries: Overview and advances | |
| KR102637750B1 (ko) | 리튬황 전지용 양극재 이를 포함하는 리튬황 전지 | |
| Rana | Functional materials to enable durable and high loading lithium-sulfur batteries | |
| Pang | Advanced electrodes and electrolytes for long-lived and high-energy-density lithium-sulfur batteries |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190906 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210115 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230210 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20230818 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230210 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |