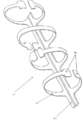
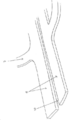
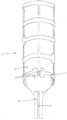
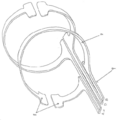
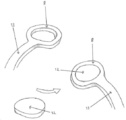
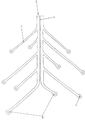
KR20190053871A - 혈관 연축의 예방 및 치료를 위한 장치 및 방법 - Google Patents
혈관 연축의 예방 및 치료를 위한 장치 및 방법 Download PDFInfo
- Publication number
- KR20190053871A KR20190053871A KR1020197009523A KR20197009523A KR20190053871A KR 20190053871 A KR20190053871 A KR 20190053871A KR 1020197009523 A KR1020197009523 A KR 1020197009523A KR 20197009523 A KR20197009523 A KR 20197009523A KR 20190053871 A KR20190053871 A KR 20190053871A
- Authority
- KR
- South Korea
- Prior art keywords
- stent structure
- vessel
- blood vessel
- struts
- electrodes
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 206010047163 Vasospasm Diseases 0.000 title claims abstract description 26
- 238000011282 treatment Methods 0.000 title claims abstract description 18
- 230000002265 prevention Effects 0.000 title claims abstract description 6
- 238000000034 method Methods 0.000 title claims description 27
- 239000004020 conductor Substances 0.000 claims abstract description 46
- 210000004204 blood vessel Anatomy 0.000 claims abstract description 43
- 210000004126 nerve fiber Anatomy 0.000 claims abstract description 28
- 238000007917 intracranial administration Methods 0.000 claims abstract description 9
- 241001465754 Metazoa Species 0.000 claims abstract description 4
- 238000003780 insertion Methods 0.000 claims description 21
- 230000037431 insertion Effects 0.000 claims description 21
- 239000012528 membrane Substances 0.000 claims description 6
- 239000003550 marker Substances 0.000 claims description 5
- 239000012777 electrically insulating material Substances 0.000 claims description 2
- 230000002792 vascular Effects 0.000 description 9
- 208000032851 Subarachnoid Hemorrhage Diseases 0.000 description 7
- 239000011248 coating agent Substances 0.000 description 6
- 238000000576 coating method Methods 0.000 description 6
- 230000008901 benefit Effects 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 239000008280 blood Substances 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 206010002329 Aneurysm Diseases 0.000 description 4
- 206010047139 Vasoconstriction Diseases 0.000 description 4
- 230000008602 contraction Effects 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 210000005036 nerve Anatomy 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 238000002604 ultrasonography Methods 0.000 description 4
- 230000025033 vasoconstriction Effects 0.000 description 4
- UIAGMCDKSXEBJQ-IBGZPJMESA-N 3-o-(2-methoxyethyl) 5-o-propan-2-yl (4s)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate Chemical group COCCOC(=O)C1=C(C)NC(C)=C(C(=O)OC(C)C)[C@H]1C1=CC=CC([N+]([O-])=O)=C1 UIAGMCDKSXEBJQ-IBGZPJMESA-N 0.000 description 3
- 229940127291 Calcium channel antagonist Drugs 0.000 description 3
- 208000032843 Hemorrhage Diseases 0.000 description 3
- 210000001367 artery Anatomy 0.000 description 3
- 230000017531 blood circulation Effects 0.000 description 3
- 238000009954 braiding Methods 0.000 description 3
- 239000000480 calcium channel blocker Substances 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 3
- 229910052737 gold Inorganic materials 0.000 description 3
- 239000010931 gold Substances 0.000 description 3
- 239000007943 implant Substances 0.000 description 3
- 238000009413 insulation Methods 0.000 description 3
- 238000003698 laser cutting Methods 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 229910001000 nickel titanium Inorganic materials 0.000 description 3
- 229960000715 nimodipine Drugs 0.000 description 3
- 230000035939 shock Effects 0.000 description 3
- 210000004686 stellate ganglion Anatomy 0.000 description 3
- 230000002889 sympathetic effect Effects 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 238000010292 electrical insulation Methods 0.000 description 2
- 239000011810 insulating material Substances 0.000 description 2
- 208000001286 intracranial vasospasm Diseases 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 206010003694 Atrophy Diseases 0.000 description 1
- 206010008190 Cerebrovascular accident Diseases 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- 208000028389 Nerve injury Diseases 0.000 description 1
- 229910001260 Pt alloy Inorganic materials 0.000 description 1
- 241000593989 Scardinius erythrophthalmus Species 0.000 description 1
- 208000034189 Sclerosis Diseases 0.000 description 1
- 208000005392 Spasm Diseases 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- 208000009443 Vascular Malformations Diseases 0.000 description 1
- WAIPAZQMEIHHTJ-UHFFFAOYSA-N [Cr].[Co] Chemical class [Cr].[Co] WAIPAZQMEIHHTJ-UHFFFAOYSA-N 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 238000002266 amputation Methods 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 230000002965 anti-thrombogenic effect Effects 0.000 description 1
- 230000008321 arterial blood flow Effects 0.000 description 1
- 230000004872 arterial blood pressure Effects 0.000 description 1
- 230000037444 atrophy Effects 0.000 description 1
- 244000052616 bacterial pathogen Species 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 210000005013 brain tissue Anatomy 0.000 description 1
- 210000001627 cerebral artery Anatomy 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 206010008118 cerebral infarction Diseases 0.000 description 1
- 208000026106 cerebrovascular disease Diseases 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000000916 dilatatory effect Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 238000002847 impedance measurement Methods 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 230000008764 nerve damage Effects 0.000 description 1
- 201000005111 ocular hyperemia Diseases 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- HWLDNSXPUQTBOD-UHFFFAOYSA-N platinum-iridium alloy Chemical compound [Ir].[Pt] HWLDNSXPUQTBOD-UHFFFAOYSA-N 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 210000002254 renal artery Anatomy 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000012781 shape memory material Substances 0.000 description 1
- 210000003625 skull Anatomy 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 210000002330 subarachnoid space Anatomy 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 201000011531 vascular cancer Diseases 0.000 description 1
- 206010055031 vascular neoplasm Diseases 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B18/1492—Probes or electrodes therefor having a flexible, catheter-like structure, e.g. for heart ablation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/0526—Head electrodes
- A61N1/0529—Electrodes for brain stimulation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N7/00—Ultrasound therapy
- A61N7/02—Localised ultrasound hyperthermia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N7/00—Ultrasound therapy
- A61N7/02—Localised ultrasound hyperthermia
- A61N7/022—Localised ultrasound hyperthermia intracavitary
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/0016—Energy applicators arranged in a two- or three dimensional array
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00214—Expandable means emitting energy, e.g. by elements carried thereon
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00053—Mechanical features of the instrument of device
- A61B2018/00214—Expandable means emitting energy, e.g. by elements carried thereon
- A61B2018/00267—Expandable means emitting energy, e.g. by elements carried thereon having a basket shaped structure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00321—Head or parts thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00345—Vascular system
- A61B2018/00404—Blood vessels other than those in or around the heart
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00345—Vascular system
- A61B2018/00404—Blood vessels other than those in or around the heart
- A61B2018/00416—Treatment of aneurisms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00315—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body for treatment of particular body parts
- A61B2018/00434—Neural system
- A61B2018/00446—Brain
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B2018/00636—Sensing and controlling the application of energy
- A61B2018/00773—Sensed parameters
- A61B2018/00875—Resistance or impedance
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B18/00—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body
- A61B18/04—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating
- A61B18/12—Surgical instruments, devices or methods for transferring non-mechanical forms of energy to or from the body by heating by passing a current through the tissue to be heated, e.g. high-frequency current
- A61B18/14—Probes or electrodes therefor
- A61B2018/1405—Electrodes having a specific shape
- A61B2018/1435—Spiral
- A61B2018/1437—Spiral whereby the windings of the spiral touch each other such as to create a continuous surface
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N7/00—Ultrasound therapy
- A61N2007/0004—Applications of ultrasound therapy
- A61N2007/0021—Neural system treatment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N7/00—Ultrasound therapy
- A61N2007/0004—Applications of ultrasound therapy
- A61N2007/0021—Neural system treatment
- A61N2007/003—Destruction of nerve tissue
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biomedical Technology (AREA)
- Radiology & Medical Imaging (AREA)
- Surgery (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Plasma & Fusion (AREA)
- Otolaryngology (AREA)
- Physics & Mathematics (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Psychology (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Electrotherapy Devices (AREA)
- Surgical Instruments (AREA)
- Prostheses (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102016116871.8A DE102016116871A1 (de) | 2016-09-08 | 2016-09-08 | Vorrichtung und Verfahren zur Vorbeugung und Behandlung eines Vasospasmus |
| DE102016116871.8 | 2016-09-08 | ||
| PCT/EP2017/072451 WO2018046592A1 (de) | 2016-09-08 | 2017-09-07 | Vorrichtung und verfahren zur vorbeugung und behandlung eines vasospasmus |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190053871A true KR20190053871A (ko) | 2019-05-20 |
Family
ID=59997306
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197009523A Ceased KR20190053871A (ko) | 2016-09-08 | 2017-09-07 | 혈관 연축의 예방 및 치료를 위한 장치 및 방법 |
Country Status (11)
| Country | Link |
|---|---|
| US (2) | US20200129228A1 (enExample) |
| EP (1) | EP3509521B1 (enExample) |
| JP (1) | JP7072751B2 (enExample) |
| KR (1) | KR20190053871A (enExample) |
| CN (1) | CN109952068B (enExample) |
| AU (1) | AU2017324399B2 (enExample) |
| BR (1) | BR112019004503A8 (enExample) |
| CA (1) | CA3035865A1 (enExample) |
| DE (1) | DE102016116871A1 (enExample) |
| ES (1) | ES2846007T3 (enExample) |
| WO (1) | WO2018046592A1 (enExample) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7267567B2 (ja) * | 2018-10-31 | 2023-05-02 | ユニチカ株式会社 | 低誘電率ポリイミド |
| EP4284309A1 (de) * | 2021-01-26 | 2023-12-06 | Phenox GmbH | Beschichtete medizinische vorrichtungen |
| DE102021102458A1 (de) | 2021-01-26 | 2022-07-28 | Phenox Gmbh | Beschichtete medizinische Vorrichtungen |
| CA3222846A1 (en) * | 2021-06-10 | 2022-12-15 | Galvanize Therapeutics, Inc. | Applying pulsed electric fields in the treatment of the vasculature |
| CN119486670A (zh) * | 2022-04-25 | 2025-02-18 | 格拉维蒂医疗技术有限公司 | 用于评价和治疗病变脉管的方法和装置 |
| CN114887220A (zh) * | 2022-04-29 | 2022-08-12 | 应脉医疗科技(上海)有限公司 | 血管内支架电极阵列及其制备方法和电刺激系统 |
| DE102022114767A1 (de) | 2022-06-13 | 2023-12-14 | Phenox Gmbh | Endovaskuläre Vorrichtung mit Führungsdraht |
| US20250241772A1 (en) | 2022-07-22 | 2025-07-31 | Phenox Gmbh | Apparatus and method for treating vasospasm |
| WO2024197304A2 (en) * | 2023-03-23 | 2024-09-26 | The Regents Of The University Of California | Advanced function stent system for chronic on-demand drug delivery, sonogenetic control of cells, and acoustic modulation of cell function |
Family Cites Families (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100316863B1 (ko) * | 1993-07-23 | 2002-09-26 | 쿠크 인코포레이티드 | 판형재료로형성된패턴을가진가요성스텐트 |
| US6602248B1 (en) * | 1995-06-07 | 2003-08-05 | Arthro Care Corp. | Methods for repairing damaged intervertebral discs |
| ATE339917T1 (de) * | 1996-10-23 | 2006-10-15 | Oratec Interventions Inc | Vorrichtung zur behandlung von zwischenwirbelscheiben |
| US7198635B2 (en) * | 2000-10-17 | 2007-04-03 | Asthmatx, Inc. | Modification of airways by application of energy |
| US20030158545A1 (en) * | 2000-09-28 | 2003-08-21 | Arthrocare Corporation | Methods and apparatus for treating back pain |
| US7653438B2 (en) * | 2002-04-08 | 2010-01-26 | Ardian, Inc. | Methods and apparatus for renal neuromodulation |
| US8774913B2 (en) * | 2002-04-08 | 2014-07-08 | Medtronic Ardian Luxembourg S.A.R.L. | Methods and apparatus for intravasculary-induced neuromodulation |
| US7231260B2 (en) * | 2004-05-06 | 2007-06-12 | Boston Scientific Scimed, Inc. | Intravascular self-anchoring electrode body with arcuate springs, spring loops, or arms |
| WO2007075593A1 (en) * | 2005-12-20 | 2007-07-05 | The Cleveland Clinic Foundation | Apparatus and method for modulating the baroreflex system |
| US7949409B2 (en) * | 2007-01-30 | 2011-05-24 | Cardiac Pacemakers, Inc. | Dual spiral lead configurations |
| US7925352B2 (en) * | 2008-03-27 | 2011-04-12 | Synecor Llc | System and method for transvascularly stimulating contents of the carotid sheath |
| US9339331B2 (en) * | 2008-12-29 | 2016-05-17 | St. Jude Medical, Atrial Fibrillation Division, Inc. | Non-contact electrode basket catheters with irrigation |
| CA2797130A1 (en) * | 2010-05-12 | 2011-11-17 | Shifamed Holdings, Llc | Low profile electrode assembly |
| US9408661B2 (en) * | 2010-07-30 | 2016-08-09 | Patrick A. Haverkost | RF electrodes on multiple flexible wires for renal nerve ablation |
| WO2012025246A1 (de) * | 2010-08-26 | 2012-03-01 | Acandis Gmbh & Co. Kg | Elektrode für medizinische anwendungen, system mit einer elektrode und verfahren zur herstellung einer elektrode |
| CN103327921B (zh) * | 2010-11-19 | 2017-02-15 | 波士顿科学西美德公司 | 肾神经检测以及消融装置 |
| EP2651500B1 (en) * | 2010-12-15 | 2016-07-20 | MED-EL Elektromedizinische Geräte GmbH | Multichannel cylindrical electrode for nerve stimulation and recording |
| EP2741815A2 (en) * | 2011-08-02 | 2014-06-18 | Samson Neurosciences Ltd. | Electrostimulation in treating cerebrovascular conditions |
| CN202637103U (zh) * | 2011-08-26 | 2013-01-02 | 王捷 | 具有肾神经标测功能的导管 |
| US20130231658A1 (en) * | 2012-03-01 | 2013-09-05 | Boston Scientific Scimed, Inc. | Expandable ablation device and methods for nerve modulation |
| US8612022B1 (en) * | 2012-09-13 | 2013-12-17 | Invatec S.P.A. | Neuromodulation catheters and associated systems and methods |
| EP2732784A1 (de) * | 2012-11-20 | 2014-05-21 | Biotronik AG | Hochfrequenz-Applikationsvorrichtung zum vaskulären Einsatz, insbesondere zur Applikation von Hochfrequenz-Energie an der Renal-Arterienwand |
| CA2913043A1 (en) * | 2013-05-20 | 2014-11-27 | Mayo Foundation For Medical Education And Research | Devices and methods for ablation of tissue |
| CN105377169B (zh) * | 2013-07-11 | 2019-04-19 | 波士顿科学国际有限公司 | 用于神经调制的装置和方法 |
| US9204929B2 (en) * | 2013-09-16 | 2015-12-08 | Biosense Webster (Israel) Ltd. | Basket catheter with deflectable spine |
| US20150105772A1 (en) * | 2013-10-14 | 2015-04-16 | Boston Scientific Scimed, Inc. | Devices and methods for nerve modulation |
| CN103750898B (zh) * | 2014-01-07 | 2016-02-17 | 先健科技(深圳)有限公司 | 一种腔内消融导管 |
| DE102014101348B4 (de) * | 2014-02-04 | 2023-04-20 | Acquandas GmbH | Medizinische Vorrichtung zur Ablation von Gewebezellen und System mit einer derartigen Vorrichtung |
| US9820664B2 (en) * | 2014-11-20 | 2017-11-21 | Biosense Webster (Israel) Ltd. | Catheter with high density electrode spine array |
-
2016
- 2016-09-08 DE DE102016116871.8A patent/DE102016116871A1/de not_active Withdrawn
-
2017
- 2017-09-07 US US16/329,869 patent/US20200129228A1/en not_active Abandoned
- 2017-09-07 WO PCT/EP2017/072451 patent/WO2018046592A1/de not_active Ceased
- 2017-09-07 AU AU2017324399A patent/AU2017324399B2/en active Active
- 2017-09-07 CN CN201780068679.XA patent/CN109952068B/zh active Active
- 2017-09-07 KR KR1020197009523A patent/KR20190053871A/ko not_active Ceased
- 2017-09-07 ES ES17777488T patent/ES2846007T3/es active Active
- 2017-09-07 JP JP2019512651A patent/JP7072751B2/ja active Active
- 2017-09-07 BR BR112019004503A patent/BR112019004503A8/pt not_active Application Discontinuation
- 2017-09-07 CA CA3035865A patent/CA3035865A1/en not_active Abandoned
- 2017-09-07 EP EP17777488.2A patent/EP3509521B1/de active Active
-
2025
- 2025-03-12 US US19/077,464 patent/US20250204981A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| DE102016116871A1 (de) | 2018-03-08 |
| US20200129228A1 (en) | 2020-04-30 |
| AU2017324399A1 (en) | 2019-05-02 |
| EP3509521A1 (de) | 2019-07-17 |
| BR112019004503A8 (pt) | 2023-03-21 |
| JP7072751B2 (ja) | 2022-05-23 |
| ES2846007T3 (es) | 2021-07-28 |
| US20250204981A1 (en) | 2025-06-26 |
| AU2017324399B2 (en) | 2022-04-07 |
| CA3035865A1 (en) | 2018-03-15 |
| WO2018046592A1 (de) | 2018-03-15 |
| JP2019531118A (ja) | 2019-10-31 |
| EP3509521B1 (de) | 2020-11-04 |
| BR112019004503A2 (pt) | 2019-05-28 |
| CN109952068B (zh) | 2022-08-09 |
| CN109952068A (zh) | 2019-06-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20250204981A1 (en) | Device and method for preventing and treating a vasospasm | |
| US20250082398A1 (en) | Shock wave balloon catheter with multiple shock wave sources | |
| JP6317678B2 (ja) | 医学的治療を支援するための装置及び方法 | |
| ES2202553T3 (es) | Conjunto de dispositivo de oclusion de aneurisma. | |
| US20190269413A1 (en) | Apparatus and method of monofilament implant delivery in a body vessel of a patient | |
| US20110264132A1 (en) | Multi-utilitarian microcatheter system and method of use | |
| KR102460929B1 (ko) | 혈관 경련 치료 | |
| JP2020049216A (ja) | 嚢状内部デバイス位置決め及び配備システム | |
| CN104487024A (zh) | 支架和支架送递装置 | |
| CN111743593A (zh) | 动脉瘤治疗装置 | |
| CN112135570A (zh) | 栓塞支架装置 | |
| CN120603540A (zh) | 具有可移动发射器的血管内碎石术导管 | |
| CN115052535A (zh) | 用于治疗动脉瘤的植入物 | |
| JP2019098177A (ja) | 結腸直腸ステント | |
| WO2010054604A1 (zh) | 用于病变血管修补的血管支架 | |
| US20250241772A1 (en) | Apparatus and method for treating vasospasm |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190402 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20200907 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20220329 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20220907 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20220329 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |