KR20190045207A - 유방 치료 장치 - Google Patents
유방 치료 장치 Download PDFInfo
- Publication number
- KR20190045207A KR20190045207A KR1020197006695A KR20197006695A KR20190045207A KR 20190045207 A KR20190045207 A KR 20190045207A KR 1020197006695 A KR1020197006695 A KR 1020197006695A KR 20197006695 A KR20197006695 A KR 20197006695A KR 20190045207 A KR20190045207 A KR 20190045207A
- Authority
- KR
- South Korea
- Prior art keywords
- breast
- tissue
- sheet
- section
- acellular
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/3604—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix characterised by the human or animal origin of the biological material, e.g. hair, fascia, fish scales, silk, shellac, pericardium, pleura, renal tissue, amniotic membrane, parenchymal tissue, fetal tissue, muscle tissue, fat tissue, enamel
- A61L27/362—Skin, e.g. dermal papillae
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/12—Mammary prostheses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/3641—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix characterised by the site of application in the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/3641—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix characterised by the site of application in the body
- A61L27/3666—Epithelial tissues other than skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/3683—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix subjected to a specific treatment prior to implantation, e.g. decellularising, demineralising, grinding, cellular disruption/non-collagenous protein removal, anti-calcification, crosslinking, supercritical fluid extraction, enzyme treatment
- A61L27/3695—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix subjected to a specific treatment prior to implantation, e.g. decellularising, demineralising, grinding, cellular disruption/non-collagenous protein removal, anti-calcification, crosslinking, supercritical fluid extraction, enzyme treatment characterised by the function or physical properties of the final product, where no specific conditions are defined to achieve this
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/56—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/0063—Implantable repair or support meshes, e.g. hernia meshes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0057—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof stretchable
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0008—Fixation appliances for connecting prostheses to the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0008—Rounded shapes, e.g. with rounded corners elliptical or oval
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0013—Horseshoe-shaped, e.g. crescent-shaped, C-shaped, U-shaped
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/04—Materials or treatment for tissue regeneration for mammary reconstruction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/34—Materials or treatment for tissue regeneration for soft tissue reconstruction
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Public Health (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Medicinal Chemistry (AREA)
- Botany (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Vascular Medicine (AREA)
- Molecular Biology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Urology & Nephrology (AREA)
- Zoology (AREA)
- Dispersion Chemistry (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662381865P | 2016-08-31 | 2016-08-31 | |
| US62/381,865 | 2016-08-31 | ||
| PCT/US2017/049516 WO2018045118A1 (en) | 2016-08-31 | 2017-08-31 | Breast treatment device |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190045207A true KR20190045207A (ko) | 2019-05-02 |
Family
ID=59858791
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197006695A Withdrawn KR20190045207A (ko) | 2016-08-31 | 2017-08-31 | 유방 치료 장치 |
Country Status (16)
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022173066A1 (ko) * | 2021-02-15 | 2022-08-18 | 주식회사 엘앤씨바이오 | 유방재건용 무세포화 피부대체재 및 그 제조방법 |
Families Citing this family (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8986377B2 (en) | 2009-07-21 | 2015-03-24 | Lifecell Corporation | Graft materials for surgical breast procedures |
| EP3081189B1 (en) | 2012-01-13 | 2018-07-04 | Lifecell Corporation | Breast prostheses and methods of manufacturing breast prostheses |
| EP3019206B1 (en) | 2013-07-11 | 2019-12-04 | Tepha, Inc. | Absorbable implants for plastic surgery |
| US10898312B2 (en) | 2013-10-28 | 2021-01-26 | The Regents Of The University Of California | Tissue grafts with fenestrations |
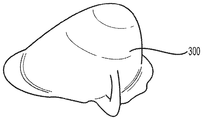
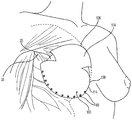
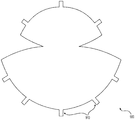
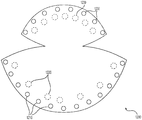
| US10842612B2 (en) | 2015-08-21 | 2020-11-24 | Lifecell Corporation | Breast treatment device |
| USD856517S1 (en) | 2016-06-03 | 2019-08-13 | Musculoskeletal Transplant Foundation | Asymmetric tissue graft |
| US10945831B2 (en) | 2016-06-03 | 2021-03-16 | Musculoskeletal Transplant Foundation | Asymmetric tissue graft |
| RU2749991C2 (ru) * | 2016-08-31 | 2021-06-21 | Лайфселл Корпорейшн | Устройство для коррекции молочной железы |
| US11426269B2 (en) | 2017-04-20 | 2022-08-30 | The Regents Of The University Of California | Systems and methods for acellular dermal matrix fenestrations in prepectoral breast reconstruction |
| WO2019156870A2 (en) | 2018-02-09 | 2019-08-15 | Tepha, Inc. | Full contour breast implant |
| USD889654S1 (en) | 2018-02-09 | 2020-07-07 | Tepha, Inc. | Three dimensional mastopexy implant |
| KR20210009302A (ko) * | 2018-03-13 | 2021-01-26 | 플라비오 나니 | 인공삽입물을 위한 생물학의 또는 생합성의 재료로 제조되고 인공삽입물에 고정을 위한 시스템을 포함하는 메쉬 또는 멤브레인 커버링, 및 해당하는 제조 방법 |
| USD892329S1 (en) | 2018-07-03 | 2020-08-04 | Tepha, Inc. | Three dimensional mastopexy implant |
| USD895812S1 (en) | 2018-09-07 | 2020-09-08 | Musculoskeletal Transplant Foundation | Soft tissue repair graft |
| US10813743B2 (en) | 2018-09-07 | 2020-10-27 | Musculoskeletal Transplant Foundation | Soft tissue repair grafts and processes for preparing and using same |
| WO2020072349A1 (en) | 2018-10-02 | 2020-04-09 | Tepha, Inc. | Medical devices to limit movement of breast implants |
| EP3920987A1 (en) | 2019-02-06 | 2021-12-15 | LifeCell Corporation | Meshed dermal tissue matrix products |
| WO2020210211A1 (en) * | 2019-04-08 | 2020-10-15 | Lifecell Corporation | Composite tissue product anchor bolster for three-dimensional biologic scaffolds and related methods |
| US11298220B2 (en) | 2019-05-03 | 2022-04-12 | Lifecell Corporation | Breast treatment device |
| EP3982878A4 (en) * | 2019-06-13 | 2022-07-20 | DR Hedén AB | MEDICAL SUPPORT |
| IT201900014193A1 (it) * | 2019-08-08 | 2021-02-08 | Gardelli Manuela | dispositivo contenitore di protesi mammaria in senologia ricostruttiva |
| US20230114297A1 (en) | 2019-09-25 | 2023-04-13 | Allosource | Pre-shaped allograft implant for reconstructive surgical use and methods of manufacture and use, and tools for forming a pre-shaped allograft implant for reconstructive surgical use |
| US20210085443A1 (en) * | 2019-09-25 | 2021-03-25 | Allosource | Pre-shaped allograft implant for reconstructive surgical use and methods of manufacture and use |
| EP4065043B1 (en) * | 2019-11-25 | 2025-12-24 | Tepha, Inc. | Breast implant wraps to limit movement of breast implants |
| JP2023523286A (ja) * | 2020-04-24 | 2023-06-02 | アラーガン、インコーポレイテッド | ドレーン対応組織拡張デバイス |
| IT202000010930A1 (it) * | 2020-05-13 | 2021-11-13 | Flavio Nanni | Rivestimento per protesi, protesi rivestita e relativo metodo di rivestimento. |
| WO2022235269A1 (en) * | 2021-05-06 | 2022-11-10 | Ott Solutions | Size adjustable device to cover and secure implantable devices in surgical applications |
| AU2022334424A1 (en) * | 2021-08-25 | 2024-04-04 | Musculoskeletal Transplant Foundation | Diversified grafts having heterogenous features and methods for making and using same |
| WO2024242981A2 (en) * | 2023-05-19 | 2024-11-28 | Davol Inc. | Implant fixation devices and related methods |
| USD1101168S1 (en) | 2023-08-09 | 2025-11-04 | Allosource | Acellular dermal matrix sheet allograft |
Family Cites Families (143)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2108205A (en) | 1936-12-01 | 1938-02-15 | Elsie L Martin | Breast pad and the like |
| US3683424A (en) | 1970-01-30 | 1972-08-15 | William J Pangman | Surgically implantable compound breast prosthesis |
| US4298998A (en) | 1980-12-08 | 1981-11-10 | Naficy Sadeque S | Breast prosthesis with biologically absorbable outer container |
| US4984585A (en) | 1983-02-17 | 1991-01-15 | Austad Eric D | Tissue expander |
| AU593267B2 (en) | 1986-07-22 | 1990-02-08 | Paul O'keeffe | Implantable fabric pouch for mammary prothesis |
| BR8705600A (pt) | 1987-10-15 | 1988-08-02 | Ricardo Alfredo Bustos | Protese mamaria |
| FR2630637B1 (fr) | 1988-04-27 | 1997-09-12 | Muller Guy Henri | Nouvelle prothese alveolaire |
| US5356429A (en) | 1991-05-16 | 1994-10-18 | Seare William J Jr | Body pocket maintenance prosthesis |
| FR2682284A1 (fr) | 1991-10-14 | 1993-04-16 | Dessapt Bernard | Prothese mammaire. |
| EP0542242B1 (de) | 1991-11-14 | 1997-06-04 | AMOENA Medizin-Orthopädie-Technik GmbH | Verfahren zum Herstellen einer Brustprothese |
| US5391203A (en) | 1992-04-13 | 1995-02-21 | Scott P. Bartlett | Method of draining and filling soft tissue implant |
| FR2709947B1 (fr) | 1993-09-13 | 1995-11-10 | Bard Sa Laboratoires | Filet prothétique galbé et son procédé de fabrication. |
| US5437824A (en) | 1993-12-23 | 1995-08-01 | Moghan Medical Corp. | Method of forming a molded silicone foam implant having open-celled interstices |
| US5658328A (en) | 1995-03-30 | 1997-08-19 | Johnson; Gerald W. | Endoscopic assisted mastopexy |
| US5733337A (en) | 1995-04-07 | 1998-03-31 | Organogenesis, Inc. | Tissue repair fabric |
| US5676161A (en) | 1995-04-24 | 1997-10-14 | Baylor College Of Medicine | Surgical procedures for mastopexy and reduction mammaplasty |
| US5584884A (en) * | 1995-07-27 | 1996-12-17 | Anthony S. Pignataro | Mammary prosthesis and method of surgically implanting same |
| JP3554412B2 (ja) | 1995-08-07 | 2004-08-18 | 株式会社メニコン | 培養皮膚用基材、培養皮膚およびその製造法 |
| FR2746298A1 (fr) | 1996-03-25 | 1997-09-26 | Bellity Philippe | Prothese de maintien de position et de forme d'un organe |
| US5755791A (en) | 1996-04-05 | 1998-05-26 | Purdue Research Foundation | Perforated submucosal tissue graft constructs |
| EP1955721B1 (en) | 1996-08-23 | 2014-10-29 | Cook Biotech, Inc. | Collagen-based graft prosthesis |
| US6666892B2 (en) | 1996-08-23 | 2003-12-23 | Cook Biotech Incorporated | Multi-formed collagenous biomaterial medical device |
| JPH10158906A (ja) | 1996-11-22 | 1998-06-16 | Torinpu Internatl Japan Kk | 女性用衣類 |
| FR2762207B1 (fr) | 1997-04-17 | 1999-07-30 | Ethnor | Perfectionnements aux protheses sous-cutanees pour plastie mammaire |
| US6638308B2 (en) | 1997-10-10 | 2003-10-28 | John D. Corbitt, Jr. | Bioabsorbable breast implant |
| US7637948B2 (en) | 1997-10-10 | 2009-12-29 | Senorx, Inc. | Tissue marking implant |
| US7658727B1 (en) | 1998-04-20 | 2010-02-09 | Medtronic, Inc | Implantable medical device with enhanced biocompatibility and biostability |
| WO1999063051A2 (en) | 1998-06-05 | 1999-12-09 | Organogenesis Inc. | Bioengineered flat sheet graft prostheses |
| US6933326B1 (en) | 1998-06-19 | 2005-08-23 | Lifecell Coporation | Particulate acellular tissue matrix |
| US6099566A (en) | 1998-09-04 | 2000-08-08 | Pmt Corporation | Prosthetic implant valve cover |
| US6723133B1 (en) | 1998-09-11 | 2004-04-20 | C. R. Bard, Inc. | Performed curved prosthesis having a reduced incidence of developing wrinkles or folds |
| US6740122B1 (en) | 1998-09-11 | 2004-05-25 | C. R. Bard, Inc. | Preformed curved prosthesis that is adapted to the external iliac vessels |
| US20030225355A1 (en) | 1998-10-01 | 2003-12-04 | Butler Charles E. | Composite material for wound repair |
| ES2402739T3 (es) | 1998-11-19 | 2013-05-08 | Organogenesis Inc. | Constructos tisulares modificados por bioingeniería y métodos para producirlos y utilizarlos |
| US20030082152A1 (en) | 1999-03-10 | 2003-05-01 | Hedrick Marc H. | Adipose-derived stem cells and lattices |
| US6777231B1 (en) | 1999-03-10 | 2004-08-17 | The Regents Of The University Of California | Adipose-derived stem cells and lattices |
| US6334868B1 (en) | 1999-10-08 | 2002-01-01 | Advanced Cardiovascular Systems, Inc. | Stent cover |
| US6203570B1 (en) | 1999-11-24 | 2001-03-20 | John L. Baeke | Breast implant with position lock |
| US20050119737A1 (en) | 2000-01-12 | 2005-06-02 | Bene Eric A. | Ocular implant and methods for making and using same |
| CA2777791A1 (en) | 2000-09-18 | 2002-03-21 | Organogenesis Inc. | Methods for treating a patient using a bioengineered flat sheet graft prostheses |
| US6913626B2 (en) | 2001-08-14 | 2005-07-05 | Mcghan Jim J. | Medical implant having bioabsorbable textured surface |
| DK1438081T3 (da) | 2001-10-26 | 2008-07-07 | Cook Biotech Inc | Medicinsk transplantatanordning med mesh mönstret struktur |
| US20110009960A1 (en) | 2001-11-16 | 2011-01-13 | Allergan, Inc. | Prosthetic fabric structure |
| US6736823B2 (en) | 2002-05-10 | 2004-05-18 | C.R. Bard, Inc. | Prosthetic repair fabric |
| US6736854B2 (en) | 2002-05-10 | 2004-05-18 | C. R. Bard, Inc. | Prosthetic repair fabric with erosion resistant edge |
| US7011688B2 (en) | 2002-05-10 | 2006-03-14 | C.R. Bard, Inc. | Prosthetic repair fabric |
| IL150151A (en) | 2002-06-11 | 2010-11-30 | Mim Minimally Invasive Mastopexy Ltd | Breast lift system |
| CA2899360A1 (en) | 2002-08-21 | 2004-04-08 | Revivicor, Inc. | Porcine animals lacking any expression of functional alpha 1,3 galactosyltransferase |
| WO2004096098A1 (en) | 2003-04-29 | 2004-11-11 | Formhold Medical Investment Holdings | Prothesis for soft tissue support |
| US7081135B2 (en) | 2003-06-09 | 2006-07-25 | Lane Fielding Smith | Mastopexy stabilization apparatus and method |
| US20040260315A1 (en) | 2003-06-17 | 2004-12-23 | Dell Jeffrey R. | Expandable tissue support member and method of forming the support member |
| US6802861B1 (en) | 2003-08-26 | 2004-10-12 | Rsh-Gs Trust | Structured breast implant |
| US20050208095A1 (en) | 2003-11-20 | 2005-09-22 | Angiotech International Ag | Polymer compositions and methods for their use |
| ITMI20032448A1 (it) | 2003-12-12 | 2005-06-13 | Angiologica B M S R L | Protesi parietale di rinforzo e metodo di realizzazione della stessa. |
| EP1734814B1 (en) | 2004-03-17 | 2015-01-21 | Revivicor, Inc. | Tissue products derived from animals lacking any expression of functional alpha 1,3 galactosyltransferase |
| CN1953657A (zh) | 2004-03-17 | 2007-04-25 | 雷维维科公司 | 来源于缺乏任何功能性α1,3半乳糖基转移酶表达的动物的组织产品 |
| ATE493091T1 (de) | 2004-03-30 | 2011-01-15 | Proxy Biomedical Ltd | Behandeltes chirurgisches mesh aus monofilen fasern |
| US7351197B2 (en) | 2004-05-07 | 2008-04-01 | Ams Research Corporation | Method and apparatus for cystocele repair |
| US8007531B2 (en) | 2004-08-06 | 2011-08-30 | Frank Robert E | Implantable prosthesis for positioning and supporting a breast implant |
| US7476249B2 (en) | 2004-08-06 | 2009-01-13 | Frank Robert E | Implantable prosthesis for positioning and supporting a breast implant |
| US7919112B2 (en) | 2004-08-26 | 2011-04-05 | Pathak Holdings, Llc | Implantable tissue compositions and method |
| US7641688B2 (en) | 2004-09-16 | 2010-01-05 | Evera Medical, Inc. | Tissue augmentation device |
| US9339403B2 (en) | 2004-11-12 | 2016-05-17 | Icon Medical Corp. | Medical adhesive for medical devices |
| RU2288674C2 (ru) | 2005-02-01 | 2006-12-10 | Федеральное государственное унитарное предприятие "Реутовский экспериментальный завод средств протезирования" Федерального агентства по здравоохранению и социальному развитию (ФГУП "РЭЗ СП" Росздрава) | Экзопротез для замещения послеоперационного дефицита тканей сохраненной молочной железы (варианты) |
| WO2006115892A2 (en) | 2005-04-28 | 2006-11-02 | Massachusetts Institute Of Technology | Tissue scaffolding comprising surface folds for tissue engeneering |
| WO2006135998A2 (en) | 2005-06-24 | 2006-12-28 | Wagenfuhr Jr J | Implantable prosthesis with double convexity |
| JP2008546498A (ja) | 2005-06-28 | 2008-12-25 | グリックスマン,アミ | ヒト用埋め込み型組織拡張器 |
| WO2007004214A2 (en) | 2005-07-04 | 2007-01-11 | Sergey Popov | Implant assembly |
| US20070038299A1 (en) | 2005-08-12 | 2007-02-15 | Arthrotek, Inc | Multilayer microperforated implant |
| WO2007070141A1 (en) | 2005-09-12 | 2007-06-21 | Proxy Biomedical Limited | Soft tissue implants and methods for making same |
| CN101309709A (zh) | 2005-09-21 | 2008-11-19 | 苏尔莫迪克斯公司 | 包含天然生物可降解的多糖的涂层和物品 |
| US20070088299A1 (en) | 2005-10-17 | 2007-04-19 | Emily Ayre | Emesis kit |
| US20070116678A1 (en) | 2005-11-23 | 2007-05-24 | Hsing-Wen Sung | Medical device with living cell sheet |
| US20080260853A1 (en) | 2006-02-22 | 2008-10-23 | Firestone Leigh H | Extracellular matrix as surgical adjunct in a lumpectomy procedure |
| ES2720582T3 (es) | 2006-05-09 | 2019-07-23 | Lifecell Corp | Tejido biológico reforzado |
| DE102006029605A1 (de) | 2006-06-26 | 2007-12-27 | Lazar, Harald, Dr. | Brustimplantat |
| US8480557B2 (en) | 2006-07-27 | 2013-07-09 | Refine, Llc | Nonaugmentive mastopexy |
| ATE529070T1 (de) | 2006-07-31 | 2011-11-15 | Organogenesis Inc | Mastopexie- und brustrekonstruktionsprothesen |
| JP2010505543A (ja) | 2006-10-03 | 2010-02-25 | アルレ メディカル,インコーポレイテッド | 低侵襲組織支持体 |
| WO2008086469A1 (en) | 2007-01-10 | 2008-07-17 | Cook Biotech Incorporated | Implantable devices useful for reinforcing a surgically created stoma |
| US8383092B2 (en) | 2007-02-16 | 2013-02-26 | Knc Ner Acquisition Sub, Inc. | Bioadhesive constructs |
| MX2009010396A (es) | 2007-03-29 | 2009-12-02 | Tyrx Pharma Inc | Cubiertas polimericas biodegradables para implantes de mama. |
| US20080281418A1 (en) | 2007-05-09 | 2008-11-13 | Leigh Hunt Firestone | Breast implant articles of multi-layered sheets of extracellular matrix or balled strips and pieces of extracellular matrix |
| US9149496B2 (en) | 2007-05-10 | 2015-10-06 | Cormatrix Cardiovascular, Inc. | ECM Constructs for treating damaged biological tissue |
| US20080281419A1 (en) | 2007-05-10 | 2008-11-13 | Matheny Robert G | Breast implants and compositions of extracellular matrix |
| CL2008001272A1 (es) | 2007-06-24 | 2008-10-03 | Gary Pierre Lauryssen | Sosten prostetico de mamas humanas que comprende una lamina continua de malla biocompatible con forma de copa que se puede asegurar entre la piel y la estructura glandular de una mama humana con una apertura a traves de la cual se ubica la estructura |
| US7875074B2 (en) | 2007-09-19 | 2011-01-25 | Ethicon, Inc. | Naturally contoured, preformed, three dimensional mesh device for breast implant support |
| US8101116B2 (en) | 2007-09-19 | 2012-01-24 | Ethicon, Inc. | Preformed support device and method and apparatus for manufacturing the same |
| AU2008313289A1 (en) * | 2007-10-16 | 2009-04-23 | Orbix Medical Ltd. | A system and method for reshaping soft tissue |
| US8313527B2 (en) | 2007-11-05 | 2012-11-20 | Allergan, Inc. | Soft prosthesis shell texturing method |
| US20110035004A1 (en) | 2007-11-14 | 2011-02-10 | Maxwell G | Interfaced medical implant |
| US8876899B2 (en) | 2007-11-14 | 2014-11-04 | G. Patrick Maxwell | Breast implant assembly |
| US8425600B2 (en) | 2007-11-14 | 2013-04-23 | G. Patrick Maxwell | Interfaced medical implant assembly |
| US20110293666A1 (en) | 2007-11-28 | 2011-12-01 | Xianyan Wang | Bioengineered Tissue Constructs and Methods for Production and Use |
| US20090198332A1 (en) | 2008-02-05 | 2009-08-06 | Hilton Becker | Method for texturing the surface of a synthetic implant |
| AU2008352542B2 (en) | 2008-03-14 | 2013-07-18 | Cook Biotech Incorporated | Graft materials and methods for staged delivery of bioactive components |
| US20100023029A1 (en) | 2008-07-23 | 2010-01-28 | Bodyaesthetic Research Center, Inc. | Mesh Device for Immediate Breast Construction and Uses Thereof |
| CN102256609B (zh) | 2008-07-30 | 2014-02-19 | 米辛瑟斯有限公司 | 衍生自前胃胞外基质的组织支架 |
| US9050184B2 (en) | 2008-08-13 | 2015-06-09 | Allergan, Inc. | Dual plane breast implant |
| US8986377B2 (en) * | 2009-07-21 | 2015-03-24 | Lifecell Corporation | Graft materials for surgical breast procedures |
| WO2011072393A1 (en) | 2009-12-17 | 2011-06-23 | Queen's University At Kingston | Decellularized adipose tissue |
| WO2011088365A1 (en) | 2010-01-14 | 2011-07-21 | Organogenesis, Inc. | Bioengineered tissue constructs and methods for producing and using thereof |
| EP2528538A2 (en) | 2010-01-28 | 2012-12-05 | Allergan, Inc. | Open celled silicone foams, implants including them and processes for making same |
| US20140257481A1 (en) | 2010-04-29 | 2014-09-11 | BioStruxs, LLC | Breast Reconstruction Device and Methods |
| US8858647B2 (en) | 2010-05-05 | 2014-10-14 | Barry Markman | Method and apparatus for a process creating an internal tissue graft for animal and human reconstructive purposes |
| ES2623475T3 (es) | 2010-05-11 | 2017-07-11 | Allergan, Inc. | Composiciones de porógenos, métodos para hacerlas y usos |
| CN103068413B (zh) | 2010-07-08 | 2015-12-16 | 生命细胞公司 | 用于使组织基质成形的方法 |
| US10195114B2 (en) | 2012-11-19 | 2019-02-05 | Guill Tool & Engineering Co., Inc | Microlayer coextrusion to create a time-release drug substance delivery product |
| IT1403996B1 (it) * | 2011-02-11 | 2013-11-08 | Baroni | Dispositivo di mantenimento morfologico applicabile ad una regione corporea sottoposta ad espansione tissutale |
| PL3199126T3 (pl) * | 2011-03-09 | 2020-05-18 | Tepha, Inc. | Układy do mastopeksji |
| US9132194B2 (en) | 2011-07-12 | 2015-09-15 | Warsaw Orthopedic, Inc. | Medical devices and methods comprising an adhesive sheet containing a drug depot |
| EP3549615B1 (en) | 2011-12-20 | 2020-12-16 | LifeCell Corporation | Sheet tissue products |
| EP3081189B1 (en) | 2012-01-13 | 2018-07-04 | Lifecell Corporation | Breast prostheses and methods of manufacturing breast prostheses |
| EP2817013B1 (en) | 2012-02-22 | 2019-05-15 | KCI Licensing, Inc. | Antimicrobial compositions, the preparation and use thereof |
| US20130273145A1 (en) | 2012-03-06 | 2013-10-17 | Kci Licensing, Inc. | Compositions, the preparation and use thereof |
| EP2863840B1 (en) | 2012-06-21 | 2017-08-16 | Lifecell Corporation | Implantable prosthesis having acellular tissue attachments |
| US9504828B2 (en) | 2012-07-26 | 2016-11-29 | Nyxoah SA | Electrical contacts on a medical device patch |
| ITTV20120038U1 (it) * | 2012-09-17 | 2014-03-18 | Deco Med S R L | Dispositivo medico, particolarmente per la ricostruzione mammaria. |
| WO2014055120A1 (en) * | 2012-10-04 | 2014-04-10 | Lifecell Corporation | Surgical template and delivery device |
| ES2409691B1 (es) | 2012-11-16 | 2013-11-21 | Federico MAYO MARTIN | Implante mamario |
| WO2014123665A1 (en) | 2013-02-06 | 2014-08-14 | Kci Licensing, Inc. | Polymers, preparation and use thereof |
| US9433489B2 (en) | 2013-03-12 | 2016-09-06 | Soft Tissue Regeneration, Inc. | Absorbable synthetic braided matrix for breast reconstruction and hernia repair |
| US9655715B2 (en) * | 2013-07-11 | 2017-05-23 | Tepha, Inc. | Absorbable implants for plastic surgery |
| CN103393482B (zh) * | 2013-08-14 | 2016-04-06 | 北京瑞健高科生物科技有限公司 | 一种基于组织基质材料的乳房假体支撑装置及其制备方法 |
| US10898312B2 (en) * | 2013-10-28 | 2021-01-26 | The Regents Of The University Of California | Tissue grafts with fenestrations |
| US10130457B2 (en) * | 2014-03-05 | 2018-11-20 | Tela Bio, Inc. | Surgical attachment device |
| WO2015148932A1 (en) * | 2014-03-28 | 2015-10-01 | Microaire Surgical Instruments, Llc | Endotine breast reconstruction devices and methods |
| US20150351889A1 (en) * | 2014-06-05 | 2015-12-10 | Vivex Biomedical Inc. | Dynamic Biometric Mesh |
| US9913711B2 (en) * | 2014-06-11 | 2018-03-13 | Robert D. Rehnke | Internal long term absorbable matrix brassiere |
| US20160228236A1 (en) * | 2015-02-11 | 2016-08-11 | Novus Scientific Ab | Breast implant support device with large back surface area |
| EP3085337B1 (en) * | 2015-04-24 | 2022-09-14 | Sofradim Production | Prosthesis for supporting a breast structure |
| EP3294211A1 (en) | 2015-05-15 | 2018-03-21 | Lifecell Corporation | Tissue matrices for plastic surgery |
| US10842612B2 (en) * | 2015-08-21 | 2020-11-24 | Lifecell Corporation | Breast treatment device |
| WO2017050837A1 (en) * | 2015-09-23 | 2017-03-30 | Novus Scientific Ab | Three-dimensional medical implant for regeneration of soft tissue |
| EP3361987B1 (en) * | 2015-10-16 | 2023-08-16 | Lifenet Health | Soft tissue grafts |
| US10945831B2 (en) * | 2016-06-03 | 2021-03-16 | Musculoskeletal Transplant Foundation | Asymmetric tissue graft |
| US10639398B2 (en) * | 2016-07-05 | 2020-05-05 | Lifecell Corporation | Tissue matrices incorporating multiple tissue types |
| RU2749991C2 (ru) * | 2016-08-31 | 2021-06-21 | Лайфселл Корпорейшн | Устройство для коррекции молочной железы |
| EP3518827A1 (en) * | 2016-10-03 | 2019-08-07 | LifeCell Corporation | Breast treatment device |
| US11426266B2 (en) * | 2017-03-29 | 2022-08-30 | Novus Scientific Ab | Medical implant |
| US11426269B2 (en) * | 2017-04-20 | 2022-08-30 | The Regents Of The University Of California | Systems and methods for acellular dermal matrix fenestrations in prepectoral breast reconstruction |
| US10813743B2 (en) * | 2018-09-07 | 2020-10-27 | Musculoskeletal Transplant Foundation | Soft tissue repair grafts and processes for preparing and using same |
| EP3920987A1 (en) * | 2019-02-06 | 2021-12-15 | LifeCell Corporation | Meshed dermal tissue matrix products |
-
2017
- 2017-08-31 RU RU2019106645A patent/RU2749991C2/ru active
- 2017-08-31 JP JP2019511490A patent/JP2019526341A/ja active Pending
- 2017-08-31 EP EP20191610.3A patent/EP3756621A1/en not_active Withdrawn
- 2017-08-31 EP EP17765528.9A patent/EP3506854B1/en active Active
- 2017-08-31 MX MX2019002263A patent/MX2019002263A/es unknown
- 2017-08-31 WO PCT/US2017/049516 patent/WO2018045118A1/en not_active Ceased
- 2017-08-31 PT PT177655289T patent/PT3506854T/pt unknown
- 2017-08-31 ES ES17765528T patent/ES2832673T3/es active Active
- 2017-08-31 AU AU2017318580A patent/AU2017318580B2/en not_active Expired - Fee Related
- 2017-08-31 KR KR1020197006695A patent/KR20190045207A/ko not_active Withdrawn
- 2017-08-31 BR BR112019004011A patent/BR112019004011A2/pt not_active Application Discontinuation
- 2017-08-31 US US15/691,865 patent/US11045579B2/en active Active
- 2017-08-31 CA CA3035256A patent/CA3035256A1/en not_active Abandoned
- 2017-08-31 DK DK17765528.9T patent/DK3506854T3/da active
- 2017-08-31 US US16/329,010 patent/US20190201580A1/en not_active Abandoned
- 2017-08-31 CN CN201780051355.5A patent/CN109640884B/zh active Active
-
2019
- 2019-02-22 CL CL2019000481A patent/CL2019000481A1/es unknown
- 2019-02-25 CO CONC2019/0001664A patent/CO2019001664A2/es unknown
-
2021
- 2021-06-28 US US17/360,770 patent/US20210322637A1/en not_active Abandoned
-
2023
- 2023-07-30 US US18/361,861 patent/US20240091408A1/en not_active Abandoned
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022173066A1 (ko) * | 2021-02-15 | 2022-08-18 | 주식회사 엘앤씨바이오 | 유방재건용 무세포화 피부대체재 및 그 제조방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2832673T3 (es) | 2021-06-10 |
| US20210322637A1 (en) | 2021-10-21 |
| EP3756621A1 (en) | 2020-12-30 |
| CL2019000481A1 (es) | 2019-06-07 |
| CA3035256A1 (en) | 2018-03-08 |
| RU2019106645A (ru) | 2020-10-01 |
| AU2017318580A1 (en) | 2019-03-07 |
| RU2019106645A3 (cg-RX-API-DMAC7.html) | 2020-12-08 |
| US11045579B2 (en) | 2021-06-29 |
| BR112019004011A2 (pt) | 2019-06-04 |
| CN109640884A (zh) | 2019-04-16 |
| US20240091408A1 (en) | 2024-03-21 |
| RU2749991C2 (ru) | 2021-06-21 |
| JP2019526341A (ja) | 2019-09-19 |
| CO2019001664A2 (es) | 2019-03-18 |
| DK3506854T3 (da) | 2020-11-23 |
| PT3506854T (pt) | 2020-11-24 |
| US20180055624A1 (en) | 2018-03-01 |
| EP3506854A1 (en) | 2019-07-10 |
| WO2018045118A1 (en) | 2018-03-08 |
| US20190201580A1 (en) | 2019-07-04 |
| CN109640884B (zh) | 2022-03-18 |
| MX2019002263A (es) | 2019-09-19 |
| AU2017318580B2 (en) | 2022-04-21 |
| EP3506854B1 (en) | 2020-08-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20190045207A (ko) | 유방 치료 장치 | |
| US20250017721A1 (en) | Breast treatment device | |
| US11219516B2 (en) | Gender-specific mesh implant with barrier for inguinal hernia repair | |
| AU2009316594B2 (en) | Method for treatment and prevention of parastomal hernias | |
| KR20100031542A (ko) | 인체 유방 인공 지지물 및 이식 방법 | |
| HK40011639A (en) | Breast treatment device | |
| HK40011639B (en) | Breast treatment device | |
| HK40081927A (en) | Gender-specific mesh implant with barrier for inguinal hernia repair | |
| HK1249391A1 (en) | Gender-specific mesh implant with barrier for inguinal hernia repair |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190307 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20200828 Comment text: Request for Examination of Application |
|
| PC1202 | Submission of document of withdrawal before decision of registration |
Comment text: [Withdrawal of Procedure relating to Patent, etc.] Withdrawal (Abandonment) Patent event code: PC12021R01D Patent event date: 20211014 |
|
| WITB | Written withdrawal of application |