KR20180101595A - 심혈관 질환 및 사건을 위한 진단학적 및 예후적 방법 - Google Patents
심혈관 질환 및 사건을 위한 진단학적 및 예후적 방법 Download PDFInfo
- Publication number
- KR20180101595A KR20180101595A KR1020187024496A KR20187024496A KR20180101595A KR 20180101595 A KR20180101595 A KR 20180101595A KR 1020187024496 A KR1020187024496 A KR 1020187024496A KR 20187024496 A KR20187024496 A KR 20187024496A KR 20180101595 A KR20180101595 A KR 20180101595A
- Authority
- KR
- South Korea
- Prior art keywords
- prognosis
- subject
- history
- biomarkers
- panel
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 178
- 208000024172 Cardiovascular disease Diseases 0.000 title claims abstract description 29
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 119
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 119
- 238000004393 prognosis Methods 0.000 claims abstract description 117
- 238000003745 diagnosis Methods 0.000 claims abstract description 89
- 239000000090 biomarker Substances 0.000 claims description 283
- 208000029078 coronary artery disease Diseases 0.000 claims description 176
- 208000010125 myocardial infarction Diseases 0.000 claims description 152
- 238000003556 assay Methods 0.000 claims description 113
- 230000034994 death Effects 0.000 claims description 91
- 230000002526 effect on cardiovascular system Effects 0.000 claims description 85
- 239000000523 sample Substances 0.000 claims description 67
- 208000006011 Stroke Diseases 0.000 claims description 63
- 238000012360 testing method Methods 0.000 claims description 61
- 108010076365 Adiponectin Proteins 0.000 claims description 44
- 238000002399 angioplasty Methods 0.000 claims description 40
- 108010092801 Midkine Proteins 0.000 claims description 39
- 230000027455 binding Effects 0.000 claims description 39
- 210000004351 coronary vessel Anatomy 0.000 claims description 39
- 230000006378 damage Effects 0.000 claims description 37
- KZMAWJRXKGLWGS-UHFFFAOYSA-N 2-chloro-n-[4-(4-methoxyphenyl)-1,3-thiazol-2-yl]-n-(3-methoxypropyl)acetamide Chemical compound S1C(N(C(=O)CCl)CCCOC)=NC(C=2C=CC(OC)=CC=2)=C1 KZMAWJRXKGLWGS-UHFFFAOYSA-N 0.000 claims description 34
- 230000000747 cardiac effect Effects 0.000 claims description 34
- 108010081689 Osteopontin Proteins 0.000 claims description 32
- 230000000414 obstructive effect Effects 0.000 claims description 32
- 210000004556 brain Anatomy 0.000 claims description 27
- 108010031374 Tissue Inhibitor of Metalloproteinase-1 Proteins 0.000 claims description 25
- 102000005353 Tissue Inhibitor of Metalloproteinase-1 Human genes 0.000 claims description 25
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 22
- 102000004890 Interleukin-8 Human genes 0.000 claims description 22
- 108090001007 Interleukin-8 Proteins 0.000 claims description 22
- 210000003734 kidney Anatomy 0.000 claims description 22
- 229910052708 sodium Inorganic materials 0.000 claims description 22
- 239000011734 sodium Substances 0.000 claims description 22
- 108010062374 Myoglobin Proteins 0.000 claims description 19
- 102000036675 Myoglobin Human genes 0.000 claims description 19
- 230000001225 therapeutic effect Effects 0.000 claims description 18
- 239000012472 biological sample Substances 0.000 claims description 17
- 239000012634 fragment Substances 0.000 claims description 17
- XKTZWUACRZHVAN-VADRZIEHSA-N interleukin-8 Chemical compound C([C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@@H](NC(C)=O)CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N1[C@H](CCC1)C(=O)N1[C@H](CCC1)C(=O)N[C@@H](C)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC(O)=CC=1)C(=O)N[C@H](CO)C(=O)N1[C@H](CCC1)C(N)=O)C1=CC=CC=C1 XKTZWUACRZHVAN-VADRZIEHSA-N 0.000 claims description 17
- 229940096397 interleukin-8 Drugs 0.000 claims description 17
- 238000013146 percutaneous coronary intervention Methods 0.000 claims description 17
- 108010015302 Matrix metalloproteinase-9 Proteins 0.000 claims description 15
- 102000004264 Osteopontin Human genes 0.000 claims description 15
- 108090001027 Troponin Proteins 0.000 claims description 15
- 102000004903 Troponin Human genes 0.000 claims description 15
- 238000001514 detection method Methods 0.000 claims description 15
- 210000000130 stem cell Anatomy 0.000 claims description 15
- 238000012544 monitoring process Methods 0.000 claims description 13
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims description 13
- 102000001776 Matrix metalloproteinase-9 Human genes 0.000 claims description 12
- 230000029142 excretion Effects 0.000 claims description 12
- 102100037840 Dehydrogenase/reductase SDR family member 2, mitochondrial Human genes 0.000 claims description 11
- 101710188053 Protein D Proteins 0.000 claims description 11
- 101710132893 Resolvase Proteins 0.000 claims description 11
- 210000004027 cell Anatomy 0.000 claims description 11
- 101000693735 Homo sapiens Prefoldin subunit 4 Proteins 0.000 claims description 10
- 102100025542 Prefoldin subunit 4 Human genes 0.000 claims description 10
- 238000001631 haemodialysis Methods 0.000 claims description 10
- 230000000322 hemodialysis Effects 0.000 claims description 10
- 239000003112 inhibitor Substances 0.000 claims description 10
- 238000012795 verification Methods 0.000 claims description 10
- 108010000134 Vascular Cell Adhesion Molecule-1 Proteins 0.000 claims description 9
- 206010012601 diabetes mellitus Diseases 0.000 claims description 9
- 238000011156 evaluation Methods 0.000 claims description 9
- 206010020772 Hypertension Diseases 0.000 claims description 8
- 102000005741 Metalloproteases Human genes 0.000 claims description 8
- 108010006035 Metalloproteases Proteins 0.000 claims description 8
- 230000000694 effects Effects 0.000 claims description 8
- 238000003384 imaging method Methods 0.000 claims description 8
- 239000000758 substrate Substances 0.000 claims description 8
- 108010067225 Cell Adhesion Molecules Proteins 0.000 claims description 7
- 102000016289 Cell Adhesion Molecules Human genes 0.000 claims description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 7
- 230000000391 smoking effect Effects 0.000 claims description 7
- 239000005541 ACE inhibitor Substances 0.000 claims description 6
- 102100027845 Pulmonary surfactant-associated protein D Human genes 0.000 claims description 6
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 claims description 6
- 239000003524 antilipemic agent Substances 0.000 claims description 6
- 239000002876 beta blocker Substances 0.000 claims description 6
- 229940097320 beta blocking agent Drugs 0.000 claims description 6
- 201000010099 disease Diseases 0.000 claims description 6
- 239000003580 lung surfactant Substances 0.000 claims description 6
- 230000002107 myocardial effect Effects 0.000 claims description 6
- -1 stem cell factor Proteins 0.000 claims description 6
- 210000005167 vascular cell Anatomy 0.000 claims description 6
- CRJYIDXYIZSNHX-UHFFFAOYSA-N Dicorin Natural products CC(O)CCC1=CC2OC(=O)C(=C)C2CC(O)C1C CRJYIDXYIZSNHX-UHFFFAOYSA-N 0.000 claims description 5
- 108010007127 Pulmonary Surfactant-Associated Protein D Proteins 0.000 claims description 5
- 235000001434 dietary modification Nutrition 0.000 claims description 5
- 210000004072 lung Anatomy 0.000 claims description 5
- 230000010412 perfusion Effects 0.000 claims description 5
- 230000005586 smoking cessation Effects 0.000 claims description 5
- 238000009662 stress testing Methods 0.000 claims description 5
- 239000000126 substance Substances 0.000 claims description 5
- 238000010968 computed tomography angiography Methods 0.000 claims description 4
- UHMKISIRZFDJRU-UHFFFAOYSA-L diethyl-methyl-[2-(1,1,6-trimethylpiperidin-1-ium-2-carbonyl)oxyethyl]azanium;diiodide Chemical compound [I-].[I-].CC[N+](C)(CC)CCOC(=O)C1CCCC(C)[N+]1(C)C UHMKISIRZFDJRU-UHFFFAOYSA-L 0.000 claims description 4
- 150000002823 nitrates Chemical class 0.000 claims description 4
- 230000004044 response Effects 0.000 claims description 4
- 238000001356 surgical procedure Methods 0.000 claims description 4
- 239000012190 activator Substances 0.000 claims description 3
- 229940127218 antiplatelet drug Drugs 0.000 claims description 3
- 239000002131 composite material Substances 0.000 claims description 3
- 239000002831 pharmacologic agent Substances 0.000 claims description 3
- 239000000106 platelet aggregation inhibitor Substances 0.000 claims description 3
- 208000031226 Hyperlipidaemia Diseases 0.000 claims description 2
- 102100036836 Natriuretic peptides B Human genes 0.000 claims description 2
- 101710187802 Natriuretic peptides B Proteins 0.000 claims description 2
- 239000002934 diuretic Substances 0.000 claims description 2
- 238000012986 modification Methods 0.000 claims description 2
- 230000004048 modification Effects 0.000 claims description 2
- 230000001452 natriuretic effect Effects 0.000 claims description 2
- 102000016776 Midkine Human genes 0.000 claims 15
- 102000011690 Adiponectin Human genes 0.000 claims 11
- 238000007887 coronary angioplasty Methods 0.000 claims 4
- 238000009007 Diagnostic Kit Methods 0.000 claims 1
- 238000000502 dialysis Methods 0.000 claims 1
- 230000001568 sexual effect Effects 0.000 claims 1
- 239000000203 mixture Substances 0.000 abstract description 4
- 235000018102 proteins Nutrition 0.000 description 91
- 238000010200 validation analysis Methods 0.000 description 72
- 238000012549 training Methods 0.000 description 51
- 102100031786 Adiponectin Human genes 0.000 description 34
- 208000031481 Pathologic Constriction Diseases 0.000 description 32
- 238000004458 analytical method Methods 0.000 description 32
- 230000036262 stenosis Effects 0.000 description 32
- 208000037804 stenosis Diseases 0.000 description 32
- 210000005166 vasculature Anatomy 0.000 description 26
- 102100030335 Midkine Human genes 0.000 description 25
- 150000001875 compounds Chemical class 0.000 description 21
- 102100040557 Osteopontin Human genes 0.000 description 20
- 230000008569 process Effects 0.000 description 20
- 201000000057 Coronary Stenosis Diseases 0.000 description 19
- 206010011089 Coronary artery stenosis Diseases 0.000 description 17
- 239000011230 binding agent Substances 0.000 description 17
- 210000004369 blood Anatomy 0.000 description 16
- 239000008280 blood Substances 0.000 description 16
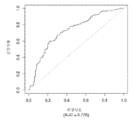
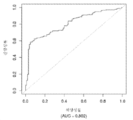
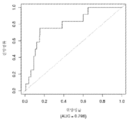
- 230000035945 sensitivity Effects 0.000 description 16
- 230000001154 acute effect Effects 0.000 description 15
- 239000003550 marker Substances 0.000 description 15
- 230000002093 peripheral effect Effects 0.000 description 15
- 238000005070 sampling Methods 0.000 description 14
- 230000002411 adverse Effects 0.000 description 13
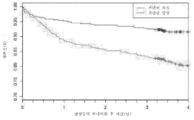
- 230000007211 cardiovascular event Effects 0.000 description 13
- 230000035882 stress Effects 0.000 description 13
- 210000001519 tissue Anatomy 0.000 description 11
- 238000009826 distribution Methods 0.000 description 10
- 208000019622 heart disease Diseases 0.000 description 10
- 108090000765 processed proteins & peptides Proteins 0.000 description 10
- 238000002583 angiography Methods 0.000 description 9
- 230000006870 function Effects 0.000 description 9
- 239000004005 microsphere Substances 0.000 description 9
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 8
- 210000002381 plasma Anatomy 0.000 description 8
- 229920001184 polypeptide Polymers 0.000 description 8
- 102000004196 processed proteins & peptides Human genes 0.000 description 8
- 208000020446 Cardiac disease Diseases 0.000 description 7
- 238000013459 approach Methods 0.000 description 7
- 238000002586 coronary angiography Methods 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 208000024891 symptom Diseases 0.000 description 7
- 201000001320 Atherosclerosis Diseases 0.000 description 6
- 238000002790 cross-validation Methods 0.000 description 6
- 238000007619 statistical method Methods 0.000 description 6
- 230000004083 survival effect Effects 0.000 description 6
- 150000001413 amino acids Chemical class 0.000 description 5
- 210000002966 serum Anatomy 0.000 description 5
- 210000002700 urine Anatomy 0.000 description 5
- 238000002965 ELISA Methods 0.000 description 4
- 206010019280 Heart failures Diseases 0.000 description 4
- 102100020880 Kit ligand Human genes 0.000 description 4
- 238000000585 Mann–Whitney U test Methods 0.000 description 4
- 230000017531 blood circulation Effects 0.000 description 4
- 238000007405 data analysis Methods 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 239000012530 fluid Substances 0.000 description 4
- 238000007726 management method Methods 0.000 description 4
- 238000010369 molecular cloning Methods 0.000 description 4
- 238000012546 transfer Methods 0.000 description 4
- 206010008479 Chest Pain Diseases 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 3
- 108090000695 Cytokines Proteins 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 102000003886 Glycoproteins Human genes 0.000 description 3
- 108090000288 Glycoproteins Proteins 0.000 description 3
- 208000010496 Heart Arrest Diseases 0.000 description 3
- 102000002274 Matrix Metalloproteinases Human genes 0.000 description 3
- 108010000684 Matrix Metalloproteinases Proteins 0.000 description 3
- 102100030412 Matrix metalloproteinase-9 Human genes 0.000 description 3
- 208000002193 Pain Diseases 0.000 description 3
- 206010062237 Renal impairment Diseases 0.000 description 3
- 102100023543 Vascular cell adhesion protein 1 Human genes 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000000740 bleeding effect Effects 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 235000012000 cholesterol Nutrition 0.000 description 3
- 238000012937 correction Methods 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 3
- 238000003018 immunoassay Methods 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 230000013016 learning Effects 0.000 description 3
- 238000007477 logistic regression Methods 0.000 description 3
- 238000004949 mass spectrometry Methods 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 238000013517 stratification Methods 0.000 description 3
- 206010003210 Arteriosclerosis Diseases 0.000 description 2
- 208000037260 Atherosclerotic Plaque Diseases 0.000 description 2
- 206010049993 Cardiac death Diseases 0.000 description 2
- 102000019034 Chemokines Human genes 0.000 description 2
- 108010012236 Chemokines Proteins 0.000 description 2
- 206010008469 Chest discomfort Diseases 0.000 description 2
- 206010011906 Death Diseases 0.000 description 2
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 2
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 102000015779 HDL Lipoproteins Human genes 0.000 description 2
- 108010010234 HDL Lipoproteins Proteins 0.000 description 2
- 102100034459 Hepatitis A virus cellular receptor 1 Human genes 0.000 description 2
- 101710185991 Hepatitis A virus cellular receptor 1 homolog Proteins 0.000 description 2
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 2
- 101000613820 Homo sapiens Osteopontin Proteins 0.000 description 2
- 101000632467 Homo sapiens Pulmonary surfactant-associated protein D Proteins 0.000 description 2
- 238000001207 Hosmer–Lemeshow test Methods 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 2
- 102100039364 Metalloproteinase inhibitor 1 Human genes 0.000 description 2
- 244000270834 Myristica fragrans Species 0.000 description 2
- 235000009421 Myristica fragrans Nutrition 0.000 description 2
- 208000008589 Obesity Diseases 0.000 description 2
- 206010057249 Phagocytosis Diseases 0.000 description 2
- 102000001708 Protein Isoforms Human genes 0.000 description 2
- 108010029485 Protein Isoforms Proteins 0.000 description 2
- 108010026552 Proteome Proteins 0.000 description 2
- 108010039445 Stem Cell Factor Proteins 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 206010000891 acute myocardial infarction Diseases 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 230000033115 angiogenesis Effects 0.000 description 2
- 239000000427 antigen Substances 0.000 description 2
- 102000036639 antigens Human genes 0.000 description 2
- 108091007433 antigens Proteins 0.000 description 2
- 206010003119 arrhythmia Diseases 0.000 description 2
- 206010003246 arthritis Diseases 0.000 description 2
- 230000001174 ascending effect Effects 0.000 description 2
- 238000002820 assay format Methods 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 238000001574 biopsy Methods 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 230000030833 cell death Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- ZPUCINDJVBIVPJ-LJISPDSOSA-N cocaine Chemical compound O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-LJISPDSOSA-N 0.000 description 2
- 230000008602 contraction Effects 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- RPLOSEMBQMPVEL-UHFFFAOYSA-N dec-1-yn-1-ol Chemical compound CCCCCCCCC#CO RPLOSEMBQMPVEL-UHFFFAOYSA-N 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- 210000002744 extracellular matrix Anatomy 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 210000004408 hybridoma Anatomy 0.000 description 2
- 230000002452 interceptive effect Effects 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 230000033001 locomotion Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 238000002552 multiple reaction monitoring Methods 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 210000004165 myocardium Anatomy 0.000 description 2
- 235000020824 obesity Nutrition 0.000 description 2
- 239000013610 patient sample Substances 0.000 description 2
- 230000008782 phagocytosis Effects 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 238000002331 protein detection Methods 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 230000002285 radioactive effect Effects 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- ROMPPAWVATWIKR-UHFFFAOYSA-N 4-[3-(4-chlorophenyl)-1,2,4-oxadiazol-5-yl]butanoic acid Chemical compound O1C(CCCC(=O)O)=NC(C=2C=CC(Cl)=CC=2)=N1 ROMPPAWVATWIKR-UHFFFAOYSA-N 0.000 description 1
- 208000009304 Acute Kidney Injury Diseases 0.000 description 1
- 206010002388 Angina unstable Diseases 0.000 description 1
- 206010003178 Arterial thrombosis Diseases 0.000 description 1
- 206010003225 Arteriospasm coronary Diseases 0.000 description 1
- 206010003658 Atrial Fibrillation Diseases 0.000 description 1
- 108050001427 Avidin/streptavidin Proteins 0.000 description 1
- 244000056139 Brassica cretica Species 0.000 description 1
- 235000003351 Brassica cretica Nutrition 0.000 description 1
- 235000003343 Brassica rupestris Nutrition 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 206010054212 Cardiac infection Diseases 0.000 description 1
- 208000031229 Cardiomyopathies Diseases 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 206010011086 Coronary artery occlusion Diseases 0.000 description 1
- IGXWBGJHJZYPQS-SSDOTTSWSA-N D-Luciferin Chemical compound OC(=O)[C@H]1CSC(C=2SC3=CC=C(O)C=C3N=2)=N1 IGXWBGJHJZYPQS-SSDOTTSWSA-N 0.000 description 1
- 108090000738 Decorin Proteins 0.000 description 1
- 102100035784 Decorin Human genes 0.000 description 1
- CYCGRDQQIOGCKX-UHFFFAOYSA-N Dehydro-luciferin Natural products OC(=O)C1=CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 CYCGRDQQIOGCKX-UHFFFAOYSA-N 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 206010014476 Elevated cholesterol Diseases 0.000 description 1
- 208000005189 Embolism Diseases 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 238000000729 Fisher's exact test Methods 0.000 description 1
- BJGNCJDXODQBOB-UHFFFAOYSA-N Fivefly Luciferin Natural products OC(=O)C1CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 BJGNCJDXODQBOB-UHFFFAOYSA-N 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 102100031000 Hepatoma-derived growth factor Human genes 0.000 description 1
- 101000775469 Homo sapiens Adiponectin Proteins 0.000 description 1
- 101001083798 Homo sapiens Hepatoma-derived growth factor Proteins 0.000 description 1
- 101001055222 Homo sapiens Interleukin-8 Proteins 0.000 description 1
- 101000669513 Homo sapiens Metalloproteinase inhibitor 1 Proteins 0.000 description 1
- 101000990990 Homo sapiens Midkine Proteins 0.000 description 1
- 101000835862 Homo sapiens Mothers against decapentaplegic homolog 1 Proteins 0.000 description 1
- 208000035150 Hypercholesterolemia Diseases 0.000 description 1
- 108091058560 IL8 Proteins 0.000 description 1
- 206010061216 Infarction Diseases 0.000 description 1
- 102100026236 Interleukin-8 Human genes 0.000 description 1
- 102000008133 Iron-Binding Proteins Human genes 0.000 description 1
- 108010035210 Iron-Binding Proteins Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- DDWFXDSYGUXRAY-UHFFFAOYSA-N Luciferin Natural products CCc1c(C)c(CC2NC(=O)C(=C2C=C)C)[nH]c1Cc3[nH]c4C(=C5/NC(CC(=O)O)C(C)C5CC(=O)O)CC(=O)c4c3C DDWFXDSYGUXRAY-UHFFFAOYSA-N 0.000 description 1
- 108090000362 Lymphotoxin-beta Proteins 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 102000012750 Membrane Glycoproteins Human genes 0.000 description 1
- 108010090054 Membrane Glycoproteins Proteins 0.000 description 1
- 108050006599 Metalloproteinase inhibitor 1 Proteins 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 102100025744 Mothers against decapentaplegic homolog 1 Human genes 0.000 description 1
- 208000029549 Muscle injury Diseases 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000037273 Pathologic Processes Diseases 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 208000005764 Peripheral Arterial Disease Diseases 0.000 description 1
- 208000030831 Peripheral arterial occlusive disease Diseases 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 101710180319 Protease 1 Proteins 0.000 description 1
- 102000016971 Proto-Oncogene Proteins c-kit Human genes 0.000 description 1
- 108010014608 Proto-Oncogene Proteins c-kit Proteins 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- 244000088415 Raphanus sativus Species 0.000 description 1
- 235000006140 Raphanus sativus var sativus Nutrition 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 208000033626 Renal failure acute Diseases 0.000 description 1
- 206010061481 Renal injury Diseases 0.000 description 1
- 206010039020 Rhabdomyolysis Diseases 0.000 description 1
- 102000001732 Small Leucine-Rich Proteoglycans Human genes 0.000 description 1
- 108010040068 Small Leucine-Rich Proteoglycans Proteins 0.000 description 1
- 101710168942 Sphingosine-1-phosphate phosphatase 1 Proteins 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 101710137710 Thioesterase 1/protease 1/lysophospholipase L1 Proteins 0.000 description 1
- 208000032109 Transient ischaemic attack Diseases 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 102000013534 Troponin C Human genes 0.000 description 1
- 102000013394 Troponin I Human genes 0.000 description 1
- 108010065729 Troponin I Proteins 0.000 description 1
- 102000004987 Troponin T Human genes 0.000 description 1
- 108090001108 Troponin T Proteins 0.000 description 1
- 208000007814 Unstable Angina Diseases 0.000 description 1
- 206010046543 Urinary incontinence Diseases 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 101710151579 Zinc metalloproteinase Proteins 0.000 description 1
- SXEHKFHPFVVDIR-UHFFFAOYSA-N [4-(4-hydrazinylphenyl)phenyl]hydrazine Chemical compound C1=CC(NN)=CC=C1C1=CC=C(NN)C=C1 SXEHKFHPFVVDIR-UHFFFAOYSA-N 0.000 description 1
- 201000011040 acute kidney failure Diseases 0.000 description 1
- 208000012998 acute renal failure Diseases 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 210000000577 adipose tissue Anatomy 0.000 description 1
- 210000005057 airway smooth muscle cell Anatomy 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 239000012062 aqueous buffer Substances 0.000 description 1
- 230000003143 atherosclerotic effect Effects 0.000 description 1
- 238000000559 atomic spectroscopy Methods 0.000 description 1
- 210000003651 basophil Anatomy 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 238000003339 best practice Methods 0.000 description 1
- 238000010256 biochemical assay Methods 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- QKSKPIVNLNLAAV-UHFFFAOYSA-N bis(2-chloroethyl) sulfide Chemical compound ClCCSCCCl QKSKPIVNLNLAAV-UHFFFAOYSA-N 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 238000009534 blood test Methods 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 230000014461 bone development Effects 0.000 description 1
- 210000004958 brain cell Anatomy 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 238000007816 calorimetric assay Methods 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 230000035605 chemotaxis Effects 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 238000003759 clinical diagnosis Methods 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 229960003920 cocaine Drugs 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 238000010668 complexation reaction Methods 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 238000003066 decision tree Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 239000000104 diagnostic biomarker Substances 0.000 description 1
- 238000007435 diagnostic evaluation Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 235000021045 dietary change Nutrition 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 230000009429 distress Effects 0.000 description 1
- 208000002173 dizziness Diseases 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 201000006549 dyspepsia Diseases 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000013020 embryo development Effects 0.000 description 1
- 230000002996 emotional effect Effects 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 210000003989 endothelium vascular Anatomy 0.000 description 1
- 238000001952 enzyme assay Methods 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 230000004133 fatty acid degradation Effects 0.000 description 1
- 230000004129 fatty acid metabolism Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 210000003714 granulocyte Anatomy 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 239000007952 growth promoter Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000024798 heartburn Diseases 0.000 description 1
- 230000011132 hemopoiesis Effects 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 229960001340 histamine Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 208000015210 hypertensive heart disease Diseases 0.000 description 1
- 230000007574 infarction Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 201000004332 intermediate coronary syndrome Diseases 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000001325 log-rank test Methods 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 210000003712 lysosome Anatomy 0.000 description 1
- 230000001868 lysosomic effect Effects 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 230000005291 magnetic effect Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 230000015654 memory Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 108091005601 modified peptides Proteins 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 230000004118 muscle contraction Effects 0.000 description 1
- 235000010460 mustard Nutrition 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 102000034288 naturally occurring fusion proteins Human genes 0.000 description 1
- 108091006048 naturally occurring fusion proteins Proteins 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 230000014511 neuron projection development Effects 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 238000012633 nuclear imaging Methods 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 210000000963 osteoblast Anatomy 0.000 description 1
- 229940043515 other immunoglobulins in atc Drugs 0.000 description 1
- 102000028703 oxygen binding proteins Human genes 0.000 description 1
- 108091009355 oxygen binding proteins Proteins 0.000 description 1
- 230000005298 paramagnetic effect Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000009054 pathological process Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 230000006461 physiological response Effects 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 235000017924 poor diet Nutrition 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000000092 prognostic biomarker Substances 0.000 description 1
- 235000019833 protease Nutrition 0.000 description 1
- 238000003498 protein array Methods 0.000 description 1
- 235000004252 protein component Nutrition 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 238000000611 regression analysis Methods 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000033458 reproduction Effects 0.000 description 1
- 230000019254 respiratory burst Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 208000004124 rheumatic heart disease Diseases 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 208000013220 shortness of breath Diseases 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 238000004557 single molecule detection Methods 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 238000005063 solubilization Methods 0.000 description 1
- 230000007928 solubilization Effects 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 230000021595 spermatogenesis Effects 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000035900 sweating Effects 0.000 description 1
- 230000002537 thrombolytic effect Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- 201000010875 transient cerebral ischemia Diseases 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 102000035160 transmembrane proteins Human genes 0.000 description 1
- 108091005703 transmembrane proteins Proteins 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 238000012285 ultrasound imaging Methods 0.000 description 1
- 230000006441 vascular event Effects 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000004393 visual impairment Effects 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/02—Detecting, measuring or recording for evaluating the cardiovascular system, e.g. pulse, heart rate, blood pressure or blood flow
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/48—Other medical applications
- A61B5/4836—Diagnosis combined with treatment in closed-loop systems or methods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/48—Other medical applications
- A61B5/4884—Other medical applications inducing physiological or psychological stress, e.g. applications for stress testing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B6/00—Apparatus or devices for radiation diagnosis; Apparatus or devices for radiation diagnosis combined with radiation therapy equipment
- A61B6/50—Apparatus or devices for radiation diagnosis; Apparatus or devices for radiation diagnosis combined with radiation therapy equipment specially adapted for specific body parts; specially adapted for specific clinical applications
- A61B6/503—Apparatus or devices for radiation diagnosis; Apparatus or devices for radiation diagnosis combined with radiation therapy equipment specially adapted for specific body parts; specially adapted for specific clinical applications for diagnosis of the heart
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/28—Neurological disorders
- G01N2800/2871—Cerebrovascular disorders, e.g. stroke, cerebral infarct, cerebral haemorrhage, transient ischemic event
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/32—Cardiovascular disorders
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/32—Cardiovascular disorders
- G01N2800/324—Coronary artery diseases, e.g. angina pectoris, myocardial infarction
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Pathology (AREA)
- Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Hematology (AREA)
- Immunology (AREA)
- Urology & Nephrology (AREA)
- Chemical & Material Sciences (AREA)
- Medical Informatics (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Surgery (AREA)
- Heart & Thoracic Surgery (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Cell Biology (AREA)
- General Physics & Mathematics (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Medicinal Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Analytical Chemistry (AREA)
- Food Science & Technology (AREA)
- Physiology (AREA)
- Cardiology (AREA)
- Dentistry (AREA)
- Oral & Maxillofacial Surgery (AREA)
- High Energy & Nuclear Physics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Optics & Photonics (AREA)
- Radiology & Medical Imaging (AREA)
- Child & Adolescent Psychology (AREA)
- Developmental Disabilities (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662289513P | 2016-02-01 | 2016-02-01 | |
| US62/289,513 | 2016-02-01 | ||
| US201662378535P | 2016-08-23 | 2016-08-23 | |
| US62/378,535 | 2016-08-23 | ||
| PCT/US2017/016081 WO2017136464A1 (en) | 2016-02-01 | 2017-02-01 | Diagnostic and prognostic methods for cardiovascular diseases and events |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180101595A true KR20180101595A (ko) | 2018-09-12 |
Family
ID=59500904
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187024496A Withdrawn KR20180101595A (ko) | 2016-02-01 | 2017-02-01 | 심혈관 질환 및 사건을 위한 진단학적 및 예후적 방법 |
Country Status (13)
| Country | Link |
|---|---|
| US (4) | US10983135B2 (enExample) |
| EP (2) | EP4574024A3 (enExample) |
| JP (4) | JP7134870B2 (enExample) |
| KR (1) | KR20180101595A (enExample) |
| CN (2) | CN108700596A (enExample) |
| AU (1) | AU2017214469A1 (enExample) |
| BR (1) | BR112018015698A2 (enExample) |
| CA (1) | CA3012192A1 (enExample) |
| MA (1) | MA43980A (enExample) |
| MX (1) | MX2018009304A (enExample) |
| RU (1) | RU2018130866A (enExample) |
| SG (1) | SG11201805906WA (enExample) |
| WO (1) | WO2017136464A1 (enExample) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7134870B2 (ja) | 2016-02-01 | 2022-09-12 | プレベンシオ, インコーポレイテッド | 心臓血管疾患及び事象のための診断及び予後方法 |
| WO2019055609A1 (en) * | 2017-09-14 | 2019-03-21 | University Of Alabama At Birmingham | BIOMARKERS AND METHODS OF RISK ASSESSMENT OF MYOCARDIAL INFARCTION AND SEVERE INFECTION IN PATIENTS WITH RHEUMATOID ARTHRITIS |
| WO2019090166A1 (en) * | 2017-11-02 | 2019-05-09 | Prevencio, Inc. | Diagnostic and prognostic methods for peripheral arterial diseases, aortic stenosis, and outcomes |
| US11009452B2 (en) | 2018-01-26 | 2021-05-18 | Viavi Solutions Inc. | Reduced false positive identification for spectroscopic quantification |
| EP3928098B1 (en) * | 2019-02-20 | 2025-01-08 | Fundació Institut d'Investigació en Ciències de la Salut Germans Trias i Pujol | In vitro method for predicting mortality risk in patients suffering from cardiogenic shock |
| CN110232975A (zh) * | 2019-05-20 | 2019-09-13 | 郑州大学第一附属医院 | 一种对糖尿病肾病患者3年内进入到肾脏替代治疗风险预测的方法 |
| CN110491512A (zh) * | 2019-08-08 | 2019-11-22 | 郑州大学第一附属医院 | 一种对肾活检确诊糖尿病肾病患者3年内进入到终末期肾脏病风险预测的方法 |
| CN110596399B (zh) * | 2019-08-29 | 2022-07-22 | 首都医科大学附属北京安贞医院 | 检测血管生成素样蛋白8含量的物质的应用 |
| CN110970131B (zh) * | 2019-10-24 | 2024-01-26 | 中国医科大学附属盛京医院 | 一种基于免疫球蛋白定量检测的肾小球疾病分类分型模型及其应用 |
| RU2726314C1 (ru) * | 2019-11-15 | 2020-07-14 | Федеральное государственное бюджетное образовательное учреждение высшего образования "Тихоокеанский государственный медицинский университет" Министерства здравоохранения Российской Федерации | Способ раннего прогнозирования риска острого повреждения почек у пациентов с ишемической болезнью сердца после реваскуляризации миокарда методом аортокоронарного шунтирования |
| RU2760445C1 (ru) * | 2020-11-13 | 2021-11-25 | Виталий Юрьевич Мишланов | Способ диагностики патологических состояний человека |
| JP7717152B2 (ja) * | 2021-03-23 | 2025-08-01 | テルモ株式会社 | 情報処理装置、情報処理方法およびプログラム |
| CN113342963B (zh) * | 2021-04-29 | 2022-03-04 | 山东大学 | 一种基于迁移学习的服务推荐方法及系统 |
| RU2770550C1 (ru) * | 2021-05-31 | 2022-04-18 | Федеральное государственное бюджетное образовательное учреждение высшего образования "Курский государственный медицинский университет" Министерства здравоохранения Российской Федерации | Способ прогнозирования сердечно-сосудистого риска с помощью определения уровня эстрогенов |
| AU2023243976A1 (en) * | 2022-03-30 | 2024-10-10 | Inflammatix, Inc. | Methods for diagnosing myocardial infarction |
| CN114974576A (zh) * | 2022-07-12 | 2022-08-30 | 曜立科技(北京)有限公司 | 一种基于元数据的心脑血管数疾病诊断管理系统 |
| CN115185936B (zh) | 2022-07-12 | 2023-02-03 | 曜立科技(北京)有限公司 | 一种基于大数据的医疗临床数据质量分析系统 |
Family Cites Families (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US20080202927A1 (en) | 2000-11-03 | 2008-08-28 | Clinical Micro Sensors, Inc. | Devices and methods for biochip multiplexing |
| US20030134433A1 (en) | 2002-01-16 | 2003-07-17 | Nanomix, Inc. | Electronic sensing of chemical and biological agents using functionalized nanostructures |
| US20060228723A1 (en) | 2002-01-16 | 2006-10-12 | Keith Bradley | System and method for electronic sensing of biomolecules |
| US8425745B2 (en) | 2006-10-06 | 2013-04-23 | Nanomix, Inc. | Electrochemical nanosensors for biomolecule detection |
| US9234867B2 (en) | 2003-05-16 | 2016-01-12 | Nanomix, Inc. | Electrochemical nanosensors for biomolecule detection |
| US8685711B2 (en) | 2004-09-28 | 2014-04-01 | Singulex, Inc. | Methods and compositions for highly sensitive detection of molecules |
| WO2006089226A1 (en) * | 2005-02-17 | 2006-08-24 | University Of Florida Research Foundation, Inc. | β-BLOCKER PHARMACOGENETICS IN HEART FAILURE |
| CN101495862A (zh) * | 2005-06-24 | 2009-07-29 | 利兰·斯坦福青年大学托管委员会 | 诊断和监测动脉粥样硬化性心血管疾病的方法和组合物 |
| US8093017B2 (en) * | 2005-12-07 | 2012-01-10 | Siemens Heathcare Diagnostics Inc. | Detection of soluble adiponectin receptor peptides and use in diagnostics and therapeutics |
| US20100331200A1 (en) * | 2006-03-31 | 2010-12-30 | Gordon Neal F | Post translational modification pattern analysis |
| WO2007146229A2 (en) | 2006-06-07 | 2007-12-21 | Tethys Bioscience, Inc. | Markers associated with arteriovascular events and methods of use thereof |
| JP5320294B2 (ja) * | 2006-10-26 | 2013-10-23 | ベー.エル.アー.ハー.エム.エス ゲーエムベーハー | プロバソプレシンまたはその断片および部分ペプチド、特にコペプチンまたはニューロフィジンiiを使用する、急性冠動脈症候群についてのリスク層化 |
| EP2147115A4 (en) * | 2007-04-16 | 2010-05-05 | CARDIOBIOINDEX / CARDIOBIOSCORE AND THE USE OF THE SPOKING PROTEOME IN CARDIOVASCULAR DIAGNOSTICS | |
| US20100119600A1 (en) | 2007-06-01 | 2010-05-13 | Joar Opheim | Substances for reducing occurrence of major cardiac events comprising red yeast rice extract and omega-3 polyunsaturated fatty acid or derivative thereof |
| GB0717637D0 (en) | 2007-09-10 | 2007-10-17 | Univ Leiden | Future cardiac event biomarkers |
| WO2009040133A1 (en) * | 2007-09-26 | 2009-04-02 | Universitätsklinikum Heidelberg | Osteopontin as novel prognostic biomarker for heart failure |
| US8241861B1 (en) * | 2008-07-08 | 2012-08-14 | Insilicos, Llc | Methods and compositions for diagnosis or prognosis of cardiovascular disease |
| EP2437652A1 (en) | 2009-06-04 | 2012-04-11 | Koninklijke Philips Electronics N.V. | Method and system for providing behavioural therapy for insomnia |
| AU2010259022B2 (en) * | 2009-06-08 | 2016-05-12 | Singulex, Inc. | Highly sensitive biomarker panels |
| WO2011022628A1 (en) | 2009-08-20 | 2011-02-24 | The Board Of Regents Of The University Of Texas System | Methods and compositions for diagnosis of acute myocardial infarction (ami) |
| EP2510116A2 (en) * | 2009-12-09 | 2012-10-17 | Aviir, Inc. | Biomarker assay for diagnosis and classification of cardiovascular disease |
| KR101256928B1 (ko) * | 2010-01-20 | 2013-04-19 | 주식회사디엔에이링크 | 비만 또는 당뇨에 관련된 단일염기다형성 및 그의 용도 |
| WO2012072595A1 (en) * | 2010-11-30 | 2012-06-07 | Universiteit Gent | Platelet factor 4 variant for the prognosis of cardiovascular outcome in patients having heart disease |
| WO2012120387A1 (en) | 2011-03-09 | 2012-09-13 | Abionic Sa | Rapid quantification of biomolecules in a selectively functionalized nanofluidic biosensor and method thereof |
| WO2012145037A1 (en) * | 2011-04-19 | 2012-10-26 | Scott & White Healthcare | Novel apoc-i isoforms and their use as biomarkers and risk factors of atherosclerotic disease |
| US20140011811A1 (en) * | 2011-10-06 | 2014-01-09 | The United States Government As Represented By The Department Of Veterans Affairs | Myosin binding protein-c for use in methods relating to diastolic heart failure |
| EP2920722B1 (en) * | 2012-11-16 | 2021-01-20 | Siemens Healthcare Diagnostics Inc. | Method to identify optimum coronary artery disease treatment |
| WO2015006713A1 (en) | 2013-07-12 | 2015-01-15 | Emory University | Diagnostic assay to predict cardiovascular risk |
| US9989526B2 (en) | 2013-07-17 | 2018-06-05 | Abionic Sa | Method and device for bioassays |
| US20150160229A1 (en) * | 2013-12-06 | 2015-06-11 | Thomas D. Schaal | Methods for detection of heart failure |
| WO2015171989A1 (en) * | 2014-05-08 | 2015-11-12 | University Of Maryland, Baltimore | Methods for assessing differential risk for developing heart failure |
| AU2015283689B2 (en) | 2014-07-03 | 2020-07-16 | Abionic Sa | Capsule for rapid molecular quantification of a fluid sample such as whole blood |
| JP7134870B2 (ja) | 2016-02-01 | 2022-09-12 | プレベンシオ, インコーポレイテッド | 心臓血管疾患及び事象のための診断及び予後方法 |
| JP7310591B2 (ja) | 2019-12-19 | 2023-07-19 | 株式会社オートネットワーク技術研究所 | 駆動装置 |
-
2017
- 2017-02-01 JP JP2018559674A patent/JP7134870B2/ja active Active
- 2017-02-01 KR KR1020187024496A patent/KR20180101595A/ko not_active Withdrawn
- 2017-02-01 CN CN201780011650.8A patent/CN108700596A/zh active Pending
- 2017-02-01 SG SG11201805906WA patent/SG11201805906WA/en unknown
- 2017-02-01 MA MA043980A patent/MA43980A/fr unknown
- 2017-02-01 CA CA3012192A patent/CA3012192A1/en active Pending
- 2017-02-01 WO PCT/US2017/016081 patent/WO2017136464A1/en not_active Ceased
- 2017-02-01 MX MX2018009304A patent/MX2018009304A/es unknown
- 2017-02-01 CN CN202210116587.7A patent/CN114441773A/zh active Pending
- 2017-02-01 US US16/073,754 patent/US10983135B2/en active Active
- 2017-02-01 EP EP25170203.1A patent/EP4574024A3/en active Pending
- 2017-02-01 BR BR112018015698A patent/BR112018015698A2/pt not_active Application Discontinuation
- 2017-02-01 EP EP17748086.0A patent/EP3411720B1/en active Active
- 2017-02-01 AU AU2017214469A patent/AU2017214469A1/en not_active Abandoned
- 2017-02-01 RU RU2018130866A patent/RU2018130866A/ru not_active Application Discontinuation
-
2020
- 2020-10-27 US US17/081,776 patent/US11977083B2/en active Active
-
2021
- 2021-06-10 JP JP2021097387A patent/JP7403498B2/ja active Active
-
2022
- 2022-09-21 JP JP2022150335A patent/JP2022171936A/ja active Pending
-
2023
- 2023-06-21 US US18/339,066 patent/US12146888B2/en active Active
-
2024
- 2024-10-11 US US18/913,852 patent/US20250060374A1/en active Pending
- 2024-12-09 JP JP2024214563A patent/JP2025029196A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| US20250060374A1 (en) | 2025-02-20 |
| JP7134870B2 (ja) | 2022-09-12 |
| MX2018009304A (es) | 2019-01-10 |
| WO2017136464A1 (en) | 2017-08-10 |
| JP2025029196A (ja) | 2025-03-05 |
| CA3012192A1 (en) | 2017-08-10 |
| BR112018015698A2 (pt) | 2018-12-26 |
| EP3411720A1 (en) | 2018-12-12 |
| RU2018130866A (ru) | 2020-03-04 |
| EP3411720B1 (en) | 2025-04-16 |
| US20230341418A1 (en) | 2023-10-26 |
| US20190369120A1 (en) | 2019-12-05 |
| MA43980A (fr) | 2018-12-12 |
| EP3411720C0 (en) | 2025-04-16 |
| JP2019507354A (ja) | 2019-03-14 |
| EP3411720A4 (en) | 2019-08-14 |
| US12146888B2 (en) | 2024-11-19 |
| JP7403498B2 (ja) | 2023-12-22 |
| SG11201805906WA (en) | 2018-08-30 |
| CN108700596A (zh) | 2018-10-23 |
| JP2021128177A (ja) | 2021-09-02 |
| JP2022171936A (ja) | 2022-11-11 |
| EP4574024A3 (en) | 2025-10-08 |
| US11977083B2 (en) | 2024-05-07 |
| AU2017214469A1 (en) | 2018-07-26 |
| US20210072260A1 (en) | 2021-03-11 |
| US10983135B2 (en) | 2021-04-20 |
| CN114441773A (zh) | 2022-05-06 |
| EP4574024A2 (en) | 2025-06-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12146888B2 (en) | Diagnostic methods for cardiovascular diseases | |
| KR101678703B1 (ko) | 갈렉틴-3 면역검정 | |
| JP7372251B2 (ja) | 末梢動脈疾患、大動脈弁狭窄症についての診断および予後の方法、ならびにアウトカム | |
| EP2834638B1 (en) | Methods for diagnosis and prognosis of sepsis | |
| CN105556308A (zh) | 用于阑尾炎的诊断和预后及腹痛病因的区分的方法和组合物 | |
| JP7447003B2 (ja) | 予後予測方法 | |
| EP3497450A1 (en) | Histones and/or proadm as markers indicating organ dysfunction | |
| CA3091531A1 (en) | Patient assessment method | |
| JP4734306B2 (ja) | ナトリウム利尿ペプチドおよび胎盤増殖因子/可溶性vegf受容体を用いた、妊婦での心疾患に関連した心機能不全と胎盤関連心機能不全とを区別するための方法 | |
| WO2020092909A1 (en) | Prognostic and diagnostic methods for risk of acute kidney injury | |
| CN111065922A (zh) | 肾上腺髓质素原作为危重病患者的肾脏替代治疗的指标 | |
| HK40074056A (en) | Diagnostic and prognostic methods for cardiovascular diseases and events | |
| HK1261993A1 (en) | Diagnostic and prognostic methods for cardiovascular diseases and events | |
| HK1261993B (en) | Diagnostic and prognostic methods for cardiovascular diseases and events | |
| EP3502691A1 (en) | Proadrenomedullin as indicator for renal replacement therapy in critically ill patients | |
| US20150104463A1 (en) | Methods and kits for predicting the risk of having a cardiovascular event in a subject |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20180824 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination |