KR20180049000A - 세포 배양 - Google Patents
세포 배양 Download PDFInfo
- Publication number
- KR20180049000A KR20180049000A KR1020187009401A KR20187009401A KR20180049000A KR 20180049000 A KR20180049000 A KR 20180049000A KR 1020187009401 A KR1020187009401 A KR 1020187009401A KR 20187009401 A KR20187009401 A KR 20187009401A KR 20180049000 A KR20180049000 A KR 20180049000A
- Authority
- KR
- South Korea
- Prior art keywords
- medium
- complete
- dimensional
- ali
- williams
- Prior art date
Links
- 238000004113 cell culture Methods 0.000 title description 17
- 210000004185 liver Anatomy 0.000 claims abstract description 275
- 230000000694 effects Effects 0.000 claims abstract description 98
- 102100031476 Cytochrome P450 1A1 Human genes 0.000 claims abstract description 79
- 108010088751 Albumins Proteins 0.000 claims abstract description 78
- 102000009027 Albumins Human genes 0.000 claims abstract description 78
- 230000028327 secretion Effects 0.000 claims abstract description 78
- 101710104049 Cytochrome P450 1A1 Proteins 0.000 claims abstract description 71
- 108050002014 Cytochrome P450 1B1 Proteins 0.000 claims abstract description 63
- 239000002609 medium Substances 0.000 claims description 597
- 210000004027 cell Anatomy 0.000 claims description 181
- 239000000203 mixture Substances 0.000 claims description 104
- 239000006143 cell culture medium Substances 0.000 claims description 102
- 210000000056 organ Anatomy 0.000 claims description 99
- 238000000034 method Methods 0.000 claims description 77
- 210000004072 lung Anatomy 0.000 claims description 76
- 102000012466 Cytochrome P450 1B1 Human genes 0.000 claims description 62
- 210000003494 hepatocyte Anatomy 0.000 claims description 59
- 239000003795 chemical substances by application Substances 0.000 claims description 58
- 239000001963 growth medium Substances 0.000 claims description 48
- 210000002919 epithelial cell Anatomy 0.000 claims description 45
- 238000003501 co-culture Methods 0.000 claims description 39
- 239000000443 aerosol Substances 0.000 claims description 38
- 210000005265 lung cell Anatomy 0.000 claims description 33
- 230000004044 response Effects 0.000 claims description 30
- 238000005259 measurement Methods 0.000 claims description 29
- 210000000130 stem cell Anatomy 0.000 claims description 24
- 230000002440 hepatic effect Effects 0.000 claims description 20
- 238000006243 chemical reaction Methods 0.000 claims description 18
- 238000012258 culturing Methods 0.000 claims description 16
- 238000000338 in vitro Methods 0.000 claims description 16
- 239000007788 liquid Substances 0.000 claims description 16
- 239000003814 drug Substances 0.000 claims description 14
- 229940079593 drug Drugs 0.000 claims description 12
- 238000009472 formulation Methods 0.000 claims description 12
- 239000000779 smoke Substances 0.000 claims description 12
- 230000021368 organ growth Effects 0.000 claims description 11
- 230000035515 penetration Effects 0.000 claims description 11
- 238000002360 preparation method Methods 0.000 claims description 9
- 230000008569 process Effects 0.000 claims description 9
- 235000019504 cigarettes Nutrition 0.000 claims description 8
- 238000000018 DNA microarray Methods 0.000 claims description 6
- 231100000041 toxicology testing Toxicity 0.000 claims description 4
- 238000007876 drug discovery Methods 0.000 claims description 3
- 102100027417 Cytochrome P450 1B1 Human genes 0.000 abstract 1
- 210000001519 tissue Anatomy 0.000 description 49
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 37
- 150000001875 compounds Chemical class 0.000 description 32
- 238000011534 incubation Methods 0.000 description 32
- 210000005229 liver cell Anatomy 0.000 description 28
- 229960003180 glutathione Drugs 0.000 description 27
- 108010053070 Glutathione Disulfide Proteins 0.000 description 22
- YPZRWBKMTBYPTK-BJDJZHNGSA-N glutathione disulfide Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@H](C(=O)NCC(O)=O)CSSC[C@@H](C(=O)NCC(O)=O)NC(=O)CC[C@H](N)C(O)=O YPZRWBKMTBYPTK-BJDJZHNGSA-N 0.000 description 22
- 230000014509 gene expression Effects 0.000 description 19
- 239000000463 material Substances 0.000 description 18
- 239000000306 component Substances 0.000 description 17
- 238000005516 engineering process Methods 0.000 description 16
- 230000004069 differentiation Effects 0.000 description 14
- 108090000765 processed proteins & peptides Proteins 0.000 description 14
- 239000013589 supplement Substances 0.000 description 14
- 238000012360 testing method Methods 0.000 description 14
- 210000005228 liver tissue Anatomy 0.000 description 13
- 230000035800 maturation Effects 0.000 description 13
- 241000208125 Nicotiana Species 0.000 description 12
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 12
- 238000003556 assay Methods 0.000 description 12
- 102000004196 processed proteins & peptides Human genes 0.000 description 12
- 108090000623 proteins and genes Proteins 0.000 description 12
- 230000002503 metabolic effect Effects 0.000 description 11
- 239000000243 solution Substances 0.000 description 11
- 108010024636 Glutathione Proteins 0.000 description 10
- 230000004060 metabolic process Effects 0.000 description 10
- 230000000391 smoking effect Effects 0.000 description 10
- HGUFODBRKLSHSI-UHFFFAOYSA-N 2,3,7,8-tetrachloro-dibenzo-p-dioxin Chemical compound O1C2=CC(Cl)=C(Cl)C=C2OC2=C1C=C(Cl)C(Cl)=C2 HGUFODBRKLSHSI-UHFFFAOYSA-N 0.000 description 9
- 239000007640 basal medium Substances 0.000 description 9
- 230000012010 growth Effects 0.000 description 9
- 230000001338 necrotic effect Effects 0.000 description 9
- 229920001184 polypeptide Polymers 0.000 description 9
- 108010074918 Cytochrome P-450 CYP1A1 Proteins 0.000 description 8
- 101000919294 Drosophila melanogaster Cytochrome P450 6a2 Proteins 0.000 description 8
- 230000004913 activation Effects 0.000 description 8
- JQXXHWHPUNPDRT-YOPQJBRCSA-N chembl1332716 Chemical compound O([C@](C1=O)(C)O\C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)/C=C\C=C(C)/C(=O)NC=2C(O)=C3C(O)=C4C)C)OC)C4=C1C3=C(O)C=2\C=N\N1CCN(C)CC1 JQXXHWHPUNPDRT-YOPQJBRCSA-N 0.000 description 8
- 238000010790 dilution Methods 0.000 description 8
- 239000012895 dilution Substances 0.000 description 8
- 230000006870 function Effects 0.000 description 8
- 229960001225 rifampicin Drugs 0.000 description 8
- 239000000758 substrate Substances 0.000 description 8
- 102000004190 Enzymes Human genes 0.000 description 7
- 108090000790 Enzymes Proteins 0.000 description 7
- -1 Here Substances 0.000 description 7
- 230000001640 apoptogenic effect Effects 0.000 description 7
- 229940088598 enzyme Drugs 0.000 description 7
- 238000002474 experimental method Methods 0.000 description 7
- 238000012423 maintenance Methods 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- YPZRWBKMTBYPTK-UHFFFAOYSA-N oxidized gamma-L-glutamyl-L-cysteinylglycine Natural products OC(=O)C(N)CCC(=O)NC(C(=O)NCC(O)=O)CSSCC(C(=O)NCC(O)=O)NC(=O)CCC(N)C(O)=O YPZRWBKMTBYPTK-UHFFFAOYSA-N 0.000 description 7
- 238000012216 screening Methods 0.000 description 7
- HJCMDXDYPOUFDY-WHFBIAKZSA-N Ala-Gln Chemical compound C[C@H](N)C(=O)N[C@H](C(O)=O)CCC(N)=O HJCMDXDYPOUFDY-WHFBIAKZSA-N 0.000 description 6
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 230000003247 decreasing effect Effects 0.000 description 6
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 6
- 150000002894 organic compounds Chemical class 0.000 description 6
- 241001465754 Metazoa Species 0.000 description 5
- 210000000424 bronchial epithelial cell Anatomy 0.000 description 5
- 238000012054 celltiter-glo Methods 0.000 description 5
- 239000012530 fluid Substances 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 230000006698 induction Effects 0.000 description 5
- 210000004379 membrane Anatomy 0.000 description 5
- 239000012528 membrane Substances 0.000 description 5
- 108091033319 polynucleotide Proteins 0.000 description 5
- 239000002157 polynucleotide Substances 0.000 description 5
- 102000040430 polynucleotide Human genes 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 230000035899 viability Effects 0.000 description 5
- 108010074922 Cytochrome P-450 CYP1A2 Proteins 0.000 description 4
- 108010020070 Cytochrome P-450 CYP2B6 Proteins 0.000 description 4
- 102000009666 Cytochrome P-450 CYP2B6 Human genes 0.000 description 4
- 102100026533 Cytochrome P450 1A2 Human genes 0.000 description 4
- GQPLMRYTRLFLPF-UHFFFAOYSA-N Nitrous Oxide Chemical compound [O-][N+]#N GQPLMRYTRLFLPF-UHFFFAOYSA-N 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 239000000427 antigen Substances 0.000 description 4
- 108091007433 antigens Proteins 0.000 description 4
- 102000036639 antigens Human genes 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 150000001720 carbohydrates Chemical class 0.000 description 4
- 235000014633 carbohydrates Nutrition 0.000 description 4
- 230000034994 death Effects 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 230000002255 enzymatic effect Effects 0.000 description 4
- 150000002632 lipids Chemical class 0.000 description 4
- 230000003278 mimic effect Effects 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 230000002685 pulmonary effect Effects 0.000 description 4
- 230000010076 replication Effects 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 231100000331 toxic Toxicity 0.000 description 4
- 230000002588 toxic effect Effects 0.000 description 4
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 102000002004 Cytochrome P-450 Enzyme System Human genes 0.000 description 3
- 108010015742 Cytochrome P-450 Enzyme System Proteins 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 3
- 108090000695 Cytokines Proteins 0.000 description 3
- 238000008157 ELISA kit Methods 0.000 description 3
- 241000196324 Embryophyta Species 0.000 description 3
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 3
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 210000001552 airway epithelial cell Anatomy 0.000 description 3
- 229910002091 carbon monoxide Inorganic materials 0.000 description 3
- 239000012881 co-culture medium Substances 0.000 description 3
- 210000000981 epithelium Anatomy 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 210000002744 extracellular matrix Anatomy 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 229960000890 hydrocortisone Drugs 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 238000004020 luminiscence type Methods 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 229930014626 natural product Natural products 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 239000002773 nucleotide Substances 0.000 description 3
- 125000003729 nucleotide group Chemical group 0.000 description 3
- 230000035699 permeability Effects 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- 210000001082 somatic cell Anatomy 0.000 description 3
- 230000000638 stimulation Effects 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 2
- VFMMPHCGEFXGIP-UHFFFAOYSA-N 7,8-Benzoflavone Chemical compound O1C2=C3C=CC=CC3=CC=C2C(=O)C=C1C1=CC=CC=C1 VFMMPHCGEFXGIP-UHFFFAOYSA-N 0.000 description 2
- HGINCPLSRVDWNT-UHFFFAOYSA-N Acrolein Chemical compound C=CC=O HGINCPLSRVDWNT-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- KAKZBPTYRLMSJV-UHFFFAOYSA-N Butadiene Chemical compound C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- LFMYNZPAVPMEGP-PIDGMYBPSA-N Fluvoxamine maleate Chemical compound OC(=O)\C=C/C(O)=O.COCCCC\C(=N/OCCN)C1=CC=C(C(F)(F)F)C=C1 LFMYNZPAVPMEGP-PIDGMYBPSA-N 0.000 description 2
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 2
- OHJKXVLJWUPWQG-PNRHKHKDSA-N Heparinsodiumsalt Chemical compound O[C@@H]1[C@@H](NS(O)(=O)=O)[C@@H](O)O[C@H](COS(O)(=O)=O)[C@H]1O[C@H]1[C@H](OS(O)(=O)=O)[C@@H](O)[C@H](O)[C@H](C(O)=O)O1 OHJKXVLJWUPWQG-PNRHKHKDSA-N 0.000 description 2
- QIGBRXMKCJKVMJ-UHFFFAOYSA-N Hydroquinone Chemical compound OC1=CC=C(O)C=C1 QIGBRXMKCJKVMJ-UHFFFAOYSA-N 0.000 description 2
- RRHGJUQNOFWUDK-UHFFFAOYSA-N Isoprene Chemical compound CC(=C)C=C RRHGJUQNOFWUDK-UHFFFAOYSA-N 0.000 description 2
- 102100033420 Keratin, type I cytoskeletal 19 Human genes 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- NBBJYMSMWIIQGU-UHFFFAOYSA-N Propionic aldehyde Chemical compound CCC=O NBBJYMSMWIIQGU-UHFFFAOYSA-N 0.000 description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- 238000011529 RT qPCR Methods 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 210000004102 animal cell Anatomy 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 210000000270 basal cell Anatomy 0.000 description 2
- 239000000560 biocompatible material Substances 0.000 description 2
- ZTQSAGDEMFDKMZ-UHFFFAOYSA-N butyric aldehyde Natural products CCCC=O ZTQSAGDEMFDKMZ-UHFFFAOYSA-N 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 230000030833 cell death Effects 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 230000003833 cell viability Effects 0.000 description 2
- 210000003850 cellular structure Anatomy 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- 210000000254 ciliated cell Anatomy 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 239000003636 conditioned culture medium Substances 0.000 description 2
- 210000000805 cytoplasm Anatomy 0.000 description 2
- 231100000135 cytotoxicity Toxicity 0.000 description 2
- 230000003013 cytotoxicity Effects 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 239000004205 dimethyl polysiloxane Substances 0.000 description 2
- 210000001671 embryonic stem cell Anatomy 0.000 description 2
- 238000013401 experimental design Methods 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 229960002107 fluvoxamine maleate Drugs 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 210000002175 goblet cell Anatomy 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 210000005260 human cell Anatomy 0.000 description 2
- 239000000017 hydrogel Substances 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 150000002431 hydrogen Chemical class 0.000 description 2
- LELOWRISYMNNSU-UHFFFAOYSA-N hydrogen cyanide Chemical compound N#C LELOWRISYMNNSU-UHFFFAOYSA-N 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 238000010166 immunofluorescence Methods 0.000 description 2
- 238000003364 immunohistochemistry Methods 0.000 description 2
- 238000010874 in vitro model Methods 0.000 description 2
- 210000004263 induced pluripotent stem cell Anatomy 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 239000001272 nitrous oxide Substances 0.000 description 2
- 210000004789 organ system Anatomy 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- 239000000813 peptide hormone Substances 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 239000011574 phosphorus Substances 0.000 description 2
- 230000035479 physiological effects, processes and functions Effects 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 2
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 2
- 239000004926 polymethyl methacrylate Substances 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 238000003127 radioimmunoassay Methods 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- GHMLBKRAJCXXBS-UHFFFAOYSA-N resorcinol Chemical compound OC1=CC=CC(O)=C1 GHMLBKRAJCXXBS-UHFFFAOYSA-N 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 229910052711 selenium Inorganic materials 0.000 description 2
- 239000011669 selenium Substances 0.000 description 2
- 238000001338 self-assembly Methods 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- LXMSZDCAJNLERA-ZHYRCANASA-N spironolactone Chemical compound C([C@@H]1[C@]2(C)CC[C@@H]3[C@@]4(C)CCC(=O)C=C4C[C@H]([C@@H]13)SC(=O)C)C[C@@]21CCC(=O)O1 LXMSZDCAJNLERA-ZHYRCANASA-N 0.000 description 2
- 239000011550 stock solution Substances 0.000 description 2
- 239000011593 sulfur Substances 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 230000001502 supplementing effect Effects 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 231100000027 toxicology Toxicity 0.000 description 2
- 231100000765 toxin Toxicity 0.000 description 2
- 108700012359 toxins Proteins 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- NLHHRLWOUZZQLW-UHFFFAOYSA-N Acrylonitrile Chemical compound C=CC#N NLHHRLWOUZZQLW-UHFFFAOYSA-N 0.000 description 1
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 1
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 1
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- 241000208199 Buxus sempervirens Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- 102000047934 Caspase-3/7 Human genes 0.000 description 1
- 108700037887 Caspase-3/7 Proteins 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 102000012422 Collagen Type I Human genes 0.000 description 1
- 108010022452 Collagen Type I Proteins 0.000 description 1
- 102000029816 Collagenase Human genes 0.000 description 1
- 102000018832 Cytochromes Human genes 0.000 description 1
- 108010052832 Cytochromes Proteins 0.000 description 1
- IGXWBGJHJZYPQS-SSDOTTSWSA-N D-Luciferin Chemical compound OC(=O)[C@H]1CSC(C=2SC3=CC=C(O)C=C3N=2)=N1 IGXWBGJHJZYPQS-SSDOTTSWSA-N 0.000 description 1
- CYCGRDQQIOGCKX-UHFFFAOYSA-N Dehydro-luciferin Natural products OC(=O)C1=CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 CYCGRDQQIOGCKX-UHFFFAOYSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 102000009123 Fibrin Human genes 0.000 description 1
- 108010073385 Fibrin Proteins 0.000 description 1
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 1
- 102000016359 Fibronectins Human genes 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- BJGNCJDXODQBOB-UHFFFAOYSA-N Fivefly Luciferin Natural products OC(=O)C1CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 BJGNCJDXODQBOB-UHFFFAOYSA-N 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 108010001103 Glutathione oxidase Proteins 0.000 description 1
- 102000002068 Glycopeptides Human genes 0.000 description 1
- 108010015899 Glycopeptides Proteins 0.000 description 1
- 229920002683 Glycosaminoglycan Polymers 0.000 description 1
- 206010019851 Hepatotoxicity Diseases 0.000 description 1
- 102000006947 Histones Human genes 0.000 description 1
- 108010033040 Histones Proteins 0.000 description 1
- 101000998011 Homo sapiens Keratin, type I cytoskeletal 19 Proteins 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- 108010066302 Keratin-19 Proteins 0.000 description 1
- DDWFXDSYGUXRAY-UHFFFAOYSA-N Luciferin Natural products CCc1c(C)c(CC2NC(=O)C(=C2C=C)C)[nH]c1Cc3[nH]c4C(=C5/NC(CC(=O)O)C(C)C5CC(=O)O)CC(=O)c4c3C DDWFXDSYGUXRAY-UHFFFAOYSA-N 0.000 description 1
- 240000004658 Medicago sativa Species 0.000 description 1
- 235000010624 Medicago sativa Nutrition 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 238000000636 Northern blotting Methods 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 101150053185 P450 gene Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 229930040373 Paraformaldehyde Natural products 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000015731 Peptide Hormones Human genes 0.000 description 1
- 108010038988 Peptide Hormones Proteins 0.000 description 1
- 108091093037 Peptide nucleic acid Proteins 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical class OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 108010057464 Prolactin Proteins 0.000 description 1
- 102000003946 Prolactin Human genes 0.000 description 1
- 102000007327 Protamines Human genes 0.000 description 1
- 108010007568 Protamines Proteins 0.000 description 1
- 102000016611 Proteoglycans Human genes 0.000 description 1
- 108010067787 Proteoglycans Proteins 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- 102000005157 Somatostatin Human genes 0.000 description 1
- 108010056088 Somatostatin Proteins 0.000 description 1
- 238000002105 Southern blotting Methods 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- 239000004809 Teflon Substances 0.000 description 1
- 229920006362 Teflon® Polymers 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 241001655291 Williamsia Species 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- IKHGUXGNUITLKF-XPULMUKRSA-N acetaldehyde Chemical compound [14CH]([14CH3])=O IKHGUXGNUITLKF-XPULMUKRSA-N 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 210000000577 adipose tissue Anatomy 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 230000029936 alkylation Effects 0.000 description 1
- 238000005804 alkylation reaction Methods 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical compound [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- 210000002821 alveolar epithelial cell Anatomy 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 229910052785 arsenic Inorganic materials 0.000 description 1
- RQNWIZPPADIBDY-UHFFFAOYSA-N arsenic atom Chemical compound [As] RQNWIZPPADIBDY-UHFFFAOYSA-N 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 210000003323 beak Anatomy 0.000 description 1
- 230000006399 behavior Effects 0.000 description 1
- 125000005605 benzo group Chemical group 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 239000003114 blood coagulation factor Substances 0.000 description 1
- 229940019700 blood coagulation factors Drugs 0.000 description 1
- 229910052796 boron Inorganic materials 0.000 description 1
- 238000013276 bronchoscopy Methods 0.000 description 1
- 230000001680 brushing effect Effects 0.000 description 1
- 229910052793 cadmium Inorganic materials 0.000 description 1
- BDOSMKKIYDKNTQ-UHFFFAOYSA-N cadmium atom Chemical compound [Cd] BDOSMKKIYDKNTQ-UHFFFAOYSA-N 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 150000003857 carboxamides Chemical class 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 210000005056 cell body Anatomy 0.000 description 1
- 239000012592 cell culture supplement Substances 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 239000013553 cell monolayer Substances 0.000 description 1
- 230000036978 cell physiology Effects 0.000 description 1
- 238000002659 cell therapy Methods 0.000 description 1
- 238000003570 cell viability assay Methods 0.000 description 1
- 230000010267 cellular communication Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000000262 chemical ionisation mass spectrometry Methods 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 235000019506 cigar Nutrition 0.000 description 1
- 210000004081 cilia Anatomy 0.000 description 1
- 239000000512 collagen gel Substances 0.000 description 1
- 239000000515 collagen sponge Substances 0.000 description 1
- 229940096422 collagen type i Drugs 0.000 description 1
- 229960002424 collagenase Drugs 0.000 description 1
- 238000004737 colorimetric analysis Methods 0.000 description 1
- 238000002485 combustion reaction Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000003271 compound fluorescence assay Methods 0.000 description 1
- 230000009073 conformational modification Effects 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229930003836 cresol Natural products 0.000 description 1
- MLUCVPSAIODCQM-NSCUHMNNSA-N crotonaldehyde Chemical compound C\C=C\C=O MLUCVPSAIODCQM-NSCUHMNNSA-N 0.000 description 1
- MLUCVPSAIODCQM-UHFFFAOYSA-N crotonaldehyde Natural products CC=CC=O MLUCVPSAIODCQM-UHFFFAOYSA-N 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000036267 drug metabolism Effects 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- YQGOJNYOYNNSMM-UHFFFAOYSA-N eosin Chemical compound [Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21 YQGOJNYOYNNSMM-UHFFFAOYSA-N 0.000 description 1
- 235000020774 essential nutrients Nutrition 0.000 description 1
- 230000034964 establishment of cell polarity Effects 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000010195 expression analysis Methods 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000007667 floating Methods 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 230000005021 gait Effects 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- ZFGMDIBRIDKWMY-PASTXAENSA-N heparin Chemical compound CC(O)=N[C@@H]1[C@@H](O)[C@H](O)[C@@H](COS(O)(=O)=O)O[C@@H]1O[C@@H]1[C@@H](C(O)=O)O[C@@H](O[C@H]2[C@@H]([C@@H](OS(O)(=O)=O)[C@@H](O[C@@H]3[C@@H](OC(O)[C@H](OS(O)(=O)=O)[C@H]3O)C(O)=O)O[C@@H]2O)CS(O)(=O)=O)[C@H](O)[C@H]1O ZFGMDIBRIDKWMY-PASTXAENSA-N 0.000 description 1
- 229960001008 heparin sodium Drugs 0.000 description 1
- 210000005161 hepatic lobe Anatomy 0.000 description 1
- 210000004024 hepatic stellate cell Anatomy 0.000 description 1
- 230000007686 hepatotoxicity Effects 0.000 description 1
- 231100000304 hepatotoxicity Toxicity 0.000 description 1
- 238000012203 high throughput assay Methods 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 238000012744 immunostaining Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000007901 in situ hybridization Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 239000011133 lead Substances 0.000 description 1
- 230000003908 liver function Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 238000007422 luminescence assay Methods 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 238000002595 magnetic resonance imaging Methods 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 1
- 229940032007 methylethyl ketone Drugs 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000004089 microcirculation Effects 0.000 description 1
- 210000004925 microvascular endothelial cell Anatomy 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 230000003562 morphometric effect Effects 0.000 description 1
- 238000013425 morphometry Methods 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229960002715 nicotine Drugs 0.000 description 1
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 125000003835 nucleoside group Chemical group 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000012634 optical imaging Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 230000036542 oxidative stress Effects 0.000 description 1
- 230000036284 oxygen consumption Effects 0.000 description 1
- 230000020477 pH reduction Effects 0.000 description 1
- 229920002866 paraformaldehyde Polymers 0.000 description 1
- 239000011236 particulate material Substances 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 239000002831 pharmacologic agent Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 210000002381 plasma Anatomy 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 210000001778 pluripotent stem cell Anatomy 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920002338 polyhydroxyethylmethacrylate Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 238000002600 positron emission tomography Methods 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 229940097325 prolactin Drugs 0.000 description 1
- 229940048914 protamine Drugs 0.000 description 1
- 238000001243 protein synthesis Methods 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000001172 regenerating effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000008521 reorganization Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 231100000812 repeated exposure Toxicity 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 238000012106 screening analysis Methods 0.000 description 1
- 238000007423 screening assay Methods 0.000 description 1
- 238000002603 single-photon emission computed tomography Methods 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000003238 somatosensory effect Effects 0.000 description 1
- NHXLMOGPVYXJNR-ATOGVRKGSA-N somatostatin Chemical compound C([C@H]1C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CSSC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(=O)N1)[C@@H](C)O)NC(=O)CNC(=O)[C@H](C)N)C(O)=O)=O)[C@H](O)C)C1=CC=CC=C1 NHXLMOGPVYXJNR-ATOGVRKGSA-N 0.000 description 1
- 229960000553 somatostatin Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000004114 suspension culture Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 238000012353 t test Methods 0.000 description 1
- BFKJFAAPBSQJPD-UHFFFAOYSA-N tetrafluoroethene Chemical compound FC(F)=C(F)F BFKJFAAPBSQJPD-UHFFFAOYSA-N 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000003039 volatile agent Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/067—Hepatocytes
- C12N5/0671—Three-dimensional culture, tissue culture or organ culture; Encapsulated cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M21/00—Bioreactors or fermenters specially adapted for specific uses
- C12M21/08—Bioreactors or fermenters specially adapted for specific uses for producing artificial tissue or for ex-vivo cultivation of tissue
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M23/00—Constructional details, e.g. recesses, hinges
- C12M23/02—Form or structure of the vessel
- C12M23/16—Microfluidic devices; Capillary tubes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0688—Cells from the lungs or the respiratory tract
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/5014—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics for testing toxicity
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/5044—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics involving specific cell types
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/5044—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics involving specific cell types
- G01N33/5067—Liver cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5008—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for testing or evaluating the effect of chemical or biological compounds, e.g. drugs, cosmetics
- G01N33/5082—Supracellular entities, e.g. tissue, organisms
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/14—Coculture with; Conditioned medium produced by hepatocytes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/27—Lung cells, respiratory tract cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2513/00—3D culture
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Immunology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- General Health & Medical Sciences (AREA)
- Cell Biology (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- General Engineering & Computer Science (AREA)
- Toxicology (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Food Science & Technology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Sustainable Development (AREA)
- Gastroenterology & Hepatology (AREA)
- Pulmonology (AREA)
- Dispersion Chemistry (AREA)
- Clinical Laboratory Science (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Abstract
Description
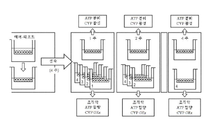
도 2. 간 구상체(GEx: 유전자 발현)의 특성화를 위한 실험 설계 및 종점의 개략적 개요.
도 3. 성숙 이후 1, 2, 3 및 4 주에 3차원 기관형 폐 배양물의 헤마톡실린 & 에오신(Hematoxylin & eosin) 및 알시안 블루(Alcian blue) 염색. 상피의 대표적인 횡단면은 20x 배율로 도시된다.
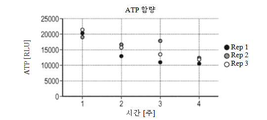
도 4. 3차원 기관형 폐 배양물의 ATP 함량은 성숙 이후 1, 2, 3 및 4 주에 측정된다. 3 가지 독립적인 측정 결과가 도시된다. Rep: 복제; RLU: 상대적인 광 유닛
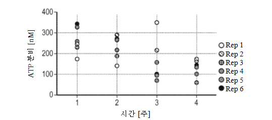
도 5. 3차원 기관형 폐 배양물의 선단 표면 액체로의 ATP 분비는 성숙 이후 1, 2, 3 및 4 주에 저장성 염류(hypotonic saline)에 의한 자극에 반응하여 측정된다. 6 가지 독립적인 측정 결과가 도시된다. Rep: 복제
도 6. 시토크롬 P450 1A1/B1 활성은 성숙 이후 1, 2, 3 및 4 주에 3차원 기관형 폐 배양물에서 측정된다. 결과는 기초 CYP 활성, TCDD 및 리팜피신으로 48 시간 처리 이후 유도된 활성, 및 α-나프토플라본에 의한 억제 이후의 유도된 활성을 도시한다. 결과는 3 가지 독립적인 실험의 평균 ± SEM으로 제시된다. 점선은 시간 지점 당 평균값에 기초한 추세를 나타낸다.
도 7. 3차원 기관형 폐 배양물에서 1상 약물 대사 효소-암호화 유전자의 발현에 대한 폴드 조절이 1, 2, 3 및 4 주에서 평가된다. 유전자 발현은 48 시간 동안 TCDD와 리팜피신(rifampicin)으로 처리된 폐 배양물(n=3)과 미처리된 배양물(n=3) 사이에서 비교되며, 폴드 변경이 ΔΔCT 방법을 사용하여 계산된다. 히트맵은 폴드 변화와 함께 20 개의 가장 위쪽(적색, 왼쪽) 및 가장 아래쪽으로 조절된 유전자(청색, 오른쪽)를 나열한다. 유전자 기호는 히트맵의 좌측에 표시된다.
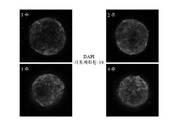
도 8. 간 구상체는 성숙 이후 1, 2, 3 및 4 주에서 시토케라틴 19 발현 동안 염색된다. 10x 배율로 의사 컬러(pseudocolor)(적색의 CK19, 청색의 핵)의 대표 이미지가 도시된다.
도 9. 간 구상체 및 그들의 조건부 배지의 총 ATP 함량은 성숙 이후 1, 2, 3 및 4 주에 측정된다. 3 가지 독립적인 측정 결과가 도시된다. Rep. 복제
도 10. 간 구상체로부터 알부민 분비는 성숙 이후 1, 2, 3 및 4 주에 정량화된다 8 가지 독립적인 측정 결과가 도시된다. 점선은 시간 지점 당 평균값에 기초한 추세를 나타낸다. Rep: 복제; 간 구상체에 의한 알파-GST 제조는 성숙 이후 1, 2, 3 및 4 주에 평가된다. 8 가지 독립적인 측정 결과가 도시된다. Rep: 복제; 시토크롬 P450 1A1/B1 활성은 성숙 이후 1, 2, 3 및 4 주에 간 구상체에서 측정된다. 결과는 기초 CYP 활성, TCDD 및 리팜피신으로 48 시간 처리 이후 유도된 활성, 및 α-나프토플라본에 의한 억제 이후의 유도된 활성을 도시한다. 결과는 3 가지 독립적인 실험의 평균 ± SEM으로 제시되며; 시토크롬 P450 1A2 활성은 성숙 이후 1, 2, 3 및 4 주에 간 구상체에서 측정된다. 결과는 기초 CYP 활성, TCDD 및 리팜피신으로 48 시간 유도 이후 유도된 활성, 및 플루복사민 말레에이트에 의한 억제 이후 유도된 활성을 도시한다. 결과는 3 가지 독립적인 실험의 평균 ± SEM으로 제시된다.
도 11. 간 구상체 및 그들의 조건부 배지의 총 ATP 함량은 윌리엄스 E와 완전한 B-ALI 배지 또는 완전한 PneumaCult-ALI 배지(StemCell Technologies, 그르노블, 프랑스)의 혼합물에서 배양 이후에 측정된다. 결과는 5 가지 독립적인 측정에 대한 평균 ± SEM으로서 제시되며; 세포 독성은 윌리엄스 E와 완전한 B-ALI 배지 또는 완전한 PneumaCult-ALI 배지의 혼합물에서 배양 이후 간 구상체에서 측정된다. 결과는 5 가지 독립적인 측정에 대한 평균 ± SEM으로서 제시된다. RFU: 상대 형광 유닛
도 12. 세포사멸은 윌리엄스 E와 완전한 B-ALI 배지 또는 완전한 PneumaCult-ALI 배지 배지의 혼합물에서 배양 이후 간 구상체에서 측정된다. 결과는 5 가지 독립적인 측정에 대한 평균 ± SEM으로서 제시되며; CYP1A1/B1 활성은 윌리엄스 E 및 완전한 B-ALI 배지 또는 완전한 PneumaCult-ALI 배지의 혼합물에서 배양 이후 간 구상체에서 측정된다. 결과는 3 가지 독립적인 측정에 대한 평균 ± SEM으로서 제시된다.
Claims (15)
- 분리된 3차원 간 구상체로서, 상기 구상체는:
구상체가 완전한 윌리엄스 E 배지에서 단독으로 배양된 3차원 간 구상체와 비교하여 증가된 ATP 함량;
완전한 윌리엄스 E 배지에서 단독으로 배양된 3차원 간 구상체와 비교하여 시토크롬 P450 1A1 및 시토크롬 P450 1B1의 동일한 또는 증가된 활성; 및
윌리엄스 E 배지에서 단독으로 배양된 3차원 간 구상체와 비교하여 증가된 알부민 분비를 가지는, 분리된 3차원 간 구상체. - 공정에 의해 얻어지거나 얻어질 수 있는 3차원 다중-기관 배양 시스템에 사용하기 위한 분리된 3차원 간 구상체로서:
상기 공정은 3차원 간 구상체를 얻는 데 충분한 기간 동안 세포 배양 배지에서 3차원 간 구상체를 배양하는 단계를 포함하며, 상기 세포 배양 배지는: (a) 완전한 PneumaCult-ALI 배지; 또는 (b) 완전한 B-ALI 배지; 또는 (c) 완전한 PneumaCult-ALI 배지 및 완전한 윌리엄스 E 배지, 또는 (d) 완전한 B-ALI 배지 및 완전한 윌리엄스 E 배지를 포함하거나, 이들로 구성되거나 필수적으로 구성되며, 상기 3차원 간 구상체에서:
ATP 함량은 완전한 윌리엄스 E 배지에서 단독으로 배양된 3차원 간 구상체와 비교하여 증가되며;
시토크롬 P450 1A1 및 시토크롬 P450 1B1 활성은 완전한 윌리엄스 E 배지에서 단독으로 배양된 3차원 간 구상체와 비교하여 동일하거나 증가되며;
알부민 분비는 윌리엄스 E 배지에서 단독으로 배양된 3차원 간 구상체와 비교하여 증가되는, 분리된 3차원 간 구상체. - 제1항 또는 제2항에 있어서, 상기 구상체가 인간 간 전구 세포주이거나 이로부터 유래되며, 적합하게, 상기 구상체가 HepaRG 세포이거나 이로부터 유래되는, 분리된 3차원 간 구상체.
- 제1항 또는 제3항에 있어서, 구상체가 세포 배양 배지에서 배양되며, 상기 세포 배양 배지가: (a) 완전한 PneumaCult-ALI 배지; 또는 (b) 완전한 B-ALI 배지; 또는 (c) 완전한 PneumaCult-ALI 배지 및 완전한 윌리엄스 E 배지, 또는 (d) 완전한 B-ALI 배지 및 완전한 윌리엄스 E 배지를 포함하거나, 이들로 구성되거나 필수적으로 구성되는, 분리된 3차원 간 구상체.
- 제1 내지 제4항 중 어느 한 항에 따른 3차원 간 구상체 및 3차원 폐 상피 세포를 포함하는 공동 배양물로서, 바람직하게,
상기 공동 배양물이 세포 배양 배지에서 유지되며, 상기 세포 배양 배지가: (a) 완전한 PneumaCult-ALI 배지; 또는 (b) 완전한 B-ALI 배지; 또는 (c) 완전한 PneumaCult-ALI 배지 및 완전한 윌리엄스 E 배지, 또는 (d) 완전한 B-ALI 배지 및 완전한 윌리엄스 E 배지를 포함하거나, 이들로 구성되거나 필수적으로 구성되는, 공동 배양물. - 3차원 기관 배양 시스템으로서, 제1항 내지 제4항 중 어느 한 항에 따른 분리된 3차원 간 구상체; 또는 제1항 내지 제4항 중 어느 한 항에 따른 분리된 3차원 간 구상체를 포함하는 3차원 다중-기관 배양 시스템을 포함하며, 적어도 하나의 다른 3차원 세포 유형 또는 제5항에 따른 공동 배양물을 더 포함하며, 바람직하게,
3차원 간 구상체가 배양 시스템에 함유된 배양 배지 내에 침지되며/침지되거나;
3차원 폐 상피 세포를 더 포함하며, 적합하게, 상기 3차원 폐 상피 세포가 3차원 다중-기관 배양 시스템의 공기 액체 계면에 있는, 3차원 기관 배양 시스템. - 3차원 다중-기관 배양 시스템으로서:
(a) 배양 배지에서 제1 3차원 세포 유형을 침지시키도록 적응되는 제1 기관 공동을 포함하는 제1 기관 성장 구간;
(b) 공기 액체 계면에서 제2 3차원 세포 유형을 배양하도록 적응되는 제2 기관 공동을 포함하고, 이때 제2 3차원 세포 유형은 제1 3차원 세포 유형과 상이한 제2 기관 성장 구간; 및
(c) 제1 기관 공동과 제2 기관 공동을 연결하여 그 사이에서 배양 배지의 유동을 허용하는 배양 배지 저장소를 포함하며; 바람직하게,
제1 기관 공동 및 제2 기관 공동은 동일한 배양 배지를 함유하며/함유하거나;
제5항에 따른 공동 배양물을 더 포함하며/포함하거나;
상기 시스템은 소형화되며/소형화되거나;
상기 시스템은 미세유체공학 장치를 포함하거나 미세유체공학 장치이며, 바람직하게 상기 시스템은 인공 생체칩인, 3차원 다중-기관 배양 시스템. - 세포 배양 배지로서: (a) 완전한 PneumaCult-ALI 배지와 완전한 윌리엄스 E 배지의 혼합물; 또는 (b) 완전한 B-ALI 배지와 완전한 윌리엄스 E 배지의 혼합물을 포함하거나, 이들로 구성되거나 필수적으로 구성되며; 바람직하게,
제1항 내지 제4항 중 어느 한 항에 따른 3차원 간 구상체 또는 제5항에 따른 공동 배양물을 더 포함하는, 세포 배양 배지. - 배양 배지를 포함하는 3차원 다중-기관 배양 시스템으로서, 상기 배양 배지는: (a) 완전한 PneumaCult-ALI 배지; 또는 (b) 완전한 B-ALI 배지; 또는 (c) 완전한 PneumaCult-ALI 배지 및 완전한 윌리엄스 E 배지, 또는 (d) 완전한 B-ALI 배지 및 완전한 윌리엄스 E 배지 중 어느 하나 또는 이의 두 개 이상의 조합을 포함하거나, 이들로 구성되거나 필수적으로 구성되는 배양 배지로 구성되는 그룹으로부터 선택되는, 3차원 다중-기관 배양 시스템.
- 3차원 기관 배양 시스템에 사용하기 위한 3차원 간 구상체를 준비하는 방법으로서:
(i) 3차원 간 구상체를 제공하는 단계;
(ii) (a) 완전한 PneumaCult-ALI 배지; 또는 (b) 완전한 B-ALI 배지; 또는 (c) 완전한 PneumaCult-ALI 배지 및 완전한 윌리엄스 E 배지, 또는 (d) 완전한 B-ALI 배지 및 완전한 윌리엄스 E 배지를 포함하거나 이들로 구성되거나 필수적으로 구성되는 배양 배지와 3차원 간 구상체를 접촉시키는 단계;
(iii) 및 3차원 기관 배양 시스템에 사용하기 위한 3차원 간 구상체를 얻는 단계를 포함하는, 3차원 기관 배양 시스템에 사용하기 위한 3차원 간 구상체를 준비하는 방법. - 3차원 다중-기관 배양 시스템에 사용하기 위한 3차원 간 구상체 및 3차원 폐 상피 세포를 포함하거나 이들로 구성되거나 필수적으로 구성되는 공동 배양물을 준비하는 방법으로서:
(i) 3차원 간 구상체 및 3차원 폐 상피 세포를 제공하는 단계;
(ii) (a) 완전한 PneumaCult-ALI 배지; 또는 (b) 완전한 B-ALI 배지; 또는 (c) 완전한 PneumaCult-ALI 배지 및 완전한 윌리엄스 E 배지, 또는 (d) 완전한 B-ALI 배지 및 완전한 윌리엄스 E 배지를 포함하거나 이들로 구성되거나 필수적으로 구성되는 배양 배지와 3차원 간 구상체 및 3차원 폐 상피 세포를 접촉시키는 단계; 및
(iii) 3차원 간 구상체 및 3차원 폐 상피 세포의 공동 배양물을 얻는 단계를 포함하는, 공동 배양물을 준비하는 방법. - 제제에 대한 3차원 간 구상체의 반응을 평가하기 위한 시험관내 방법으로서:
(i) 적어도 하나의 제제와 제1항 내지 제4항 중 어느 한 항에 따른 3차원 간 구상체 또는 제5항에 따른 공동 배양물 또는 제6항에 따른 3차원 기관 배양 시스템, 또는 제7항 또는 제9항에 따른 3차원 다중-기관 배양 시스템을 접촉시키는 단계; 및
(ii) 적어도 하나의 제제와 접촉한 후 3차원 간 구상체 또는 공동 배양물 또는 3차원 기관 배양 시스템 또는 3차원 다중-기관 배양 시스템의 하나 이상의 반응을 측정하는 단계를 포함하며;
적어도 하나의 제제와의 접촉 전후의 적어도 하나의 반응의 차이는 제제가 세포의 반응을 조절하는 표시인, 제에 대한 3차원 간 구상체의 반응을 평가하기 위한 시험관내 방법. - 제제에 대한 3차원 간 구상체 및 3차원 폐 상피 세포의 반응을 평가하는 시험관내 방법으로서:
(i) 적어도 하나의 제제와 제5항에 따른 공동 배양물 또는 제6항에 따른 3차원 기관 배양 시스템 또는 제7항 내지 제9항 중 어느 한 항에 따른 3차원 다중-기관 배양 시스템을 접촉시키는 단계; 및
(ii) 적어도 하나의 제제와의 접촉 후 공동 배양물 또는 3차원 기관 배양 시스템 또는 3차원 다중-기관 배양 시스템의 하나 이상의 반응을 측정하는 단계를 포함하며;
적어도 하나의 제제와의 접촉 전후의 하나 이상의 반응의 차이는 제제가 세포의 반응을 조절하는 표시이며; 바람직하게,
상기 단계 (ii)는 3차원 폐 상피 세포 내로 적어도 하나의 제제 침투를 측정하는 것을 포함하며/포함하거나;
상기 방법은: (iii) 3차원 간 구상체에서 적어도 하나의 제제의 생체-활성화를 측정하는 단계를 더 포함하며; 상기 단계 (ii) 및 (iii)에서의 측정은 동시에 수행되거나 단계 (iii)에서의 측정은 단계 (ii)에서의 측정 후에 수행되며/수행되거나;
상기 제제는 에어로졸이며, 적합하게 상기 에어로졸은 연기, 적합하게 담배 연기이거나 그로부터 유래하는, 제제에 대한 3차원 간 구상체 및 3차원 폐 상피 세포의 반응을 평가하는 시험관내 방법. - 세포 배양 배지의 용도로서: 3차원 간 구상체 또는 3차원 폐 상피 세포를 배양하거나 3차원 간 구상체 및 3차원 폐 상피 세포를 공동 배양하기 위한, (a) 완전한 PneumaCult-ALI 배지; 또는 (b) 완전한 B-ALI 배지; (c) 완전한 PneumaCult-ALI 배지 및 완전한 윌리엄스 E 배지, 또는 (d) 완전한 B-ALI 배지 및 완전한 윌리엄스 E 배지를 포함하거나 이들로 구성되거나 필수적으로 구성되는, 세포 배양 배지의 용도.
- 독성 시험을 위한 또는 약물 발견을 위한, 또는 폐 세포 내로의 약물 침투를 결정하기 위한 및/또는 적합하게 제제가 에어로졸인, 간세포에서 제제의 생체-활성화를 결정하기 위한 제6항에 따른 3차원 기관 배양 시스템 또는 제7항 또는 제9항에 따른 3차원 다중-기관 배양 시스템의 용도.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP16185725.5A EP3287521B1 (en) | 2016-08-25 | 2016-08-25 | Cell culture |
| EP16185725.5 | 2016-08-25 | ||
| PCT/EP2017/070881 WO2018036910A1 (en) | 2016-08-25 | 2017-08-17 | Cell culture |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180049000A true KR20180049000A (ko) | 2018-05-10 |
Family
ID=56842661
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187009401A KR20180049000A (ko) | 2016-08-25 | 2017-08-17 | 세포 배양 |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US11041145B2 (ko) |
| EP (2) | EP3940059A1 (ko) |
| JP (2) | JP6810742B2 (ko) |
| KR (1) | KR20180049000A (ko) |
| CN (1) | CN108138135A (ko) |
| PL (1) | PL3287521T3 (ko) |
| RU (1) | RU2018111219A (ko) |
| WO (1) | WO2018036910A1 (ko) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20210189346A1 (en) | 2018-05-16 | 2021-06-24 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Method of culturing proliferative hepatocytes |
| WO2020172337A1 (en) * | 2019-02-19 | 2020-08-27 | Iontox, Llc | Methods to predict repeated dose toxicity using an integrated organ platform |
| CN113699202A (zh) * | 2020-05-21 | 2021-11-26 | 华子昂 | 一种用混合细胞与人工细胞培养巢制备胶原蛋白的方法 |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2748812B1 (fr) | 1996-05-20 | 1999-06-18 | Sumitomo Chemical Co | Trousse de diagnostic et procede pour determiner la concentration d'un cytochrome p450 |
| GB9913979D0 (en) | 1999-06-17 | 1999-08-18 | Univ Wales Medicine | Spheroid preparation |
| US7351584B2 (en) | 2002-02-26 | 2008-04-01 | In Vitro Technologies, Inc. | Hepatocyte bioreactor system for long term culture of functional hepatocyte spheroids |
| US7220839B2 (en) | 2002-03-25 | 2007-05-22 | Japan Science And Technology Agency | Antibody recognizing proliferative human liver cells, proliferative human liver cells and functional human liver cells |
| US7753192B2 (en) | 2005-12-23 | 2010-07-13 | The Coca-Cola Company | Apparatus and method for orienting spheroidal containers and packaging beverages in spheroidal containers |
| JP5217220B2 (ja) | 2007-04-12 | 2013-06-19 | 株式会社日立製作所 | 細胞分離装置 |
| JP2010534065A (ja) * | 2007-07-20 | 2010-11-04 | セルアーティス アーベー | ヒト胚盤胞幹細胞に、胚体内胚葉(DE‐hep)を介して由来する新規な肝細胞の集団 |
| WO2010042669A2 (en) | 2008-10-07 | 2010-04-15 | Ipierian, Inc. | Co-culture compositions and methods |
| KR101370222B1 (ko) | 2009-01-08 | 2014-03-05 | 가부시키가이샤 히타치세이사쿠쇼 | 동물 간 세포의 배양 방법 |
| GB201104511D0 (en) * | 2011-03-17 | 2011-05-04 | Univ Cardiff | Cell based screening assay |
| JP5812816B2 (ja) | 2011-11-15 | 2015-11-17 | コバレントマテリアル株式会社 | 細胞培養担体及びその製造方法 |
| DE102011121317A1 (de) | 2011-12-16 | 2013-06-20 | Universitätsklinikum Hamburg-Eppendorf | Gerüstfreie Leberzellaggregate zur Verwendung bei der Transplantation |
| CN102559581B (zh) * | 2012-01-20 | 2014-09-10 | 四川大学华西医院 | 一种无血清肝细胞培养基 |
| US10677783B2 (en) | 2012-09-27 | 2020-06-09 | Corning Incorporated | Method for evaluating effect of cytokine on metabolic activity of cytochrome P450, and drug screening method |
| US20140106356A1 (en) | 2012-10-12 | 2014-04-17 | National Taiwan University | Kit for drug metabolism determination and toxicity prediction |
| US9442105B2 (en) * | 2013-03-15 | 2016-09-13 | Organovo, Inc. | Engineered liver tissues, arrays thereof, and methods of making the same |
| US11447743B2 (en) | 2014-04-22 | 2022-09-20 | Nippon Shokubai Co., Ltd. | Cell culture substrate comprising fluorine-containing polymer on its surface |
| JP6681664B2 (ja) | 2014-09-05 | 2020-04-15 | 株式会社日本触媒 | 含フッ素ポリイミドを含む酸素ガス透過性の細胞培養用基材、該基材を備えた細胞培養用容器、及び、該基材を用いた細胞培養方法 |
-
2016
- 2016-08-25 EP EP21180293.9A patent/EP3940059A1/en active Pending
- 2016-08-25 PL PL16185725T patent/PL3287521T3/pl unknown
- 2016-08-25 EP EP16185725.5A patent/EP3287521B1/en active Active
-
2017
- 2017-08-17 CN CN201780003424.5A patent/CN108138135A/zh active Pending
- 2017-08-17 KR KR1020187009401A patent/KR20180049000A/ko not_active Application Discontinuation
- 2017-08-17 US US15/757,415 patent/US11041145B2/en active Active
- 2017-08-17 RU RU2018111219A patent/RU2018111219A/ru unknown
- 2017-08-17 JP JP2018519751A patent/JP6810742B2/ja active Active
- 2017-08-17 WO PCT/EP2017/070881 patent/WO2018036910A1/en active Application Filing
-
2020
- 2020-12-11 JP JP2020205752A patent/JP7097942B2/ja active Active
-
2021
- 2021-05-12 US US17/318,056 patent/US11981926B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| WO2018036910A1 (en) | 2018-03-01 |
| PL3287521T3 (pl) | 2021-12-20 |
| JP7097942B2 (ja) | 2022-07-08 |
| EP3287521B1 (en) | 2021-06-23 |
| US11041145B2 (en) | 2021-06-22 |
| JP2019506134A (ja) | 2019-03-07 |
| EP3287521A1 (en) | 2018-02-28 |
| EP3940059A1 (en) | 2022-01-19 |
| US20210269774A1 (en) | 2021-09-02 |
| US11981926B2 (en) | 2024-05-14 |
| US20190119647A1 (en) | 2019-04-25 |
| RU2018111219A (ru) | 2019-09-30 |
| CN108138135A (zh) | 2018-06-08 |
| JP6810742B2 (ja) | 2021-01-06 |
| RU2018111219A3 (ko) | 2019-09-30 |
| JP2021048865A (ja) | 2021-04-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Rödling et al. | 3D models of the hematopoietic stem cell niche under steady-state and active conditions | |
| Bray et al. | Addressing patient specificity in the engineering of tumor models | |
| JP7097942B2 (ja) | 細胞培養 | |
| JP2021019602A (ja) | 操作した肝臓組織、そのアレイ、およびそれを製造する方法 | |
| JP6694512B2 (ja) | 幹細胞由来ヒト肝細胞を使用した微小組織形成 | |
| CA2968655C (en) | Methods for generation of podocytes from pluripotent stem cells and cells produced by the same | |
| CN111032853B (zh) | 用于体外暴露的细胞培养板、装置和方法 | |
| JP7427589B2 (ja) | 改善された細胞培養装置 | |
| Yang et al. | Flourishing tumor organoids: History, emerging technology, and application | |
| WO2014200997A2 (en) | Method for preparing three-dimensional, organotypic cell cultures and uses thereof | |
| WO2013138348A1 (en) | Systems and methods for studying inflammation-drug interactions | |
| JP2022517179A (ja) | 穿孔構造 | |
| WO2014188888A1 (ja) | 細胞培養方法、粒子状培養担体、及び粒子包含細胞凝集体 | |
| CN118613577A (zh) | 类器官的制备方法及其用途 | |
| US20130130374A1 (en) | Cellular compositions and methods for their preparation and use | |
| RU2810805C2 (ru) | Перфорированная конструкция | |
| JP2020505076A (ja) | 肝細胞の共培養を含む組成物および方法 | |
| WO2021221166A1 (ja) | 多列繊毛気道上皮細胞、分化誘導方法および製造方法 | |
| Greier | Metabolic Stress and Epithelial-Mesenchymal Transition in Head and Neck Cancer | |
| Yang et al. | Effects of degree of cell-cell contact on liver specific functions of rat primary hepatocytes | |
| Baptista | c12) United States Patent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20180403 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20180418 Comment text: Request for Examination of Application |
|
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20190315 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20190521 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20190315 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |