KR20170102050A - 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 - Google Patents
알파-멜라노사이트 자극 호르몬의 펩티드 유사체 Download PDFInfo
- Publication number
- KR20170102050A KR20170102050A KR1020177023883A KR20177023883A KR20170102050A KR 20170102050 A KR20170102050 A KR 20170102050A KR 1020177023883 A KR1020177023883 A KR 1020177023883A KR 20177023883 A KR20177023883 A KR 20177023883A KR 20170102050 A KR20170102050 A KR 20170102050A
- Authority
- KR
- South Korea
- Prior art keywords
- xaa
- msh
- ser
- ala
- peptide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 230
- WHNFPRLDDSXQCL-UAZQEYIDSA-N α-msh Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(N)=O)NC(=O)[C@H](CO)NC(C)=O)C1=CC=C(O)C=C1 WHNFPRLDDSXQCL-UAZQEYIDSA-N 0.000 title abstract description 290
- 102100027467 Pro-opiomelanocortin Human genes 0.000 title description 29
- 101800001751 Melanocyte-stimulating hormone alpha Proteins 0.000 title description 8
- 238000011282 treatment Methods 0.000 claims abstract description 85
- 102000008314 Type 1 Melanocortin Receptor Human genes 0.000 claims abstract description 84
- 108010021428 Type 1 Melanocortin Receptor Proteins 0.000 claims abstract description 83
- 238000000034 method Methods 0.000 claims abstract description 42
- 150000001875 compounds Chemical class 0.000 claims description 88
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 70
- QNAYBMKLOCPYGJ-UWTATZPHSA-N D-alanine Chemical compound C[C@@H](N)C(O)=O QNAYBMKLOCPYGJ-UWTATZPHSA-N 0.000 claims description 68
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 67
- 201000010099 disease Diseases 0.000 claims description 65
- MTCFGRXMJLQNBG-UWTATZPHSA-N D-Serine Chemical compound OC[C@@H](N)C(O)=O MTCFGRXMJLQNBG-UWTATZPHSA-N 0.000 claims description 63
- -1 D-Nle Chemical compound 0.000 claims description 47
- 229920001184 polypeptide Polymers 0.000 claims description 45
- KDXKERNSBIXSRK-RXMQYKEDSA-N D-lysine Chemical compound NCCCC[C@@H](N)C(O)=O KDXKERNSBIXSRK-RXMQYKEDSA-N 0.000 claims description 31
- AGPKZVBTJJNPAG-RFZPGFLSSA-N D-Isoleucine Chemical compound CC[C@@H](C)[C@@H](N)C(O)=O AGPKZVBTJJNPAG-RFZPGFLSSA-N 0.000 claims description 30
- COLNVLDHVKWLRT-MRVPVSSYSA-N D-phenylalanine Chemical compound OC(=O)[C@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-MRVPVSSYSA-N 0.000 claims description 30
- 230000001225 therapeutic effect Effects 0.000 claims description 30
- 206010061218 Inflammation Diseases 0.000 claims description 28
- 230000004054 inflammatory process Effects 0.000 claims description 26
- 201000001441 melanoma Diseases 0.000 claims description 26
- 239000008194 pharmaceutical composition Substances 0.000 claims description 26
- ODKSFYDXXFIFQN-SCSAIBSYSA-N D-arginine Chemical compound OC(=O)[C@H](N)CCCNC(N)=N ODKSFYDXXFIFQN-SCSAIBSYSA-N 0.000 claims description 25
- HNDVDQJCIGZPNO-RXMQYKEDSA-N D-histidine Chemical compound OC(=O)[C@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-RXMQYKEDSA-N 0.000 claims description 25
- QIVBCDIJIAJPQS-SECBINFHSA-N D-tryptophane Chemical compound C1=CC=C2C(C[C@@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-SECBINFHSA-N 0.000 claims description 24
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 20
- ONIBWKKTOPOVIA-SCSAIBSYSA-N D-Proline Chemical compound OC(=O)[C@H]1CCCN1 ONIBWKKTOPOVIA-SCSAIBSYSA-N 0.000 claims description 19
- 239000003795 chemical substances by application Substances 0.000 claims description 19
- KZSNJWFQEVHDMF-SCSAIBSYSA-N D-valine Chemical compound CC(C)[C@@H](N)C(O)=O KZSNJWFQEVHDMF-SCSAIBSYSA-N 0.000 claims description 17
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 17
- 150000003839 salts Chemical class 0.000 claims description 17
- WHUUTDBJXJRKMK-GSVOUGTGSA-N D-glutamic acid Chemical compound OC(=O)[C@H](N)CCC(O)=O WHUUTDBJXJRKMK-GSVOUGTGSA-N 0.000 claims description 15
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 15
- FFEARJCKVFRZRR-SCSAIBSYSA-N D-methionine Chemical compound CSCC[C@@H](N)C(O)=O FFEARJCKVFRZRR-SCSAIBSYSA-N 0.000 claims description 14
- OUYCCCASQSFEME-MRVPVSSYSA-N D-tyrosine Chemical compound OC(=O)[C@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-MRVPVSSYSA-N 0.000 claims description 13
- AHLPHDHHMVZTML-SCSAIBSYSA-N D-Ornithine Chemical compound NCCC[C@@H](N)C(O)=O AHLPHDHHMVZTML-SCSAIBSYSA-N 0.000 claims description 12
- 229940127089 cytotoxic agent Drugs 0.000 claims description 12
- 238000000338 in vitro Methods 0.000 claims description 12
- 208000023275 Autoimmune disease Diseases 0.000 claims description 11
- 150000008575 L-amino acids Chemical class 0.000 claims description 11
- RHGKLRLOHDJJDR-SCSAIBSYSA-N D-citrulline Chemical compound OC(=O)[C@H](N)CCCNC(N)=O RHGKLRLOHDJJDR-SCSAIBSYSA-N 0.000 claims description 9
- 201000004982 autoimmune uveitis Diseases 0.000 claims description 9
- 210000004899 c-terminal region Anatomy 0.000 claims description 9
- CKLJMWTZIZZHCS-UHFFFAOYSA-N D-OH-Asp Natural products OC(=O)C(N)CC(O)=O CKLJMWTZIZZHCS-UHFFFAOYSA-N 0.000 claims description 8
- CKLJMWTZIZZHCS-UWTATZPHSA-N D-aspartic acid Chemical compound OC(=O)[C@H](N)CC(O)=O CKLJMWTZIZZHCS-UWTATZPHSA-N 0.000 claims description 8
- 230000002401 inhibitory effect Effects 0.000 claims description 8
- LAXXPOJCFVMVAX-SSDOTTSWSA-N (2r)-2-azaniumyl-4-butylsulfanylbutanoate Chemical compound CCCCSCC[C@@H]([NH3+])C([O-])=O LAXXPOJCFVMVAX-SSDOTTSWSA-N 0.000 claims description 7
- 108091005804 Peptidases Proteins 0.000 claims description 6
- 239000004365 Protease Substances 0.000 claims description 6
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 claims description 6
- 230000015556 catabolic process Effects 0.000 claims description 6
- 238000006731 degradation reaction Methods 0.000 claims description 6
- 239000003053 toxin Substances 0.000 claims description 6
- 231100000765 toxin Toxicity 0.000 claims description 6
- 230000001093 anti-cancer Effects 0.000 claims description 5
- 230000008878 coupling Effects 0.000 claims description 5
- 238000010168 coupling process Methods 0.000 claims description 5
- 238000005859 coupling reaction Methods 0.000 claims description 5
- QADCERNTBWTXFV-JSGCOSHPSA-N Arg-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCCNC(N)=N)N)C(O)=O)=CNC2=C1 QADCERNTBWTXFV-JSGCOSHPSA-N 0.000 claims description 4
- 206010020751 Hypersensitivity Diseases 0.000 claims description 4
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 4
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 claims description 4
- 230000007815 allergy Effects 0.000 claims description 4
- 239000002246 antineoplastic agent Substances 0.000 claims description 4
- 206010003246 arthritis Diseases 0.000 claims description 4
- 201000006417 multiple sclerosis Diseases 0.000 claims description 4
- 206010039073 rheumatoid arthritis Diseases 0.000 claims description 4
- 208000032467 Aplastic anaemia Diseases 0.000 claims description 3
- UGJLILSJKSBVIR-ZFWWWQNUSA-N Arg-Trp-Gly Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCCN=C(N)N)N)C(=O)NCC(O)=O)=CNC2=C1 UGJLILSJKSBVIR-ZFWWWQNUSA-N 0.000 claims description 3
- 201000001320 Atherosclerosis Diseases 0.000 claims description 3
- 208000015943 Coeliac disease Diseases 0.000 claims description 3
- 208000011231 Crohn disease Diseases 0.000 claims description 3
- 208000007882 Gastritis Diseases 0.000 claims description 3
- 208000015023 Graves' disease Diseases 0.000 claims description 3
- YSPZCHGIWAQVKQ-AVGNSLFASA-N Lys-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCCN YSPZCHGIWAQVKQ-AVGNSLFASA-N 0.000 claims description 3
- PQPMMGQTRQFSDA-SRVKXCTJSA-N Met-Glu-His Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CNC=N1)C(O)=O PQPMMGQTRQFSDA-SRVKXCTJSA-N 0.000 claims description 3
- 201000004681 Psoriasis Diseases 0.000 claims description 3
- 206010028417 myasthenia gravis Diseases 0.000 claims description 3
- 208000031225 myocardial ischemia Diseases 0.000 claims description 3
- 201000008482 osteoarthritis Diseases 0.000 claims description 3
- 108010071207 serylmethionine Proteins 0.000 claims description 3
- 238000002054 transplantation Methods 0.000 claims description 3
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 2
- 208000023328 Basedow disease Diseases 0.000 claims description 2
- 125000000734 D-serino group Chemical group [H]N([H])[C@@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 claims description 2
- 208000026935 allergic disease Diseases 0.000 claims description 2
- 239000003504 photosensitizing agent Substances 0.000 claims description 2
- 239000002534 radiation-sensitizing agent Substances 0.000 claims description 2
- GVGLGOZIDCSQPN-PVHGPHFFSA-N Heroin Chemical compound O([C@H]1[C@H](C=C[C@H]23)OC(C)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4OC(C)=O GVGLGOZIDCSQPN-PVHGPHFFSA-N 0.000 claims 1
- 102400000740 Melanocyte-stimulating hormone alpha Human genes 0.000 abstract description 259
- 101710200814 Melanotropin alpha Proteins 0.000 abstract description 251
- 239000000825 pharmaceutical preparation Substances 0.000 abstract description 2
- 239000000637 Melanocyte-Stimulating Hormone Substances 0.000 abstract 1
- 241000699670 Mus sp. Species 0.000 description 111
- 210000004027 cell Anatomy 0.000 description 63
- 125000005647 linker group Chemical group 0.000 description 58
- 239000002953 phosphate buffered saline Substances 0.000 description 55
- 241000699666 Mus <mouse, genus> Species 0.000 description 52
- 230000000694 effects Effects 0.000 description 47
- 230000027455 binding Effects 0.000 description 40
- 239000000203 mixture Substances 0.000 description 40
- 230000002354 daily effect Effects 0.000 description 39
- 239000003814 drug Substances 0.000 description 35
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 34
- 229920001223 polyethylene glycol Polymers 0.000 description 29
- 102100040247 Tumor necrosis factor Human genes 0.000 description 28
- 235000001014 amino acid Nutrition 0.000 description 28
- 229940024606 amino acid Drugs 0.000 description 28
- 150000001413 amino acids Chemical class 0.000 description 27
- 108010007013 Melanocyte-Stimulating Hormones Proteins 0.000 description 26
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 25
- 102000003814 Interleukin-10 Human genes 0.000 description 25
- 108090000174 Interleukin-10 Proteins 0.000 description 25
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 25
- 239000002158 endotoxin Substances 0.000 description 25
- 229940076144 interleukin-10 Drugs 0.000 description 25
- 239000002202 Polyethylene glycol Substances 0.000 description 24
- 206010046851 Uveitis Diseases 0.000 description 24
- 108020004999 messenger RNA Proteins 0.000 description 24
- 210000000952 spleen Anatomy 0.000 description 24
- 229920006008 lipopolysaccharide Polymers 0.000 description 23
- ORQXBVXKBGUSBA-UHFFFAOYSA-N cyclohexyl D-alanine Natural products OC(=O)C(N)CC1CCCCC1 ORQXBVXKBGUSBA-UHFFFAOYSA-N 0.000 description 22
- 201000002491 encephalomyelitis Diseases 0.000 description 20
- 210000002966 serum Anatomy 0.000 description 18
- 102000004127 Cytokines Human genes 0.000 description 16
- 108090000695 Cytokines Proteins 0.000 description 16
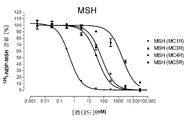
- 102000004378 Melanocortin Receptors Human genes 0.000 description 16
- 108090000950 Melanocortin Receptors Proteins 0.000 description 16
- 229940079593 drug Drugs 0.000 description 16
- 238000009472 formulation Methods 0.000 description 16
- 230000014509 gene expression Effects 0.000 description 16
- 235000002639 sodium chloride Nutrition 0.000 description 16
- 230000008685 targeting Effects 0.000 description 16
- 229940124597 therapeutic agent Drugs 0.000 description 16
- 210000002540 macrophage Anatomy 0.000 description 15
- 108090000623 proteins and genes Proteins 0.000 description 15
- 102100021943 C-C motif chemokine 2 Human genes 0.000 description 14
- 101710155857 C-C motif chemokine 2 Proteins 0.000 description 14
- 238000002347 injection Methods 0.000 description 14
- 239000007924 injection Substances 0.000 description 14
- 235000018102 proteins Nutrition 0.000 description 14
- 102000004169 proteins and genes Human genes 0.000 description 14
- 102000005962 receptors Human genes 0.000 description 14
- 108020003175 receptors Proteins 0.000 description 14
- 230000002829 reductive effect Effects 0.000 description 14
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 13
- 229960003957 dexamethasone Drugs 0.000 description 13
- 230000002757 inflammatory effect Effects 0.000 description 13
- 239000000126 substance Substances 0.000 description 13
- 238000011740 C57BL/6 mouse Methods 0.000 description 12
- 150000008574 D-amino acids Chemical class 0.000 description 12
- 241001529936 Murinae Species 0.000 description 12
- 239000003253 alpha intermedin derivative Substances 0.000 description 12
- 230000006698 induction Effects 0.000 description 12
- 238000007912 intraperitoneal administration Methods 0.000 description 12
- 229920000642 polymer Polymers 0.000 description 12
- 238000006467 substitution reaction Methods 0.000 description 12
- 241001465754 Metazoa Species 0.000 description 11
- 239000003153 chemical reaction reagent Substances 0.000 description 11
- 210000001519 tissue Anatomy 0.000 description 11
- OHCQJHSOBUTRHG-KGGHGJDLSA-N FORSKOLIN Chemical compound O=C([C@@]12O)C[C@](C)(C=C)O[C@]1(C)[C@@H](OC(=O)C)[C@@H](O)[C@@H]1[C@]2(C)[C@@H](O)CCC1(C)C OHCQJHSOBUTRHG-KGGHGJDLSA-N 0.000 description 10
- 238000004458 analytical method Methods 0.000 description 10
- 230000003110 anti-inflammatory effect Effects 0.000 description 10
- 230000004044 response Effects 0.000 description 10
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 9
- 108020004414 DNA Proteins 0.000 description 9
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 9
- 108090001005 Interleukin-6 Proteins 0.000 description 9
- 102000004889 Interleukin-6 Human genes 0.000 description 9
- 108010000410 MSH receptor Proteins 0.000 description 9
- 238000000684 flow cytometry Methods 0.000 description 9
- 230000002519 immonomodulatory effect Effects 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 230000002441 reversible effect Effects 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- 241000282414 Homo sapiens Species 0.000 description 8
- 108090000189 Neuropeptides Proteins 0.000 description 8
- UIGMAMGZOJVTDN-WHFBIAKZSA-N Ser-Gly-Ser Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O UIGMAMGZOJVTDN-WHFBIAKZSA-N 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 8
- 239000002254 cytotoxic agent Substances 0.000 description 8
- 231100000599 cytotoxic agent Toxicity 0.000 description 8
- 230000003247 decreasing effect Effects 0.000 description 8
- 239000000839 emulsion Substances 0.000 description 8
- 238000001727 in vivo Methods 0.000 description 8
- 230000003834 intracellular effect Effects 0.000 description 8
- 230000003902 lesion Effects 0.000 description 8
- 230000002207 retinal effect Effects 0.000 description 8
- 239000000758 substrate Substances 0.000 description 8
- 239000003981 vehicle Substances 0.000 description 8
- 239000002253 acid Substances 0.000 description 7
- 230000000670 limiting effect Effects 0.000 description 7
- 238000003753 real-time PCR Methods 0.000 description 7
- 230000009467 reduction Effects 0.000 description 7
- 239000004094 surface-active agent Substances 0.000 description 7
- 125000003396 thiol group Chemical group [H]S* 0.000 description 7
- SHAPQBYCPZRQIP-RXMQYKEDSA-N (2r)-2-(thiophen-2-ylamino)propanoic acid Chemical compound OC(=O)[C@@H](C)NC1=CC=CS1 SHAPQBYCPZRQIP-RXMQYKEDSA-N 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 108010008364 Melanocortins Proteins 0.000 description 6
- 102100038247 Retinol-binding protein 3 Human genes 0.000 description 6
- 238000010521 absorption reaction Methods 0.000 description 6
- 230000037396 body weight Effects 0.000 description 6
- 230000008859 change Effects 0.000 description 6
- 239000003937 drug carrier Substances 0.000 description 6
- 150000002148 esters Chemical class 0.000 description 6
- 239000000194 fatty acid Substances 0.000 description 6
- 230000001976 improved effect Effects 0.000 description 6
- 230000005764 inhibitory process Effects 0.000 description 6
- 108010048996 interstitial retinol-binding protein Proteins 0.000 description 6
- 150000002632 lipids Chemical class 0.000 description 6
- 239000002865 melanocortin Substances 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
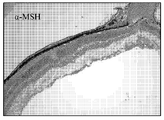
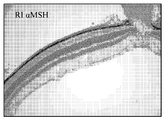
- 210000001328 optic nerve Anatomy 0.000 description 6
- 239000012071 phase Substances 0.000 description 6
- 230000000069 prophylactic effect Effects 0.000 description 6
- 210000000278 spinal cord Anatomy 0.000 description 6
- 239000006228 supernatant Substances 0.000 description 6
- 239000000725 suspension Substances 0.000 description 6
- 208000024891 symptom Diseases 0.000 description 6
- 101150013553 CD40 gene Proteins 0.000 description 5
- SUZLHDUTVMZSEV-UHFFFAOYSA-N Deoxycoleonol Natural products C12C(=O)CC(C)(C=C)OC2(C)C(OC(=O)C)C(O)C2C1(C)C(O)CCC2(C)C SUZLHDUTVMZSEV-UHFFFAOYSA-N 0.000 description 5
- 206010061818 Disease progression Diseases 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 5
- 108090000790 Enzymes Proteins 0.000 description 5
- 102000013462 Interleukin-12 Human genes 0.000 description 5
- 108010065805 Interleukin-12 Proteins 0.000 description 5
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 5
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 5
- 241000124008 Mammalia Species 0.000 description 5
- 108010081690 Pertussis Toxin Proteins 0.000 description 5
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 5
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 5
- 235000004279 alanine Nutrition 0.000 description 5
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 5
- 125000000539 amino acid group Chemical group 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- OHCQJHSOBUTRHG-UHFFFAOYSA-N colforsin Natural products OC12C(=O)CC(C)(C=C)OC1(C)C(OC(=O)C)C(O)C1C2(C)C(O)CCC1(C)C OHCQJHSOBUTRHG-UHFFFAOYSA-N 0.000 description 5
- 239000007859 condensation product Substances 0.000 description 5
- 229920001577 copolymer Polymers 0.000 description 5
- 235000014113 dietary fatty acids Nutrition 0.000 description 5
- 230000005750 disease progression Effects 0.000 description 5
- 231100000673 dose–response relationship Toxicity 0.000 description 5
- 229940088598 enzyme Drugs 0.000 description 5
- 229930195729 fatty acid Natural products 0.000 description 5
- 238000005755 formation reaction Methods 0.000 description 5
- 125000000524 functional group Chemical group 0.000 description 5
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 5
- 208000027866 inflammatory disease Diseases 0.000 description 5
- 210000001165 lymph node Anatomy 0.000 description 5
- 108010064235 lysylglycine Proteins 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 210000001616 monocyte Anatomy 0.000 description 5
- 239000003921 oil Substances 0.000 description 5
- 235000019198 oils Nutrition 0.000 description 5
- 210000000056 organ Anatomy 0.000 description 5
- 230000036961 partial effect Effects 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- 239000003755 preservative agent Substances 0.000 description 5
- 210000001525 retina Anatomy 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- 108700012359 toxins Proteins 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- BVAUMRCGVHUWOZ-ZETCQYMHSA-N (2s)-2-(cyclohexylazaniumyl)propanoate Chemical compound OC(=O)[C@H](C)NC1CCCCC1 BVAUMRCGVHUWOZ-ZETCQYMHSA-N 0.000 description 4
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 4
- 108010053187 Diphtheria Toxin Proteins 0.000 description 4
- 102000016607 Diphtheria Toxin Human genes 0.000 description 4
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 4
- NTBOEZICHOSJEE-YUMQZZPRSA-N Gly-Lys-Ser Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O NTBOEZICHOSJEE-YUMQZZPRSA-N 0.000 description 4
- LCRDMSSAKLTKBU-ZDLURKLDSA-N Gly-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN LCRDMSSAKLTKBU-ZDLURKLDSA-N 0.000 description 4
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 101000946889 Homo sapiens Monocyte differentiation antigen CD14 Proteins 0.000 description 4
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 4
- 239000004472 Lysine Substances 0.000 description 4
- 108010088565 Melanocortin 5 receptor Proteins 0.000 description 4
- 102000030612 Melanocortin 5 receptor Human genes 0.000 description 4
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 description 4
- 101000756628 Mus musculus Actin, cytoplasmic 1 Proteins 0.000 description 4
- 108010057466 NF-kappa B Proteins 0.000 description 4
- 102000003945 NF-kappa B Human genes 0.000 description 4
- 206010033799 Paralysis Diseases 0.000 description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 4
- 229930006000 Sucrose Natural products 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 239000013543 active substance Substances 0.000 description 4
- 239000002671 adjuvant Substances 0.000 description 4
- 238000012867 alanine scanning Methods 0.000 description 4
- 125000003275 alpha amino acid group Chemical group 0.000 description 4
- 125000003277 amino group Chemical group 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 4
- 230000021615 conjugation Effects 0.000 description 4
- 239000003431 cross linking reagent Substances 0.000 description 4
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 4
- 235000018417 cysteine Nutrition 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 150000004665 fatty acids Chemical class 0.000 description 4
- 239000012634 fragment Substances 0.000 description 4
- 239000000499 gel Substances 0.000 description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 4
- 235000014304 histidine Nutrition 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 235000018977 lysine Nutrition 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 238000010172 mouse model Methods 0.000 description 4
- 210000003024 peritoneal macrophage Anatomy 0.000 description 4
- 239000013641 positive control Substances 0.000 description 4
- 230000037452 priming Effects 0.000 description 4
- 210000004988 splenocyte Anatomy 0.000 description 4
- 239000005720 sucrose Substances 0.000 description 4
- 239000003765 sweetening agent Substances 0.000 description 4
- 239000012581 transferrin Substances 0.000 description 4
- BQWBEDSJTMWJAE-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-[(2-iodoacetyl)amino]benzoate Chemical compound C1=CC(NC(=O)CI)=CC=C1C(=O)ON1C(=O)CCC1=O BQWBEDSJTMWJAE-UHFFFAOYSA-N 0.000 description 3
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 3
- JKFYKCYQEWQPTM-SSDOTTSWSA-N (2r)-2-amino-2-(4-fluorophenyl)acetic acid Chemical compound OC(=O)[C@H](N)C1=CC=C(F)C=C1 JKFYKCYQEWQPTM-SSDOTTSWSA-N 0.000 description 3
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- IGFJVXOATGZTHD-UHFFFAOYSA-N Arg-Phe-His Natural products NC(CCNC(=N)N)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc2c[nH]cn2)C(=O)O IGFJVXOATGZTHD-UHFFFAOYSA-N 0.000 description 3
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 3
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 3
- FXTUGWXZTFMTIV-GJZGRUSLSA-N Gly-Trp-Arg Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)CN FXTUGWXZTFMTIV-GJZGRUSLSA-N 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- 101001046686 Homo sapiens Integrin alpha-M Proteins 0.000 description 3
- 101000978431 Homo sapiens Melanocortin receptor 3 Proteins 0.000 description 3
- 102100022338 Integrin alpha-M Human genes 0.000 description 3
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 3
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 3
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 3
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 3
- 102100023726 Melanocortin receptor 3 Human genes 0.000 description 3
- 102000003979 Mineralocorticoid Receptors Human genes 0.000 description 3
- 108090000375 Mineralocorticoid Receptors Proteins 0.000 description 3
- 101001033265 Mus musculus Interleukin-10 Proteins 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 102000003797 Neuropeptides Human genes 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- YRBGKVIWMNEVCZ-WDSKDSINSA-N Ser-Glu-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O YRBGKVIWMNEVCZ-WDSKDSINSA-N 0.000 description 3
- 210000001744 T-lymphocyte Anatomy 0.000 description 3
- 229920001615 Tragacanth Polymers 0.000 description 3
- 102000004338 Transferrin Human genes 0.000 description 3
- 108090000901 Transferrin Proteins 0.000 description 3
- 108090000631 Trypsin Proteins 0.000 description 3
- 102000004142 Trypsin Human genes 0.000 description 3
- BGXVHVMJZCSOCA-AVGNSLFASA-N Val-Pro-Lys Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)O)N BGXVHVMJZCSOCA-AVGNSLFASA-N 0.000 description 3
- IEDXPSOJFSVCKU-HOKPPMCLSA-N [4-[[(2S)-5-(carbamoylamino)-2-[[(2S)-2-[6-(2,5-dioxopyrrolidin-1-yl)hexanoylamino]-3-methylbutanoyl]amino]pentanoyl]amino]phenyl]methyl N-[(2S)-1-[[(2S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]-N-methylcarbamate Chemical compound CC[C@H](C)[C@@H]([C@@H](CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1)OC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCc1ccc(NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@@H](NC(=O)CCCCCN2C(=O)CCC2=O)C(C)C)cc1)C(C)C IEDXPSOJFSVCKU-HOKPPMCLSA-N 0.000 description 3
- 239000004480 active ingredient Substances 0.000 description 3
- 239000007900 aqueous suspension Substances 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
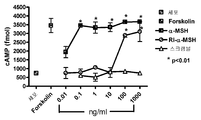
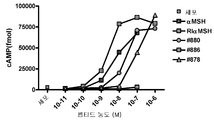
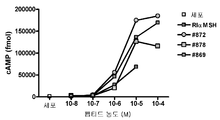
- 238000013262 cAMP assay Methods 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 239000003086 colorant Substances 0.000 description 3
- 238000012875 competitive assay Methods 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- VGBVAARMQYYITG-DESRROFGSA-N des-acetyl msh Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CO)C1=CC=C(O)C=C1 VGBVAARMQYYITG-DESRROFGSA-N 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 235000019441 ethanol Nutrition 0.000 description 3
- 230000003203 everyday effect Effects 0.000 description 3
- 239000000796 flavoring agent Substances 0.000 description 3
- 235000003599 food sweetener Nutrition 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 230000001506 immunosuppresive effect Effects 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 238000010348 incorporation Methods 0.000 description 3
- 210000004969 inflammatory cell Anatomy 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 239000007928 intraperitoneal injection Substances 0.000 description 3
- 238000010253 intravenous injection Methods 0.000 description 3
- 239000000787 lecithin Substances 0.000 description 3
- 235000010445 lecithin Nutrition 0.000 description 3
- 229940067606 lecithin Drugs 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 229930182817 methionine Natural products 0.000 description 3
- 239000004005 microsphere Substances 0.000 description 3
- 108010093470 monomethyl auristatin E Proteins 0.000 description 3
- 238000006452 multicomponent reaction Methods 0.000 description 3
- 210000005036 nerve Anatomy 0.000 description 3
- 210000000440 neutrophil Anatomy 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 150000007523 nucleic acids Chemical class 0.000 description 3
- 102000039446 nucleic acids Human genes 0.000 description 3
- 108020004707 nucleic acids Proteins 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 230000006320 pegylation Effects 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 238000004007 reversed phase HPLC Methods 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- HHSGWIABCIVPJT-UHFFFAOYSA-M sodium;1-[4-[(2-iodoacetyl)amino]benzoyl]oxy-2,5-dioxopyrrolidine-3-sulfonate Chemical compound [Na+].O=C1C(S(=O)(=O)[O-])CC(=O)N1OC(=O)C1=CC=C(NC(=O)CI)C=C1 HHSGWIABCIVPJT-UHFFFAOYSA-M 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 239000000600 sorbitol Substances 0.000 description 3
- 235000010356 sorbitol Nutrition 0.000 description 3
- 125000006850 spacer group Chemical group 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 230000000638 stimulation Effects 0.000 description 3
- 238000010254 subcutaneous injection Methods 0.000 description 3
- 239000007929 subcutaneous injection Substances 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 238000012384 transportation and delivery Methods 0.000 description 3
- 239000012588 trypsin Substances 0.000 description 3
- 235000015112 vegetable and seed oil Nutrition 0.000 description 3
- 239000008158 vegetable oil Substances 0.000 description 3
- LLXVXPPXELIDGQ-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-(2,5-dioxopyrrol-1-yl)benzoate Chemical compound C=1C=CC(N2C(C=CC2=O)=O)=CC=1C(=O)ON1C(=O)CCC1=O LLXVXPPXELIDGQ-UHFFFAOYSA-N 0.000 description 2
- JWDFQMWEFLOOED-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-(pyridin-2-yldisulfanyl)propanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCSSC1=CC=CC=N1 JWDFQMWEFLOOED-UHFFFAOYSA-N 0.000 description 2
- PMJWDPGOWBRILU-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-[4-(2,5-dioxopyrrol-1-yl)phenyl]butanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCCC(C=C1)=CC=C1N1C(=O)C=CC1=O PMJWDPGOWBRILU-UHFFFAOYSA-N 0.000 description 2
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 2
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 2
- MAWFYTWZTJWMFB-UHFFFAOYSA-N 1-[2-[2-[2-(2,5-dioxopyrrol-1-yl)ethoxy]propan-2-yloxy]ethyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1CCOC(C)(C)OCCN1C(=O)C=CC1=O MAWFYTWZTJWMFB-UHFFFAOYSA-N 0.000 description 2
- DIYPCWKHSODVAP-UHFFFAOYSA-N 1-[3-(2,5-dioxopyrrol-1-yl)benzoyl]oxy-2,5-dioxopyrrolidine-3-sulfonic acid Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)C1=CC=CC(N2C(C=CC2=O)=O)=C1 DIYPCWKHSODVAP-UHFFFAOYSA-N 0.000 description 2
- FPKVOQKZMBDBKP-UHFFFAOYSA-N 1-[4-[(2,5-dioxopyrrol-1-yl)methyl]cyclohexanecarbonyl]oxy-2,5-dioxopyrrolidine-3-sulfonic acid Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)C1CCC(CN2C(C=CC2=O)=O)CC1 FPKVOQKZMBDBKP-UHFFFAOYSA-N 0.000 description 2
- VHYRLCJMMJQUBY-UHFFFAOYSA-N 1-[4-[4-(2,5-dioxopyrrol-1-yl)phenyl]butanoyloxy]-2,5-dioxopyrrolidine-3-sulfonic acid Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)CCCC1=CC=C(N2C(C=CC2=O)=O)C=C1 VHYRLCJMMJQUBY-UHFFFAOYSA-N 0.000 description 2
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 2
- ASNTZYQMIUCEBV-UHFFFAOYSA-N 2,5-dioxo-1-[6-[3-(pyridin-2-yldisulfanyl)propanoylamino]hexanoyloxy]pyrrolidine-3-sulfonic acid Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)CCCCCNC(=O)CCSSC1=CC=CC=N1 ASNTZYQMIUCEBV-UHFFFAOYSA-N 0.000 description 2
- VPFUWHKTPYPNGT-UHFFFAOYSA-N 3-(3,4-dihydroxyphenyl)-1-(5-hydroxy-2,2-dimethylchromen-6-yl)propan-1-one Chemical compound OC1=C2C=CC(C)(C)OC2=CC=C1C(=O)CCC1=CC=C(O)C(O)=C1 VPFUWHKTPYPNGT-UHFFFAOYSA-N 0.000 description 2
- OBKXEAXTFZPCHS-UHFFFAOYSA-N 4-phenylbutyric acid Chemical compound OC(=O)CCCC1=CC=CC=C1 OBKXEAXTFZPCHS-UHFFFAOYSA-N 0.000 description 2
- 108010066676 Abrin Proteins 0.000 description 2
- 102000007469 Actins Human genes 0.000 description 2
- 108010085238 Actins Proteins 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- 239000004475 Arginine Substances 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 208000032116 Autoimmune Experimental Encephalomyelitis Diseases 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 102000019034 Chemokines Human genes 0.000 description 2
- 108010012236 Chemokines Proteins 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- AYUOWUNWZGTNKB-ULQDDVLXSA-N His-Phe-Arg Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AYUOWUNWZGTNKB-ULQDDVLXSA-N 0.000 description 2
- 206010062767 Hypophysitis Diseases 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- HGNRJCINZYHNOU-LURJTMIESA-N Lys-Gly Chemical compound NCCCC[C@H](N)C(=O)NCC(O)=O HGNRJCINZYHNOU-LURJTMIESA-N 0.000 description 2
- 101150004219 MCR1 gene Proteins 0.000 description 2
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- 101000648740 Mus musculus Tumor necrosis factor Proteins 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 2
- 108010001267 Protein Subunits Proteins 0.000 description 2
- 101000762949 Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) Exotoxin A Proteins 0.000 description 2
- 241000283984 Rodentia Species 0.000 description 2
- 101100206347 Schizosaccharomyces pombe (strain 972 / ATCC 24843) pmh1 gene Proteins 0.000 description 2
- VVKVHAOOUGNDPJ-SRVKXCTJSA-N Ser-Tyr-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O VVKVHAOOUGNDPJ-SRVKXCTJSA-N 0.000 description 2
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 101710137302 Surface antigen S Proteins 0.000 description 2
- 206010052779 Transplant rejections Diseases 0.000 description 2
- LCPVBXOHXMBLFW-JSGCOSHPSA-N Trp-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)=CNC2=C1 LCPVBXOHXMBLFW-JSGCOSHPSA-N 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 206010047115 Vasculitis Diseases 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000010933 acylation Effects 0.000 description 2
- 238000005917 acylation reaction Methods 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 2
- 235000009697 arginine Nutrition 0.000 description 2
- 235000003704 aspartic acid Nutrition 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 2
- 230000001588 bifunctional effect Effects 0.000 description 2
- 239000012148 binding buffer Substances 0.000 description 2
- 229920002988 biodegradable polymer Polymers 0.000 description 2
- 239000004621 biodegradable polymer Substances 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 230000001593 cAMP accumulation Effects 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 150000001735 carboxylic acids Chemical group 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 238000011284 combination treatment Methods 0.000 description 2
- 230000009137 competitive binding Effects 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 210000004087 cornea Anatomy 0.000 description 2
- 239000003246 corticosteroid Substances 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- 230000016396 cytokine production Effects 0.000 description 2
- 239000008121 dextrose Substances 0.000 description 2
- 239000008298 dragée Substances 0.000 description 2
- YQGOJNYOYNNSMM-UHFFFAOYSA-N eosin Chemical compound [Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21 YQGOJNYOYNNSMM-UHFFFAOYSA-N 0.000 description 2
- 208000012997 experimental autoimmune encephalomyelitis Diseases 0.000 description 2
- 235000013355 food flavoring agent Nutrition 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- 108010015792 glycyllysine Proteins 0.000 description 2
- 238000007490 hematoxylin and eosin (H&E) staining Methods 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- 229920002674 hyaluronan Polymers 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 210000002865 immune cell Anatomy 0.000 description 2
- 230000028993 immune response Effects 0.000 description 2
- 230000003053 immunization Effects 0.000 description 2
- 238000002649 immunization Methods 0.000 description 2
- 238000003018 immunoassay Methods 0.000 description 2
- 239000003018 immunosuppressive agent Substances 0.000 description 2
- 230000008595 infiltration Effects 0.000 description 2
- 238000001764 infiltration Methods 0.000 description 2
- 230000028709 inflammatory response Effects 0.000 description 2
- 210000000936 intestine Anatomy 0.000 description 2
- 238000010255 intramuscular injection Methods 0.000 description 2
- 239000007927 intramuscular injection Substances 0.000 description 2
- 230000009545 invasion Effects 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 206010025135 lupus erythematosus Diseases 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000010534 mechanism of action Effects 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- ZGEGCLOFRBLKSE-UHFFFAOYSA-N methylene hexane Natural products CCCCCC=C ZGEGCLOFRBLKSE-UHFFFAOYSA-N 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 239000000346 nonvolatile oil Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 239000004006 olive oil Substances 0.000 description 2
- 235000008390 olive oil Nutrition 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- 238000002823 phage display Methods 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 239000011574 phosphorus Substances 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 108091008695 photoreceptors Proteins 0.000 description 2
- 210000003635 pituitary gland Anatomy 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 2
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 230000001323 posttranslational effect Effects 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 239000000651 prodrug Substances 0.000 description 2
- 229940002612 prodrug Drugs 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 230000017854 proteolysis Effects 0.000 description 2
- 210000003289 regulatory T cell Anatomy 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
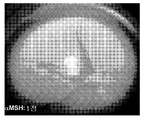
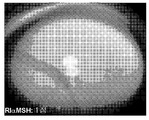
- 230000004256 retinal image Effects 0.000 description 2
- 235000004400 serine Nutrition 0.000 description 2
- 210000004927 skin cell Anatomy 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 235000015424 sodium Nutrition 0.000 description 2
- 235000010413 sodium alginate Nutrition 0.000 description 2
- 239000000661 sodium alginate Substances 0.000 description 2
- 229940005550 sodium alginate Drugs 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000012439 solid excipient Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000008174 sterile solution Substances 0.000 description 2
- 150000003431 steroids Chemical class 0.000 description 2
- 230000004936 stimulating effect Effects 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 230000004797 therapeutic response Effects 0.000 description 2
- 239000002562 thickening agent Substances 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 235000002374 tyrosine Nutrition 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 239000000341 volatile oil Substances 0.000 description 2
- 230000004580 weight loss Effects 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- VQZYZXLBKBUOHE-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-(pyridin-2-yldisulfanyl)butanoate Chemical compound C=1C=CC=NC=1SSC(C)CC(=O)ON1C(=O)CCC1=O VQZYZXLBKBUOHE-UHFFFAOYSA-N 0.000 description 1
- VYEKJNGRMIAFMH-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-(1-pyridin-2-ylsulfanylethyl)benzoate Chemical compound C=1C=C(C(=O)ON2C(CCC2=O)=O)C=CC=1C(C)SC1=CC=CC=N1 VYEKJNGRMIAFMH-UHFFFAOYSA-N 0.000 description 1
- JSHOVKSMJRQOGY-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-(pyridin-2-yldisulfanyl)butanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCCSSC1=CC=CC=N1 JSHOVKSMJRQOGY-UHFFFAOYSA-N 0.000 description 1
- GKSPIZSKQWTXQG-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-[1-(pyridin-2-yldisulfanyl)ethyl]benzoate Chemical compound C=1C=C(C(=O)ON2C(CCC2=O)=O)C=CC=1C(C)SSC1=CC=CC=N1 GKSPIZSKQWTXQG-UHFFFAOYSA-N 0.000 description 1
- QYEAAMBIUQLHFQ-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 6-[3-(pyridin-2-yldisulfanyl)propanoylamino]hexanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCCCCNC(=O)CCSSC1=CC=CC=N1 QYEAAMBIUQLHFQ-UHFFFAOYSA-N 0.000 description 1
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- DQJCDTNMLBYVAY-ZXXIYAEKSA-N (2S,5R,10R,13R)-16-{[(2R,3S,4R,5R)-3-{[(2S,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5-(ethylamino)-6-hydroxy-2-(hydroxymethyl)oxan-4-yl]oxy}-5-(4-aminobutyl)-10-carbamoyl-2,13-dimethyl-4,7,12,15-tetraoxo-3,6,11,14-tetraazaheptadecan-1-oic acid Chemical compound NCCCC[C@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)CC[C@H](C(N)=O)NC(=O)[C@@H](C)NC(=O)C(C)O[C@@H]1[C@@H](NCC)C(O)O[C@H](CO)[C@H]1O[C@H]1[C@H](NC(C)=O)[C@@H](O)[C@H](O)[C@@H](CO)O1 DQJCDTNMLBYVAY-ZXXIYAEKSA-N 0.000 description 1
- WTKYBFQVZPCGAO-ZCFIWIBFSA-N (2r)-2-(pyridin-3-ylamino)propanoic acid Chemical compound OC(=O)[C@@H](C)NC1=CC=CN=C1 WTKYBFQVZPCGAO-ZCFIWIBFSA-N 0.000 description 1
- GAUUPDQWKHTCAX-SECBINFHSA-N (2r)-2-amino-3-(1-benzothiophen-3-yl)propanoic acid Chemical compound C1=CC=C2C(C[C@@H](N)C(O)=O)=CSC2=C1 GAUUPDQWKHTCAX-SECBINFHSA-N 0.000 description 1
- JVJGCCBAOOWGEO-RUTPOYCXSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-4-amino-2-[[(2s,3s)-2-[[(2s,3s)-2-[[(2s)-2-azaniumyl-3-hydroxypropanoyl]amino]-3-methylpentanoyl]amino]-3-methylpentanoyl]amino]-4-oxobutanoyl]amino]-3-phenylpropanoyl]amino]-4-carboxylatobutanoyl]amino]-6-azaniumy Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=CC=C1 JVJGCCBAOOWGEO-RUTPOYCXSA-N 0.000 description 1
- HKZAAJSTFUZYTO-LURJTMIESA-N (2s)-2-[[2-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]acetyl]amino]-3-hydroxypropanoic acid Chemical compound NCC(=O)NCC(=O)NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O HKZAAJSTFUZYTO-LURJTMIESA-N 0.000 description 1
- QMCOCIWNMHBIIA-LROMGURASA-N (2s)-n-tert-butyl-1-[(2s)-1-[(2s)-2-[[(2s)-2-[[(2s)-2-(dimethylamino)-3-methylbutanoyl]amino]-3-methylbutanoyl]-methylamino]-3-methylbutanoyl]pyrrolidine-2-carbonyl]pyrrolidine-2-carboxamide Chemical compound CC(C)[C@H](N(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(=O)NC(C)(C)C)CCC1 QMCOCIWNMHBIIA-LROMGURASA-N 0.000 description 1
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 1
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 description 1
- BDNKZNFMNDZQMI-UHFFFAOYSA-N 1,3-diisopropylcarbodiimide Chemical compound CC(C)N=C=NC(C)C BDNKZNFMNDZQMI-UHFFFAOYSA-N 0.000 description 1
- JLPULHDHAOZNQI-ZTIMHPMXSA-N 1-hexadecanoyl-2-(9Z,12Z-octadecadienoyl)-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCC\C=C/C\C=C/CCCCC JLPULHDHAOZNQI-ZTIMHPMXSA-N 0.000 description 1
- IIRFMIKBXCMIPE-UHFFFAOYSA-N 2,5-dioxo-1-[6-[[2-[1-(pyridin-2-yldisulfanyl)ethyl]benzoyl]amino]hexanoyloxy]pyrrolidine-3-sulfonic acid Chemical compound C=1C=CC=C(C(=O)NCCCCCC(=O)ON2C(C(CC2=O)S(O)(=O)=O)=O)C=1C(C)SSC1=CC=CC=N1 IIRFMIKBXCMIPE-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- FBUTXZSKZCQABC-UHFFFAOYSA-N 2-amino-1-methyl-7h-purine-6-thione Chemical compound S=C1N(C)C(N)=NC2=C1NC=N2 FBUTXZSKZCQABC-UHFFFAOYSA-N 0.000 description 1
- LAXXPOJCFVMVAX-UHFFFAOYSA-N 2-azaniumyl-4-butylsulfanylbutanoate Chemical compound CCCCSCCC(N)C(O)=O LAXXPOJCFVMVAX-UHFFFAOYSA-N 0.000 description 1
- KIUMMUBSPKGMOY-UHFFFAOYSA-N 3,3'-Dithiobis(6-nitrobenzoic acid) Chemical compound C1=C([N+]([O-])=O)C(C(=O)O)=CC(SSC=2C=C(C(=CC=2)[N+]([O-])=O)C(O)=O)=C1 KIUMMUBSPKGMOY-UHFFFAOYSA-N 0.000 description 1
- JMUAKWNHKQBPGJ-UHFFFAOYSA-N 3-(pyridin-2-yldisulfanyl)-n-[4-[3-(pyridin-2-yldisulfanyl)propanoylamino]butyl]propanamide Chemical compound C=1C=CC=NC=1SSCCC(=O)NCCCCNC(=O)CCSSC1=CC=CC=N1 JMUAKWNHKQBPGJ-UHFFFAOYSA-N 0.000 description 1
- LYUQWQRTDLVQGA-UHFFFAOYSA-N 3-phenylpropylamine Chemical group NCCCC1=CC=CC=C1 LYUQWQRTDLVQGA-UHFFFAOYSA-N 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N 4-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- YJEMXOZWENRSFC-UHFFFAOYSA-N 5,6-dichlorotriazine-4-carboxylic acid Chemical compound OC(=O)C1=NN=NC(Cl)=C1Cl YJEMXOZWENRSFC-UHFFFAOYSA-N 0.000 description 1
- ODHCTXKNWHHXJC-VKHMYHEASA-N 5-oxo-L-proline Chemical compound OC(=O)[C@@H]1CCC(=O)N1 ODHCTXKNWHHXJC-VKHMYHEASA-N 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- 230000005730 ADP ribosylation Effects 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- NHLAEBFGWPXFGI-WHFBIAKZSA-N Ala-Gly-Asn Chemical compound C[C@@H](C(=O)NCC(=O)N[C@@H](CC(=O)N)C(=O)O)N NHLAEBFGWPXFGI-WHFBIAKZSA-N 0.000 description 1
- OMDNCNKNEGFOMM-BQBZGAKWSA-N Ala-Met-Gly Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)NCC(O)=O OMDNCNKNEGFOMM-BQBZGAKWSA-N 0.000 description 1
- 208000031873 Animal Disease Models Diseases 0.000 description 1
- 235000003911 Arachis Nutrition 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- DNLQVHBBMPZUGJ-BQBZGAKWSA-N Arg-Ser-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O DNLQVHBBMPZUGJ-BQBZGAKWSA-N 0.000 description 1
- VYZBPPBKFCHCIS-WPRPVWTQSA-N Arg-Val-Gly Chemical compound OC(=O)CNC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCN=C(N)N VYZBPPBKFCHCIS-WPRPVWTQSA-N 0.000 description 1
- 108010011485 Aspartame Proteins 0.000 description 1
- 101000669426 Aspergillus restrictus Ribonuclease mitogillin Proteins 0.000 description 1
- 208000030767 Autoimmune encephalitis Diseases 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 241000588832 Bordetella pertussis Species 0.000 description 1
- 208000007204 Brain death Diseases 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 102000014914 Carrier Proteins Human genes 0.000 description 1
- 102000004225 Cathepsin B Human genes 0.000 description 1
- 108090000712 Cathepsin B Proteins 0.000 description 1
- 102000003908 Cathepsin D Human genes 0.000 description 1
- 108090000258 Cathepsin D Proteins 0.000 description 1
- 102000005600 Cathepsins Human genes 0.000 description 1
- 108010084457 Cathepsins Proteins 0.000 description 1
- 229930186147 Cephalosporin Natural products 0.000 description 1
- 208000002691 Choroiditis Diseases 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- 108700032819 Croton tiglium crotin II Proteins 0.000 description 1
- DZLQXIFVQFTFJY-BYPYZUCNSA-N Cys-Gly-Gly Chemical compound SC[C@H](N)C(=O)NCC(=O)NCC(O)=O DZLQXIFVQFTFJY-BYPYZUCNSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 description 1
- 229930182818 D-methionine Natural products 0.000 description 1
- LRQKBLKVPFOOQJ-RXMQYKEDSA-N D-norleucine Chemical compound CCCC[C@@H](N)C(O)=O LRQKBLKVPFOOQJ-RXMQYKEDSA-N 0.000 description 1
- 229930182832 D-phenylalanine Natural products 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- 208000016192 Demyelinating disease Diseases 0.000 description 1
- 206010012305 Demyelination Diseases 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 108010013369 Enteropeptidase Proteins 0.000 description 1
- 102100029727 Enteropeptidase Human genes 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 206010015943 Eye inflammation Diseases 0.000 description 1
- 108010074860 Factor Xa Proteins 0.000 description 1
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 1
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 1
- 108700004714 Gelonium multiflorum GEL Proteins 0.000 description 1
- 208000032612 Glial tumor Diseases 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- IGNGBUVODQLMRJ-CIUDSAMLSA-N Gln-Ala-Met Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(O)=O IGNGBUVODQLMRJ-CIUDSAMLSA-N 0.000 description 1
- KRGZZKWSBGPLKL-IUCAKERBSA-N Glu-Gly-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCC(=O)O)N KRGZZKWSBGPLKL-IUCAKERBSA-N 0.000 description 1
- RAUDKMVXNOWDLS-WDSKDSINSA-N Glu-Gly-Ser Chemical compound OC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O RAUDKMVXNOWDLS-WDSKDSINSA-N 0.000 description 1
- QOXDAWODGSIDDI-GUBZILKMSA-N Glu-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)O)N QOXDAWODGSIDDI-GUBZILKMSA-N 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- IDOGEHIWMJMAHT-BYPYZUCNSA-N Gly-Gly-Cys Chemical compound NCC(=O)NCC(=O)N[C@@H](CS)C(O)=O IDOGEHIWMJMAHT-BYPYZUCNSA-N 0.000 description 1
- WDEHMRNSGHVNOH-VHSXEESVSA-N Gly-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)CN)C(=O)O WDEHMRNSGHVNOH-VHSXEESVSA-N 0.000 description 1
- FGPLUIQCSKGLTI-WDSKDSINSA-N Gly-Ser-Glu Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O FGPLUIQCSKGLTI-WDSKDSINSA-N 0.000 description 1
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 1
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Polymers OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 1
- 102000002068 Glycopeptides Human genes 0.000 description 1
- 108010015899 Glycopeptides Proteins 0.000 description 1
- 208000003807 Graves Disease Diseases 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- SQUHHTBVTRBESD-UHFFFAOYSA-N Hexa-Ac-myo-Inositol Natural products CC(=O)OC1C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O SQUHHTBVTRBESD-UHFFFAOYSA-N 0.000 description 1
- 240000004153 Hibiscus sabdariffa Species 0.000 description 1
- 235000001018 Hibiscus sabdariffa Nutrition 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- CUEQQFOGARVNHU-VGDYDELISA-N His-Ser-Ile Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O CUEQQFOGARVNHU-VGDYDELISA-N 0.000 description 1
- 101000978418 Homo sapiens Melanocortin receptor 4 Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- PFPUFNLHBXKPHY-HTFCKZLJSA-N Ile-Ile-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)O)N PFPUFNLHBXKPHY-HTFCKZLJSA-N 0.000 description 1
- PXKACEXYLPBMAD-JBDRJPRFSA-N Ile-Ser-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PXKACEXYLPBMAD-JBDRJPRFSA-N 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- JKGHDYGZRDWHGA-SRVKXCTJSA-N Leu-Asn-Leu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O JKGHDYGZRDWHGA-SRVKXCTJSA-N 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 101150015860 MC1R gene Proteins 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-L Malonate Chemical compound [O-]C(=O)CC([O-])=O OFOBLEOULBTSOW-UHFFFAOYSA-L 0.000 description 1
- 229930126263 Maytansine Natural products 0.000 description 1
- 102000017351 Melanocortin 3 receptors Human genes 0.000 description 1
- 108050005365 Melanocortin 3 receptors Proteins 0.000 description 1
- 102100023724 Melanocortin receptor 4 Human genes 0.000 description 1
- SOAYQFDWEIWPPR-IHRRRGAJSA-N Met-Ser-Tyr Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O SOAYQFDWEIWPPR-IHRRRGAJSA-N 0.000 description 1
- 101710151803 Mitochondrial intermediate peptidase 2 Proteins 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 101001095809 Mus musculus Ribonuclease inhibitor Proteins 0.000 description 1
- 241000187479 Mycobacterium tuberculosis Species 0.000 description 1
- 241001049988 Mycobacterium tuberculosis H37Ra Species 0.000 description 1
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 1
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical class ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 1
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 1
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- ISYSEOWLRQKQEQ-JYJNAYRXSA-N Phe-His-Glu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(O)=O ISYSEOWLRQKQEQ-JYJNAYRXSA-N 0.000 description 1
- MYQCCQSMKNCNKY-KKUMJFAQSA-N Phe-His-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)N[C@@H](CO)C(=O)O)N MYQCCQSMKNCNKY-KKUMJFAQSA-N 0.000 description 1
- 208000012641 Pigmentation disease Diseases 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 229920001214 Polysorbate 60 Polymers 0.000 description 1
- 208000003971 Posterior uveitis Diseases 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 102000002067 Protein Subunits Human genes 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 206010038848 Retinal detachment Diseases 0.000 description 1
- 108010039491 Ricin Proteins 0.000 description 1
- 235000005291 Rumex acetosa Nutrition 0.000 description 1
- 108010084592 Saporins Proteins 0.000 description 1
- QVOGDCQNGLBNCR-FXQIFTODSA-N Ser-Arg-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O QVOGDCQNGLBNCR-FXQIFTODSA-N 0.000 description 1
- HBTCFCHYALPXME-HTFCKZLJSA-N Ser-Ile-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HBTCFCHYALPXME-HTFCKZLJSA-N 0.000 description 1
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 1
- FPCGZYMRFFIYIH-CIUDSAMLSA-N Ser-Lys-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O FPCGZYMRFFIYIH-CIUDSAMLSA-N 0.000 description 1
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 108090000787 Subtilisin Proteins 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 230000006052 T cell proliferation Effects 0.000 description 1
- 101150088517 TCTA gene Proteins 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 108020004566 Transfer RNA Proteins 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- DTQVDTLACAAQTR-UHFFFAOYSA-M Trifluoroacetate Chemical compound [O-]C(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-M 0.000 description 1
- 235000021307 Triticum Nutrition 0.000 description 1
- 244000098338 Triticum aestivum Species 0.000 description 1
- BGDKAVGWHJFAGW-UHFFFAOYSA-N Tropicamide Chemical group C=1C=CC=CC=1C(CO)C(=O)N(CC)CC1=CC=NC=C1 BGDKAVGWHJFAGW-UHFFFAOYSA-N 0.000 description 1
- AOAMKFFPFOPMLX-BVSLBCMMSA-N Trp-Arg-Phe Chemical compound C([C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)N)C(O)=O)C1=CC=CC=C1 AOAMKFFPFOPMLX-BVSLBCMMSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 238000010162 Tukey test Methods 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 206010047571 Visual impairment Diseases 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- WERKSKAQRVDLDW-ANOHMWSOSA-N [(2s,3r,4r,5r)-2,3,4,5,6-pentahydroxyhexyl] (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO WERKSKAQRVDLDW-ANOHMWSOSA-N 0.000 description 1
- BTXLWRWDYZKCPQ-UHFFFAOYSA-N [nitro(phenyl)methyl] carbonochloridate Chemical compound ClC(=O)OC([N+](=O)[O-])C1=CC=CC=C1 BTXLWRWDYZKCPQ-UHFFFAOYSA-N 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 208000038016 acute inflammation Diseases 0.000 description 1
- 230000006022 acute inflammation Effects 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- IBVAQQYNSHJXBV-UHFFFAOYSA-N adipic acid dihydrazide Chemical compound NNC(=O)CCCCC(=O)NN IBVAQQYNSHJXBV-UHFFFAOYSA-N 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- UAHFGYDRQSXQEB-LEBBXHLNSA-N afamelanotide Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(N)=O)NC(=O)[C@H](CO)NC(C)=O)C1=CC=C(O)C=C1 UAHFGYDRQSXQEB-LEBBXHLNSA-N 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- HAXFWIACAGNFHA-UHFFFAOYSA-N aldrithiol Chemical compound C=1C=CC=NC=1SSC1=CC=CC=N1 HAXFWIACAGNFHA-UHFFFAOYSA-N 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 108010001818 alpha-sarcin Proteins 0.000 description 1
- 230000001668 ameliorated effect Effects 0.000 description 1
- 230000009435 amidation Effects 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 238000003277 amino acid sequence analysis Methods 0.000 description 1
- 125000006295 amino methylene group Chemical group [H]N(*)C([H])([H])* 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 238000011558 animal model by disease Methods 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- MWPLVEDNUUSJAV-UHFFFAOYSA-N anthracene Chemical compound C1=CC=CC2=CC3=CC=CC=C3C=C21 MWPLVEDNUUSJAV-UHFFFAOYSA-N 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000030741 antigen processing and presentation Effects 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000010516 arginylation Effects 0.000 description 1
- 238000003491 array Methods 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 239000000605 aspartame Substances 0.000 description 1
- IAOZJIPTCAWIRG-QWRGUYRKSA-N aspartame Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 IAOZJIPTCAWIRG-QWRGUYRKSA-N 0.000 description 1
- 235000010357 aspartame Nutrition 0.000 description 1
- 229960003438 aspartame Drugs 0.000 description 1
- 238000003149 assay kit Methods 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 239000000305 astragalus gummifer gum Substances 0.000 description 1
- 210000001130 astrocyte Anatomy 0.000 description 1
- 108010044540 auristatin Proteins 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 229910052797 bismuth Inorganic materials 0.000 description 1
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 238000006664 bond formation reaction Methods 0.000 description 1
- 208000025698 brain inflammatory disease Diseases 0.000 description 1
- 210000005013 brain tissue Anatomy 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 230000003491 cAMP production Effects 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 1
- 229930195731 calicheamicin Natural products 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 229940124587 cephalosporin Drugs 0.000 description 1
- 150000001780 cephalosporins Chemical class 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 238000001311 chemical methods and process Methods 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 229960001338 colchicine Drugs 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 208000010247 contact dermatitis Diseases 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 230000001351 cycling effect Effects 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000017858 demethylation Effects 0.000 description 1
- 238000010520 demethylation reaction Methods 0.000 description 1
- 210000001787 dendrite Anatomy 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 229940097957 dexamethasone 2 mg Drugs 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- AFOSIXZFDONLBT-UHFFFAOYSA-N divinyl sulfone Chemical compound C=CS(=O)(=O)C=C AFOSIXZFDONLBT-UHFFFAOYSA-N 0.000 description 1
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 1
- 229940043264 dodecyl sulfate Drugs 0.000 description 1
- AMRJKAQTDDKMCE-UHFFFAOYSA-N dolastatin Chemical compound CC(C)C(N(C)C)C(=O)NC(C(C)C)C(=O)N(C)C(C(C)C)C(OC)CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(C=1SC=CN=1)CC1=CC=CC=C1 AMRJKAQTDDKMCE-UHFFFAOYSA-N 0.000 description 1
- 229930188854 dolastatin Natural products 0.000 description 1
- 230000002222 downregulating effect Effects 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 230000002888 effect on disease Effects 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 210000003162 effector t lymphocyte Anatomy 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 239000003974 emollient agent Substances 0.000 description 1
- 206010014599 encephalitis Diseases 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 108010028531 enomycin Proteins 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- AEOCXXJPGCBFJA-UHFFFAOYSA-N ethionamide Chemical compound CCC1=CC(C(N)=S)=CC=N1 AEOCXXJPGCBFJA-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 208000030533 eye disease Diseases 0.000 description 1
- 150000002191 fatty alcohols Chemical class 0.000 description 1
- 125000001924 fatty-acyl group Chemical group 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 150000002211 flavins Chemical class 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 210000003194 forelimb Anatomy 0.000 description 1
- 230000022244 formylation Effects 0.000 description 1
- 238000006170 formylation reaction Methods 0.000 description 1
- 239000012737 fresh medium Substances 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 230000006251 gamma-carboxylation Effects 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 108010079547 glutamylmethionine Proteins 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 1
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 1
- 108010050848 glycylleucine Proteins 0.000 description 1
- 239000008202 granule composition Substances 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 150000003278 haem Chemical group 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 208000006454 hepatitis Diseases 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- FBPFZTCFMRRESA-UHFFFAOYSA-N hexane-1,2,3,4,5,6-hexol Chemical compound OCC(O)C(O)C(O)C(O)CO FBPFZTCFMRRESA-UHFFFAOYSA-N 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 208000010726 hind limb paralysis Diseases 0.000 description 1
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 description 1
- 238000010562 histological examination Methods 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 230000008629 immune suppression Effects 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 229960003444 immunosuppressant agent Drugs 0.000 description 1
- 229940124589 immunosuppressive drug Drugs 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000012606 in vitro cell culture Methods 0.000 description 1
- 230000004968 inflammatory condition Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 1
- 229960000367 inositol Drugs 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 230000026045 iodination Effects 0.000 description 1
- 238000006192 iodination reaction Methods 0.000 description 1
- 210000004153 islets of langerhan Anatomy 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 239000008274 jelly Substances 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 208000027905 limb weakness Diseases 0.000 description 1
- 231100000861 limb weakness Toxicity 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 238000001294 liquid chromatography-tandem mass spectrometry Methods 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 208000018191 liver inflammation Diseases 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 230000002132 lysosomal effect Effects 0.000 description 1
- 108010009298 lysylglutamic acid Proteins 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- WKPWGQKGSOKKOO-RSFHAFMBSA-N maytansine Chemical compound CO[C@@H]([C@@]1(O)C[C@](OC(=O)N1)([C@H]([C@@H]1O[C@@]1(C)[C@@H](OC(=O)[C@H](C)N(C)C(C)=O)CC(=O)N1C)C)[H])\C=C\C=C(C)\CC2=CC(OC)=C(Cl)C1=C2 WKPWGQKGSOKKOO-RSFHAFMBSA-N 0.000 description 1
- 210000002752 melanocyte Anatomy 0.000 description 1
- 230000037323 metabolic rate Effects 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 108010010621 modeccin Proteins 0.000 description 1
- 238000001823 molecular biology technique Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 229940087766 mydriacyl Drugs 0.000 description 1
- 230000007498 myristoylation Effects 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 1
- 210000004897 n-terminal region Anatomy 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 239000002858 neurotransmitter agent Substances 0.000 description 1
- 125000006502 nitrobenzyl group Chemical group 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- GYCKQBWUSACYIF-UHFFFAOYSA-N o-hydroxybenzoic acid ethyl ester Natural products CCOC(=O)C1=CC=CC=C1O GYCKQBWUSACYIF-UHFFFAOYSA-N 0.000 description 1
- 239000007764 o/w emulsion Substances 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical group CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 231100000327 ocular toxicity Toxicity 0.000 description 1
- 239000012053 oil suspension Substances 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 239000013307 optical fiber Substances 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 239000006072 paste Substances 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 210000004197 pelvis Anatomy 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- 108010076042 phenomycin Proteins 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- NMHMNPHRMNGLLB-UHFFFAOYSA-N phloretic acid Chemical compound OC(=O)CCC1=CC=C(O)C=C1 NMHMNPHRMNGLLB-UHFFFAOYSA-N 0.000 description 1
- 150000003905 phosphatidylinositols Chemical class 0.000 description 1
- 230000006461 physiological response Effects 0.000 description 1
- 230000019612 pigmentation Effects 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 239000002745 poly(ortho ester) Substances 0.000 description 1
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920000056 polyoxyethylene ether Polymers 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 235000012015 potatoes Nutrition 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 244000144977 poultry Species 0.000 description 1
- 230000013823 prenylation Effects 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000009696 proliferative response Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 108010029020 prolylglycine Proteins 0.000 description 1
- 125000006239 protecting group Chemical group 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 230000009145 protein modification Effects 0.000 description 1
- 230000020978 protein processing Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 229940043131 pyroglutamate Drugs 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000001525 receptor binding assay Methods 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004264 retinal detachment Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 235000019204 saccharin Nutrition 0.000 description 1
- 229940081974 saccharin Drugs 0.000 description 1
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 1
- 238000007665 sagging Methods 0.000 description 1
- 210000001991 scapula Anatomy 0.000 description 1
- 230000002000 scavenging effect Effects 0.000 description 1
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- UQDJGEHQDNVPGU-UHFFFAOYSA-N serine phosphoethanolamine Chemical compound [NH3+]CCOP([O-])(=O)OCC([NH3+])C([O-])=O UQDJGEHQDNVPGU-UHFFFAOYSA-N 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 235000003513 sheep sorrel Nutrition 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 238000010532 solid phase synthesis reaction Methods 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000001593 sorbitan monooleate Substances 0.000 description 1
- 235000011069 sorbitan monooleate Nutrition 0.000 description 1
- 229940035049 sorbitan monooleate Drugs 0.000 description 1
- 229940083466 soybean lecithin Drugs 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 210000001845 splenic macrophage Anatomy 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 238000013179 statistical model Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 230000019635 sulfation Effects 0.000 description 1
- 238000005670 sulfation reaction Methods 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 238000012385 systemic delivery Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 231100000057 systemic toxicity Toxicity 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 108010029464 tasidotin Proteins 0.000 description 1
- 229950010740 tasidotin Drugs 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- 150000003536 tetrazoles Chemical class 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 235000008521 threonine Nutrition 0.000 description 1
- 229960004072 thrombin Drugs 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 1
- 125000002221 trityl group Chemical group [H]C1=C([H])C([H])=C([H])C([H])=C1C([*])(C1=C(C(=C(C(=C1[H])[H])[H])[H])[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 230000006433 tumor necrosis factor production Effects 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 208000029257 vision disease Diseases 0.000 description 1
- 230000004393 visual impairment Effects 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229910052727 yttrium Inorganic materials 0.000 description 1
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 description 1
- ORQXBVXKBGUSBA-QMMMGPOBSA-N β-cyclohexyl-alanine Chemical group OC(=O)[C@@H](N)CC1CCCCC1 ORQXBVXKBGUSBA-QMMMGPOBSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/665—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans derived from pro-opiomelanocortin, pro-enkephalin or pro-dynorphin
- C07K14/68—Melanocyte-stimulating hormone [MSH]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/665—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans derived from pro-opiomelanocortin, pro-enkephalin or pro-dynorphin
- C07K14/68—Melanocyte-stimulating hormone [MSH]
- C07K14/685—Alpha-melanotropin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/33—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans derived from pro-opiomelanocortin, pro-enkephalin or pro-dynorphin
- A61K38/34—Melanocyte stimulating hormone [MSH], e.g. alpha- or beta-melanotropin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/0004—Screening or testing of compounds for diagnosis of disorders, assessment of conditions, e.g. renal clearance, gastric emptying, testing for diabetes, allergy, rheuma, pancreas functions
- A61K49/0008—Screening agents using (non-human) animal models or transgenic animal models or chimeric hosts, e.g. Alzheimer disease animal model, transgenic model for heart failure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/04—Drugs for disorders of the muscular or neuromuscular system for myasthenia gravis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/10—Tetrapeptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/10—Tetrapeptides
- C07K5/1021—Tetrapeptides with the first amino acid being acidic
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/04—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing only normal peptide links
- C07K5/10—Tetrapeptides
- C07K5/1024—Tetrapeptides with the first amino acid being heterocyclic
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
- C07K7/04—Linear peptides containing only normal peptide links
- C07K7/08—Linear peptides containing only normal peptide links having 12 to 20 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pharmacology & Pharmacy (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Immunology (AREA)
- Endocrinology (AREA)
- Gastroenterology & Hepatology (AREA)
- Epidemiology (AREA)
- Zoology (AREA)
- Diabetes (AREA)
- Toxicology (AREA)
- Rheumatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Pathology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Neurosurgery (AREA)
- Transplantation (AREA)
- Vascular Medicine (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US5637308P | 2008-05-27 | 2008-05-27 | |
| US61/056,373 | 2008-05-27 | ||
| PCT/US2009/037809 WO2009151708A2 (en) | 2008-05-27 | 2009-03-20 | Peptide analogs of alpha-melanocyte stimulating hormone |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167020183A Division KR101810110B1 (ko) | 2008-05-27 | 2009-03-20 | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187030624A Division KR20180118250A (ko) | 2008-05-27 | 2009-03-20 | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170102050A true KR20170102050A (ko) | 2017-09-06 |
Family
ID=41278142
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177023883A Ceased KR20170102050A (ko) | 2008-05-27 | 2009-03-20 | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 |
| KR1020187030624A Ceased KR20180118250A (ko) | 2008-05-27 | 2009-03-20 | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 |
| KR1020167020183A Expired - Fee Related KR101810110B1 (ko) | 2008-05-27 | 2009-03-20 | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 |
| KR1020107029335A Expired - Fee Related KR101666631B1 (ko) | 2008-05-27 | 2009-03-20 | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187030624A Ceased KR20180118250A (ko) | 2008-05-27 | 2009-03-20 | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 |
| KR1020167020183A Expired - Fee Related KR101810110B1 (ko) | 2008-05-27 | 2009-03-20 | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 |
| KR1020107029335A Expired - Fee Related KR101666631B1 (ko) | 2008-05-27 | 2009-03-20 | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 |
Country Status (16)
| Country | Link |
|---|---|
| US (4) | US8440793B2 (cg-RX-API-DMAC7.html) |
| EP (2) | EP2816055B1 (cg-RX-API-DMAC7.html) |
| JP (4) | JP5624027B2 (cg-RX-API-DMAC7.html) |
| KR (4) | KR20170102050A (cg-RX-API-DMAC7.html) |
| CN (2) | CN107098957A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2009258054B2 (cg-RX-API-DMAC7.html) |
| BR (1) | BRPI0912141A2 (cg-RX-API-DMAC7.html) |
| CA (2) | CA2998948A1 (cg-RX-API-DMAC7.html) |
| DK (2) | DK2297178T3 (cg-RX-API-DMAC7.html) |
| ES (2) | ES2715329T3 (cg-RX-API-DMAC7.html) |
| IL (3) | IL209369A (cg-RX-API-DMAC7.html) |
| MX (3) | MX356295B (cg-RX-API-DMAC7.html) |
| PT (2) | PT2297178E (cg-RX-API-DMAC7.html) |
| RU (1) | RU2496786C2 (cg-RX-API-DMAC7.html) |
| TR (1) | TR201903610T4 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2009151708A2 (cg-RX-API-DMAC7.html) |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2816055B1 (en) | 2008-05-27 | 2018-12-19 | Genzyme Corporation | Peptide analogs of alpha-melanocyte stimulating hormons |
| CN101914138A (zh) * | 2010-07-13 | 2010-12-15 | 华东师范大学 | 一种用偶联剂制得的肝癌靶向肽-阿霉素及合成方法 |
| CN102008732B (zh) * | 2010-11-08 | 2012-10-24 | 武汉华耀生物医药有限公司 | 一种叶酸偶联抗体药物及其制备方法与应用 |
| WO2012158960A2 (en) | 2011-05-17 | 2012-11-22 | H. Lee Moffitt Cancer Center & Research Institute, Inc. | Melanocortin 1 receptor ligands and methods of use |
| AU2013348043B2 (en) | 2012-11-21 | 2017-11-02 | University Of Cincinnati | Skin care compositions and methods comprising selective agonists of melanocortin 1 receptor |
| WO2015067503A1 (en) * | 2013-11-07 | 2015-05-14 | Clinuvel Ag | Alpha-msh analogues for use in the treatment of psoriasis |
| EP3212220A1 (en) * | 2014-10-28 | 2017-09-06 | Clinuvel AG | Inflammatory disease |
| AU2016298175B2 (en) | 2015-07-28 | 2022-01-06 | University Of Iowa Research Foundation | Compositions and methods of treating cancer |
| SG10201506686WA (en) * | 2015-08-24 | 2017-03-30 | Agency Science Tech & Res | Conjugates |
| CN106905432B (zh) * | 2015-12-18 | 2018-12-21 | 兰州大学 | 一种α促黑素细胞激素的融合蛋白及其制备方法和应用 |
| EP3448872A4 (en) * | 2016-04-27 | 2019-12-11 | The Regents of the University of California | PREPARATION OF FUNCTIONAL HOMOCYSTEINRESTES IN POLYPEPTIDES AND PEPTIDES |
| US20220072092A1 (en) * | 2016-06-24 | 2022-03-10 | University Of Iowa Research Foundation | Compositions and methods of treating melanoma |
| US11179484B2 (en) | 2016-06-24 | 2021-11-23 | University Of Iowa Research Foundation | Compositions and methods of treating melanoma |
| CN109803979B (zh) * | 2016-10-04 | 2023-01-31 | 帝斯曼知识产权资产管理有限公司 | 黑皮质素-1-受体激动剂 |
| KR101957014B1 (ko) * | 2017-04-18 | 2019-06-20 | 순천대학교 산학협력단 | 항염증 활성을 나타내는 펩타이드 및 이를 유효성분으로 포함하는 항염증용 조성물 |
| MX2022003446A (es) | 2019-09-23 | 2022-07-13 | Dds Res Inc | Composiciones de vesiculas lipidicas con agentes potenciadores de la penetracion. |
| WO2021157973A1 (ko) * | 2020-02-07 | 2021-08-12 | 이화여자대학교 산학협력단 | 페길화된 ige-의존적 히스타민 방출인자(hrf) 결합 펩타이드 및 이의 용도 |
| US11529335B2 (en) | 2020-07-31 | 2022-12-20 | University Of Iowa Research Foundation | Compositions and methods for treating cancer |
| RU2766340C1 (ru) * | 2021-02-10 | 2022-03-15 | Игорь Закванович Зайцев | Пептидный толерогенный компаунд |
| MX2023011157A (es) | 2021-03-24 | 2024-01-25 | Glo Pharma Inc | Peptidos y metodos para reducir la pigmentacion de la piel. |
| WO2023091689A1 (en) | 2021-11-19 | 2023-05-25 | University Of Iowa Research Foundation | Combined use of mcr1-directed radiotherapy and immune checkpoint inhibition in the treatment of melanoma |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4894443A (en) | 1984-02-08 | 1990-01-16 | Cetus Corporation | Toxin conjugates |
| US4542225A (en) | 1984-08-29 | 1985-09-17 | Dana-Farber Cancer Institute, Inc. | Acid-cleavable compound |
| US4952394A (en) | 1987-11-23 | 1990-08-28 | Bristol-Myers Company | Drug-monoclonal antibody conjugates |
| US5194425A (en) * | 1988-06-23 | 1993-03-16 | Anergen, Inc. | Mhc-mediated toxic conjugates useful in ameliorating autoimmunity |
| US5137877B1 (en) * | 1990-05-14 | 1996-01-30 | Bristol Myers Squibb Co | Bifunctional linking compounds conjugates and methods for their production |
| US5618528A (en) * | 1994-02-28 | 1997-04-08 | Sterling Winthrop Inc. | Biologically compatible linear block copolymers of polyalkylene oxide and peptide units |
| US5612474A (en) | 1994-06-30 | 1997-03-18 | Eli Lilly And Company | Acid labile immunoconjugate intermediates |
| AU3374795A (en) | 1994-08-29 | 1996-03-22 | Prizm Pharmaceuticals, Inc. | Conjugates of vascular endothelial growth factor with targeted agents |
| US5716928A (en) | 1995-06-07 | 1998-02-10 | Avmax, Inc. | Use of essential oils to increase bioavailability of oral pharmaceutical compounds |
| US5858401A (en) * | 1996-01-22 | 1999-01-12 | Sidmak Laboratories, Inc. | Pharmaceutical composition for cyclosporines |
| JP3647881B2 (ja) * | 1996-07-12 | 2005-05-18 | イムノメディクス,インコーポレイテッド | 放射性金属元素結合ペプチドの類似化合物 |
| WO1999021571A1 (en) | 1997-10-27 | 1999-05-06 | Trega Biosciences, Inc. | Melanocortin receptor ligands and methods of using same |
| US7157418B1 (en) | 1998-07-22 | 2007-01-02 | Osprey Pharmaceuticals, Ltd. | Methods and compositions for treating secondary tissue damage and other inflammatory conditions and disorders |
| US20020193332A1 (en) * | 2001-02-12 | 2002-12-19 | Hedley Mary Lynne | Methods of treating bladder disorders |
| US6969702B2 (en) * | 2002-11-20 | 2005-11-29 | Neuronova Ab | Compounds and methods for increasing neurogenesis |
| WO2005120588A2 (en) * | 2004-05-26 | 2005-12-22 | The Curators Of The University Of Missouri | Peptides delivered to cell nuclei |
| CN105837661B (zh) * | 2005-07-08 | 2024-10-15 | 益普生制药股份有限公司 | 黑皮质素受体配体 |
| MX2008012721A (es) * | 2006-04-05 | 2009-02-20 | Unversity Of California | Composiciones y metodos para el analisis y tratamiento de sindrome de consuncion por sida y disfuncion de celulas inmunes. |
| FR2914646A1 (fr) * | 2007-04-04 | 2008-10-10 | Neorphys Soc Par Actions Simpl | Analogues peptidiques des recepteurs des melanocortines |
| US8069129B2 (en) * | 2007-04-10 | 2011-11-29 | Ab Initio Technology Llc | Editing and compiling business rules |
| CN101302245B (zh) * | 2007-05-09 | 2011-11-16 | 中国人民解放军军事医学科学院毒物药物研究所 | 黑色素皮质激素受体四肽类激动剂及其制备方法和用途 |
| EP2816055B1 (en) * | 2008-05-27 | 2018-12-19 | Genzyme Corporation | Peptide analogs of alpha-melanocyte stimulating hormons |
-
2009
- 2009-03-20 EP EP14178968.5A patent/EP2816055B1/en not_active Not-in-force
- 2009-03-20 KR KR1020177023883A patent/KR20170102050A/ko not_active Ceased
- 2009-03-20 CN CN201611160601.4A patent/CN107098957A/zh active Pending
- 2009-03-20 CA CA2998948A patent/CA2998948A1/en not_active Abandoned
- 2009-03-20 KR KR1020187030624A patent/KR20180118250A/ko not_active Ceased
- 2009-03-20 KR KR1020167020183A patent/KR101810110B1/ko not_active Expired - Fee Related
- 2009-03-20 DK DK09763002.4T patent/DK2297178T3/da active
- 2009-03-20 MX MX2013012525A patent/MX356295B/es unknown
- 2009-03-20 WO PCT/US2009/037809 patent/WO2009151708A2/en not_active Ceased
- 2009-03-20 JP JP2011511661A patent/JP5624027B2/ja not_active Expired - Fee Related
- 2009-03-20 DK DK14178968.5T patent/DK2816055T3/en active
- 2009-03-20 AU AU2009258054A patent/AU2009258054B2/en not_active Ceased
- 2009-03-20 KR KR1020107029335A patent/KR101666631B1/ko not_active Expired - Fee Related
- 2009-03-20 RU RU2010153217/04A patent/RU2496786C2/ru not_active IP Right Cessation
- 2009-03-20 BR BRPI0912141A patent/BRPI0912141A2/pt not_active IP Right Cessation
- 2009-03-20 ES ES14178968T patent/ES2715329T3/es active Active
- 2009-03-20 MX MX2010012990A patent/MX2010012990A/es active IP Right Grant
- 2009-03-20 CA CA2726076A patent/CA2726076C/en not_active Expired - Fee Related
- 2009-03-20 PT PT97630024T patent/PT2297178E/pt unknown
- 2009-03-20 PT PT14178968T patent/PT2816055T/pt unknown
- 2009-03-20 ES ES09763002.4T patent/ES2492670T3/es active Active
- 2009-03-20 CN CN200980120892.6A patent/CN102143970B/zh not_active Expired - Fee Related
- 2009-03-20 TR TR2019/03610T patent/TR201903610T4/tr unknown
- 2009-03-20 US US12/408,560 patent/US8440793B2/en active Active
- 2009-03-20 EP EP09763002.4A patent/EP2297178B1/en not_active Not-in-force
-
2010
- 2010-11-17 IL IL209369A patent/IL209369A/en not_active IP Right Cessation
- 2010-11-26 MX MX2018006299A patent/MX2018006299A/es unknown
-
2013
- 2013-05-08 US US13/890,039 patent/US9115174B2/en active Active
-
2014
- 2014-07-24 JP JP2014150428A patent/JP6170019B2/ja not_active Expired - Fee Related
-
2015
- 2015-08-19 US US14/830,572 patent/US9738698B2/en active Active
-
2016
- 2016-05-29 IL IL245906A patent/IL245906A0/en unknown
-
2017
- 2017-01-05 JP JP2017000343A patent/JP6585099B2/ja not_active Expired - Fee Related
- 2017-05-01 IL IL252058A patent/IL252058A0/en unknown
- 2017-07-10 US US15/645,974 patent/US10385108B2/en active Active
-
2019
- 2019-02-04 JP JP2019017752A patent/JP2019065053A/ja not_active Withdrawn
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101810110B1 (ko) | 알파-멜라노사이트 자극 호르몬의 펩티드 유사체 | |
| AU2013347975B2 (en) | Improved peptide pharmaceuticals for insulin resistance | |
| HK1160656A (zh) | α-促黑素細胞激素的肽類似物 | |
| HK1160656B (en) | Peptide analogs of alpha-melanocyte stimulating hormone | |
| RU2820132C2 (ru) | Способ улучшения симптомов со стороны нижних мочевыводящих путей |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20170825 Application number text: 1020167020183 Filing date: 20160722 |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20170922 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20171207 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20180823 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20171207 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| A107 | Divisional application of patent | ||
| J201 | Request for trial against refusal decision | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20181023 Application number text: 1020167020183 Filing date: 20160722 |
|
| PJ0201 | Trial against decision of rejection |
Patent event date: 20181023 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20180823 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Decision date: 20191227 Appeal identifier: 2018101004382 Request date: 20181023 |
|
| J301 | Trial decision |
Free format text: TRIAL NUMBER: 2018101004382; TRIAL DECISION FOR APPEAL AGAINST DECISION TO DECLINE REFUSAL REQUESTED 20181023 Effective date: 20191227 |
|
| PJ1301 | Trial decision |
Patent event code: PJ13011S01D Patent event date: 20191227 Comment text: Trial Decision on Objection to Decision on Refusal Appeal kind category: Appeal against decision to decline refusal Request date: 20181023 Decision date: 20191227 Appeal identifier: 2018101004382 |