KR20170100039A - 사이토메갈로바이러스 항원 및 이의 용도 - Google Patents
사이토메갈로바이러스 항원 및 이의 용도 Download PDFInfo
- Publication number
- KR20170100039A KR20170100039A KR1020177023123A KR20177023123A KR20170100039A KR 20170100039 A KR20170100039 A KR 20170100039A KR 1020177023123 A KR1020177023123 A KR 1020177023123A KR 20177023123 A KR20177023123 A KR 20177023123A KR 20170100039 A KR20170100039 A KR 20170100039A
- Authority
- KR
- South Korea
- Prior art keywords
- leu
- protein
- fragment
- complex
- pro
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 241000701022 Cytomegalovirus Species 0.000 title claims abstract description 128
- 239000000427 antigen Substances 0.000 title description 22
- 102000036639 antigens Human genes 0.000 title description 22
- 108091007433 antigens Proteins 0.000 title description 22
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 195
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 193
- 239000012634 fragment Substances 0.000 claims description 161
- 230000035772 mutation Effects 0.000 claims description 95
- 108091005804 Peptidases Proteins 0.000 claims description 59
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 claims description 59
- 239000004365 Protease Substances 0.000 claims description 58
- 239000000203 mixture Substances 0.000 claims description 51
- 125000000539 amino acid group Chemical group 0.000 claims description 49
- 102000039446 nucleic acids Human genes 0.000 claims description 39
- 108020004707 nucleic acids Proteins 0.000 claims description 39
- 150000007523 nucleic acids Chemical class 0.000 claims description 39
- 241000700605 Viruses Species 0.000 claims description 23
- 238000003776 cleavage reaction Methods 0.000 claims description 23
- 230000007017 scission Effects 0.000 claims description 23
- 230000028993 immune response Effects 0.000 claims description 22
- 241000701074 Human alphaherpesvirus 2 Species 0.000 claims description 20
- 230000002163 immunogen Effects 0.000 claims description 20
- 241000701085 Human alphaherpesvirus 3 Species 0.000 claims description 19
- 238000012217 deletion Methods 0.000 claims description 19
- 230000037430 deletion Effects 0.000 claims description 19
- 241001529453 unidentified herpesvirus Species 0.000 claims description 19
- 241000700588 Human alphaherpesvirus 1 Species 0.000 claims description 17
- 238000007792 addition Methods 0.000 claims description 16
- 238000006467 substitution reaction Methods 0.000 claims description 15
- 239000002671 adjuvant Substances 0.000 claims description 10
- 230000001939 inductive effect Effects 0.000 claims description 9
- 108091033319 polynucleotide Proteins 0.000 claims description 9
- 102000040430 polynucleotide Human genes 0.000 claims description 9
- 239000002157 polynucleotide Substances 0.000 claims description 9
- 238000003259 recombinant expression Methods 0.000 claims description 5
- 241001492322 Bovine alphaherpesvirus 5 Species 0.000 claims description 3
- 235000018102 proteins Nutrition 0.000 description 172
- 210000004027 cell Anatomy 0.000 description 108
- 235000019419 proteases Nutrition 0.000 description 53
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 39
- 108090000765 processed proteins & peptides Proteins 0.000 description 34
- 238000000034 method Methods 0.000 description 29
- 102000004196 processed proteins & peptides Human genes 0.000 description 29
- 229920001184 polypeptide Polymers 0.000 description 27
- 241000701024 Human betaherpesvirus 5 Species 0.000 description 25
- 229960005486 vaccine Drugs 0.000 description 25
- 150000001413 amino acids Chemical class 0.000 description 23
- 238000004519 manufacturing process Methods 0.000 description 21
- 235000001014 amino acid Nutrition 0.000 description 20
- 108020004414 DNA Proteins 0.000 description 19
- 229940024606 amino acid Drugs 0.000 description 17
- 108010092854 aspartyllysine Proteins 0.000 description 17
- 230000002829 reductive effect Effects 0.000 description 17
- IBMVEYRWAWIOTN-UHFFFAOYSA-N L-Leucyl-L-Arginyl-L-Proline Natural products CC(C)CC(N)C(=O)NC(CCCN=C(N)N)C(=O)N1CCCC1C(O)=O IBMVEYRWAWIOTN-UHFFFAOYSA-N 0.000 description 16
- WUHBLPVELFTPQK-KKUMJFAQSA-N Leu-Tyr-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O WUHBLPVELFTPQK-KKUMJFAQSA-N 0.000 description 16
- 238000001262 western blot Methods 0.000 description 16
- 241000710929 Alphavirus Species 0.000 description 15
- 108010076504 Protein Sorting Signals Proteins 0.000 description 15
- 108010047857 aspartylglycine Proteins 0.000 description 15
- 239000013598 vector Substances 0.000 description 15
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 14
- 230000003472 neutralizing effect Effects 0.000 description 14
- YRTOMUMWSTUQAX-FXQIFTODSA-N Asn-Pro-Asp Chemical compound NC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O YRTOMUMWSTUQAX-FXQIFTODSA-N 0.000 description 13
- MTAOBYXRYJZRGQ-WDSKDSINSA-N Glu-Gly-Asp Chemical compound OC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O MTAOBYXRYJZRGQ-WDSKDSINSA-N 0.000 description 13
- CLODWIOAKCSBAN-BQBZGAKWSA-N Gly-Arg-Asp Chemical compound NC(N)=NCCC[C@H](NC(=O)CN)C(=O)N[C@@H](CC(O)=O)C(O)=O CLODWIOAKCSBAN-BQBZGAKWSA-N 0.000 description 13
- HFPVRZWORNJRRC-UWVGGRQHSA-N Gly-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CN HFPVRZWORNJRRC-UWVGGRQHSA-N 0.000 description 13
- -1 aromatic amino acids Chemical class 0.000 description 13
- NGYHSXDNNOFHNE-AVGNSLFASA-N Arg-Pro-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O NGYHSXDNNOFHNE-AVGNSLFASA-N 0.000 description 12
- XYLSGAWRCZECIQ-JYJNAYRXSA-N Lys-Tyr-Glu Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCC(O)=O)C(O)=O)CC1=CC=C(O)C=C1 XYLSGAWRCZECIQ-JYJNAYRXSA-N 0.000 description 12
- LHALYDBUDCWMDY-CIUDSAMLSA-N Pro-Glu-Ala Chemical compound C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1)C(O)=O LHALYDBUDCWMDY-CIUDSAMLSA-N 0.000 description 12
- FVFUOQIYDPAIJR-XIRDDKMYSA-N Ser-Trp-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CO)N FVFUOQIYDPAIJR-XIRDDKMYSA-N 0.000 description 12
- DBOXBUDEAJVKRE-LSJOCFKGSA-N Val-Asn-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](C(C)C)C(=O)O)N DBOXBUDEAJVKRE-LSJOCFKGSA-N 0.000 description 12
- ZBLQIYPCUWZSRZ-QEJZJMRPSA-N Ala-Phe-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@H](C)N)CC1=CC=CC=C1 ZBLQIYPCUWZSRZ-QEJZJMRPSA-N 0.000 description 11
- VUGWHBXPMAHEGZ-SRVKXCTJSA-N Arg-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCN=C(N)N VUGWHBXPMAHEGZ-SRVKXCTJSA-N 0.000 description 11
- CWJQMCPYXNVMBS-STECZYCISA-N Ile-Arg-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N CWJQMCPYXNVMBS-STECZYCISA-N 0.000 description 11
- ILJREDZFPHTUIE-GUBZILKMSA-N Leu-Asp-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O ILJREDZFPHTUIE-GUBZILKMSA-N 0.000 description 11
- OJPHFSOMBZKQKQ-GUBZILKMSA-N Ser-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CO OJPHFSOMBZKQKQ-GUBZILKMSA-N 0.000 description 11
- 108010053037 kyotorphin Proteins 0.000 description 11
- 210000004899 c-terminal region Anatomy 0.000 description 10
- 208000015181 infectious disease Diseases 0.000 description 10
- 238000004113 cell culture Methods 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- 210000002919 epithelial cell Anatomy 0.000 description 9
- 239000002245 particle Substances 0.000 description 9
- 238000012163 sequencing technique Methods 0.000 description 9
- 241000272186 Falco columbarius Species 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 238000003780 insertion Methods 0.000 description 8
- 230000037431 insertion Effects 0.000 description 8
- 210000004962 mammalian cell Anatomy 0.000 description 8
- 108010087924 alanylproline Proteins 0.000 description 7
- 239000003814 drug Substances 0.000 description 7
- 230000013595 glycosylation Effects 0.000 description 7
- 238000006206 glycosylation reaction Methods 0.000 description 7
- 241000701161 unidentified adenovirus Species 0.000 description 7
- 230000003612 virological effect Effects 0.000 description 7
- 241000699802 Cricetulus griseus Species 0.000 description 6
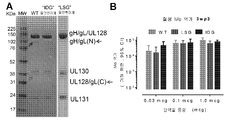
- 101150088904 UL130 gene Proteins 0.000 description 6
- 108010068380 arginylarginine Proteins 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- 230000000295 complement effect Effects 0.000 description 6
- 210000002889 endothelial cell Anatomy 0.000 description 6
- 230000002209 hydrophobic effect Effects 0.000 description 6
- 230000003053 immunization Effects 0.000 description 6
- 238000002649 immunization Methods 0.000 description 6
- 230000005847 immunogenicity Effects 0.000 description 6
- 239000008194 pharmaceutical composition Substances 0.000 description 6
- 210000002966 serum Anatomy 0.000 description 6
- 210000002845 virion Anatomy 0.000 description 6
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 5
- JSNWZMFSLIWAHS-HJGDQZAQSA-N Asp-Thr-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O JSNWZMFSLIWAHS-HJGDQZAQSA-N 0.000 description 5
- 241000282472 Canis lupus familiaris Species 0.000 description 5
- CLVUXCBGKUECIT-HJGDQZAQSA-N Leu-Asp-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CLVUXCBGKUECIT-HJGDQZAQSA-N 0.000 description 5
- ZDJQVSIPFLMNOX-RHYQMDGZSA-N Leu-Thr-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N ZDJQVSIPFLMNOX-RHYQMDGZSA-N 0.000 description 5
- 241000124008 Mammalia Species 0.000 description 5
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 5
- MQUZANJDFOQOBX-SRVKXCTJSA-N Ser-Phe-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O MQUZANJDFOQOBX-SRVKXCTJSA-N 0.000 description 5
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 5
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 5
- 229910002091 carbon monoxide Inorganic materials 0.000 description 5
- 125000002091 cationic group Chemical group 0.000 description 5
- 238000006386 neutralization reaction Methods 0.000 description 5
- 239000007764 o/w emulsion Substances 0.000 description 5
- 239000006228 supernatant Substances 0.000 description 5
- 108010015385 valyl-prolyl-proline Proteins 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- NYDBKUNVSALYPX-NAKRPEOUSA-N Ala-Ile-Arg Chemical compound C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CCCN=C(N)N NYDBKUNVSALYPX-NAKRPEOUSA-N 0.000 description 4
- QPBSRMDNJOTFAL-AICCOOGYSA-N Ala-Leu-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QPBSRMDNJOTFAL-AICCOOGYSA-N 0.000 description 4
- FFZJHQODAYHGPO-KZVJFYERSA-N Ala-Pro-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N FFZJHQODAYHGPO-KZVJFYERSA-N 0.000 description 4
- VDCIPFYVCICPEC-FXQIFTODSA-N Asn-Arg-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O VDCIPFYVCICPEC-FXQIFTODSA-N 0.000 description 4
- ULRPXVNMIIYDDJ-ACZMJKKPSA-N Asn-Glu-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(=O)N)N ULRPXVNMIIYDDJ-ACZMJKKPSA-N 0.000 description 4
- NCXTYSVDWLAQGZ-ZKWXMUAHSA-N Asn-Ser-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O NCXTYSVDWLAQGZ-ZKWXMUAHSA-N 0.000 description 4
- RXBGWGRSWXOBGK-KKUMJFAQSA-N Asp-Lys-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O RXBGWGRSWXOBGK-KKUMJFAQSA-N 0.000 description 4
- 206010011831 Cytomegalovirus infection Diseases 0.000 description 4
- NJCALAAIGREHDR-WDCWCFNPSA-N Glu-Leu-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O NJCALAAIGREHDR-WDCWCFNPSA-N 0.000 description 4
- RXGLHDWAZQECBI-SRVKXCTJSA-N Leu-Leu-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O RXGLHDWAZQECBI-SRVKXCTJSA-N 0.000 description 4
- RGUXWMDNCPMQFB-YUMQZZPRSA-N Leu-Ser-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O RGUXWMDNCPMQFB-YUMQZZPRSA-N 0.000 description 4
- OZVXDDFYCQOPFD-XQQFMLRXSA-N Lys-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N OZVXDDFYCQOPFD-XQQFMLRXSA-N 0.000 description 4
- 241000710961 Semliki Forest virus Species 0.000 description 4
- 241000710960 Sindbis virus Species 0.000 description 4
- IGROJMCBGRFRGI-YTLHQDLWSA-N Thr-Ala-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O IGROJMCBGRFRGI-YTLHQDLWSA-N 0.000 description 4
- WFUAUEQXPVNAEF-ZJDVBMNYSA-N Thr-Arg-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)O)C(O)=O)CCCN=C(N)N WFUAUEQXPVNAEF-ZJDVBMNYSA-N 0.000 description 4
- JYVKKBDANPZIAW-AVGNSLFASA-N Val-Arg-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C(C)C)N JYVKKBDANPZIAW-AVGNSLFASA-N 0.000 description 4
- 241000710959 Venezuelan equine encephalitis virus Species 0.000 description 4
- 230000005875 antibody response Effects 0.000 description 4
- 108010093581 aspartyl-proline Proteins 0.000 description 4
- 238000010790 dilution Methods 0.000 description 4
- 239000012895 dilution Substances 0.000 description 4
- 239000000539 dimer Substances 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- 210000002950 fibroblast Anatomy 0.000 description 4
- 108010073628 glutamyl-valyl-phenylalanine Proteins 0.000 description 4
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 4
- 108010087823 glycyltyrosine Proteins 0.000 description 4
- 230000006698 induction Effects 0.000 description 4
- 108010051673 leucyl-glycyl-phenylalanine Proteins 0.000 description 4
- 238000012544 monitoring process Methods 0.000 description 4
- 239000002773 nucleotide Substances 0.000 description 4
- 125000003729 nucleotide group Chemical group 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 239000001397 quillaja saponaria molina bark Substances 0.000 description 4
- 229930182490 saponin Natural products 0.000 description 4
- 150000007949 saponins Chemical class 0.000 description 4
- 238000002864 sequence alignment Methods 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 241000894007 species Species 0.000 description 4
- BRPMXFSTKXXNHF-IUCAKERBSA-N (2s)-1-[2-[[(2s)-pyrrolidine-2-carbonyl]amino]acetyl]pyrrolidine-2-carboxylic acid Chemical compound OC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H]1NCCC1 BRPMXFSTKXXNHF-IUCAKERBSA-N 0.000 description 3
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 3
- YLTKNGYYPIWKHZ-ACZMJKKPSA-N Ala-Ala-Glu Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O YLTKNGYYPIWKHZ-ACZMJKKPSA-N 0.000 description 3
- IXTPACPAXIOCRG-ACZMJKKPSA-N Ala-Glu-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CS)C(=O)O)N IXTPACPAXIOCRG-ACZMJKKPSA-N 0.000 description 3
- CBCCCLMNOBLBSC-XVYDVKMFSA-N Ala-His-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(O)=O CBCCCLMNOBLBSC-XVYDVKMFSA-N 0.000 description 3
- 108010011170 Ala-Trp-Arg-His-Pro-Gln-Phe-Gly-Gly Proteins 0.000 description 3
- XCIGOVDXZULBBV-DCAQKATOSA-N Ala-Val-Lys Chemical compound CC(C)[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](CCCCN)C(O)=O XCIGOVDXZULBBV-DCAQKATOSA-N 0.000 description 3
- KJGNDQCYBNBXDA-GUBZILKMSA-N Arg-Arg-Cys Chemical compound C(C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CS)C(=O)O)N)CN=C(N)N KJGNDQCYBNBXDA-GUBZILKMSA-N 0.000 description 3
- NVCIXQYNWYTLDO-IHRRRGAJSA-N Arg-His-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCCN=C(N)N)N NVCIXQYNWYTLDO-IHRRRGAJSA-N 0.000 description 3
- OVQJAKFLFTZDNC-GUBZILKMSA-N Arg-Pro-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(O)=O OVQJAKFLFTZDNC-GUBZILKMSA-N 0.000 description 3
- QMQZYILAWUOLPV-JYJNAYRXSA-N Arg-Tyr-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)CC1=CC=C(O)C=C1 QMQZYILAWUOLPV-JYJNAYRXSA-N 0.000 description 3
- VLIJAPRTSXSGFY-STQMWFEESA-N Arg-Tyr-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=C(O)C=C1 VLIJAPRTSXSGFY-STQMWFEESA-N 0.000 description 3
- JLNFZLNDHONLND-GARJFASQSA-N Asn-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)N)N JLNFZLNDHONLND-GARJFASQSA-N 0.000 description 3
- XOQYDFCQPWAMSA-KKHAAJSZSA-N Asn-Val-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XOQYDFCQPWAMSA-KKHAAJSZSA-N 0.000 description 3
- AITKTFCQOBRJTG-CIUDSAMLSA-N Asp-Leu-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)O)N AITKTFCQOBRJTG-CIUDSAMLSA-N 0.000 description 3
- MNQMTYSEKZHIDF-GCJQMDKQSA-N Asp-Thr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O MNQMTYSEKZHIDF-GCJQMDKQSA-N 0.000 description 3
- PQHYZJPCYRDYNE-QWRGUYRKSA-N Cys-Gly-Phe Chemical compound [H]N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PQHYZJPCYRDYNE-QWRGUYRKSA-N 0.000 description 3
- DGQJGBDBFVGLGL-ZKWXMUAHSA-N Cys-Val-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CS)N DGQJGBDBFVGLGL-ZKWXMUAHSA-N 0.000 description 3
- 102000053602 DNA Human genes 0.000 description 3
- 102220510722 Dual specificity tyrosine-phosphorylation-regulated kinase 2_S98G_mutation Human genes 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- JSYULGSPLTZDHM-NRPADANISA-N Gln-Ala-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O JSYULGSPLTZDHM-NRPADANISA-N 0.000 description 3
- QBLMTCRYYTVUQY-GUBZILKMSA-N Gln-Leu-Asp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O QBLMTCRYYTVUQY-GUBZILKMSA-N 0.000 description 3
- PSERKXGRRADTKA-MNXVOIDGSA-N Gln-Leu-Ile Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O PSERKXGRRADTKA-MNXVOIDGSA-N 0.000 description 3
- WVWZIPOJECFDAG-AVGNSLFASA-N Glu-Phe-Cys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)O)N WVWZIPOJECFDAG-AVGNSLFASA-N 0.000 description 3
- FVGOGEGGQLNZGH-DZKIICNBSA-N Glu-Val-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 FVGOGEGGQLNZGH-DZKIICNBSA-N 0.000 description 3
- GWCRIHNSVMOBEQ-BQBZGAKWSA-N Gly-Arg-Ser Chemical compound [H]NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O GWCRIHNSVMOBEQ-BQBZGAKWSA-N 0.000 description 3
- WZSHYFGOLPXPLL-RYUDHWBXSA-N Gly-Phe-Glu Chemical compound NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O WZSHYFGOLPXPLL-RYUDHWBXSA-N 0.000 description 3
- ABPRMMYHROQBLY-NKWVEPMBSA-N Gly-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)CN)C(=O)O ABPRMMYHROQBLY-NKWVEPMBSA-N 0.000 description 3
- GNNJKUYDWFIBTK-QWRGUYRKSA-N Gly-Tyr-Asp Chemical compound [H]NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O GNNJKUYDWFIBTK-QWRGUYRKSA-N 0.000 description 3
- 102000003886 Glycoproteins Human genes 0.000 description 3
- 108090000288 Glycoproteins Proteins 0.000 description 3
- FFYYUUWROYYKFY-IHRRRGAJSA-N His-Val-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O FFYYUUWROYYKFY-IHRRRGAJSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- VEPIBPGLTLPBDW-URLPEUOOSA-N Ile-Phe-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N VEPIBPGLTLPBDW-URLPEUOOSA-N 0.000 description 3
- KBDIBHQICWDGDL-PPCPHDFISA-N Ile-Thr-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)O)N KBDIBHQICWDGDL-PPCPHDFISA-N 0.000 description 3
- SENJXOPIZNYLHU-UHFFFAOYSA-N L-leucyl-L-arginine Natural products CC(C)CC(N)C(=O)NC(C(O)=O)CCCN=C(N)N SENJXOPIZNYLHU-UHFFFAOYSA-N 0.000 description 3
- KFKWRHQBZQICHA-STQMWFEESA-N L-leucyl-L-phenylalanine Natural products CC(C)C[C@H](N)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 KFKWRHQBZQICHA-STQMWFEESA-N 0.000 description 3
- FJUKMPUELVROGK-IHRRRGAJSA-N Leu-Arg-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N FJUKMPUELVROGK-IHRRRGAJSA-N 0.000 description 3
- YOKVEHGYYQEQOP-QWRGUYRKSA-N Leu-Leu-Gly Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O YOKVEHGYYQEQOP-QWRGUYRKSA-N 0.000 description 3
- ZGUMORRUBUCXEH-AVGNSLFASA-N Leu-Lys-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZGUMORRUBUCXEH-AVGNSLFASA-N 0.000 description 3
- DPURXCQCHSQPAN-AVGNSLFASA-N Leu-Pro-Pro Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DPURXCQCHSQPAN-AVGNSLFASA-N 0.000 description 3
- SQUFDMCWMFOEBA-KKUMJFAQSA-N Leu-Ser-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 SQUFDMCWMFOEBA-KKUMJFAQSA-N 0.000 description 3
- FDBTVENULFNTAL-XQQFMLRXSA-N Leu-Val-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N FDBTVENULFNTAL-XQQFMLRXSA-N 0.000 description 3
- DSWOTZCVCBEPOU-IUCAKERBSA-N Met-Arg-Gly Chemical compound CSCC[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CCCNC(N)=N DSWOTZCVCBEPOU-IUCAKERBSA-N 0.000 description 3
- JUXONJROIXKHEV-GUBZILKMSA-N Met-Cys-Arg Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCNC(N)=N JUXONJROIXKHEV-GUBZILKMSA-N 0.000 description 3
- ZBLSZPYQQRIHQU-RCWTZXSCSA-N Met-Thr-Val Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O ZBLSZPYQQRIHQU-RCWTZXSCSA-N 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 3
- JIYJYFIXQTYDNF-YDHLFZDLSA-N Phe-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CC=CC=C1)N JIYJYFIXQTYDNF-YDHLFZDLSA-N 0.000 description 3
- GCFNFKNPCMBHNT-IRXDYDNUSA-N Phe-Tyr-Gly Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)NCC(=O)O)N GCFNFKNPCMBHNT-IRXDYDNUSA-N 0.000 description 3
- NUZHSNLQJDYSRW-BZSNNMDCSA-N Pro-Arg-Trp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O NUZHSNLQJDYSRW-BZSNNMDCSA-N 0.000 description 3
- WPQKSRHDTMRSJM-CIUDSAMLSA-N Pro-Asp-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1 WPQKSRHDTMRSJM-CIUDSAMLSA-N 0.000 description 3
- NMELOOXSGDRBRU-YUMQZZPRSA-N Pro-Glu-Gly Chemical compound OC(=O)CNC(=O)[C@H](CCC(=O)O)NC(=O)[C@@H]1CCCN1 NMELOOXSGDRBRU-YUMQZZPRSA-N 0.000 description 3
- XYSXOCIWCPFOCG-IHRRRGAJSA-N Pro-Leu-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O XYSXOCIWCPFOCG-IHRRRGAJSA-N 0.000 description 3
- FKYKZHOKDOPHSA-DCAQKATOSA-N Pro-Leu-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O FKYKZHOKDOPHSA-DCAQKATOSA-N 0.000 description 3
- SUENWIFTSTWUKD-AVGNSLFASA-N Pro-Leu-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O SUENWIFTSTWUKD-AVGNSLFASA-N 0.000 description 3
- LNICFEXCAHIJOR-DCAQKATOSA-N Pro-Ser-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O LNICFEXCAHIJOR-DCAQKATOSA-N 0.000 description 3
- FIODMZKLZFLYQP-GUBZILKMSA-N Pro-Val-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O FIODMZKLZFLYQP-GUBZILKMSA-N 0.000 description 3
- YDTUEBLEAVANFH-RCWTZXSCSA-N Pro-Val-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 YDTUEBLEAVANFH-RCWTZXSCSA-N 0.000 description 3
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 3
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 3
- JNQZPAWOPBZGIX-RCWTZXSCSA-N Thr-Arg-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)[C@@H](C)O)CCCN=C(N)N JNQZPAWOPBZGIX-RCWTZXSCSA-N 0.000 description 3
- AIISTODACBDQLW-WDSOQIARSA-N Trp-Leu-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 AIISTODACBDQLW-WDSOQIARSA-N 0.000 description 3
- DLZKEQQWXODGGZ-KWQFWETISA-N Tyr-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 DLZKEQQWXODGGZ-KWQFWETISA-N 0.000 description 3
- PZXUIGWOEWWFQM-SRVKXCTJSA-N Tyr-Asn-Asn Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O PZXUIGWOEWWFQM-SRVKXCTJSA-N 0.000 description 3
- ZRPLVTZTKPPSBT-AVGNSLFASA-N Tyr-Glu-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZRPLVTZTKPPSBT-AVGNSLFASA-N 0.000 description 3
- QFXVAFIHVWXXBJ-AVGNSLFASA-N Tyr-Ser-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O QFXVAFIHVWXXBJ-AVGNSLFASA-N 0.000 description 3
- 101150032047 UL131 gene Proteins 0.000 description 3
- ZLFHAAGHGQBQQN-GUBZILKMSA-N Val-Ala-Pro Natural products CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O ZLFHAAGHGQBQQN-GUBZILKMSA-N 0.000 description 3
- NZYNRRGJJVSSTJ-GUBZILKMSA-N Val-Ser-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NZYNRRGJJVSSTJ-GUBZILKMSA-N 0.000 description 3
- BGTDGENDNWGMDQ-KJEVXHAQSA-N Val-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](C(C)C)N)O BGTDGENDNWGMDQ-KJEVXHAQSA-N 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 239000004599 antimicrobial Substances 0.000 description 3
- 108010077245 asparaginyl-proline Proteins 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 108010060199 cysteinylproline Proteins 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 230000003511 endothelial effect Effects 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 230000004927 fusion Effects 0.000 description 3
- 108010072405 glycyl-aspartyl-glycine Proteins 0.000 description 3
- 108010050848 glycylleucine Proteins 0.000 description 3
- 108010077515 glycylproline Proteins 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 239000000411 inducer Substances 0.000 description 3
- 229940079322 interferon Drugs 0.000 description 3
- 238000007918 intramuscular administration Methods 0.000 description 3
- 210000003734 kidney Anatomy 0.000 description 3
- 108010034529 leucyl-lysine Proteins 0.000 description 3
- 108010044056 leucyl-phenylalanine Proteins 0.000 description 3
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 3
- 108010000761 leucylarginine Proteins 0.000 description 3
- 108010057821 leucylproline Proteins 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 239000007908 nanoemulsion Substances 0.000 description 3
- 108010051242 phenylalanylserine Proteins 0.000 description 3
- 108010014614 prolyl-glycyl-proline Proteins 0.000 description 3
- 230000010076 replication Effects 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 3
- 229910052717 sulfur Inorganic materials 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 229940044616 toll-like receptor 7 agonist Drugs 0.000 description 3
- 108010087967 type I signal peptidase Proteins 0.000 description 3
- 108010003137 tyrosyltyrosine Proteins 0.000 description 3
- 230000029812 viral genome replication Effects 0.000 description 3
- YYGNTYWPHWGJRM-UHFFFAOYSA-N (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene Chemical compound CC(C)=CCCC(C)=CCCC(C)=CCCC=C(C)CCC=C(C)CCC=C(C)C YYGNTYWPHWGJRM-UHFFFAOYSA-N 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- SOBIAADAMRHGKH-CIUDSAMLSA-N Ala-Leu-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O SOBIAADAMRHGKH-CIUDSAMLSA-N 0.000 description 2
- 108010011667 Ala-Phe-Ala Proteins 0.000 description 2
- BVLPIIBTWIYOML-ZKWXMUAHSA-N Ala-Val-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O BVLPIIBTWIYOML-ZKWXMUAHSA-N 0.000 description 2
- KMSHNDWHPWXPEC-BQBZGAKWSA-N Arg-Asp-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O KMSHNDWHPWXPEC-BQBZGAKWSA-N 0.000 description 2
- XRLOBFSLPCHYLQ-ULQDDVLXSA-N Arg-Tyr-His Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O XRLOBFSLPCHYLQ-ULQDDVLXSA-N 0.000 description 2
- NECWUSYTYSIFNC-DLOVCJGASA-N Asp-Ala-Phe Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 NECWUSYTYSIFNC-DLOVCJGASA-N 0.000 description 2
- MFMJRYHVLLEMQM-DCAQKATOSA-N Asp-Arg-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(=O)O)N MFMJRYHVLLEMQM-DCAQKATOSA-N 0.000 description 2
- PXLNPFOJZQMXAT-BYULHYEWSA-N Asp-Asp-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(O)=O PXLNPFOJZQMXAT-BYULHYEWSA-N 0.000 description 2
- VAWNQIGQPUOPQW-ACZMJKKPSA-N Asp-Glu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O VAWNQIGQPUOPQW-ACZMJKKPSA-N 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 241000282465 Canis Species 0.000 description 2
- 208000003322 Coinfection Diseases 0.000 description 2
- NITLUESFANGEIW-BQBZGAKWSA-N Cys-Pro-Gly Chemical compound [H]N[C@@H](CS)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O NITLUESFANGEIW-BQBZGAKWSA-N 0.000 description 2
- 108010041986 DNA Vaccines Proteins 0.000 description 2
- 229940021995 DNA vaccine Drugs 0.000 description 2
- 241000702421 Dependoparvovirus Species 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 206010066919 Epidemic polyarthritis Diseases 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 2
- NSNUZSPSADIMJQ-WDSKDSINSA-N Gln-Gly-Asp Chemical compound NC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O NSNUZSPSADIMJQ-WDSKDSINSA-N 0.000 description 2
- ORYMMTRPKVTGSJ-XVKPBYJWSA-N Gln-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O ORYMMTRPKVTGSJ-XVKPBYJWSA-N 0.000 description 2
- FCKPEGOCSVZPNC-WHOFXGATSA-N Gly-Ile-Phe Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 FCKPEGOCSVZPNC-WHOFXGATSA-N 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- 101001090901 Homo sapiens Retroelement silencing factor 1 Proteins 0.000 description 2
- 101000864837 Homo sapiens SIN3-HDAC complex-associated factor Proteins 0.000 description 2
- 241000701041 Human betaherpesvirus 7 Species 0.000 description 2
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 2
- 241000701027 Human herpesvirus 6 Species 0.000 description 2
- HPCFRQWLTRDGHT-AJNGGQMLSA-N Ile-Leu-Leu Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O HPCFRQWLTRDGHT-AJNGGQMLSA-N 0.000 description 2
- XLXPYSDGMXTTNQ-UHFFFAOYSA-N Ile-Phe-Leu Natural products CCC(C)C(N)C(=O)NC(C(=O)NC(CC(C)C)C(O)=O)CC1=CC=CC=C1 XLXPYSDGMXTTNQ-UHFFFAOYSA-N 0.000 description 2
- YHFPHRUWZMEOIX-CYDGBPFRSA-N Ile-Val-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)O)N YHFPHRUWZMEOIX-CYDGBPFRSA-N 0.000 description 2
- 108020004684 Internal Ribosome Entry Sites Proteins 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- 241000880493 Leptailurus serval Species 0.000 description 2
- BQSLGJHIAGOZCD-CIUDSAMLSA-N Leu-Ala-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O BQSLGJHIAGOZCD-CIUDSAMLSA-N 0.000 description 2
- FIJMQLGQLBLBOL-HJGDQZAQSA-N Leu-Asn-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O FIJMQLGQLBLBOL-HJGDQZAQSA-N 0.000 description 2
- KGCLIYGPQXUNLO-IUCAKERBSA-N Leu-Gly-Glu Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(O)=O KGCLIYGPQXUNLO-IUCAKERBSA-N 0.000 description 2
- KOSWSHVQIVTVQF-ZPFDUUQYSA-N Leu-Ile-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O KOSWSHVQIVTVQF-ZPFDUUQYSA-N 0.000 description 2
- DSFYPIUSAMSERP-IHRRRGAJSA-N Leu-Leu-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N DSFYPIUSAMSERP-IHRRRGAJSA-N 0.000 description 2
- PDQDCFBVYXEFSD-SRVKXCTJSA-N Leu-Leu-Asp Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O PDQDCFBVYXEFSD-SRVKXCTJSA-N 0.000 description 2
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 2
- YUTNOGOMBNYPFH-XUXIUFHCSA-N Leu-Pro-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O YUTNOGOMBNYPFH-XUXIUFHCSA-N 0.000 description 2
- ADJWHHZETYAAAX-SRVKXCTJSA-N Leu-Ser-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N ADJWHHZETYAAAX-SRVKXCTJSA-N 0.000 description 2
- 101710175625 Maltose/maltodextrin-binding periplasmic protein Proteins 0.000 description 2
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 2
- LXUJDHOKVUYHRC-KKUMJFAQSA-N Phe-Cys-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CC1=CC=CC=C1)N LXUJDHOKVUYHRC-KKUMJFAQSA-N 0.000 description 2
- YKUGPVXSDOOANW-KKUMJFAQSA-N Phe-Leu-Asp Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O YKUGPVXSDOOANW-KKUMJFAQSA-N 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- 102100034981 Retroelement silencing factor 1 Human genes 0.000 description 2
- 241000710942 Ross River virus Species 0.000 description 2
- 102100030066 SIN3-HDAC complex-associated factor Human genes 0.000 description 2
- 229920002684 Sepharose Polymers 0.000 description 2
- AEGUWTFAQQWVLC-BQBZGAKWSA-N Ser-Gly-Arg Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O AEGUWTFAQQWVLC-BQBZGAKWSA-N 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 108010090804 Streptavidin Proteins 0.000 description 2
- BHEOSNUKNHRBNM-UHFFFAOYSA-N Tetramethylsqualene Natural products CC(=C)C(C)CCC(=C)C(C)CCC(C)=CCCC=C(C)CCC(C)C(=C)CCC(C)C(C)=C BHEOSNUKNHRBNM-UHFFFAOYSA-N 0.000 description 2
- 108010028230 Trp-Ser- His-Pro-Gln-Phe-Glu-Lys Proteins 0.000 description 2
- TVOGEPLDNYTAHD-CQDKDKBSSA-N Tyr-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 TVOGEPLDNYTAHD-CQDKDKBSSA-N 0.000 description 2
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 2
- DVLWZWNAQUBZBC-ZNSHCXBVSA-N Val-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](C(C)C)N)O DVLWZWNAQUBZBC-ZNSHCXBVSA-N 0.000 description 2
- 108010087302 Viral Structural Proteins Proteins 0.000 description 2
- 208000036142 Viral infection Diseases 0.000 description 2
- 238000001261 affinity purification Methods 0.000 description 2
- 108010011559 alanylphenylalanine Proteins 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 230000003190 augmentative effect Effects 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 230000021615 conjugation Effects 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 2
- 238000006471 dimerization reaction Methods 0.000 description 2
- PRAKJMSDJKAYCZ-UHFFFAOYSA-N dodecahydrosqualene Natural products CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C PRAKJMSDJKAYCZ-UHFFFAOYSA-N 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 108020001507 fusion proteins Proteins 0.000 description 2
- 102000037865 fusion proteins Human genes 0.000 description 2
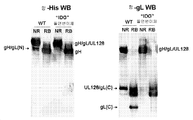
- 101150015940 gL gene Proteins 0.000 description 2
- 108010049041 glutamylalanine Proteins 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 108010050343 histidyl-alanyl-glutamine Proteins 0.000 description 2
- 108010092114 histidylphenylalanine Proteins 0.000 description 2
- 230000028996 humoral immune response Effects 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 239000002955 immunomodulating agent Substances 0.000 description 2
- 229940121354 immunomodulator Drugs 0.000 description 2
- 230000003308 immunostimulating effect Effects 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000005342 ion exchange Methods 0.000 description 2
- 230000021633 leukocyte mediated immunity Effects 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 244000052769 pathogen Species 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 108010070643 prolylglutamic acid Proteins 0.000 description 2
- 108010090894 prolylleucine Proteins 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 235000004252 protein component Nutrition 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000001177 retroviral effect Effects 0.000 description 2
- 102200012486 rs111033669 Human genes 0.000 description 2
- 229940031439 squalene Drugs 0.000 description 2
- TUHBEKDERLKLEC-UHFFFAOYSA-N squalene Natural products CC(=CCCC(=CCCC(=CCCC=C(/C)CCC=C(/C)CC=C(C)C)C)C)C TUHBEKDERLKLEC-UHFFFAOYSA-N 0.000 description 2
- 238000012916 structural analysis Methods 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 238000011282 treatment Methods 0.000 description 2
- 108010027345 wheylin-1 peptide Proteins 0.000 description 2
- GJLXVWOMRRWCIB-MERZOTPQSA-N (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-acetamido-5-(diaminomethylideneamino)pentanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanamide Chemical compound C([C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(N)=O)C1=CC=C(O)C=C1 GJLXVWOMRRWCIB-MERZOTPQSA-N 0.000 description 1
- BVAUMRCGVHUWOZ-ZETCQYMHSA-N (2s)-2-(cyclohexylazaniumyl)propanoate Chemical compound OC(=O)[C@H](C)NC1CCCCC1 BVAUMRCGVHUWOZ-ZETCQYMHSA-N 0.000 description 1
- LDUWTIUXPVCEQF-LURJTMIESA-N (2s)-2-(cyclopentylamino)propanoic acid Chemical compound OC(=O)[C@H](C)NC1CCCC1 LDUWTIUXPVCEQF-LURJTMIESA-N 0.000 description 1
- IYKLZBIWFXPUCS-VIFPVBQESA-N (2s)-2-(naphthalen-1-ylamino)propanoic acid Chemical compound C1=CC=C2C(N[C@@H](C)C(O)=O)=CC=CC2=C1 IYKLZBIWFXPUCS-VIFPVBQESA-N 0.000 description 1
- IGXNPQWXIRIGBF-KEOOTSPTSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-amino-3-(1h-imidazol-5-yl)propanoyl]amino]-3-(1h-imidazol-5-yl)propanoyl]amino]-3-(1h-imidazol-5-yl)propanoyl]amino]-3-(1h-imidazol-5-yl)propanoyl]amino]-3-(1h-imidazol-5-yl)propanoic acid Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1NC=NC=1)C(O)=O)C1=CN=CN1 IGXNPQWXIRIGBF-KEOOTSPTSA-N 0.000 description 1
- RSPOGBIHKNKRFJ-FSPLSTOPSA-N (2s,3s)-2-amino-2,3-dimethylpentanoic acid Chemical compound CC[C@H](C)[C@](C)(N)C(O)=O RSPOGBIHKNKRFJ-FSPLSTOPSA-N 0.000 description 1
- NUUYZLOGRAXQBP-UHFFFAOYSA-N 1,1-diphenoxyethanol Chemical compound C=1C=CC=CC=1OC(O)(C)OC1=CC=CC=C1 NUUYZLOGRAXQBP-UHFFFAOYSA-N 0.000 description 1
- PIDRBUDUWHBYSR-UHFFFAOYSA-N 1-[2-[[2-[(2-amino-4-methylpentanoyl)amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carboxylic acid Chemical compound CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O PIDRBUDUWHBYSR-UHFFFAOYSA-N 0.000 description 1
- ZADWXFSZEAPBJS-JTQLQIEISA-N 1-methyl-L-tryptophan Chemical compound C1=CC=C2N(C)C=C(C[C@H](N)C(O)=O)C2=C1 ZADWXFSZEAPBJS-JTQLQIEISA-N 0.000 description 1
- KSXTUUUQYQYKCR-LQDDAWAPSA-M 2,3-bis[[(z)-octadec-9-enoyl]oxy]propyl-trimethylazanium;chloride Chemical compound [Cl-].CCCCCCCC\C=C/CCCCCCCC(=O)OCC(C[N+](C)(C)C)OC(=O)CCCCCCC\C=C/CCCCCCCC KSXTUUUQYQYKCR-LQDDAWAPSA-M 0.000 description 1
- ARSWQPLPYROOBG-ZETCQYMHSA-N 2-methylleucine Chemical compound CC(C)C[C@](C)(N)C(O)=O ARSWQPLPYROOBG-ZETCQYMHSA-N 0.000 description 1
- 108010036211 5-HT-moduline Proteins 0.000 description 1
- AAQGRPOPTAUUBM-ZLUOBGJFSA-N Ala-Ala-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O AAQGRPOPTAUUBM-ZLUOBGJFSA-N 0.000 description 1
- CXRCVCURMBFFOL-FXQIFTODSA-N Ala-Ala-Pro Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O CXRCVCURMBFFOL-FXQIFTODSA-N 0.000 description 1
- FXKNPWNXPQZLES-ZLUOBGJFSA-N Ala-Asn-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O FXKNPWNXPQZLES-ZLUOBGJFSA-N 0.000 description 1
- YIGLXQRFQVWFEY-NRPADANISA-N Ala-Gln-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O YIGLXQRFQVWFEY-NRPADANISA-N 0.000 description 1
- BGNLUHXLSAQYRQ-FXQIFTODSA-N Ala-Glu-Gln Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O BGNLUHXLSAQYRQ-FXQIFTODSA-N 0.000 description 1
- PCIFXPRIFWKWLK-YUMQZZPRSA-N Ala-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@H](C)N PCIFXPRIFWKWLK-YUMQZZPRSA-N 0.000 description 1
- VNYMOTCMNHJGTG-JBDRJPRFSA-N Ala-Ile-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O VNYMOTCMNHJGTG-JBDRJPRFSA-N 0.000 description 1
- YHKANGMVQWRMAP-DCAQKATOSA-N Ala-Leu-Arg Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N YHKANGMVQWRMAP-DCAQKATOSA-N 0.000 description 1
- LBYMZCVBOKYZNS-CIUDSAMLSA-N Ala-Leu-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O LBYMZCVBOKYZNS-CIUDSAMLSA-N 0.000 description 1
- MEFILNJXAVSUTO-JXUBOQSCSA-N Ala-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MEFILNJXAVSUTO-JXUBOQSCSA-N 0.000 description 1
- XRUJOVRWNMBAAA-NHCYSSNCSA-N Ala-Phe-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)C)CC1=CC=CC=C1 XRUJOVRWNMBAAA-NHCYSSNCSA-N 0.000 description 1
- CQJHFKKGZXKZBC-BPNCWPANSA-N Ala-Pro-Tyr Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 CQJHFKKGZXKZBC-BPNCWPANSA-N 0.000 description 1
- ARHJJAAWNWOACN-FXQIFTODSA-N Ala-Ser-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O ARHJJAAWNWOACN-FXQIFTODSA-N 0.000 description 1
- YNOCMHZSWJMGBB-GCJQMDKQSA-N Ala-Thr-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O YNOCMHZSWJMGBB-GCJQMDKQSA-N 0.000 description 1
- BOKLLPVAQDSLHC-FXQIFTODSA-N Ala-Val-Cys Chemical compound C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(=O)O)N BOKLLPVAQDSLHC-FXQIFTODSA-N 0.000 description 1
- REWSWYIDQIELBE-FXQIFTODSA-N Ala-Val-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O REWSWYIDQIELBE-FXQIFTODSA-N 0.000 description 1
- 239000012103 Alexa Fluor 488 Substances 0.000 description 1
- 229920000856 Amylose Polymers 0.000 description 1
- 101001073212 Arabidopsis thaliana Peroxidase 33 Proteins 0.000 description 1
- DPNHSNLIULPOBH-GUBZILKMSA-N Arg-Asn-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCN=C(N)N)N DPNHSNLIULPOBH-GUBZILKMSA-N 0.000 description 1
- IGULQRCJLQQPSM-DCAQKATOSA-N Arg-Cys-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O IGULQRCJLQQPSM-DCAQKATOSA-N 0.000 description 1
- FEZJJKXNPSEYEV-CIUDSAMLSA-N Arg-Gln-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O FEZJJKXNPSEYEV-CIUDSAMLSA-N 0.000 description 1
- VDBKFYYIBLXEIF-GUBZILKMSA-N Arg-Gln-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O VDBKFYYIBLXEIF-GUBZILKMSA-N 0.000 description 1
- OHYQKYUTLIPFOX-ZPFDUUQYSA-N Arg-Glu-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O OHYQKYUTLIPFOX-ZPFDUUQYSA-N 0.000 description 1
- ZZZWQALDSQQBEW-STQMWFEESA-N Arg-Gly-Tyr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZZZWQALDSQQBEW-STQMWFEESA-N 0.000 description 1
- OCDJOVKIUJVUMO-SRVKXCTJSA-N Arg-His-Gln Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N OCDJOVKIUJVUMO-SRVKXCTJSA-N 0.000 description 1
- YKBHOXLMMPZPHQ-GMOBBJLQSA-N Arg-Ile-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O YKBHOXLMMPZPHQ-GMOBBJLQSA-N 0.000 description 1
- NMRHDSAOIURTNT-RWMBFGLXSA-N Arg-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N NMRHDSAOIURTNT-RWMBFGLXSA-N 0.000 description 1
- OISWSORSLQOGFV-AVGNSLFASA-N Arg-Met-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CCCN=C(N)N OISWSORSLQOGFV-AVGNSLFASA-N 0.000 description 1
- URAUIUGLHBRPMF-NAKRPEOUSA-N Arg-Ser-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O URAUIUGLHBRPMF-NAKRPEOUSA-N 0.000 description 1
- BXLDDWZOTGGNOJ-SZMVWBNQSA-N Arg-Trp-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CCCN=C(N)N)N BXLDDWZOTGGNOJ-SZMVWBNQSA-N 0.000 description 1
- PSUXEQYPYZLNER-QXEWZRGKSA-N Arg-Val-Asn Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O PSUXEQYPYZLNER-QXEWZRGKSA-N 0.000 description 1
- 241000238421 Arthropoda Species 0.000 description 1
- CMLGVVWQQHUXOZ-GHCJXIJMSA-N Asn-Ala-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O CMLGVVWQQHUXOZ-GHCJXIJMSA-N 0.000 description 1
- KWQPAXYXVMHJJR-AVGNSLFASA-N Asn-Gln-Tyr Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 KWQPAXYXVMHJJR-AVGNSLFASA-N 0.000 description 1
- SEKBHZJLARBNPB-GHCJXIJMSA-N Asn-Ile-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O SEKBHZJLARBNPB-GHCJXIJMSA-N 0.000 description 1
- MVXJBVVLACEGCG-PCBIJLKTSA-N Asn-Phe-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O MVXJBVVLACEGCG-PCBIJLKTSA-N 0.000 description 1
- VLDRQOHCMKCXLY-SRVKXCTJSA-N Asn-Ser-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O VLDRQOHCMKCXLY-SRVKXCTJSA-N 0.000 description 1
- UXHYOWXTJLBEPG-GSSVUCPTSA-N Asn-Thr-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O UXHYOWXTJLBEPG-GSSVUCPTSA-N 0.000 description 1
- DATSKXOXPUAOLK-KKUMJFAQSA-N Asn-Tyr-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(O)=O DATSKXOXPUAOLK-KKUMJFAQSA-N 0.000 description 1
- KVMPVNGOKHTUHZ-GCJQMDKQSA-N Asp-Ala-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KVMPVNGOKHTUHZ-GCJQMDKQSA-N 0.000 description 1
- SBHUBSDEZQFJHJ-CIUDSAMLSA-N Asp-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(O)=O SBHUBSDEZQFJHJ-CIUDSAMLSA-N 0.000 description 1
- BFOYULZBKYOKAN-OLHMAJIHSA-N Asp-Asp-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O BFOYULZBKYOKAN-OLHMAJIHSA-N 0.000 description 1
- ZSVJVIOVABDTTL-YUMQZZPRSA-N Asp-Gly-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)O)N ZSVJVIOVABDTTL-YUMQZZPRSA-N 0.000 description 1
- POTCZYQVVNXUIG-BQBZGAKWSA-N Asp-Gly-Pro Chemical compound OC(=O)C[C@H](N)C(=O)NCC(=O)N1CCC[C@H]1C(O)=O POTCZYQVVNXUIG-BQBZGAKWSA-N 0.000 description 1
- SNDBKTFJWVEVPO-WHFBIAKZSA-N Asp-Gly-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(O)=O SNDBKTFJWVEVPO-WHFBIAKZSA-N 0.000 description 1
- ICZWAZVKLACMKR-CIUDSAMLSA-N Asp-His-Ser Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CN=CN1 ICZWAZVKLACMKR-CIUDSAMLSA-N 0.000 description 1
- JNNVNVRBYUJYGS-CIUDSAMLSA-N Asp-Leu-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O JNNVNVRBYUJYGS-CIUDSAMLSA-N 0.000 description 1
- UJGRZQYSNYTCAX-SRVKXCTJSA-N Asp-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(O)=O UJGRZQYSNYTCAX-SRVKXCTJSA-N 0.000 description 1
- UMHUHHJMEXNSIV-CIUDSAMLSA-N Asp-Leu-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(O)=O UMHUHHJMEXNSIV-CIUDSAMLSA-N 0.000 description 1
- GYWQGGUCMDCUJE-DLOVCJGASA-N Asp-Phe-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(O)=O GYWQGGUCMDCUJE-DLOVCJGASA-N 0.000 description 1
- BKOIIURTQAJHAT-GUBZILKMSA-N Asp-Pro-Pro Chemical compound OC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 BKOIIURTQAJHAT-GUBZILKMSA-N 0.000 description 1
- YODBPLSWNJMZOJ-BPUTZDHNSA-N Asp-Trp-Arg Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CC(=O)O)N YODBPLSWNJMZOJ-BPUTZDHNSA-N 0.000 description 1
- PLNJUJGNLDSFOP-UWJYBYFXSA-N Asp-Tyr-Ala Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O PLNJUJGNLDSFOP-UWJYBYFXSA-N 0.000 description 1
- 102100021277 Beta-secretase 2 Human genes 0.000 description 1
- 101710150190 Beta-secretase 2 Proteins 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 101100342815 Caenorhabditis elegans lec-1 gene Proteins 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 241000724252 Cucumber mosaic virus Species 0.000 description 1
- ZOLXQKZHYOHHMD-DLOVCJGASA-N Cys-Ala-Phe Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CS)N ZOLXQKZHYOHHMD-DLOVCJGASA-N 0.000 description 1
- CEZSLNCYQUFOSL-BQBZGAKWSA-N Cys-Arg-Gly Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O CEZSLNCYQUFOSL-BQBZGAKWSA-N 0.000 description 1
- ZIKWRNJXFIQECJ-CIUDSAMLSA-N Cys-Cys-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O ZIKWRNJXFIQECJ-CIUDSAMLSA-N 0.000 description 1
- LMXOUGMSGHFLRX-CIUDSAMLSA-N Cys-Gln-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CS)N LMXOUGMSGHFLRX-CIUDSAMLSA-N 0.000 description 1
- HHABWQIFXZPZCK-ACZMJKKPSA-N Cys-Gln-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N HHABWQIFXZPZCK-ACZMJKKPSA-N 0.000 description 1
- WVLZTXGTNGHPBO-SRVKXCTJSA-N Cys-Leu-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O WVLZTXGTNGHPBO-SRVKXCTJSA-N 0.000 description 1
- MBRWOKXNHTUJMB-CIUDSAMLSA-N Cys-Pro-Glu Chemical compound [H]N[C@@H](CS)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O MBRWOKXNHTUJMB-CIUDSAMLSA-N 0.000 description 1
- XBELMDARIGXDKY-GUBZILKMSA-N Cys-Pro-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CS)N XBELMDARIGXDKY-GUBZILKMSA-N 0.000 description 1
- YNJBLTDKTMKEET-ZLUOBGJFSA-N Cys-Ser-Ser Chemical compound SC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O YNJBLTDKTMKEET-ZLUOBGJFSA-N 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 229920002271 DEAE-Sepharose Polymers 0.000 description 1
- GUBGYTABKSRVRQ-WFVLMXAXSA-N DEAE-cellulose Chemical compound OC1C(O)C(O)C(CO)O[C@H]1O[C@@H]1C(CO)OC(O)C(O)C1O GUBGYTABKSRVRQ-WFVLMXAXSA-N 0.000 description 1
- 230000004543 DNA replication Effects 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- XZWYTXMRWQJBGX-VXBMVYAYSA-N FLAG peptide Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)CC1=CC=C(O)C=C1 XZWYTXMRWQJBGX-VXBMVYAYSA-N 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- YNNXQZDEOCYJJL-CIUDSAMLSA-N Gln-Arg-Asp Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)CN=C(N)N YNNXQZDEOCYJJL-CIUDSAMLSA-N 0.000 description 1
- TWHDOEYLXXQYOZ-FXQIFTODSA-N Gln-Asn-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N TWHDOEYLXXQYOZ-FXQIFTODSA-N 0.000 description 1
- WLODHVXYKYHLJD-ACZMJKKPSA-N Gln-Asp-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CO)C(=O)O)N WLODHVXYKYHLJD-ACZMJKKPSA-N 0.000 description 1
- COYGBRTZEVWZBW-XKBZYTNZSA-N Gln-Cys-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CCC(N)=O COYGBRTZEVWZBW-XKBZYTNZSA-N 0.000 description 1
- NKCZYEDZTKOFBG-GUBZILKMSA-N Gln-Gln-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NKCZYEDZTKOFBG-GUBZILKMSA-N 0.000 description 1
- KVXVVDFOZNYYKZ-DCAQKATOSA-N Gln-Gln-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O KVXVVDFOZNYYKZ-DCAQKATOSA-N 0.000 description 1
- PNENQZWRFMUZOM-DCAQKATOSA-N Gln-Glu-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O PNENQZWRFMUZOM-DCAQKATOSA-N 0.000 description 1
- HDUDGCZEOZEFOA-KBIXCLLPSA-N Gln-Ile-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CCC(=O)N)N HDUDGCZEOZEFOA-KBIXCLLPSA-N 0.000 description 1
- HWEINOMSWQSJDC-SRVKXCTJSA-N Gln-Leu-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O HWEINOMSWQSJDC-SRVKXCTJSA-N 0.000 description 1
- UWMDGPFFTKDUIY-HJGDQZAQSA-N Gln-Pro-Thr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O UWMDGPFFTKDUIY-HJGDQZAQSA-N 0.000 description 1
- XIYWAJQIWLXXAF-XKBZYTNZSA-N Gln-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O XIYWAJQIWLXXAF-XKBZYTNZSA-N 0.000 description 1
- OUBUHIODTNUUTC-WDCWCFNPSA-N Gln-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O OUBUHIODTNUUTC-WDCWCFNPSA-N 0.000 description 1
- VDMABHYXBULDGN-LAEOZQHASA-N Gln-Val-Asp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O VDMABHYXBULDGN-LAEOZQHASA-N 0.000 description 1
- UTKUTMJSWKKHEM-WDSKDSINSA-N Glu-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O UTKUTMJSWKKHEM-WDSKDSINSA-N 0.000 description 1
- ITYRYNUZHPNCIK-GUBZILKMSA-N Glu-Ala-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O ITYRYNUZHPNCIK-GUBZILKMSA-N 0.000 description 1
- ATRHMOJQJWPVBQ-DRZSPHRISA-N Glu-Ala-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O ATRHMOJQJWPVBQ-DRZSPHRISA-N 0.000 description 1
- RSUVOPBMWMTVDI-XEGUGMAKSA-N Glu-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C)C(O)=O)=CNC2=C1 RSUVOPBMWMTVDI-XEGUGMAKSA-N 0.000 description 1
- GCYFUZJHAXJKKE-KKUMJFAQSA-N Glu-Arg-Tyr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O GCYFUZJHAXJKKE-KKUMJFAQSA-N 0.000 description 1
- PAQUJCSYVIBPLC-AVGNSLFASA-N Glu-Asp-Phe Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 PAQUJCSYVIBPLC-AVGNSLFASA-N 0.000 description 1
- ZZIFPJZQHRJERU-WDSKDSINSA-N Glu-Cys-Gly Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CS)C(=O)NCC(O)=O ZZIFPJZQHRJERU-WDSKDSINSA-N 0.000 description 1
- CUXJIASLBRJOFV-LAEOZQHASA-N Glu-Gly-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O CUXJIASLBRJOFV-LAEOZQHASA-N 0.000 description 1
- VSRCAOIHMGCIJK-SRVKXCTJSA-N Glu-Leu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VSRCAOIHMGCIJK-SRVKXCTJSA-N 0.000 description 1
- MWMJCGBSIORNCD-AVGNSLFASA-N Glu-Leu-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O MWMJCGBSIORNCD-AVGNSLFASA-N 0.000 description 1
- YGLCLCMAYUYZSG-AVGNSLFASA-N Glu-Lys-His Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 YGLCLCMAYUYZSG-AVGNSLFASA-N 0.000 description 1
- TZXOPHFCAATANZ-QEJZJMRPSA-N Glu-Ser-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)O)N TZXOPHFCAATANZ-QEJZJMRPSA-N 0.000 description 1
- GPSHCSTUYOQPAI-JHEQGTHGSA-N Glu-Thr-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O GPSHCSTUYOQPAI-JHEQGTHGSA-N 0.000 description 1
- YQAQQKPWFOBSMU-WDCWCFNPSA-N Glu-Thr-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O YQAQQKPWFOBSMU-WDCWCFNPSA-N 0.000 description 1
- CQGBSALYGOXQPE-HTUGSXCWSA-N Glu-Thr-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CCC(=O)O)N)O CQGBSALYGOXQPE-HTUGSXCWSA-N 0.000 description 1
- UUTGYDAKPISJAO-JYJNAYRXSA-N Glu-Tyr-Leu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 UUTGYDAKPISJAO-JYJNAYRXSA-N 0.000 description 1
- WGYHAAXZWPEBDQ-IFFSRLJSSA-N Glu-Val-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGYHAAXZWPEBDQ-IFFSRLJSSA-N 0.000 description 1
- MFVQGXGQRIXBPK-WDSKDSINSA-N Gly-Ala-Glu Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O MFVQGXGQRIXBPK-WDSKDSINSA-N 0.000 description 1
- KKBWDNZXYLGJEY-UHFFFAOYSA-N Gly-Arg-Pro Natural products NCC(=O)NC(CCNC(=N)N)C(=O)N1CCCC1C(=O)O KKBWDNZXYLGJEY-UHFFFAOYSA-N 0.000 description 1
- FSPVILZGHUJOHS-QWRGUYRKSA-N Gly-His-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CNC=N1 FSPVILZGHUJOHS-QWRGUYRKSA-N 0.000 description 1
- HAXARWKYFIIHKD-ZKWXMUAHSA-N Gly-Ile-Ser Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O HAXARWKYFIIHKD-ZKWXMUAHSA-N 0.000 description 1
- UUYBFNKHOCJCHT-VHSXEESVSA-N Gly-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN UUYBFNKHOCJCHT-VHSXEESVSA-N 0.000 description 1
- QAMMIGULQSIRCD-IRXDYDNUSA-N Gly-Phe-Tyr Chemical compound C([C@H](NC(=O)C[NH3+])C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C([O-])=O)C1=CC=CC=C1 QAMMIGULQSIRCD-IRXDYDNUSA-N 0.000 description 1
- HJARVELKOSZUEW-YUMQZZPRSA-N Gly-Pro-Gln Chemical compound [H]NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O HJARVELKOSZUEW-YUMQZZPRSA-N 0.000 description 1
- WNGHUXFWEWTKAO-YUMQZZPRSA-N Gly-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN WNGHUXFWEWTKAO-YUMQZZPRSA-N 0.000 description 1
- CUVBTVWFVIIDOC-YEPSODPASA-N Gly-Thr-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)CN CUVBTVWFVIIDOC-YEPSODPASA-N 0.000 description 1
- BAYQNCWLXIDLHX-ONGXEEELSA-N Gly-Val-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)CN BAYQNCWLXIDLHX-ONGXEEELSA-N 0.000 description 1
- 208000007514 Herpes zoster Diseases 0.000 description 1
- 241000700586 Herpesviridae Species 0.000 description 1
- MWWOPNQSBXEUHO-ULQDDVLXSA-N His-Arg-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CN=CN1 MWWOPNQSBXEUHO-ULQDDVLXSA-N 0.000 description 1
- ZZLWLWSUIBSMNP-CIUDSAMLSA-N His-Asp-Ser Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZZLWLWSUIBSMNP-CIUDSAMLSA-N 0.000 description 1
- VFBZWZXKCVBTJR-SRVKXCTJSA-N His-Leu-Asp Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N VFBZWZXKCVBTJR-SRVKXCTJSA-N 0.000 description 1
- BXOLYFJYQQRQDJ-MXAVVETBSA-N His-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1=CN=CN1)N BXOLYFJYQQRQDJ-MXAVVETBSA-N 0.000 description 1
- YAALVYQFVJNXIV-KKUMJFAQSA-N His-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 YAALVYQFVJNXIV-KKUMJFAQSA-N 0.000 description 1
- FHKZHRMERJUXRJ-DCAQKATOSA-N His-Ser-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CN=CN1 FHKZHRMERJUXRJ-DCAQKATOSA-N 0.000 description 1
- CUEQQFOGARVNHU-VGDYDELISA-N His-Ser-Ile Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O CUEQQFOGARVNHU-VGDYDELISA-N 0.000 description 1
- FFKJUTZARGRVTH-KKUMJFAQSA-N His-Ser-Tyr Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O FFKJUTZARGRVTH-KKUMJFAQSA-N 0.000 description 1
- NBWATNYAUVSAEQ-ZEILLAHLSA-N His-Thr-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N)O NBWATNYAUVSAEQ-ZEILLAHLSA-N 0.000 description 1
- XGBVLRJLHUVCNK-DCAQKATOSA-N His-Val-Ser Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O XGBVLRJLHUVCNK-DCAQKATOSA-N 0.000 description 1
- 101001123325 Homo sapiens Peroxisome proliferator-activated receptor gamma coactivator 1-beta Proteins 0.000 description 1
- 241001135569 Human adenovirus 5 Species 0.000 description 1
- 241001502974 Human gammaherpesvirus 8 Species 0.000 description 1
- VZIFYHYNQDIPLI-HJWJTTGWSA-N Ile-Arg-Phe Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N VZIFYHYNQDIPLI-HJWJTTGWSA-N 0.000 description 1
- YPQDTQJBOFOTJQ-SXTJYALSSA-N Ile-Asn-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N YPQDTQJBOFOTJQ-SXTJYALSSA-N 0.000 description 1
- LEDRIAHEWDJRMF-CFMVVWHZSA-N Ile-Asn-Tyr Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 LEDRIAHEWDJRMF-CFMVVWHZSA-N 0.000 description 1
- NKRJALPCDNXULF-BYULHYEWSA-N Ile-Asp-Gly Chemical compound [H]N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O NKRJALPCDNXULF-BYULHYEWSA-N 0.000 description 1
- AQTWDZDISVGCAC-CFMVVWHZSA-N Ile-Asp-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N AQTWDZDISVGCAC-CFMVVWHZSA-N 0.000 description 1
- HOLOYAZCIHDQNS-YVNDNENWSA-N Ile-Gln-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N HOLOYAZCIHDQNS-YVNDNENWSA-N 0.000 description 1
- WUKLZPHVWAMZQV-UKJIMTQDSA-N Ile-Glu-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](C(C)C)C(=O)O)N WUKLZPHVWAMZQV-UKJIMTQDSA-N 0.000 description 1
- MQFGXJNSUJTXDT-QSFUFRPTSA-N Ile-Gly-Ile Chemical compound N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)O MQFGXJNSUJTXDT-QSFUFRPTSA-N 0.000 description 1
- IOVUXUSIGXCREV-DKIMLUQUSA-N Ile-Leu-Phe Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 IOVUXUSIGXCREV-DKIMLUQUSA-N 0.000 description 1
- GVKKVHNRTUFCCE-BJDJZHNGSA-N Ile-Leu-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)O)N GVKKVHNRTUFCCE-BJDJZHNGSA-N 0.000 description 1
- YCKPUHHMCFSUMD-IUKAMOBKSA-N Ile-Thr-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N YCKPUHHMCFSUMD-IUKAMOBKSA-N 0.000 description 1
- NAFIFZNBSPWYOO-RWRJDSDZSA-N Ile-Thr-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N NAFIFZNBSPWYOO-RWRJDSDZSA-N 0.000 description 1
- UYODHPPSCXBNCS-XUXIUFHCSA-N Ile-Val-Leu Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC(C)C UYODHPPSCXBNCS-XUXIUFHCSA-N 0.000 description 1
- JZBVBOKASHNXAD-NAKRPEOUSA-N Ile-Val-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N JZBVBOKASHNXAD-NAKRPEOUSA-N 0.000 description 1
- 101710110377 Immediate early protein IE1 Proteins 0.000 description 1
- 101710205424 Immediate-early protein 1 Proteins 0.000 description 1
- 206010061598 Immunodeficiency Diseases 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 1
- JTTHKOPSMAVJFE-VIFPVBQESA-N L-homophenylalanine Chemical compound OC(=O)[C@@H](N)CCC1=CC=CC=C1 JTTHKOPSMAVJFE-VIFPVBQESA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- LZDNBBYBDGBADK-UHFFFAOYSA-N L-valyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C(C)C)C(O)=O)=CNC2=C1 LZDNBBYBDGBADK-UHFFFAOYSA-N 0.000 description 1
- NHTGHBARYWONDQ-JTQLQIEISA-N L-α-methyl-Tyrosine Chemical compound OC(=O)[C@](N)(C)CC1=CC=C(O)C=C1 NHTGHBARYWONDQ-JTQLQIEISA-N 0.000 description 1
- 241000446313 Lamella Species 0.000 description 1
- 101710192606 Latent membrane protein 2 Proteins 0.000 description 1
- 241000713666 Lentivirus Species 0.000 description 1
- CQQGCWPXDHTTNF-GUBZILKMSA-N Leu-Ala-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O CQQGCWPXDHTTNF-GUBZILKMSA-N 0.000 description 1
- PBCHMHROGNUXMK-DLOVCJGASA-N Leu-Ala-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 PBCHMHROGNUXMK-DLOVCJGASA-N 0.000 description 1
- QPRQGENIBFLVEB-BJDJZHNGSA-N Leu-Ala-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O QPRQGENIBFLVEB-BJDJZHNGSA-N 0.000 description 1
- HBJZFCIVFIBNSV-DCAQKATOSA-N Leu-Arg-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(N)=O)C(O)=O HBJZFCIVFIBNSV-DCAQKATOSA-N 0.000 description 1
- GRZSCTXVCDUIPO-SRVKXCTJSA-N Leu-Arg-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O GRZSCTXVCDUIPO-SRVKXCTJSA-N 0.000 description 1
- HASRFYOMVPJRPU-SRVKXCTJSA-N Leu-Arg-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O HASRFYOMVPJRPU-SRVKXCTJSA-N 0.000 description 1
- IBMVEYRWAWIOTN-RWMBFGLXSA-N Leu-Arg-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(O)=O IBMVEYRWAWIOTN-RWMBFGLXSA-N 0.000 description 1
- IGUOAYLTQJLPPD-DCAQKATOSA-N Leu-Asn-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IGUOAYLTQJLPPD-DCAQKATOSA-N 0.000 description 1
- BPANDPNDMJHFEV-CIUDSAMLSA-N Leu-Asp-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(O)=O BPANDPNDMJHFEV-CIUDSAMLSA-N 0.000 description 1
- YKNBJXOJTURHCU-DCAQKATOSA-N Leu-Asp-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N YKNBJXOJTURHCU-DCAQKATOSA-N 0.000 description 1
- MMEDVBWCMGRKKC-GARJFASQSA-N Leu-Asp-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N MMEDVBWCMGRKKC-GARJFASQSA-N 0.000 description 1
- PNUCWVAGVNLUMW-CIUDSAMLSA-N Leu-Cys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O PNUCWVAGVNLUMW-CIUDSAMLSA-N 0.000 description 1
- RVVBWTWPNFDYBE-SRVKXCTJSA-N Leu-Glu-Arg Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O RVVBWTWPNFDYBE-SRVKXCTJSA-N 0.000 description 1
- YVKSMSDXKMSIRX-GUBZILKMSA-N Leu-Glu-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O YVKSMSDXKMSIRX-GUBZILKMSA-N 0.000 description 1
- VZBIUJURDLFFOE-IHRRRGAJSA-N Leu-His-Arg Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O VZBIUJURDLFFOE-IHRRRGAJSA-N 0.000 description 1
- BKTXKJMNTSMJDQ-AVGNSLFASA-N Leu-His-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N BKTXKJMNTSMJDQ-AVGNSLFASA-N 0.000 description 1
- AVEGDIAXTDVBJS-XUXIUFHCSA-N Leu-Ile-Arg Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AVEGDIAXTDVBJS-XUXIUFHCSA-N 0.000 description 1
- HNDWYLYAYNBWMP-AJNGGQMLSA-N Leu-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(C)C)N HNDWYLYAYNBWMP-AJNGGQMLSA-N 0.000 description 1
- QLDHBYRUNQZIJQ-DKIMLUQUSA-N Leu-Ile-Phe Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O QLDHBYRUNQZIJQ-DKIMLUQUSA-N 0.000 description 1
- JNDYEOUZBLOVOF-AVGNSLFASA-N Leu-Leu-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O JNDYEOUZBLOVOF-AVGNSLFASA-N 0.000 description 1
- QNBVTHNJGCOVFA-AVGNSLFASA-N Leu-Leu-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCC(O)=O QNBVTHNJGCOVFA-AVGNSLFASA-N 0.000 description 1
- FOBUGKUBUJOWAD-IHPCNDPISA-N Leu-Leu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FOBUGKUBUJOWAD-IHPCNDPISA-N 0.000 description 1
- WXUOJXIGOPMDJM-SRVKXCTJSA-N Leu-Lys-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O WXUOJXIGOPMDJM-SRVKXCTJSA-N 0.000 description 1
- RZXLZBIUTDQHJQ-SRVKXCTJSA-N Leu-Lys-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O RZXLZBIUTDQHJQ-SRVKXCTJSA-N 0.000 description 1
- DDVHDMSBLRAKNV-IHRRRGAJSA-N Leu-Met-Leu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(O)=O DDVHDMSBLRAKNV-IHRRRGAJSA-N 0.000 description 1
- ZDBMWELMUCLUPL-QEJZJMRPSA-N Leu-Phe-Ala Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C)C(O)=O)CC1=CC=CC=C1 ZDBMWELMUCLUPL-QEJZJMRPSA-N 0.000 description 1
- PJWOOBTYQNNRBF-BZSNNMDCSA-N Leu-Phe-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCCN)C(=O)O)N PJWOOBTYQNNRBF-BZSNNMDCSA-N 0.000 description 1
- UHNQRAFSEBGZFZ-YESZJQIVSA-N Leu-Phe-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N UHNQRAFSEBGZFZ-YESZJQIVSA-N 0.000 description 1
- WMIOEVKKYIMVKI-DCAQKATOSA-N Leu-Pro-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O WMIOEVKKYIMVKI-DCAQKATOSA-N 0.000 description 1
- AKVBOOKXVAMKSS-GUBZILKMSA-N Leu-Ser-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O AKVBOOKXVAMKSS-GUBZILKMSA-N 0.000 description 1
- MVHXGBZUJLWZOH-BJDJZHNGSA-N Leu-Ser-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O MVHXGBZUJLWZOH-BJDJZHNGSA-N 0.000 description 1
- AMSSKPUHBUQBOQ-SRVKXCTJSA-N Leu-Ser-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N AMSSKPUHBUQBOQ-SRVKXCTJSA-N 0.000 description 1
- BCUVPZLLSRMPJL-XIRDDKMYSA-N Leu-Trp-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CS)C(=O)O)N BCUVPZLLSRMPJL-XIRDDKMYSA-N 0.000 description 1
- LFXSPAIBSZSTEM-PMVMPFDFSA-N Leu-Trp-Phe Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC3=CC=CC=C3)C(=O)O)N LFXSPAIBSZSTEM-PMVMPFDFSA-N 0.000 description 1
- SUYRAPCRSCCPAK-VFAJRCTISA-N Leu-Trp-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SUYRAPCRSCCPAK-VFAJRCTISA-N 0.000 description 1
- RDFIVFHPOSOXMW-ACRUOGEOSA-N Leu-Tyr-Phe Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O RDFIVFHPOSOXMW-ACRUOGEOSA-N 0.000 description 1
- AXVIGSRGTMNSJU-YESZJQIVSA-N Leu-Tyr-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N2CCC[C@@H]2C(=O)O)N AXVIGSRGTMNSJU-YESZJQIVSA-N 0.000 description 1
- FBNPMTNBFFAMMH-UHFFFAOYSA-N Leu-Val-Arg Natural products CC(C)CC(N)C(=O)NC(C(C)C)C(=O)NC(C(O)=O)CCCN=C(N)N FBNPMTNBFFAMMH-UHFFFAOYSA-N 0.000 description 1
- XZNJZXJZBMBGGS-NHCYSSNCSA-N Leu-Val-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O XZNJZXJZBMBGGS-NHCYSSNCSA-N 0.000 description 1
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 108010028921 Lipopeptides Proteins 0.000 description 1
- YIBOAHAOAWACDK-QEJZJMRPSA-N Lys-Ala-Phe Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 YIBOAHAOAWACDK-QEJZJMRPSA-N 0.000 description 1
- WXJKFRMKJORORD-DCAQKATOSA-N Lys-Arg-Ala Chemical compound NC(=N)NCCC[C@@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@@H](N)CCCCN WXJKFRMKJORORD-DCAQKATOSA-N 0.000 description 1
- HIIZIQUUHIXUJY-GUBZILKMSA-N Lys-Asp-Gln Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HIIZIQUUHIXUJY-GUBZILKMSA-N 0.000 description 1
- LMVOVCYVZBBWQB-SRVKXCTJSA-N Lys-Asp-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN LMVOVCYVZBBWQB-SRVKXCTJSA-N 0.000 description 1
- HEWWNLVEWBJBKA-WDCWCFNPSA-N Lys-Gln-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCCN HEWWNLVEWBJBKA-WDCWCFNPSA-N 0.000 description 1
- QKXZCUCBFPEXNK-KKUMJFAQSA-N Lys-Leu-His Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 QKXZCUCBFPEXNK-KKUMJFAQSA-N 0.000 description 1
- TVOOGUNBIWAURO-KATARQTJSA-N Lys-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCCN)N)O TVOOGUNBIWAURO-KATARQTJSA-N 0.000 description 1
- IEVXCWPVBYCJRZ-IXOXFDKPSA-N Lys-Thr-His Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IEVXCWPVBYCJRZ-IXOXFDKPSA-N 0.000 description 1
- YKBSXQFZWFXFIB-VOAKCMCISA-N Lys-Thr-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O YKBSXQFZWFXFIB-VOAKCMCISA-N 0.000 description 1
- FPQMQEOVSKMVMA-ACRUOGEOSA-N Lys-Tyr-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)NC(=O)[C@H](CCCCN)N)O FPQMQEOVSKMVMA-ACRUOGEOSA-N 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 241000699673 Mesocricetus auratus Species 0.000 description 1
- WYEXWKAWMNJKPN-UBHSHLNASA-N Met-Ala-Phe Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CCSC)N WYEXWKAWMNJKPN-UBHSHLNASA-N 0.000 description 1
- ZAJNRWKGHWGPDQ-SDDRHHMPSA-N Met-Arg-Pro Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(=O)O)N ZAJNRWKGHWGPDQ-SDDRHHMPSA-N 0.000 description 1
- USBFEVBHEQBWDD-AVGNSLFASA-N Met-Leu-Val Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O USBFEVBHEQBWDD-AVGNSLFASA-N 0.000 description 1
- GGXZOTSDJJTDGB-GUBZILKMSA-N Met-Ser-Val Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O GGXZOTSDJJTDGB-GUBZILKMSA-N 0.000 description 1
- WOGNGBROIHHFAO-JYJNAYRXSA-N Met-Tyr-Met Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCSC)C(=O)O)N WOGNGBROIHHFAO-JYJNAYRXSA-N 0.000 description 1
- 108060004795 Methyltransferase Proteins 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 101100518501 Mus musculus Spp1 gene Proteins 0.000 description 1
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 1
- AUEJLPRZGVVDNU-UHFFFAOYSA-N N-L-tyrosyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CC1=CC=C(O)C=C1 AUEJLPRZGVVDNU-UHFFFAOYSA-N 0.000 description 1
- 230000004988 N-glycosylation Effects 0.000 description 1
- 108010066427 N-valyltryptophan Proteins 0.000 description 1
- BQVUABVGYYSDCJ-UHFFFAOYSA-N Nalpha-L-Leucyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)CC(C)C)C(O)=O)=CNC2=C1 BQVUABVGYYSDCJ-UHFFFAOYSA-N 0.000 description 1
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 102100028961 Peroxisome proliferator-activated receptor gamma coactivator 1-beta Human genes 0.000 description 1
- OPEVYHFJXLCCRT-AVGNSLFASA-N Phe-Gln-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O OPEVYHFJXLCCRT-AVGNSLFASA-N 0.000 description 1
- KJJROSNFBRWPHS-JYJNAYRXSA-N Phe-Glu-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O KJJROSNFBRWPHS-JYJNAYRXSA-N 0.000 description 1
- PBXYXOAEQQUVMM-ULQDDVLXSA-N Phe-His-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CC2=CC=CC=C2)N PBXYXOAEQQUVMM-ULQDDVLXSA-N 0.000 description 1
- RORUIHAWOLADSH-HJWJTTGWSA-N Phe-Ile-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CC1=CC=CC=C1 RORUIHAWOLADSH-HJWJTTGWSA-N 0.000 description 1
- KBVJZCVLQWCJQN-KKUMJFAQSA-N Phe-Leu-Asn Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O KBVJZCVLQWCJQN-KKUMJFAQSA-N 0.000 description 1
- YCCUXNNKXDGMAM-KKUMJFAQSA-N Phe-Leu-Ser Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YCCUXNNKXDGMAM-KKUMJFAQSA-N 0.000 description 1
- HQPWNHXERZCIHP-PMVMPFDFSA-N Phe-Leu-Trp Chemical compound C([C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)C1=CC=CC=C1 HQPWNHXERZCIHP-PMVMPFDFSA-N 0.000 description 1
- QRUOLOPKCOEZKU-HJWJTTGWSA-N Phe-Met-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC1=CC=CC=C1)N QRUOLOPKCOEZKU-HJWJTTGWSA-N 0.000 description 1
- IAOZOFPONWDXNT-IXOXFDKPSA-N Phe-Ser-Thr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O IAOZOFPONWDXNT-IXOXFDKPSA-N 0.000 description 1
- BSTPNLNKHKBONJ-HTUGSXCWSA-N Phe-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N)O BSTPNLNKHKBONJ-HTUGSXCWSA-N 0.000 description 1
- APZNYJFGVAGFCF-JYJNAYRXSA-N Phe-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)C(O)=O APZNYJFGVAGFCF-JYJNAYRXSA-N 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- OOLOTUZJUBOMAX-GUBZILKMSA-N Pro-Ala-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O OOLOTUZJUBOMAX-GUBZILKMSA-N 0.000 description 1
- OLHDPZMYUSBGDE-GUBZILKMSA-N Pro-Arg-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CS)C(=O)O OLHDPZMYUSBGDE-GUBZILKMSA-N 0.000 description 1
- ORPZXBQTEHINPB-SRVKXCTJSA-N Pro-Arg-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(O)=O ORPZXBQTEHINPB-SRVKXCTJSA-N 0.000 description 1
- INXAPZFIOVGHSV-CIUDSAMLSA-N Pro-Asn-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]1CCCN1 INXAPZFIOVGHSV-CIUDSAMLSA-N 0.000 description 1
- KPDRZQUWJKTMBP-DCAQKATOSA-N Pro-Asp-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@@H]1CCCN1 KPDRZQUWJKTMBP-DCAQKATOSA-N 0.000 description 1
- HXOLCSYHGRNXJJ-IHRRRGAJSA-N Pro-Asp-Phe Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HXOLCSYHGRNXJJ-IHRRRGAJSA-N 0.000 description 1
- XZONQWUEBAFQPO-HJGDQZAQSA-N Pro-Gln-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XZONQWUEBAFQPO-HJGDQZAQSA-N 0.000 description 1
- YSUZKYSRAFNLRB-ULQDDVLXSA-N Pro-Gln-Trp Chemical compound N([C@@H](CCC(=O)N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)C(=O)[C@@H]1CCCN1 YSUZKYSRAFNLRB-ULQDDVLXSA-N 0.000 description 1
- KIPIKSXPPLABPN-CIUDSAMLSA-N Pro-Glu-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 KIPIKSXPPLABPN-CIUDSAMLSA-N 0.000 description 1
- NXEYSLRNNPWCRN-SRVKXCTJSA-N Pro-Glu-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O NXEYSLRNNPWCRN-SRVKXCTJSA-N 0.000 description 1
- JUJGNDZIKKQMDJ-IHRRRGAJSA-N Pro-His-His Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CNC=N1)C(O)=O JUJGNDZIKKQMDJ-IHRRRGAJSA-N 0.000 description 1
- XQHGISDMVBTGAL-ULQDDVLXSA-N Pro-His-Phe Chemical compound C([C@@H](C(=O)[O-])NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1[NH2+]CCC1)C1=CC=CC=C1 XQHGISDMVBTGAL-ULQDDVLXSA-N 0.000 description 1
- LPGSNRSLPHRNBW-AVGNSLFASA-N Pro-His-Val Chemical compound C([C@@H](C(=O)N[C@@H](C(C)C)C([O-])=O)NC(=O)[C@H]1[NH2+]CCC1)C1=CN=CN1 LPGSNRSLPHRNBW-AVGNSLFASA-N 0.000 description 1
- AQGUSRZKDZYGGV-GMOBBJLQSA-N Pro-Ile-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O AQGUSRZKDZYGGV-GMOBBJLQSA-N 0.000 description 1
- CLJLVCYFABNTHP-DCAQKATOSA-N Pro-Leu-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O CLJLVCYFABNTHP-DCAQKATOSA-N 0.000 description 1
- FXGIMYRVJJEIIM-UWVGGRQHSA-N Pro-Leu-Gly Chemical compound OC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1 FXGIMYRVJJEIIM-UWVGGRQHSA-N 0.000 description 1
- RPLMFKUKFZOTER-AVGNSLFASA-N Pro-Met-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1 RPLMFKUKFZOTER-AVGNSLFASA-N 0.000 description 1
- JLMZKEQFMVORMA-SRVKXCTJSA-N Pro-Pro-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 JLMZKEQFMVORMA-SRVKXCTJSA-N 0.000 description 1
- KBUAPZAZPWNYSW-SRVKXCTJSA-N Pro-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 KBUAPZAZPWNYSW-SRVKXCTJSA-N 0.000 description 1
- POQFNPILEQEODH-FXQIFTODSA-N Pro-Ser-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O POQFNPILEQEODH-FXQIFTODSA-N 0.000 description 1
- PRKWBYCXBBSLSK-GUBZILKMSA-N Pro-Ser-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O PRKWBYCXBBSLSK-GUBZILKMSA-N 0.000 description 1
- GZNYIXWOIUFLGO-ZJDVBMNYSA-N Pro-Thr-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GZNYIXWOIUFLGO-ZJDVBMNYSA-N 0.000 description 1
- 108020005091 Replication Origin Proteins 0.000 description 1
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- ZUGXSSFMTXKHJS-ZLUOBGJFSA-N Ser-Ala-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O ZUGXSSFMTXKHJS-ZLUOBGJFSA-N 0.000 description 1
- WTWGOQRNRFHFQD-JBDRJPRFSA-N Ser-Ala-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WTWGOQRNRFHFQD-JBDRJPRFSA-N 0.000 description 1
- WDXYVIIVDIDOSX-DCAQKATOSA-N Ser-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CCCN=C(N)N WDXYVIIVDIDOSX-DCAQKATOSA-N 0.000 description 1
- WXUBSIDKNMFAGS-IHRRRGAJSA-N Ser-Arg-Tyr Chemical compound NC(N)=NCCC[C@H](NC(=O)[C@H](CO)N)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 WXUBSIDKNMFAGS-IHRRRGAJSA-N 0.000 description 1
- FIDMVVBUOCMMJG-CIUDSAMLSA-N Ser-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO FIDMVVBUOCMMJG-CIUDSAMLSA-N 0.000 description 1
- BGOWRLSWJCVYAQ-CIUDSAMLSA-N Ser-Asp-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O BGOWRLSWJCVYAQ-CIUDSAMLSA-N 0.000 description 1
- KJMOINFQVCCSDX-XKBZYTNZSA-N Ser-Gln-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KJMOINFQVCCSDX-XKBZYTNZSA-N 0.000 description 1
- SMIDBHKWSYUBRZ-ACZMJKKPSA-N Ser-Glu-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O SMIDBHKWSYUBRZ-ACZMJKKPSA-N 0.000 description 1
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 1
- JEHPKECJCALLRW-CUJWVEQBSA-N Ser-His-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JEHPKECJCALLRW-CUJWVEQBSA-N 0.000 description 1
- HEUVHBXOVZONPU-BJDJZHNGSA-N Ser-Leu-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HEUVHBXOVZONPU-BJDJZHNGSA-N 0.000 description 1
- VZQRNAYURWAEFE-KKUMJFAQSA-N Ser-Leu-Phe Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 VZQRNAYURWAEFE-KKUMJFAQSA-N 0.000 description 1
- PPNPDKGQRFSCAC-CIUDSAMLSA-N Ser-Lys-Asp Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CC(O)=O)C(O)=O PPNPDKGQRFSCAC-CIUDSAMLSA-N 0.000 description 1
- GDUZTEQRAOXYJS-SRVKXCTJSA-N Ser-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GDUZTEQRAOXYJS-SRVKXCTJSA-N 0.000 description 1
- NUEHQDHDLDXCRU-GUBZILKMSA-N Ser-Pro-Arg Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O NUEHQDHDLDXCRU-GUBZILKMSA-N 0.000 description 1
- PPCZVWHJWJFTFN-ZLUOBGJFSA-N Ser-Ser-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O PPCZVWHJWJFTFN-ZLUOBGJFSA-N 0.000 description 1
- PYTKULIABVRXSC-BWBBJGPYSA-N Ser-Ser-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PYTKULIABVRXSC-BWBBJGPYSA-N 0.000 description 1
- NADLKBTYNKUJEP-KATARQTJSA-N Ser-Thr-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O NADLKBTYNKUJEP-KATARQTJSA-N 0.000 description 1
- VLMIUSLQONKLDV-HEIBUPTGSA-N Ser-Thr-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VLMIUSLQONKLDV-HEIBUPTGSA-N 0.000 description 1
- BDMWLJLPPUCLNV-XGEHTFHBSA-N Ser-Thr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O BDMWLJLPPUCLNV-XGEHTFHBSA-N 0.000 description 1
- WMZVVNLPHFSUPA-BPUTZDHNSA-N Ser-Trp-Arg Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CO)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 WMZVVNLPHFSUPA-BPUTZDHNSA-N 0.000 description 1
- FHXGMDRKJHKLKW-QWRGUYRKSA-N Ser-Tyr-Gly Chemical compound OC[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=C(O)C=C1 FHXGMDRKJHKLKW-QWRGUYRKSA-N 0.000 description 1
- PLQWGQUNUPMNOD-KKUMJFAQSA-N Ser-Tyr-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(O)=O PLQWGQUNUPMNOD-KKUMJFAQSA-N 0.000 description 1
- YEDSOSIKVUMIJE-DCAQKATOSA-N Ser-Val-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O YEDSOSIKVUMIJE-DCAQKATOSA-N 0.000 description 1
- ANOQEBQWIAYIMV-AEJSXWLSSA-N Ser-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CO)N ANOQEBQWIAYIMV-AEJSXWLSSA-N 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 108020004682 Single-Stranded DNA Proteins 0.000 description 1
- 108091027544 Subgenomic mRNA Proteins 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 101710109576 Terminal protein Proteins 0.000 description 1
- LVHHEVGYAZGXDE-KDXUFGMBSA-N Thr-Ala-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)N1CCC[C@@H]1C(=O)O)N)O LVHHEVGYAZGXDE-KDXUFGMBSA-N 0.000 description 1
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 1
- VFEHSAJCWWHDBH-RHYQMDGZSA-N Thr-Arg-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O VFEHSAJCWWHDBH-RHYQMDGZSA-N 0.000 description 1
- OHAJHDJOCKKJLV-LKXGYXEUSA-N Thr-Asp-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O OHAJHDJOCKKJLV-LKXGYXEUSA-N 0.000 description 1
- ASJDFGOPDCVXTG-KATARQTJSA-N Thr-Cys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O ASJDFGOPDCVXTG-KATARQTJSA-N 0.000 description 1
- WDFPMSHYMRBLKM-NKIYYHGXSA-N Thr-Glu-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N)O WDFPMSHYMRBLKM-NKIYYHGXSA-N 0.000 description 1
- YDWLCDQXLCILCZ-BWAGICSOSA-N Thr-His-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O YDWLCDQXLCILCZ-BWAGICSOSA-N 0.000 description 1
- PRNGXSILMXSWQQ-OEAJRASXSA-N Thr-Leu-Phe Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PRNGXSILMXSWQQ-OEAJRASXSA-N 0.000 description 1
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 1
- PUEWAXRPXOEQOW-HJGDQZAQSA-N Thr-Met-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(N)=O)C(O)=O PUEWAXRPXOEQOW-HJGDQZAQSA-N 0.000 description 1
- DNCUODYZAMHLCV-XGEHTFHBSA-N Thr-Pro-Cys Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)O)N)O DNCUODYZAMHLCV-XGEHTFHBSA-N 0.000 description 1
- JAJOFWABAUKAEJ-QTKMDUPCSA-N Thr-Pro-His Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N)O JAJOFWABAUKAEJ-QTKMDUPCSA-N 0.000 description 1
- SGAOHNPSEPVAFP-ZDLURKLDSA-N Thr-Ser-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SGAOHNPSEPVAFP-ZDLURKLDSA-N 0.000 description 1
- AHERARIZBPOMNU-KATARQTJSA-N Thr-Ser-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O AHERARIZBPOMNU-KATARQTJSA-N 0.000 description 1
- VBMOVTMNHWPZJR-SUSMZKCASA-N Thr-Thr-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O VBMOVTMNHWPZJR-SUSMZKCASA-N 0.000 description 1
- BBPCSGKKPJUYRB-UVOCVTCTSA-N Thr-Thr-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O BBPCSGKKPJUYRB-UVOCVTCTSA-N 0.000 description 1
- KPMIQCXJDVKWKO-IFFSRLJSSA-N Thr-Val-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O KPMIQCXJDVKWKO-IFFSRLJSSA-N 0.000 description 1
- SBYQHZCMVSPQCS-RCWTZXSCSA-N Thr-Val-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O SBYQHZCMVSPQCS-RCWTZXSCSA-N 0.000 description 1
- QNXZCKMXHPULME-ZNSHCXBVSA-N Thr-Val-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@@H]1C(=O)O)N)O QNXZCKMXHPULME-ZNSHCXBVSA-N 0.000 description 1
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 1
- 108091036066 Three prime untranslated region Proteins 0.000 description 1
- 241000710924 Togaviridae Species 0.000 description 1
- 102000002689 Toll-like receptor Human genes 0.000 description 1
- 108020000411 Toll-like receptor Proteins 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- PKZVWAGGKFAVKR-UBHSHLNASA-N Trp-Cys-Cys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)O)N PKZVWAGGKFAVKR-UBHSHLNASA-N 0.000 description 1
- BOESUSAIMQGVJD-RYQLBKOJSA-N Trp-Met-Pro Chemical compound CSCC[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CNC3=CC=CC=C32)N BOESUSAIMQGVJD-RYQLBKOJSA-N 0.000 description 1
- DTPWXZXGFAHEKL-NWLDYVSISA-N Trp-Thr-Glu Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O DTPWXZXGFAHEKL-NWLDYVSISA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- CRWOSTCODDFEKZ-HRCADAONSA-N Tyr-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC2=CC=C(C=C2)O)N)C(=O)O CRWOSTCODDFEKZ-HRCADAONSA-N 0.000 description 1
- IIJWXEUNETVJPV-IHRRRGAJSA-N Tyr-Arg-Ser Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(=O)O)N)O IIJWXEUNETVJPV-IHRRRGAJSA-N 0.000 description 1
- DKKHULUSOSWGHS-UWJYBYFXSA-N Tyr-Asn-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CC=C(C=C1)O)N DKKHULUSOSWGHS-UWJYBYFXSA-N 0.000 description 1
- MBFJIHUHHCJBSN-AVGNSLFASA-N Tyr-Asn-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O MBFJIHUHHCJBSN-AVGNSLFASA-N 0.000 description 1
- PEVVXUGSAKEPEN-AVGNSLFASA-N Tyr-Asn-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O PEVVXUGSAKEPEN-AVGNSLFASA-N 0.000 description 1
- SCCKSNREWHMKOJ-SRVKXCTJSA-N Tyr-Asn-Ser Chemical compound N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O SCCKSNREWHMKOJ-SRVKXCTJSA-N 0.000 description 1
- RCLOWEZASFJFEX-KKUMJFAQSA-N Tyr-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 RCLOWEZASFJFEX-KKUMJFAQSA-N 0.000 description 1
- MNMYOSZWCKYEDI-JRQIVUDYSA-N Tyr-Asp-Thr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MNMYOSZWCKYEDI-JRQIVUDYSA-N 0.000 description 1
- NQJDICVXXIMMMB-XDTLVQLUSA-N Tyr-Glu-Ala Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O NQJDICVXXIMMMB-XDTLVQLUSA-N 0.000 description 1
- KOVXHANYYYMBRF-IRIUXVKKSA-N Tyr-Glu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O KOVXHANYYYMBRF-IRIUXVKKSA-N 0.000 description 1
- PMDWYLVWHRTJIW-STQMWFEESA-N Tyr-Gly-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC1=CC=C(O)C=C1 PMDWYLVWHRTJIW-STQMWFEESA-N 0.000 description 1
- KCPFDGNYAMKZQP-KBPBESRZSA-N Tyr-Gly-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(O)=O KCPFDGNYAMKZQP-KBPBESRZSA-N 0.000 description 1
- KHCSOLAHNLOXJR-BZSNNMDCSA-N Tyr-Leu-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KHCSOLAHNLOXJR-BZSNNMDCSA-N 0.000 description 1
- DAOREBHZAKCOEN-ULQDDVLXSA-N Tyr-Leu-Met Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(O)=O DAOREBHZAKCOEN-ULQDDVLXSA-N 0.000 description 1
- RGYCVIZZTUBSSG-JYJNAYRXSA-N Tyr-Pro-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O RGYCVIZZTUBSSG-JYJNAYRXSA-N 0.000 description 1
- QFHRUCJIRVILCK-YJRXYDGGSA-N Tyr-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O QFHRUCJIRVILCK-YJRXYDGGSA-N 0.000 description 1
- AOIZTZRWMSPPAY-KAOXEZKKSA-N Tyr-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N)O AOIZTZRWMSPPAY-KAOXEZKKSA-N 0.000 description 1
- UUJHRSTVQCFDPA-UFYCRDLUSA-N Tyr-Tyr-Val Chemical compound C([C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 UUJHRSTVQCFDPA-UFYCRDLUSA-N 0.000 description 1
- DJIJBQYBDKGDIS-JYJNAYRXSA-N Tyr-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(O)=O DJIJBQYBDKGDIS-JYJNAYRXSA-N 0.000 description 1
- 206010046865 Vaccinia virus infection Diseases 0.000 description 1
- YFOCMOVJBQDBCE-NRPADANISA-N Val-Ala-Glu Chemical compound C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N YFOCMOVJBQDBCE-NRPADANISA-N 0.000 description 1
- KKHRWGYHBZORMQ-NHCYSSNCSA-N Val-Arg-Glu Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N KKHRWGYHBZORMQ-NHCYSSNCSA-N 0.000 description 1
- HZYOWMGWKKRMBZ-BYULHYEWSA-N Val-Asp-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N HZYOWMGWKKRMBZ-BYULHYEWSA-N 0.000 description 1
- UEHRGZCNLSWGHK-DLOVCJGASA-N Val-Glu-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UEHRGZCNLSWGHK-DLOVCJGASA-N 0.000 description 1
- BEGDZYNDCNEGJZ-XVKPBYJWSA-N Val-Gly-Gln Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(N)=O BEGDZYNDCNEGJZ-XVKPBYJWSA-N 0.000 description 1
- HQYVQDRYODWONX-DCAQKATOSA-N Val-His-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CO)C(=O)O)N HQYVQDRYODWONX-DCAQKATOSA-N 0.000 description 1
- UMPVMAYCLYMYGA-ONGXEEELSA-N Val-Leu-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O UMPVMAYCLYMYGA-ONGXEEELSA-N 0.000 description 1
- AEMPCGRFEZTWIF-IHRRRGAJSA-N Val-Leu-Lys Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O AEMPCGRFEZTWIF-IHRRRGAJSA-N 0.000 description 1
- RFKJNTRMXGCKFE-FHWLQOOXSA-N Val-Leu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)CC(C)C)C(O)=O)=CNC2=C1 RFKJNTRMXGCKFE-FHWLQOOXSA-N 0.000 description 1
- IJGPOONOTBNTFS-GVXVVHGQSA-N Val-Lys-Glu Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O IJGPOONOTBNTFS-GVXVVHGQSA-N 0.000 description 1
- QRVPEKJBBRYISE-XUXIUFHCSA-N Val-Lys-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C(C)C)N QRVPEKJBBRYISE-XUXIUFHCSA-N 0.000 description 1
- YLRAFVVWZRSZQC-DZKIICNBSA-N Val-Phe-Glu Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N YLRAFVVWZRSZQC-DZKIICNBSA-N 0.000 description 1
- XBJKAZATRJBDCU-GUBZILKMSA-N Val-Pro-Ala Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O XBJKAZATRJBDCU-GUBZILKMSA-N 0.000 description 1
- DOFAQXCYFQKSHT-SRVKXCTJSA-N Val-Pro-Pro Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DOFAQXCYFQKSHT-SRVKXCTJSA-N 0.000 description 1
- VIKZGAUAKQZDOF-NRPADANISA-N Val-Ser-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O VIKZGAUAKQZDOF-NRPADANISA-N 0.000 description 1
- UJMCYJKPDFQLHX-XGEHTFHBSA-N Val-Ser-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](C(C)C)N)O UJMCYJKPDFQLHX-XGEHTFHBSA-N 0.000 description 1
- YQYFYUSYEDNLSD-YEPSODPASA-N Val-Thr-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O YQYFYUSYEDNLSD-YEPSODPASA-N 0.000 description 1
- GTACFKZDQFTVAI-STECZYCISA-N Val-Tyr-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)CC1=CC=C(O)C=C1 GTACFKZDQFTVAI-STECZYCISA-N 0.000 description 1
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 1
- LLJLBRRXKZTTRD-GUBZILKMSA-N Val-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N LLJLBRRXKZTTRD-GUBZILKMSA-N 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 108700005077 Viral Genes Proteins 0.000 description 1
- 108010067390 Viral Proteins Proteins 0.000 description 1
- 108020000999 Viral RNA Proteins 0.000 description 1
- 229940123627 Viral replication inhibitor Drugs 0.000 description 1
- ISXSJGHXHUZXNF-LXZPIJOJSA-N [(3s,8s,9s,10r,13r,14s,17r)-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-3-yl] n-[2-(dimethylamino)ethyl]carbamate;hydrochloride Chemical compound Cl.C1C=C2C[C@@H](OC(=O)NCCN(C)C)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 ISXSJGHXHUZXNF-LXZPIJOJSA-N 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 238000005903 acid hydrolysis reaction Methods 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 108010045023 alanyl-prolyl-tyrosine Proteins 0.000 description 1
- 108010041407 alanylaspartic acid Proteins 0.000 description 1
- 108010005233 alanylglutamic acid Proteins 0.000 description 1
- 108010070944 alanylhistidine Proteins 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 1
- 150000001371 alpha-amino acids Chemical class 0.000 description 1
- 235000008206 alpha-amino acids Nutrition 0.000 description 1
- HRRYYCWYCMJNGA-ZETCQYMHSA-N alpha-methyl-L-histidine Chemical compound OC(=O)[C@](N)(C)CC1=CN=CN1 HRRYYCWYCMJNGA-ZETCQYMHSA-N 0.000 description 1
- HYOWVAAEQCNGLE-JTQLQIEISA-N alpha-methyl-L-phenylalanine Chemical compound OC(=O)[C@](N)(C)CC1=CC=CC=C1 HYOWVAAEQCNGLE-JTQLQIEISA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical class [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical compound O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 239000004037 angiogenesis inhibitor Substances 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 230000001740 anti-invasion Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 239000000935 antidepressant agent Substances 0.000 description 1
- 229940005513 antidepressants Drugs 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 108010060035 arginylproline Proteins 0.000 description 1
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 1
- 108010038633 aspartylglutamate Proteins 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- SWJXWSAKHXBQSY-UHFFFAOYSA-N benzo(c)cinnoline Chemical compound C1=CC=C2C3=CC=CC=C3N=NC2=C1 SWJXWSAKHXBQSY-UHFFFAOYSA-N 0.000 description 1
- SQVRNKJHWKZAKO-UHFFFAOYSA-N beta-N-Acetyl-D-neuraminic acid Natural products CC(=O)NC1C(O)CC(O)(C(O)=O)OC1C(O)C(O)CO SQVRNKJHWKZAKO-UHFFFAOYSA-N 0.000 description 1
- 239000012148 binding buffer Substances 0.000 description 1
- 239000000227 bioadhesive Substances 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000004900 c-terminal fragment Anatomy 0.000 description 1
- 102220427461 c.289A>G Human genes 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- SQQXRXKYTKFFSM-UHFFFAOYSA-N chembl1992147 Chemical compound OC1=C(OC)C(OC)=CC=C1C1=C(C)C(C(O)=O)=NC(C=2N=C3C4=NC(C)(C)N=C4C(OC)=C(O)C3=CC=2)=C1N SQQXRXKYTKFFSM-UHFFFAOYSA-N 0.000 description 1
- 235000013330 chicken meat Nutrition 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 238000011490 co-immunoprecipitation assay Methods 0.000 description 1
- 230000008045 co-localization Effects 0.000 description 1
- 230000000536 complexating effect Effects 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- NKLPQNGYXWVELD-UHFFFAOYSA-M coomassie brilliant blue Chemical compound [Na+].C1=CC(OCC)=CC=C1NC1=CC=C(C(=C2C=CC(C=C2)=[N+](CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=2C=CC(=CC=2)N(CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=C1 NKLPQNGYXWVELD-UHFFFAOYSA-M 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 210000004748 cultured cell Anatomy 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 108010004073 cysteinylcysteine Proteins 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- 238000000432 density-gradient centrifugation Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 239000012149 elution buffer Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 210000001723 extracellular space Anatomy 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 230000037433 frameshift Effects 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 108010078144 glutaminyl-glycine Proteins 0.000 description 1
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 1
- 108010079547 glutamylmethionine Proteins 0.000 description 1
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 1
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 1
- YMAWOPBAYDPSLA-UHFFFAOYSA-N glycylglycine Chemical compound [NH3+]CC(=O)NCC([O-])=O YMAWOPBAYDPSLA-UHFFFAOYSA-N 0.000 description 1
- 108010037850 glycylvaline Proteins 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 239000003966 growth inhibitor Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 108010036413 histidylglycine Proteins 0.000 description 1
- 108010025306 histidylleucine Proteins 0.000 description 1
- 239000012510 hollow fiber Substances 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000001165 hydrophobic group Chemical group 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- HOPZBJPSUKPLDT-UHFFFAOYSA-N imidazo[4,5-h]quinolin-2-one Chemical class C1=CN=C2C3=NC(=O)N=C3C=CC2=C1 HOPZBJPSUKPLDT-UHFFFAOYSA-N 0.000 description 1
- 229940124669 imidazoquinoline Drugs 0.000 description 1
- DOUYETYNHWVLEO-UHFFFAOYSA-N imiquimod Chemical compound C1=CC=CC2=C3N(CC(C)C)C=NC3=C(N)N=C21 DOUYETYNHWVLEO-UHFFFAOYSA-N 0.000 description 1
- 229960002751 imiquimod Drugs 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000001571 immunoadjuvant effect Effects 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 239000000568 immunological adjuvant Substances 0.000 description 1
- 229960003444 immunosuppressant agent Drugs 0.000 description 1
- 230000001861 immunosuppressant effect Effects 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 1
- 108010078274 isoleucylvaline Proteins 0.000 description 1
- 210000003292 kidney cell Anatomy 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 108010012058 leucyltyrosine Proteins 0.000 description 1
- 238000012417 linear regression Methods 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 108010064235 lysylglycine Proteins 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 210000004779 membrane envelope Anatomy 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 108010005942 methionylglycine Proteins 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 230000003232 mucoadhesive effect Effects 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 125000001446 muramyl group Chemical group N[C@@H](C=O)[C@@H](O[C@@H](C(=O)*)C)[C@H](O)[C@H](O)CO 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 210000004898 n-terminal fragment Anatomy 0.000 description 1
- 210000004897 n-terminal region Anatomy 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 239000002077 nanosphere Substances 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 229960000470 omalizumab Drugs 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229940023041 peptide vaccine Drugs 0.000 description 1
- 238000009521 phase II clinical trial Methods 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 108010073101 phenylalanylleucine Proteins 0.000 description 1
- 108010073025 phenylalanylphenylalanine Proteins 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 229920002627 poly(phosphazenes) Polymers 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920000056 polyoxyethylene ether Polymers 0.000 description 1
- 229940051841 polyoxyethylene ether Drugs 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 108010077112 prolyl-proline Proteins 0.000 description 1
- 108010031719 prolyl-serine Proteins 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 108010053725 prolylvaline Proteins 0.000 description 1
- 229940021993 prophylactic vaccine Drugs 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 239000002510 pyrogen Substances 0.000 description 1
- ZADWXFSZEAPBJS-UHFFFAOYSA-N racemic N-methyl tryptophan Natural products C1=CC=C2N(C)C=C(CC(N)C(O)=O)C2=C1 ZADWXFSZEAPBJS-UHFFFAOYSA-N 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 229960000329 ribavirin Drugs 0.000 description 1
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 description 1
- 102200017868 rs121434557 Human genes 0.000 description 1
- 102220088226 rs756434530 Human genes 0.000 description 1
- 210000003079 salivary gland Anatomy 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 108010069117 seryl-lysyl-aspartic acid Proteins 0.000 description 1
- 108010026333 seryl-proline Proteins 0.000 description 1
- 125000005629 sialic acid group Chemical group 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 150000003584 thiosemicarbazones Chemical class 0.000 description 1
- 108010061238 threonyl-glycine Proteins 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 239000013638 trimer Substances 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 108010080629 tryptophan-leucine Proteins 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 108010078580 tyrosylleucine Proteins 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 208000007089 vaccinia Diseases 0.000 description 1
- 108010073969 valyllysine Proteins 0.000 description 1
- 108010009962 valyltyrosine Proteins 0.000 description 1
- 210000003501 vero cell Anatomy 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 239000000277 virosome Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 229910052727 yttrium Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/245—Herpetoviridae, e.g. herpes simplex virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/081—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from DNA viruses
- C07K16/082—Hepadnaviridae, e.g. hepatitis B virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/525—Virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55566—Emulsions, e.g. Freund's adjuvant, MF59
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/245—Herpetoviridae, e.g. herpes simplex virus
- A61K39/25—Varicella-zoster virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/42—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum viral
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/08—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses
- C07K16/081—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from viruses from DNA viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16111—Cytomegalovirus, e.g. human herpesvirus 5
- C12N2710/16122—New viral proteins or individual genes, new structural or functional aspects of known viral proteins or genes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16111—Cytomegalovirus, e.g. human herpesvirus 5
- C12N2710/16134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16111—Cytomegalovirus, e.g. human herpesvirus 5
- C12N2710/16151—Methods of production or purification of viral material
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Virology (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Molecular Biology (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Gastroenterology & Hepatology (AREA)
- Communicable Diseases (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Oncology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biotechnology (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15152221.6 | 2015-01-22 | ||
| EP15152221.6A EP3048114A1 (en) | 2015-01-22 | 2015-01-22 | Cytomegalovirus antigens and uses thereof |
| PCT/IB2016/050335 WO2016116904A1 (en) | 2015-01-22 | 2016-01-22 | Cytomegalovirus antigens and uses thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170100039A true KR20170100039A (ko) | 2017-09-01 |
Family
ID=52434568
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177023123A Ceased KR20170100039A (ko) | 2015-01-22 | 2016-01-22 | 사이토메갈로바이러스 항원 및 이의 용도 |
Country Status (24)
| Country | Link |
|---|---|
| US (2) | US10167321B2 (enExample) |
| EP (3) | EP3048114A1 (enExample) |
| JP (1) | JP6717836B2 (enExample) |
| KR (1) | KR20170100039A (enExample) |
| CN (1) | CN107531761B (enExample) |
| AU (2) | AU2016210548B2 (enExample) |
| BE (1) | BE1023087B1 (enExample) |
| BR (1) | BR112017015567A2 (enExample) |
| CA (1) | CA2974041C (enExample) |
| DK (1) | DK3247722T5 (enExample) |
| EA (1) | EA038250B1 (enExample) |
| ES (1) | ES2937959T3 (enExample) |
| FI (1) | FI3247722T3 (enExample) |
| HR (1) | HRP20230177T1 (enExample) |
| HU (1) | HUE061175T2 (enExample) |
| IL (1) | IL253366B (enExample) |
| LT (1) | LT3247722T (enExample) |
| MX (1) | MX382518B (enExample) |
| PL (1) | PL3247722T3 (enExample) |
| PT (1) | PT3247722T (enExample) |
| SG (1) | SG11201705740UA (enExample) |
| SI (1) | SI3247722T1 (enExample) |
| WO (1) | WO2016116904A1 (enExample) |
| ZA (1) | ZA201704912B (enExample) |
Families Citing this family (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TR201802741T4 (tr) * | 2010-05-14 | 2018-03-21 | Univ Oregon Health & Science | Rekombinant hcmv ve rhcmv vektörleri ve bunların kullanımları. |
| PT2691530T (pt) | 2011-06-10 | 2018-05-10 | Univ Oregon Health & Science | Glicoproteínas e vectores recombinantes cmv |
| US10023626B2 (en) | 2013-09-30 | 2018-07-17 | Modernatx, Inc. | Polynucleotides encoding immune modulating polypeptides |
| CN113528466B (zh) | 2014-07-16 | 2024-12-24 | 俄勒冈健康与科学大学 | 包含外源抗原的人巨细胞病毒 |
| SG11201706454VA (en) | 2015-02-10 | 2017-09-28 | Univ Oregon Health & Science | Methods and compositions useful in generating non canonical cd8+ t cell responses |
| US11364292B2 (en) | 2015-07-21 | 2022-06-21 | Modernatx, Inc. | CHIKV RNA vaccines |
| EP4218805A1 (en) | 2015-07-21 | 2023-08-02 | ModernaTX, Inc. | Infectious disease vaccines |
| WO2017070624A1 (en) | 2015-10-22 | 2017-04-27 | Modernatx, Inc. | Tropical disease vaccines |
| MA46316A (fr) | 2015-10-22 | 2021-03-24 | Modernatx Inc | Vaccin contre le cytomégalovirus humain |
| CN108474003A (zh) | 2015-11-20 | 2018-08-31 | 俄勒冈健康与科学大学 | 包含微小rna识别元件的cmv载体 |
| US11318197B2 (en) | 2016-03-03 | 2022-05-03 | Duke University | Compositions and methods for inducing HIV-1 antibodies |
| WO2017152146A2 (en) | 2016-03-03 | 2017-09-08 | Duke University | Compositions and methods for inducing hiv-1 antibodies |
| WO2018067580A1 (en) | 2016-10-03 | 2018-04-12 | Duke University | Methods to identify immunogens by targeting improbable mutations |
| BR112019007982A2 (pt) | 2016-10-18 | 2019-07-02 | Univ Oregon Health & Science | vetores de citomegalovírus elicitando células t restringidas por moléculas do complexo de histocompatibilidade e principal |
| WO2018075980A1 (en) | 2016-10-21 | 2018-04-26 | Modernatx, Inc. | Human cytomegalovirus vaccine |
| MA47515A (fr) | 2017-02-16 | 2019-12-25 | Modernatx Inc | Compositions immunogènes très puissantes |
| CA3060019A1 (en) | 2017-04-19 | 2018-10-25 | Glaxosmithkline Biologicals Sa | Modified cytomegalovirus proteins and stabilized complexes |
| MA50253A (fr) | 2017-09-14 | 2020-07-22 | Modernatx Inc | Vaccins à arn contre le virus zika |
| US11773144B2 (en) | 2017-10-02 | 2023-10-03 | Duke University | Mosaic HIV-1 envelopes to induce ADCC responses |
| AU2019228551B2 (en) | 2018-02-28 | 2025-07-24 | University Of Washington | Self-assembling nanostructure vaccines |
| EP3773708A2 (en) | 2018-04-03 | 2021-02-17 | Sanofi | Antigenic epstein barr virus polypeptides |
| US12138304B2 (en) | 2018-10-01 | 2024-11-12 | Duke University | HIV-1 envelope stabilizing mutations |
| CA3116175A1 (en) | 2018-10-17 | 2020-04-23 | Glaxosmithkline Biologicals Sa | Modified cytomegalovirus proteins and stabilized complexes |
| CN109627330A (zh) * | 2018-12-18 | 2019-04-16 | 马鞍山史记动物健康管理有限公司 | 一种猪伪狂犬病毒高效价阳性血清制备方法 |
| MA55321A (fr) | 2019-03-15 | 2022-01-19 | Modernatx Inc | Vaccins à base d'arn contre le vih |
| EP4004018A1 (en) | 2019-07-24 | 2022-06-01 | GlaxoSmithKline Biologicals SA | Modified human cytomegalovirus proteins |
| US11406703B2 (en) | 2020-08-25 | 2022-08-09 | Modernatx, Inc. | Human cytomegalovirus vaccine |
| WO2023144665A1 (en) | 2022-01-28 | 2023-08-03 | Glaxosmithkline Biologicals Sa | Modified human cytomegalovirus proteins |
| CN118580343B (zh) * | 2022-10-21 | 2025-08-19 | 珠海泰诺麦博制药股份有限公司 | 抗人巨细胞病毒抗体及其用途 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US5219740A (en) | 1987-02-13 | 1993-06-15 | Fred Hutchinson Cancer Research Center | Retroviral gene transfer into diploid fibroblasts for gene therapy |
| WO1991006667A1 (en) | 1989-11-06 | 1991-05-16 | Cell Genesys, Inc. | Production of proteins using homologous recombination |
| CA2654563A1 (en) | 2006-06-07 | 2007-12-21 | The Trustees Of Princeton University | Cytomegalovirus surface protein complex for use in vaccines and as a drug target |
| EP2352759B1 (en) * | 2008-07-16 | 2017-11-01 | Institute for Research in Biomedicine | Human cytomegalovirus neutralizing antibodies and use thereof |
| ES2443952T3 (es) | 2009-09-02 | 2014-02-21 | Novartis Ag | Composiciones inmunógenas que incluyen moduladores de la actividad de TLR |
| ES2649896T3 (es) | 2010-07-06 | 2018-01-16 | Glaxosmithkline Biologicals Sa | Emulsiones catiónicas de aceite en agua |
| CA2814386C (en) | 2010-10-11 | 2019-08-20 | Novartis Ag | Antigen delivery platforms |
| MX350258B (es) | 2011-07-06 | 2017-08-31 | Novartis Ag | Emulsiones cationicas de aceite en agua. |
| TWI570240B (zh) * | 2011-09-09 | 2017-02-11 | 默沙東公司 | 作為細胞巨大病毒疫苗之條件式複製cmv |
| MX378747B (es) | 2012-07-06 | 2025-03-10 | Glaxosmithkline Biologicals S A Star | Complejos de proteínas de citomegalovirus. |
| US20150359879A1 (en) * | 2012-10-30 | 2015-12-17 | Redvax Gmbh | Recombinant particle based vaccines against human cytomegalovirus infection |
| MX373329B (es) | 2013-12-20 | 2020-05-21 | Novartis Ag | Células eucariotas novedosas y métodos para la expresión recombinante de un producto de interés. |
| LT3083676T (lt) | 2013-12-20 | 2019-11-25 | Novartis Ag | Naujos eukariotinės ląstelės, ir dominančio polipeptido rekombinantinės raiškos būdai |
| EP3015475A1 (en) | 2014-10-31 | 2016-05-04 | Novartis AG | Mammalian cells expressing cytomegalovirus antigens |
| EP3047856A1 (en) | 2015-01-23 | 2016-07-27 | Novartis AG | Cmv antigens and uses thereof |
-
2015
- 2015-01-22 EP EP15152221.6A patent/EP3048114A1/en not_active Withdrawn
-
2016
- 2016-01-22 EP EP22203406.8A patent/EP4180056A1/en active Pending
- 2016-01-22 FI FIEP16702210.2T patent/FI3247722T3/fi active
- 2016-01-22 SI SI201631665T patent/SI3247722T1/sl unknown
- 2016-01-22 DK DK16702210.2T patent/DK3247722T5/da active
- 2016-01-22 EA EA201791562A patent/EA038250B1/ru unknown
- 2016-01-22 ES ES16702210T patent/ES2937959T3/es active Active
- 2016-01-22 AU AU2016210548A patent/AU2016210548B2/en not_active Ceased
- 2016-01-22 HR HRP20230177TT patent/HRP20230177T1/hr unknown
- 2016-01-22 JP JP2017538387A patent/JP6717836B2/ja active Active
- 2016-01-22 CA CA2974041A patent/CA2974041C/en active Active
- 2016-01-22 SG SG11201705740UA patent/SG11201705740UA/en unknown
- 2016-01-22 BR BR112017015567A patent/BR112017015567A2/pt not_active Application Discontinuation
- 2016-01-22 US US15/544,730 patent/US10167321B2/en active Active
- 2016-01-22 KR KR1020177023123A patent/KR20170100039A/ko not_active Ceased
- 2016-01-22 MX MX2017009538A patent/MX382518B/es unknown
- 2016-01-22 WO PCT/IB2016/050335 patent/WO2016116904A1/en not_active Ceased
- 2016-01-22 EP EP16702210.2A patent/EP3247722B1/en active Active
- 2016-01-22 LT LTEPPCT/IB2016/050335T patent/LT3247722T/lt unknown
- 2016-01-22 HU HUE16702210A patent/HUE061175T2/hu unknown
- 2016-01-22 BE BE2016/5050A patent/BE1023087B1/fr not_active IP Right Cessation
- 2016-01-22 PL PL16702210.2T patent/PL3247722T3/pl unknown
- 2016-01-22 CN CN201680017610.XA patent/CN107531761B/zh active Active
- 2016-01-22 PT PT167022102T patent/PT3247722T/pt unknown
-
2017
- 2017-07-09 IL IL253366A patent/IL253366B/en unknown
- 2017-07-19 ZA ZA2017/04912A patent/ZA201704912B/en unknown
-
2018
- 2018-09-10 AU AU2018226521A patent/AU2018226521B2/en not_active Ceased
- 2018-11-15 US US16/192,347 patent/US20190276498A1/en not_active Abandoned
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2018226521B2 (en) | Cytomegalovirus antigens and uses thereof | |
| EP3230305B1 (en) | Cytomegalovirus antigens | |
| WO2015181142A1 (en) | Cytomegalovirus complexes and uses thereof | |
| EP3047856A1 (en) | Cmv antigens and uses thereof | |
| CN113151184A (zh) | 基于细胞膜展示冠状病毒免疫原以诱导中和抗体的方法 | |
| JP2023523423A (ja) | SARS-CoV-2に対するワクチン及びその調製物 | |
| HK40087930A (en) | Cytomegalovirus antigens and uses thereof | |
| US20070003577A1 (en) | Purified trimeric S protein as vaccine against severe acute respiratory syndrome virus infections | |
| KR20250024845A (ko) | 복제 결함형 헤르페스 심플렉스 바이러스 1형바이러스 백신 | |
| CN117062843A (zh) | 用于预防感染与治疗长期新冠肺炎的针对SARS-CoV-2变体的疫苗组合物 | |
| US20190031723A1 (en) | Truncated glycoprotein g of herpes simplex virus 2 | |
| Ambagala | Interference with major histocompatibility complex class I pathway by animal alphaherpesviruses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20170818 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210112 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230525 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20231011 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230525 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |