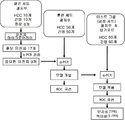
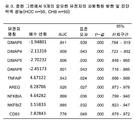
KR20150058465A - 피험체에서 간암을 진단하는 방법 및 간암을 진단하기 위한 키트 - Google Patents
피험체에서 간암을 진단하는 방법 및 간암을 진단하기 위한 키트 Download PDFInfo
- Publication number
- KR20150058465A KR20150058465A KR1020157010267A KR20157010267A KR20150058465A KR 20150058465 A KR20150058465 A KR 20150058465A KR 1020157010267 A KR1020157010267 A KR 1020157010267A KR 20157010267 A KR20157010267 A KR 20157010267A KR 20150058465 A KR20150058465 A KR 20150058465A
- Authority
- KR
- South Korea
- Prior art keywords
- gene
- hcc
- leu
- subject
- ala
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 68
- 208000014018 liver neoplasm Diseases 0.000 title claims abstract description 43
- 201000007270 liver cancer Diseases 0.000 title claims abstract description 42
- 208000019425 cirrhosis of liver Diseases 0.000 claims abstract description 13
- 108090000623 proteins and genes Proteins 0.000 claims description 185
- 206010073071 hepatocellular carcinoma Diseases 0.000 claims description 106
- 231100000844 hepatocellular carcinoma Toxicity 0.000 claims description 104
- 230000014509 gene expression Effects 0.000 claims description 58
- 102000007299 Amphiregulin Human genes 0.000 claims description 45
- 108010033760 Amphiregulin Proteins 0.000 claims description 45
- 239000003550 marker Substances 0.000 claims description 44
- 102000004169 proteins and genes Human genes 0.000 claims description 36
- 239000000523 sample Substances 0.000 claims description 32
- 108091034117 Oligonucleotide Proteins 0.000 claims description 19
- 210000000265 leukocyte Anatomy 0.000 claims description 19
- 108060008682 Tumor Necrosis Factor Proteins 0.000 claims description 17
- 238000003753 real-time PCR Methods 0.000 claims description 17
- 102100024413 GTPase IMAP family member 5 Human genes 0.000 claims description 16
- 102000003390 tumor necrosis factor Human genes 0.000 claims description 16
- 101710190497 GTPase IMAP family member 5 Proteins 0.000 claims description 15
- 101150048713 Tnfaip3 gene Proteins 0.000 claims description 15
- 238000012360 testing method Methods 0.000 claims description 15
- 238000003199 nucleic acid amplification method Methods 0.000 claims description 14
- 101150036244 AREG gene Proteins 0.000 claims description 13
- 230000003321 amplification Effects 0.000 claims description 13
- 108020004707 nucleic acids Proteins 0.000 claims description 13
- 102000039446 nucleic acids Human genes 0.000 claims description 13
- 150000007523 nucleic acids Chemical class 0.000 claims description 13
- 241000282414 Homo sapiens Species 0.000 claims description 12
- 102000013446 GTP Phosphohydrolases Human genes 0.000 claims description 10
- 108091006109 GTPases Proteins 0.000 claims description 10
- 238000004458 analytical method Methods 0.000 claims description 10
- 206010016654 Fibrosis Diseases 0.000 claims description 8
- 230000007882 cirrhosis Effects 0.000 claims description 8
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 claims description 7
- 230000000295 complement effect Effects 0.000 claims description 7
- 239000013068 control sample Substances 0.000 claims description 7
- 238000003556 assay Methods 0.000 claims description 6
- 239000002773 nucleotide Substances 0.000 claims description 5
- 125000003729 nucleotide group Chemical group 0.000 claims description 5
- 230000002250 progressing effect Effects 0.000 claims description 5
- 108020005187 Oligonucleotide Probes Proteins 0.000 claims description 4
- 210000000601 blood cell Anatomy 0.000 claims description 4
- 210000005228 liver tissue Anatomy 0.000 claims description 4
- 238000012544 monitoring process Methods 0.000 claims description 4
- 239000002751 oligonucleotide probe Substances 0.000 claims description 4
- 239000007801 affinity label Substances 0.000 claims description 3
- 241000894007 species Species 0.000 claims description 3
- 102000004190 Enzymes Human genes 0.000 claims description 2
- 108090000790 Enzymes Proteins 0.000 claims description 2
- 239000007850 fluorescent dye Substances 0.000 claims description 2
- 230000002285 radioactive effect Effects 0.000 claims description 2
- 101000830570 Homo sapiens Tumor necrosis factor alpha-induced protein 3 Proteins 0.000 claims 5
- 102100024596 Tumor necrosis factor alpha-induced protein 3 Human genes 0.000 claims 5
- 208000016350 chronic hepatitis B virus infection Diseases 0.000 claims 1
- 206010008909 Chronic Hepatitis Diseases 0.000 abstract description 9
- 208000006454 hepatitis Diseases 0.000 abstract description 9
- 208000002672 hepatitis B Diseases 0.000 description 32
- 235000018102 proteins Nutrition 0.000 description 32
- 208000000419 Chronic Hepatitis B Diseases 0.000 description 30
- 102000013529 alpha-Fetoproteins Human genes 0.000 description 22
- 108010026331 alpha-Fetoproteins Proteins 0.000 description 22
- 230000035945 sensitivity Effects 0.000 description 21
- 108020004414 DNA Proteins 0.000 description 20
- 102100024418 GTPase IMAP family member 8 Human genes 0.000 description 20
- 101710190494 GTPase IMAP family member 8 Proteins 0.000 description 20
- 102100024421 GTPase IMAP family member 6 Human genes 0.000 description 19
- 238000012549 training Methods 0.000 description 19
- 102100024412 GTPase IMAP family member 4 Human genes 0.000 description 18
- 101710190481 GTPase IMAP family member 4 Proteins 0.000 description 18
- 101000961071 Homo sapiens NF-kappa-B inhibitor alpha Proteins 0.000 description 15
- 101001076431 Homo sapiens NF-kappa-B inhibitor zeta Proteins 0.000 description 15
- 102100039337 NF-kappa-B inhibitor alpha Human genes 0.000 description 15
- 102100026009 NF-kappa-B inhibitor zeta Human genes 0.000 description 15
- 102100035793 CD83 antigen Human genes 0.000 description 14
- 101000946856 Homo sapiens CD83 antigen Proteins 0.000 description 14
- 101000833389 Homo sapiens GTPase IMAP family member 6 Proteins 0.000 description 14
- 238000003745 diagnosis Methods 0.000 description 14
- 229920001184 polypeptide Polymers 0.000 description 12
- 102000004196 processed proteins & peptides Human genes 0.000 description 12
- 108090000765 processed proteins & peptides Proteins 0.000 description 12
- 101150090724 3 gene Proteins 0.000 description 11
- 206010028980 Neoplasm Diseases 0.000 description 11
- 239000003623 enhancer Substances 0.000 description 11
- 230000002441 reversible effect Effects 0.000 description 10
- 229940123121 B-cell inhibitor Drugs 0.000 description 9
- 201000011510 cancer Diseases 0.000 description 9
- 238000002493 microarray Methods 0.000 description 9
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 8
- 238000001514 detection method Methods 0.000 description 8
- 210000002966 serum Anatomy 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 7
- 239000008280 blood Substances 0.000 description 7
- 201000010099 disease Diseases 0.000 description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 7
- 230000002068 genetic effect Effects 0.000 description 7
- 238000012216 screening Methods 0.000 description 7
- 210000001519 tissue Anatomy 0.000 description 7
- 238000011161 development Methods 0.000 description 6
- 108010034529 leucyl-lysine Proteins 0.000 description 6
- 238000007477 logistic regression Methods 0.000 description 6
- 101710190496 GTPase IMAP family member 6 Proteins 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 238000013211 curve analysis Methods 0.000 description 5
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 5
- 238000002604 ultrasonography Methods 0.000 description 5
- 238000010200 validation analysis Methods 0.000 description 5
- 101000833376 Homo sapiens GTPase IMAP family member 5 Proteins 0.000 description 4
- 108010063628 acarboxyprothrombin Proteins 0.000 description 4
- 108010087924 alanylproline Proteins 0.000 description 4
- 239000002299 complementary DNA Substances 0.000 description 4
- 238000003018 immunoassay Methods 0.000 description 4
- 210000004185 liver Anatomy 0.000 description 4
- 238000007726 management method Methods 0.000 description 4
- JTJMJGYZQZDUJJ-UHFFFAOYSA-N phencyclidine Chemical compound C1CCCCN1C1(C=2C=CC=CC=2)CCCCC1 JTJMJGYZQZDUJJ-UHFFFAOYSA-N 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- -1 GIMAP5 Proteins 0.000 description 3
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 3
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 3
- 108010047495 alanylglycine Proteins 0.000 description 3
- 239000000090 biomarker Substances 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 210000004027 cell Anatomy 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 108010050848 glycylleucine Proteins 0.000 description 3
- 108010015792 glycyllysine Proteins 0.000 description 3
- 238000009396 hybridization Methods 0.000 description 3
- 208000019423 liver disease Diseases 0.000 description 3
- 108020004999 messenger RNA Proteins 0.000 description 3
- 210000005259 peripheral blood Anatomy 0.000 description 3
- 239000011886 peripheral blood Substances 0.000 description 3
- 108010029020 prolylglycine Proteins 0.000 description 3
- 238000011002 quantification Methods 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- NMWKYTGJWUAZPZ-WWHBDHEGSA-N (4S)-4-[[(4R,7S,10S,16S,19S,25S,28S,31R)-31-[[(2S)-2-[[(1R,6R,9S,12S,18S,21S,24S,27S,30S,33S,36S,39S,42R,47R,53S,56S,59S,62S,65S,68S,71S,76S,79S,85S)-47-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-methylbutanoyl]amino]-3-methylbutanoyl]amino]-3-hydroxypropanoyl]amino]-3-(1H-imidazol-4-yl)propanoyl]amino]-3-phenylpropanoyl]amino]-4-oxobutanoyl]amino]-3-carboxypropanoyl]amino]-18-(4-aminobutyl)-27,68-bis(3-amino-3-oxopropyl)-36,71,76-tribenzyl-39-(3-carbamimidamidopropyl)-24-(2-carboxyethyl)-21,56-bis(carboxymethyl)-65,85-bis[(1R)-1-hydroxyethyl]-59-(hydroxymethyl)-62,79-bis(1H-imidazol-4-ylmethyl)-9-methyl-33-(2-methylpropyl)-8,11,17,20,23,26,29,32,35,38,41,48,54,57,60,63,66,69,72,74,77,80,83,86-tetracosaoxo-30-propan-2-yl-3,4,44,45-tetrathia-7,10,16,19,22,25,28,31,34,37,40,49,55,58,61,64,67,70,73,75,78,81,84,87-tetracosazatetracyclo[40.31.14.012,16.049,53]heptaoctacontane-6-carbonyl]amino]-3-methylbutanoyl]amino]-7-(3-carbamimidamidopropyl)-25-(hydroxymethyl)-19-[(4-hydroxyphenyl)methyl]-28-(1H-imidazol-4-ylmethyl)-10-methyl-6,9,12,15,18,21,24,27,30-nonaoxo-16-propan-2-yl-1,2-dithia-5,8,11,14,17,20,23,26,29-nonazacyclodotriacontane-4-carbonyl]amino]-5-[[(2S)-1-[[(2S)-1-[[(2S)-3-carboxy-1-[[(2S)-1-[[(2S)-1-[[(1S)-1-carboxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-5-oxopentanoic acid Chemical compound CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@@H]3NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]4CCCN4C(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](Cc4c[nH]cn4)NC(=O)[C@H](Cc4ccccc4)NC3=O)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N3CCC[C@H]3C(=O)N[C@@H](C)C(=O)N2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)C(=O)N[C@@H](C)C(O)=O NMWKYTGJWUAZPZ-WWHBDHEGSA-N 0.000 description 2
- DQVAZKGVGKHQDS-UHFFFAOYSA-N 2-[[1-[2-[(2-amino-4-methylpentanoyl)amino]-4-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-4-methylpentanoic acid Chemical compound CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(=O)NC(CC(C)C)C(O)=O DQVAZKGVGKHQDS-UHFFFAOYSA-N 0.000 description 2
- AUFHLLPVPSMEOG-YUMQZZPRSA-N Arg-Gly-Glu Chemical compound NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O AUFHLLPVPSMEOG-YUMQZZPRSA-N 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 102400001368 Epidermal growth factor Human genes 0.000 description 2
- 101800003838 Epidermal growth factor Proteins 0.000 description 2
- 206010073069 Hepatic cancer Diseases 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- LHSGPCFBGJHPCY-UHFFFAOYSA-N L-leucine-L-tyrosine Natural products CC(C)CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 LHSGPCFBGJHPCY-UHFFFAOYSA-N 0.000 description 2
- UCOCBWDBHCUPQP-DCAQKATOSA-N Leu-Arg-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O UCOCBWDBHCUPQP-DCAQKATOSA-N 0.000 description 2
- WQWSMEOYXJTFRU-GUBZILKMSA-N Leu-Glu-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O WQWSMEOYXJTFRU-GUBZILKMSA-N 0.000 description 2
- PTRKPHUGYULXPU-KKUMJFAQSA-N Leu-Phe-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O PTRKPHUGYULXPU-KKUMJFAQSA-N 0.000 description 2
- DFXQCCBKGUNYGG-GUBZILKMSA-N Lys-Gln-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCCN DFXQCCBKGUNYGG-GUBZILKMSA-N 0.000 description 2
- 108010087066 N2-tryptophyllysine Proteins 0.000 description 2
- 102000004264 Osteopontin Human genes 0.000 description 2
- 108010081689 Osteopontin Proteins 0.000 description 2
- 239000013614 RNA sample Substances 0.000 description 2
- WDXYVIIVDIDOSX-DCAQKATOSA-N Ser-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CCCN=C(N)N WDXYVIIVDIDOSX-DCAQKATOSA-N 0.000 description 2
- JMZKMSTYXHFYAK-VEVYYDQMSA-N Thr-Arg-Asn Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)O JMZKMSTYXHFYAK-VEVYYDQMSA-N 0.000 description 2
- TZKPNGDGUVREEB-FOHZUACHSA-N Thr-Asn-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O TZKPNGDGUVREEB-FOHZUACHSA-N 0.000 description 2
- PRTHQBSMXILLPC-XGEHTFHBSA-N Thr-Ser-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O PRTHQBSMXILLPC-XGEHTFHBSA-N 0.000 description 2
- 102000006747 Transforming Growth Factor alpha Human genes 0.000 description 2
- 101800004564 Transforming growth factor alpha Proteins 0.000 description 2
- VFOHXOLPLACADK-GVXVVHGQSA-N Val-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](C(C)C)N VFOHXOLPLACADK-GVXVVHGQSA-N 0.000 description 2
- DAVNYIUELQBTAP-XUXIUFHCSA-N Val-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N DAVNYIUELQBTAP-XUXIUFHCSA-N 0.000 description 2
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 2
- WDIWOIRFNMLNKO-ULQDDVLXSA-N Val-Leu-Tyr Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 WDIWOIRFNMLNKO-ULQDDVLXSA-N 0.000 description 2
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 2
- 229940024606 amino acid Drugs 0.000 description 2
- 235000001014 amino acid Nutrition 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 2
- 229960000723 ampicillin Drugs 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 108010009111 arginyl-glycyl-glutamic acid Proteins 0.000 description 2
- 108010068380 arginylarginine Proteins 0.000 description 2
- 108010062796 arginyllysine Proteins 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 231100000504 carcinogenesis Toxicity 0.000 description 2
- 208000006990 cholangiocarcinoma Diseases 0.000 description 2
- 108010060199 cysteinylproline Proteins 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 108010054813 diprotin B Proteins 0.000 description 2
- 229940116977 epidermal growth factor Drugs 0.000 description 2
- 210000003743 erythrocyte Anatomy 0.000 description 2
- 239000012997 ficoll-paque Substances 0.000 description 2
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 2
- 108010079413 glycyl-prolyl-glutamic acid Proteins 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 108010040030 histidinoalanine Proteins 0.000 description 2
- 108010025306 histidylleucine Proteins 0.000 description 2
- 108010057821 leucylproline Proteins 0.000 description 2
- 238000007834 ligase chain reaction Methods 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 108010056582 methionylglutamic acid Proteins 0.000 description 2
- 238000010208 microarray analysis Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- VBUNOIXRZNJNAD-UHFFFAOYSA-N ponazuril Chemical group CC1=CC(N2C(N(C)C(=O)NC2=O)=O)=CC=C1OC1=CC=C(S(=O)(=O)C(F)(F)F)C=C1 VBUNOIXRZNJNAD-UHFFFAOYSA-N 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 108010061238 threonyl-glycine Proteins 0.000 description 2
- 108010073969 valyllysine Proteins 0.000 description 2
- 238000012795 verification Methods 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- 101150033839 4 gene Proteins 0.000 description 1
- 101150106774 9 gene Proteins 0.000 description 1
- LGQPPBQRUBVTIF-JBDRJPRFSA-N Ala-Ala-Ile Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LGQPPBQRUBVTIF-JBDRJPRFSA-N 0.000 description 1
- FJVAQLJNTSUQPY-CIUDSAMLSA-N Ala-Ala-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCCN FJVAQLJNTSUQPY-CIUDSAMLSA-N 0.000 description 1
- PIPTUBPKYFRLCP-NHCYSSNCSA-N Ala-Ala-Phe Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 PIPTUBPKYFRLCP-NHCYSSNCSA-N 0.000 description 1
- NXSFUECZFORGOG-CIUDSAMLSA-N Ala-Asn-Leu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O NXSFUECZFORGOG-CIUDSAMLSA-N 0.000 description 1
- GORKKVHIBWAQHM-GCJQMDKQSA-N Ala-Asn-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GORKKVHIBWAQHM-GCJQMDKQSA-N 0.000 description 1
- CSAHOYQKNHGDHX-ACZMJKKPSA-N Ala-Gln-Asn Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O CSAHOYQKNHGDHX-ACZMJKKPSA-N 0.000 description 1
- FUSPCLTUKXQREV-ACZMJKKPSA-N Ala-Glu-Ala Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O FUSPCLTUKXQREV-ACZMJKKPSA-N 0.000 description 1
- FBHOPGDGELNWRH-DRZSPHRISA-N Ala-Glu-Phe Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O FBHOPGDGELNWRH-DRZSPHRISA-N 0.000 description 1
- ZVFVBBGVOILKPO-WHFBIAKZSA-N Ala-Gly-Ala Chemical compound C[C@H](N)C(=O)NCC(=O)N[C@@H](C)C(O)=O ZVFVBBGVOILKPO-WHFBIAKZSA-N 0.000 description 1
- FAJIYNONGXEXAI-CQDKDKBSSA-N Ala-His-Phe Chemical compound C([C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CNC=N1 FAJIYNONGXEXAI-CQDKDKBSSA-N 0.000 description 1
- PNALXAODQKTNLV-JBDRJPRFSA-N Ala-Ile-Ala Chemical compound C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O PNALXAODQKTNLV-JBDRJPRFSA-N 0.000 description 1
- WUHJHHGYVVJMQE-BJDJZHNGSA-N Ala-Leu-Ile Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WUHJHHGYVVJMQE-BJDJZHNGSA-N 0.000 description 1
- AWZKCUCQJNTBAD-SRVKXCTJSA-N Ala-Leu-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCCCN AWZKCUCQJNTBAD-SRVKXCTJSA-N 0.000 description 1
- SOBIAADAMRHGKH-CIUDSAMLSA-N Ala-Leu-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O SOBIAADAMRHGKH-CIUDSAMLSA-N 0.000 description 1
- LDLSENBXQNDTPB-DCAQKATOSA-N Ala-Lys-Arg Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N LDLSENBXQNDTPB-DCAQKATOSA-N 0.000 description 1
- IPZQNYYAYVRKKK-FXQIFTODSA-N Ala-Pro-Ala Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O IPZQNYYAYVRKKK-FXQIFTODSA-N 0.000 description 1
- FQNILRVJOJBFFC-FXQIFTODSA-N Ala-Pro-Asp Chemical compound C[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(=O)O)C(=O)O)N FQNILRVJOJBFFC-FXQIFTODSA-N 0.000 description 1
- IORKCNUBHNIMKY-CIUDSAMLSA-N Ala-Pro-Glu Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O IORKCNUBHNIMKY-CIUDSAMLSA-N 0.000 description 1
- ADSGHMXEAZJJNF-DCAQKATOSA-N Ala-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N ADSGHMXEAZJJNF-DCAQKATOSA-N 0.000 description 1
- DCVYRWFAMZFSDA-ZLUOBGJFSA-N Ala-Ser-Ala Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O DCVYRWFAMZFSDA-ZLUOBGJFSA-N 0.000 description 1
- NHWYNIZWLJYZAG-XVYDVKMFSA-N Ala-Ser-His Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N NHWYNIZWLJYZAG-XVYDVKMFSA-N 0.000 description 1
- HOVPGJUNRLMIOZ-CIUDSAMLSA-N Ala-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)N HOVPGJUNRLMIOZ-CIUDSAMLSA-N 0.000 description 1
- UCDOXFBTMLKASE-HERUPUMHSA-N Ala-Ser-Trp Chemical compound C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N UCDOXFBTMLKASE-HERUPUMHSA-N 0.000 description 1
- XMIAMUXIMWREBJ-HERUPUMHSA-N Ala-Trp-Asn Chemical compound C[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CC(=O)N)C(=O)O)N XMIAMUXIMWREBJ-HERUPUMHSA-N 0.000 description 1
- BGGAIXWIZCIFSG-XDTLVQLUSA-N Ala-Tyr-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O BGGAIXWIZCIFSG-XDTLVQLUSA-N 0.000 description 1
- ZJLORAAXDAJLDC-CQDKDKBSSA-N Ala-Tyr-Leu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(O)=O ZJLORAAXDAJLDC-CQDKDKBSSA-N 0.000 description 1
- DHONNEYAZPNGSG-UBHSHLNASA-N Ala-Val-Phe Chemical compound C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 DHONNEYAZPNGSG-UBHSHLNASA-N 0.000 description 1
- OMSKGWFGWCQFBD-KZVJFYERSA-N Ala-Val-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OMSKGWFGWCQFBD-KZVJFYERSA-N 0.000 description 1
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 1
- 108010082126 Alanine transaminase Proteins 0.000 description 1
- SBVJJNJLFWSJOV-UBHSHLNASA-N Arg-Ala-Phe Chemical compound NC(=N)NCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 SBVJJNJLFWSJOV-UBHSHLNASA-N 0.000 description 1
- GIVATXIGCXFQQA-FXQIFTODSA-N Arg-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N GIVATXIGCXFQQA-FXQIFTODSA-N 0.000 description 1
- BIOCIVSVEDFKDJ-GUBZILKMSA-N Arg-Arg-Asp Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O BIOCIVSVEDFKDJ-GUBZILKMSA-N 0.000 description 1
- MUXONAMCEUBVGA-DCAQKATOSA-N Arg-Arg-Gln Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(O)=O MUXONAMCEUBVGA-DCAQKATOSA-N 0.000 description 1
- OVVUNXXROOFSIM-SDDRHHMPSA-N Arg-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O OVVUNXXROOFSIM-SDDRHHMPSA-N 0.000 description 1
- DPXDVGDLWJYZBH-GUBZILKMSA-N Arg-Asn-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O DPXDVGDLWJYZBH-GUBZILKMSA-N 0.000 description 1
- QPOARHANPULOTM-GMOBBJLQSA-N Arg-Asn-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCN=C(N)N)N QPOARHANPULOTM-GMOBBJLQSA-N 0.000 description 1
- YUGFLWBWAJFGKY-BQBZGAKWSA-N Arg-Cys-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CS)C(=O)NCC(O)=O YUGFLWBWAJFGKY-BQBZGAKWSA-N 0.000 description 1
- OANWAFQRNQEDSY-DCAQKATOSA-N Arg-Cys-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCCN=C(N)N)N OANWAFQRNQEDSY-DCAQKATOSA-N 0.000 description 1
- MTANSHNQTWPZKP-KKUMJFAQSA-N Arg-Gln-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCCN=C(N)N)N)O MTANSHNQTWPZKP-KKUMJFAQSA-N 0.000 description 1
- NYZGVTGOMPHSJW-CIUDSAMLSA-N Arg-Glu-Cys Chemical compound C(C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CS)C(=O)O)N)CN=C(N)N NYZGVTGOMPHSJW-CIUDSAMLSA-N 0.000 description 1
- PBSOQGZLPFVXPU-YUMQZZPRSA-N Arg-Glu-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O PBSOQGZLPFVXPU-YUMQZZPRSA-N 0.000 description 1
- OHYQKYUTLIPFOX-ZPFDUUQYSA-N Arg-Glu-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O OHYQKYUTLIPFOX-ZPFDUUQYSA-N 0.000 description 1
- GOWZVQXTHUCNSQ-NHCYSSNCSA-N Arg-Glu-Val Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O GOWZVQXTHUCNSQ-NHCYSSNCSA-N 0.000 description 1
- UBCPNBUIQNMDNH-NAKRPEOUSA-N Arg-Ile-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O UBCPNBUIQNMDNH-NAKRPEOUSA-N 0.000 description 1
- UZGFHWIJWPUPOH-IHRRRGAJSA-N Arg-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N UZGFHWIJWPUPOH-IHRRRGAJSA-N 0.000 description 1
- JEOCWTUOMKEEMF-RHYQMDGZSA-N Arg-Leu-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JEOCWTUOMKEEMF-RHYQMDGZSA-N 0.000 description 1
- RTDZQOFEGPWSJD-AVGNSLFASA-N Arg-Leu-Val Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O RTDZQOFEGPWSJD-AVGNSLFASA-N 0.000 description 1
- RIIVUOJDDQXHRV-SRVKXCTJSA-N Arg-Lys-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O RIIVUOJDDQXHRV-SRVKXCTJSA-N 0.000 description 1
- CLICCYPMVFGUOF-IHRRRGAJSA-N Arg-Lys-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O CLICCYPMVFGUOF-IHRRRGAJSA-N 0.000 description 1
- BTJVOUQWFXABOI-IHRRRGAJSA-N Arg-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCNC(N)=N BTJVOUQWFXABOI-IHRRRGAJSA-N 0.000 description 1
- VEAIMHJZTIDCIH-KKUMJFAQSA-N Arg-Phe-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O VEAIMHJZTIDCIH-KKUMJFAQSA-N 0.000 description 1
- DPLFNLDACGGBAK-KKUMJFAQSA-N Arg-Phe-Glu Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N DPLFNLDACGGBAK-KKUMJFAQSA-N 0.000 description 1
- FIQKRDXFTANIEJ-ULQDDVLXSA-N Arg-Phe-His Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N FIQKRDXFTANIEJ-ULQDDVLXSA-N 0.000 description 1
- IGFJVXOATGZTHD-UHFFFAOYSA-N Arg-Phe-His Natural products NC(CCNC(=N)N)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc2c[nH]cn2)C(=O)O IGFJVXOATGZTHD-UHFFFAOYSA-N 0.000 description 1
- UIUXXFIKWQVMEX-UFYCRDLUSA-N Arg-Phe-Tyr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O UIUXXFIKWQVMEX-UFYCRDLUSA-N 0.000 description 1
- DNLQVHBBMPZUGJ-BQBZGAKWSA-N Arg-Ser-Gly Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O DNLQVHBBMPZUGJ-BQBZGAKWSA-N 0.000 description 1
- JQHASVQBAKRJKD-GUBZILKMSA-N Arg-Ser-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)N JQHASVQBAKRJKD-GUBZILKMSA-N 0.000 description 1
- HRCIIMCTUIAKQB-XGEHTFHBSA-N Arg-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O HRCIIMCTUIAKQB-XGEHTFHBSA-N 0.000 description 1
- ZJBUILVYSXQNSW-YTWAJWBKSA-N Arg-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O ZJBUILVYSXQNSW-YTWAJWBKSA-N 0.000 description 1
- OGZBJJLRKQZRHL-KJEVXHAQSA-N Arg-Thr-Tyr Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 OGZBJJLRKQZRHL-KJEVXHAQSA-N 0.000 description 1
- ZUVMUOOHJYNJPP-XIRDDKMYSA-N Arg-Trp-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZUVMUOOHJYNJPP-XIRDDKMYSA-N 0.000 description 1
- IZSMEUDYADKZTJ-KJEVXHAQSA-N Arg-Tyr-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O IZSMEUDYADKZTJ-KJEVXHAQSA-N 0.000 description 1
- ULBHWNVWSCJLCO-NHCYSSNCSA-N Arg-Val-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCN=C(N)N ULBHWNVWSCJLCO-NHCYSSNCSA-N 0.000 description 1
- PFOYSEIHFVKHNF-FXQIFTODSA-N Asn-Ala-Arg Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O PFOYSEIHFVKHNF-FXQIFTODSA-N 0.000 description 1
- SLKLLQWZQHXYSV-CIUDSAMLSA-N Asn-Ala-Lys Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O SLKLLQWZQHXYSV-CIUDSAMLSA-N 0.000 description 1
- XQQVCUIBGYFKDC-OLHMAJIHSA-N Asn-Asp-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XQQVCUIBGYFKDC-OLHMAJIHSA-N 0.000 description 1
- NKTLGLBAGUJEGA-BIIVOSGPSA-N Asn-Cys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CS)NC(=O)[C@H](CC(=O)N)N)C(=O)O NKTLGLBAGUJEGA-BIIVOSGPSA-N 0.000 description 1
- XWFPGQVLOVGSLU-CIUDSAMLSA-N Asn-Gln-Arg Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N XWFPGQVLOVGSLU-CIUDSAMLSA-N 0.000 description 1
- BZWRLDPIWKOVKB-ZPFDUUQYSA-N Asn-Leu-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BZWRLDPIWKOVKB-ZPFDUUQYSA-N 0.000 description 1
- JEEFEQCRXKPQHC-KKUMJFAQSA-N Asn-Leu-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O JEEFEQCRXKPQHC-KKUMJFAQSA-N 0.000 description 1
- NCFJQJRLQJEECD-NHCYSSNCSA-N Asn-Leu-Val Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O NCFJQJRLQJEECD-NHCYSSNCSA-N 0.000 description 1
- RZNAMKZJPBQWDJ-SRVKXCTJSA-N Asn-Lys-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)N)N RZNAMKZJPBQWDJ-SRVKXCTJSA-N 0.000 description 1
- AYOAHKWVQLNPDM-HJGDQZAQSA-N Asn-Lys-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O AYOAHKWVQLNPDM-HJGDQZAQSA-N 0.000 description 1
- YUOXLJYVSZYPBJ-CIUDSAMLSA-N Asn-Pro-Glu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O YUOXLJYVSZYPBJ-CIUDSAMLSA-N 0.000 description 1
- GZXOUBTUAUAVHD-ACZMJKKPSA-N Asn-Ser-Glu Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O GZXOUBTUAUAVHD-ACZMJKKPSA-N 0.000 description 1
- MKJBPDLENBUHQU-CIUDSAMLSA-N Asn-Ser-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O MKJBPDLENBUHQU-CIUDSAMLSA-N 0.000 description 1
- HCZQKHSRYHCPSD-IUKAMOBKSA-N Asn-Thr-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HCZQKHSRYHCPSD-IUKAMOBKSA-N 0.000 description 1
- AMGQTNHANMRPOE-LKXGYXEUSA-N Asn-Thr-Ser Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O AMGQTNHANMRPOE-LKXGYXEUSA-N 0.000 description 1
- PWUHPMMGQFPCFG-UBHSHLNASA-N Asn-Trp-Cys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(=O)N)N PWUHPMMGQFPCFG-UBHSHLNASA-N 0.000 description 1
- XZFONYMRYTVLPL-NHCYSSNCSA-N Asn-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(=O)N)N XZFONYMRYTVLPL-NHCYSSNCSA-N 0.000 description 1
- AXXCUABIFZPKPM-BQBZGAKWSA-N Asp-Arg-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O AXXCUABIFZPKPM-BQBZGAKWSA-N 0.000 description 1
- VZNOVQKGJQJOCS-SRVKXCTJSA-N Asp-Asp-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O VZNOVQKGJQJOCS-SRVKXCTJSA-N 0.000 description 1
- JUWZKMBALYLZCK-WHFBIAKZSA-N Asp-Gly-Asn Chemical compound OC(=O)C[C@H](N)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O JUWZKMBALYLZCK-WHFBIAKZSA-N 0.000 description 1
- WSXDIZFNQYTUJB-SRVKXCTJSA-N Asp-His-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O WSXDIZFNQYTUJB-SRVKXCTJSA-N 0.000 description 1
- SPKCGKRUYKMDHP-GUDRVLHUSA-N Asp-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)O)N SPKCGKRUYKMDHP-GUDRVLHUSA-N 0.000 description 1
- CJUKAWUWBZCTDQ-SRVKXCTJSA-N Asp-Leu-Lys Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O CJUKAWUWBZCTDQ-SRVKXCTJSA-N 0.000 description 1
- QNMKWNONJGKJJC-NHCYSSNCSA-N Asp-Leu-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O QNMKWNONJGKJJC-NHCYSSNCSA-N 0.000 description 1
- FQHBAQLBIXLWAG-DCAQKATOSA-N Asp-Lys-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N FQHBAQLBIXLWAG-DCAQKATOSA-N 0.000 description 1
- BKOIIURTQAJHAT-GUBZILKMSA-N Asp-Pro-Pro Chemical compound OC(=O)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 BKOIIURTQAJHAT-GUBZILKMSA-N 0.000 description 1
- NBKLEMWHDLAUEM-CIUDSAMLSA-N Asp-Ser-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(=O)O)N NBKLEMWHDLAUEM-CIUDSAMLSA-N 0.000 description 1
- MGSVBZIBCCKGCY-ZLUOBGJFSA-N Asp-Ser-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O MGSVBZIBCCKGCY-ZLUOBGJFSA-N 0.000 description 1
- JSHWXQIZOCVWIA-ZKWXMUAHSA-N Asp-Ser-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O JSHWXQIZOCVWIA-ZKWXMUAHSA-N 0.000 description 1
- UXRVDHVARNBOIO-QSFUFRPTSA-N Asp-Val-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(=O)O)N UXRVDHVARNBOIO-QSFUFRPTSA-N 0.000 description 1
- QPDUWAUSSWGJSB-NGZCFLSTSA-N Asp-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC(=O)O)N QPDUWAUSSWGJSB-NGZCFLSTSA-N 0.000 description 1
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 1
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 1
- 206010004593 Bile duct cancer Diseases 0.000 description 1
- 101100380241 Caenorhabditis elegans arx-2 gene Proteins 0.000 description 1
- 208000005623 Carcinogenesis Diseases 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- SZQCDCKIGWQAQN-FXQIFTODSA-N Cys-Arg-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(O)=O SZQCDCKIGWQAQN-FXQIFTODSA-N 0.000 description 1
- GEEXORWTBTUOHC-FXQIFTODSA-N Cys-Arg-Ser Chemical compound C(C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N)CN=C(N)N GEEXORWTBTUOHC-FXQIFTODSA-N 0.000 description 1
- OIMUAKUQOUEPCZ-WHFBIAKZSA-N Cys-Asn-Gly Chemical compound SC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O OIMUAKUQOUEPCZ-WHFBIAKZSA-N 0.000 description 1
- KPENUVBHAKRDQR-GUBZILKMSA-N Cys-His-Glu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(O)=O KPENUVBHAKRDQR-GUBZILKMSA-N 0.000 description 1
- PRHGYQOSEHLDRW-VGDYDELISA-N Cys-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CS)N PRHGYQOSEHLDRW-VGDYDELISA-N 0.000 description 1
- IZUNQDRIAOLWCN-YUMQZZPRSA-N Cys-Leu-Gly Chemical compound CC(C)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CS)N IZUNQDRIAOLWCN-YUMQZZPRSA-N 0.000 description 1
- VTBGVPWSWJBERH-DCAQKATOSA-N Cys-Leu-Met Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CS)N VTBGVPWSWJBERH-DCAQKATOSA-N 0.000 description 1
- LHMSYHSAAJOEBL-CIUDSAMLSA-N Cys-Lys-Asn Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O LHMSYHSAAJOEBL-CIUDSAMLSA-N 0.000 description 1
- VDUPGIDTWNQAJD-CIUDSAMLSA-N Cys-Lys-Cys Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)CS)C(=O)N[C@@H](CS)C(O)=O VDUPGIDTWNQAJD-CIUDSAMLSA-N 0.000 description 1
- POSRGGKLRWCUBE-CIUDSAMLSA-N Cys-Met-Glu Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CS)N POSRGGKLRWCUBE-CIUDSAMLSA-N 0.000 description 1
- LKHMGNHQULEPFY-ACZMJKKPSA-N Cys-Ser-Glu Chemical compound SC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O LKHMGNHQULEPFY-ACZMJKKPSA-N 0.000 description 1
- WVWRADGCZPIJJR-IHRRRGAJSA-N Cys-Val-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CS)N WVWRADGCZPIJJR-IHRRRGAJSA-N 0.000 description 1
- SHIBSTMRCDJXLN-UHFFFAOYSA-N Digoxigenin Natural products C1CC(C2C(C3(C)CCC(O)CC3CC2)CC2O)(O)C2(C)C1C1=CC(=O)OC1 SHIBSTMRCDJXLN-UHFFFAOYSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- SHWNNYZBHZIQQV-UHFFFAOYSA-J EDTA monocalcium diisodium salt Chemical compound [Na+].[Na+].[Ca+2].[O-]C(=O)CN(CC([O-])=O)CCN(CC([O-])=O)CC([O-])=O SHWNNYZBHZIQQV-UHFFFAOYSA-J 0.000 description 1
- 102000001301 EGF receptor Human genes 0.000 description 1
- 108060006698 EGF receptor Proteins 0.000 description 1
- 101710142246 External core antigen Proteins 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- YJIUYQKQBBQYHZ-ACZMJKKPSA-N Gln-Ala-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O YJIUYQKQBBQYHZ-ACZMJKKPSA-N 0.000 description 1
- DTCCMDYODDPHBG-ACZMJKKPSA-N Gln-Ala-Cys Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CS)C(O)=O DTCCMDYODDPHBG-ACZMJKKPSA-N 0.000 description 1
- KVYVOGYEMPEXBT-GUBZILKMSA-N Gln-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(N)=O KVYVOGYEMPEXBT-GUBZILKMSA-N 0.000 description 1
- XXLBHPPXDUWYAG-XQXXSGGOSA-N Gln-Ala-Thr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XXLBHPPXDUWYAG-XQXXSGGOSA-N 0.000 description 1
- JFOKLAPFYCTNHW-SRVKXCTJSA-N Gln-Arg-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(=O)N)N JFOKLAPFYCTNHW-SRVKXCTJSA-N 0.000 description 1
- JESJDAAGXULQOP-CIUDSAMLSA-N Gln-Arg-Ser Chemical compound C(C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)CN=C(N)N JESJDAAGXULQOP-CIUDSAMLSA-N 0.000 description 1
- RRYLMJWPWBJFPZ-ACZMJKKPSA-N Gln-Asn-Asp Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N RRYLMJWPWBJFPZ-ACZMJKKPSA-N 0.000 description 1
- TWHDOEYLXXQYOZ-FXQIFTODSA-N Gln-Asn-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N TWHDOEYLXXQYOZ-FXQIFTODSA-N 0.000 description 1
- PONUFVLSGMQFAI-AVGNSLFASA-N Gln-Asn-Phe Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PONUFVLSGMQFAI-AVGNSLFASA-N 0.000 description 1
- UICOTGULOUGGLC-NUMRIWBASA-N Gln-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N)O UICOTGULOUGGLC-NUMRIWBASA-N 0.000 description 1
- XFKUFUJECJUQTQ-CIUDSAMLSA-N Gln-Gln-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O XFKUFUJECJUQTQ-CIUDSAMLSA-N 0.000 description 1
- LLRJEFPKIIBGJP-DCAQKATOSA-N Gln-Glu-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)N)N LLRJEFPKIIBGJP-DCAQKATOSA-N 0.000 description 1
- PNENQZWRFMUZOM-DCAQKATOSA-N Gln-Glu-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O PNENQZWRFMUZOM-DCAQKATOSA-N 0.000 description 1
- IKFZXRLDMYWNBU-YUMQZZPRSA-N Gln-Gly-Arg Chemical compound NC(=O)CC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCN=C(N)N IKFZXRLDMYWNBU-YUMQZZPRSA-N 0.000 description 1
- HVQCEQTUSWWFOS-WDSKDSINSA-N Gln-Gly-Cys Chemical compound C(CC(=O)N)[C@@H](C(=O)NCC(=O)N[C@@H](CS)C(=O)O)N HVQCEQTUSWWFOS-WDSKDSINSA-N 0.000 description 1
- DQPOBSRQNWOBNA-GUBZILKMSA-N Gln-His-Asn Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O DQPOBSRQNWOBNA-GUBZILKMSA-N 0.000 description 1
- SBHVGKBYOQKAEA-SDDRHHMPSA-N Gln-His-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CCC(=O)N)N)C(=O)O SBHVGKBYOQKAEA-SDDRHHMPSA-N 0.000 description 1
- HWEINOMSWQSJDC-SRVKXCTJSA-N Gln-Leu-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O HWEINOMSWQSJDC-SRVKXCTJSA-N 0.000 description 1
- VUVKKXPCKILIBD-AVGNSLFASA-N Gln-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CCC(=O)N)N VUVKKXPCKILIBD-AVGNSLFASA-N 0.000 description 1
- HPCOBEHVEHWREJ-DCAQKATOSA-N Gln-Lys-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O HPCOBEHVEHWREJ-DCAQKATOSA-N 0.000 description 1
- DSRVQBZAMPGEKU-AVGNSLFASA-N Gln-Phe-Cys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)N)N DSRVQBZAMPGEKU-AVGNSLFASA-N 0.000 description 1
- XQDGOJPVMSWZSO-SRVKXCTJSA-N Gln-Pro-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(=O)N)N XQDGOJPVMSWZSO-SRVKXCTJSA-N 0.000 description 1
- OTQSTOXRUBVWAP-NRPADANISA-N Gln-Ser-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O OTQSTOXRUBVWAP-NRPADANISA-N 0.000 description 1
- VEYGCDYMOXHJLS-GVXVVHGQSA-N Gln-Val-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O VEYGCDYMOXHJLS-GVXVVHGQSA-N 0.000 description 1
- GJLXZITZLUUXMJ-NHCYSSNCSA-N Gln-Val-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CCC(=O)N)N GJLXZITZLUUXMJ-NHCYSSNCSA-N 0.000 description 1
- SOEXCCGNHQBFPV-DLOVCJGASA-N Gln-Val-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O SOEXCCGNHQBFPV-DLOVCJGASA-N 0.000 description 1
- LKDIBBOKUAASNP-FXQIFTODSA-N Glu-Ala-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O LKDIBBOKUAASNP-FXQIFTODSA-N 0.000 description 1
- JJKKWYQVHRUSDG-GUBZILKMSA-N Glu-Ala-Lys Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O JJKKWYQVHRUSDG-GUBZILKMSA-N 0.000 description 1
- OJGLIOXAKGFFDW-SRVKXCTJSA-N Glu-Arg-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(=O)O)N OJGLIOXAKGFFDW-SRVKXCTJSA-N 0.000 description 1
- SRZLHYPAOXBBSB-HJGDQZAQSA-N Glu-Arg-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SRZLHYPAOXBBSB-HJGDQZAQSA-N 0.000 description 1
- YYOBUPFZLKQUAX-FXQIFTODSA-N Glu-Asn-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O YYOBUPFZLKQUAX-FXQIFTODSA-N 0.000 description 1
- CKRUHITYRFNUKW-WDSKDSINSA-N Glu-Asn-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O CKRUHITYRFNUKW-WDSKDSINSA-N 0.000 description 1
- ZXQPJYWZSFGWJB-AVGNSLFASA-N Glu-Cys-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(=O)O)N ZXQPJYWZSFGWJB-AVGNSLFASA-N 0.000 description 1
- MUSGDMDGNGXULI-DCAQKATOSA-N Glu-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O MUSGDMDGNGXULI-DCAQKATOSA-N 0.000 description 1
- QIQABBIDHGQXGA-ZPFDUUQYSA-N Glu-Ile-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O QIQABBIDHGQXGA-ZPFDUUQYSA-N 0.000 description 1
- BKRQSECBKKCCKW-HVTMNAMFSA-N Glu-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CCC(=O)O)N BKRQSECBKKCCKW-HVTMNAMFSA-N 0.000 description 1
- GXMXPCXXKVWOSM-KQXIARHKSA-N Glu-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N GXMXPCXXKVWOSM-KQXIARHKSA-N 0.000 description 1
- VSRCAOIHMGCIJK-SRVKXCTJSA-N Glu-Leu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VSRCAOIHMGCIJK-SRVKXCTJSA-N 0.000 description 1
- RBXSZQRSEGYDFG-GUBZILKMSA-N Glu-Lys-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O RBXSZQRSEGYDFG-GUBZILKMSA-N 0.000 description 1
- XEKAJTCACGEBOK-KKUMJFAQSA-N Glu-Met-Phe Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CCC(=O)O)N XEKAJTCACGEBOK-KKUMJFAQSA-N 0.000 description 1
- UMHRCVCZUPBBQW-GARJFASQSA-N Glu-Met-Pro Chemical compound CSCC[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N UMHRCVCZUPBBQW-GARJFASQSA-N 0.000 description 1
- KXTAGESXNQEZKB-DZKIICNBSA-N Glu-Phe-Val Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C(C)C)C(O)=O)CC1=CC=CC=C1 KXTAGESXNQEZKB-DZKIICNBSA-N 0.000 description 1
- AAJHGGDRKHYSDH-GUBZILKMSA-N Glu-Pro-Gln Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCC(=O)O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O AAJHGGDRKHYSDH-GUBZILKMSA-N 0.000 description 1
- GMVCSRBOSIUTFC-FXQIFTODSA-N Glu-Ser-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O GMVCSRBOSIUTFC-FXQIFTODSA-N 0.000 description 1
- IDEODOAVGCMUQV-GUBZILKMSA-N Glu-Ser-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O IDEODOAVGCMUQV-GUBZILKMSA-N 0.000 description 1
- JWNZHMSRZXXGTM-XKBZYTNZSA-N Glu-Ser-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JWNZHMSRZXXGTM-XKBZYTNZSA-N 0.000 description 1
- BDISFWMLMNBTGP-NUMRIWBASA-N Glu-Thr-Asp Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O BDISFWMLMNBTGP-NUMRIWBASA-N 0.000 description 1
- GPSHCSTUYOQPAI-JHEQGTHGSA-N Glu-Thr-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O GPSHCSTUYOQPAI-JHEQGTHGSA-N 0.000 description 1
- UMZHHILWZBFPGL-LOKLDPHHSA-N Glu-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N)O UMZHHILWZBFPGL-LOKLDPHHSA-N 0.000 description 1
- JDAYMLXPUJRSDJ-XIRDDKMYSA-N Glu-Trp-Arg Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 JDAYMLXPUJRSDJ-XIRDDKMYSA-N 0.000 description 1
- VJVAQZYGLMJPTK-QEJZJMRPSA-N Glu-Trp-Asp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CCC(=O)O)N VJVAQZYGLMJPTK-QEJZJMRPSA-N 0.000 description 1
- UUTGYDAKPISJAO-JYJNAYRXSA-N Glu-Tyr-Leu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 UUTGYDAKPISJAO-JYJNAYRXSA-N 0.000 description 1
- RLFSBAPJTYKSLG-WHFBIAKZSA-N Gly-Ala-Asp Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(O)=O RLFSBAPJTYKSLG-WHFBIAKZSA-N 0.000 description 1
- FKJQNJCQTKUBCD-XPUUQOCRSA-N Gly-Ala-His Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)O FKJQNJCQTKUBCD-XPUUQOCRSA-N 0.000 description 1
- LJPIRKICOISLKN-WHFBIAKZSA-N Gly-Ala-Ser Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O LJPIRKICOISLKN-WHFBIAKZSA-N 0.000 description 1
- AIJAPFVDBFYNKN-WHFBIAKZSA-N Gly-Asn-Asp Chemical compound C([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)CN)C(=O)N AIJAPFVDBFYNKN-WHFBIAKZSA-N 0.000 description 1
- IWAXHBCACVWNHT-BQBZGAKWSA-N Gly-Asp-Arg Chemical compound NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IWAXHBCACVWNHT-BQBZGAKWSA-N 0.000 description 1
- LLXVQPKEQQCISF-YUMQZZPRSA-N Gly-Asp-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)CN LLXVQPKEQQCISF-YUMQZZPRSA-N 0.000 description 1
- IXKRSKPKSLXIHN-YUMQZZPRSA-N Gly-Cys-Leu Chemical compound [H]NCC(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O IXKRSKPKSLXIHN-YUMQZZPRSA-N 0.000 description 1
- BIRKKBCSAIHDDF-WDSKDSINSA-N Gly-Glu-Cys Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(O)=O BIRKKBCSAIHDDF-WDSKDSINSA-N 0.000 description 1
- BUEFQXUHTUZXHR-LURJTMIESA-N Gly-Gly-Pro zwitterion Chemical compound NCC(=O)NCC(=O)N1CCC[C@H]1C(O)=O BUEFQXUHTUZXHR-LURJTMIESA-N 0.000 description 1
- VIIBEIQMLJEUJG-LAEOZQHASA-N Gly-Ile-Gln Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(O)=O VIIBEIQMLJEUJG-LAEOZQHASA-N 0.000 description 1
- XVYKMNXXJXQKME-XEGUGMAKSA-N Gly-Ile-Tyr Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 XVYKMNXXJXQKME-XEGUGMAKSA-N 0.000 description 1
- UUYBFNKHOCJCHT-VHSXEESVSA-N Gly-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN UUYBFNKHOCJCHT-VHSXEESVSA-N 0.000 description 1
- LHYJCVCQPWRMKZ-WEDXCCLWSA-N Gly-Leu-Thr Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LHYJCVCQPWRMKZ-WEDXCCLWSA-N 0.000 description 1
- VLIJYPMATZSOLL-YUMQZZPRSA-N Gly-Lys-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)CN VLIJYPMATZSOLL-YUMQZZPRSA-N 0.000 description 1
- QVDGHDFFYHKJPN-QWRGUYRKSA-N Gly-Phe-Cys Chemical compound NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CS)C(O)=O QVDGHDFFYHKJPN-QWRGUYRKSA-N 0.000 description 1
- JPVGHHQGKPQYIL-KBPBESRZSA-N Gly-Phe-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CC1=CC=CC=C1 JPVGHHQGKPQYIL-KBPBESRZSA-N 0.000 description 1
- JJGBXTYGTKWGAT-YUMQZZPRSA-N Gly-Pro-Glu Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O JJGBXTYGTKWGAT-YUMQZZPRSA-N 0.000 description 1
- IRJWAYCXIYUHQE-WHFBIAKZSA-N Gly-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)CN IRJWAYCXIYUHQE-WHFBIAKZSA-N 0.000 description 1
- IALQAMYQJBZNSK-WHFBIAKZSA-N Gly-Ser-Asn Chemical compound [H]NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O IALQAMYQJBZNSK-WHFBIAKZSA-N 0.000 description 1
- LCRDMSSAKLTKBU-ZDLURKLDSA-N Gly-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN LCRDMSSAKLTKBU-ZDLURKLDSA-N 0.000 description 1
- ZZWUYQXMIFTIIY-WEDXCCLWSA-N Gly-Thr-Leu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O ZZWUYQXMIFTIIY-WEDXCCLWSA-N 0.000 description 1
- 102100021181 Golgi phosphoprotein 3 Human genes 0.000 description 1
- RVKIPWVMZANZLI-UHFFFAOYSA-N H-Lys-Trp-OH Natural products C1=CC=C2C(CC(NC(=O)C(N)CCCCN)C(O)=O)=CNC2=C1 RVKIPWVMZANZLI-UHFFFAOYSA-N 0.000 description 1
- 206010019695 Hepatic neoplasm Diseases 0.000 description 1
- 241000700721 Hepatitis B virus Species 0.000 description 1
- 206010019799 Hepatitis viral Diseases 0.000 description 1
- AWASVTXPTOLPPP-MBLNEYKQSA-N His-Ala-Thr Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O AWASVTXPTOLPPP-MBLNEYKQSA-N 0.000 description 1
- TVQGUFGDVODUIF-LSJOCFKGSA-N His-Arg-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC1=CN=CN1)N TVQGUFGDVODUIF-LSJOCFKGSA-N 0.000 description 1
- LBQAHBIVXQSBIR-HVTMNAMFSA-N His-Ile-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CN=CN1)N LBQAHBIVXQSBIR-HVTMNAMFSA-N 0.000 description 1
- SKYULSWNBYAQMG-IHRRRGAJSA-N His-Leu-Arg Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SKYULSWNBYAQMG-IHRRRGAJSA-N 0.000 description 1
- OQDLKDUVMTUPPG-AVGNSLFASA-N His-Leu-Glu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O OQDLKDUVMTUPPG-AVGNSLFASA-N 0.000 description 1
- PGRPSOUCWRBWKZ-DLOVCJGASA-N His-Lys-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC1=CN=CN1 PGRPSOUCWRBWKZ-DLOVCJGASA-N 0.000 description 1
- BSVLMPMIXPQNKC-KBPBESRZSA-N His-Phe-Gly Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(O)=O BSVLMPMIXPQNKC-KBPBESRZSA-N 0.000 description 1
- SVVULKPWDBIPCO-BZSNNMDCSA-N His-Phe-Leu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(O)=O SVVULKPWDBIPCO-BZSNNMDCSA-N 0.000 description 1
- ZFDKSLBEWYCOCS-BZSNNMDCSA-N His-Phe-Lys Chemical compound C([C@@H](C(=O)N[C@@H](CCCCN)C(O)=O)NC(=O)[C@@H](N)CC=1NC=NC=1)C1=CC=CC=C1 ZFDKSLBEWYCOCS-BZSNNMDCSA-N 0.000 description 1
- KAXZXLSXFWSNNZ-XVYDVKMFSA-N His-Ser-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O KAXZXLSXFWSNNZ-XVYDVKMFSA-N 0.000 description 1
- WKEABZIITNXXQZ-CIUDSAMLSA-N His-Ser-Cys Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N WKEABZIITNXXQZ-CIUDSAMLSA-N 0.000 description 1
- BFOGZWSSGMLYKV-DCAQKATOSA-N His-Ser-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC1=CN=CN1)N BFOGZWSSGMLYKV-DCAQKATOSA-N 0.000 description 1
- PBJOQLUVSGXRSW-YTQUADARSA-N His-Trp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CNC3=CC=CC=C32)NC(=O)[C@H](CC4=CN=CN4)N)C(=O)O PBJOQLUVSGXRSW-YTQUADARSA-N 0.000 description 1
- 101001040734 Homo sapiens Golgi phosphoprotein 3 Proteins 0.000 description 1
- NKVZTQVGUNLLQW-JBDRJPRFSA-N Ile-Ala-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)O)N NKVZTQVGUNLLQW-JBDRJPRFSA-N 0.000 description 1
- TZCGZYWNIDZZMR-UHFFFAOYSA-N Ile-Arg-Ala Natural products CCC(C)C(N)C(=O)NC(C(=O)NC(C)C(O)=O)CCCN=C(N)N TZCGZYWNIDZZMR-UHFFFAOYSA-N 0.000 description 1
- VZIFYHYNQDIPLI-HJWJTTGWSA-N Ile-Arg-Phe Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N VZIFYHYNQDIPLI-HJWJTTGWSA-N 0.000 description 1
- DURWCDDDAWVPOP-JBDRJPRFSA-N Ile-Cys-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O)N DURWCDDDAWVPOP-JBDRJPRFSA-N 0.000 description 1
- WZDCVAWMBUNDDY-KBIXCLLPSA-N Ile-Glu-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](C)C(=O)O)N WZDCVAWMBUNDDY-KBIXCLLPSA-N 0.000 description 1
- HUWYGQOISIJNMK-SIGLWIIPSA-N Ile-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N HUWYGQOISIJNMK-SIGLWIIPSA-N 0.000 description 1
- KLBVGHCGHUNHEA-BJDJZHNGSA-N Ile-Leu-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)O)N KLBVGHCGHUNHEA-BJDJZHNGSA-N 0.000 description 1
- FZWVCYCYWCLQDH-NHCYSSNCSA-N Ile-Leu-Gly Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)O)N FZWVCYCYWCLQDH-NHCYSSNCSA-N 0.000 description 1
- PHRWFSFCNJPWRO-PPCPHDFISA-N Ile-Leu-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N PHRWFSFCNJPWRO-PPCPHDFISA-N 0.000 description 1
- UOPBQSJRBONRON-STECZYCISA-N Ile-Met-Tyr Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 UOPBQSJRBONRON-STECZYCISA-N 0.000 description 1
- WYUHAXJAMDTOAU-IAVJCBSLSA-N Ile-Phe-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N WYUHAXJAMDTOAU-IAVJCBSLSA-N 0.000 description 1
- LRAUKBMYHHNADU-DKIMLUQUSA-N Ile-Phe-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)[C@@H](C)CC)CC1=CC=CC=C1 LRAUKBMYHHNADU-DKIMLUQUSA-N 0.000 description 1
- XQLGNKLSPYCRMZ-HJWJTTGWSA-N Ile-Phe-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C(C)C)C(=O)O)N XQLGNKLSPYCRMZ-HJWJTTGWSA-N 0.000 description 1
- KCTIFOCXAIUQQK-QXEWZRGKSA-N Ile-Pro-Gly Chemical compound CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O KCTIFOCXAIUQQK-QXEWZRGKSA-N 0.000 description 1
- PBWMCUAFLPMYPF-ZQINRCPSSA-N Ile-Trp-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N PBWMCUAFLPMYPF-ZQINRCPSSA-N 0.000 description 1
- YJRSIJZUIUANHO-NAKRPEOUSA-N Ile-Val-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)O)N YJRSIJZUIUANHO-NAKRPEOUSA-N 0.000 description 1
- BCISUQVFDGYZBO-QSFUFRPTSA-N Ile-Val-Asp Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC(O)=O BCISUQVFDGYZBO-QSFUFRPTSA-N 0.000 description 1
- WIYDLTIBHZSPKY-HJWJTTGWSA-N Ile-Val-Phe Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 WIYDLTIBHZSPKY-HJWJTTGWSA-N 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 108010065920 Insulin Lispro Proteins 0.000 description 1
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 1
- SITWEMZOJNKJCH-UHFFFAOYSA-N L-alanine-L-arginine Natural products CC(N)C(=O)NC(C(O)=O)CCCNC(N)=N SITWEMZOJNKJCH-UHFFFAOYSA-N 0.000 description 1
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 1
- SENJXOPIZNYLHU-UHFFFAOYSA-N L-leucyl-L-arginine Natural products CC(C)CC(N)C(=O)NC(C(O)=O)CCCN=C(N)N SENJXOPIZNYLHU-UHFFFAOYSA-N 0.000 description 1
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 1
- 241000880493 Leptailurus serval Species 0.000 description 1
- GRZSCTXVCDUIPO-SRVKXCTJSA-N Leu-Arg-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O GRZSCTXVCDUIPO-SRVKXCTJSA-N 0.000 description 1
- YOZCKMXHBYKOMQ-IHRRRGAJSA-N Leu-Arg-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)O)N YOZCKMXHBYKOMQ-IHRRRGAJSA-N 0.000 description 1
- TWQIYNGNYNJUFM-NHCYSSNCSA-N Leu-Asn-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O TWQIYNGNYNJUFM-NHCYSSNCSA-N 0.000 description 1
- ILJREDZFPHTUIE-GUBZILKMSA-N Leu-Asp-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O ILJREDZFPHTUIE-GUBZILKMSA-N 0.000 description 1
- DLCOFDAHNMMQPP-SRVKXCTJSA-N Leu-Asp-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O DLCOFDAHNMMQPP-SRVKXCTJSA-N 0.000 description 1
- MMEDVBWCMGRKKC-GARJFASQSA-N Leu-Asp-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N MMEDVBWCMGRKKC-GARJFASQSA-N 0.000 description 1
- DKEZVKFLETVJFY-CIUDSAMLSA-N Leu-Cys-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)N)C(=O)O)N DKEZVKFLETVJFY-CIUDSAMLSA-N 0.000 description 1
- PIHFVNPEAHFNLN-KKUMJFAQSA-N Leu-Cys-Tyr Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N PIHFVNPEAHFNLN-KKUMJFAQSA-N 0.000 description 1
- ZYLJULGXQDNXDK-GUBZILKMSA-N Leu-Gln-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O ZYLJULGXQDNXDK-GUBZILKMSA-N 0.000 description 1
- KUEVMUXNILMJTK-JYJNAYRXSA-N Leu-Gln-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 KUEVMUXNILMJTK-JYJNAYRXSA-N 0.000 description 1
- CCQLQKZTXZBXTN-NHCYSSNCSA-N Leu-Gly-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O CCQLQKZTXZBXTN-NHCYSSNCSA-N 0.000 description 1
- UCDHVOALNXENLC-KBPBESRZSA-N Leu-Gly-Tyr Chemical compound CC(C)C[C@H]([NH3+])C(=O)NCC(=O)N[C@H](C([O-])=O)CC1=CC=C(O)C=C1 UCDHVOALNXENLC-KBPBESRZSA-N 0.000 description 1
- USLNHQZCDQJBOV-ZPFDUUQYSA-N Leu-Ile-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O USLNHQZCDQJBOV-ZPFDUUQYSA-N 0.000 description 1
- KOSWSHVQIVTVQF-ZPFDUUQYSA-N Leu-Ile-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O KOSWSHVQIVTVQF-ZPFDUUQYSA-N 0.000 description 1
- LXKNSJLSGPNHSK-KKUMJFAQSA-N Leu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)O)N LXKNSJLSGPNHSK-KKUMJFAQSA-N 0.000 description 1
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 1
- RZXLZBIUTDQHJQ-SRVKXCTJSA-N Leu-Lys-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O RZXLZBIUTDQHJQ-SRVKXCTJSA-N 0.000 description 1
- HDHQQEDVWQGBEE-DCAQKATOSA-N Leu-Met-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(O)=O HDHQQEDVWQGBEE-DCAQKATOSA-N 0.000 description 1
- WMIOEVKKYIMVKI-DCAQKATOSA-N Leu-Pro-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O WMIOEVKKYIMVKI-DCAQKATOSA-N 0.000 description 1
- KWLWZYMNUZJKMZ-IHRRRGAJSA-N Leu-Pro-Leu Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O KWLWZYMNUZJKMZ-IHRRRGAJSA-N 0.000 description 1
- CHJKEDSZNSONPS-DCAQKATOSA-N Leu-Pro-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O CHJKEDSZNSONPS-DCAQKATOSA-N 0.000 description 1
- MVHXGBZUJLWZOH-BJDJZHNGSA-N Leu-Ser-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O MVHXGBZUJLWZOH-BJDJZHNGSA-N 0.000 description 1
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 1
- AMSSKPUHBUQBOQ-SRVKXCTJSA-N Leu-Ser-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N AMSSKPUHBUQBOQ-SRVKXCTJSA-N 0.000 description 1
- JGKHAFUAPZCCDU-BZSNNMDCSA-N Leu-Tyr-Leu Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C([O-])=O)CC1=CC=C(O)C=C1 JGKHAFUAPZCCDU-BZSNNMDCSA-N 0.000 description 1
- UFPLDOKWDNTTRP-ULQDDVLXSA-N Leu-Tyr-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(C)C)CC1=CC=C(O)C=C1 UFPLDOKWDNTTRP-ULQDDVLXSA-N 0.000 description 1
- FBNPMTNBFFAMMH-UHFFFAOYSA-N Leu-Val-Arg Natural products CC(C)CC(N)C(=O)NC(C(C)C)C(=O)NC(C(O)=O)CCCN=C(N)N FBNPMTNBFFAMMH-UHFFFAOYSA-N 0.000 description 1
- XOEDPXDZJHBQIX-ULQDDVLXSA-N Leu-Val-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 XOEDPXDZJHBQIX-ULQDDVLXSA-N 0.000 description 1
- QESXLSQLQHHTIX-RHYQMDGZSA-N Leu-Val-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QESXLSQLQHHTIX-RHYQMDGZSA-N 0.000 description 1
- MSFITIBEMPWCBD-ULQDDVLXSA-N Leu-Val-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 MSFITIBEMPWCBD-ULQDDVLXSA-N 0.000 description 1
- XFIHDSBIPWEYJJ-YUMQZZPRSA-N Lys-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN XFIHDSBIPWEYJJ-YUMQZZPRSA-N 0.000 description 1
- IRNSXVOWSXSULE-DCAQKATOSA-N Lys-Ala-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN IRNSXVOWSXSULE-DCAQKATOSA-N 0.000 description 1
- CLBGMWIYPYAZPR-AVGNSLFASA-N Lys-Arg-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O CLBGMWIYPYAZPR-AVGNSLFASA-N 0.000 description 1
- JGAMUXDWYSXYLM-SRVKXCTJSA-N Lys-Arg-Glu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O JGAMUXDWYSXYLM-SRVKXCTJSA-N 0.000 description 1
- SJNZALDHDUYDBU-IHRRRGAJSA-N Lys-Arg-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(O)=O SJNZALDHDUYDBU-IHRRRGAJSA-N 0.000 description 1
- CKSXSQUVEYCDIW-AVGNSLFASA-N Lys-Arg-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCCN)N CKSXSQUVEYCDIW-AVGNSLFASA-N 0.000 description 1
- SWWCDAGDQHTKIE-RHYQMDGZSA-N Lys-Arg-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SWWCDAGDQHTKIE-RHYQMDGZSA-N 0.000 description 1
- DNEJSAIMVANNPA-DCAQKATOSA-N Lys-Asn-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O DNEJSAIMVANNPA-DCAQKATOSA-N 0.000 description 1
- YKIRNDPUWONXQN-GUBZILKMSA-N Lys-Asn-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N YKIRNDPUWONXQN-GUBZILKMSA-N 0.000 description 1
- HQVDJTYKCMIWJP-YUMQZZPRSA-N Lys-Asn-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O HQVDJTYKCMIWJP-YUMQZZPRSA-N 0.000 description 1
- SVJRVFPSHPGWFF-DCAQKATOSA-N Lys-Cys-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SVJRVFPSHPGWFF-DCAQKATOSA-N 0.000 description 1
- AIPHUKOBUXJNKM-KKUMJFAQSA-N Lys-Cys-Phe Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O AIPHUKOBUXJNKM-KKUMJFAQSA-N 0.000 description 1
- WTZUSCUIVPVCRH-SRVKXCTJSA-N Lys-Gln-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N WTZUSCUIVPVCRH-SRVKXCTJSA-N 0.000 description 1
- GJJQCBVRWDGLMQ-GUBZILKMSA-N Lys-Glu-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O GJJQCBVRWDGLMQ-GUBZILKMSA-N 0.000 description 1
- LPAJOCKCPRZEAG-MNXVOIDGSA-N Lys-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCCCN LPAJOCKCPRZEAG-MNXVOIDGSA-N 0.000 description 1
- WGLAORUKDGRINI-WDCWCFNPSA-N Lys-Glu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGLAORUKDGRINI-WDCWCFNPSA-N 0.000 description 1
- ISHNZELVUVPCHY-ZETCQYMHSA-N Lys-Gly-Gly Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)NCC(O)=O ISHNZELVUVPCHY-ZETCQYMHSA-N 0.000 description 1
- PBLLTSKBTAHDNA-KBPBESRZSA-N Lys-Gly-Phe Chemical compound [H]N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O PBLLTSKBTAHDNA-KBPBESRZSA-N 0.000 description 1
- VLMNBMFYRMGEMB-QWRGUYRKSA-N Lys-His-Gly Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CNC=N1 VLMNBMFYRMGEMB-QWRGUYRKSA-N 0.000 description 1
- MXMDJEJWERYPMO-XUXIUFHCSA-N Lys-Ile-Arg Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O MXMDJEJWERYPMO-XUXIUFHCSA-N 0.000 description 1
- NJNRBRKHOWSGMN-SRVKXCTJSA-N Lys-Leu-Asn Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O NJNRBRKHOWSGMN-SRVKXCTJSA-N 0.000 description 1
- AIRZWUMAHCDDHR-KKUMJFAQSA-N Lys-Leu-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O AIRZWUMAHCDDHR-KKUMJFAQSA-N 0.000 description 1
- YPLVCBKEPJPBDQ-MELADBBJSA-N Lys-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCCN)N YPLVCBKEPJPBDQ-MELADBBJSA-N 0.000 description 1
- HVAUKHLDSDDROB-KKUMJFAQSA-N Lys-Lys-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O HVAUKHLDSDDROB-KKUMJFAQSA-N 0.000 description 1
- WBSCNDJQPKSPII-KKUMJFAQSA-N Lys-Lys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O WBSCNDJQPKSPII-KKUMJFAQSA-N 0.000 description 1
- URGPVYGVWLIRGT-DCAQKATOSA-N Lys-Met-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(O)=O URGPVYGVWLIRGT-DCAQKATOSA-N 0.000 description 1
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 1
- UQJOKDAYFULYIX-AVGNSLFASA-N Lys-Pro-Pro Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 UQJOKDAYFULYIX-AVGNSLFASA-N 0.000 description 1
- DNWBUCHHMRQWCZ-GUBZILKMSA-N Lys-Ser-Gln Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(N)=O DNWBUCHHMRQWCZ-GUBZILKMSA-N 0.000 description 1
- YRNRVKTYDSLKMD-KKUMJFAQSA-N Lys-Ser-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O YRNRVKTYDSLKMD-KKUMJFAQSA-N 0.000 description 1
- PLOUVAYOMTYJRG-JXUBOQSCSA-N Lys-Thr-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O PLOUVAYOMTYJRG-JXUBOQSCSA-N 0.000 description 1
- VKCPHIOZDWUFSW-ONGXEEELSA-N Lys-Val-Gly Chemical compound OC(=O)CNC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN VKCPHIOZDWUFSW-ONGXEEELSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 238000000585 Mann–Whitney U test Methods 0.000 description 1
- ONGCSGVHCSAATF-CIUDSAMLSA-N Met-Ala-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O ONGCSGVHCSAATF-CIUDSAMLSA-N 0.000 description 1
- QEVRUYFHWJJUHZ-DCAQKATOSA-N Met-Ala-Leu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(C)C QEVRUYFHWJJUHZ-DCAQKATOSA-N 0.000 description 1
- DLAFCQWUMFMZSN-GUBZILKMSA-N Met-Arg-Ala Chemical compound CSCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C)C(O)=O)CCCN=C(N)N DLAFCQWUMFMZSN-GUBZILKMSA-N 0.000 description 1
- FRWZTWWOORIIBA-FXQIFTODSA-N Met-Asn-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N FRWZTWWOORIIBA-FXQIFTODSA-N 0.000 description 1
- MVBZBRKNZVJEKK-DTWKUNHWSA-N Met-Gly-Pro Chemical compound CSCC[C@@H](C(=O)NCC(=O)N1CCC[C@@H]1C(=O)O)N MVBZBRKNZVJEKK-DTWKUNHWSA-N 0.000 description 1
- QZPXMHVKPHJNTR-DCAQKATOSA-N Met-Leu-Asn Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O QZPXMHVKPHJNTR-DCAQKATOSA-N 0.000 description 1
- UROWNMBTQGGTHB-DCAQKATOSA-N Met-Leu-Asp Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O UROWNMBTQGGTHB-DCAQKATOSA-N 0.000 description 1
- WPTHAGXMYDRPFD-SRVKXCTJSA-N Met-Lys-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O WPTHAGXMYDRPFD-SRVKXCTJSA-N 0.000 description 1
- LCPUWQLULVXROY-RHYQMDGZSA-N Met-Lys-Thr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LCPUWQLULVXROY-RHYQMDGZSA-N 0.000 description 1
- AOFZWWDTTJLHOU-ULQDDVLXSA-N Met-Lys-Tyr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 AOFZWWDTTJLHOU-ULQDDVLXSA-N 0.000 description 1
- FBLBCGLSRXBANI-KKUMJFAQSA-N Met-Phe-Glu Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N FBLBCGLSRXBANI-KKUMJFAQSA-N 0.000 description 1
- LQTGGXSOMDSWTQ-UNQGMJICSA-N Met-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CCSC)N)O LQTGGXSOMDSWTQ-UNQGMJICSA-N 0.000 description 1
- YLDSJJOGQNEQJK-AVGNSLFASA-N Met-Pro-Leu Chemical compound CSCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O YLDSJJOGQNEQJK-AVGNSLFASA-N 0.000 description 1
- CULGJGUDIJATIP-STQMWFEESA-N Met-Tyr-Gly Chemical compound CSCC[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=C(O)C=C1 CULGJGUDIJATIP-STQMWFEESA-N 0.000 description 1
- KPVLLNDCBYXKNV-CYDGBPFRSA-N Met-Val-Ile Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O KPVLLNDCBYXKNV-CYDGBPFRSA-N 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 108700011259 MicroRNAs Proteins 0.000 description 1
- 101100014651 Mus musculus Gimap5 gene Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- WUGMRIBZSVSJNP-UHFFFAOYSA-N N-L-alanyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)C)C(O)=O)=CNC2=C1 WUGMRIBZSVSJNP-UHFFFAOYSA-N 0.000 description 1
- XZFYRXDAULDNFX-UHFFFAOYSA-N N-L-cysteinyl-L-phenylalanine Natural products SCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XZFYRXDAULDNFX-UHFFFAOYSA-N 0.000 description 1
- AJHCSUXXECOXOY-UHFFFAOYSA-N N-glycyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)CN)C(O)=O)=CNC2=C1 AJHCSUXXECOXOY-UHFFFAOYSA-N 0.000 description 1
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 1
- 108010057466 NF-kappa B Proteins 0.000 description 1
- 102000003945 NF-kappa B Human genes 0.000 description 1
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- BJEYSVHMGIJORT-NHCYSSNCSA-N Phe-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=CC=C1 BJEYSVHMGIJORT-NHCYSSNCSA-N 0.000 description 1
- HHOOEUSPFGPZFP-QWRGUYRKSA-N Phe-Asn-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O HHOOEUSPFGPZFP-QWRGUYRKSA-N 0.000 description 1
- VUYCNYVLKACHPA-KKUMJFAQSA-N Phe-Asp-His Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N VUYCNYVLKACHPA-KKUMJFAQSA-N 0.000 description 1
- KJJROSNFBRWPHS-JYJNAYRXSA-N Phe-Glu-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O KJJROSNFBRWPHS-JYJNAYRXSA-N 0.000 description 1
- LWPMGKSZPKFKJD-DZKIICNBSA-N Phe-Glu-Val Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O LWPMGKSZPKFKJD-DZKIICNBSA-N 0.000 description 1
- ZLGQEBCCANLYRA-RYUDHWBXSA-N Phe-Gly-Glu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O ZLGQEBCCANLYRA-RYUDHWBXSA-N 0.000 description 1
- XEXSSIBQYNKFBX-KBPBESRZSA-N Phe-Gly-His Chemical compound C([C@H](N)C(=O)NCC(=O)N[C@@H](CC=1N=CNC=1)C(O)=O)C1=CC=CC=C1 XEXSSIBQYNKFBX-KBPBESRZSA-N 0.000 description 1
- QPVFUAUFEBPIPT-CDMKHQONSA-N Phe-Gly-Thr Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(O)=O QPVFUAUFEBPIPT-CDMKHQONSA-N 0.000 description 1
- KRYSMKKRRRWOCZ-QEWYBTABSA-N Phe-Ile-Glu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O KRYSMKKRRRWOCZ-QEWYBTABSA-N 0.000 description 1
- YTILBRIUASDGBL-BZSNNMDCSA-N Phe-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 YTILBRIUASDGBL-BZSNNMDCSA-N 0.000 description 1
- MSHZERMPZKCODG-ACRUOGEOSA-N Phe-Leu-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 MSHZERMPZKCODG-ACRUOGEOSA-N 0.000 description 1
- MJAYDXWQQUOURZ-JYJNAYRXSA-N Phe-Lys-Gln Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O MJAYDXWQQUOURZ-JYJNAYRXSA-N 0.000 description 1
- KLXQWABNAWDRAY-ACRUOGEOSA-N Phe-Lys-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 KLXQWABNAWDRAY-ACRUOGEOSA-N 0.000 description 1
- AXIOGMQCDYVTNY-ACRUOGEOSA-N Phe-Phe-Leu Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 AXIOGMQCDYVTNY-ACRUOGEOSA-N 0.000 description 1
- DEZCWWXTRAKZKJ-UFYCRDLUSA-N Phe-Phe-Met Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(O)=O DEZCWWXTRAKZKJ-UFYCRDLUSA-N 0.000 description 1
- VDTYRPWRWRCROL-UFYCRDLUSA-N Phe-Val-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 VDTYRPWRWRCROL-UFYCRDLUSA-N 0.000 description 1
- VIIRRNQMMIHYHQ-XHSDSOJGSA-N Phe-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CC=CC=C2)N VIIRRNQMMIHYHQ-XHSDSOJGSA-N 0.000 description 1
- GAMLAXHLYGLQBJ-UFYCRDLUSA-N Phe-Val-Tyr Chemical compound N[C@H](C(=O)N[C@H](C(=O)N[C@H](C(=O)O)CC1=CC=C(C=C1)O)C(C)C)CC1=CC=CC=C1 GAMLAXHLYGLQBJ-UFYCRDLUSA-N 0.000 description 1
- APZNYJFGVAGFCF-JYJNAYRXSA-N Phe-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)C(O)=O APZNYJFGVAGFCF-JYJNAYRXSA-N 0.000 description 1
- ALJGSKMBIUEJOB-FXQIFTODSA-N Pro-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@@H]1CCCN1 ALJGSKMBIUEJOB-FXQIFTODSA-N 0.000 description 1
- ZSKJPKFTPQCPIH-RCWTZXSCSA-N Pro-Arg-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O ZSKJPKFTPQCPIH-RCWTZXSCSA-N 0.000 description 1
- LUGOKRWYNMDGTD-FXQIFTODSA-N Pro-Cys-Asn Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(=O)N)C(=O)O LUGOKRWYNMDGTD-FXQIFTODSA-N 0.000 description 1
- JFNPBBOGGNMSRX-CIUDSAMLSA-N Pro-Gln-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O JFNPBBOGGNMSRX-CIUDSAMLSA-N 0.000 description 1
- HJSCRFZVGXAGNG-SRVKXCTJSA-N Pro-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1 HJSCRFZVGXAGNG-SRVKXCTJSA-N 0.000 description 1
- LQZZPNDMYNZPFT-KKUMJFAQSA-N Pro-Gln-Phe Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LQZZPNDMYNZPFT-KKUMJFAQSA-N 0.000 description 1
- KIPIKSXPPLABPN-CIUDSAMLSA-N Pro-Glu-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 KIPIKSXPPLABPN-CIUDSAMLSA-N 0.000 description 1
- MGDFPGCFVJFITQ-CIUDSAMLSA-N Pro-Glu-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O MGDFPGCFVJFITQ-CIUDSAMLSA-N 0.000 description 1
- QNZLIVROMORQFH-BQBZGAKWSA-N Pro-Gly-Cys Chemical compound C1C[C@H](NC1)C(=O)NCC(=O)N[C@@H](CS)C(=O)O QNZLIVROMORQFH-BQBZGAKWSA-N 0.000 description 1
- AUQGUYPHJSMAKI-CYDGBPFRSA-N Pro-Ile-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]1CCCN1 AUQGUYPHJSMAKI-CYDGBPFRSA-N 0.000 description 1
- FMLRRBDLBJLJIK-DCAQKATOSA-N Pro-Leu-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1 FMLRRBDLBJLJIK-DCAQKATOSA-N 0.000 description 1
- BRJGUPWVFXKBQI-XUXIUFHCSA-N Pro-Leu-Ile Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BRJGUPWVFXKBQI-XUXIUFHCSA-N 0.000 description 1
- XYSXOCIWCPFOCG-IHRRRGAJSA-N Pro-Leu-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O XYSXOCIWCPFOCG-IHRRRGAJSA-N 0.000 description 1
- YAZNFQUKPUASKB-DCAQKATOSA-N Pro-Lys-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)O YAZNFQUKPUASKB-DCAQKATOSA-N 0.000 description 1
- HBBBLSVBQGZKOZ-GUBZILKMSA-N Pro-Met-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(O)=O HBBBLSVBQGZKOZ-GUBZILKMSA-N 0.000 description 1
- XZBYTHCRAVAXQQ-DCAQKATOSA-N Pro-Met-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(O)=O XZBYTHCRAVAXQQ-DCAQKATOSA-N 0.000 description 1
- JIWJRKNYLSHONY-KKUMJFAQSA-N Pro-Phe-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O JIWJRKNYLSHONY-KKUMJFAQSA-N 0.000 description 1
- GFHXZNVJIKMAGO-IHRRRGAJSA-N Pro-Phe-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O GFHXZNVJIKMAGO-IHRRRGAJSA-N 0.000 description 1
- SPLBRAKYXGOFSO-UNQGMJICSA-N Pro-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@@H]2CCCN2)O SPLBRAKYXGOFSO-UNQGMJICSA-N 0.000 description 1
- KDBHVPXBQADZKY-GUBZILKMSA-N Pro-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 KDBHVPXBQADZKY-GUBZILKMSA-N 0.000 description 1
- RCYUBVHMVUHEBM-RCWTZXSCSA-N Pro-Pro-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O RCYUBVHMVUHEBM-RCWTZXSCSA-N 0.000 description 1
- QKDIHFHGHBYTKB-IHRRRGAJSA-N Pro-Ser-Phe Chemical compound N([C@@H](CO)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C(=O)[C@@H]1CCCN1 QKDIHFHGHBYTKB-IHRRRGAJSA-N 0.000 description 1
- SNGZLPOXVRTNMB-LPEHRKFASA-N Pro-Ser-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N2CCC[C@@H]2C(=O)O SNGZLPOXVRTNMB-LPEHRKFASA-N 0.000 description 1
- MKGIILKDUGDRRO-FXQIFTODSA-N Pro-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 MKGIILKDUGDRRO-FXQIFTODSA-N 0.000 description 1
- PRKWBYCXBBSLSK-GUBZILKMSA-N Pro-Ser-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O PRKWBYCXBBSLSK-GUBZILKMSA-N 0.000 description 1
- YHUBAXGAAYULJY-ULQDDVLXSA-N Pro-Tyr-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(O)=O YHUBAXGAAYULJY-ULQDDVLXSA-N 0.000 description 1
- FIODMZKLZFLYQP-GUBZILKMSA-N Pro-Val-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O FIODMZKLZFLYQP-GUBZILKMSA-N 0.000 description 1
- FHJQROWZEJFZPO-SRVKXCTJSA-N Pro-Val-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 FHJQROWZEJFZPO-SRVKXCTJSA-N 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 238000002123 RNA extraction Methods 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- ZUGXSSFMTXKHJS-ZLUOBGJFSA-N Ser-Ala-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O ZUGXSSFMTXKHJS-ZLUOBGJFSA-N 0.000 description 1
- LVVBAKCGXXUHFO-ZLUOBGJFSA-N Ser-Ala-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(O)=O LVVBAKCGXXUHFO-ZLUOBGJFSA-N 0.000 description 1
- HBZBPFLJNDXRAY-FXQIFTODSA-N Ser-Ala-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O HBZBPFLJNDXRAY-FXQIFTODSA-N 0.000 description 1
- COAHUSQNSVFYBW-FXQIFTODSA-N Ser-Asn-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O COAHUSQNSVFYBW-FXQIFTODSA-N 0.000 description 1
- HEQPKICPPDOSIN-SRVKXCTJSA-N Ser-Asp-Tyr Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HEQPKICPPDOSIN-SRVKXCTJSA-N 0.000 description 1
- COLJZWUVZIXSSS-CIUDSAMLSA-N Ser-Cys-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CO)N COLJZWUVZIXSSS-CIUDSAMLSA-N 0.000 description 1
- OJPHFSOMBZKQKQ-GUBZILKMSA-N Ser-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CO OJPHFSOMBZKQKQ-GUBZILKMSA-N 0.000 description 1
- VDVYTKZBMFADQH-AVGNSLFASA-N Ser-Gln-Tyr Chemical compound OC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 VDVYTKZBMFADQH-AVGNSLFASA-N 0.000 description 1
- UOLGINIHBRIECN-FXQIFTODSA-N Ser-Glu-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O UOLGINIHBRIECN-FXQIFTODSA-N 0.000 description 1
- BRGQQXQKPUCUJQ-KBIXCLLPSA-N Ser-Glu-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BRGQQXQKPUCUJQ-KBIXCLLPSA-N 0.000 description 1
- GRSLLFZTTLBOQX-CIUDSAMLSA-N Ser-Glu-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CO)N GRSLLFZTTLBOQX-CIUDSAMLSA-N 0.000 description 1
- GZBKRJVCRMZAST-XKBZYTNZSA-N Ser-Glu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GZBKRJVCRMZAST-XKBZYTNZSA-N 0.000 description 1
- IXCHOHLPHNGFTJ-YUMQZZPRSA-N Ser-Gly-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N IXCHOHLPHNGFTJ-YUMQZZPRSA-N 0.000 description 1
- WSTIOCFMWXNOCX-YUMQZZPRSA-N Ser-Gly-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CO)N WSTIOCFMWXNOCX-YUMQZZPRSA-N 0.000 description 1
- KDGARKCAKHBEDB-NKWVEPMBSA-N Ser-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CO)N)C(=O)O KDGARKCAKHBEDB-NKWVEPMBSA-N 0.000 description 1
- VMLONWHIORGALA-SRVKXCTJSA-N Ser-Leu-Leu Chemical compound CC(C)C[C@@H](C([O-])=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]([NH3+])CO VMLONWHIORGALA-SRVKXCTJSA-N 0.000 description 1
- XXNYYSXNXCJYKX-DCAQKATOSA-N Ser-Leu-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(O)=O XXNYYSXNXCJYKX-DCAQKATOSA-N 0.000 description 1
- JLPMFVAIQHCBDC-CIUDSAMLSA-N Ser-Lys-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CO)N JLPMFVAIQHCBDC-CIUDSAMLSA-N 0.000 description 1
- PTWIYDNFWPXQSD-GARJFASQSA-N Ser-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)N)C(=O)O PTWIYDNFWPXQSD-GARJFASQSA-N 0.000 description 1
- FKYWFUYPVKLJLP-DCAQKATOSA-N Ser-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO FKYWFUYPVKLJLP-DCAQKATOSA-N 0.000 description 1
- FZXOPYUEQGDGMS-ACZMJKKPSA-N Ser-Ser-Gln Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O FZXOPYUEQGDGMS-ACZMJKKPSA-N 0.000 description 1
- GYDFRTRSSXOZCR-ACZMJKKPSA-N Ser-Ser-Glu Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O GYDFRTRSSXOZCR-ACZMJKKPSA-N 0.000 description 1
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 1
- CUXJENOFJXOSOZ-BIIVOSGPSA-N Ser-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CO)N)C(=O)O CUXJENOFJXOSOZ-BIIVOSGPSA-N 0.000 description 1
- OLKICIBQRVSQMA-SRVKXCTJSA-N Ser-Ser-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O OLKICIBQRVSQMA-SRVKXCTJSA-N 0.000 description 1
- SNXUIBACCONSOH-BWBBJGPYSA-N Ser-Thr-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CO)C(O)=O SNXUIBACCONSOH-BWBBJGPYSA-N 0.000 description 1
- HAYADTTXNZFUDM-IHRRRGAJSA-N Ser-Tyr-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(O)=O HAYADTTXNZFUDM-IHRRRGAJSA-N 0.000 description 1
- PCMZJFMUYWIERL-ZKWXMUAHSA-N Ser-Val-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O PCMZJFMUYWIERL-ZKWXMUAHSA-N 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- GFDUZZACIWNMPE-KZVJFYERSA-N Thr-Ala-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(O)=O GFDUZZACIWNMPE-KZVJFYERSA-N 0.000 description 1
- DWYAUVCQDTZIJI-VZFHVOOUSA-N Thr-Ala-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O DWYAUVCQDTZIJI-VZFHVOOUSA-N 0.000 description 1
- PKXHGEXFMIZSER-QTKMDUPCSA-N Thr-Arg-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N)O PKXHGEXFMIZSER-QTKMDUPCSA-N 0.000 description 1
- WFUAUEQXPVNAEF-ZJDVBMNYSA-N Thr-Arg-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)O)C(O)=O)CCCN=C(N)N WFUAUEQXPVNAEF-ZJDVBMNYSA-N 0.000 description 1
- JXKMXEBNZCKSDY-JIOCBJNQSA-N Thr-Asp-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N)O JXKMXEBNZCKSDY-JIOCBJNQSA-N 0.000 description 1
- XDARBNMYXKUFOJ-GSSVUCPTSA-N Thr-Asp-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XDARBNMYXKUFOJ-GSSVUCPTSA-N 0.000 description 1
- LOHBIDZYHQQTDM-IXOXFDKPSA-N Thr-Cys-Phe Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LOHBIDZYHQQTDM-IXOXFDKPSA-N 0.000 description 1
- LIXBDERDAGNVAV-XKBZYTNZSA-N Thr-Gln-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O LIXBDERDAGNVAV-XKBZYTNZSA-N 0.000 description 1
- UDQBCBUXAQIZAK-GLLZPBPUSA-N Thr-Glu-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O UDQBCBUXAQIZAK-GLLZPBPUSA-N 0.000 description 1
- KBBRNEDOYWMIJP-KYNKHSRBSA-N Thr-Gly-Thr Chemical compound C[C@H]([C@@H](C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O KBBRNEDOYWMIJP-KYNKHSRBSA-N 0.000 description 1
- ODXKUIGEPAGKKV-KATARQTJSA-N Thr-Leu-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(=O)O)N)O ODXKUIGEPAGKKV-KATARQTJSA-N 0.000 description 1
- MECLEFZMPPOEAC-VOAKCMCISA-N Thr-Leu-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)O)N)O MECLEFZMPPOEAC-VOAKCMCISA-N 0.000 description 1
- SPVHQURZJCUDQC-VOAKCMCISA-N Thr-Lys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O SPVHQURZJCUDQC-VOAKCMCISA-N 0.000 description 1
- LHNNQVXITHUCAB-QTKMDUPCSA-N Thr-Met-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N)O LHNNQVXITHUCAB-QTKMDUPCSA-N 0.000 description 1
- WVVOFCVMHAXGLE-LFSVMHDDSA-N Thr-Phe-Ala Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(O)=O WVVOFCVMHAXGLE-LFSVMHDDSA-N 0.000 description 1
- VGYVVSQFSSKZRJ-OEAJRASXSA-N Thr-Phe-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)[C@H](O)C)CC1=CC=CC=C1 VGYVVSQFSSKZRJ-OEAJRASXSA-N 0.000 description 1
- MXDOAJQRJBMGMO-FJXKBIBVSA-N Thr-Pro-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O MXDOAJQRJBMGMO-FJXKBIBVSA-N 0.000 description 1
- QJIODPFLAASXJC-JHYOHUSXSA-N Thr-Thr-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O QJIODPFLAASXJC-JHYOHUSXSA-N 0.000 description 1
- DXDMNBJJEXYMLA-UBHSHLNASA-N Trp-Asn-Asp Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O)=CNC2=C1 DXDMNBJJEXYMLA-UBHSHLNASA-N 0.000 description 1
- MWHOLXNKRKRQQH-XIRDDKMYSA-N Trp-Asp-His Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC3=CN=CN3)C(=O)O)N MWHOLXNKRKRQQH-XIRDDKMYSA-N 0.000 description 1
- VTHNLRXALGUDBS-BPUTZDHNSA-N Trp-Gln-Glu Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N VTHNLRXALGUDBS-BPUTZDHNSA-N 0.000 description 1
- UDCHKDYNMRJYMI-QEJZJMRPSA-N Trp-Glu-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O UDCHKDYNMRJYMI-QEJZJMRPSA-N 0.000 description 1
- WSGPBCAGEGHKQJ-BBRMVZONSA-N Trp-Gly-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC1=CNC2=CC=CC=C21)N WSGPBCAGEGHKQJ-BBRMVZONSA-N 0.000 description 1
- GQHAIUPYZPTADF-FDARSICLSA-N Trp-Ile-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 GQHAIUPYZPTADF-FDARSICLSA-N 0.000 description 1
- KPEVFMGKBCMTJF-SZMVWBNQSA-N Trp-Val-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N KPEVFMGKBCMTJF-SZMVWBNQSA-N 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- VCXWRWYFJLXITF-AUTRQRHGSA-N Tyr-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 VCXWRWYFJLXITF-AUTRQRHGSA-N 0.000 description 1
- DLZKEQQWXODGGZ-KWQFWETISA-N Tyr-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 DLZKEQQWXODGGZ-KWQFWETISA-N 0.000 description 1
- TVOGEPLDNYTAHD-CQDKDKBSSA-N Tyr-Ala-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 TVOGEPLDNYTAHD-CQDKDKBSSA-N 0.000 description 1
- HTHCZRWCFXMENJ-KKUMJFAQSA-N Tyr-Arg-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O HTHCZRWCFXMENJ-KKUMJFAQSA-N 0.000 description 1
- DYEGCOJHFNJBKB-UFYCRDLUSA-N Tyr-Arg-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 DYEGCOJHFNJBKB-UFYCRDLUSA-N 0.000 description 1
- DANHCMVVXDXOHN-SRVKXCTJSA-N Tyr-Asp-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 DANHCMVVXDXOHN-SRVKXCTJSA-N 0.000 description 1
- TZXFLDNBYYGLKA-BZSNNMDCSA-N Tyr-Asp-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 TZXFLDNBYYGLKA-BZSNNMDCSA-N 0.000 description 1
- XBWKCYFGRXKWGO-SRVKXCTJSA-N Tyr-Cys-Asn Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O XBWKCYFGRXKWGO-SRVKXCTJSA-N 0.000 description 1
- QOIKZODVIPOPDD-AVGNSLFASA-N Tyr-Cys-Gln Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(O)=O QOIKZODVIPOPDD-AVGNSLFASA-N 0.000 description 1
- HVHJYXDXRIWELT-RYUDHWBXSA-N Tyr-Glu-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O HVHJYXDXRIWELT-RYUDHWBXSA-N 0.000 description 1
- ILTXFANLDMJWPR-SIUGBPQLSA-N Tyr-Ile-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N ILTXFANLDMJWPR-SIUGBPQLSA-N 0.000 description 1
- ZOBLBMGJKVJVEV-BZSNNMDCSA-N Tyr-Lys-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)O)N)O ZOBLBMGJKVJVEV-BZSNNMDCSA-N 0.000 description 1
- AVFGBGGRZOKSFS-KJEVXHAQSA-N Tyr-Met-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O AVFGBGGRZOKSFS-KJEVXHAQSA-N 0.000 description 1
- KZOZXAYPVKKDIO-UFYCRDLUSA-N Tyr-Met-Tyr Chemical compound C([C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 KZOZXAYPVKKDIO-UFYCRDLUSA-N 0.000 description 1
- WTTRJMAZPDHPGS-KKXDTOCCSA-N Tyr-Phe-Ala Chemical compound C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O WTTRJMAZPDHPGS-KKXDTOCCSA-N 0.000 description 1
- RGYCVIZZTUBSSG-JYJNAYRXSA-N Tyr-Pro-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O RGYCVIZZTUBSSG-JYJNAYRXSA-N 0.000 description 1
- ZPFLBLFITJCBTP-QWRGUYRKSA-N Tyr-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O ZPFLBLFITJCBTP-QWRGUYRKSA-N 0.000 description 1
- NHOVZGFNTGMYMI-KKUMJFAQSA-N Tyr-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NHOVZGFNTGMYMI-KKUMJFAQSA-N 0.000 description 1
- PAPWZOJOLKZEFR-AVGNSLFASA-N Val-Arg-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)O)N PAPWZOJOLKZEFR-AVGNSLFASA-N 0.000 description 1
- VMRFIKXKOFNMHW-GUBZILKMSA-N Val-Arg-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(=O)O)N VMRFIKXKOFNMHW-GUBZILKMSA-N 0.000 description 1
- DNOOLPROHJWCSQ-RCWTZXSCSA-N Val-Arg-Thr Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O DNOOLPROHJWCSQ-RCWTZXSCSA-N 0.000 description 1
- UDLYXGYWTVOIKU-QXEWZRGKSA-N Val-Asn-Arg Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N UDLYXGYWTVOIKU-QXEWZRGKSA-N 0.000 description 1
- XEYUMGGWQCIWAR-XVKPBYJWSA-N Val-Gln-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)NCC(=O)O)N XEYUMGGWQCIWAR-XVKPBYJWSA-N 0.000 description 1
- IWZYXFRGWKEKBJ-GVXVVHGQSA-N Val-Gln-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N IWZYXFRGWKEKBJ-GVXVVHGQSA-N 0.000 description 1
- PYXQBKJPHNCTNW-CYDGBPFRSA-N Val-Ile-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](C(C)C)N PYXQBKJPHNCTNW-CYDGBPFRSA-N 0.000 description 1
- OVBMCNDKCWAXMZ-NAKRPEOUSA-N Val-Ile-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](C(C)C)N OVBMCNDKCWAXMZ-NAKRPEOUSA-N 0.000 description 1
- APQIVBCUIUDSMB-OSUNSFLBSA-N Val-Ile-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](C(C)C)N APQIVBCUIUDSMB-OSUNSFLBSA-N 0.000 description 1
- SJLVYVZBFDTRCG-DCAQKATOSA-N Val-Lys-Cys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)O)N SJLVYVZBFDTRCG-DCAQKATOSA-N 0.000 description 1
- LJSZPMSUYKKKCP-UBHSHLNASA-N Val-Phe-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C)C(O)=O)CC1=CC=CC=C1 LJSZPMSUYKKKCP-UBHSHLNASA-N 0.000 description 1
- NHXZRXLFOBFMDM-AVGNSLFASA-N Val-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C NHXZRXLFOBFMDM-AVGNSLFASA-N 0.000 description 1
- DEGUERSKQBRZMZ-FXQIFTODSA-N Val-Ser-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O DEGUERSKQBRZMZ-FXQIFTODSA-N 0.000 description 1
- GBIUHAYJGWVNLN-AEJSXWLSSA-N Val-Ser-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N GBIUHAYJGWVNLN-AEJSXWLSSA-N 0.000 description 1
- GBIUHAYJGWVNLN-UHFFFAOYSA-N Val-Ser-Pro Natural products CC(C)C(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O GBIUHAYJGWVNLN-UHFFFAOYSA-N 0.000 description 1
- HWNYVQMOLCYHEA-IHRRRGAJSA-N Val-Ser-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N HWNYVQMOLCYHEA-IHRRRGAJSA-N 0.000 description 1
- 101710185494 Zinc finger protein Proteins 0.000 description 1
- 102100023597 Zinc finger protein 816 Human genes 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 101150092805 actc1 gene Proteins 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 108010045350 alanyl-tyrosyl-alanine Proteins 0.000 description 1
- 108010041407 alanylaspartic acid Proteins 0.000 description 1
- 108010011559 alanylphenylalanine Proteins 0.000 description 1
- 108010070783 alanyltyrosine Proteins 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 108010008355 arginyl-glutamine Proteins 0.000 description 1
- 108010001271 arginyl-glutamyl-arginine Proteins 0.000 description 1
- 108010059459 arginyl-threonyl-phenylalanine Proteins 0.000 description 1
- 108010060035 arginylproline Proteins 0.000 description 1
- 108010077245 asparaginyl-proline Proteins 0.000 description 1
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 1
- 108010038633 aspartylglutamate Proteins 0.000 description 1
- 108010047857 aspartylglycine Proteins 0.000 description 1
- 108010092854 aspartyllysine Proteins 0.000 description 1
- 238000002820 assay format Methods 0.000 description 1
- 210000001130 astrocyte Anatomy 0.000 description 1
- 238000012550 audit Methods 0.000 description 1
- 230000003305 autocrine Effects 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 208000026900 bile duct neoplasm Diseases 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 230000036952 cancer formation Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 101150071738 chb gene Proteins 0.000 description 1
- 238000000546 chi-square test Methods 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000003759 clinical diagnosis Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 230000000875 corresponding effect Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 108010016616 cysteinylglycine Proteins 0.000 description 1
- 108010069495 cysteinyltyrosine Proteins 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 231100000517 death Toxicity 0.000 description 1
- 238000000432 density-gradient centrifugation Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 230000009274 differential gene expression Effects 0.000 description 1
- 230000001079 digestive effect Effects 0.000 description 1
- QONQRTHLHBTMGP-UHFFFAOYSA-N digitoxigenin Natural products CC12CCC(C3(CCC(O)CC3CC3)C)C3C11OC1CC2C1=CC(=O)OC1 QONQRTHLHBTMGP-UHFFFAOYSA-N 0.000 description 1
- SHIBSTMRCDJXLN-KCZCNTNESA-N digoxigenin Chemical compound C1([C@@H]2[C@@]3([C@@](CC2)(O)[C@H]2[C@@H]([C@@]4(C)CC[C@H](O)C[C@H]4CC2)C[C@H]3O)C)=CC(=O)OC1 SHIBSTMRCDJXLN-KCZCNTNESA-N 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000010195 expression analysis Methods 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 238000011223 gene expression profiling Methods 0.000 description 1
- 230000004547 gene signature Effects 0.000 description 1
- 102000054767 gene variant Human genes 0.000 description 1
- 108010013768 glutamyl-aspartyl-proline Proteins 0.000 description 1
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 1
- 108010049041 glutamylalanine Proteins 0.000 description 1
- 108010079547 glutamylmethionine Proteins 0.000 description 1
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 1
- HPAIKDPJURGQLN-UHFFFAOYSA-N glycyl-L-histidyl-L-phenylalanine Natural products C=1C=CC=CC=1CC(C(O)=O)NC(=O)C(NC(=O)CN)CC1=CN=CN1 HPAIKDPJURGQLN-UHFFFAOYSA-N 0.000 description 1
- 108010072405 glycyl-aspartyl-glycine Proteins 0.000 description 1
- 108010082286 glycyl-seryl-alanine Proteins 0.000 description 1
- 108010020688 glycylhistidine Proteins 0.000 description 1
- 108010087823 glycyltyrosine Proteins 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 208000006359 hepatoblastoma Diseases 0.000 description 1
- 108010045383 histidyl-glycyl-glutamic acid Proteins 0.000 description 1
- 108010036413 histidylglycine Proteins 0.000 description 1
- 108010028295 histidylhistidine Proteins 0.000 description 1
- 108010092114 histidylphenylalanine Proteins 0.000 description 1
- 108010018006 histidylserine Proteins 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000003100 immobilizing effect Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 238000002649 immunization Methods 0.000 description 1
- 230000003053 immunization Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 1
- 108010000761 leucylarginine Proteins 0.000 description 1
- 108010091871 leucylmethionine Proteins 0.000 description 1
- 108010012058 leucyltyrosine Proteins 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 108010016686 methionyl-alanyl-serine Proteins 0.000 description 1
- 239000002679 microRNA Substances 0.000 description 1
- 239000003226 mitogen Substances 0.000 description 1
- 238000002966 oligonucleotide array Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000012753 partial hepatectomy Methods 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 239000008194 pharmaceutical composition Substances 0.000 description 1
- 108010073101 phenylalanylleucine Proteins 0.000 description 1
- 108010051242 phenylalanylserine Proteins 0.000 description 1
- 108010083476 phenylalanyltryptophan Proteins 0.000 description 1
- 239000002953 phosphate buffered saline Substances 0.000 description 1
- 108010031719 prolyl-serine Proteins 0.000 description 1
- 108010053725 prolylvaline Proteins 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000012502 risk assessment Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 230000000405 serological effect Effects 0.000 description 1
- 108010048818 seryl-histidine Proteins 0.000 description 1
- 108010071207 serylmethionine Proteins 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- ZEYOIOAKZLALAP-UHFFFAOYSA-M sodium amidotrizoate Chemical compound [Na+].CC(=O)NC1=C(I)C(NC(C)=O)=C(I)C(C([O-])=O)=C1I ZEYOIOAKZLALAP-UHFFFAOYSA-M 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 108010072986 threonyl-seryl-lysine Proteins 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 108010044292 tryptophyltyrosine Proteins 0.000 description 1
- 108010005834 tyrosyl-alanyl-glycine Proteins 0.000 description 1
- 108010020532 tyrosyl-proline Proteins 0.000 description 1
- 108010009962 valyltyrosine Proteins 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 201000001862 viral hepatitis Diseases 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 108010027345 wheylin-1 peptide Proteins 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57438—Specifically defined cancers of liver, pancreas or kidney
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/158—Expression markers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/50—Determining the risk of developing a disease
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Pathology (AREA)
- Analytical Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Urology & Nephrology (AREA)
- Oncology (AREA)
- General Health & Medical Sciences (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Hematology (AREA)
- Hospice & Palliative Care (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Biochemistry (AREA)
- Biomedical Technology (AREA)
- Physics & Mathematics (AREA)
- Genetics & Genomics (AREA)
- Gastroenterology & Hepatology (AREA)
- General Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Cell Biology (AREA)
- Biophysics (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261704425P | 2012-09-21 | 2012-09-21 | |
| US61/704,425 | 2012-09-21 | ||
| PCT/SG2013/000414 WO2014046623A1 (en) | 2012-09-21 | 2013-09-23 | Methods of diagnosing liver cancer in a subject and a kit for diagnosing liver cancer |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150058465A true KR20150058465A (ko) | 2015-05-28 |
Family
ID=50341782
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157010267A Withdrawn KR20150058465A (ko) | 2012-09-21 | 2013-09-23 | 피험체에서 간암을 진단하는 방법 및 간암을 진단하기 위한 키트 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20150232943A1 (enExample) |
| EP (1) | EP2898099A4 (enExample) |
| JP (1) | JP2015530094A (enExample) |
| KR (1) | KR20150058465A (enExample) |
| CN (1) | CN104812914B (enExample) |
| SG (1) | SG11201502089QA (enExample) |
| WO (1) | WO2014046623A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018208091A2 (ko) | 2017-05-10 | 2018-11-15 | 서울대학교산학협력단 | 간암 고위험군의 간암 발병 모니터링 또는 진단용 바이오마커 및 그 용도 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101843173B1 (ko) * | 2014-10-24 | 2018-03-29 | 씨비에스바이오사이언스 주식회사 | 혈관 내피 세포 성장인자 수용체 억제제에 대한 감수성 예측 방법 |
| CN108048422A (zh) * | 2017-11-30 | 2018-05-18 | 天津市湖滨盘古基因科学发展有限公司 | 一种gtp酶,imap家族成员7突变蛋白及其应用 |
| CN110241198A (zh) * | 2019-05-30 | 2019-09-17 | 成都吉诺迈尔生物科技有限公司 | 一种表征hHRD同源重组缺陷的基因组重组指纹及其鉴定方法 |
| CN111610262A (zh) * | 2020-05-19 | 2020-09-01 | 上海鹿明生物科技有限公司 | 一种用于肝胆疾病诊断的代谢标志物 |
| WO2025111814A1 (zh) * | 2023-11-28 | 2025-06-05 | 何牮 | 一种b细胞的生物标志物组及其应用 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2003214604A1 (en) * | 2002-03-21 | 2003-10-08 | Hadasit Medical Research Services And Development Ltd. | Peripheral blood cell markers useful for diagnosing multiple sclerosis and methods and kits utilizing same |
| WO2005043163A2 (en) * | 2003-11-04 | 2005-05-12 | Roche Diagnostics Gmbh | Method for distinguishing who classified aml subtypes |
| EP1830289A1 (en) * | 2005-11-30 | 2007-09-05 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Methods for hepatocellular carninoma classification and prognosis |
| WO2008138578A2 (en) * | 2007-05-11 | 2008-11-20 | Medical Prognosis Institute | Methods, kits, and devices for identifying biomarkers of treatment response and use thereof to predict treatment efficacy |
| EP2687609B1 (en) * | 2008-11-10 | 2017-01-04 | The United States of America, as represented by The Secretary, Department of Health and Human Services | Method for treating solid tumor |
-
2013
- 2013-09-23 US US14/429,317 patent/US20150232943A1/en not_active Abandoned
- 2013-09-23 WO PCT/SG2013/000414 patent/WO2014046623A1/en not_active Ceased
- 2013-09-23 KR KR1020157010267A patent/KR20150058465A/ko not_active Withdrawn
- 2013-09-23 SG SG11201502089QA patent/SG11201502089QA/en unknown
- 2013-09-23 JP JP2015533022A patent/JP2015530094A/ja active Pending
- 2013-09-23 CN CN201380060446.7A patent/CN104812914B/zh active Active
- 2013-09-23 EP EP13838539.8A patent/EP2898099A4/en not_active Withdrawn
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018208091A2 (ko) | 2017-05-10 | 2018-11-15 | 서울대학교산학협력단 | 간암 고위험군의 간암 발병 모니터링 또는 진단용 바이오마커 및 그 용도 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2014046623A1 (en) | 2014-03-27 |
| JP2015530094A (ja) | 2015-10-15 |
| EP2898099A1 (en) | 2015-07-29 |
| CN104812914B (zh) | 2020-07-24 |
| US20150232943A1 (en) | 2015-08-20 |
| SG11201502089QA (en) | 2015-06-29 |
| CN104812914A (zh) | 2015-07-29 |
| EP2898099A4 (en) | 2016-05-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Jia et al. | Diagnosis accuracy of serum glypican-3 in patients with hepatocellular carcinoma: a systematic review with meta-analysis | |
| Qiao et al. | Serum gp73 is also a biomarker for diagnosing cirrhosis in population with chronic HBV infection | |
| US12391992B2 (en) | Kit for early screening of liver cell cancer and preparation method and use thereof | |
| CN111172279B (zh) | 外周血甲基化基因及idh1联合检测诊断肺癌模型 | |
| KR20150058465A (ko) | 피험체에서 간암을 진단하는 방법 및 간암을 진단하기 위한 키트 | |
| US10859573B2 (en) | Nourin molecular biomarkers diagnose angina patients with negative troponin | |
| JP6250600B2 (ja) | 非小細胞肺癌バイオマーカーを検出する方法 | |
| JP2012526544A5 (enExample) | ||
| TWI408370B (zh) | 胰臟癌之血清生物檢測標誌及其應用 | |
| JP2002515591A (ja) | 結腸癌を診断し、モニターし、そして病期決定する新規方法 | |
| CN114107509B (zh) | 一种肝癌预后标记物及其应用 | |
| JP6876301B2 (ja) | 肝細胞がん患者の再発リスク予測を補助する方法、装置、コンピュータプログラム製品及びキット | |
| JP2002515263A (ja) | 前立腺がんを診断し、モニターし、そして病期決定する新規な方法 | |
| Xie et al. | Clinical significance of MiR-27a expression in serum exosomes in patients with heart failure | |
| JP2008167714A (ja) | 川崎病発症のリスク因子 | |
| CN113631723B (zh) | 结核病标志物在结核病诊断和疗效评估中的应用 | |
| El-Sharkawy et al. | Circulating tumor cells in breast cancer: a step toward precision medicine for real-time monitoring of metastasis | |
| CN103487580A (zh) | Dkk1作为诊断标志物的用途 | |
| CN117957445A (zh) | 慢性肝疾病中的肝癌发生的诊断标志物 | |
| WO2007053659A2 (en) | Method of screening for hepatocellular carcinoma | |
| US20130225437A1 (en) | Biomarkers of cancer | |
| CN102010903A (zh) | Il-23在肝癌早期病变诊断中的应用 | |
| Xin et al. | Fibrinogen Like Protein 1 as a Potential Biomarker for Hepatitis B Virus Related Hepatocellular Carcinoma | |
| JP2025142656A (ja) | アベマシクリブまたはその薬学的に許容可能な塩による肝障害発現リスクの検査方法 | |
| Kwon et al. | Studying the Impact of Golgi Protein 73 Serving as a Candidate Biomarker in Early Diagnosis for Hepatocellular Carcinoma among Saudi Patients |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20150421 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |